Quantitative Elasticity Mapping of Submicron Silica Hollow Particles by PeakForce QNM AFM Mode
Abstract
1. Introduction
- Since the force loading and detection in AFM is based on cantilever bending, and the cantilever is inclined relative to the sample in the cantilever holder, a straight vertical indentation is impossible for the entire force range. Therefore, special care of the force range and cantilever stiffness is required for quantitative measurements of mechanical properties in AFM experiments. Moreover, because of a non-linear relationship between the detected signal and the actual deflection of the AFM cantilever, optical lever sensitivity calibration of the AFM setup should be carried out in the whole applied force range [65].
- If the surface normal of the sample is not aligned with the vertical Z-axis of the AFM scanner, the AFM tip contacts the surface at an angle not anticipated by most contact mechanics models affecting the accuracy of the mechanical measurements [66,67]. Such samples as individual particles or capsules immobilized on a solid substrate present significant variations of the local slope (up to ) resulting in great changes in the tip–sample contact area. Moreover, non-vertical contact between the AFM tip and the sample produces lateral forces acting on the cantilever in addition to the normal force. While the normal stiffness of the tip–surface contact is usually described with the contact mechanics models, the lateral forces strongly depend on the type of contact (e.g., free sliding, pinning, friction) that occurs between the tip and the sample, which is difficult to predict. These lateral forces lead to an additional contribution to the normal deflection of the cantilever which can result in both underestimation and overestimation of the measured stiffness depending on the surface slope and AFM tip geometry [66,67]. To circumvent this issue, many studies choose regions of interest from relatively flat sections of the sample surface.
- Another important factor is the mechanical stress related to the AFM probe apex radius. It was demonstrated that the use of sharp commercial AFM probes with a tip radius of about 20 nm led to a non-linear stress–strain response in relatively soft polymeric samples (e.g., polyurethanes, polystyrene), resulting in overestimation of the Young’s modulus [60,61]. This effect disappears when dull probes with a radius of several hundreds of nm were used. Moreover, the measured moduli were independent of the indentation depth and close to those evaluated by nanoindentation and DMA measurements when dull AFM probes were used. Similar results were reported for AFM measurements of elasticity for rigid materials (e.g., fused silica, HOPG, Si, Au) [63]. The measurements using a sharp tip with 40 nm radius resulted in a large deviation (up to 122%) of the Young’s moduli from their nominal values, whereas this deviation was only up to 13% when a blunt tip with 200 nm radius was used. The authors suggested that plastic deformations were the main reason for this difference. Regarding the mechanical property measurements of spherical shells, it was demonstrated that the use of sharp commercial AFM probes could result in puncturing of the thin silica shells at relatively low indentations, seriously limiting the applied force range [46]. Moreover, finite-element analysis of elastic shell indentation with an AFM tip of different geometry reveals an effect of the tip radius on the shape of the force–distance curves [68,69].
- Nowadays, various contact mechanics models have been developed to evaluate the elastic modulus from force–distance measurements, e.g., the Hertz [70], Sneddon [71], Reissner [72], Johnson–Kendall–Roberts (JKR) [73], and Derjaguin–Muller–Toporov (DMT) [74] models. These models’ validity is strongly dependent on the AFM probe, sample size and geometry, material stiffness, and adhesion between the AFM probe and sample. Correct description of the contact mechanics is obviously crucial for accurate evaluation of the elastic modulus.
- One more factor affecting the accuracy of the mechanical properties measurements of hollow and solid particles is the effect of the rigid substrate (also known as the stress-interaction effect [75]) because during indentation experiments the upper part of the particles is deformed by the AFM probe, whereas the bottom part is simultaneously deformed by the substrate [76,77]. Interestingly, if the substrate effect is not accounted for, the indentation of particles and capsules against a substrate leads to an underestimation of the material elastic modulus, in contrast to an overestimation of the mechanical properties during indentation of thin films [78].
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Silica Hollow Particles
2.2.1. Synthesis of PEOS
2.2.2. Synthesis of PEOS-PEG
2.2.3. Conversion of PEOS-PEG to Silica Hollow Particles
2.3. Characterization Methods
2.4. PeakForce Tapping QNM
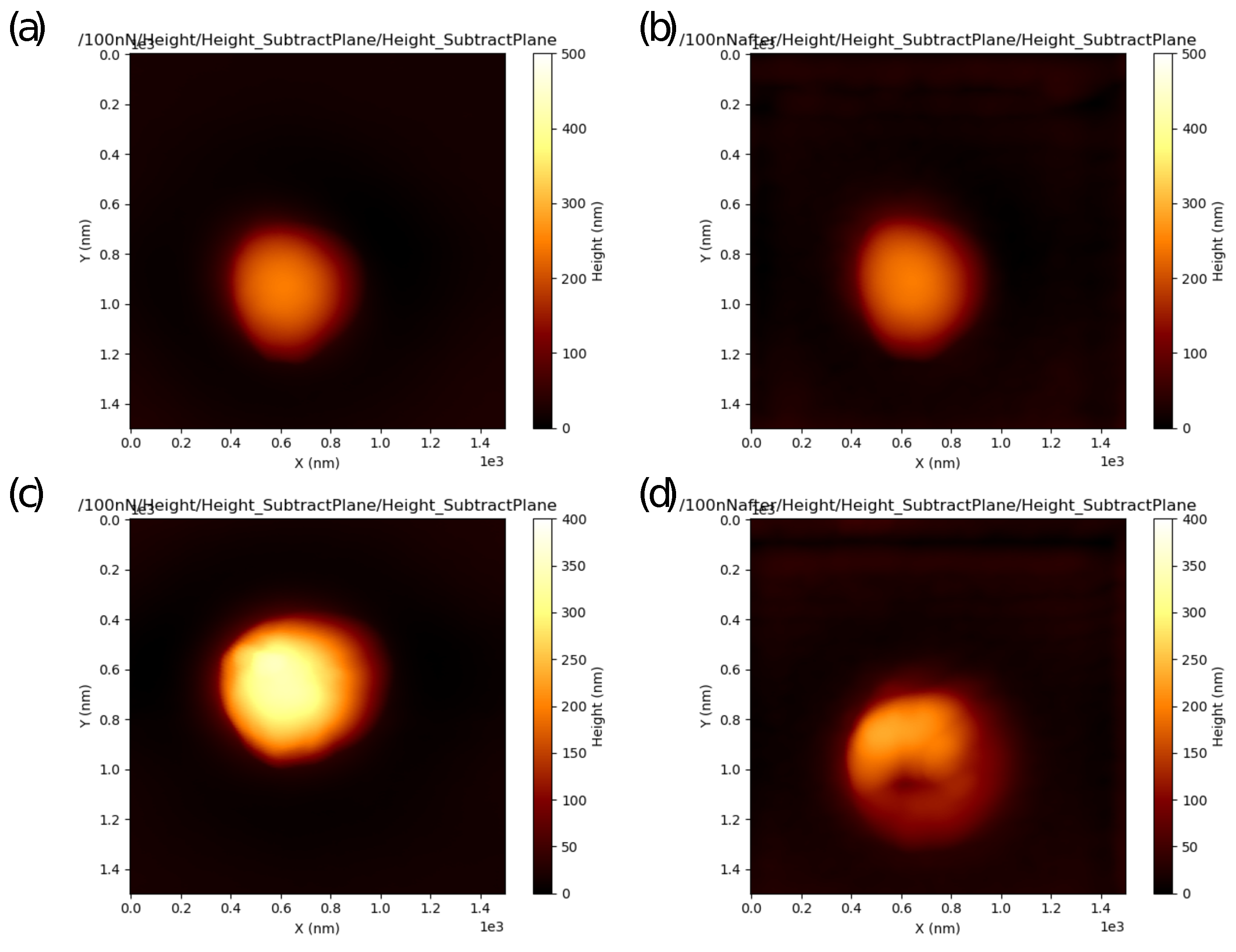
2.4.1. Sample Preparation for AFM Measurements
2.4.2. AFM Probes
2.4.3. PeakForce QNM Calibration
2.4.4. Automated AFM PeakForce Curves Processing
- The extraction of the raw data and division of the data array to 65,536 pairs (approach and retract) of the force–displacement curves for the AFM image with a resolution of pixels using the Sync. Distance value evaluated at calibration.
- The recalculation of the force–displacement curves as the AFM cantilever deflection (d in nm instead of in V in the raw data) vs. the Z-piezo position (Z in nm instead of time in time steps) using the calibration results.
- The baseline correction. Force–displacement curve measurements may be affected by optical interference, thermal drift, electrostatic long-range forces, hydrodynamic force due to viscous friction of the cantilever, etc. As a consequence, a non-zero cantilever deflection is measured in the pre-contact region between the probe and the sample surface. Thus, a proper baseline correction should be performed before the data analysis. In our program, an option allows the user to select a number of points defining the baseline. Afterwards, a linear baseline was calculated using linear regression in the user-selected non-contact range for each force–displacement curve and subtracted from the initial data.
- Correction for the cantilever bending to convert the Z-piezo position (Z in nm) to distance ( in nm) by subtraction of the cantilever deflection d.
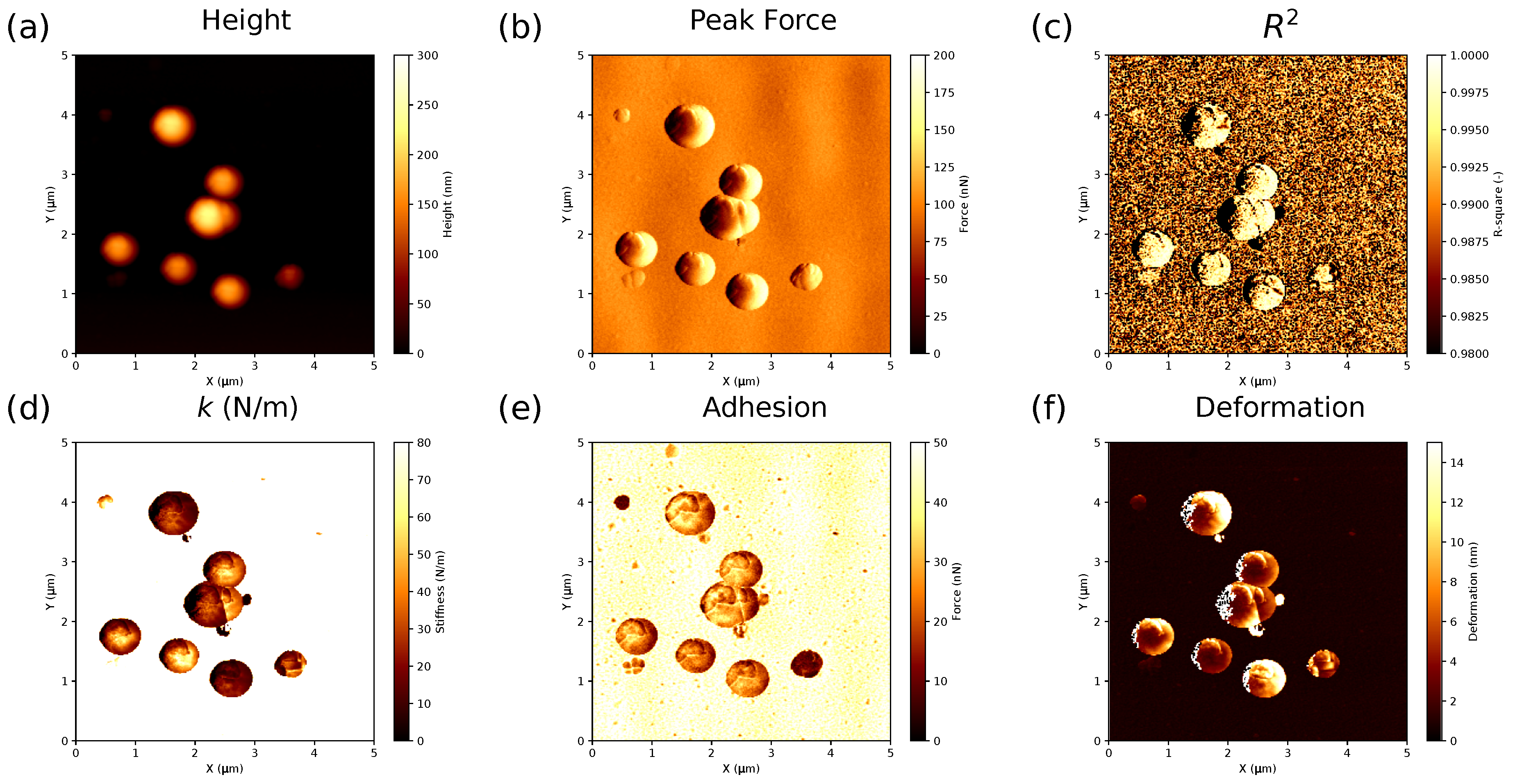
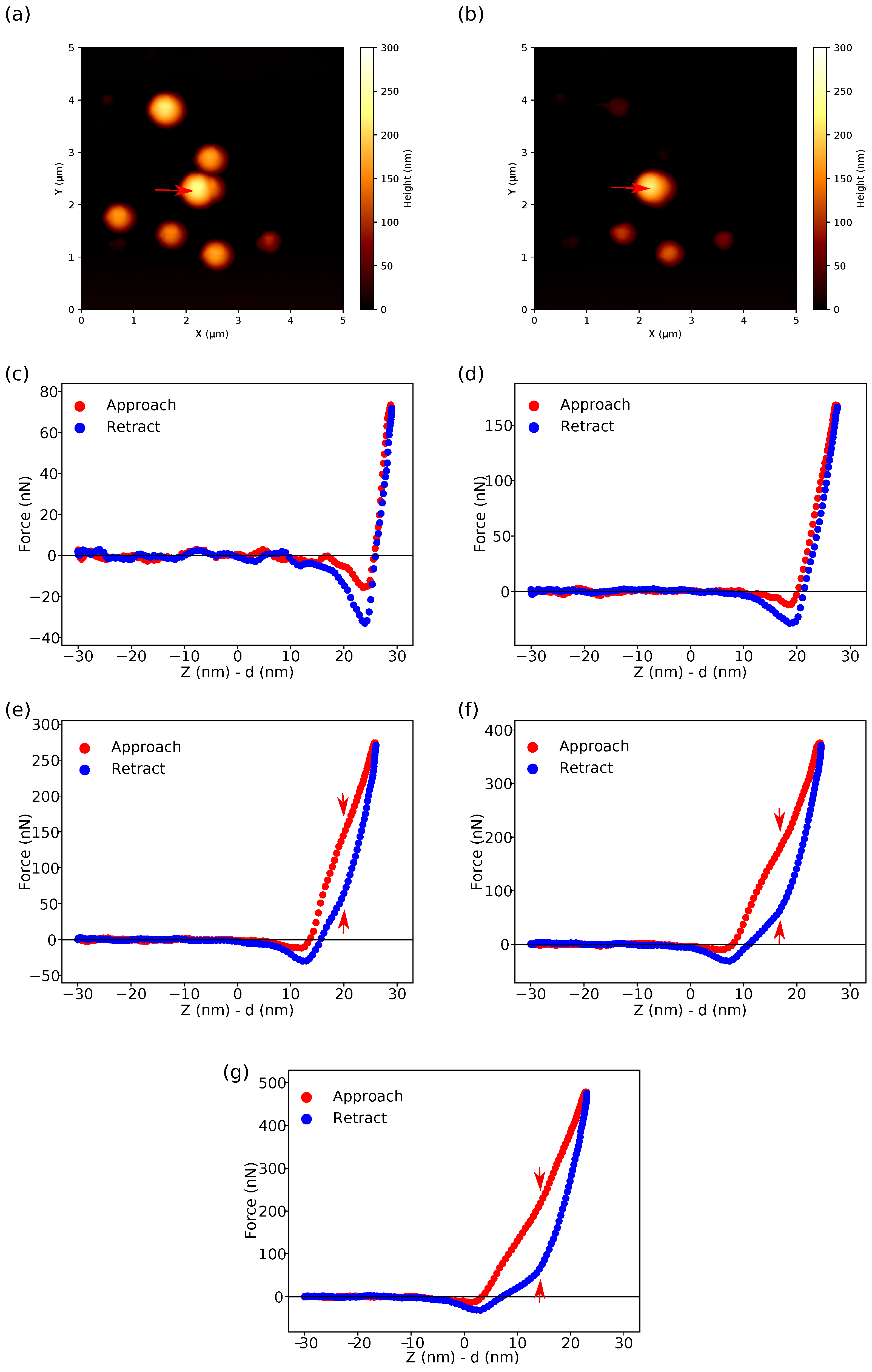
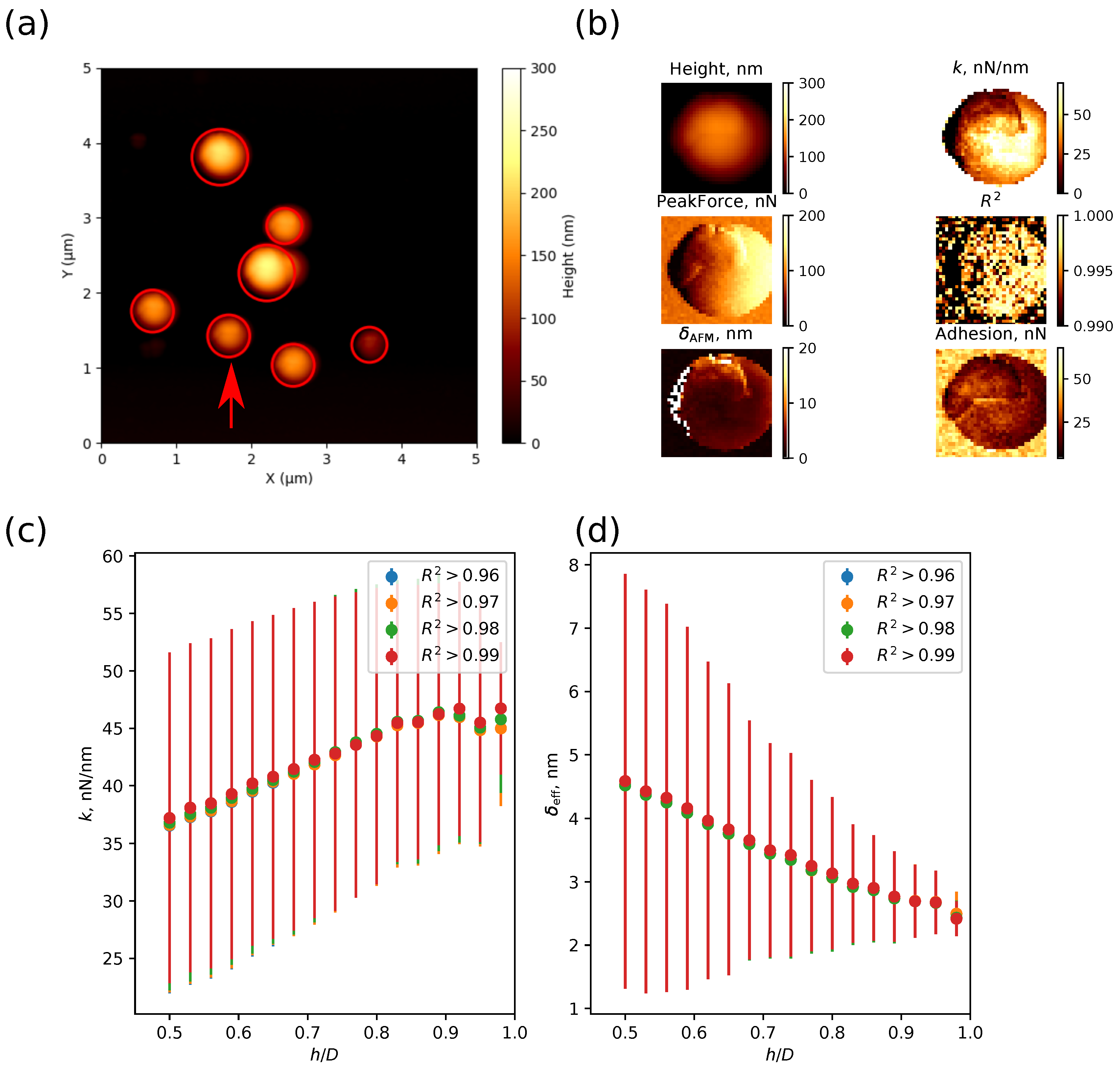
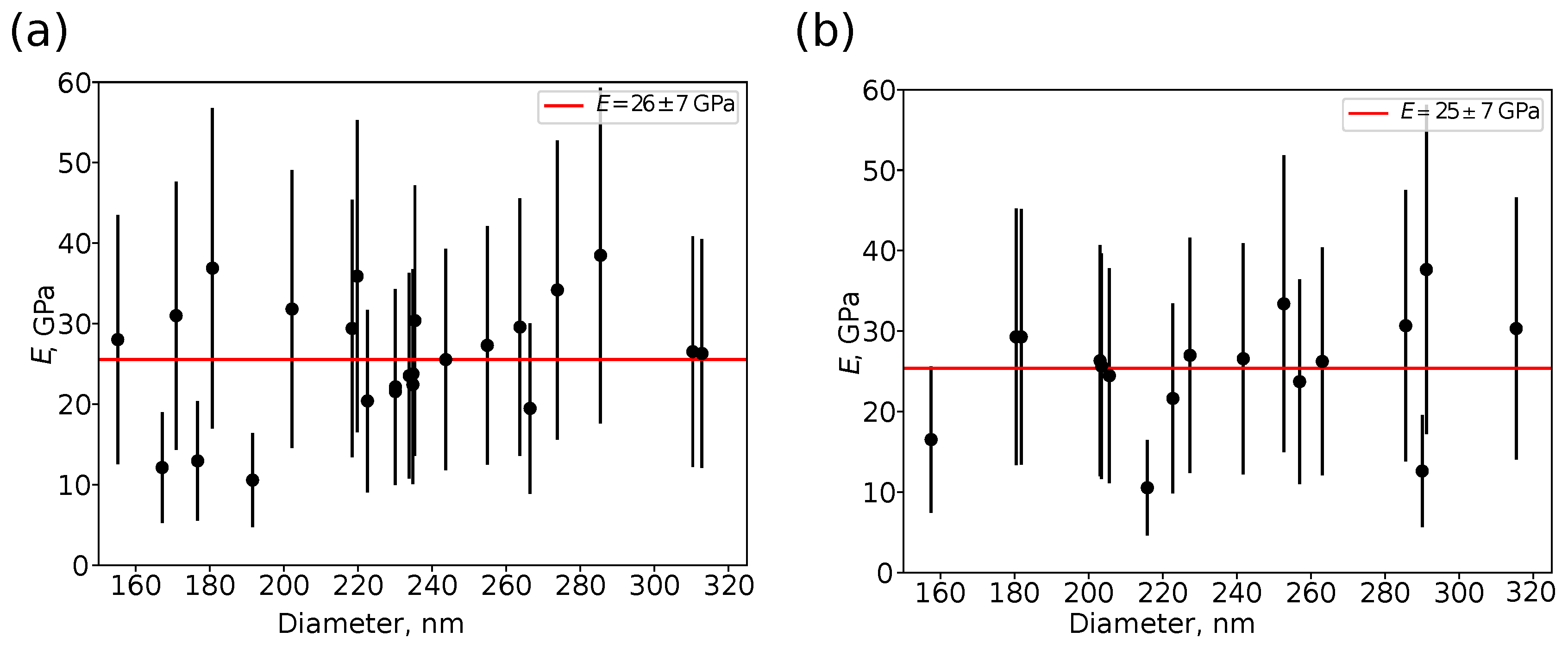
- Fitting the curves using a selected contact mechanics model (e.g., the Hertz, DMT, JKR, Reissner, Berry model) in the user-defined fitting region of the force–distance curve (Figure 2) and visualization of the resolved spatial map of the Young’s modulus, local stiffness, indentation, adhesion force, etc. depending on the model used (Figure 3). The coefficient of determination of the linear regression for the approximation of the experimental data by the model used was also calculated for each force–distance curve to estimate the quality of the fit.
2.5. Contact Mechanics Models for Small Elastic Deformations of Hollow Spheres
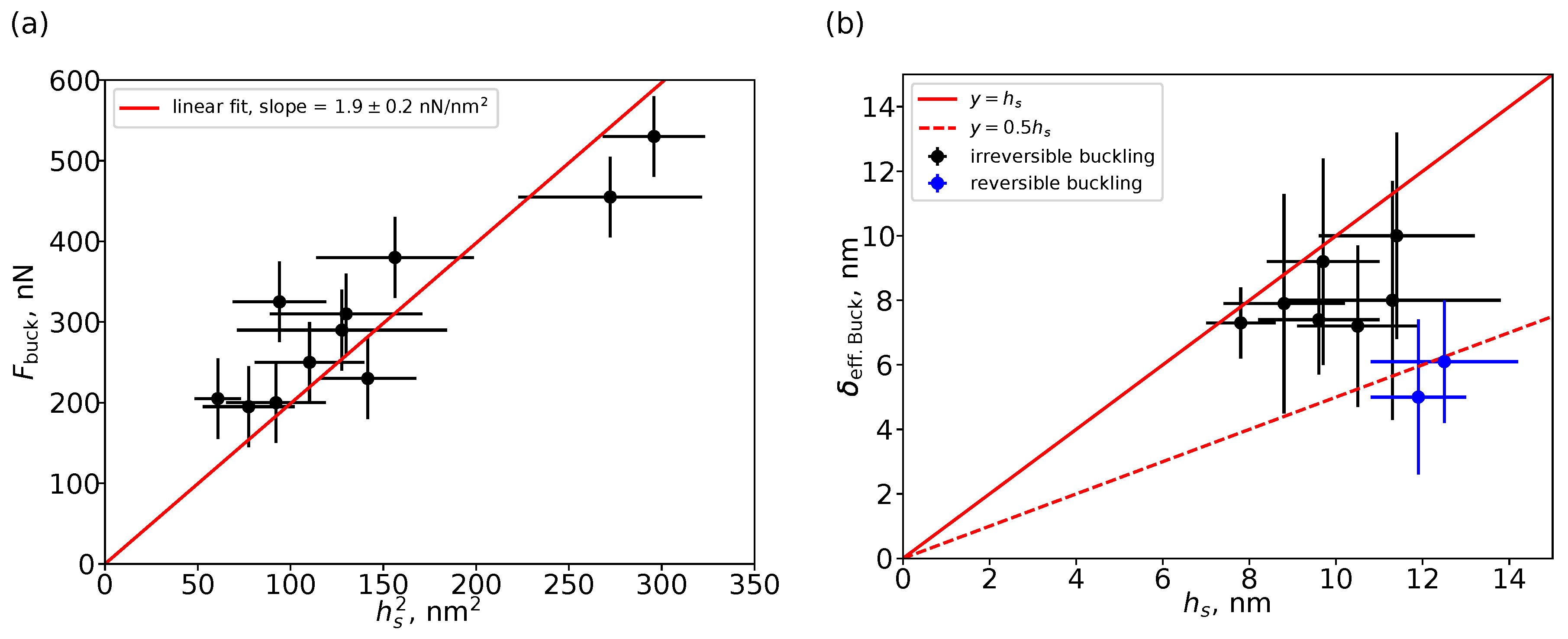
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bysell, H.; Månsson, R.; Hansson, P.; Malmsten, M. Microgels and microcapsules in peptide and protein drug delivery. Adv. Drug Deliv. Rev. 2011, 63, 1172–1185. [Google Scholar] [CrossRef]
- Mora-Huertas, C.; Fessi, H.; Elaissari, A. Polymer-based nanocapsules for drug delivery. Int. J. Pharm. 2010, 385, 113–142. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yan, K.; Hu, C.; Zhao, Y.; Chen, Z.; Zhu, X.; Möller, M. Encapsulation of enzymes in silica nanocapsules formed by an amphiphilic precursor polymer in water. J. Mater. Chem. B 2015, 3, 1261–1267. [Google Scholar] [CrossRef]
- Popat, A.; Hartono, S.B.; Stahr, F.; Liu, J.; Qiao, S.Z.; Lu, G.Q.M. Mesoporous silica nanoparticles for bioadsorption, enzyme immobilisation, and delivery carriers. Nanoscale 2011, 3, 2801–2818. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Liang, Q.; Zang, Q.; Lv, Z.; Meng, X.; Feng, J. Construction of prochloraz-loaded hollow mesoporous silica nanoparticles coated with metal–phenolic networks for precise release and improved biosafety of pesticides. Nanomaterials 2022, 12, 2885. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Matthews, C.C.; Yang, K.; Odarczenko, M.T.; White, S.R.; Sottos, N.R. Autonomous Indication of Mechanical Damage in Polymeric Coatings. Adv. Mater. 2016, 28, 2189–2194. [Google Scholar] [CrossRef]
- Zheng, X.; Wang, Q.; Li, Y.; Luan, J.; Wang, N. Microcapsule-Based Visualization Smart Sensors for Damage Detection: Principles and Applications. Adv. Mater. Technol. 2020, 5, 1900832. [Google Scholar] [CrossRef]
- Samadzadeh, M.; Boura, S.H.; Peikari, M.; Kasiriha, S.; Ashrafi, A. A review on self-healing coatings based on micro/nanocapsules. Prog. Org. Coat. 2010, 68, 159–164. [Google Scholar] [CrossRef]
- Zhu, D.Y.; Rong, M.Z.; Zhang, M.Q. Self-healing polymeric materials based on microencapsulated healing agents: From design to preparation. Prog. Polym. Sci. 2015, 49–50, 175–220. [Google Scholar] [CrossRef]
- Hofmeister, I.; Landfester, K.; Taden, A. pH-Sensitive Nanocapsules with Barrier Properties: Fragrance Encapsulation and Controlled Release. Macromolecules 2014, 47, 5768–5773. [Google Scholar] [CrossRef]
- Cao, Z.; Xu, C.; Ding, X.; Zhu, S.; Chen, H.; Qi, D. Synthesis of fragrance/silica nanocapsules through a sol–gel process in miniemulsions and their application as aromatic finishing agents. Colloid Polym. Sci. 2015, 293, 1129–1139. [Google Scholar] [CrossRef]
- Liu, C.; Liang, B.; Shi, G.; Li, Z.; Zheng, X.; Huang, Y.; Lin, L. Preparation and characteristics of nanocapsules containing essential oil for textile application. Flavour Fragr. J. 2015, 30, 295–301. [Google Scholar] [CrossRef]
- Fernandes, P.A.; Delcea, M.; Skirtach, A.G.; Möhwald, H.; Fery, A. Quantification of release from microcapsules upon mechanical deformation with AFM. Soft Matter 2010, 6, 1879–1883. [Google Scholar] [CrossRef]
- Datta, S.S.; Abbaspourrad, A.; Amstad, E.; Fan, J.; Kim, S.H.; Romanowsky, M.; Shum, H.C.; Sun, B.; Utada, A.S.; Windbergs, M.; et al. 25th Anniversary Article: Double Emulsion Templated Solid Microcapsules: Mechanics And Controlled Release. Adv. Mater. 2014, 26, 2205–2218. [Google Scholar] [CrossRef] [PubMed]
- Elsner, N.; Dubreuil, F.; Fery, A. Tuning of microcapsule adhesion by varying the capsule-wall thickness. Phys. Rev. E 2004, 69, 031802. [Google Scholar] [CrossRef]
- Graf, P.; Finken, R.; Seifert, U. Adhesion of Microcapsules. Langmuir 2006, 22, 7117–7119. [Google Scholar] [CrossRef] [PubMed]
- Hui, Y.; Wibowo, D.; Liu, Y.; Ran, R.; Wang, H.F.; Seth, A.; Middelberg, A.P.J.; Zhao, C.X. Understanding the Effects of Nanocapsular Mechanical Property on Passive and Active Tumor Targeting. ACS Nano 2018, 12, 2846–2857. [Google Scholar] [CrossRef]
- Hui, Y.; Yi, X.; Hou, F.; Wibowo, D.; Zhang, F.; Zhao, D.; Gao, H.; Zhao, C.X. Role of Nanoparticle Mechanical Properties in Cancer Drug Delivery. ACS Nano 2019, 13, 7410–7424. [Google Scholar] [CrossRef]
- Li, Z.; Xiao, C.; Yong, T.; Li, Z.; Gan, L.; Yang, X. Influence of nanomedicine mechanical properties on tumor targeting delivery. Chem. Soc. Rev. 2020, 49, 2273–2290. [Google Scholar] [CrossRef]
- Fery, A.; Weinkamer, R. Mechanical properties of micro- and nanocapsules: Single-capsule measurements. Polymer 2007, 48, 7221–7235. [Google Scholar] [CrossRef]
- Neubauer, M.P.; Poehlmann, M.; Fery, A. Microcapsule mechanics: From stability to function. Adv. Colloid Interface Sci. 2014, 207, 65–80. [Google Scholar] [CrossRef] [PubMed]
- Poncelet, D.; Neufeld, R.J. Shear breakage of nylon membrane microcapsules in a turbine reactor. Biotechnol. Bioeng. 1989, 33, 95–103. [Google Scholar] [CrossRef]
- Ramanujan, S.; Pozrikidis, C. Deformation of liquid capsules enclosed by elastic membranes in simple shear flow: Large deformations and the effect of fluid viscosities. J. Fluid Mech. 1998, 361, 117–143. [Google Scholar] [CrossRef]
- Koleva, I.; Rehage, H. A comparison of different experimental methods for investigating the mechanical properties of plane polysiloxane membranes and capsule walls. Soft Matter 2012, 8, 7672–7682. [Google Scholar] [CrossRef]
- Gross, M.; Krüger, T.; Varnik, F. Rheology of dense suspensions of elastic capsules: Normal stresses, yield stress, jamming and confinement effects. Soft Matter 2014, 10, 4360–4372. [Google Scholar] [CrossRef]
- Datta, S.S.; Kim, S.H.; Paulose, J.; Abbaspourrad, A.; Nelson, D.R.; Weitz, D.A. Delayed Buckling and Guided Folding of Inhomogeneous Capsules. Phys. Rev. Lett. 2012, 109, 134302. [Google Scholar] [CrossRef]
- Gao, C.; Leporatti, S.; Moya, S.; Donath, E.; Möhwald, H. Stability and Mechanical Properties of Polyelectrolyte Capsules Obtained by Stepwise Assembly of Poly(styrenesulfonate sodium salt) and Poly(diallyldimethyl ammonium) Chloride onto Melamine Resin Particles. Langmuir 2001, 17, 3491–3495. [Google Scholar] [CrossRef]
- Zhu, R.; Avsievich, T.; Popov, A.; Meglinski, I. Optical Tweezers in Studies of Red Blood Cells. Cells 2020, 9, 545. [Google Scholar] [CrossRef]
- Magazzú, A.; Marcuello, C. Investigation of Soft Matter Nanomechanics by Atomic Force Microscopy and Optical Tweezers: A Comprehensive Review. Nanomaterials 2023, 13, 963. [Google Scholar] [CrossRef]
- Leick, S.; Henning, S.; Degen, P.; Suter, D.; Rehage, H. Deformation of liquid-filled calcium alginate capsules in a spinning drop apparatus. Phys. Chem. Chem. Phys. 2010, 12, 2950–2958. [Google Scholar] [CrossRef]
- Kleinberger, R.M.; Burke, N.A.; Dalnoki-Veress, K.; Stöver, H.D. Systematic study of alginate-based microcapsules by micropipette aspiration and confocal fluorescence microscopy. Mater. Sci. Eng. C 2013, 33, 4295–4304. [Google Scholar] [CrossRef]
- Mabrouk, E.; Cuvelier, D.; Pontani, L.L.; Xu, B.; Lévy, D.; Keller, P.; Brochard-Wyart, F.; Nassoy, P.; Li, M.H. Formation and material properties of giant liquid crystal polymersomes. Soft Matter 2009, 5, 1870–1878. [Google Scholar] [CrossRef]
- Su, J.F.; Wang, X.Y.; Dong, H. Micromechanical properties of melamine–formaldehyde microcapsules by nanoindentation: Effect of size and shell thickness. Mater. Lett. 2012, 89, 1–4. [Google Scholar] [CrossRef]
- Ciriminna, R.; Sciortino, M.; Alonzo, G.; Schrijver, A.d.; Pagliaro, M. From Molecules to Systems: Sol-Gel Microencapsulation in Silica-Based Materials. Chem. Rev. 2011, 111, 765–789. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Shi, C.; Wang, T.; Li, X.; Ma, J. Recent progress in hollow silica: Template synthesis, morphologies and applications. Microporous Mesoporous Mater. 2016, 227, 121–136. [Google Scholar] [CrossRef]
- Sharma, J.; Polizos, G. Hollow Silica Particles: Recent Progress and Future Perspectives. Nanomaterials 2020, 10, 1599. [Google Scholar] [CrossRef]
- Jeelani, P.G.; Mulay, P.; Venkat, R.; Ramalingam, C. Multifaceted application of silica nanoparticles. A review. Silicon 2020, 12, 1337–1354. [Google Scholar] [CrossRef]
- Wu, S.H.; Mou, C.Y.; Lin, H.P. Synthesis of mesoporous silica nanoparticles. Chem. Soc. Rev. 2013, 42, 3862–3875. [Google Scholar] [CrossRef]
- Tang, F.; Li, L.; Chen, D. Mesoporous Silica Nanoparticles: Synthesis, Biocompatibility and Drug Delivery. Adv. Mater. 2012, 24, 1504–1534. [Google Scholar] [CrossRef] [PubMed]
- Al-Khafaji, M.A.; Gaál, A.; Jezsó, B.; Mihály, J.; Bartczak, D.; Goenaga-Infante, H.; Varga, Z. Synthesis of Porous Hollow Organosilica Particles with Tunable Shell Thickness. Nanomaterials 2022, 12, 1172. [Google Scholar] [CrossRef]
- Woignier, T.; Reynes, J.; Hafidi Alaoui, A.; Beurroies, I.; Phalippou, J. Different kinds of structure in aerogels: Relationships with the mechanical properties. J. Non-Cryst. Solids 1998, 241, 45–52. [Google Scholar] [CrossRef]
- Saxena, R.; Rodriguez, O.; Cho, W.; Gill, W.; Plawsky, J.; Baklanov, M.; Mogilnikov, K. Internal matrix structure of low-k mesoporous silica and its relation to mechanical properties. J. Non-Cryst. Solids 2004, 349, 189–199. [Google Scholar] [CrossRef]
- Ovchinnikov, I.S.; Vishnevskiy, A.S.; Seregin, D.S.; Rezvanov, A.A.; Schneider, D.; Sigov, A.S.; Vorotilov, K.A.; Baklanov, M.R. Evaluation of Mechanical Properties of Porous OSG Films by PFQNM AFM and Benchmarking with Traditional Instrumentation. Langmuir 2020, 36, 9377–9387. [Google Scholar] [CrossRef]
- Marszewski, M.; Dashti, A.; McNeil, P.E.; Fox, M.; Wall, V.; Butts, D.M.; King, S.C.; Kashanchi, G.N.; Tolbert, S.H.; Dunn, B.; et al. Elastic and plastic mechanical properties of nanoparticle-based silica aerogels and xerogels. Microporous Mesoporous Mater. 2022, 330, 111569. [Google Scholar] [CrossRef]
- Zhang, L.; D’Acunzi, M.; Kappl, M.; Auernhammer, G.K.; Vollmer, D.; van Kats, C.M.; van Blaaderen, A. Hollow Silica Spheres: Synthesis and Mechanical Properties. Langmuir 2009, 25, 2711–2717. [Google Scholar] [CrossRef]
- Zhang, L.; D’Acunzi, M.; Kappl, M.; Imhof, A.; Blaaderen, A.V.; Butt, H.J.; Graf, R.; Vollmer, D. Tuning the mechanical properties of silica microcapsules. Phys. Chem. Chem. Phys. 2010, 12, 15392–15398. [Google Scholar] [CrossRef] [PubMed]
- Uebel, F.; Thérien-Aubin, H.; Landfester, K. Tailoring the mechanoresponsive release from silica nanocapsules. Nanoscale 2021, 13, 15415–15421. [Google Scholar] [CrossRef]
- Romeis, S.; Paul, J.; Hanisch, M.; Marthala, V.R.R.; Hartmann, M.; Taylor, R.N.K.; Schmidt, J.; Peukert, W. Correlation of Enhanced Strength and Internal Structure for Heat-Treated Submicron Stöber Silica Particles. Part. Part. Syst. Charact. 2014, 31, 664–674. [Google Scholar] [CrossRef]
- Paul, J.; Romeis, S.; Tomas, J.; Peukert, W. A review of models for single particle compression and their application to silica microspheres. Adv. Powder Technol. 2014, 25, 136–153. [Google Scholar] [CrossRef]
- Paul, J.; Romeis, S.; Mačković, M.; Marthala, V.; Herre, P.; Przybilla, T.; Hartmann, M.; Spiecker, E.; Schmidt, J.; Peukert, W. In situ cracking of silica beads in the SEM and TEM — Effect of particle size on structure–property correlations. Powder Technol. 2015, 270, 337–347. [Google Scholar] [CrossRef]
- Wang, H.; Agrawal, G.; Tsarkova, L.; Zhu, X.; Möller, M. Self-Templating Amphiphilic Polymer Precursors for Fabricating Mesostructured Silica Particles: A Water-Based Facile and Universal Method. Adv. Mater. 2013, 25, 1017–1021. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, Z.; Zhu, X.; Möller, M. Silica nanoparticles catalyse the formation of silica nanocapsules in a surfactant-free emulsion system. J. Mater. Chem. A 2015, 3, 24428–24436. [Google Scholar] [CrossRef]
- Agrawal, G.; Ülpenich, A.; Zhu, X.; Möller, M.; Pich, A. Microgel-Based Adaptive Hybrid Capsules with Tunable Shell Permeability. Chem. Mater. 2014, 26, 5882–5891. [Google Scholar] [CrossRef]
- Mercadé-Prieto, R.; Zhang, Z. Mechanical characterization of microspheres—capsules, cells and beads: A review. J. Microencapsul. 2012, 29, 277–285. [Google Scholar] [CrossRef]
- Herre, P.; Romeis, S.; Mačković, M.; Przybilla, T.; Paul, J.; Schwenger, J.; Torun, B.; Grundmeier, G.; Spiecker, E.; Peukert, W. Deformation behavior of nanocrystalline titania particles accessed by complementary in situ electron microscopy techniques. J. Amer. Ceram. Soc. 2017, 100, 5709–5722. [Google Scholar] [CrossRef]
- Radmacher, M.; Cleveland, J.P.; Fritz, M.; Hansma, H.G.; Hansma, P.K. Mapping interaction forces with the atomic force microscope. Biophys. J. 1994, 66, 2159–2165. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, B.; Erina, N.; Su, C. Quantitative mechanical property mapping at the nanoscale with PeakForce QNM. Appl. Note Veeco Instruments Inc 2010, 1, 1–11. [Google Scholar]
- Chopinet, L.; Formosa, C.; Rols, M.; Duval, R.; Dague, E. Imaging living cells surface and quantifying its properties at high resolution using AFM in QI™ mode. Micron 2013, 48, 26–33. [Google Scholar] [CrossRef]
- Magonov, S.; Belikov, S.; Surtchev, M.; Leesment, S.; Malovichko, I. High-Resolution Mapping of Quantitative Elastic Modulus of Polymers. Microsc. Microanal. 2015, 21, 2183–2184. [Google Scholar] [CrossRef]
- Dokukin, M.E.; Sokolov, I. On the Measurements of Rigidity Modulus of Soft Materials in Nanoindentation Experiments at Small Depth. Macromolecules 2012, 45, 4277–4288. [Google Scholar] [CrossRef]
- Dokukin, M.E.; Sokolov, I. Quantitative Mapping of the Elastic Modulus of Soft Materials with HarmoniX and PeakForce QNM AFM Modes. Langmuir 2012, 28, 16060–16071. [Google Scholar] [CrossRef]
- Kilpatrick, J.I.; Revenko, I.; Rodriguez, B.J. Nanomechanics of Cells and Biomaterials Studied by Atomic Force Microscopy. Adv. Healthc. Mater. 2015, 4, 2456–2474. [Google Scholar] [CrossRef] [PubMed]
- Zeng, G.; Dirscherl, K.; Garnæs, J. Toward Accurate Quantitative Elasticity Mapping of Rigid Nanomaterials by Atomic Force Microscopy: Effect of Acquisition Frequency, Loading Force, and Tip Geometry. Nanomaterials 2018, 8, 616. [Google Scholar] [CrossRef]
- Liu, Y.; Sokolov, I.; Dokukin, M.E.; Xiong, Y.; Peng, P. Can AFM be used to measure absolute values of Young’s modulus of nanocomposite materials down to the nanoscale? Nanoscale 2020, 12, 12432–12443. [Google Scholar] [CrossRef]
- Thormann, E.; Pettersson, T.; Claesson, P.M. How to measure forces with atomic force microscopy without significant influence from nonlinear optical lever sensitivity. Rev. Sci. Instrum. 2009, 80, 093701. [Google Scholar] [CrossRef]
- Stan, G.; Cook, R.F. Mapping the elastic properties of granular Au films by contact resonance atomic force microscopy. Nanotechnology 2008, 19, 235701. [Google Scholar] [CrossRef]
- Heinze, K.; Arnould, O.; Delenne, J.Y.; Lullien-Pellerin, V.; Ramonda, M.; George, M. On the effect of local sample slope during modulus measurements by contact-resonance atomic force microscopy. Ultramicroscopy 2018, 194, 78–88. [Google Scholar] [CrossRef]
- Nasto, A.; Ajdari, A.; Lazarus, A.; Vaziri, A.; Reis, P.M. Localization of deformation in thin shells under indentation. Soft Matter 2013, 9, 6796–6803. [Google Scholar] [CrossRef]
- Gibbons, M.M.; Klug, W.S. Nonlinear finite-element analysis of nanoindentation of viral capsids. Phys. Rev. E 2007, 75, 031901. [Google Scholar] [CrossRef]
- Hertz, H. On the contact of elastic solids. In Miscellaneous Papers; Macmillan and Co.: London, UK, 1896; pp. 146–162. [Google Scholar]
- Sneddon, I.N. The relation between load and penetration in the axisymmetric Boussinesq problem for a punch of arbitrary profile. Int. J. Eng. Sci. 1965, 3, 47–57. [Google Scholar] [CrossRef]
- Reissner, E. Stresses and small displacements of shallow spherical shells. II. J. Math. Phys. 1946, 25, 279–300. [Google Scholar] [CrossRef]
- Johnson, K.L.; Kendall, K.; Roberts, A.D.; Tabor, D. Surface energy and the contact of elastic solids. Proc. R. Soc. A Math. Phys. Eng. Sci. 1971, 324, 301–313. [Google Scholar] [CrossRef]
- Derjaguin, B.V.; Muller, V.M.; Toporov, Y.P. Effect of contact deformations on the adhesion of particles. J. Colloid Interface Sci. 1975, 53, 314–326. [Google Scholar] [CrossRef]
- Collinson, D.W.; Sheridan, R.J.; Palmeri, M.J.; Brinson, L.C. Best practices and recommendations for accurate nanomechanical characterization of heterogeneous polymer systems with atomic force microscopy. Prog. Polym. Sci. 2021, 119, 101420. [Google Scholar] [CrossRef]
- Glaubitz, M.; Medvedev, N.; Pussak, D.; Hartmann, L.; Schmidt, S.; Helm, C.A.; Delcea, M. A novel contact model for AFM indentation experiments on soft spherical cell-like particles. Soft Matter 2014, 10, 6732–6741. [Google Scholar] [CrossRef]
- Berry, J.D.; Mettu, S.; Dagastine, R.R. Precise measurements of capsule mechanical properties using indentation. Soft Matter 2017, 13, 1943–1947. [Google Scholar] [CrossRef]
- Dimitriadis, E.K.; Horkay, F.; Maresca, J.; Kachar, B.; Chadwick, R.S. Determination of Elastic Moduli of Thin Layers of Soft Material Using the Atomic Force Microscope. Biophys. J. 2002, 82, 2798–2810. [Google Scholar] [CrossRef]
- Zhu, X.; Jaumann, M.; Peter, K.; Möller, M.; Melian, C.; Adams-Buda, A.; Demco, D.E.; Blümich, B. One-Pot Synthesis of Hyperbranched Polyethoxysiloxanes. Macromolecules 2006, 39, 1701–1708. [Google Scholar] [CrossRef]
- Howarter, J.A.; Youngblood, J.P. Optimization of Silica Silanization by 3-Aminopropyltriethoxysilane. Langmuir 2006, 22, 11142–11147. [Google Scholar] [CrossRef]
- Hüttl, G.; Klemm, V.; Popp, R.; Simon, F.; Müller, E. Tailored colloidal AFM probes and their TEM investigation. Surf. Interface Anal. 2002, 33, 50–53. [Google Scholar] [CrossRef]
- Wagner, R.; Moon, R.; Pratt, J.; Shaw, G.; Raman, A. Uncertainty quantification in nanomechanical measurements using the atomic force microscope. Nanotechnology 2011, 22, 455703. [Google Scholar] [CrossRef] [PubMed]
- Sheridan, R.J.; Collinson, D.W.; Brinson, L.C. Vanishing Cantilever Calibration Error with Magic Ratio Atomic Force Microscopy. Adv. Theory Simul. 2020, 3, 2000090. [Google Scholar] [CrossRef]
- Williams, P.M.; Shakesheff, K.M.; Davies, M.C.; Jackson, D.E.; Roberts, C.J.; Tendler, S.J.B. Blind reconstruction of scanning probe image data. J. Vac. Sci. Technol. B 1996, 14, 1557–1562. [Google Scholar] [CrossRef]
- Villarrubia, J.S. Algorithms for scanned probe microscope image simulation, surface reconstruction, and tip estimation. J. Res. Natl. Inst. Stand. Technol. 1997, 102, 425. [Google Scholar] [CrossRef]
- Sader, J.E.; Borgani, R.; Gibson, C.T.; Haviland, D.B.; Higgins, M.J.; Kilpatrick, J.I.; Lu, J.; Mulvaney, P.; Shearer, C.J.; Slattery, A.D.; et al. A virtual instrument to standardise the calibration of atomic force microscope cantilevers. Rev. Sci. Instrum. 2016, 87, 093711. [Google Scholar] [CrossRef]
- Somnath, S.; Smith, C.R.; Laanait, N.; Vasudevan, R.K.; Jesse, S. USID and Pycroscopy – Open Source Frameworks for Storing and Analyzing Imaging and Spectroscopy Data. Microsc. Microanal. 2019, 25, 220–221. [Google Scholar] [CrossRef]
- Pogorelov, A. Bendings of Surfaces and Stability of Shells; American Mathematical Society: Providence, RI, USA, 1988. [Google Scholar]
- Mercadé-Prieto, R.; Allen, R.; York, D.; Preece, J.A.; Goodwin, T.E.; Zhang, Z. Compression of elastic–perfectly plastic microcapsules using micromanipulation and finite element modelling: Determination of the yield stress. Chem. Eng. Sci. 2011, 66, 1835–1843. [Google Scholar] [CrossRef]
- Mercadé-Prieto, R.; Allen, R.; Zhang, Z.; York, D.; Preece, J.A.; Goodwin, T.E. Failure of elastic-plastic core–shell microcapsules under compression. AIChE J. 2012, 58, 2674–2681. [Google Scholar] [CrossRef]
- Hutchinson, J.W. Buckling of spherical shells revisited. Proc. Math. Phys. Eng. Sci. 2016, 472, 20160577. [Google Scholar] [CrossRef]
- Hutchinson, J.W.; Thompson, J.M.T. Imperfections and energy barriers in shell buckling. Int. J. Solids Struct. 2018, 148, 157–168. [Google Scholar] [CrossRef]
- Marthelot, J.; López Jiménez, F.; Lee, A.; Hutchinson, J.W.; Reis, P.M. Buckling of a Pressurized Hemispherical Shell Subjected to a Probing Force. J. Appl. Mech. 2017, 84, 121005. [Google Scholar] [CrossRef]
- Lytra, A.; Sboros, V.; Giannakopoulos, A.; Pelekasis, N. Modeling atomic force microscopy and shell mechanical properties estimation of coated microbubbles. Soft Matter 2020, 16, 4661–4681. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Streltsov, D.R.; Borisov, K.M.; Kalinina, A.A.; Muzafarov, A.M. Quantitative Elasticity Mapping of Submicron Silica Hollow Particles by PeakForce QNM AFM Mode. Nanomaterials 2023, 13, 1916. https://doi.org/10.3390/nano13131916
Streltsov DR, Borisov KM, Kalinina AA, Muzafarov AM. Quantitative Elasticity Mapping of Submicron Silica Hollow Particles by PeakForce QNM AFM Mode. Nanomaterials. 2023; 13(13):1916. https://doi.org/10.3390/nano13131916
Chicago/Turabian StyleStreltsov, Dmitry R., Kirill M. Borisov, Aleksandra A. Kalinina, and Aziz M. Muzafarov. 2023. "Quantitative Elasticity Mapping of Submicron Silica Hollow Particles by PeakForce QNM AFM Mode" Nanomaterials 13, no. 13: 1916. https://doi.org/10.3390/nano13131916
APA StyleStreltsov, D. R., Borisov, K. M., Kalinina, A. A., & Muzafarov, A. M. (2023). Quantitative Elasticity Mapping of Submicron Silica Hollow Particles by PeakForce QNM AFM Mode. Nanomaterials, 13(13), 1916. https://doi.org/10.3390/nano13131916






