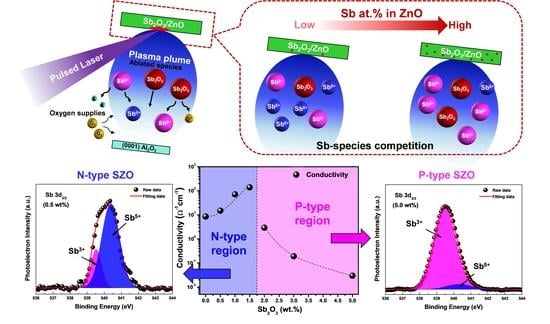
Influence of Antimony Species on Electrical Properties of Sb-Doped Zinc Oxide Thin Films Prepared by Pulsed Laser Deposition
Abstract
1. Introduction
2. Experimental Details
2.1. Preparation of Sb2O3:ZnO Composite Targets with Different Contents of Sb2O3
2.2. Preparation of ZnO and SZO Thin Film via Pulsed Laser Deposition
2.3. Strategy to Qualitatively Control Sb Species during the Pulsed Laser Deposition
2.4. Characterization of Specific Properties of ZnO and SZO Thin Films
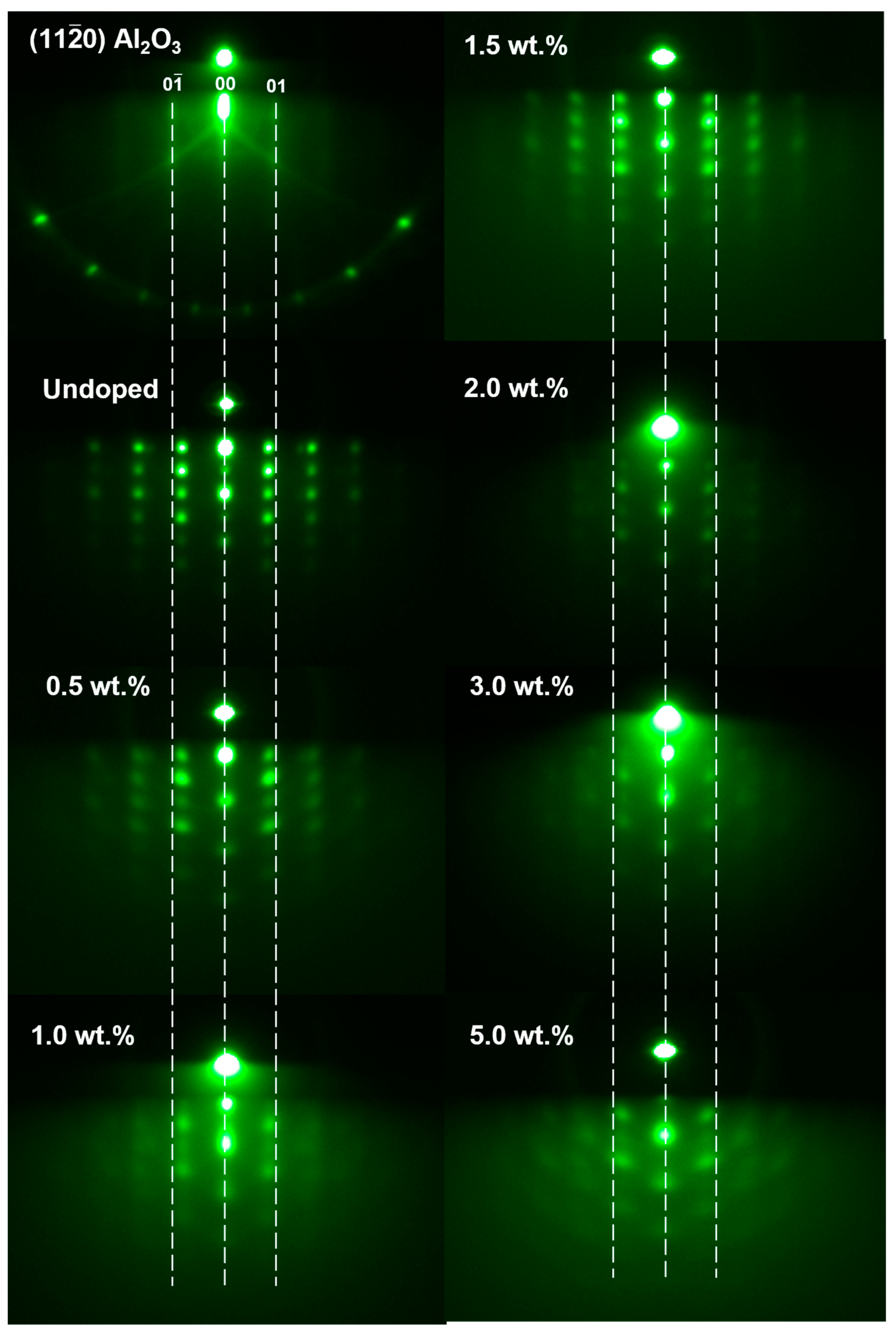
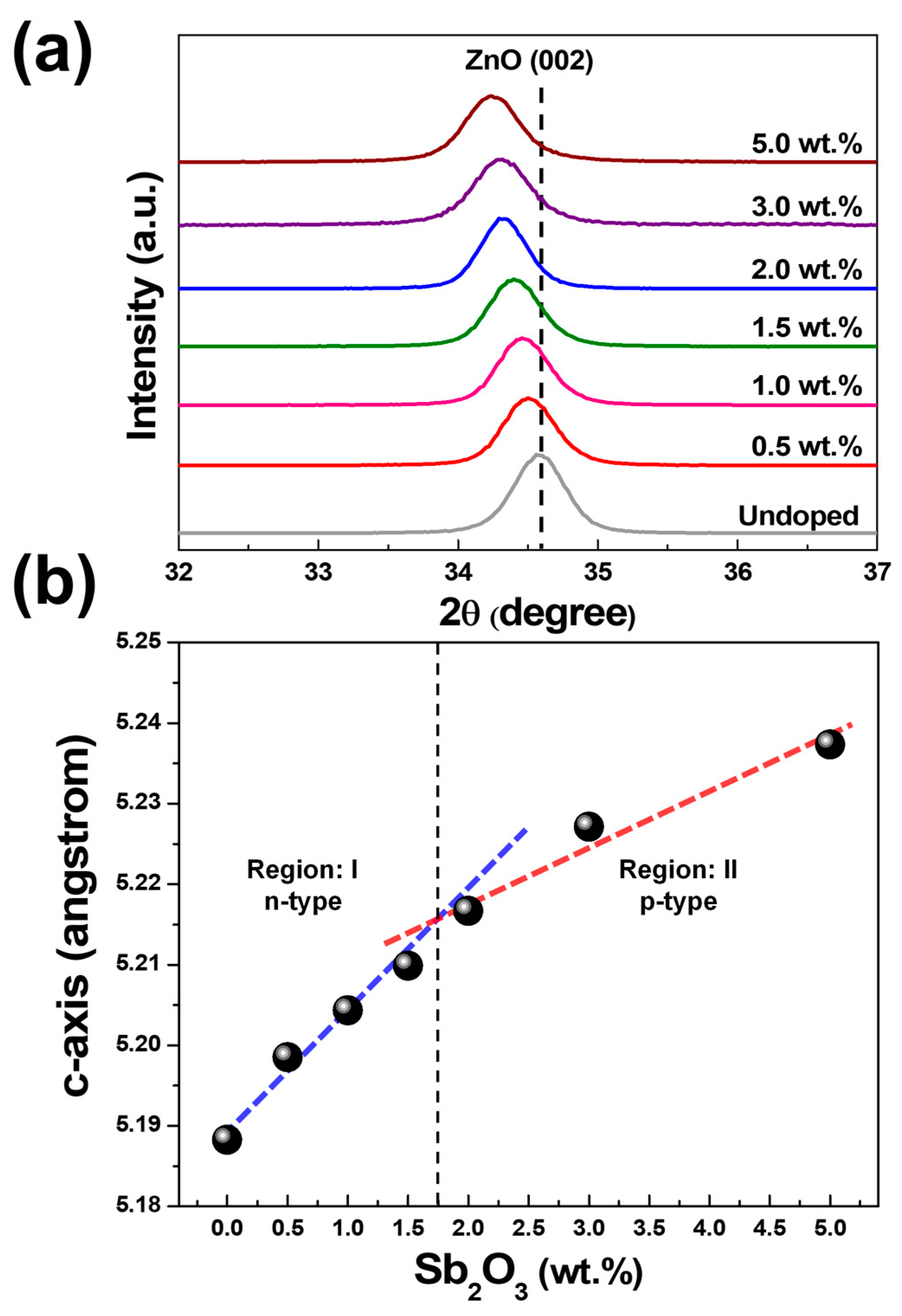
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, K.; Sakurai, M.; Aono, M. ZnO-Based Ultraviolet Photodetectors. Sensors 2010, 10, 8604–8634. [Google Scholar] [CrossRef]
- Yang, Z.; Chu, S.; Chen, W.V.; Li, L.; Kong, J.; Ren, J.; Yu, P.K.L.; Liu, J. ZnO:Sb/ZnO:Ga Light Emitting Diode onc-Plane Sapphire by Molecular Beam Epitaxy. Appl. Phys. Express 2010, 3, 032101. [Google Scholar] [CrossRef]
- Pearton, S.J.; Ren, F. Advances in ZnO-based materials for light emitting diodes. Curr. Opin. Chem. Eng. 2014, 3, 51–55. [Google Scholar] [CrossRef]
- Vittal, R.; Ho, K.-C. Zinc oxide based dye-sensitized solar cells: A review. Renew. Sustain. Energ. Rev. 2017, 70, 920–935. [Google Scholar] [CrossRef]
- da Silva-Neto, M.L.; de Oliveira, M.C.A.; Dominguez, C.T.; Lins, R.E.M.; Rakov, N.; de Araujo, C.B.; Menezes, L.S.; de Oliveira, H.P.; Gomes, A.S.L. UV random laser emission from flexible ZnO-Ag-enriched electrospun cellulose acetate fiber matrix. Sci. Rep. 2019, 9, 11765. [Google Scholar] [CrossRef] [PubMed]
- Özgür, Ü.; Alivov, Y.I.; Liu, C.; Teke, A.; Reshchikov, M.A.; Doğan, S.; Avrutin, V.; Cho, S.J.; Morkoç, H. A comprehensive review of ZnO materials and devices. J. Appl. Phys. 2005, 98, 041301. [Google Scholar] [CrossRef]
- McCluskey, M.D.; Jokela, S.J. Defects in ZnO. J. Appl. Phys. 2009, 106, 071101. [Google Scholar] [CrossRef]
- Van de Walle, C.G. Hydrogen as a Cause of Doping in Zinc Oxide. Phys. Rev. Lett. 2000, 85, 1012–1015. [Google Scholar] [CrossRef]
- Zhang, S.B.; Wei, S.H.; Zunger, A. Intrinsicn-type versusp-type doping asymmetry and the defect physics of ZnO. Phys. Rev. B 2001, 63, 075205. [Google Scholar] [CrossRef]
- Ilyas, U.; Rawat, R.S.; Tan, T.L.; Lee, P.; Chen, R.; Sun, H.D.; Fengji, L.; Zhang, S. Oxygen rich p-type ZnO thin films using wet chemical route with enhanced carrier concentration by temperature-dependent tuning of acceptor defects. J. Appl. Phys. 2011, 110, 093522. [Google Scholar] [CrossRef]
- Chen, J.Y.; Zhang, H.T.; Chen, Q.; Husian, F.; Cherng, J.-S. Stable p-type nitrogen-doped zinc oxide films prepared by magnetron sputtering. Vacuu 2020, 180, 109576. [Google Scholar] [CrossRef]
- Cao, L.; Zhu, L.; Jiang, J.; Li, Y.; Zhang, Y.; Ye, Z. Preparation and properties of p-type Ag-doped ZnMgO thin films by pulsed laser deposition. J. Alloys Compd. 2012, 516, 157–160. [Google Scholar] [CrossRef]
- Sáaedi, A.; Yousefi, R.; Jamali-Sheini, F.; Cheraghizade, M.; Khorsand Zak, A.; Huang, N.M. Optical and electrical properties of p-type Li-doped ZnO nanowires. Superlattices Microstruct. 2013, 61, 91–96. [Google Scholar] [CrossRef]
- Lin, S.S.; Lu, J.G.; Ye, Z.Z.; He, H.P.; Gu, X.Q.; Chen, L.X.; Huang, J.Y.; Zhao, B.H. p-type behavior in Na-doped ZnO films and ZnO homojunction light-emitting diodes. Solid State Commun. 2008, 148, 25–28. [Google Scholar] [CrossRef]
- Limpijumnong, S.; Zhang, S.B.; Wei, S.H.; Park, C.H. Doping by large-size-mismatched impurities: The microscopic origin of arsenic- or antimony-doped p-type zinc oxide. Phys. Rev. Lett. 2004, 92, 155504. [Google Scholar] [CrossRef] [PubMed]
- Xiu, F.X.; Yang, Z.; Mandalapu, L.J.; Zhao, D.T.; Liu, J.L.; Beyermann, W.P. High-mobility Sb-doped p-type ZnO by molecular-beam epitaxy. Appl. Phys. Lett. 2005, 87, 152101. [Google Scholar] [CrossRef]
- Qin, J.M.; Yao, B.; Yan, Y.; Zhang, J.Y.; Jia, X.P.; Zhang, Z.Z.; Li, B.H.; Shan, C.X.; Shen, D.Z. Formation of stable and reproducible low resistivity and high carrier concentration p-type ZnO doped at high pressure with Sb. Appl. Phys. Lett. 2009, 95, 022101. [Google Scholar] [CrossRef]
- Przezdziecka, E.; Paradowska, K.M.; Lisowski, W.; Wierzbicka, A.; Jakiela, R.; Zielony, E.; Gumienny, Z.; Placzek-Popko, E.; Kozanecki, A. ZnO:Sb MBE layers with different Sb content-optical, electronic and structural analysis. J. Alloys Compd. 2019, 797, 1163–1172. [Google Scholar] [CrossRef]
- Luo, C.; Ho, L.-P.; Azad, F.; Anwand, W.; Butterling, M.; Wagner, A.; Kuznetsov, A.; Zhu, H.; Su, S.; Ling, F.C.-C. Sb-related defects in Sb-doped ZnO thin film grown by pulsed laser deposition. J. Appl. Phys. 2018, 123, 161525. [Google Scholar] [CrossRef]
- Petretto, G.; Bruneval, F. Comprehensive Ab Initio Study of Doping in Bulk ZnO with Group-V Elements. Phys. Rev. Appl. 2014, 1, 024005. [Google Scholar] [CrossRef]
- Cheng, Y.; Yang, K.; Chen, J.; Che, L.; Zhang, X. Influence of molar ratio of Sb/Zn on the crystal, electrical and optical properties of Sb-doped ZnO films. J. Alloys Compd. 2017, 699, 690–694. [Google Scholar] [CrossRef]
- Nasser, R.; Song, J.-M.; Elhouichet, H. Epitaxial growth and properties study of p-type doped ZnO:Sb by PLD. Superlattices Microstruct. 2021, 155, 106908. [Google Scholar] [CrossRef]
- Chen, Y.; Bagnall, D.M.; Koh, H.-J.; Park, K.-T.; Hiraga, K.; Zhu, Z.; Yao, T. Plasma assisted molecular beam epitaxy of ZnO on c -plane sapphire: Growth and characterization. J. Appl. Phys. 1998, 84, 3912–3918. [Google Scholar] [CrossRef]
- Chen, Y.; Bagnall, D.M.; Zhu, Z.; Sekiuchi, T.; Park, K.-t.; Hiraga, K.; Yao, T.; Koyama, S.; Shen, M.Y.; Goto, T. Growth of ZnO single crystal thin films on c-plane (0 0 0 1) sapphire by plasma enhanced molecular beam epitaxy. J. Cryst. Growth 1997, 181, 165–169. [Google Scholar] [CrossRef]
- Greczynski, G.; Hultman, L. Compromising Science by Ignorant Instrument Calibration—Need to Revisit Half a Century of Published XPS Data. Angew. Chem. Int. Ed. 2020, 59, 5002–5006. [Google Scholar] [CrossRef]
- Chen, S.-N.; Chen, C.-H.; Lin, Z.-H.; Tsao, Y.-H.; Liu, C.-P. On enhancing capability of tribocharge transfer of ZnO nanorod arrays by Sb doping for anomalous output performance improvement of triboelectric nanogenerators. Nano Energy 2018, 45, 311–318. [Google Scholar] [CrossRef]
- Zhu, B.L.; Zhu, S.J.; Zhao, X.Z.; Su, F.H.; Li, G.H.; Wu, X.G.; Wu, J. Characteristics of undoped and Sb-doped ZnO thin films prepared in different atmospheres by pulsed laser deposition. Phys. Status Solidi A 2011, 208, 843–850. [Google Scholar] [CrossRef]
- Nasser, R.; Othmen, W.B.H.; Elhouichet, H.; Férid, M. Preparation, characterization of Sb-doped ZnO nanocrystals and their excellent solar light driven photocatalytic activity. Appl. Surf. Sci. 2017, 393, 486–495. [Google Scholar] [CrossRef]
- Luo, C.-Q.; Ho, L.-P.; Chi-Chung Ling, F. The origin of additional modes in Raman spectra of ZnO:Sb films. Phys. B Condens. Matter 2020, 593, 412256. [Google Scholar] [CrossRef]
- Lu, J.G.; Fujita, S.; Kawaharamura, T.; Nishinaka, H.; Kamada, Y.; Ohshima, T.; Ye, Z.Z.; Zeng, Y.J.; Zhang, Y.Z.; Zhu, L.P.; et al. Carrier concentration dependence of band gap shift in n-type ZnO:Al films. J. Appl. Phys. 2007, 101, 083705. [Google Scholar] [CrossRef]
- Saw, K.G.; Aznan, N.M.; Yam, F.K.; Ng, S.S.; Pung, S.Y. New Insights on the Burstein-Moss Shift and Band Gap Narrowing in Indium-Doped Zinc Oxide Thin Films. PLoS ONE 2015, 10, e0141180. [Google Scholar] [CrossRef]
- Rao, T.P.; Kumar, M.C.S.; Angayarkanni, S.A.; Ashok, M. Effect of stress on optical band gap of ZnO thin films with substrate temperature by spray pyrolysis. J. Alloys Compd. 2009, 485, 413–417. [Google Scholar] [CrossRef]
- Jessadaluk, S.; Khemasiri, N.; Rattanawarinchai, P.; Kayunkid, N.; Rahong, S.; Rangkasikorn, A.; Wirunchit, S.; Klamchuen, A.; Nukeaw, J. Electroreflectance study of antimony doped ZnO thin films grown by pulsed laser deposition. Opt. Mater. 2021, 120, 111461. [Google Scholar] [CrossRef]
- Liang, H.; Chen, Y.; Xia, X.; Feng, Q.; Liu, Y.; Shen, R.; Luo, Y.; Du, G. Influence of Sb valency on the conductivity type of Sb-doped ZnO. Thin Solid Film. 2015, 589, 199–202. [Google Scholar] [CrossRef]
- Saáedi, A.; Yousefi, R.; Jamali-Sheini, F.; Zak, A.K.; Cheraghizade, M.; Mahmoudian, M.R.; Baghchesara, M.A.; Dezaki, A.S. XPS studies and photocurrent applications of alkali-metals-doped ZnO nanoparticles under visible illumination conditions. Phys. E Low-Dimens. Syst. Nanostruct. 2016, 79, 113–118. [Google Scholar] [CrossRef]
- Zeng, D.W.; Xie, C.S.; Zhu, B.L.; Song, W.L.; Wang, A.H. Synthesis and characteristics of Sb-doped ZnO nanoparticles. Mater. Sci. Eng. B 2003, 104, 68–72. [Google Scholar] [CrossRef]
- Pandey, S.K.; Kumar Pandey, S.; Awasthi, V.; Gupta, M.; Deshpande, U.P.; Mukherjee, S. Influence of in-situ annealing ambient on p-type conduction in dual ion beam sputtered Sb-doped ZnO thin films. Appl. Phys. Lett. 2013, 103, 072109. [Google Scholar] [CrossRef]
- Caglar, Y.; Caglar, M.; Ilican, S. XRD, SEM, XPS studies of Sb doped ZnO films and electrical properties of its based Schottky diodes. Optik 2018, 164, 424–432. [Google Scholar] [CrossRef]
- Nasser, R.; Elhouichet, H. Production of acceptor complexes in sol-gel ZnO thin films by Sb doping. J. Lumin. 2018, 196, 11–19. [Google Scholar] [CrossRef]
- Friedrich, F.; Sieber, I.; Klimm, C.; Klaus, M.; Genzel, C.; Nickel, N.H. Sb-doping of ZnO: Phase segregation and its impact on p-type doping. Appl. Phys. Lett. 2011, 98, 131902. [Google Scholar] [CrossRef]
- Wang, F.; Li, X.; Jiang, G.; Liu, W.; Zhu, C. Origin of (103) plane of ZnO films deposited by RF magnetron sputtering. J. Mater. Sci. Mater. Electron. 2013, 24, 3764–3767. [Google Scholar] [CrossRef]
- Khemasiri, N.; Kayunkid, N.; Soyeux, N.; Rattanawarinchai, P.; Jessadaluk, S.; Wirunchit, S.; Rangkasikorn, A.; Rahong, S.; Klamchuen, A.; Nukeaw, J. Influence of aluminum-doped zinc oxide seeding film on morphological properties of hydrothermally-grown zinc oxide nanorods. Jpn. J. Appl. Phys. 2020, 59, 035502. [Google Scholar] [CrossRef]
- Adhikari, A.; Przezdziecka, E.; Mishra, S.; Sybilski, P.; Sajkowski, J.; Guziewicz, E. Optical Properties of ZnO Deposited by Atomic Layer Deposition on Sapphire: A Comparison of Thin and Thick Films. Phys. Status Solidi A 2021, 218, 2000669. [Google Scholar] [CrossRef]
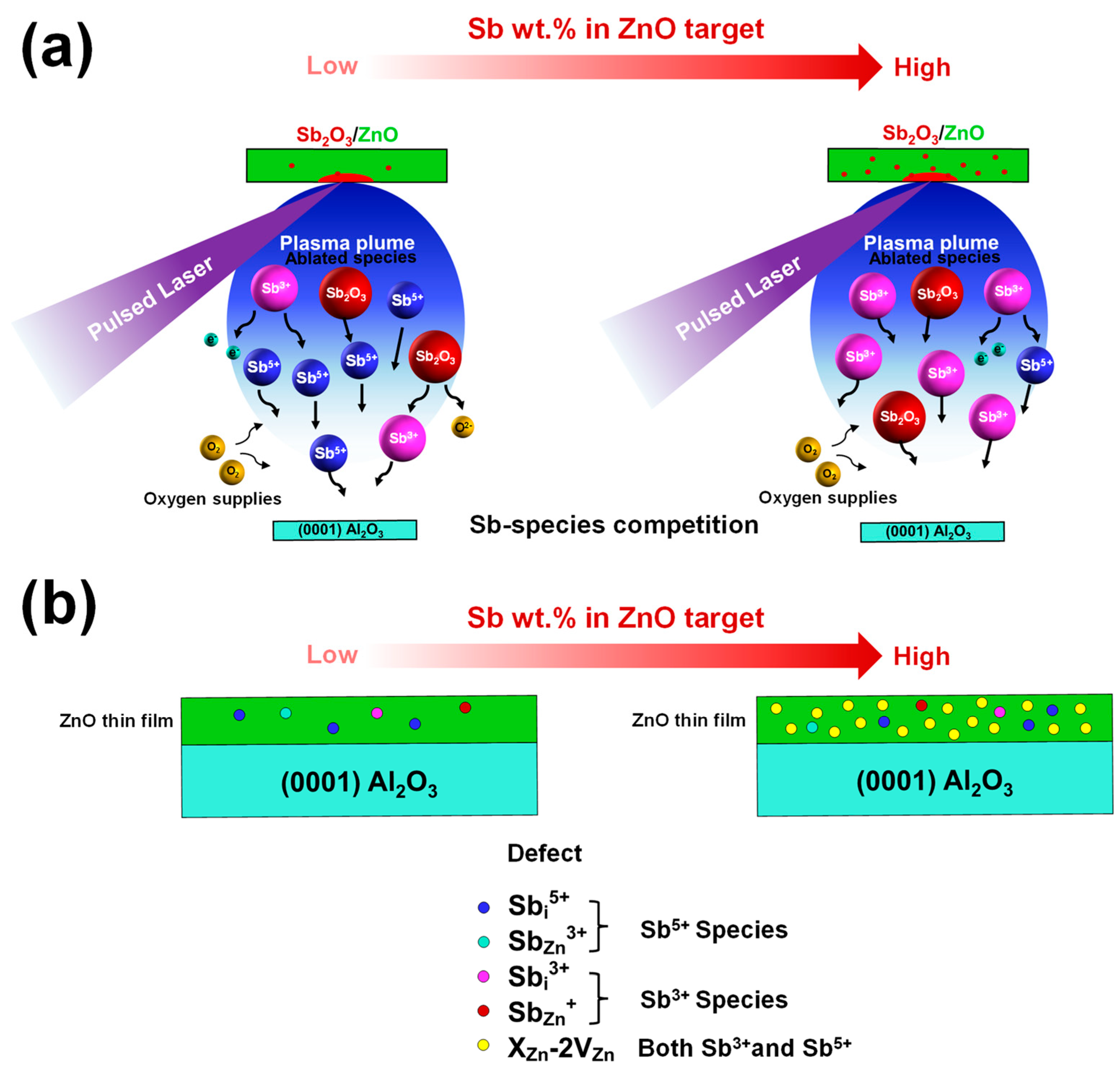
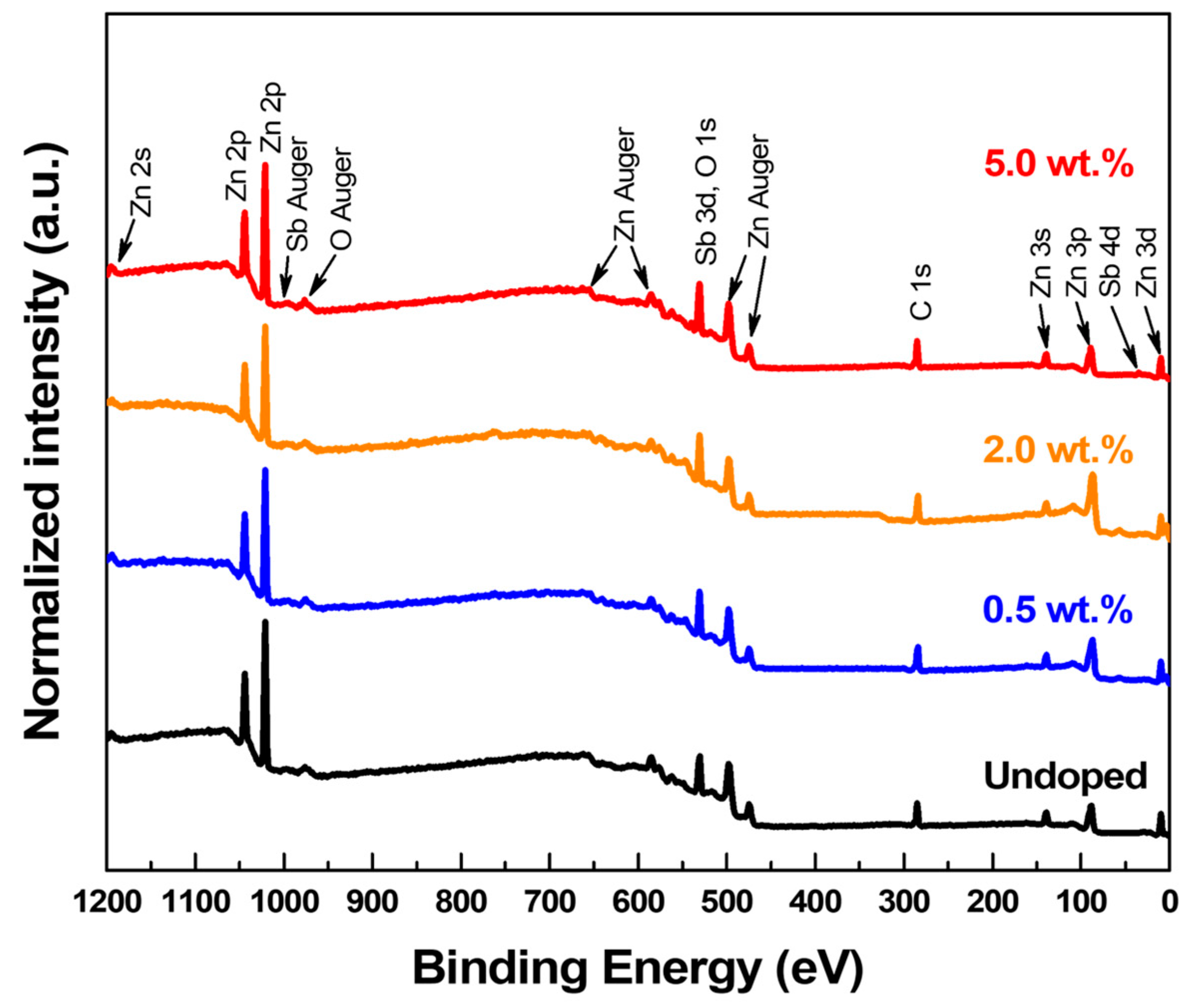
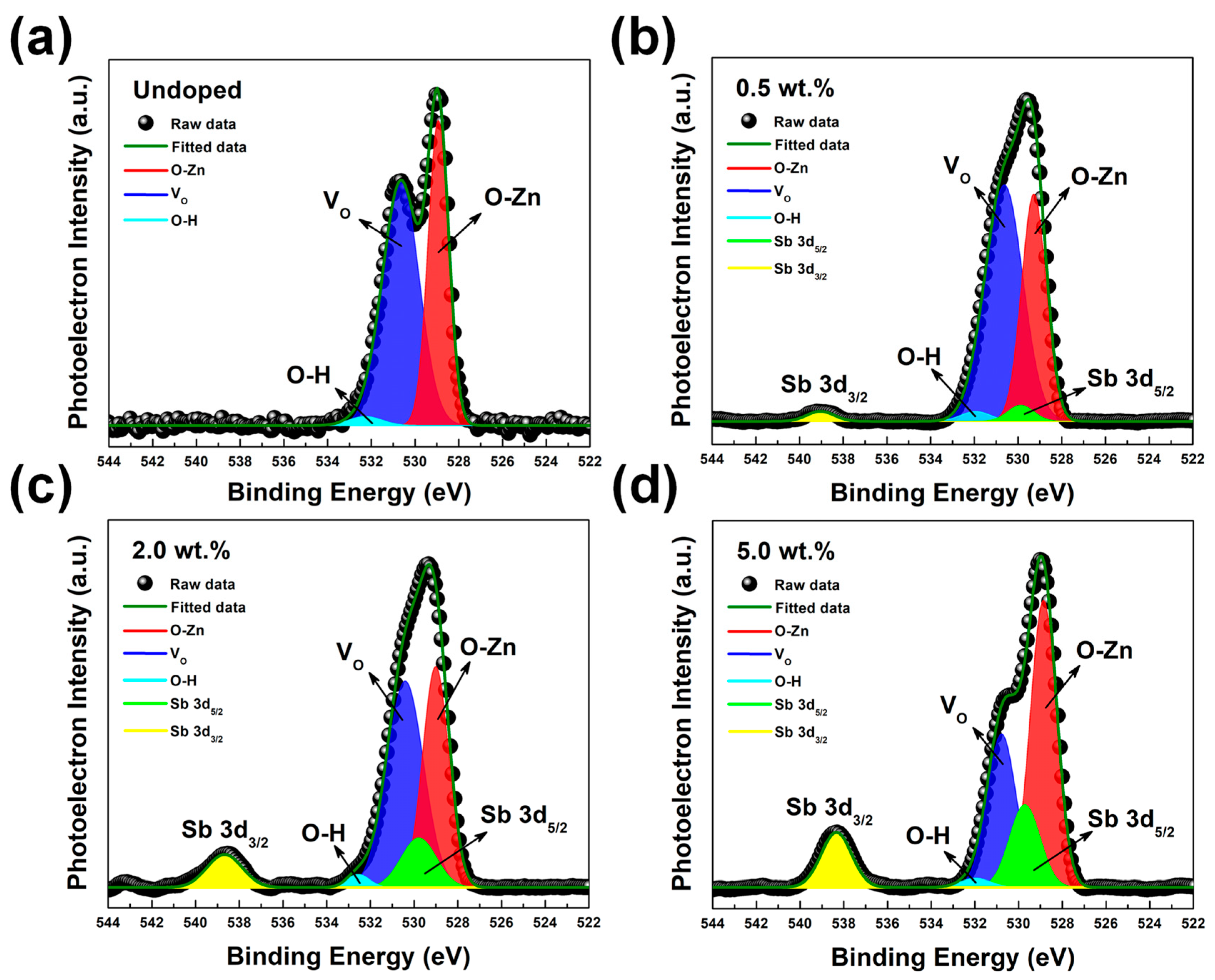
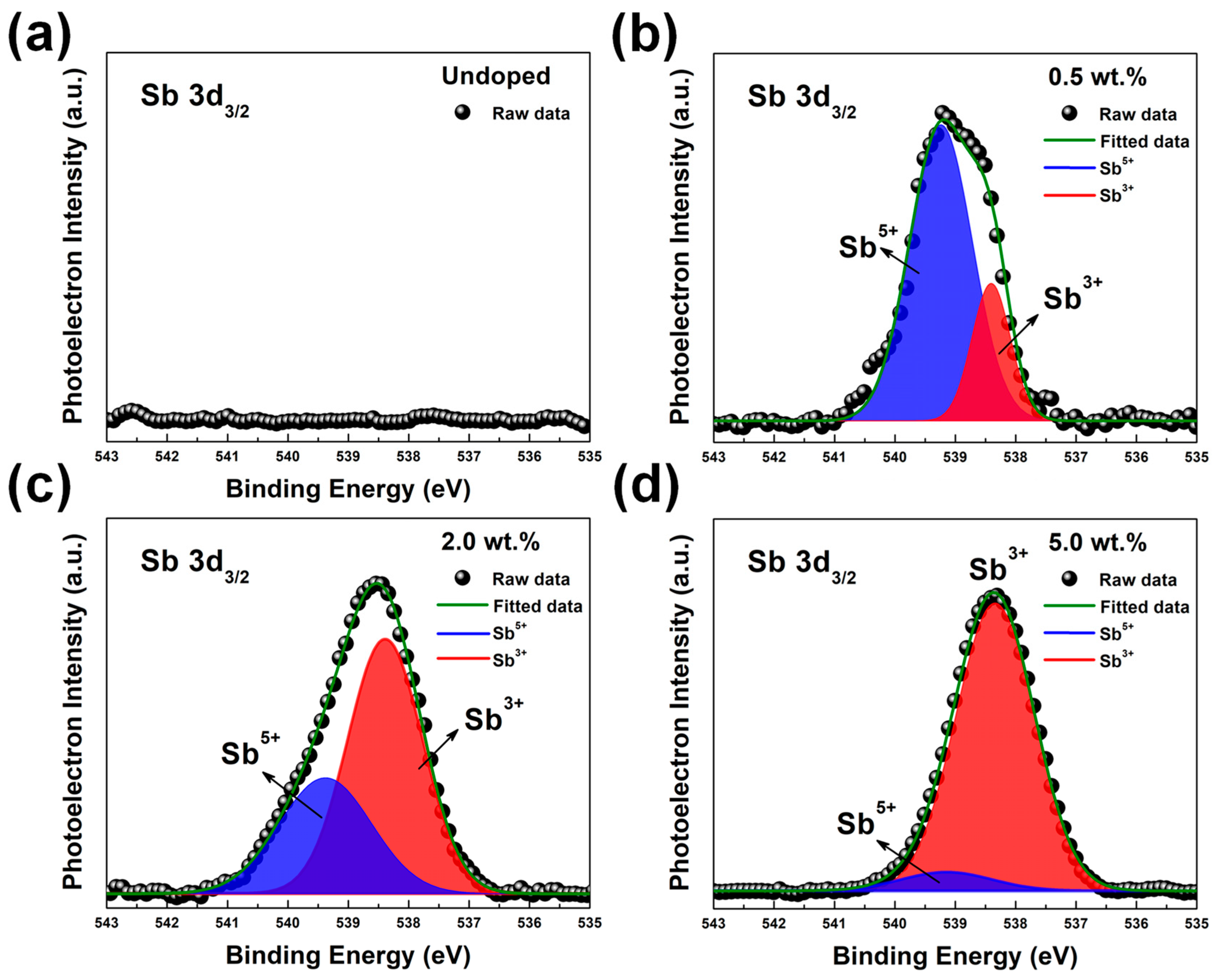
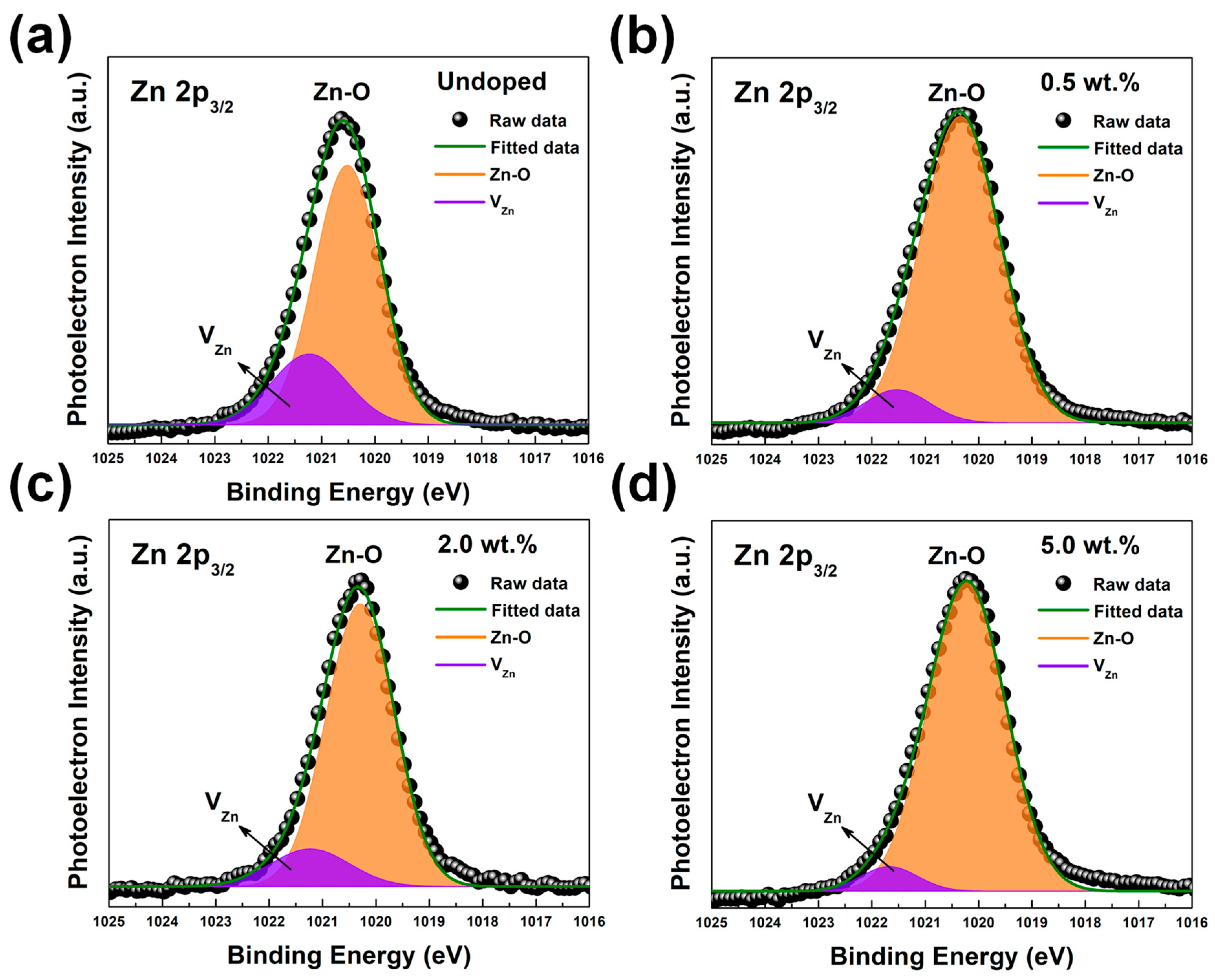
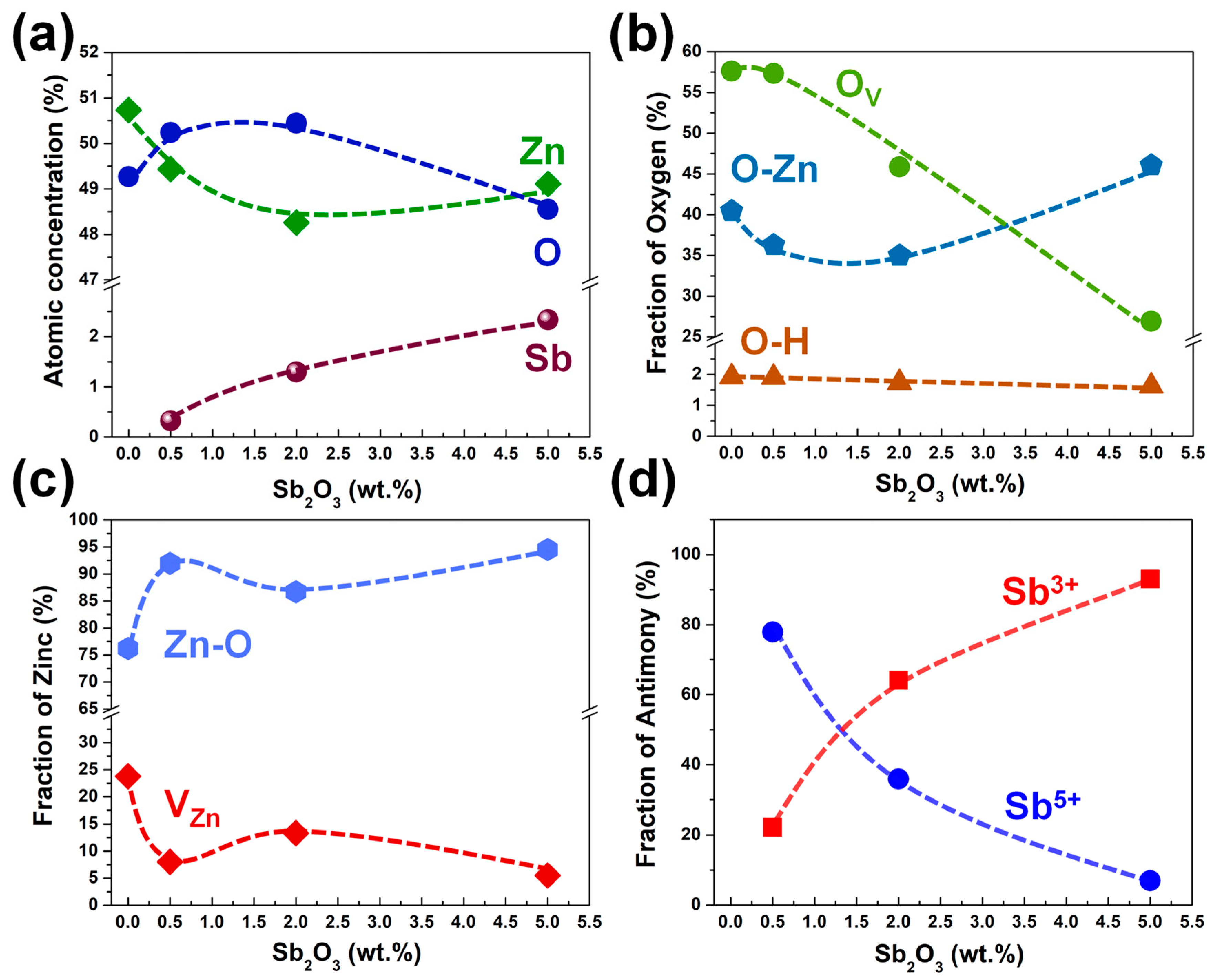
| Category | Possible Defect | Effect on Lattice Spacing | Conductivity Type |
|---|---|---|---|
| Interstitials | Sbi3+ | Expanded | n |
| Sbi5+ | Expanded | n | |
| Substitutes | SbZn+ | Expanded | n |
| SbZn3+ | Reduced | n | |
| Complexes | XZn–2VZn | Expanded | p |
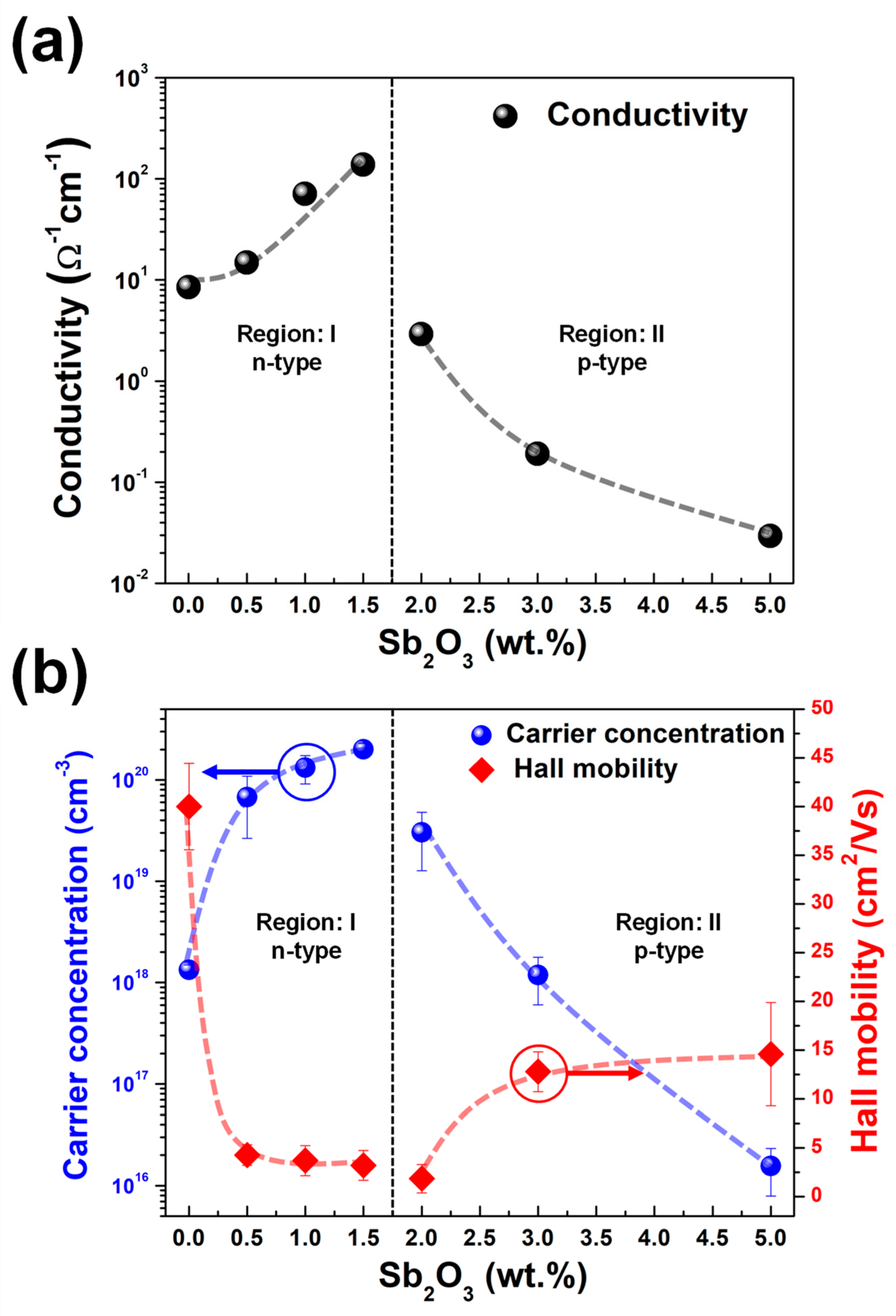
| Sample Name | Conductivity (Ω−1 cm−1) | Conductivity Type | Carrier Concentration (cm−3) | Hall Mobility (cm2/Vs) | Region |
|---|---|---|---|---|---|
| Undoped | 8.49 | n | 1.34 × 1018 | 40.00 | region I |
| 0.5 wt.% Sb2O3 | 14.90 | n | 6.75 × 1019 | 4.26 | |
| 1.0 wt.% Sb2O3 | 70.92 | n | 1.33 × 1020 | 3.70 | |
| 1.5 wt.% Sb2O3 | 138.27 | n | 2.01 × 1020 | 3.21 | |
| 2.0 wt.% Sb2O3 | 2.91 | p | 3.03 × 1019 | 1.85 | region II |
| 3.0 wt.% Sb2O3 | 0.19 | p | 1.19 × 1018 | 12.79 | |
| 5.0 wt.% Sb2O3 | 0.03 | p | 1.56 × 1016 | 14.59 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jessadaluk, S.; Khemasiri, N.; Kayunkid, N.; Rangkasikorn, A.; Wirunchit, S.; Tammarugwattana, N.; Mano, K.; Chananonnawathorn, C.; Horprathum, M.; Klamchuen, A.; et al. Influence of Antimony Species on Electrical Properties of Sb-Doped Zinc Oxide Thin Films Prepared by Pulsed Laser Deposition. Nanomaterials 2023, 13, 1799. https://doi.org/10.3390/nano13111799
Jessadaluk S, Khemasiri N, Kayunkid N, Rangkasikorn A, Wirunchit S, Tammarugwattana N, Mano K, Chananonnawathorn C, Horprathum M, Klamchuen A, et al. Influence of Antimony Species on Electrical Properties of Sb-Doped Zinc Oxide Thin Films Prepared by Pulsed Laser Deposition. Nanomaterials. 2023; 13(11):1799. https://doi.org/10.3390/nano13111799
Chicago/Turabian StyleJessadaluk, Sukittaya, Narathon Khemasiri, Navaphun Kayunkid, Adirek Rangkasikorn, Supamas Wirunchit, Narin Tammarugwattana, Kitipong Mano, Chanunthorn Chananonnawathorn, Mati Horprathum, Annop Klamchuen, and et al. 2023. "Influence of Antimony Species on Electrical Properties of Sb-Doped Zinc Oxide Thin Films Prepared by Pulsed Laser Deposition" Nanomaterials 13, no. 11: 1799. https://doi.org/10.3390/nano13111799
APA StyleJessadaluk, S., Khemasiri, N., Kayunkid, N., Rangkasikorn, A., Wirunchit, S., Tammarugwattana, N., Mano, K., Chananonnawathorn, C., Horprathum, M., Klamchuen, A., Rahong, S., & Nukeaw, J. (2023). Influence of Antimony Species on Electrical Properties of Sb-Doped Zinc Oxide Thin Films Prepared by Pulsed Laser Deposition. Nanomaterials, 13(11), 1799. https://doi.org/10.3390/nano13111799