Features of Copper and Gold Nanoparticle Translocation in Petroselinum crispum Segments
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experiment
2.3. Analytical Techniques
2.4. Biochemical Analysis and Antioxidant Activity
2.5. Data Evaluation
2.6. Statistical Analysis
3. Results
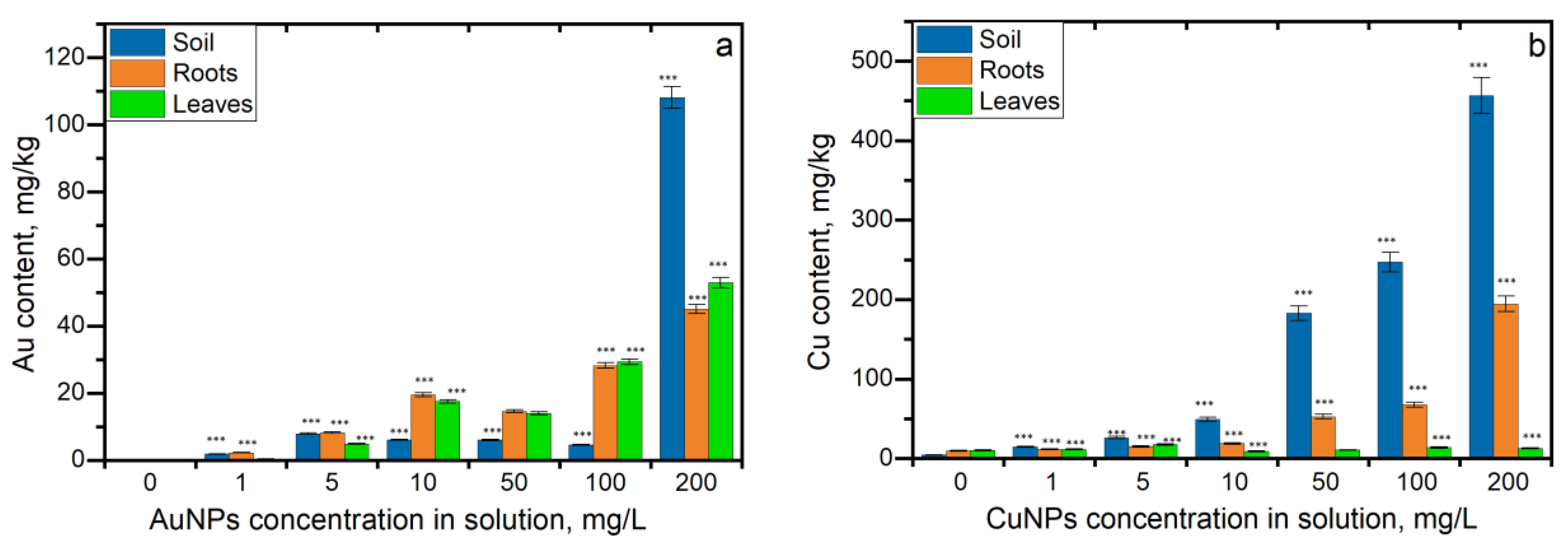
3.1. Gold and Copper Uptake in Parsley Segments
3.2. Translocation Coefficient (TF) and the Bioconcentration Factor (BCF)
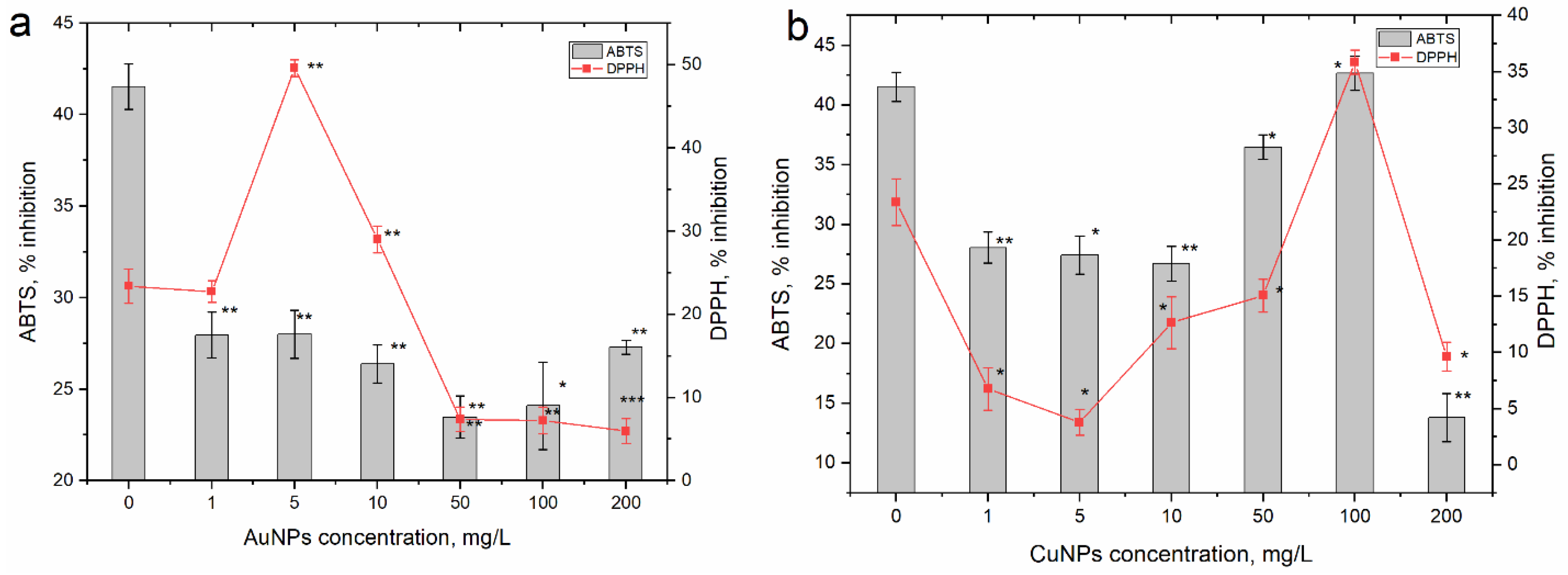
3.3. Effects of AuNPs and CuNPs on Parsley’s Biochemical Parameters and Antioxidant Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rubilar, O.; Rai, M.; Tortella, G.; Diez, M.C.; Seabra, A.B.; Durán, N. Biogenic Nanoparticles: Copper, Copper Oxides, Copper Sulphides, Complex Copper Nanostructures and Their Applications. Biotechnol. Lett. 2013, 35, 1365–1375. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, C.; Lin, Y.; Hu, P.; Shen, Y.; Wang, K.; Meng, S.; Chai, Y.; Dai, X.; Liu, X.; et al. Nanocomposite Membranes Enhance Bone Regeneration Through Restoring Physiological Electric Microenvironment. ACS Nano 2016, 10, 7279–7286. [Google Scholar] [CrossRef] [PubMed]
- Shnoudeh, A.J.; Hamad, I.; Abdo, R.W.; Qadumii, L.; Jaber, A.Y.; Surchi, H.S.; Alkelany, S.Z. Synthesis, Characterization, and Applications of Metal Nanoparticles. In Biomaterials and Bionanotechnology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 527–612. ISBN 9780128144282. [Google Scholar]
- Li, Y.; Liang, J.; Tao, Z.; Chen, J. CuO Particles and Plates: Synthesis and Gas-Sensor Application. Mater. Res. Bull. 2008, 43, 2380–2385. [Google Scholar] [CrossRef]
- Genedy, M.; Kandil, U.F.; Matteo, E.N.; Stormont, J.; Reda Taha, M.M. A New Polymer Nanocomposite Repair Material for Restoring Wellbore Seal Integrity. Int. J. Greenh. Gas Control 2017, 58, 290–298. [Google Scholar] [CrossRef]
- Tao, B.; Lin, C.; Deng, Y.; Yuan, Z.; Shen, X.; Chen, M.; He, Y.; Peng, Z.; Hu, Y.; Cai, K. Copper-Nanoparticle-Embedded Hydrogel for Killing Bacteria and Promoting Wound Healing with Photothermal Therapy. J. Mater. Chem. B 2019, 7, 2534–2548. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Iwakuma, N.; Sharma, P.; Moudgil, B.M.; Wu, C.; McNeill, J.; Jiang, H.; Grobmyer, S.R. Gold Nanoparticles as a Contrast Agent for Invivo Tumor Imaging with Photoacoustic Tomography. Nanotechnology 2009, 20, 395102. [Google Scholar] [CrossRef]
- Zhang, M.; Shao, S.; Yue, H.; Wang, X.; Zhang, W.; Chen, F.; Zheng, L.; Xing, J.; Qin, Y. High Stability Au Nps: From Design to Application in Nanomedicine. Int. J. Nanomed. 2021, 16, 6067–6094. [Google Scholar] [CrossRef]
- Chithrani, D.B.; Dunne, M.; Stewart, J.; Allen, C.; Jaffray, D.A. Cellular Uptake and Transport of Gold Nanoparticles Incorporated in a Liposomal Carrier. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 161–169. [Google Scholar] [CrossRef]
- Paciotti, G.F.; Myer, L.; Weinreich, D.; Goia, D.; Pavel, N.; McLaughlin, R.E.; Tamarkin, L. Colloidal Gold: A Novel Nanoparticle Vector for Tumor Directed Drug Delivery. Drug Deliv. J. Deliv. Target. Ther. Agents 2004, 11, 169–183. [Google Scholar] [CrossRef]
- Gawande, M.B.; Goswami, A.; Felpin, F.X.; Asefa, T.; Huang, X.; Silva, R.; Zou, X.; Zboril, R.; Varma, R.S. Cu and Cu-Based Nanoparticles: Synthesis and Applications in Catalysis. Chem. Rev. 2016, 116, 3722–3811. [Google Scholar] [CrossRef]
- Xu, V.W.; Nizami, M.Z.I.; Yin, I.X.; Yu, O.Y.; Lung, C.Y.K.; Chu, C.H. Application of Copper Nanoparticles in Dentistry. Nanomaterials 2022, 12, 805. [Google Scholar] [CrossRef] [PubMed]
- Al-Hakkani, M.F. Biogenic Copper Nanoparticles and Their Applications: A Review. SN Appl. Sci. 2020, 2, 505. [Google Scholar] [CrossRef]
- Klaine, S.J.; Alvarez, P.J.J.; Batley, G.E.; Fernandes, T.F.; Handy, R.D.; Lyon, D.Y.; Mahendra, S.; McLaughlin, M.J.; Lead, J.R. Nanomaterials in the Environment: Behavior, Fate, Bioavailability, and Effects. Environ. Toxicol. Chem. 2008, 27, 1825–1851. [Google Scholar] [CrossRef]
- Gao, M.; Chang, J.; Wang, Z.; Zhang, H.; Wang, T. Advances in Transport and Toxicity of Nanoparticles in Plants. J. Nanobiotechnol. 2023, 21, 75. [Google Scholar] [CrossRef] [PubMed]
- Tou, F.; Yang, Y.; Feng, J.; Niu, Z.; Pan, H.; Qin, Y.; Guo, X.; Meng, X.; Liu, M.; Hochella, M.F. Environmental Risk Implications of Metals in Sludges from Waste Water Treatment Plants: The Discovery of Vast Stores of Metal-Containing Nanoparticles. Environ. Sci. Technol. 2017, 51, 4831–4840. [Google Scholar] [CrossRef]
- Gottschalk, F.; Nowack, B. The Release of Engineered Nanomaterials to the Environment. J. Environ. Monit. 2011, 13, 1145–1155. [Google Scholar] [CrossRef]
- Moreno-Martín, G.; Gómez-Gómez, B.; León-González, M.E.; Madrid, Y. Characterization of AgNPs and AuNPs in Sewage Sludge by Single Particle Inductively Coupled Plasma-Mass Spectrometry. Talanta 2022, 238, 123033. [Google Scholar] [CrossRef]
- Singh, A. A Review of Wastewater Irrigation: Environmental Implications. Resour. Conserv. Recycl. 2021, 168, 105454. [Google Scholar] [CrossRef]
- Gottschalk, F.; Sonderer, T.; Scholz, R.W.; Nowack, B. Modeled Environmental Concentrations of Engineered Nanomaterials (TiO2, ZnO, Ag, CNT, Fullerenes) for Different Regions. Environ. Sci. Technol. 2009, 43, 9216–9222. [Google Scholar] [CrossRef]
- Bakshi, M.; Kumar, A. Copper-Based Nanoparticles in the Soil-Plant Environment: Assessing Their Applications, Interactions, Fate and Toxicity. Chemosphere 2021, 281, 130940. [Google Scholar] [CrossRef]
- Masarovicová, E.; Králová, K. Metal Nanoparticles and Plants. Ecol. Chem. Eng. S 2013, 20, 9–22. [Google Scholar] [CrossRef]
- Monica, R.C.; Cremonini, R. Nanoparticles and Higher Plants. Caryologia 2009, 62, 161–165. [Google Scholar] [CrossRef]
- Rattan, S.; Partap, M.; Kanika; Kumar, S.; Warghat, A.R. Nutrient Feeding Approach Enhances the Vegetative Growth Biomass, Volatile Oil Composition, and Myristicin Content in Hydroponically Cultivated Petroselinum crispum (Mill.) Nyman. J. Appl. Res. Med. Aromat. Plants 2022, 26, 100359. [Google Scholar] [CrossRef]
- Farzaei, M.H.; Abbasabadi, Z.; Ardekani, M.R.S.; Rahimi, R.; Farzaei, F. Parsley: A Review of Ethnopharmacology, Phytochemistry and Biological Activities. J. Tradit. Chin. Med. 2013, 33, 815–826. [Google Scholar] [CrossRef]
- Tang, E.L.H.; Rajarajeswaran, J.; Fung, S.; Kanthimathi, M.S. Petroselinum Crispum Has Antioxidant Properties, Protects against DNA Damage and Inhibits Proliferation and Migration of Cancer Cells. J. Sci. Food Agric. 2015, 95, 2763–2771. [Google Scholar] [CrossRef]
- Kuete, V. Medicinal Spices and Vegetables from Africa: Therapeutic Potential against Metabolic, Inflammatory, Infectious and Systemic Diseases; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Marthe, F. Petroselinum crispum (Mill.) Nyman (Parsley). In Medicinal, Aromatic and Stimulant Plants; Springer: Cham, Switzerland, 2020; pp. 435–466. [Google Scholar]
- Dehkourdi, E.H.; Mosavi, M. Effect of Anatase Nanoparticles (TiO2) on Parsley Seed Germination (Petroselinum crispum) in Vitro. Biol. Trace Elem. Res. 2013, 155, 283–286. [Google Scholar] [CrossRef] [PubMed]
- Mondal, K.K.; Mani, C. Investigation of the Antibacterial Properties of Nanocopper against Xanthomonas Axonopodis Pv. Punicae, the Incitant of Pomegranate Bacterial Blight. Ann. Microbiol. 2012, 62, 889–893. [Google Scholar] [CrossRef]
- Bramhanwade, K.; Shende, S.; Bonde, S.; Gade, A.; Rai, M. Fungicidal Activity of Cu Nanoparticles against Fusarium Causing Crop Diseases. Environ. Chem. Lett. 2016, 14, 229–235. [Google Scholar] [CrossRef]
- Seregina, T.; Chernikova, O.; Mazhaysky, Y.; Ampleeva, L. Features of the Influence of Copper Nanoparticles and Copper Oxide on the Formation of Barley Crop. Agron. Res. 2020, 18, 1010–1017. [Google Scholar] [CrossRef]
- Rai, M.; Ingle, A.P.; Pandit, R.; Paralikar, P.; Shende, S.; Gupta, I.; Biswas, J.K.; Da Silva, S.S. Copper and Copper Nanoparticles: Role in Management of Insect-Pests and Pathogenic Microbes. Nanotechnol. Rev. 2018, 7, 303–315. [Google Scholar] [CrossRef]
- Judy, J.D.; Unrine, J.M.; Rao, W.; Wirick, S.; Bertsch, P.M. Bioavailability of Gold Nanomaterials to Plants: Importance of Particle Size and Surface Coating. Environ. Sci. Technol. 2012, 46, 8467–8474. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, A.; Zivcak, M.; Sytar, O.; Kalaji, H.M.; He, X.; Mbarki, S.; Brestic, M. Impact of Metal and Metal Oxide Nanoparticles on Plant: A Critical Review. Front. Chem. 2017, 5, 78. [Google Scholar] [CrossRef] [PubMed]
- Faizan, M.; Faraz, A.; Hayat, S. Effective Use of Zinc Oxide Nanoparticles through Root Dipping on the Performance of Growth, Quality, Photosynthesis and Antioxidant System in Tomato. J. Plant Biochem. Biotechnol. 2020, 29, 553–567. [Google Scholar] [CrossRef]
- Hong, H.J.; Ryu, J. Synthesis of Copper Nanoparticles from Cu2+-Spiked Wastewater via Adsorptive Separation and Subsequent Chemical Reduction. Nanomaterials 2021, 11, 2051. [Google Scholar] [CrossRef]
- Popovich, H.B.; Malinina, A.O. Formation of Salad and Cabbage Seedlings under the Influence of Additional Lightning. Plant Soil Sci. 2019, 10, 58–66. [Google Scholar] [CrossRef]
- Zhu, Z.J.; Wang, H.; Yan, B.; Zheng, H.; Jiang, Y.; Miranda, O.R.; Rotello, V.M.; Xing, B.; Vachet, R.W. Effect of Surface Charge on the Uptake and Distribution of Gold Nanoparticles in Four Plant Species. Environ. Sci. Technol. 2012, 46, 12391–12398. [Google Scholar] [CrossRef]
- Wei, S.; Zhou, Q.; Saha, U.K. Hyperaccumulative Characteristics of Weed Species to Heavy Metals. Water. Air. Soil Pollut. 2008, 192, 173–181. [Google Scholar] [CrossRef]
- Sabo-Attwood, T.; Unrine, J.M.; Stone, J.W.; Murphy, C.J.; Ghoshroy, S.; Blom, D.; Bertsch, P.M.; Newman, L.A. Uptake, Distribution and Toxicity of Gold Nanoparticles in Tobacco (Nicotiana Xanthi) Seedlings. Nanotoxicology 2012, 6, 353–360. [Google Scholar] [CrossRef]
- Malejko, J.; Godlewska-Żyłkiewicz, B.; Vanek, T.; Landa, P.; Nath, J.; Dror, I.; Berkowitz, B. Uptake, Translocation, Weathering and Speciation of Gold Nanoparticles in Potato, Radish, Carrot and Lettuce Crops. J. Hazard. Mater. 2021, 418, 126219. [Google Scholar] [CrossRef]
- Feichtmeier, N.S.; Walther, P.; Leopold, K. Uptake, Effects, and Regeneration of Barley Plants Exposed to Gold Nanoparticles. Environ. Sci. Pollut. Res. 2015, 22, 8549–8558. [Google Scholar] [CrossRef]
- Zhao, L.; Ortiz, C.; Adeleye, A.S.; Hu, Q.; Zhou, H.; Huang, Y.; Keller, A.A. Metabolomics to Detect Response of Lettuce (Lactuca sativa) to Cu(OH)2 Nanopesticides: Oxidative Stress Response and Detoxification Mechanisms. Environ. Sci. Technol. 2016, 50, 9697–9707. [Google Scholar] [CrossRef]
- Tamez, C.; Morelius, E.W.; Hernandez-Viezcas, J.A.; Peralta-Videa, J.R.; Gardea-Torresdey, J. Biochemical and Physiological Effects of Copper Compounds/Nanoparticles on Sugarcane (Saccharum officinarum). Sci. Total Environ. 2019, 649, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Mosa, K.A.; El-Naggar, M.; Ramamoorthy, K.; Alawadhi, H.; Elnaggar, A.; Wartanian, S.; Ibrahim, E.; Hani, H. Copper Nanoparticles Induced Genotoxicty, Oxidative Stress, and Changes in Superoxide Dismutase (SOD) Gene Expression in Cucumber (Cucumis sativus) Plants. Front. Plant Sci. 2018, 9, 872. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Huang, Y.; Hu, J.; Zhou, H.; Adeleye, A.S.; Keller, A.A. 1H NMR and GC-MS Based Metabolomics Reveal Defense and Detoxification Mechanism of Cucumber Plant under Nano-Cu Stress. Environ. Sci. Technol. 2016, 50, 2000–2010. [Google Scholar] [CrossRef]
- Ali, S.; Mehmood, A.; Khan, N. Uptake, Translocation, and Consequences of Nanomaterials on Plant Growth and Stress Adaptation. J. Nanomater. 2021, 2021, 6677616. [Google Scholar] [CrossRef]
- Wang, S.; Fu, Y.; Zheng, S.; Xu, Y.; Sun, Y. Phytotoxicity and Accumulation of Copper-Based Nanoparticles in Brassica under Cadmium Stress. Nanomaterials 2022, 12, 1497. [Google Scholar] [CrossRef]
- Ma, Y.; Kuang, L.; He, X.; Bai, W.; Ding, Y.; Zhang, Z.; Zhao, Y.; Chai, Z. Effects of Rare Earth Oxide Nanoparticles on Root Elongation of Plants. Chemosphere 2010, 78, 273–279. [Google Scholar] [CrossRef]
- Khan, M.R.; Adam, V.; Rizvi, T.F.; Zhang, B.; Ahamad, F.; Jośko, I.; Zhu, Y.; Yang, M.; Mao, C. Nanoparticle–Plant Interactions: Two-Way Traffic. Small 2019, 15, 1901794. [Google Scholar] [CrossRef]
- Lee, W.; An, Y.; Yoon, H.; Kweon, H. Toxicity and Bioavailability of Copper Nanoparticles to the Terrestrial Plants Mung Bean (Phaseolus radiatus) and Wheat (Triticum aestivum): Plant Agar Test for Water-insoluble Nanoparticles. Environ. Toxicol. Chem. An Int. J. 2008, 27, 1915–1921. [Google Scholar] [CrossRef]
- Perreault, F.; Samadani, M.; Dewez, D. Effect of Soluble Copper Released from Copper Oxide Nanoparticles Solubilisation on Growth and Photosynthetic Processes of Lemna gibba L. Nanotoxicology 2014, 8, 374–382. [Google Scholar] [CrossRef]
- Da Costa, M.V.J.; Sharma, P.K. Effect of Copper Oxide Nanoparticles on Growth, Morphology, Photosynthesis, and Antioxidant Response in Oryza Sativa. Photosynthetica 2016, 54, 110–119. [Google Scholar] [CrossRef]
- Nekrasova, G.F.; Ushakova, O.S.; Ermakov, A.E.; Uimin, M.A.; Byzov, I.V. Effects of Copper(II) Ions and Copper Oxide Nanoparticles on Elodea Densa Planch. Russ. J. Ecol. 2011, 42, 458–463. [Google Scholar] [CrossRef]
- Arora, S.; Sharma, P.; Kumar, S.; Nayan, R.; Khanna, P.K.; Zaidi, M.G.H. Gold-Nanoparticle Induced Enhancement in Growth and Seed Yield of Brassica Juncea. Plant Growth Regul. 2012, 66, 303–310. [Google Scholar] [CrossRef]
- Barrena, R.; Casals, E.; Colón, J.; Font, X.; Sánchez, A.; Puntes, V. Evaluation of the Ecotoxicity of Model Nanoparticles. Chemosphere 2009, 75, 850–857. [Google Scholar] [CrossRef] [PubMed]


| Concentration of NPs in Solution, mg/L | BCF | TF | |
|---|---|---|---|
| Roots | Leaves | ||
| CuNPs | |||
| 1 | 0.78 | 0.8 | 1.03 |
| 5 | 0.57 | 0.67 | 1.17 |
| 10 | 0.38 | 0.18 | 0.48 |
| 50 | 0.29 | 0.06 | 0.2 |
| 100 | 0.27 | 0.06 | 0.2 |
| 200 | 0.43 | 0.03 | 0.06 |
| AuNPs | |||
| 1 | 1.2 | 0.24 | 0.2 |
| 5 | 1.04 | 0.62 | 0.6 |
| 10 | 3.21 | 2.88 | 0.9 |
| 50 | 2.41 | 2.31 | 0.96 |
| 100 | 6.08 | 6.32 | 1.04 |
| 200 | 0.42 | 0.49 | 1.17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peshkova, A.; Zinicovscaia, I.; Cepoi, L.; Rudi, L.; Chiriac, T.; Yushin, N.; Sohatsky, A. Features of Copper and Gold Nanoparticle Translocation in Petroselinum crispum Segments. Nanomaterials 2023, 13, 1754. https://doi.org/10.3390/nano13111754
Peshkova A, Zinicovscaia I, Cepoi L, Rudi L, Chiriac T, Yushin N, Sohatsky A. Features of Copper and Gold Nanoparticle Translocation in Petroselinum crispum Segments. Nanomaterials. 2023; 13(11):1754. https://doi.org/10.3390/nano13111754
Chicago/Turabian StylePeshkova, Alexandra, Inga Zinicovscaia, Liliana Cepoi, Ludmila Rudi, Tatiana Chiriac, Nikita Yushin, and Alexander Sohatsky. 2023. "Features of Copper and Gold Nanoparticle Translocation in Petroselinum crispum Segments" Nanomaterials 13, no. 11: 1754. https://doi.org/10.3390/nano13111754
APA StylePeshkova, A., Zinicovscaia, I., Cepoi, L., Rudi, L., Chiriac, T., Yushin, N., & Sohatsky, A. (2023). Features of Copper and Gold Nanoparticle Translocation in Petroselinum crispum Segments. Nanomaterials, 13(11), 1754. https://doi.org/10.3390/nano13111754











