Print-Light-Synthesis for Single-Step Metal Nanoparticle Synthesis and Patterned Electrode Production
Abstract
:1. Introduction
2. Fundamentals on Printing and Light-Induced Synthesis of Nanoparticles and Nanoparticle-Decorated Electrodes
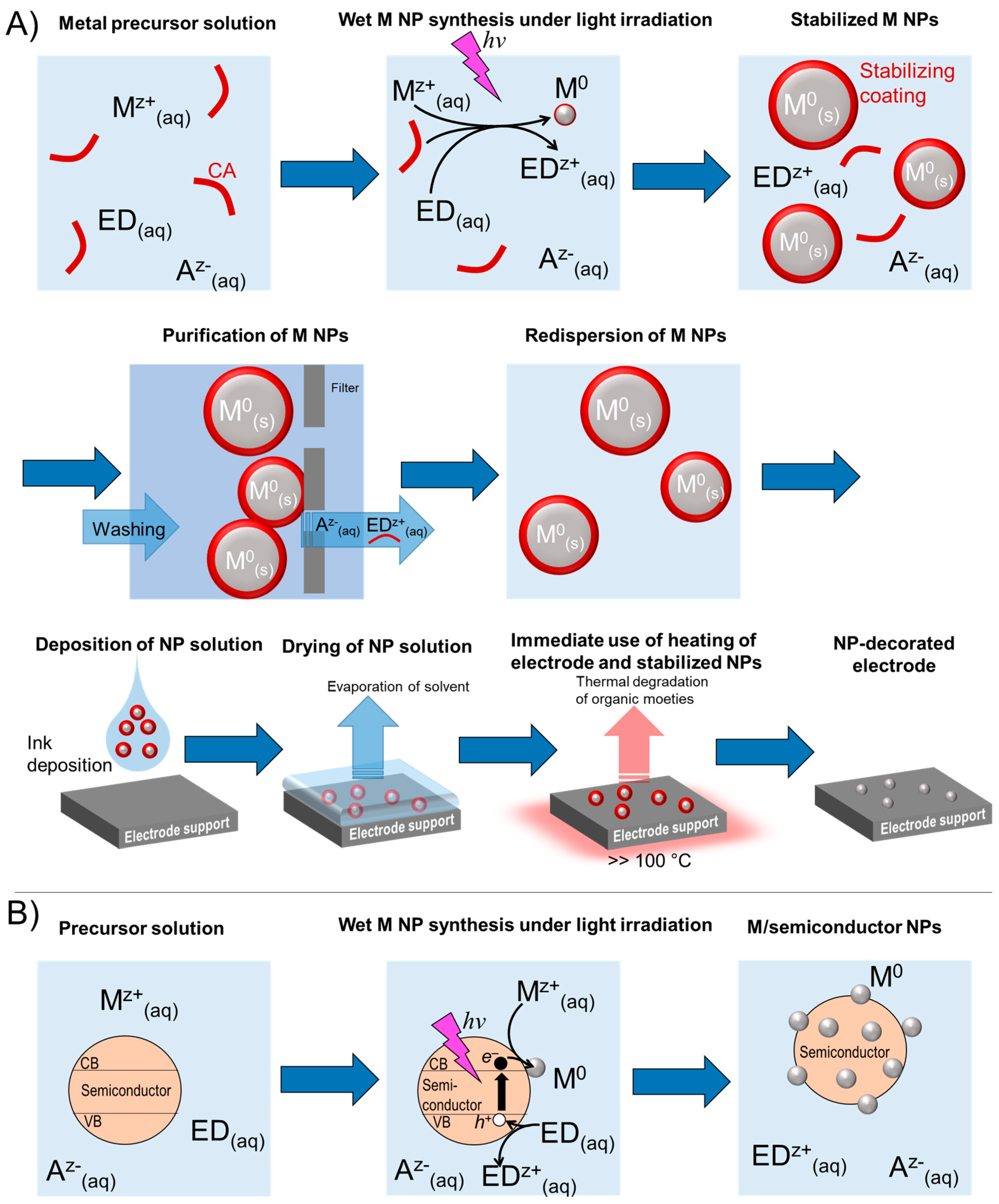
2.1. Irradition-Free and Irradiation-Induced Nanoparticle Synthesis
2.2. Most Common Printing Techniques Suitable for Print-Light-Synthesis
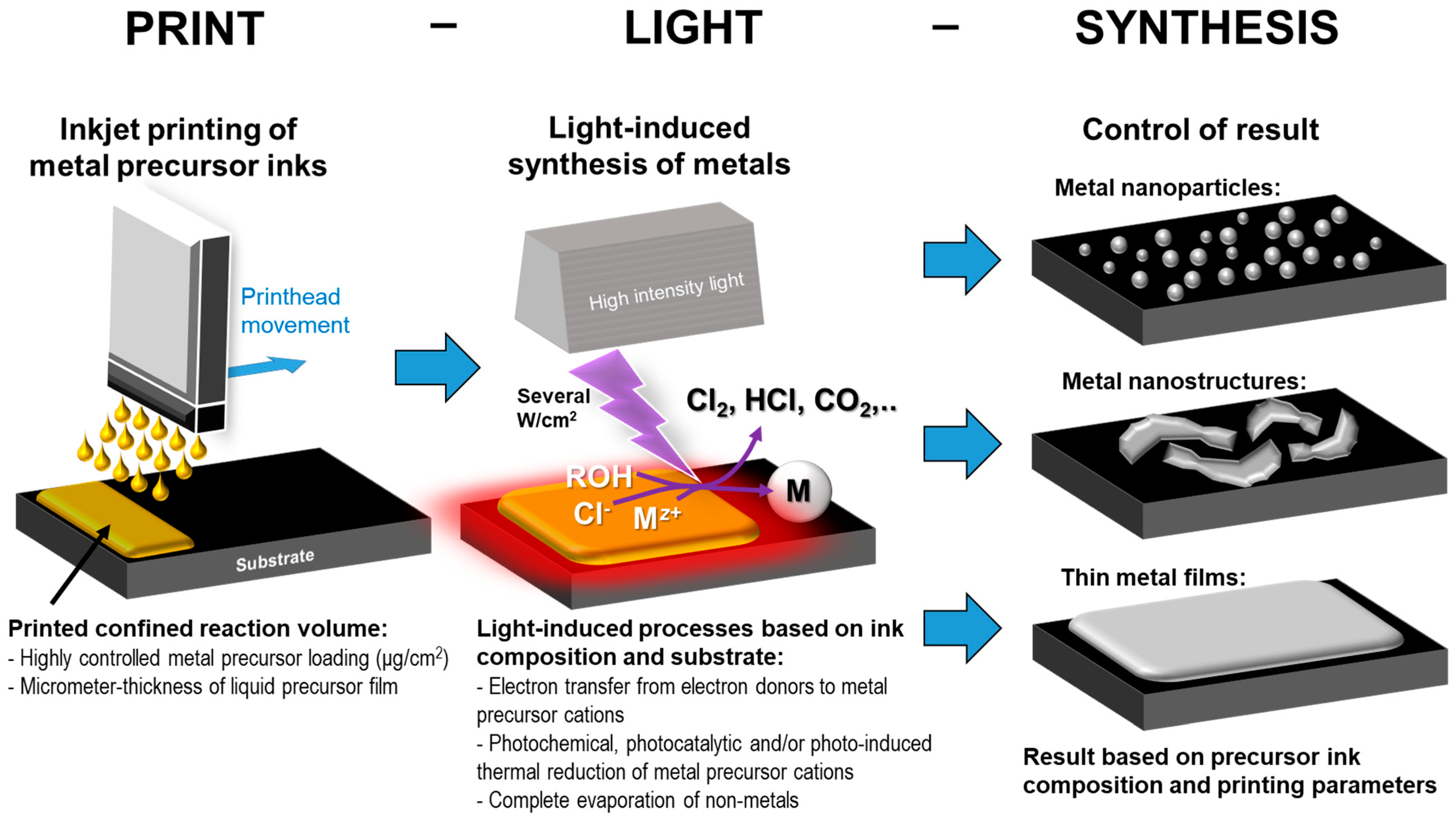
3. Print-Light-Synthesis of Electrodes
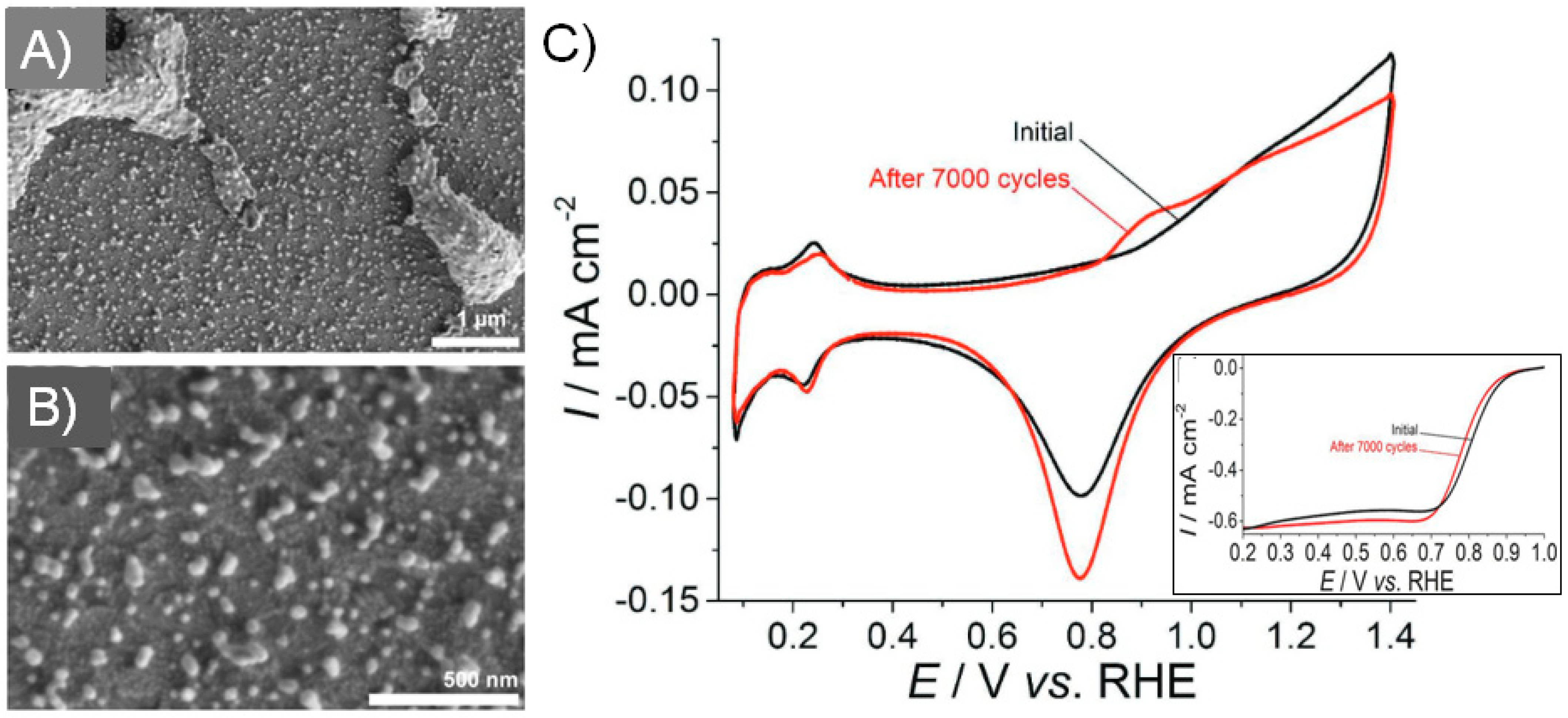
3.1. Print-Light-Synthesis of Metal Nanoparticles, Nanostructures and Films
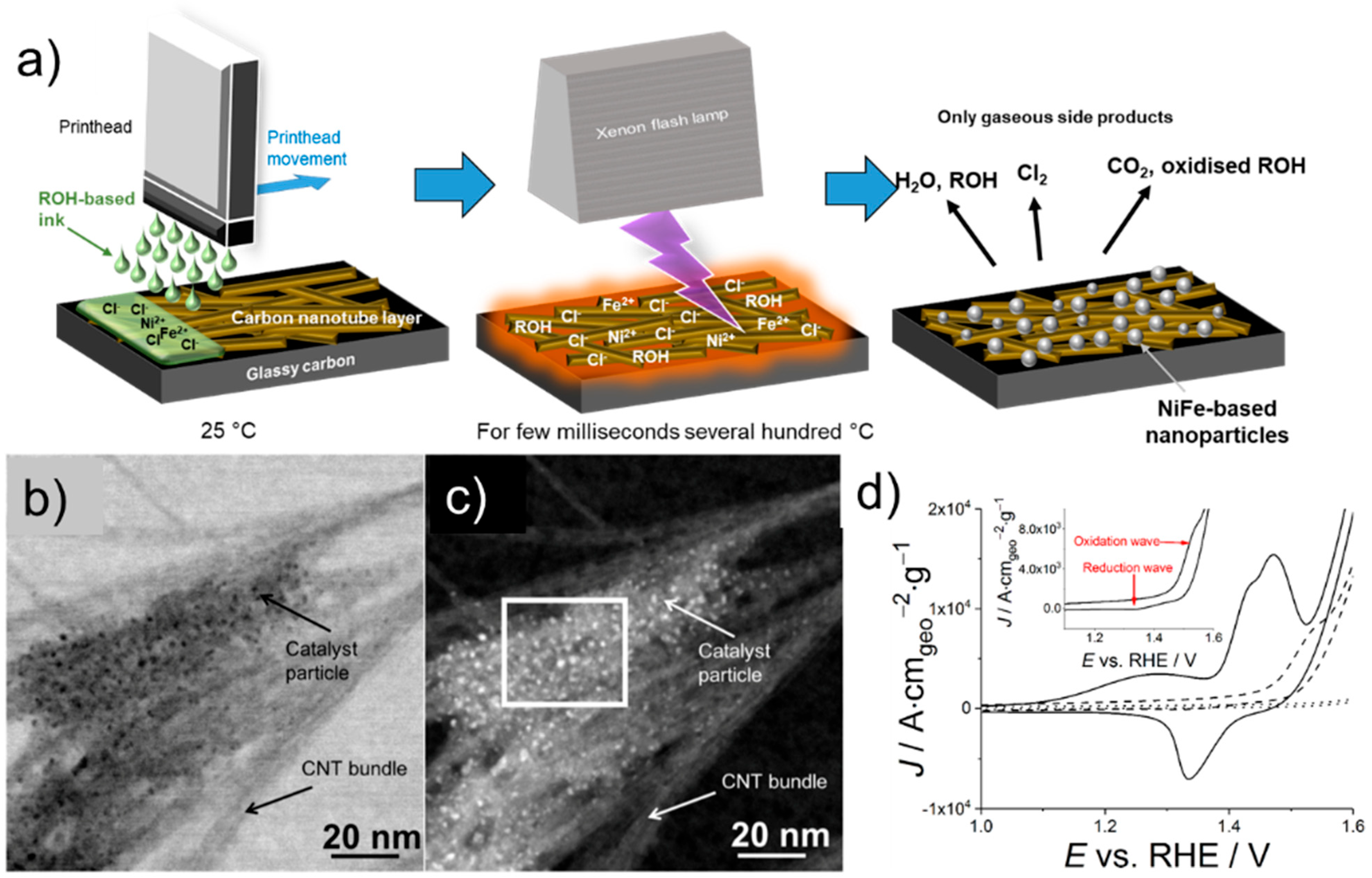
3.2. Print-Light-Synthesis of Mixed Metal Nanoparticles
3.3. Oxidative Print-Light-Synthesis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A. Experimental Details
References
- Baig, N.; Kammakakam, I.; Falath, W.; Kammakakam, I. Nanomaterials: A review of synthesis methods, properties, recent progress, and challenges. Mater. Adv. 2021, 2, 1821–1871. [Google Scholar] [CrossRef]
- Abdelhafiz, A.; Tanvir, A.N.M.; Zeng, M.; Wang, B.; Ren, Z.; Harutyunyan, A.R.; Zhang, Y.; Li, J. Pulsed Light Synthesis of High Entropy Nanocatalysts with Enhanced Catalytic Activity and Prolonged Stability for Oxygen Evolution Reaction. Adv. Sci. 2023, 2300426. [Google Scholar] [CrossRef] [PubMed]
- Lesch, A. Print-Light-Synthesis of Platinum Nanostructured Indium-Tin-Oxide Electrodes for Energy Research. Adv. Mater. Technol. 2018, 3, 1700201. [Google Scholar] [CrossRef] [Green Version]
- Shariq, M.; Friedrich, B.; Budic, B.; Hodnik, N.; Ruiz-Zepeda, F.; Majerič, P.; Rudolf, R. Successful Synthesis of Gold Nanoparticles through Ultrasonic Spray Pyrolysis from a Gold(III) Nitrate Precursor and Their Interaction with a High Electron Beam. ChemistryOpen 2018, 7, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, K.; Routsis, K.; Bett, J.A.S. The thermal decomposition of platinum(II) and (IV) complexes. Thermochim. Acta 1974, 10, 109–117. [Google Scholar] [CrossRef]
- Stern, K.H. High Temperature Properties and Decomposition of Inorganic Salts Part 3, Nitrates and Nitrites. J. Phys. Chem. Ref. Data 1972, 1, 747–772. [Google Scholar] [CrossRef] [Green Version]
- Kanungo, S.B.; Mishra, S.K. Thermal dehydration and decomposition of FeCl3·xH2O. J. Therm. Anal. 1996, 46, 1487–1500. [Google Scholar] [CrossRef]
- Reddington, E.; Sapienza, A.; Gurau, B.; Viswanathan, R.; Sarangapani, S.; Smotkin, E.S.; Mallouk, T.E. Combinatorial electrochemistry: A highly parallel, optical screening method for discovery of better electrocatalysts. Science 1998, 280, 1735–1737. [Google Scholar] [CrossRef] [Green Version]
- Reiser, A.; Koch, L.; Dunn, K.A.; Matsuura, T.; Iwata, F.; Fogel, O.; Kotler, Z.; Zhou, N.; Charipar, K.; Piqué, A.; et al. Metals by Micro-Scale Additive Manufacturing: Comparison of Microstructure and Mechanical Properties. Adv. Funct. Mater. 2020, 30, 1910491. [Google Scholar] [CrossRef]
- Sakamoto, M.; Fujistuka, M.; Majima, T. Light as a construction tool of metal nanoparticles: Synthesis and mechanism. J. Photochem. Photobiol. C Photochem. Rev. 2009, 10, 33–56. [Google Scholar] [CrossRef]
- Kacenauskaite, L.; Swane, A.A.; Kirkensgaard, J.J.K.; Fleige, M.; Kunz, S.; Vosch, T.; Arenz, M. Synthesis mechanism and influence of light on unprotected platinum nanoparticles synthesis at room temperature. ChemNanoMat 2016, 2, 104–107. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, X.; Khakhulin, D.; Su, P.; Wulff, M.; Baudelet, F.; Weng, T.C.; Kong, Q.; Sun, Y. Deciphering Photochemical Reaction Pathways of Aqueous Tetrachloroauric Acid by X-ray Transient Absorption Spectroscopy. J. Phys. Chem. Lett. 2022, 13, 8921–8927. [Google Scholar] [CrossRef]
- Kurihara, K.; Fendler, J.H.; Kizling, J.; Stenius, P. Laser and Pulse Radiolytically Induced Colloidal Gold Formation in Water and in Water-in-Oil Microemulsions. J. Am. Chem. Soc. 1983, 105, 2574–2579. [Google Scholar] [CrossRef]
- Frias Batista, L.M.; Meader, V.K.; Romero, K.; Kunzler, K.; Kabir, F.; Bullock, A.; Tibbetts, K.M. Kinetic Control of [AuCl4]− Photochemical Reduction and Gold Nanoparticle Size with Hydroxyl Radical Scavengers. J. Phys. Chem. B 2019, 123, 7204–7213. [Google Scholar] [CrossRef]
- Jang, Y.R.; Joo, S.J.; Chu, J.H.; Uhm, H.J.; Park, J.W.; Ryu, C.H.; Yu, M.H.; Kim, H.S. A Review on Intense Pulsed Light Sintering Technologies for Conductive Electrodes in Printed Electronics. Int. J. Precis. Eng. Manuf.-Green Technol. 2021, 8, 327–363. [Google Scholar] [CrossRef]
- Jović, M.; Zhu, Y.; Lesch, A.; Bondarenko, A.; Cortés-Salazar, F.; Gumy, F.; Girault, H.H. Inkjet-printed microtiter plates for portable electrochemical immunoassays. J. Electroanal. Chem. 2017, 786, 69–76. [Google Scholar] [CrossRef] [Green Version]
- Rosen, Y.S.; Yakushenko, A.; Offenhäusser, A.; Magdassi, S. Self-Reducing Copper Precursor Inks and Photonic Additive Yield Conductive Patterns under Intense Pulsed Light. ACS Omega 2017, 2, 573–581. [Google Scholar] [CrossRef]
- Costa Bassetto, V.; Oliveira Silva, W.; Pereira, C.M.; Girault, H.H. Flash light synthesis of noble metal nanoparticles for electrochemical applications: Silver, gold, and their alloys. J. Solid State Electrochem. 2020, 24, 1781–1788. [Google Scholar] [CrossRef]
- Reynard, D.; Nagar, B.; Girault, H. Photonic flash synthesis of Mo2C/graphene electrocatalyst for the hydrogen evolution reaction. ACS Catal. 2021, 11, 5865–5872. [Google Scholar] [CrossRef]
- Hu, Q.; Sun, X.Z.; Parmenter, C.D.J.; Fay, M.W.; Smith, E.F.; Rance, G.A.; He, Y.; Zhang, F.; Liu, Y.; Irvine, D.; et al. Additive manufacture of complex 3D Au-containing nanocomposites by simultaneous two-photon polymerisation and photoreduction. Sci. Rep. 2017, 7, 17150. [Google Scholar] [CrossRef] [Green Version]
- Lemarchand, J.; Bridonneau, N.; Battaglini, N.; Carn, F.; Mattana, G.; Piro, B.; Zrig, S.; Noël, V. Challenges, Prospects, and Emerging Applications of Inkjet-Printed Electronics: A Chemist’s Point of View. Angew. Chem. Int. Ed. 2022, 61, e202200166. [Google Scholar] [CrossRef]
- Mubarak, S.; Dhamodharan, D.; Byun, H.S. Recent advances in 3D printed electrode materials for electrochemical energy storage devices. J. Energy Chem. 2023, 81, 272–312. [Google Scholar] [CrossRef]
- Derby, B. Inkjet printing of functional and structural materials: Fluid property requirements, feature stability, and resolution. Annu. Rev. Mater. Res. 2010, 40, 395–414. [Google Scholar] [CrossRef]
- Yang, W.; List-Kratochvil, E.J.W.; Wang, C. Metal particle-free inks for printed flexible electronics. J. Mater. Chem. C 2019, 7, 15098–15117. [Google Scholar] [CrossRef]
- Lesch, A.; Momotenko, D.; Cortés-Salazar, F.; Wirth, I.; Tefashe, U.M.; Meiners, F.; Vaske, B.; Girault, H.H.; Wittstock, G. Fabrication of soft gold microelectrode arrays as probes for scanning electrochemical microscopy. J. Electroanal. Chem. 2012, 666, 52–61. [Google Scholar] [CrossRef] [Green Version]
- Maiorano, E.; Gianvittorio, S.; Lanzi, M.; Tonelli, D.; Pick, H.; Lesch, A. Print-Light-Synthesis of Gold Thin Film Electrodes for Electrochemical Sensing. Adv. Mater. Technol. 2023, 2202039. [Google Scholar] [CrossRef]
- Valeton, J.J.P.; Hermans, K.; Bastiaansen, C.W.M.; Broer, D.J.; Perelaer, J.; Schubert, U.S.; Crawford, G.P.; Smith, P.J. Room temperature preparation of conductive silver features using spin-coating and inkjet printing. J. Mater. Chem. 2010, 20, 543–546. [Google Scholar] [CrossRef]
- Zope, K.R.; Cormier, D.; Williams, S.A. Reactive Silver Oxalate Ink Composition with Enhanced Curing Conditions for Flexible Substrates. ACS Appl. Mater. Interfaces 2018, 10, 3830–3837. [Google Scholar] [CrossRef]
- Araki, T.; Sugahara, T.; Jiu, J.; Nagao, S.; Nogi, M.; Koga, H.; Uchida, H.; Shinozaki, K.; Suganuma, K. Cu salt ink formulation for printed electronics using photonic sintering. Langmuir 2013, 29, 11192–11197. [Google Scholar] [CrossRef]
- Song, S.M.; Cho, S.M. Copper Ion Inks Capable of Screen Printing and Intense Pulsed-Light Sintering on PET Substrates. ACS Appl. Electron. Mater. 2022, 4, 1882–1890. [Google Scholar] [CrossRef]
- Deore, B.; Paquet, C.; Kell, A.J.; Lacelle, T.; Liu, X.; Mozenson, O.; Lopinski, G.; Brzezina, G.; Guo, C.; Lafrenière, S.; et al. Formulation of Screen-Printable Cu Molecular Ink for Conductive/Flexible/Solderable Cu Traces. ACS Appl. Mater. Interfaces 2019, 11, 38880–38894. [Google Scholar] [CrossRef]
- Kell, A.J.; Paquet, C.; Mozenson, O.; Djavani-Tabrizi, I.; Deore, B.; Liu, X.; Lopinski, G.P.; James, R.; Hettak, K.; Shaker, J.; et al. Versatile Molecular Silver Ink Platform for Printed Flexible Electronics. ACS Appl. Mater. Interfaces 2017, 9, 17226–17237. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.Y.; Yoo, T.H.; Song, Y.W.; Lim, D.S.; Oh, Y.J. Cu ion ink for a flexible substrate and highly conductive patterning by intensive pulsed light sintering. ACS Appl. Mater. Interfaces 2013, 5, 4113–4119. [Google Scholar] [CrossRef]
- Blasco, E.; Müller, J.; Müller, P.; Trouillet, V.; Schön, M.; Scherer, T.; Barner-Kowollik, C.; Wegener, M. Fabrication of Conductive 3D Gold-Containing Microstructures via Direct Laser Writing. Adv. Mater. 2016, 28, 3592–3595. [Google Scholar] [CrossRef]
- Cao, Y.Y.; Takeyasu, N.; Tanaka, T.; Duan, X.M.; Kawata, S. 3D metallic nanostructure fabrication by surfactant-assisted multiphoton-induced reduction. Small 2009, 5, 1144–1148. [Google Scholar] [CrossRef]
- Szalkowski, M.; Sulowska, K.; Jönsson-Niedziółka, M.; Wiwatowski, K.; Niedziółka-Jönsson, J.; Maćkowski, S.; Piatkowski, D. Photochemical Printing of Plasmonically Active Silver Nanostructures. Int. J. Mol. Sci. 2020, 21, 2006. [Google Scholar] [CrossRef] [Green Version]
- Ji, S.Y.; Kim, H.Y.; Cho, S.H.; Chang, W.S. Photochemical reduction of silver precursor and elastomer composite for flexible and conductive patterning. Materials 2019, 12, 33809. [Google Scholar] [CrossRef] [Green Version]
- Rosenbaum, C.; Murphy, M.; Lawrence, P.T.; Sirkoch, C.; Schneeberg, S.R.; Zigner, K.; Morris, S.; Richman, E.; Anyanwu, C.; Will, E.; et al. Electro- and photochemical studies of gold (III) bromide towards a novel laser-based method of gold patterning. Int. J. Extrem. Manuf. 2022, 4, 035001. [Google Scholar] [CrossRef]
- Kumaran, A.U.S.; Miyawaki, T.; Ichimura, M. Photochemical deposition of patterned gold thin films. Jpn. J. Appl. Phys. Part 2 2006, 45, L1283–L1285. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Q.; Ouyang, X.; Lei, D.Y.; Zhang, A.P.; Tam, H.Y. Ultrafast Light-Controlled Growth of Silver Nanoparticles for Direct Plasmonic Color Printing. ACS Nano 2018, 12, 9913–9921. [Google Scholar] [CrossRef]
- Zhang, Y.; Liang, Z.; Zhang, A.P.; Tam, H.Y. Direct Printing of Micropatterned Plasmonic Substrates of Size-Controlled Gold Nanoparticles by Precision Photoreduction. Adv. Opt. Mater. 2021, 9, 2001368. [Google Scholar] [CrossRef]
- Zuo, P.; Jiang, L.; Li, X.; Li, B.; Ran, P.; Li, X.; Qu, L.; Lu, Y. Metal (Ag, Pt)-MoS2 Hybrids Greenly Prepared Through Photochemical Reduction of Femtosecond Laser Pulses for SERS and HER. ACS Sustain. Chem. Eng. 2018, 6, 7704–7714. [Google Scholar] [CrossRef]
- Yang, X.; Sun, M.; Bian, Y.; He, X. A Room-Temperature High-Conductivity Metal Printing Paradigm with Visible-Light Projection Lithography. Adv. Funct. Mater. 2019, 29, 1807615. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.; Bai, J.; Yao, Y.; Wang, C. Printing continuous metal structures via polymer-assisted photochemical deposition. Mater. Today 2020, 37, 10–17. [Google Scholar] [CrossRef]
- Choi, S.; Zhao, Z.; Zuo, J.; Faruque, H.M.R.; Yao, Y.; Wang, C. Structural color printing via polymer-assisted photochemical deposition. Light Sci. Appl. 2022, 11, 84. [Google Scholar] [CrossRef]
- Wu, D.; Yao, B.; Wu, S.; Hingorani, H.; Cui, Q.; Hua, M.; Frenkel, I.; Du, Y.; Hsiai, T.K.; He, X. Room-Temperature Annealing-Free Gold Printing via Anion-Assisted Photochemical Deposition. Adv. Mater. 2022, 34, 2201772. [Google Scholar] [CrossRef]
- Wang, X.; Cui, K.; Xuan, Q.; Zhu, C.; Zhao, N.; Xu, J. Blue Laser Projection Printing of Conductive Complex 2D and 3D Metallic Structures from Photosensitive Precursors. ACS Appl. Mater. Interfaces 2019, 11, 21668–21674. [Google Scholar] [CrossRef]
- Liu, X.; Shen, Y.; Yang, R.; Zou, S.; Ji, X.; Shi, L.; Zhang, Y.; Liu, D.; Xiao, L.; Zheng, X.; et al. Inkjet printing assisted synthesis of multicomponent mesoporous metal oxides for ultrafast catalyst exploration. Nano Lett. 2012, 12, 5733–5739. [Google Scholar] [CrossRef]
- Haber, J.A.; Cai, Y.; Jung, S.; Xiang, C.; Mitrovic, S.; Jin, J.; Bell, A.T.; Gregoire, J.M. Discovering Ce-rich oxygen evolution catalysts, from high throughput screening to water electrolysis. Energy Environ. Sci. 2014, 7, 682–688. [Google Scholar] [CrossRef] [Green Version]
- Costa Bassetto, V.; Mensi, M.; Oveisi, E.; Girault, H.H.; Lesch, A. Print-Light-Synthesis of Ni and NiFe-Nanoscale Catalysts for Oxygen Evolution. ACS Appl. Energy Mater. 2019, 2, 6322–6331. [Google Scholar] [CrossRef]
- Zhao, L.; Liu, Z.; Chen, D.; Liu, F.; Yang, Z.; Li, X.; Yu, H.; Liu, H.; Zhou, W. Laser Synthesis and Microfabrication of Micro/Nanostructured Materials Toward Energy Conversion and Storage. Nano-Micro Lett. 2021, 13, 49. [Google Scholar] [CrossRef]
- Zhou, X.; Guo, W.; Zhu, Y.; Peng, P. The laser writing of highly conductive and anti-oxidative copper structures in liquid. Nanoscale 2020, 12, 563–571. [Google Scholar] [CrossRef]
- Silva, W.O.; Costa Bassetto, V.; Baster, D.; Mensi, M.; Oveisi, E.; Girault, H.H. Oxidative Print Light Synthesis Thin Film Deposition of Prussian Blue. ACS Appl. Electron. Mater. 2020, 2, 927–935. [Google Scholar] [CrossRef]
- Kindle, C.; Castonguay, A.; McGee, S.; Tomko, J.A.; Hopkins, P.E.; Zarzar, L.D. Direct Laser Writing from Aqueous Precursors for Nano to Microscale Topographical Control, Integration, and Synthesis of Nanocrystalline Mixed Metal Oxides. ACS Appl. Nano Mater. 2019, 2, 2581–2586. [Google Scholar] [CrossRef]
- McGee, S.; Fest, A.; Chandler, C.; Nova, N.N.; Lei, Y.; Goff, J.; Sinnott, S.B.; Dabo, I.; Terrones, M.; Zarzar, L.D. Direct Laser Writing of Multimetal Bifunctional Catalysts for Overall Water Splitting. ACS Appl. Energy Mater. 2023, 6, 3756–3768. [Google Scholar] [CrossRef]
- Castonguay, A.C.; Yi, N.; Li, B.; Zhao, J.; Li, H.; Gao, Y.; Nova, N.N.; Tiwari, N.; Zarzar, L.D.; Cheng, H. Direct Laser Writing of Microscale Metal Oxide Gas Sensors from Liquid Precursors. ACS Appl. Mater. Interfaces 2022, 14, 28163–28173. [Google Scholar] [CrossRef]
- Bae, G.; Kim, M.; Jang, M.; Kwon, Y.M.; Yim, S.; Song, W.; Myung, S.; Lee, S.S.; Hasan, T.; An, K.S. Photon-Pen Writing for Metal Oxide-Based Versatile Nanosensors. Adv. Funct. Mater. 2022, 32, 2204821. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gianvittorio, S.; Tonelli, D.; Lesch, A. Print-Light-Synthesis for Single-Step Metal Nanoparticle Synthesis and Patterned Electrode Production. Nanomaterials 2023, 13, 1915. https://doi.org/10.3390/nano13131915
Gianvittorio S, Tonelli D, Lesch A. Print-Light-Synthesis for Single-Step Metal Nanoparticle Synthesis and Patterned Electrode Production. Nanomaterials. 2023; 13(13):1915. https://doi.org/10.3390/nano13131915
Chicago/Turabian StyleGianvittorio, Stefano, Domenica Tonelli, and Andreas Lesch. 2023. "Print-Light-Synthesis for Single-Step Metal Nanoparticle Synthesis and Patterned Electrode Production" Nanomaterials 13, no. 13: 1915. https://doi.org/10.3390/nano13131915
APA StyleGianvittorio, S., Tonelli, D., & Lesch, A. (2023). Print-Light-Synthesis for Single-Step Metal Nanoparticle Synthesis and Patterned Electrode Production. Nanomaterials, 13(13), 1915. https://doi.org/10.3390/nano13131915






