Abstract
SF6 gas is an arc extinguishing medium that is widely used in gas insulated switchgear (GIS). When insulation failure occurs in GIS, it leads to the decomposition of SF6 in partial discharge (PD) and other environments. The detection of the main decomposition components of SF6 is an effective method to diagnose the type and degree of discharge fault. In this paper, Mg-MOF-74 is proposed as a gas sensing nanomaterial for detecting the main decomposition components of SF6. The adsorption of SF6, CF4, CS2, H2S, SO2, SO2F2 and SOF2 on Mg-MOF-74 was calculated by Gaussian16 simulation software based on density functional theory. The analysis includes parameters of the adsorption process such as binding energy, charge transfer, and adsorption distance, as well as the change in bond length, bond angle, density of states, and frontier orbital of the gas molecules. The results show that Mg-MOF-74 has different degrees of adsorption for seven gases, and chemical adsorption will lead to changes in the conductivity of the system; therefore, it can be used as a gas sensing material for the preparation of SF6 decomposition component gas sensors.
1. Introduction
Sulfur hexafluoride gas is an arc extinguishing medium widely used in gas insulated switch gear (GIS). However, insulation defects inside GIS can lead to partial discharge (PD) and other faults. SF6 decomposes into extremely unstable low-fluorine sulfides (SFn, n = 1~5) in an overheated environment with long-term failure [1,2,3,4,5]. Although SFn can collide with F atoms in the environment to recover to SF6 molecules, in the presence of micro-amounts of O2 and H2O, SFn further reacts to form SO2, SOF2, SO2F2, H2S and other major products [6,7,8]; if the fault occurs on the basin insulator, SFn can react with electrode materials, insulation materials, etc., to generate characteristic gases CF4, CS2, etc. [9,10]; therefore, the types of the above decomposition products are closely related to the fault types. Detecting the main decomposition components of SF6 in GIS equipment by the gas sensor method is an effective method to diagnose discharge faults and types [11,12,13,14], and guarantees the safe operation of electrical insulation equipment.
The core of gas sensors is gas sensitive material. At present, the gas sensitive materials used to detect SF6 decomposition gas mainly include: noble metal surface modified inorganic materials (including metal oxides [15,16,17,18,19,20], metal sulfides [21,22,23,24], metal selenides [25,26,27,28]) series; metal-doped carbon-based materials (including graphene [29,30,31,32,33], carbon nanotubes [34,35,36,37]) series; and composite materials [38,39,40] compounded by inorganic materials and carbon-based materials. These modified nanomaterials exhibit good gas sensing properties, but the limited design and flexibility of inorganic materials limit the development space of the above materials.
Metal-organic frameworks (MOFs) are inorganic-organic hybrid crystalline porous materials formed by coordination bonds among metal ions or metal clusters as nodes and organic ligands [41]; they have the characteristics of design flexibility, structural diversity, adjustable pore size and high specific surface area. Therefore, by changing the types of metal ions or metal clusters and the type, structure and chain length of organic ligands, the pore size and spatial morphology of MOFs can be controlled to synthesize MOFs materials with different three-dimensional structures and different pore sizes [42,43,44,45]. These unique and novel materials have been rapidly developed in the past two decades, and have become an ideal platform for various advanced functional materials and applications, making them important in the aspects of gas storage, adsorption and separation, catalysis, analysis and detection, drug delivery and so on [46,47,48,49,50]. However, the research and application of gas sensitive materials for detecting the main decomposition components of SF6 are rarely reported.
In the study of inorganic metal oxide and sulfide gas sensing materials, Peng, S. et al. first studied the gas sensing properties of metal oxide (ZnO) gas sensors for SF6 decomposition components (SO2, SOF2, SO2F2). The results showed that the response of flower-like ZnO to SO2 is better at 270–300 °C, while the response of SOF2 and SO2F2 is weaker [51]. Li, B.L. and other scholars have studied scandium-doped molybdenum sulfide materials (Sc-MoS2). Based on density functional theory, the adsorption properties of intrinsic MoS2 and Sc-MoS2 materials on four typical SF6 decomposition gas molecules (H2S, SO2, SOF2, SO2F2) were studied [52]. In the study of gas adsorption in metal-organic frameworks, Lee, K. et al. studied the Van der Waals adsorption of isomorphic M-MOF-74 (M = Mg, Ti, V, Cr, Mn, Fe, Co, Ni, Cu, Zn) on H2, CO, CO2, H2O, H2S, N2, NH3, and SO2 based on the DFT principle [53]. Liu, J. et al. studied the adsorption properties of 11 MOFs materials (including Mg-MOF-74) for H2S, and then separated H2S from H2S/CO2 [54]. Li, S. et al. studied and compared the adsorption parameters of benzene ring and Cu on H2S and SO2 gas molecules in MOF-505, and the results showed that the adsorption of gas molecules on Cu was greater than that on benzene ring [55].
Mg-MOF-74 (CPO-27-Mg) is a new type of nanoscale MOFs material with Mg2+ as the central ion and 2,5-dihydroxy-1,4-benzenedicarboxylic acid as the ligand. Henkelis, S.E. et al. introduced the synthesis method of this MOF in detail [56]. The crystal structure used in this article is generated by single crystal X-ray analysis, and is currently recorded in the Cambridge structure database (doi: 10.5517/ccdc.csd.cc20k4pf). By analyzing the crystal information file (.cif) in CCDC 1863524, the metal ions/ligands ratio in a single unit cell of Mg-MOF-74 is 9:7. Another useful method for calculating the metal ions/ligands ratio which was mentioned by Visa, A. et al. in the article [57]: the relationship of the ratio between the number of metal ions and ligands can be deducted by gradually building a 3D supramolecular network throughout the HyperChem 7.52 package.
As a gas sensing material, the pore size of 1.2 nm (4 × 4 × 4 super cell shown in Figure 1) provides the structural premise for SF6, CF4, CS2, H2S, SO2, SO2F2 and SOF2 to enter the pores of the material. The unsaturated coordinated Mg2+ in the pores provides the possibility for gas adsorption. In this paper, through the simulation research based on the DFT principle, by selecting a more accurate functional, the chemical adsorption characteristics of Mg-MOF-74 clusters on SF6 and CF4, CS2, H2S, SO2, SO2F2 and SOF2 produced by SF6 discharge decomposition in GIS equipment are analyzed more accurately. By introducing the frontier molecular orbital theory, the energy gap change of the cluster after adsorbing gas is analyzed, and then the influence of adsorption behavior on the conductivity of the material is speculated. The application prospect of Mg-MOF-74 as a gas sensing material for on-line detection of SF6 discharge decomposition products is predicted theoretically. It provides a new perspective for the research and application of MOFs as new gas sensitive materials.
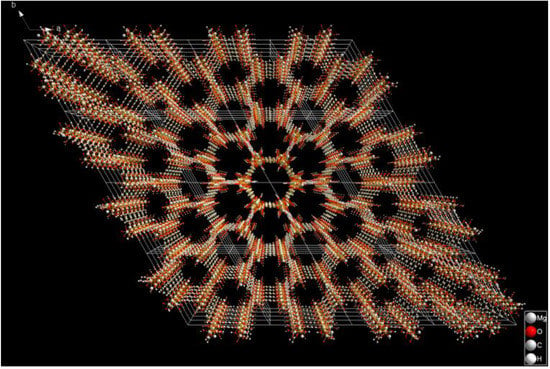
Figure 1.
4 × 4 × 4 super cell of Mg-MOF-74.
2. Calculation Parameter Setting and Model Construction
The modeling involved in this paper is completed in GaussView, and the structural optimization and single point calculation are completed in Gaussian 16 software. In the application of Gaussian series quantum chemistry simulation software, Sciortino, G. et al. found that the PBE0 functional and the def2-tzvp basis set have extremely high accuracy in the calculation of Ni(II) complexes [58]. PBE0 functional together with def-tzvp is the best-performing method, and is excellent in the study of platinum-catalyzed chemical reaction mechanism based on DFT principle [59].
Therefore, when dealing with the exchange correlation term of electrons, we also use the PBE0 hybrid functional with higher calculation accuracy than LDA, GGA and meta-GGA. Because the sensitivity of geometric structure optimization to the basis set is much lower than that of single point energy calculation, and the time consumption of these tasks is much higher than that of single point calculation, the def2-svp (2-zeta) basis set with appropriate accuracy is selected for structural optimization, and the def2-tzvp (3-zeta) basis set with higher accuracy is used in single point energy calculation; the GD3BJ algorithm is used to correct the Van der Waals effect, and sets the charge to 0 and the spin multiplicity to 1.
By reading the crystallographic information (.cif) file, each periodic structural unit of Mg-MOF-74 contains 638 atoms, 704 chemical bonds and 18 polyhedra. Due to the application of high-precision functional, the computational power cannot meet the optimization of the overall periodic structure and the single point calculation of adsorption. It can be seen from Figure 2 that the smallest repeating unit in Mg-MOF-74 is a cluster containing four Mg atoms, three benzene rings, three hydroxyl groups, three carboxyl groups and one coordinated water (inside the white dotted box). Therefore, this paper uses this cluster (without bound water) as the adsorption substrate to simulate the adsorption characteristics of seven gas molecules on this segment, and then infers the macroscopic adsorption characteristics of the material on gases.
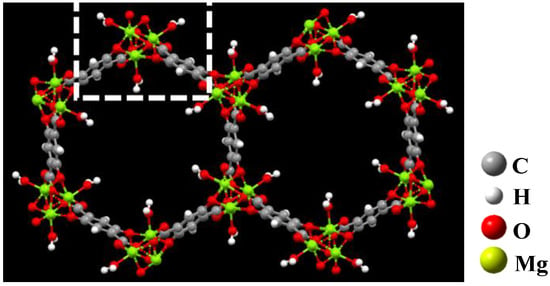
Figure 2.
The extracted Mg-MOF-74 clusters.
The optimized Mg-MOF-74 cluster and gas molecular model are shown in Figure 3. In order to represent the total energy change of gas molecules adsorbed on Mg-MOF-74, the binding energy during adsorption is defined as:
Ebinding = EMOF-gas − EMOF − Egas- + EBSSE,
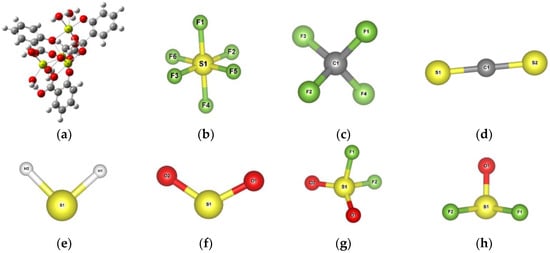
Figure 3.
Mg-MOF-74 clusters and gas molecular models after structure optimization. (a) Mg-MOF-74clusters, (b) SF6, (c) CF4, (d) CS2, (e) H2S, (f) SO2, (g) SO2F2, (h) SOF2.
In Equation (1): EMOF-gas is the total energy of the system after Mg-MOF-74 adsorbs gas molecules, EMOF is the total energy of Mg-MOF-74 clusters before adsorption, and Egas is the total energy of gas molecules before adsorption. The basis set superposition error (BSSE) is corrected using the counterpoise method proposed by Boys and Bernardi [60], and EBSSE is the correction value.
The charge transfer amount is the number of charge transfer obtained by analyzing the charge population through the Hirshfeld charge model.
ΔQ = Q1 − Q2,
In Equation (2): ΔQ is the charge transfer amount of the system, Q1 is the charge of gas molecules after adsorption, and Q2 is the charge of gas molecules before adsorption.
The adsorption distance is defined as the distance between the gas molecule and the adsorption site of Mg-MOF-74. The Van der Waals radius is 1/2 of the distance between two adjacent nuclei when atoms interact with each other through Van der Waals force. The covalent radius is 1/2 of the nucleus spacing when the atoms of the same element form diatomic molecules.
The change of charge density is analyzed by the distribution of the yellow region (atom has electron loss property) and the blue region (atom has electron gain property) in the differential charge density diagram.
In this paper, the discrete orbital occupation diagram is broadened by Gaussian function to obtain the density of states (DOS) curve, and the chemical adsorption of Mg-MOF-74 on gas is further analyzed by the total density of states, gas density of states and local density of states.
3. Results
The adsorption calculation in this paper makes the SF6, CF4, CS2, H2S, SO2, SO2F2 andSOF2 gas molecules vertically close to the unsaturated sites on the Mg-MOF-74 material; after reaching the most stable state, by extracting the adsorption parameters (binding energy, charge transfer amount, adsorption distance), the change of bond length and bond angle of gas analysis after adsorption is measured, and the adsorption capacity of Mg-MOF-74 to seven gases is comprehensively judged by analyzing the change of orbital occupancy.
3.1. Parameters of Adsorption Behavior
- Adsorption Model:
The adsorption model of SF6, CF4, CS2, H2S, SO2, SO2F2 and SOF2 gas molecules on Mg-MOF-74 material is shown in Figure 3a–g.
It can be seen that the F atoms in CF4 and SF6 interact with the Mg atoms in the adsorbed substrate, Figure 4a,b. The S atoms in CS2 and H2S interact with the Mg atoms in the adsorption substrate, Figure 3c,d. The O atom and S atom in SO2 gas interact with the Mg atom and O atom in the adsorbed substrate, respectively, Figure 3e. The O atoms in SO2F2 and SOF2 interact with the Mg atoms in the adsorption substrate, Figure 3f,g.
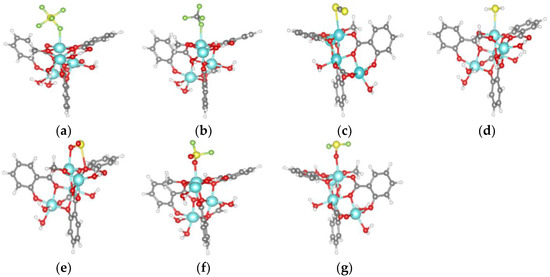
Figure 4.
Adsorption models of gas molecules on Mg-MOF-74 material. (a) SF6, (b) CF4, (c) CS2, (d) H2S, (e) SO2, (f) SO2F2, (g) SOF2.
- 2.
- Parameters of adsorption behavior:
The adsorption energies, charge transfer and adsorption distance in the adsorption process of seven gas molecules SF6, CF4, CS2, H2S, SO2, SO2F2 and SOF2 on Mg-MOF-74 are listed in Table 1.

Table 1.
The adsorption energy and charge transfer amount of Mg-MOF-74 for gas molecules.
Combined with Formula (1), the adsorption energy of Mg-MOF-74 material to each gas molecule is less than 0, indicating that the adsorption process system reaches a lower energy stable state after heat-releasing; the relationship among the adsorption capacity of the material to each gas is: H2S > SO2 > SOF2 > SO2F2 > CS2 > SF6 > CF4.
According to Formula (2) in this paper, the gas molecules lose electrons during the adsorption process, and the substrate material Mg-MOF-74 obtains electrons.
The adsorption distance among each gas molecule and the substrate is less than the sum of Van der Waals radius and larger than the sum of covalent radius (shown in Supplemental material Table S1). Therefore, according to the adsorption distance, it can be inferred that the strength of the chemical bond formed by the adsorption of seven gases by Mg atoms on Mg-MOF-74 material belongs to weaker chemical action.
- 3.
- Differential charge density:
Figure 5a–g shows the charge density difference in the adsorption process. The yellow regions show the electron losing property and the blue regions show the electron gaining property.
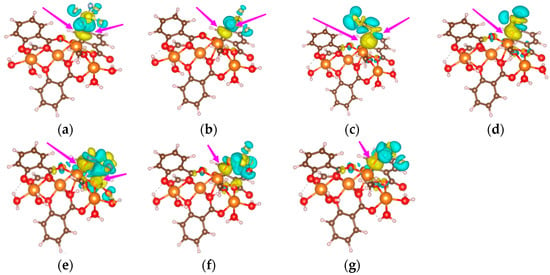
Figure 5.
Differential charge density diagrams of gas molecules adsorbed on Mg-MOF-74 material. (a) SF6, (b) CF4, (c) CS2, (d) H2S, (e) SO2, (f) SO2F2, (g) SOF2.
The charge distribution near the F atom of SF6 and CF4 is relatively uniform, and the charge distribution inside the molecule does not change significantly. The charge distribution near the C atom in CS2 is uniform, and the Mg atom bonded to the S atom in CS2 is wrapped by the yellow regions. The charge distribution near the H atom in H2S is uniform, and the Mg atom bonded to the S atom in H2S is surrounded by the yellow regions. The Mg atom bonded to the O atom in SO2 is wrapped by the yellow regions. The charge distribution near the S atom and F atom in SO2F2 is uniform, and the Mg atom bonded to the O atom in SO2F2 is wrapped by the yellow regions. The charge distribution near the S and F atoms in SOF2 is uniform, and the Mg atom bonded to the O atom in SOF2 is wrapped by the yellow regions. The above results show that the Mg atoms on the Mg-MOF-74 material exhibit electron-gaining properties during the adsorption process, while the gas molecules exhibit electron-losing properties.
3.2. The Change of Bond Length and Bond Angle of Gas Molecules after Adsorption
The bond length changes of SF6, CF4, CS2, H2S, SO2, SO2F2 and SOF2 molecules before and after adsorption and the bond angle changes are shown in Supplementary Materials Tables S1–S8.
The bond length of SF6, CF4, CS2, H2S, SO2, SO2F2 and SOF2 gas molecules changes slightly due to adsorption; the obvious changes in the bond angle (atomic number shown in Figure 2b–g) are as follow: SF6 molecules F(1)-S(1)-F(4) and F(2)-S(1)-F(3) decrease by 1.28° and 1.20°, respectively; CF4 molecules F(1)-C(1)-F(3) decrease by 1.28°; SO2 molecules O(1)-S(1)-O(2) decrease by 2.13°; SO2F2 molecules F(1)-S(1)-O(2) decrease by 2.2°; SOF2 molecules F(1)-S(1)-F(2) increase by 1°; for CS2 and H2S, the bond angle change is less than 1°. All the bond length and bong angle changes are shown in Supplementary Materials Tables S2–S8.
The changes of bond length and bond angle before and after adsorption of the above seven gas molecules, together with the adsorption energy, adsorption distance, charge transfer amount and differential charge density diagram, strongly prove that there are certain interactions among gas molecules and the adsorption substrate.
3.3. The Orbital Occupation Changes of Each System before and after Gas Adsorption
The orbital occupation calculation results of Gaussian 16 software are imported into Multiwfn software to obtain discrete orbital occupation information under different energies, as indicated by the blue arrow in Figure 6a.
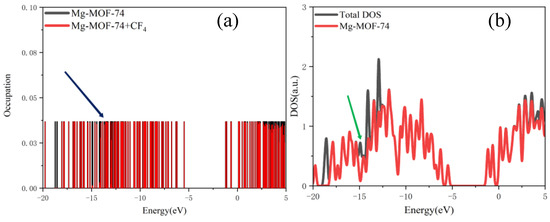
Figure 6.
(a) Discrete orbital occupation information, (b) broadening.
The Gaussian function is used for broadening, and the half-peak width is set to 0.01 eV to obtain a continuous density of states diagram (as indicated by the green arrow in Figure 6b, which can clearly and intuitively analyze the orbital occupation change. According to the extra-nuclear electron arrangement of Mg atoms, there are no filled electrons in the 3p orbital of Mg atoms, and Mg atoms form Mg2+ by losing two electrons in the 3s orbital. Therefore, the bonding interaction during adsorption is analyzed by the overlap of the 3s and 3p orbital curves of Mg atoms on Mg-MOF-74 material with the outer orbital curves of SF6, CF4, CS2, H2S, SO2, SO2F2 and SOF2 gas molecules.
- The orbital occupation of Mg-MOF-74 after adsorbing SF6 gas is shown in Figure 7a–c.
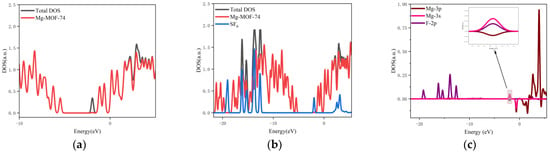 Figure 7. The orbital occupancy information of Mg-MOF-74 after adsorbing SF6. (a) The orbital occupation comparison of Mg-MOF-74 after adsorbing SF6, (b) Comparison of orbital occupancy between materials and gas, (c) The occupation analysis of 3s,3p orbital of Magnesium atom and 2p orbital of Fluorine atom.
Figure 7. The orbital occupancy information of Mg-MOF-74 after adsorbing SF6. (a) The orbital occupation comparison of Mg-MOF-74 after adsorbing SF6, (b) Comparison of orbital occupancy between materials and gas, (c) The occupation analysis of 3s,3p orbital of Magnesium atom and 2p orbital of Fluorine atom.
By observing Figure 7a, it can be found that after adsorbing SF6 gas, a new orbital occupation appears near the position of −1.95 eV, and the other energy positions do not change significantly. Compared with the total orbital occupation, it can be seen that the orbital occupation changes near the energy of −1.95 eV, 0.44 eV and 1.65 eV after adsorption of SF6 are contributed by SF6, as shown in Figure 7b. By analyzing the 3s, 3p orbitals of the adsorbed substrate Mg atom and the 2p orbital occupation of the SF6 gas molecule F atom, it can be clearly found that the 2p orbital of the F atom and the 3s orbital occupation broadening curve of the Mg atom overlap near the energy −1.95 eV position (as shown in Figure 7c), which indicates that the Mg-MOF-74 material has bonding adsorption effect on the SF6 gas.
- 2.
- The orbital occupation of Mg-MOF-74 after adsorbing CF4 gas is shown in Figure 8a–c.
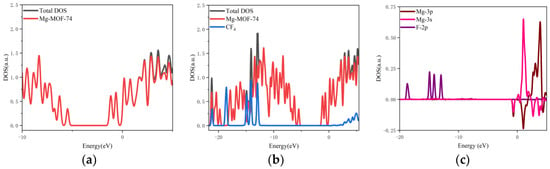 Figure 8. The orbital occupancy information of Mg-MOF-74 after adsorbing CF4. (a) The orbital occupation comparison of Mg-MOF-74 after adsorbing CF4, (b) Comparison of orbital occupancy between materials and gas, (c)The occupation analysis of 3s,3p orbital of Magnesium atom and 2p orbital of Fluorine atom.
Figure 8. The orbital occupancy information of Mg-MOF-74 after adsorbing CF4. (a) The orbital occupation comparison of Mg-MOF-74 after adsorbing CF4, (b) Comparison of orbital occupancy between materials and gas, (c)The occupation analysis of 3s,3p orbital of Magnesium atom and 2p orbital of Fluorine atom.
By observing Figure 8a, it can be found that there is no new orbital occupation after adsorption of CF4 gas. Compared with the total orbital occupation, it can be seen that the orbital occupation changes near the energy of 0.42 eV, 1.16 eV and 2.21 eV after CF4 adsorption are contributed by CF4, as shown in Figure 8b.
By analyzing the 3s and 3p orbitals of the Mg atom of the adsorption substrate and the 2p orbital occupation of the F atom of the CF4 gas molecule, it can be clearly found that the 2p orbital of the F atom does not overlap with the 3s and 3p orbital occupation broadening curves of the Mg atom (as shown in Figure 8c), which indicates that the Mg-MOF-74 material barely has bonding adsorption effect on the CF4 gas.
- 3.
- The orbital occupation of Mg-MOF-74 after adsorbing CS2 gas is shown in Figure 9a–c.
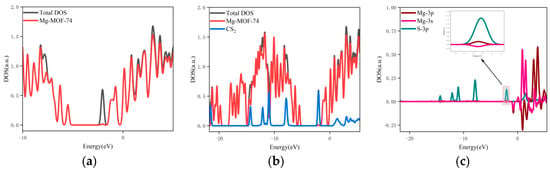 Figure 9. The orbital occupancy information of Mg-MOF-74 after adsorbing CS2. (a) The orbital occupation comparison of Mg-MOF-74 after adsorbing CS2, (b) Comparison of orbital occupancy between materials and gas, (c) The occupation analysis of 3s,3p orbital of Magnesium atom and 3p orbital of Sulfur atom.
Figure 9. The orbital occupancy information of Mg-MOF-74 after adsorbing CS2. (a) The orbital occupation comparison of Mg-MOF-74 after adsorbing CS2, (b) Comparison of orbital occupancy between materials and gas, (c) The occupation analysis of 3s,3p orbital of Magnesium atom and 3p orbital of Sulfur atom.
It can be seen from Figure 9a that after adsorbing CS2 gas, a new orbital occupation appears near the position of −2.03 eV, and the other energy positions do not change significantly. Compared with the total orbital occupancy, it can be seen that the orbital occupancy changes near the energy of −2.03 eV, 0.84 eV and 3.69 eV after adsorption of CS2 are contributed by CS2, as shown in Figure 9b. By analyzing the 3s and 3p orbitals of the Mg atom of the adsorption substrate and the 3p orbital occupation of the S atom of the CS2 gas molecule, it can be clearly found that the 3p orbital of the S atom and the 3p orbital occupation broadening curve of the Mg atom overlap near the energy −2.03 eV position (as shown in Figure 9c), which indicates that the Mg-MOF-74 material has bonding adsorption effect on the CS2 gas.
- 4.
- The orbital occupation of Mg-MOF-74 after adsorbing H2S gas is shown in Figure 10a–c.
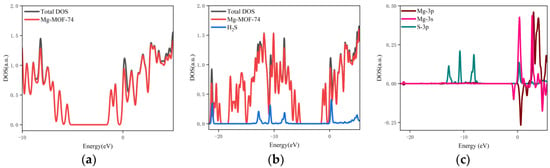 Figure 10. The orbital occupancy information of Mg-MOF-74 after adsorbing H2S. (a) The orbital occupation comparison of Mg-MOF-74 after adsorbing H2S, (b) Comparison of orbital occupancy between materials and gas, (c) The occupation analysis of 3s,3p orbital of Magnesium atom and 3p orbital of Sulfur atom.
Figure 10. The orbital occupancy information of Mg-MOF-74 after adsorbing H2S. (a) The orbital occupation comparison of Mg-MOF-74 after adsorbing H2S, (b) Comparison of orbital occupancy between materials and gas, (c) The occupation analysis of 3s,3p orbital of Magnesium atom and 3p orbital of Sulfur atom.
It can be found from Figure 10a that there is no new orbital occupation after adsorption of H2S gas. Compared with the total orbital occupation, it can be seen that the orbital occupation changes of the system near the energy of 0.35 eV, −6.95 eV and −7.47 eV after adsorption of H2S are contributed by H2S, as shown in Figure 10b.
By analyzing the 3s and 3p orbitals of the Mg atom of the adsorption substrate and the 3p orbital occupation of the S atom of the H2S gas molecule, it can be clearly found that the 3p orbital of the S atom and the 3s orbital occupation broadening curve of the Mg atom overlap near the energy of 0.35 eV (as shown in Figure 10c), which indicates that the Mg-MOF-74 material has bonding adsorption effect on the H2S gas.
- 5.
- The orbital occupation of Mg-MOF-74 after adsorbing SO2 gas is shown in Figure 11a–c.
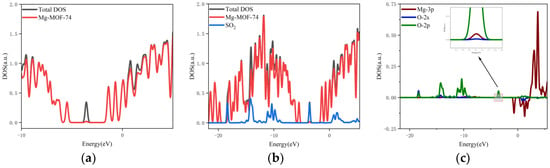 Figure 11. The orbital occupancy information of Mg-MOF-74 after adsorbing SO2. (a) The orbital occupation comparison of Mg-MOF-74 after adsorbing SO2, (b) Comparison of orbital occupancy between materials and gas, (c)The occupation analysis of 3p orbital of Magnesium atom and 2s, 2p orbital of Oxygen atom.
Figure 11. The orbital occupancy information of Mg-MOF-74 after adsorbing SO2. (a) The orbital occupation comparison of Mg-MOF-74 after adsorbing SO2, (b) Comparison of orbital occupancy between materials and gas, (c)The occupation analysis of 3p orbital of Magnesium atom and 2s, 2p orbital of Oxygen atom.
It can be seen from Figure 11a that after the adsorption of SO2 gas, a new orbital occupation occurs near −3.56 eV, and the remaining energy positions do not change significantly. Compared with the total orbital occupation, it can be seen that the change of orbital occupation near the energy of −3.56 eV, −10.35 eV and 1.38 eV after SO2 adsorption is contributed by SO2, as shown in Figure 11b. By analyzing the 3s and 3p orbitals of the Mg atom of the adsorption substrate and the 2p orbital occupation of the O atom of the SO2 gas molecule, it can be clearly found that the 2p orbital of the O atom and the 3p orbital occupation broadening curve of the Mg atom overlap near the energy −3.56 eV position (as shown in Figure 11c), which indicates that the Mg-MOF-74 material has bonding adsorption effect on the SO2 gas.
- 6.
- The orbital occupation of Mg-MOF-74 after adsorbing SO2F2 gas is shown in Figure 12a–c.
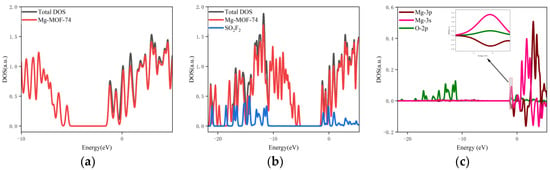 Figure 12. The orbital occupancy information of Mg-MOF-74 after adsorbing SO2F2. (a) The orbital occupation comparison of Mg-MOF-74 after adsorbing SO2F2, (b) Comparison of orbital occupancy between materials and gas, (c)The occupation analysis of 3s,3p orbital of Magnesium atom and 2p orbital of Oxygen atom.
Figure 12. The orbital occupancy information of Mg-MOF-74 after adsorbing SO2F2. (a) The orbital occupation comparison of Mg-MOF-74 after adsorbing SO2F2, (b) Comparison of orbital occupancy between materials and gas, (c)The occupation analysis of 3s,3p orbital of Magnesium atom and 2p orbital of Oxygen atom.
By observing Figure 11a, it can be found that there is no new orbital occupation after adsorption of SO2F2 gas. Compared with the total orbital occupation, it can be seen that the change of orbital occupation near the energy of −1.02 eV, −0.1 eV and 0.89 eV after adsorption of SO2F2 is contributed by SO2F2, as shown in Figure 12b.
By analyzing the 3s and 3p orbitals of the Mg atom of the adsorption substrate and the 2p orbital occupation of the O atom of the SO2F2 gas molecule, it can be clearly found that the 2p orbital of the O atom and the 3p orbital occupation broadening curve of the Mg atom overlap near the energy −1.02 eV position (as shown in Figure 12c), which indicates that the Mg-MOF-74 material has bonding adsorption effect on the SO2F2 gas.
- 7.
- The orbital occupation of Mg-MOF-74 after adsorbing SOF2 gas is shown in Figure 13a–c.
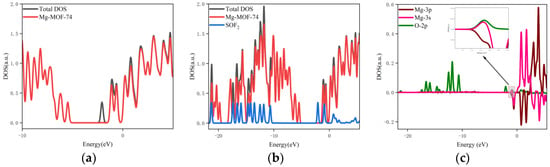 Figure 13. The orbital occupancy information of Mg-MOF-74 after adsorbing SOF2. (a) The orbital occupation comparison of Mg-MOF-74 after adsorbing SOF2, (b) Comparison of orbital occupancy between materials and gas, (c)The occupation analysis of 3s,3p orbital of Magnesium atom and 2p orbital of Oxygen atom.
Figure 13. The orbital occupancy information of Mg-MOF-74 after adsorbing SOF2. (a) The orbital occupation comparison of Mg-MOF-74 after adsorbing SOF2, (b) Comparison of orbital occupancy between materials and gas, (c)The occupation analysis of 3s,3p orbital of Magnesium atom and 2p orbital of Oxygen atom.
It can be found from Figure 13a that after the adsorption of SOF2 gas, a new orbital occupation appears near −2.07 eV after adsorption, and the other energy positions do not change significantly. Compared with the total orbital occupation, it can be seen that the orbital occupation changes near the energy of −2.07 eV, −0.93 eV and 0.77 eV after adsorption of SOF2 are contributed by SOF2, as shown in Figure 13b.
By analyzing the 3s and 3p orbitals of the Mg atom of the adsorption substrate and the 2p orbital occupation of the O atom of the SOF2 gas molecule, it can be clearly found that the 2p orbital of the O atom and the 3p orbital occupation broadening curve of the Mg atom overlap near the energy −0.93 eV position (as shown in Figure 13c), which indicates that the Mg-MOF-74 material has bonding adsorption effect on the SOF2 gas.
3.4. Conductivity Analysis after Adsorption
Pham, H.Q et al. have systematically studied the electronic band structure of a series of reticular metal-organic framework materials based on density functional theory [61]. By calculating the HOMO-LUMO gap under different types, different numbers of substituents and different CAr-CAr-C=O dihedral angle models, it is revealed that the band gap energy can be predicted by the HOMO-LUMO gap of MOFs organic ligands. The results show that the electronic band structure of MOFs can be calculated by first-principles calculations of organic linkers instead of complex and time-consuming calculations on periodic systems. In this section, by introducing the frontier molecular orbital theory to calculate the energy gap, the conductivity change of the cluster after adsorbing gas is qualitatively analyzed. It indicates the effect of gas adsorption on the conductivity of Mg-MOF-74 material.
The frontier molecular orbital distribution and energy of Mg-MOF-74 before and after adsorption of seven gases are shown in Figure 14. From the diagram, it can be seen that the highest occupied molecular orbital (HOMO) of the Mg-MOF-74 cluster and the system after adsorbing SF6, CF4, CS2, H2S, SO2, SO2F2 and SOF2 are mainly distributed on the surface of the benzene ring, which is also the same as the law in the literature [61]. The lowest unoccupied molecular orbital (LUMO) of SF6, CS2, SO2 and SO2F2 systems are mainly distributed on the surface of gas molecules. The LUMO of CF4, H2S and SO2F2 systems are mainly distributed on the surface of the other benzene ring of the cluster. With the change of frontier orbital distribution, the HOMO and LUMO energies of the seven systems have different degrees of increase and decrease, respectively. For HOMO, the change of SO2F2 system is the most obvious, and for LUMO, the change of SO2 system is the most obvious.
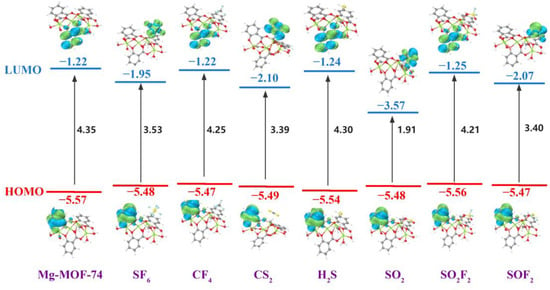
Figure 14.
The frontier molecular orbital distribution of Mg-MOF-74 after adsorption gas.
The energy gap’s change demonstrates that when the Mg-MOF-74 cluster only has chemical adsorption for CF4, SF6, CS2, H2S, SO2, SO2F2 and SOF2 gases, the conductivity of each system has different degrees of improvement, and the order of promotion is: SO2 > CS2 > SOF2 > SF6 > SO2F2 > CF4 > H2S. Therefore, in the application, it is possible to analyze the gas composition by comparing the resistance value response difference of the material to seven gases under the same sensor electrode preparation, the same gas flow rate, and the same measurement temperature, and then determine whether SF6 decomposes or not.
4. Conclusions
In this paper, GaussView software is used to construct the cluster of Mg-MOF-74 material and seven gas molecular models of SF6, CF4, CS2, H2S, SO2, SO2F2 and SOF2. The adsorption properties of Mg-MOF-74 clusters to gases are calculated by Gaussian16 software based on the DFT principle. The main conclusions are as follows:
When the adsorption of each gas molecule by Mg-MOF-74 material reaches a stable state, the relationship among the adsorption capacity of each gas is: H2S > SO2 > SOF2 > SO2F2 > CS2 > SF6 > CF4.During the adsorption process, the metal atoms in the MOFs gain electrons, the gas molecules lose electrons, and the adsorption process leads to changes in the bond length and bond angle of the gas molecules.
The density of states curve obtained by orbital occupation broadening shows that the adsorption of CF4 by Mg-MOF-74 belongs to physical adsorption, and the adsorption of the other six gases belongs to chemical adsorption.
The frontier molecular orbital analysis shows that the chemical adsorption of Mg-MOF-74 on gas causes the change of conductivity of the system. Therefore, the response difference of the material to the gas in GIS and pure SF6 gas can be used to determine whether there is a fault inside the equipment.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nano13111705/s1, Table S1: The sum of Van der waals radius and covalent radius; Table S2: Changes of bond length and bond angle after SF6 molecules adsorption; Table S3: Changes of bond length and bond angle after CF4 molecules adsorption; Table S4: Changes of bond length and bond angle after CS2 molecules adsorption; Table S5: Changes of bond length and bond angle after H2S molecules adsorption; Table S6: Changes of bond length and bond angle after SO2 molecules adsorption; Table S7: Changes of bond length and bond angle after SO2F2 molecules adsorption; Table S8: Changes of bond length and bond angle after SOF2 molecules adsorption.
Author Contributions
Methodology, visualization, formal analysis and writing—original draft, T.L.; supervision, F.L.; validation and investigation, B.J.; conceptualization and supervision, T.L. and X.F.; visualization and supervision, F.L. and X.F.; software and validation, T.L. and F.L.; supervision, writing—review and editing, F.L. and B.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Research and Application of SF6 Decomposition Compone- nt Detection Sensor Technology Based on Modified Nanomaterials, Electric Power Research Institute of Hainan Power Grid Co., Ltd.(730002021030103HH00008).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study is available on request from the corresponding authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Riechert, U.; Holaus, W. Ultra high-voltage gas-insulated switchgear—A technology milestone. Eur. Trans. Electr. Power 2012, 22, 60–82. [Google Scholar] [CrossRef]
- Zhuang, Y.; Hu, X.; Tang, B.; Wang, S.; Cui, A.; Hou, K.; He, Y.; Zhu, L.; Li, W.; Chu, J. Effects of SF6 decomposition components and concentrations on the discharge faults and insulation defects in GIS equipment. Sci. Rep. 2020, 10, 15039. [Google Scholar] [CrossRef] [PubMed]
- Rao, X.; Tang, J.; Zeng, F.; Li, D.; Xia, X.; Su, Y.; Lu, Y. Mechanism of Trace O2 on SF6 Characteristic Decomposed Components Under Spark Discharge. Plasma Chem. Plasma Proc. 2020, 40, 469–481. [Google Scholar] [CrossRef]
- Cho, Y.S.; Hong, T.Y.; Youn, Y.W.; Sun, J.H.; Lee, S.-H. Study on the Correlation between Partial Discharge Energy and SF6 Decomposition Gas Generation. Energies 2020, 13, 4655. [Google Scholar] [CrossRef]
- Zeng, F.; Lei, Z.; Yang, X.; Tang, J.; Yao, Q.; Miao, Y. Evaluating DC Partial Discharge with SF6 Decomposition Characteristics. IEEE Trans. Power Deliv. 2019, 34, 1383–1392. [Google Scholar] [CrossRef]
- Zhang, X.; Tian, S.; Xiao, S.; Huang, Y.; Liu, F. Partial discharge decomposition characteristics of typical defects in the gas chamber of SF6 insulated ring network cabinet. IEEE Trans. Dielectr. Electr. Insul. 2017, 24, 1794–1801. [Google Scholar] [CrossRef]
- Liu, M. Decomposing Mechanism of SF6 under Positive DC Partial Discharge in the Presence of Trace H2O. ACS Omega 2020, 5, 13389–13395. [Google Scholar] [CrossRef]
- Tang, J.; Zeng, F.; Zhang, X.; Pan, J.; Yao, Q.; Hou, X.; Tang, Y. Relationship between Decomposition Gas Ratios and Partial Discharge Energy in GIS, and the Influence of Residual Water and Oxygen. IEEE Trans. Dielectr. Electr. Insul. 2014, 21, 1226–1234. [Google Scholar] [CrossRef]
- Beyer, C.; Jenett, H.; Klockow, D. Influence of Reactive SFx, Gases on Electrode Surfaces After Electrical Discharges Under SF6, Atmosphere. IEEE Trans. Dielectr. Electr. Insul. 2000, 7, 234–240. [Google Scholar] [CrossRef]
- Tang, J.; Yang, X.; Ye, G.; Yao, Q.; Miao, Y.; Zeng, F. Decomposition Characteristics of SF6 and Partial Discharge Recognition under Negative DC Conditions. Energies 2017, 10, 556. [Google Scholar] [CrossRef]
- Ammar, S.M.; Zulkurnain, A.-M.; Rai, N.A. SF6 Decomposed Component Analysis for Partial Discharge Diagnosis in GIS: A Review. IEEE Access 2022, 10, 27270–27288. [Google Scholar]
- Tang, J.; Yang, X.; Yang, D.; Yao, Q.; Miao, Y.; Zhang, C.; Zeng, F. Using SF6 Decomposed Component Analysis for the Diagnosis of Partial Discharge Severity Initiated by Free Metal Particle Defect. Energies 2017, 10, 1119. [Google Scholar] [CrossRef]
- Zeng, F.; Tang, J.; Zhang, X.; Xie, Y.; Yao, Q.; Miao, Y. Reconstructing and extracting information on SF6 decomposition characteristic components induced by partial overthermal fault in GIE. IEEE Trans. Dielectr. Electr. Insul. 2016, 23, 183–193. [Google Scholar] [CrossRef]
- Xiao, S.; Zhang, J.; Zhang, X.; Cui, H. Pt-doped single-walled CNT as a superior media for evaluating the operation status of insulation devices: A first-principle study. AIP Adv. 2018, 8, 105101. [Google Scholar] [CrossRef]
- Liu, M. Adsorption Behavior of Ni-Doped ZnO Monolayer upon SF6 Decomposed Components and Effect of the Applied Electric Field. ACS Omega 2020, 5, 24118–24124. [Google Scholar] [CrossRef]
- Yang, A.; Li, W.; Chu, J.; Wang, D.; Yuan, H.; Zhu, J.; Wang, X.; Rong, M. Enhanced sensing of sulfur hexafluoride decompo- sition components based on noble-metal-functionalized cerium oxide. Mater. Des. 2020, 187, 08391. [Google Scholar] [CrossRef]
- Sun, H.; Gui, Y.; Wei, H.; Long, Y.; Wang, Q.; Tang, C. DFT study of SF6 decomposed products on Pd-TiO2: Gas sensing mechanism study. Adsorption 2019, 25, 1643–1653. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, Q.; Zeng, W. Competitive adsorption of SF6 decompositions on Ni-doped ZnO (100) surface: Computational and experimental study. Appl. Surf. Sci. 2019, 479, 185–197. [Google Scholar] [CrossRef]
- Wang, Y.; Gui, Y.; Ji, C.; Tang, C.; Zhou, Q.; Li, J.; Zhang, X. Adsorption of SF6 decomposition components on Pt3-TiO2 (101) surface: A DFT study. Appl. Surf. Sci. 2018, 459, 242–248. [Google Scholar] [CrossRef]
- Wu, P.; Zhang, X.; Chen, D.; Tang, J. Adsorption of SF6 Decomposed Products on ZnO-Modified C3N: A Theoretical Study. Nanoscale Res. Lett. 2020, 15, 186. [Google Scholar] [CrossRef]
- Hou, W.; Liu, Y.; Zeng, W.; Zhou, Q. Theoretical screening into Ag-Embedded HfS2 monolayers as gas sensor for detecting SF6 decomposition gases. J. Mater. Res. Technol. 2022, 18, 1991–2000. [Google Scholar] [CrossRef]
- Liu, H.; Wang, F.; Hu, K.; Li, T.; Yan, Y.; Li, J. The adsorption and sensing performances of Ir-modified MoS2 monolayer toward SF6 decomposition products: A DFT study. Nanomaterials 2021, 11, 100. [Google Scholar] [CrossRef] [PubMed]
- Gui, X.; Zhou, Q.; Peng, S.; Xu, L.; Zeng, W. Adsorption behavior of Rh-doped Molybdenum disulfide monolayer towards Sulfur dioxide, Thionyl fluoride, Sulfuryl fluoride based on DFT study. Phys. E 2020, 122, 114224. [Google Scholar] [CrossRef]
- Gui, Y.; Wang, Y.; Duan, S.; Tang, C.; Zhou, Q.; Xu, L.; Zhang, X. Ab Initio Study of SOF2 and SO2F2 Adsorption on Co-MoS2. ACS Omega 2019, 4, 2517–2522. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Gui, Y.; Li, W.; Li, Q.; Chen, X. Gas-sensing properties of Ptn-doped WSe2 to SF6 de- composition products. J. Ind. Eng. Chem. 2021, 97, 452–459. [Google Scholar] [CrossRef]
- Qian, H.; Deng, J.; Xie, Z.; Pan, Z.; Zhang, J.; Zhou, H. Adsorption and Gas Sensing Properties of the Pt3-MoSe2 Monolayer to SOF2 and SO2F2. ACS Omega 2020, 5, 7722–7728. [Google Scholar] [CrossRef]
- Wang, X.; Liao, Y. Selective detection of SO2 in SF6 insulation devices by Rh-doped HfSe2 monolayer: A first-principles study. Appl. Phys. A -Mater. 2019, 125, 468. [Google Scholar] [CrossRef]
- Cui, H.; Feng, Z.; Wang, W.; Peng, X.; Hu, J. Adsorption Behavior of Pd-Doped PtS2 Monolayer Upon SF6 Decomposed Species and the Effect of Applied Electric Field. IEEE Sens. J. 2022, 22, 6764–6771. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, X.; Tang, J.; Cui, H.; Chen, Z.; Li, Y. Different doping of penta-graphene as adsor-bent and gas sensing material for scavenging and detecting SF6 decomposed species. Sustain. Mater. Technol. 2019, 21, e00100. [Google Scholar]
- Peng, X.; Liu, D.; Zhao, F.; Tang, C. Gas sensing properties of Mg-doped graphene for H2S, SO2, SOF2, and SO2F2 based on DFT. Int. J. Quantum Chem. 2022, 122, e26989. [Google Scholar] [CrossRef]
- Chen, D.; Tang, J.; Zhang, X.; Fang, J.; Li, Y.; Zhuo, R. Detecting decompo-sitions of sulfur hexa-fluoride using reduced graphene oxide decorated with Pt nanoparticles. J. Phys. D-Appl. Phys. 2018, 51, 185304. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, L.; Wu, X.; Hu, W. Experimental Sensing and Density Functional Theory Study of H2S and SOF2 Adsorption on Au-Modified Graphene. Adv. Sci. 2015, 2, 1500101. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Huang, R.; Gui, Y.; Zeng, H. Gas sensing analysis of Ag-decorated graphene for sulfur hexafluoride decomposition products based on the density functional theory. Sensors 2016, 16, 1830. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Cui, H.; Gui, Y.; Tang, J. Mechanism and Application of Carbon Nanotube Sensors in SF6 Decomposed Production Detection: A Review. Nanoscale Res. Lett. 2017, 12, 177. [Google Scholar] [CrossRef]
- Zhang, X.; Cui, H.; Dong, X.; Chen, D.; Tang, J. Adsorption performance of Rh decorated SWCNT upon SF6 decomposed components based on DFT method. Appl. Surf. Sci. 2017, 420, 825–832. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, X.; Chen, D.; Tang, J. Pt & Pd decorated CNT as a workable media for SOF2 sensing: A DFT study. Appl. Surf. Sci. 2019, 4, 335–341. [Google Scholar]
- Zhang, X.; Gui, Y.; Xiao, H.; Zhang, Y. Analysis of adsorption properties of typical partial discharge gases on Ni-SWCNTs using density functional theory. Appl. Surf. Sci. 2016, 379, 47–54. [Google Scholar] [CrossRef]
- Zhang, Q.; Gui, Y.; Qiao, H.; Chen, X.; Cao, L. Theoretical study of SF6 decomposition products adsorption on metal oxide cluster-modified single-layer graphene. J. Ind. Eng. Chem. 2022, 105, 278–290. [Google Scholar] [CrossRef]
- Pi, S.; Zhang, X.; Cui, H.; Chen, D.; Zhang, G.; Xiao, S.; Tang, J. Facile fabrication of Au nanoparticles/tin oxide/reduced graphene oxide ternary nanocomposite and its high-performance SF6 decomposition components sensing. Front. Chem. 2019, 7, 476. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, X.; Cheng, X.; Xu, Y.; Gao, S.; Zhao, H.; Huo, L. Hierarchical NiO Cube/Nitrogen-doped reduced graphene oxide composite with enhanced H2S sensing properties at low temperature. ACS Appl. Mater. Inter. 2017, 9, 26293–26303. [Google Scholar] [CrossRef]
- Yaghi, O.M.; O’keeffe, M.; Ockwig, N.W.; Chae, H.K.; Eddaoudi, M.; Kim, J. Reticular synthesis and the design of new materials. Nature 2003, 423, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Eddaoudi, M.; Kim, J.; Rosi, N.; Vodak, D.; Wachter, J.; O’Keeffe, M.; Yaghi, O.M. Systematic design of pore size and functio nality in isoreticular MOFs and their application in methane storage. Science 2002, 295, 469–472. [Google Scholar] [CrossRef]
- Karadeniz, B.; Žili, D.; Huskic, I.; Germann, L.S.; Fidelli, A.M.; Muratović, S.; Lončarić, I.; Etter, M.; Dinnebier, R.E.; Barišić, D.; et al. Controllingthe polymorphism and topology transfor- mation in porphyrinic zirconiummetal-organic frameworks via mechanochemistry. J. Am. Chem. Soc. 2019, 141, 19214–19220. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, M.I.; Turkiewicz, A.B.; Darago, L.E.; Oktawiec, J.; Bustillo, K.; Grandjean, F.; Long, G.J.; Long, J.R. Confinement of atomically defined-metal halide sheets in a metal-organic framework. Nature 2020, 577, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Dhaka, S.; Kumar, R.; Deep, A.; Kurade, M.B.; Ji, S.-W.; Jeon, B.-H. Metal-organic frameworks (MOFs) for the removal of emerging contaminants from aquatic environments. Coordin. Chem. Rev. 2019, 380, 330–352. [Google Scholar] [CrossRef]
- Janghouri, M.; Hosseini, H. Water-Soluble Metal-Organic Framework Hybrid Electron Injection Layer for Organic Light-Emitting Devices. J. Inorg. Organomet. Polym. Mater. 2017, 27, 800–805. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Yang, H.; Zhao, W.Y.; Wang, J.; Yang, Q.S. A weakly luminescent Tb-MOF-based “turn-on” sensor for the highly selective and sensitive sensing of an anthrax bio- marker. Dalton Trans. 2021, 50, 1300–1306. [Google Scholar] [CrossRef]
- Li, H.; Lv, N.; Xue, L.; Liu, B.; Feng, J.; Ren, X.; Guo, T.; Chen, D.; Stoddart, J.F.; Gref, R.; et al. Composite CD-MOF nanocrystals-containing micro-spheres for sustained drug delivery. Nanoscale 2017, 9, 7454–7463. [Google Scholar] [CrossRef]
- Song, Y.; He, M.; Zhao, J.; Jin, W. Structural manipulation of ZIF-8-based membranes for high- efficiency molecular separation. Sep. Purif. Technol. 2021, 270, 118722. [Google Scholar] [CrossRef]
- Reddy, R.C.K.; Lin, J.; Chen, Y.; Zeng, C.; Lin, X.; Cai, Y.; Su, C.-Y. Progress of nanostructured metal oxides de-rived from metal-organic frameworks as anode materials for lithium-ion Batte-ries. Coordin. Chem. Rev. 2020, 420, 213434. [Google Scholar] [CrossRef]
- Peng, S.; Wu, G.; Song, W.; Wang, Q. Application of Flower-Like ZnO Nanorods Gas Sensor Detecting Decomposition Products. J. Nanomater. 2013, 2013, 875. [Google Scholar] [CrossRef]
- Li, B.; Zhou, Q.; Peng, R.; Liao, Y.; Zeng, W. Adsorption of SF6 decomposition gases (H2S, SO2, SOF2 and SO2F2) on Sc-doped MoS2 surface: A DFT study. Appl. Surf. Sci. 2021, 549, 149271. [Google Scholar] [CrossRef]
- Lee, K.; Howe, J.D.; Lin, L.-C.; Smit, B.; Neaton, J.B. Small-Molecule Adsorption in Open-Site Metal-Organic Frameworks: A Systematic Density Functional Theory Study for Rational Design. Chem. Mater. 2015, 27, 668–678. [Google Scholar] [CrossRef]
- Liu, J.; Wei, Y.; Li, P.; Zhao, Y.; Zou, R. Selective H2S/CO2 Separation by Metal-Organic Frameworks Based on Chemical-Physical Adsorption. J. Phys. Chem. C 2017, 121, 13249–13255. [Google Scholar] [CrossRef]
- Li, S.; Zhu, S.; Zhou, Q.; Gui, Y.; Wei, X. Adsorption mechanism of decomposition gas of SF6 circuit breaker on MOF-505 analogue. Vacuum 2021, 183, 109816. [Google Scholar] [CrossRef]
- Henkelis, S.E.; Vornholt, S.M.; Cordes, D.B.; Slawin, A.M.Z.; Wheatley, P.S.; Morris, R.E. A single crystal study of CPO-27 and UTSA-74 for nitric oxide storage and release. CrystEngComm 2019, 21, 1857. [Google Scholar] [CrossRef]
- Visa, A.; Mracec, M.; Maranescu, B.; Maranescu, V.; Ilia, G.; Popa, A.; Mracec, M. Structure simulation into a lamellar supramolecular network and calculation of the metal ions/ligands ratio. Chem. Cent. J. 2012, 6, 91. [Google Scholar] [CrossRef]
- Sciortino, G.; Lihi, N.; Czine, T.; Maréchal, J.-D.; Lledós, A.; Garribba, E. Accurate prediction of vertical electronic transitions of Ni(II) coordination compounds via time dependent density functional theory. Int. J. Quantum Chem. 2018, 118, e25655. [Google Scholar] [CrossRef]
- Alongamo, C.I.L.; Tasheh, S.N.; Nkungli, N.K.; Bine, F.K.; Ghogomu, J.N. Structural, Electronic, and Charge Transport Properties of New Materials based on 2-(5-Mercapto-1,3,4-Oxadiazol-2-yl) Phenol for Organic Solar Cells and Light Emitting Diodes by DFT and TD-DFT. J. Chem. 2022, 2022, 1802826. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, J.; Yan, H.; Chen, H.; Mao, W.; Dai, G. Insight into the nature of the noncovalent interactions of furan, pyridine, and pyrazine with AtX. J. Mol. Model. 2023, 29, 13. [Google Scholar] [CrossRef]
- Pham, H.Q.; Mai, T.; Pham-Tran, N.-N. Engineering of Band Gap in Metal-Organic Frameworks by Functionalizing Organic Linker: A Systematic Density Functional Theory Investigation. J. Phys. Chem. C 2014, 118, 4567–4577. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).