Structure and Optical Anisotropy of Spider Scales and Silk: The Use of Chromaticity and Azimuth Colors to Optically Characterize Complex Biological Structures
Abstract
1. Introduction
2. Materials and Methods
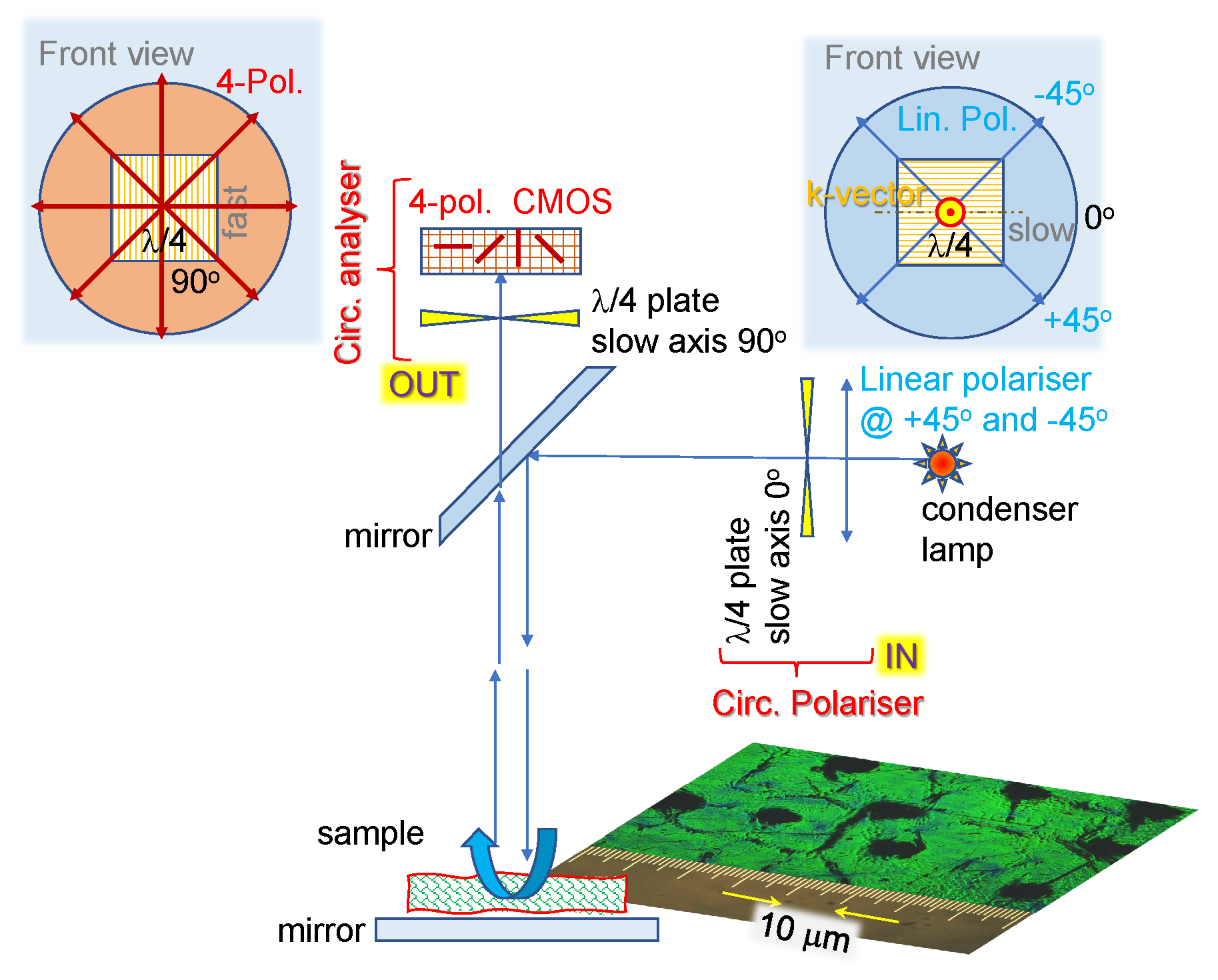
2.1. Optical Imaging Techniques
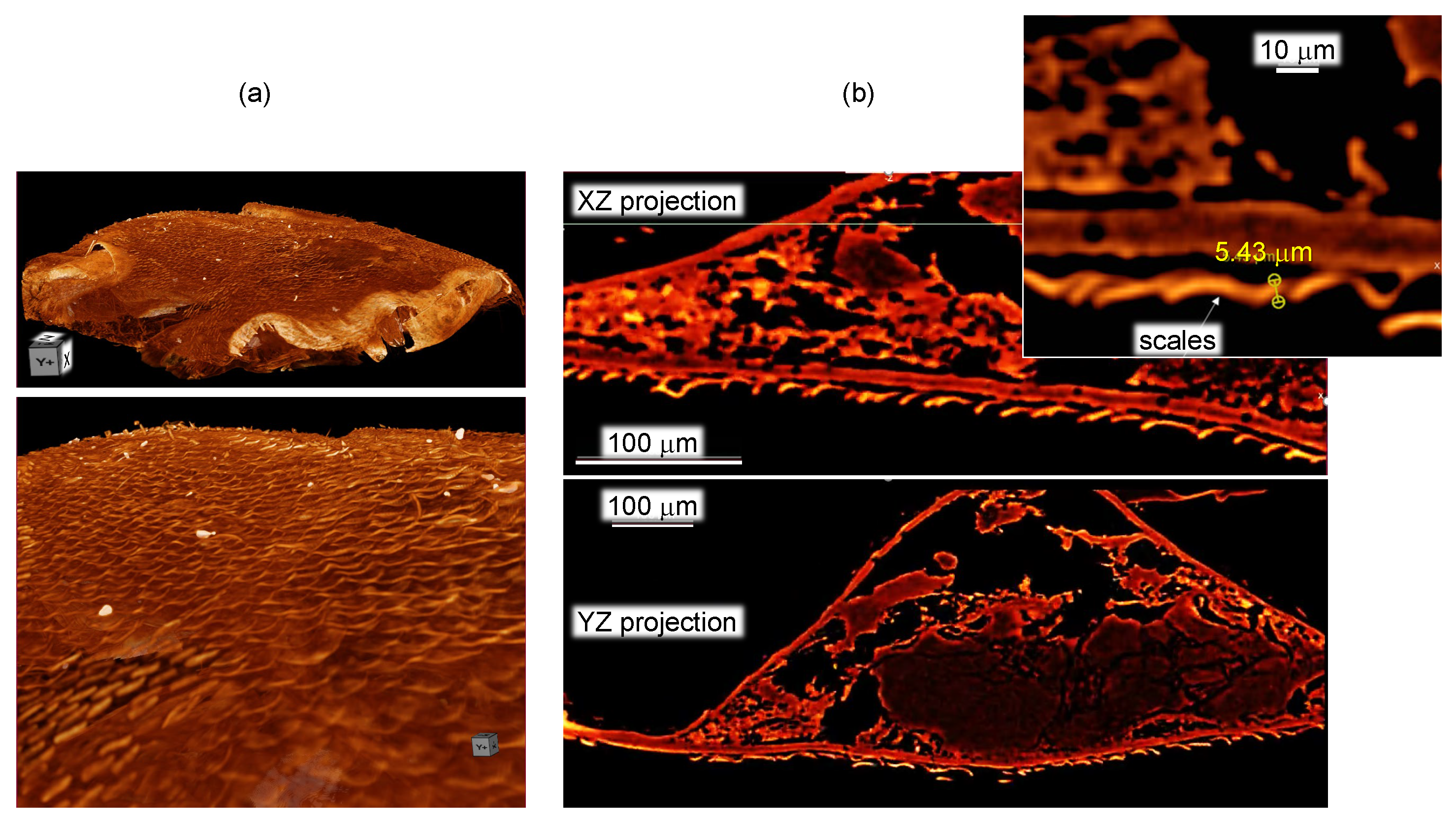
2.2. X-ray Tomography
3. Results
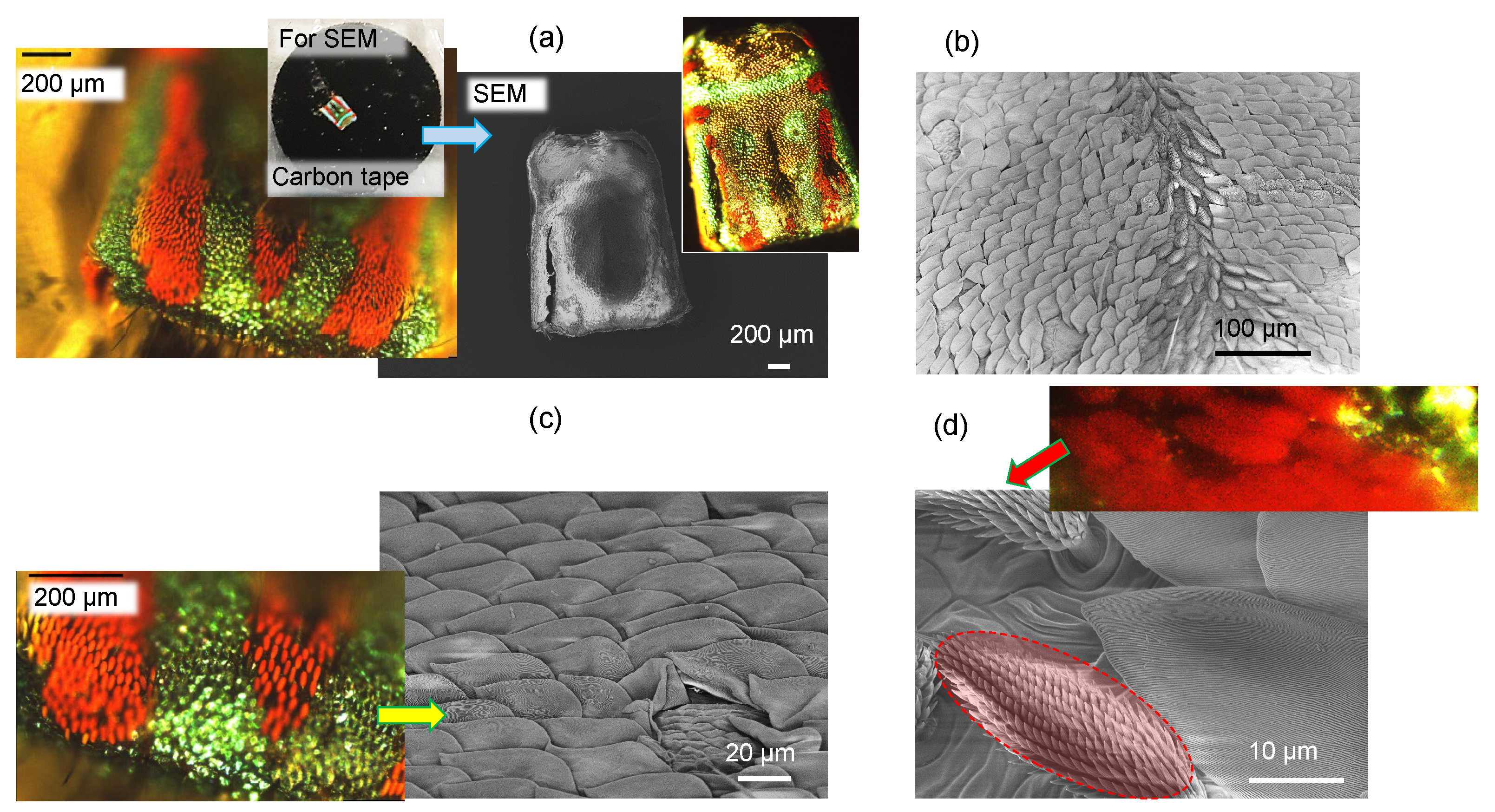
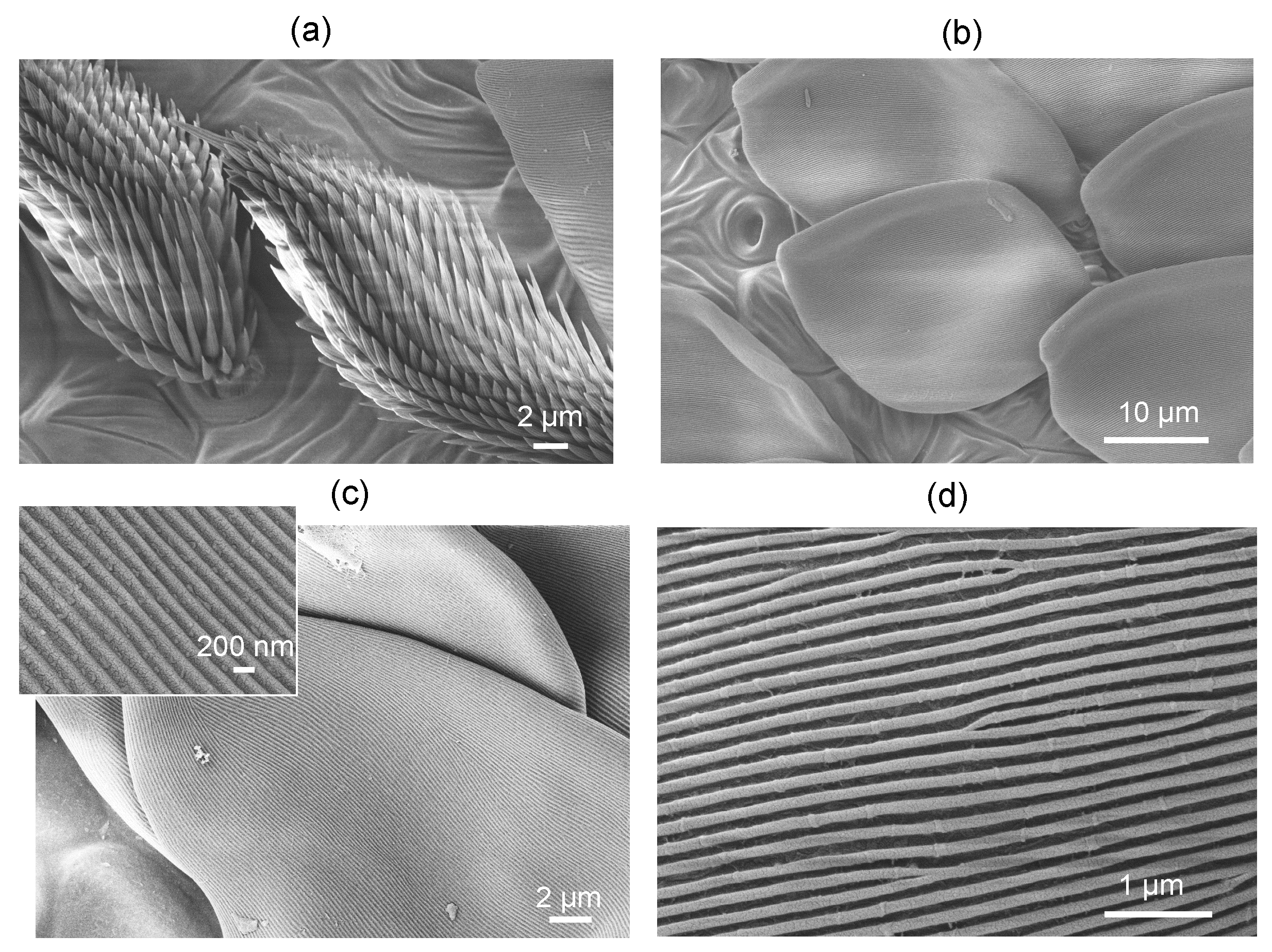
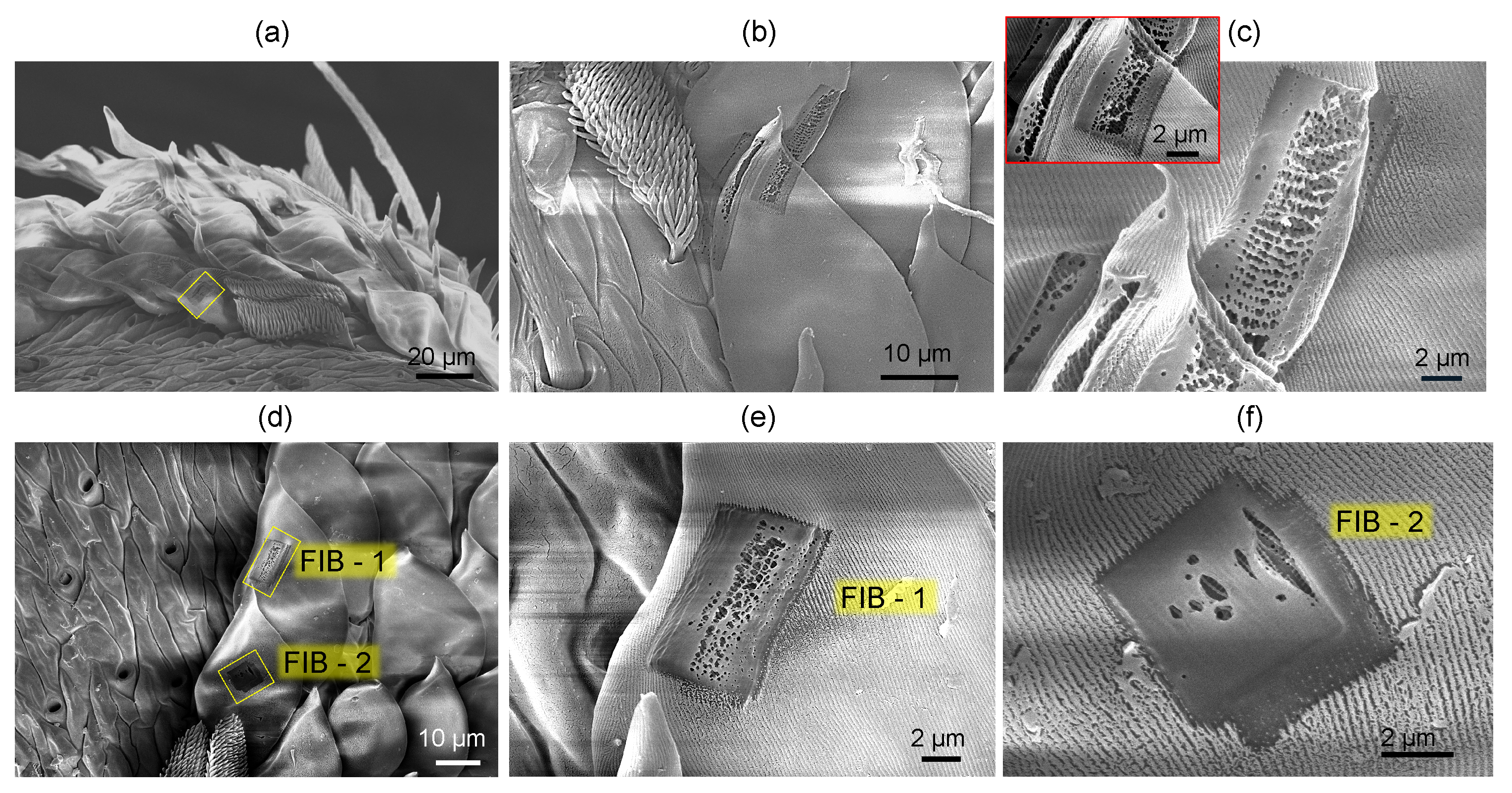
3.1. Spider Scales: Peacock Spider
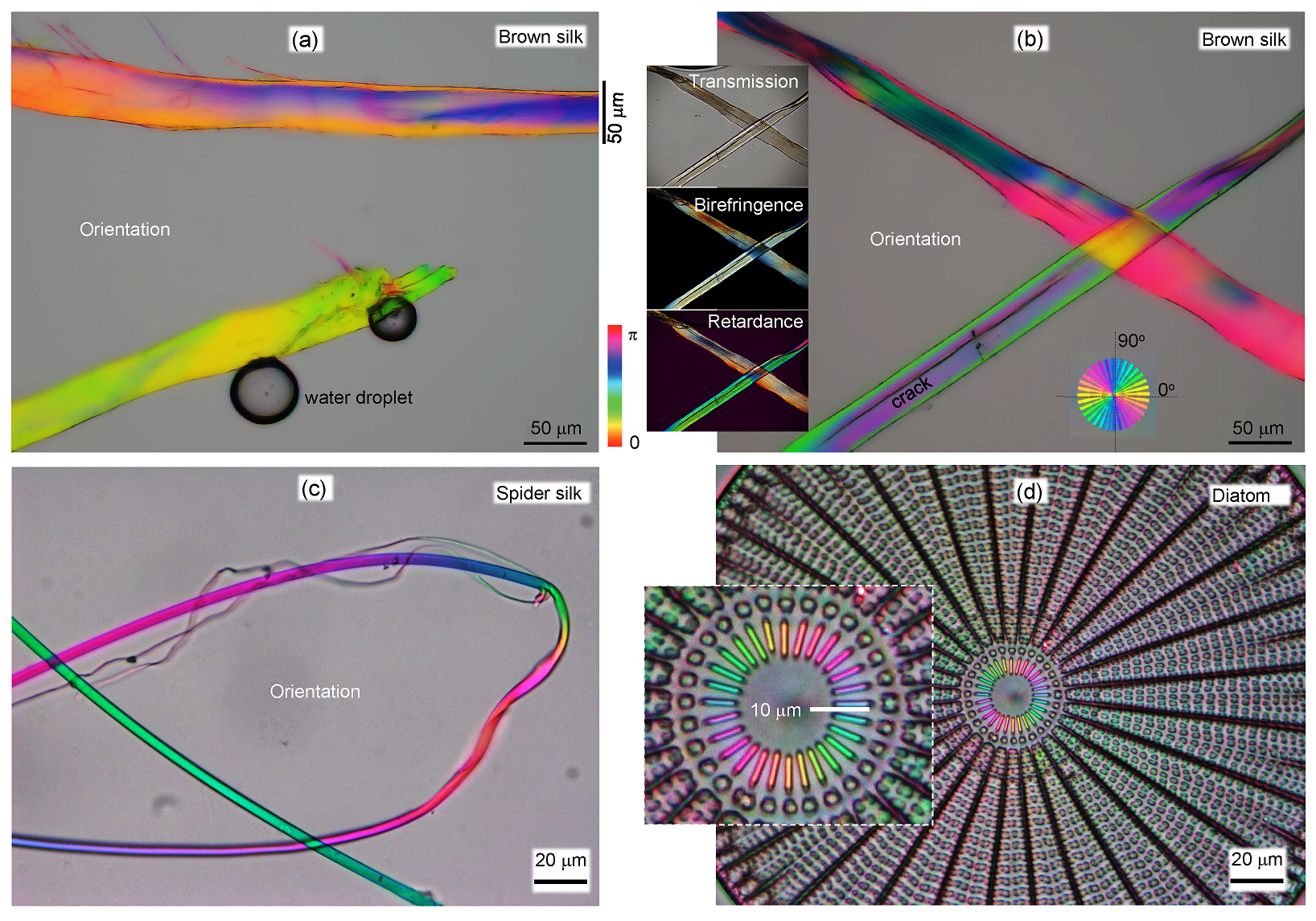
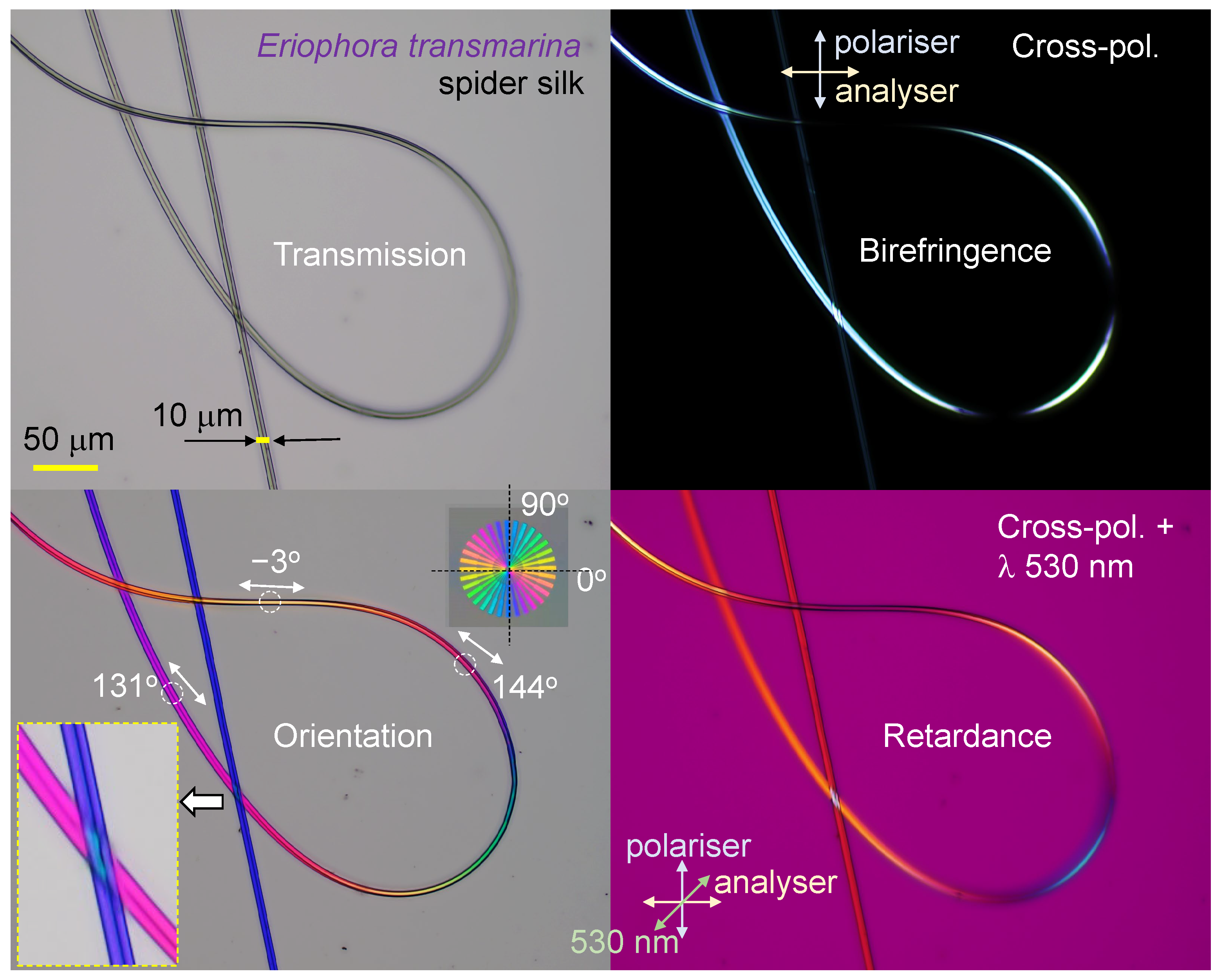
3.2. Spider Silk
4. Discussion
4.1. Polychromatic Polarizing Module
4.2. Four-Polarization Camera
4.3. Stokes Parameters from 4-pol. Imaging
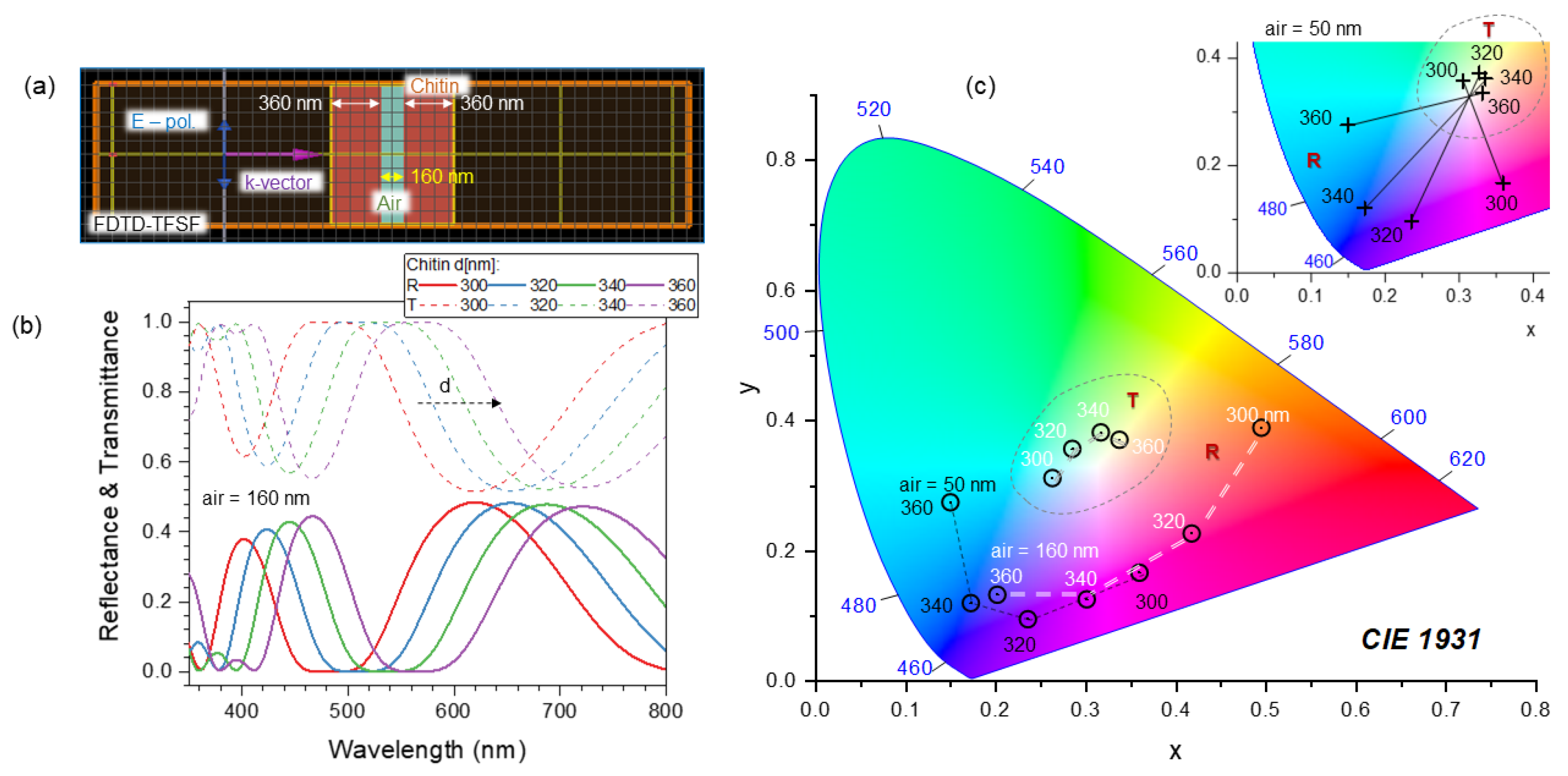
4.4. Nanotextured Surfaces for Analysis of Polarization Anisotropy in Reflection
5. Conclusions and Outlook
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Complex and Larger Fibers


Appendix B. Model: Spider Scale Illuminated at an Angle
Appendix C. Sub-100 nm Surface Texturing

Appendix D. Spider Silk Test in Outer Space

References
- Rothammer, M.; Zollfrank, C.; Busch, K.; von Freymann, G. Tailored Disorder in Photonics: Learning from Nature. Adv. Optical Mater. 2021, 9, 2100787. [Google Scholar] [CrossRef]
- Parker, A.; Hegedus, Z. Diffractive optics in spiders. J. Opt. A Pure Appl. Opt. 2003, 5, S111. [Google Scholar] [CrossRef]
- Natural History Museum Bern. World Spider Catalog. Version 24. Available online: https://wsc.nmbe.ch/ (accessed on 26 March 2023).
- Kariko, S.; Timonen, J.; Weaver, J.; Gur, D.; Marks, C.; Leiserowitz, L.; Kolle, M.; Li, L. Structural origins of coloration in the spider Phoroncidia rubroargentea Berland, 1913 (Araneae: Theridiidae) from Madagascar. J. R. Soc. Interface 2018, 15, 20170930. [Google Scholar] [CrossRef] [PubMed]
- John, M.; Thomas, S. Biofibres and biocomposites. Carbohydr. Polym. 2008, 71, 343–364. [Google Scholar] [CrossRef]
- Ryu, M.; Honda, R.; Reich, A.; Cernescu, A.; Li, J.L.; Hu, J.; Juodkazis, S.; Morikawa, J. Near-Field IR Orientational Spectroscopy of Silk. Appl. Sci. 2019, 9, 3991. [Google Scholar] [CrossRef]
- Fujisawa, H.; Ryu, M.; Lundgaard, S.; Linklater, D.; Ivanova, E.; Nishijima, Y.; Juodkazis, S.; Morikawa, J. Direct Measurement of Temperature Diffusivity of Nanocellulose-Doped Biodegradable Composite Films. Micromachines 2020, 11, 738. [Google Scholar] [CrossRef]
- Ryu, M.; Balčytis, A.; Wang, X.; Vongsvivut, J.; Hikima, Y.; Li, J.; Tobin, M.J.; Juodkazis, S.; Morikawa, J. Orientational Mapping Augmented Sub-Wavelength Hyper-Spectral Imaging of Silk. Sci. Rep. 2017, 7, 7419. [Google Scholar] [CrossRef]
- Ryu, M.; Kobayashi, H.; Balcytis, A.; Wang, X.; Vongsvivut, J.; Li, J.; Urayama, N.; Mizeikis, V.; Tobin, M.; Juodkazis, S. Nanoscale chemical mapping of laser-solubilized silk. Mater. Res. Express 2017, 4, 115028. [Google Scholar] [CrossRef]
- Ryu, M.; Honda, R.; Cernescu, A.; Vailionis, A.; Balcytis, A.; Vongsvivut, J.; Li, J.L.; Linklater, D.; Ivanova, E.; Mizeikis, V.; et al. Nanoscale optical and structural characterisation of silk. Beilstein J. Nanotechnol. 2019, 10, 922–929. [Google Scholar] [CrossRef]
- Moestopo, W.P.; Shaker, S.; Deng, W.; Greer, J.R. Knots are not for naught: Design, properties, and topology of hierarchical intertwined microarchitected materials. Sci. Adv. 2023, 9, eade6725. [Google Scholar] [CrossRef]
- Blamires, S.; Nobbs, M.; Martens, P.; Tso, I.; Chuang, W.; Chang, C.; Sheu, H. Multiscale mechanisms of nutritionally-induced property variation in spider silk. PLoS ONE 2018, 13, e0192005. [Google Scholar] [CrossRef]
- Blamires, S.; Little, D.; White, T.; Kane, D. Photoreflectance/scattering measurements of spider silks informed by standard optics. R. Soc. Open Sci. 2020, 7, 192174. [Google Scholar] [CrossRef]
- Blamires, S.; Cerexhe, G.; White, T.; Herberstein, M.; Kasumovic, M. Spider silk colouration co-varies with thermal properties but not protein structure. J. R. Soc. Interface 2019, 16, 20190199. [Google Scholar] [CrossRef]
- Balčytis, A.; Ryu, M.; Wang, X.; Novelli, F.; Seniutinas, G.; Du, S.; Wang, X.; Li, J.; Davis, J.; Appadoo, D.; et al. Silk: Optical Properties over 12.6 Octaves THz-IR-Visible-UV Range. Materials 2017, 10, 356. [Google Scholar] [CrossRef]
- Rajabi, M.; Lavrentovich, O.; Shribak, M. Instantaneous mapping of liquid crystal orientation using a polychromatic polarizing microscope. Liquid Crystals 2023, 50, 181–190. [Google Scholar] [CrossRef]
- Ingram, A.; Parker, A. Diffractive optics in spiders. Philos. Trans. R Soc. Lond. B Biol. Sci. 2008, 363, 2465–2480. [Google Scholar] [CrossRef]
- Saito, A.; Yonezawa, M.; Murase, J.; Juodkazis, S.; Mizeikis, V.; Akai-Kasaya, M.; Kuwahara, Y. Numerical analysis on the optical role of nanometer scale randomness on the Morpho butterfly’s scale. J. Nanosci. Nanotechnol. 2011, 11, 2785–2792. [Google Scholar] [CrossRef]
- Saito, A.; Yamashita1, K.; Hattori, T.; Kuwahara, Y. Novel optical applications inspired by the Morpho butterfly’s coloration: Technology transfer from reflection to transmission. Jpn. J. Appl. Phys. 2022, 61, SD0801. [Google Scholar] [CrossRef]
- Stavenga, D.; Otto, J.; Wilts, B. Splendid coloration of the peacock spider Maratus splendens. J. R. Soc. Interface 2016, 2016, 20160437. [Google Scholar] [CrossRef]
- McCoy, D.; McCoy, V.; Mandsberg, N.; Shneidman, A.; Aizenberg, J.; Prum, R.; Haig, D. Structurally assisted super black in colourful peacock spiders. Proc. Biol. Sci. 2019, 286, 20190589. [Google Scholar] [CrossRef]
- Han, M.; Smith, D.; Ng, S.H.; Vilagosh, Z.; Anand, V.; Katkus, T.; Reklaitis, I.; Mu, H.; Ryu, M.; Morikawa, J.; et al. THz Filters Made by Laser Ablation of Stainless Steel and Kapton Film. Micromachines 2022, 13, 1170. [Google Scholar] [CrossRef] [PubMed]
- Honda, R.; Ryu, M.; Li, J.L.; Mizeikis, V.; Juodkazis, S.; Morikawa, J. Simple multi-wavelength imaging of birefringence:case study of silk. Sci. Rep. 2018, 8, 17652. [Google Scholar] [CrossRef] [PubMed]
- Shimotsuma, Y.; Kazansky, P.; Qiu, J.; Hirao, K. Self-Organized Nanogratings in Glass Irradiated by Ultrashort Light Pulses. Phys. Rev. Lett. 2003, 91, 247405. [Google Scholar] [CrossRef] [PubMed]
- Ryu, M.; Nishijima, Y.; To, S.M.N.; Hashizume, T.; Matsubara, R.; Kubono, A.; Hu, J.; Ng, S.; Juodkazis, S.; Morikawa, J. Hyperspectral Molecular Orientation Mapping in Metamaterials. Appl. Sci. 2021, 11, 1544. [Google Scholar] [CrossRef]
- Stavenga, G.; Wilts, B.; Leertouwer, H.; Hariyama, T. Polarized iridescence of the multilayered elytra of the Japanese jewel beetle, Chrysochroa fulgidissima. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2011, 366, 709–723. [Google Scholar] [CrossRef]
- Hikima, Y.; Morikawa, J.; Hashimoto, T. FT-IR Image Processing Algorithms for In-Plane Orientation Function and Azimuth Angle of Uniaxially Drawn Polyethylene Composite Film. Macromolecules 2011, 44, 3950–3957. [Google Scholar] [CrossRef]
- Honda, R.; Ryu, M.; Moritake, M.; Balcytis, A.; Mizeikis, V.; Vongsvivut, J.; Tobin, M.J.; Appadoo, D.; Li, J.L.; Ng, S.H.; et al. Hyperspectral mapping of anisotropy. Nanoscale Horizons 2019, 4, 1443–1449. [Google Scholar]
- Honda, R.; Ryu, M.; Balcytis, A.; Vongsvivut, J.; Tobin, M.J.; Juodkazis, S.; Morikawa, J. Paracetamol micro-structure analysis by optical mapping. Appl. Surf. Sci. 2019, 473, 127–132. [Google Scholar] [CrossRef]
- Collett, E. Polarization, 3rd ed.; SPIE Press, Field Guides: Bellingham, UK, 2005. [Google Scholar]
- Honda, R.; Ryu, M.; Moritake, M.; Balcytis, A.; Mizeikis, V.; Vongsvivut, J.; Tobin, M.J.; Appadoo, D.; Li, J.L.; Ng, S.H.; et al. Infrared Polariscopy Imaging of Linear Polymeric Patterns with a Focal Plane Array. Nanomaterials 2019, 9, 732. [Google Scholar] [CrossRef]
- Ng, S.; Allan, B.; Ierodiaconou, D.; Anand, V.; Babanin, A.; Juodkazis, S. Drone Polariscopy—Towards Remote Sensing Applications. Eng. Proc. 2021, 11, 46. [Google Scholar]
- Brasselet, E.; Gervinskas, G.; Seniutinas, G.; Juodkazis, S. Topological shaping of light by closed-path nanoslits. Phys. Rev. Lett. 2013, 111, 193901. [Google Scholar] [CrossRef]
- Nishijima, Y.; Komatsu, R.; Ota, S.; Seniutinas, G.; Balčytis, A.; Juodkazis, S. Anti-reflective surfaces: Cascading nano/microstructuring. Appl. Phys. Lett. Photonics 2016, 1, 076104. [Google Scholar] [CrossRef]
- Ivanova, E.P.; Hasan, J.; Webb, H.K.; Gervinskas, G.; Juodkazis, S.; Truong, V.K.; Wu, A.H.F.; Lamb, R.N.; Baulin, V.; Watson, G.S.; et al. Bactericidal activity of nanostructured black silicon. Nature Commun. 2013, 4, 2838. [Google Scholar] [CrossRef]
- Nagai, T.; Yuyama, K.I.; Shoji, T.; Matsumura, Y.; Linklater, D.; Ivanova, E.; Juodkazis, S.; Tsuboi, Y. Wavelength-Sensitive Optical Tweezers Using Black-Si Nanospikes for Controlling the Internal Polarity of a Polymer Droplet. ACS Appl. Nano Mater. 2023, 6, 180–189. [Google Scholar] [CrossRef]
- Ryu, M.; Ng, S.; Han, M.; Anand, V.; Katkus, T.; Vongsvivut, J.; Appadoo, D.; Nishijima, Y.; Juodkazis, S.; Morikawa, J. Polariscopy with optical near-fields. Nanoscale Horiz. 2022, 7, 1047–1053. [Google Scholar]
- Ryu, M.; Ng, S.; Anand, V.; Lundgaard, S.; Hu, J.; Katkus, T.; Appadoo, D.; Vilagosh, Z.; Wood, A.; Juodkazis, S.; et al. Attenuated Total Reflection at THz Wavelengths: Prospective Use of Total Internal Reflection and Polariscopy. Appl. Sci. 2021, 11, 7632. [Google Scholar] [CrossRef]
- Linklater, D.; Juodkazis, S.; Ivanova, E. Nanofabrication of mechano-bactericidal surfaces. Nanoscale 2017, 9, 16564–16585. [Google Scholar] [CrossRef]
- Linklater, D.; Nguyen, H.; Bhadra, C.; Juodkazis, S.; Ivanova, E. Influence of nanoscale topology on bactericidal efficiency of black silicon surfaces. Nanotechnology 2017, 28, 469501. [Google Scholar] [CrossRef]
- Linklater, D.; Baulin, V.; Juodkazis, S.; Crawford, R.; Stoodley, P.; Ivanova, E. Mechano-bactericidal actions of nanostructured surfaces. Nat. Rev. Microbiol. 2021, 19, 8–22. [Google Scholar] [CrossRef]
- Linklater, D.; Saita, S.; Murata, T.; Yanagishita, T.; Dekiwadia, C.; Crawford, R.; Masuda, H.; Kusaka, H.; Ivanova, E. Nanopillar Polymer Films as Antibacterial Packaging Materials. ACS Appl. Nano Mater. 2022, 5, 2578–2591. [Google Scholar] [CrossRef]
- Salimon, A.I.; Sapozhnikov, P.V.; Everaerts, J.; Kalinina, O.Y.; Besnard, C.; Papadaki, C.; Cvjetinovic, J.; Statnik, E.S.; Kan, Y.; Korsunsky, A.M.; et al. A Mini-Atlas of diatom frustule electron microscopy images at different magnifications. Mater. Today Proc. 2020, 33, 1924–1933. [Google Scholar] [CrossRef]
- Masuda, H.; Fukuda, K. Ordered metal nanohole arrays made by a two-step replication of honeycomb structures of anodic alumina. Science 1995, 268, 1466. [Google Scholar] [CrossRef] [PubMed]
- Masuda, H.; Satoh, M. Fabrication of gold nanodot array using anodic porous alumina as an evaporation mask. Jpn. J. Appl. Phys. Part 2 Lett. 1996, 35, 126. [Google Scholar] [CrossRef]
- Kondo, T.; Fukushima, T.; Nishio, K.; Masuda, H. Surface-Enhanced Raman Scattering in Hierarchical Structures of Au Formed Using Templates by Site-Controlled Tunnel Etching of Al. Appl. Phys. Express 2009, 2, 125001. [Google Scholar] [CrossRef]
- Masuda, H.; Yanagishita, T.; Kondo, T. Encyclopedia of Interfacial Chemistry: Surface Science and Electrochemistry; Elsevier: Amsterdam, The Netherlands, 2018; Chapter Fabrication of Anodic Porous Alumina; pp. 226–235. [Google Scholar]
- ASTM E595-15; Standard Test Method for Total Mass Loss and Collected Volatile Condensable Materials from Outgassing in a Vacuum Environment. ASTM: West Conshohocken, PA, USA, 2021.




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Linklater, D.; Vailionis, A.; Ryu, M.; Kamegaki, S.; Morikawa, J.; Mu, H.; Smith, D.; Maasoumi, P.; Ford, R.; Katkus, T.; et al. Structure and Optical Anisotropy of Spider Scales and Silk: The Use of Chromaticity and Azimuth Colors to Optically Characterize Complex Biological Structures. Nanomaterials 2023, 13, 1894. https://doi.org/10.3390/nano13121894
Linklater D, Vailionis A, Ryu M, Kamegaki S, Morikawa J, Mu H, Smith D, Maasoumi P, Ford R, Katkus T, et al. Structure and Optical Anisotropy of Spider Scales and Silk: The Use of Chromaticity and Azimuth Colors to Optically Characterize Complex Biological Structures. Nanomaterials. 2023; 13(12):1894. https://doi.org/10.3390/nano13121894
Chicago/Turabian StyleLinklater, Denver, Arturas Vailionis, Meguya Ryu, Shuji Kamegaki, Junko Morikawa, Haoran Mu, Daniel Smith, Pegah Maasoumi, Rohan Ford, Tomas Katkus, and et al. 2023. "Structure and Optical Anisotropy of Spider Scales and Silk: The Use of Chromaticity and Azimuth Colors to Optically Characterize Complex Biological Structures" Nanomaterials 13, no. 12: 1894. https://doi.org/10.3390/nano13121894
APA StyleLinklater, D., Vailionis, A., Ryu, M., Kamegaki, S., Morikawa, J., Mu, H., Smith, D., Maasoumi, P., Ford, R., Katkus, T., Blamires, S., Kondo, T., Nishijima, Y., Moraru, D., Shribak, M., O’Connor, A., Ivanova, E. P., Ng, S. H., Masuda, H., & Juodkazis, S. (2023). Structure and Optical Anisotropy of Spider Scales and Silk: The Use of Chromaticity and Azimuth Colors to Optically Characterize Complex Biological Structures. Nanomaterials, 13(12), 1894. https://doi.org/10.3390/nano13121894









