Abstract
The continuous consumption of fossil energy and excessive emissions of carbon dioxide (CO2) have caused a serious energy crisis and led to the greenhouse effect. Using natural resources to convert CO2 into fuel or high-value chemicals is considered to be an effective solution. Photoelectrochemical (PEC) catalysis utilizes abundant solar energy resources, combined with the advantages of photocatalysis (PC) and electrocatalysis (EC), to achieve efficient CO2 conversion. In this review, the basic principles and evaluation criteria, of PEC catalytic reduction to CO2 (PEC CO2RR), are introduced. Next, the recent research progress on typical kinds of photocathode materials for CO2 reduction are reviewed, and the structure–function relationships between material composition/structure and activity/selectivity are discussed. Finally, the possible catalytic mechanisms and the challenges of using PEC to reduce CO2 are proposed.
1. Introduction
With the increasing demand for energy in modern society, the crisis of fossil fuels (such as oil, natural gas, etc.) and the greenhouse effect have ensued [1,2,3,4,5]. Therefore, the need for the development of clean and renewable energy is urgent. Solar energy, as a kind of renewable, clean energy, has limitless potential [6]. Using solar energy converts carbon dioxide (CO2) into fuel or high-value chemicals, such as carbon monoxide (CO), formic acid (HCOOH), methanol (CH3OH), ethanol (CH3CH2OH), acetic acid (CH3COOH), and so on, and is considered an effective means of solving the energy crisis and mitigating the greenhouse effect [7,8,9,10,11].
Photoelectrochemical (PEC) characteristics of semiconductor nanoparticles convert and store solar energy in the form of chemical bonds, making them an effective catalyst for the carbon dioxide reduction reaction (CO2RR) [12,13]. Compared with photocatalysis (PC) [14,15] or electrocatalysis (EC) [16,17], PEC has obvious advantages in CO2RR. For instance, PEC CO2RR can not only promote the separation of photo-generated charges by an external voltage, efficiently overcoming the energy barrier and achieving higher solar energy conversion efficiency [18,19], but can also decrease the overpotential under the action of solar energy to save energy. In other words, the synergistic effects of light and the applied bias potential could jointly reduce the CO2 activation energy and facilitate the reduction of CO2 [20].
Generally, PEC CO2RR includes three processes: (i) the generation of electron-hole pairs on the semiconductor under photo-excitation, and the adsorption of CO2 on the surface of the catalyst; (ii) the separation and transfer of electron-hole pairs on the semiconductor; (iii) the surface catalytic reaction, including H2O oxidation by holes and CO2 reduction by electrons [21,22]. Some p-type semiconductor materials have been applied for PEC CO2RR, such as CuO2 [23,24,25], GaP [26,27,28], TiO2 [29,30], etc. An ideal p-type semiconductor should meet certain conditions for its conduction band (CB) and valence band (VB) positions, such as the energy level of the CB being more negative than that of CO2 reduction, and the bandwidth being within the range of light absorption. Although PEC has made significant progress in CO2RR, there are still significant challenges ahead, mainly including further improving the current density and Faraday efficiency (FE), overcoming the high redox potential to activate CO2 and break the C=O bond [31,32], and inhibiting the hydrogen evolution reaction (HER) competing with CO2RR [33].
2. Basic Configuration of PEC Devices
PEC devices for CO2 reduction are generally divided into two types: single chamber electrolytic cell and H-type electrolytic cell, which are closed containers made of Teflon and quartz glass, respectively.
As shown in Figure 1 [34], in the single-chamber cell, the working electrode, the counter electrode, and the reference electrode are in the same reaction compartment. When CO2 is reduced, some of the reduction products (such as HCOOH, CH3OH, etc.) can be transferred to the counter electrode and oxidized, resulting in a significant decline in the yield, which is one of the most fatal drawbacks of the single reactor.
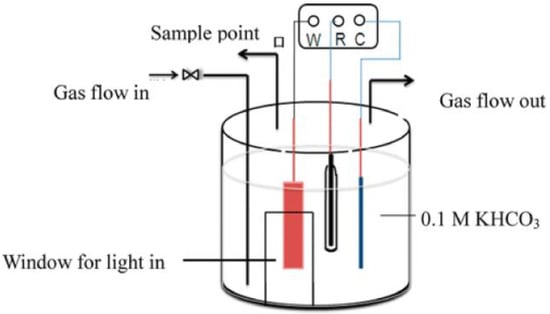
Figure 1.
Schematic diagram of the single-chamber reactor. (W, R, and C present the working electrode, reference electrode, and counter electrode, respectively) Adapted with permission from Ref. [34]. Copyright 2014, American Chemical Society.
In the H-type electrolytic cell, the working electrode and the reference electrode are in one compartment, the counter electrode is in another compartment, and the two compartments are separated by a proton membrane. Compared with the single chamber cell, the oxidation reaction occurs in the anode compartment, while the reduction reaction occurs in the cathode compartment, which can effectively prevent the reduction products from being oxidized and can significantly improve the yield of reduction products. However, the transfer of hydrogen protons in the H-type electrolytic cell will be weakened. The three-electrode system of H-type electrolytic cells usually includes the following four types: (1) photocathode (PC, generally p-type semiconductor) and dark anode (A, Figure 2a); (2) photoanode (PA, generally n-type semiconductor) and dark cathode (C, Figure 2b); (3) PA and PC (Figure 2c); (4) photovoltaic cells and electrodes containing electrochemical catalysts (Figure 2d). Each of the above four electrolytic cells also contains a reference electrode (RE), and the electrolyte can be pure water or a salt solution.
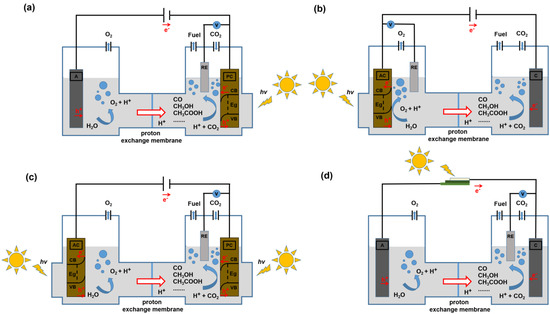
Figure 2.
Schematic diagrams of an electrolysis cell for PEC CO2RR in a three-electrode system. (a) PC, A; (b) PA, C; (c) PA, PC; (d) A, C; and each has a RE.
3. General Parameters to Evaluate the PEC CO2 Reduction
The PEC CO2RR efficiency on different catalysts can be evaluated by the following specific standards:
3.1. Faraday Efficiency (FE)
FE is one of the important standards to evaluate CO2 reduction performance, which can be expressed as the ratio of the actual product yield to the theoretical product yield. FE is a more intuitive way to illustrate the selectivity of a catalyst, and its calculation formula is shown in Equation (1) [35,36,37]:
In Equation (1): FEp is the Faraday efficiency of p (p represents the reduction product of CO2, such as HCOOH); Ip is the current density of p; It is the total current density. np is the amount of the substance; N is the number of electrons that are created for every mole of the corresponding substance. F is Faraday constant (F = 96,485 C/mol). Q is the total amount of charge (Q = It).
The higher the FE value, the better the selectivity of the catalyst for a specific product. Its value is influenced by multiple factors, such as temperature, electrolyte concentration, applied voltage, solution acidity, and even electrode material purity.
3.2. Solar to Fuel Efficiency (STF)
STF is the conversion efficiency of the PEC reduction of CO2 system under light irradiation when the action potential between the working electrode and the counter electrode is zero [38,39]. There are two different calculation methods for STF, such as Equations (2) and (3) [35,38,40]:
In Equation (2): Cfuel is the amount of fuel produced per unit of time; ΔG0 is Gibbs free energy that converts CO2 into fuel; Psolar is the light power density of incident light and Area is the effective area of the working electrode exposed by the incident light.
In Equation (3): Jsc is photocurrent density under short circuit conditions; ΔE0 is the thermodynamic potential of CO2 converted to fuel; FE is the Faraday efficiency; Psolar is the light power density of incident light.
3.3. Current Density
Current density is the ratio of the tested current to the geometric area of the working electrode, as calculated in Equation (4), which is related to the yield of the product.
In Equation (4): Jcd is current density (mA/cm2); I (A) represents the total current of the i-t test, and Area is the geometric area of the working electrode.
Based on the total current density and the FE of the corresponding product (such as HCOOH), the partial current density of the corresponding product can be calculated, as shown in Equation (5).
In Equation (5): Jpcd is the partial current density of the corresponding product; FEp is the Faraday efficiency corresponding to the product.
3.4. Linear Sweep Voltammetry Curve (LSV)
The onset potential and overpotential of the reaction can be obtained from the LSV curve, which is also an important performance for catalysts.
3.5. Incident Photon to Current Efficiency (IPCE)
IPCE is the conversion efficiency of the incident photon into photocurrent under irradiation of incident light with a certain wavelength, which is also called external quantum efficiency [41,42]. IPCE can be calculated by the ratio of the total energy of the converted electrons to the total energy of the incident photons, as shown in Equation (6).
In Equation (6): Jsc is photocurrent density under short circuit conditions; λ is the wavelength of incident light; P is the light intensity of incident light at a certain wavelength.
3.6. Absorbed Photon-Current Conversion Efficiency (APCE)
APCE is the conversion efficiency of absorbed photons into the photocurrent under light irradiation, which can evaluate the recombination efficiency of photo-generated carriers on the semiconductors as shown in Equation (7) [38,43].
In Equation (7): A(λ) is the absorbance.
3.7. Turnover Number (TON) and Turnover Frequency (TOF)
TON and TOF are used to characterize the catalytic activity and stability of the catalyst [44]. TON is the mole number of the conversion substrate divided by the mole number of the catalytic activity center, which can also be expressed as the conversion rate of the active site within a certain period. TOF is the number of conversions of a single active site per unit time. In the photoelectrochemical reduction of CO2, TON and TOF are defined as follows (Equations (8) and (9)) [19,24]:
In Equations (8) and (9), npro is the mole number of the product; ncat is the mole number of the catalyst; t is reaction time.
3.8. Tafel Slope
Tafel slope reflects the relationship between overpotential and current density. The smaller the Tafel slope, the lower the overpotential under the same kinetic current density, indicating better catalytic performance. Moreover, the Tafel slope is also often used to explain reaction mechanisms [45].
3.9. Reaction Stability
Stability testing is necessary because most semiconductors are unstable due to photo-corrosion. The amperometric i-t curve is generally used to describe the stability of the catalyst. In the test, when the current density decreases, the stability attenuates.
4. Photocathode Materials for CO2RR
Generally, CO2RR involves many proton and electron reactions which can produce various reduction products, including CO, CH4, HCOOH, CH3OH, CH3COOH, CH3CH2OH, etc. To date, metal oxides, metal sulfides, phosphates, etc. have been reported as photoelectrochemical catalysts for PEC CO2RR [46,47]. Moreover, a variety of strategies have been proposed to further improve the activity, stability, and selectivity of the catalyst in PEC CO2RR, such as the modification of the co-catalyst, and the formation of the protective layer [48]. In this section, we mainly focus on the recent progress of representative photocathode materials to convert CO2 into different C products, and their performance enhancement strategies.
4.1. Types of Catalysts Commonly Used in PEC CO2RR
The development core of PEC technology lies in the development of highly active photoelectric-catalysts, and there are many types of catalysts reported so far for CO2 reduction. Among them, metal/oxides (Pt, Au, Fe2O3, CuFeO2, Cu2O, TiO2, etc.), metal sulfides (CuS, etc.), metal phosphates (GaP, InP, etc.), MOF-based materials (ZIF9-Co3O4, etc.), and non-metallic carbon materials (g-C3N4, etc.) have been widely studied. To overcome the overpotential for CO2 reduction and drive the reaction with sufficient electrical potential, the conduction band edge of the semiconductor should be much more negative than the potential for CO2 reduction. Due to the sufficient negative CB positions, II-VI group materials in the periodic table have received extensive research in the early stages, mainly focusing on the properties of quantum dot materials such as CdTe, ZnTe, CdSe, and CdSeTe. These catalysts typically also have appropriate band gap width and band guide position, which are beneficial for absorbing visible light and reducing CO2. In IV group materials, Si has good absorption of ultraviolet light, visible light, and even infrared light. Although the bandgap position of Si satisfies the multi-electron reduction potential of CO2RR, it is easily oxidized and the recombination probability of photogenerated carriers is high, making it often used as a substrate. Apart from Si-based catalysts, GaP, InP, Cu2O, and CuFeO2 are also usually adopted as photocathode substrates.
Based on different semiconductors and reaction systems, the products of PEC CO2RR also gradually shift from C1 products (CH4, CH3OH, CO, HCOOH, etc.) to C2 products (C2H4, CH3CH2OH, CH3COOH, etc.) and multicarbon (C2+) products (acetone, polyols, etc.). Table 1 summarizes the representative catalysts or active sites for PEC CO2 reduction according to the types of CO2-reduced products [49]. Similar to the situation in electrocatalytic CO2 reduction, Au, Ag-based and Bi, Sn, In-based materials are excellent catalysts for the photocatalytic reduction of CO2 to CO and HCOOH in aqueous electrolytes, respectively. The highest FEs for PEC CO and HCOOH production, using these metal-based catalysts, are close to 100%. For example, Fe-based catalysts have been studied extensively for CO2RR. The element dopants, together with the local coordination environment of the Fe center, play remarkable roles in affecting the electronic structures and catalytic performances [50]. The reported Fe-based catalysts mainly produce CO with high Fes up to 90% [51,52,53]. However, there are few reports about the selective formation of multicarbon (C2+) products over Fe-based catalysts, due to their low FE and low current density. As a comparison, though Cu-based electrocatalysts exhibit unique advantages in electrocatalytic CO2 reduction, as they can reduce CO2 to various carbon products (CH3OH, CH4, or C2+ chemicals) through multiple electron transfer reactions, it is still a huge challenge to improve the selectivity of a single product, due to the complex and diverse properties. Biological catalysts, including molecular catalysts and enzymes, are also applied in PEC CO2 reduction, where the corresponding photocathodes have achieved a record FE of 100% for CO or HCOOH production [54]. In recent years, graphitic carbon nitride (g-C3N4), as a non-metallic semiconductor material, has shown broad application prospects in CO2 reduction research, due to its excellent catalytic performance, which comes from its sufficiently negative CB. Research has shown that pyridine N enriched g-C3N4, as an active site, can contribute to C-C coupling in PEC CO2RR to produce CH3CH2OH. In addition, vacancy-rich TiO2 [55], oxygen-doped CdS, CuFeO2, and carbon/Cu2O composites are also explored as active sites for PEC CO2 reduction to produce CH3OH, CH3COOH, or CH3CH2OH, but most of them showed low selectivity for their primary products during the PEC CO2 reduction process. To sum up, it remains a significant challenge for electrocatalytic CO2RR to produce C2+ products efficiently over non-copper catalysts, and there is a lack of photocathodes using metal-based or biological co-catalysts for PEC CO2 reduction to produce CH3OH, CH4, or C2+ chemicals with high FEs (>90%).

Table 1.
Summary of the catalysts or active sites for PEC CO2 reduction to different products.
4.2. Strategies for Enhancing the Performance of Photocathodes in PEC CO2RR
In recent years, numerous achievements have been made in the research of photocatalytic CO2 reduction in photocathode materials. Despite significant progress, there are still many challenges in the efficient reduction of the CO2 reaction process. This review is based on the aforementioned PEC CO2RR process and briefly elaborates on the basic strategies for enhancing the efficiency of PEC CO2RR from three perspectives: light absorption, carrier separation, and surface reaction kinetics.
4.2.1. Improving Light Absorption
The light absorption capacity of semiconductors is closely related to their band structure, therefore, optimizing the band structure of semiconductors is a major strategy for enhancing the absorption performance of photocathodes. In addition, loading light absorbing material onto catalysts is an effective method to enhance their light absorption performance.
Self-doping or doping with other elements can alter the band structure of catalytic materials, thereby improving their light absorption performance. At the same time, the conductivity of the photocathodes is greatly improved, and the current density increases. With regard to PEC systems, there are only a few studies on the doping of photoelectrodes, including Mg-doped CuFeO2 [56], B-doped C3N4 [57], N-doped ZnTe [58], and S, N-co-doped nanoporous carbon [59] photocathodes for PEC reduction of CO2.
Plasma metal nanostructures can also improve the efficiency of light energy collection and energy conversion, and have been widely used in semiconductors [60]. Under the action of an incident light wave electric field, the outer free electrons of metal nanoparticles are polarized and move, generating a new electric field that applies a linear internal restoring force within the original system. This type of electronic dipole oscillation, limited to the interior of metal nanoparticles, is known as localized surface plasmon resonance (LSPR). Common precious metals with LSPR effects include Au, Ag, Pt, etc., while non-precious metals mainly include Cu. In catalytic reactions, the abovementioned metal cocatalysts exhibit excellent performance in PEC CO2RR.
Incorporating dye molecules and quantum dots into the catalyst can enhance its light absorption ability. Xu et al. [30] utilized multifunctional TiO2 thin films as photocathodes to convert CO2 into CO, and lower CH3CH2OH through PEC. The light absorption ability of the photocathodes is improved by combining the dye molecule, Eosin Y Disodium, with TiO2, and Pd nanoparticles and amine ligands are introduced to capture protons and CO2, respectively.
4.2.2. Enhancing the Separation Efficiency of Photogenerated Carriers
In the process of PEC CO2 reduction, a large amount of recombination of photogenerated carriers occurs on the catalyst body and surface, greatly reducing the catalytic reaction efficiency. Therefore, enhancing the separation efficiency of photogenerated carriers is an important means of improving the photoelectrocatalytic efficiency.
Constructing homo/heterojunctions is considered a highly effective strategy for improving the separation of photogenerated electrons and holes, as well as enhancing the selectivity for specific products in PEC CO2 reduction [61]. The heterojunction photocathode is usually composed of two or more semiconductors with alternating band structures, such as ZnO/ZnTe [62], CdTe/ZnTe [63], CuO/Cu2O [64], and CuFeO2/CuO [65], etc.
4.2.3. Accelerating Surface Reaction Kinetics
Interface reaction is a crucial step in the PEC process. As mentioned above, after the generation and separation of photogenerated carriers, they ultimately need to be transferred to the surface of the catalyst to participate in the redox reaction. For the photoelectric reduction of the CO2 system, when the photogenerated carriers reach the catalyst surface, electrons participate in the CO2RR, and holes participate in the oxidation reaction of H2O. Research has shown that strengthening the interface reaction process is crucial for improving the efficiency of PEC CO2RR.
Controlling the surface morphology of catalysts is an effective way to accelerate interfacial reactions. By adjusting the surface morphology of the catalyst, it has a high specific surface area and exposes a specific crystal plane, thus promoting the absorption of CO2 at its surface active site. In addition, precise control of the morphology of the co catalyst can be an effective method to ensure illumination on the photocathode.
Modifying semiconductor surfaces, using different techniques, is another effective strategy for accelerating interface reactions. The sluggish reaction kinetics of CO2 reduction, as a result of the high activation energy or overpotential, largely impedes the reaction process. By using techniques such as chemical etching, electrochemical treatment, or chemical vapor deposition (CVD), catalyst deposition (noble/non-noble metals, MOFs [66], molecular complexes [67], polymers, carbon materials [68]), and passivation with thin protective materials on the surface of photocathodes, the surface of photocathodes can be functionalized, which help to accelerate interface reaction kinetics (accelerate multi-electron reactions, reduce reaction overpotential, and promote the transfer of photogenerated charge carriers to the catalyst surface). In some cases, surface catalysts can also improve the stability of the photoelectrodes by rapidly consuming the surface charge carriers. Furthermore, specific functional catalysts can increase the selectivity for the desired products by manipulating the adsorption strengths of certain reaction intermediates.
4.3. Representative Photocathode Materials and Their Performance Enhancement Strategies
4.3.1. Zinc (Zn)-Based Photocathodes
Zinc-based photocathodes, such as ZnTe and ZnO, used in PEC CO2RR, mainly produce CO. ZnTe is a p-type semiconductor with a direct bandgap of 2.3 eV, and the conduction band position of ZnTe is relatively negative, which is conducive to transport interface electrons [69,70]. Jang et al. [71] first reported a new Zn/ZnO/ZnTe photocathode for PEC CO2RR (Figure 3a). An obvious photoelectric reaction can be observed on the Zn/ZnO/ZnTe photocathode, and under the Hg lamp (with >420 nm cutoff filter), the photocurrent density of the Zn/ZnO/ZnTe photocathode approaches 20 mA·cm−2 at −0.7 V vs. the reversible hydrogen electrode (RHE) (Figure 3b). Compared with Zn/ZnO, Zn/ZnO/ZnTe showed significantly improved visible light absorption performance, which is consistent with the result that the light absorption edge can be extended to approximately 570 nm (Figure 3c) [62]. However, due to the competitive reaction of HER, the FE of CO at −0.7 V vs. RHE is as low as 22.9%, and the stability of the Zn/ZnO/ZnTe photocathode also needs to be enhanced. To further improve its performance, Jang et al. coupled Au nanoparticles with ZnTe/ZnO/Zn (ZOZT) to obtain a ZOZT-Au photocathode, as shown in Figure 3d. ZOZT-Au not only further enhanced photocurrent density, but also improved the FE of CO to 58% at −0.7 V vs. RHE, in comparison with ZOZT (Figure 3e–f).
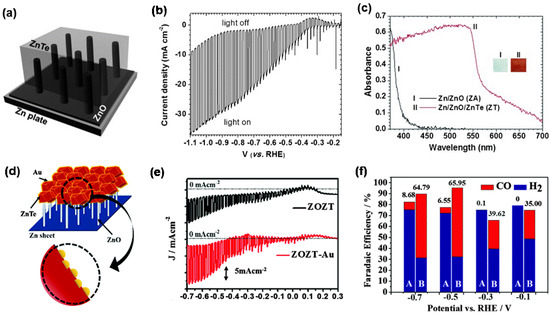
Figure 3.
(a) Diagram of the Zn/ZnO/ZnTe structure; (b) LSV curves of Zn/ZnO/ZnTe with the light on and off; (c) UV-Vis absorption spectra of Zn/ZnO and Zn/ZnO/ZnTe. Adapted with permission from Ref. [71]. Copyright 2014, Wiley-VCH. (d) Diagram of Au-coupled ZnTe/ZnO/Zn structure; (e) LSVs of ZOZT and ZOZT-Au with the light on and off; (f) FE for ZOZT (A) and ZOZT-Au (B) (the value above the cylinder indicates the selectivity of CO, concerning H2 during H2O reduction). Adapted with permission from Ref. [62]. Copyright 2016, Royal Society of Chemistry.
This work offers an effective strategy by using a highly conductive metal substrate and heterojunction structure, which can improve the current density and facilitate the separation of photogenerated carriers. In particular, the deposition of Au nanoparticles could further improve the selectivity for CO and inhibit the competitive H2 evolution, as well as promote the electron transfer by forming a Schottky junction with ZnTe at the interface. In addition, Au enhances the stability of the electrode. Coupling metal electrocatalysts with semiconductors or forming heterojunctions between semiconductors can effectively increase current density and promote carrier separation, which is worth learning in the design of optoelectronic materials.
4.3.2. Cobalt (Co)-Based Photocathodes
The most common catalyst in Co-based photocathodes is Co3O4, which is also a semiconductor material with a band gap of 2.07 eV and a conduction band of −1.05 eV [72,73], and the appropriate band gap width and band guide position are beneficial for absorbing visible light and reducing CO2. Zhao et al. carried out a systematic study on Co3O4 for PEC CO2RR. Firstly, the hierarchical porous Co3O4 (HA-Co3O4) was designed for PEC CO2RR to HCOOH [74], which showed the top micro-flowers and the bottom rhombus structure (Figure 4a–c). This typical hierarchical structure added the active site of photocatalysis and electrochemistry, making the photocurrent density on the HA-Co3O4 electrode much higher than that on the Co3O4 rhombus nanorod electrode (NR-Co3O4) and the Co3O4 nanoparticle-coated electrode (NP-Co3O4) (Figure 4d), and the HCOO− yield reached 384.8 ± 7.4 μmol in 8 h, which is higher than the yield of pure EC, PC and EC + PC (Figure 4e). Secondly, the metal Cu nanoparticles were modified on the Co3O4 nanotube arrays to form a metal/metal oxide composite material (Cu-Co3O4) for PEC CO2RR (Figure 4f) [75]. The Co3O4 nanotube arrays obtained by in situ growth in cobalt foil, through anodizing technology, can not only reduce the resistance between the substrate and the nanotube arrays, but also provide more active sites to promote light absorption. (Figure 4g). In addition, Cu nanoparticles can also promote the adsorption of reducing intermediates, thereby improving the selectivity of formic acid, resulting in a yield of 6.75 mmol·L−1·cm−2 of HCOOH in PEC CO2RR, with selectivity approaching 100% within 8 h (Figure 4h). The successful application of Cu-Co3O4 in PEC CO2RR once again proves that the coupling between metal and semiconductor is a very desirable design in PEC CO2RR.
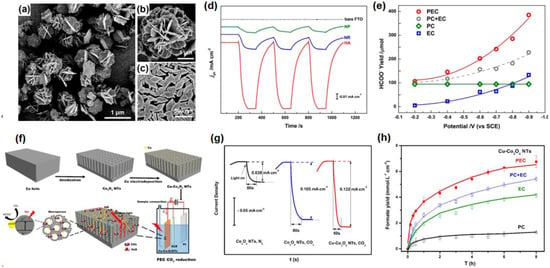
Figure 4.
(a–c) SEM images of HA-Co3O4 structure; (d) i-t curves of bare FTO, NP-Co3O4, NR-Co3O4, and HA-Co3O4 with the light on/off; (e) Formate yields of the sample HA-Co3O4 in PEC CO2RR (red circle), EC (blue square), PC (green rhombus) and PC + EC (gray circle). Adapted with permission from Ref. [74]. Copyright 2013, American Chemical Society. (f) Schematic mechanism of the Cu-Co3O4 NTs fabrication and PEC CO2RR; (g) i-t curves of Co3O4 NTs and Cu-Co3O4 NTs in N2 and CO2-saturated electrolyte; (h) Formate yields of the sample Cu-Co3O4 in PEC CO2RR (red), EC (green), PC (black) and PC + EC (blue). Adapted with permission from Ref. [75]. Copyright 2015, American Chemical Society.
The composite Ru(bpy)2dppz-Co3O4/carbon aerogel (Ru(bpy)2dppz-Co3O4/CA) (Figure 5a), prepared by solvothermal reaction and droplet casting method, also showed superior PEC CO2RR performance [72]. The photocurrent response (Figure 5b) and the yield of HCOO− (Figure 5c) on the sample Ru(bpy)2dppz-Co3O4/CA is significantly higher than that of Co3O4 and Co3O4/CA, such as the FE of HCOO− is 86% at −0.6V vs. normal hydrogen electrode (NHE). Such a good activity is mainly ascribed to the following aspects: (1) carbon aerogel has rich micropores and a large specific surface area, which can enhance the adsorption of CO2; (2) Ru(bpy)2dppz can be used as an electron transfer medium and CO2 activator, and the synergistic effect between bpy and dppz can also effectively achieve the rapid transfer of electrons. It is believed that modifying the metal/oxide catalysts with organic molecules or linkers, to construct a microenvironment for CO2 activation and reduction, will be a new strategy to regulate the photocathode activity or product selectivity.

Figure 5.
(a) Schematic illustration structure of Ru(bpy)2dppz-Co3O4/CA and PEC CO2RR; (b) i-t curves of Co3O4/FTO (dotted line), Co3O4/CA (dashed line) and Ru(bpy)2dppz-Co3O4/CA (solid line) with the light on and off; (c) the yield of HCOO− on Co3O4/FTO (gray triangle), Ru(bpy)2dppz/CA (green rhombus), Co3O4/CA (cyan square) and Ru(bpy)2dppz-Co3O4/CA (red circle). Adapted with permission from Ref. [72]. Copyright 2016, Royal Society of Chemistry. (d) Preparation schematic diagram of ZIF9-Co3O4 NWs and proposed mechanism of PEC CO2RR; (e) formate yields on the samples, ZIF9, Co3O4 NWs, and ZIF9-Co3O4 NWs electrode in PEC CO2RR; (f) SOMO orbit of the bent CO2-ZIF9 molecule; and (g) its corresponding changes in Gibbs energetic Adapted with permission from Ref. [76]. Copyright 2016, Elsevier.
Additionally, the Co3O4 nanowires (Co3O4 NWs), combined with Co-MOF (ZIF9), are conducive to the adsorption, and the capture of CO2 has also been applied in PEC CO2RR (Figure 5d) [76]. Under light illumination, the yield of formic acid is 578.7 mol·L−1·cm−2 and the FE of HCOOH is 70.6% in 8 h at −0.9V vs. saturated calomel electrode (SCE) (Figure 5e). DFT theoretical calculation results showed that when CO2 was absorbed into the micropores of ZIF9, the structure of linear CO2 would change and the bond angle would reduce, due to bending, which could promote the activation of CO2 (Figure 5f–g). It has been concluded that the structure of Co3O4 NWs can enhance light capture, while ZIF9 can improve the adsorption performance of CO2. Moreover, the p-p type heterojunction between Co3O4 NWs and ZIF9 is highly beneficial in promoting the separation of photogenerated electron-hole pairs, showing great potential in CO2 reduction. In brief, the research progresses on Co-based photocathodes has paved the way for the development of non-precious metals to achieve PEC reduction of CO2.
4.3.3. Silicon (Si)-Based Photocathodes
Si, as a potential p-type semiconductor, has a narrow energy bandgap and is widely used in PEC. Moreover, Si is abundant on Earth and has great industrial value [77]. Bradley et al. first reported p-Si photocathode materials in 1982 [78]. In PEC CO2RR, p-Si is usually used for substrates and compounded with metal or other semiconductor catalysts. This is because, although Si has strong light capture ability, its CO2 reduction activity is weak and it has high selectivity for hydrogen. It has been reported that Si photoelectrodes modified with nanoporous Au thin film mesh, formed by electrochemical reduction of the anodized gold thin film, can be used for PEC CO2RR (Figure 6a) [77]. Compared with pure Si photoelectrodes, when the potential is greater than 1.1 V vs. RHE, the photocurrent density of Si photoelectrodes, with nanoporous Au thin film mesh, is reduced, but the initial potential shifts to a positive potential (Figure 6b). Additionally, the FE of CO on Si photoelectrode, with reduced anodic Au (w/RA Au), was 91% at −0.03 V vs. RHE, which is higher than that of pure Si and Si photoelectrode with untreated Au (Figure 6c). The excellent PEC CO2RR of the w/RA Au was ascribed to the Au co-catalyst and p-n heterojunctions formed on Si, which jointly facilitated the separation of the photogenerated carrier.
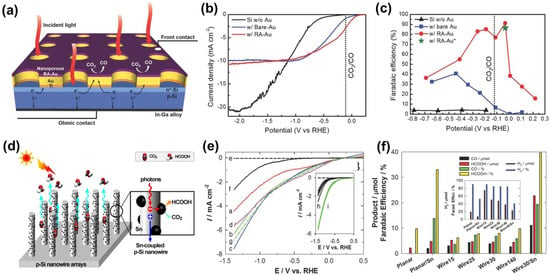
Figure 6.
(a) Schematic of Au3Cu NP/Si NW photoelectrode design for CO2 reduction; (b) PEC CO2RR LSV curves; (c) FE of CO; (bare Si—black; Si photoelectrodes of the Au mesh without RA treatment—blue; Si photoelectrode of the Au mesh with RA treatment—red; Green star symbol indicates FE of CO on Si with the Au mesh after the RA treatment). Adapted with permission from Ref. [77]. Copyright 2016, Wiley-VCH. (d) Vertically aligned, free-standing p-Si nanowire arrays of varying lengths are grown on p-Si wafers and coupled with Sn nanoparticles for solar CO2 conversion; (e) LSVs of the irradiated p-Si wire arrays etched for 1, 3, 5, and 10 h (a–d, respectively), planar Si (e—dark; f—irradiated), and p-Si wire array coupled with g—Sn nanoparticles. Inset: 5-times repeated LSVs of the h—irradiated planar, and i—wire electrodes; (f) Comparison of the products (CO and formate) and their FE. Inset: the amount of H2 produced and FE. Adapted with permission from Ref. [79]. Copyright 2014, Wiley-VCH.
In addition, Choi et al. [79] reported that Ag-assisted etchants were mixed with HF and H2O2 to form Si nanowires by etching the Si surface and combined with co-catalyst Sn to convert CO2 into HCOOH through PEC CO2RR (Figure 6d). Under AM 1.5G light (100 mW·cm−2), p-Si wire arrays have higher photocurrent density, more positive onset potential, and better stability. As the etching time increases, the photocurrent first increases and then remains unchanged (Figure 6e). The length of nanowires and the coupling of Sn have an effect on the selectivity of PEC CO2RR (Figure 6f). In addition, the FE for HCOOH in the H-type electrolytic cell reached 88.4%, which is higher than that in the single-chamber electrolytic cell. Although p-Si wire arrays can promote photon absorption and carrier separation, due to their high geometric optical path and low reflectivity, the preparation process generally requires highly corrosive hydrofluoric acid or complex etching technologies, making it unsuitable for large-scale operation.
A p-Si/metal oxide (nitrides)/metal composite was also used for PEC CO2RR. Sheng et al. [80] photo-deposited ZnO and Cu on the GaN/n+-p Si substrate to convert CO2 to syngas using PEC, in which the FE of CO was as high as 70% at −0.2 V vs. RHE in CO2-saturated 0.5 M KHCO3 solution (pH = 8) (Figure 7a–b). The photocurrent density and FE of CO have no obvious attenuations at −0.2 V vs. RHE under illumination for 10 h (Figure 7c), indicating their high stability, mainly ascribed to the photoabsorption of p-n Si heterojunction, effective electron extraction by GaN, and the fast surface kinetics of the Cu-ZnO co-catalyst. LSV results show that Cu-ZnO/GaN/n+-p Si has the highest photocurrent density among all presented samples (Figure 7d), which could be attributed to the ZnO as a cocatalyst to absorb and activate CO2 molecules, leading to the bend of the C=O bond and the decrease in CO2 chemical stability (Figure 7e). In the Cu-ZnO/GaN/n+-p Si photocathode, Si serves as the substrate for light capture, and GaN promotes the separation of photogenerated carriers. Combined with the Cu-ZnO cocatalyst, PEC reduction of CO2 can be effectively achieved, which demonstrates the combination of photocatalysts and electrocatalysts, fully achieving the synergistic effects of photo and electricity in material design.

Figure 7.
(a) Schematic illustration of Cu-ZnO/GaN/n+-p Si photoelectrode structure and PEC CO2RR; (b) relationship between the FE (CO and H2) of Cu-ZnO/GaN/ n+-p Si photocathode and the potential; (c) the stability and FE of Cu-ZnO/GaN/n+-p Si photocathode at −0.2 V vs. RHE for CO and H2; (d) LSV curves of different samples; (e) possible reaction mechanism for the PEC CO2RR to produce CO on Cu-ZnO cocatalysts. Adapted with permission from Ref. [80]. Copyright 2016, Wiley-VCH.
4.3.4. Iron (Fe)-Based Photocathodes
Fe-based photocathode materials mainly include CuFeO2 and Fe2O3. Among them, the bandwidth of CuFeO2 is 1.5~1.6 eV, which is beneficial to photon absorption. Furthermore, CuFeO2 has the structure of delafossite, and it has been reported that the conductivity of the delafossite structure can be improved by adding trivalent or bivalent metals [56,81]. For example, Gu et al. [56] synthesized Mg-doped CuFeO2 by traditional solid-state methods, which showed a high photocurrent density. However, the FE of HCOO− is as low as 10% on the photocathode Mg-doped CuFeO2 at −0.9 V vs. SCE, due to the serious hydrogen evolution competitive reaction. Interestingly, CuFeO2 and CuO mixed p-type catalysts were prepared to improve the PEC CO2RR activity, and the selectivity of HCOO− is close to 90% under simulated light irradiation [65]. More importantly, the activity can last for a week. Moreover, the onset potential of CuFeO2/CuO is +0.9 V vs. RHE, which is a very positive potential in PEC CO2RR.
The bandwidth of transition metal oxide Fe2O3 is 2.20 eV, therefore it has good visible light absorption performance [82]. Compared with the pure TiO2 nanotubes, the TiO2 nanotubes modified with Fe2O3 (Fe2O3/TiO2 NTs) not only expanded the optical absorption region (Figure 8a) but also achieved good PEC CO2RR for HCOOH [83]. At an incident wavelength of 500 nm and a bias voltage of −1.0 V vs. RHE, the selectivity and yield of HCOOH can reach 99.89% (Figure 8b) and 74,896.13 nmol·h−1·cm−2 (Figure 8c), respectively. As shown in Figure 8d, Fe2O3/TiO2 NTs have an embedded heterojunction, and electrons in the VB of TiO2 gradually transfer to the CB of Fe2O3 under incident light irradiation. Meanwhile, the Fe2O3 can absorb CO2 to form CO2·− (ads) and further obtain [FeIIICOOH]2+ under the effect of electrons and H2O. Finally, the HCOOH was formed during the transformation process of O-FeIII and O-FeII. Since Fe-based photocathodes are beneficial for future large-scale applications, the development of efficient non-noble-metal-based co-catalysts should receive much more attention.
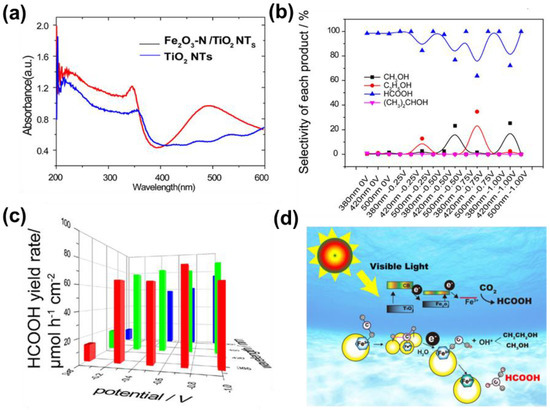
Figure 8.
(a) UV-DRS of TiO2 NTs and Fe2O3/TiO2 NTs; (b) selectivity of CO2RR products under PC (0 V) and PEC conditions on Fe2O3/TiO2 NTs; (c) yield rate of HCOOH on Fe2O3/TiO2 NTs; (d) proposed reaction pathways for the PEC CO2RR on Fe2O3/TiO2 NTs. Adapted with permission from Ref. [83]. Copyright 2018, American Chemical Society.
4.3.5. Copper (Cu)-Based Photocathodes
Among the non-noble metals, many recent studies on CO2 reduction have primarily focused on designing Cu-based materials due to their high abundance, low toxicity, unique catalytic activity, and good stability. There are two main forms of copper oxides, CuO and Cu2O. Cu-based photocathodes, especially Cu2O, are the most common photoelectrochemical catalysts, due to their good light absorption performance [84,85]. The bandwidth of Cu2O is 1.9–2.2 eV, and the CB position is conducive to the reduction of CO2 [86]. Additionally, the maximum photocurrent density of Cu2O can reach 14.7 mA·cm−2 under the illumination of AM 1.5 G light [87]. However, the biggest disadvantage of Cu2O is its susceptibility to light corrosion. Therefore, heterojunction and core–shell structure, etc. are often used to effectively avoid photo-corrosion. Additionally, CuO has also a narrow bandwidth of 1.3–1.6 eV, leading to a high absorption coefficient in the solar spectrum [88]. Moreover, Cu-based photocathodes have complex and diverse properties, thus it is challenging to improve the selectivity of a single product.
The CuO-Cu2O nanorod arrays, as a photocathode, have been prepared on a Cu substrate by a thermal oxidized and electrodeposition process for the reduction of CO2 (Figure 9a) [64]. By changing the time of Cu2O electrodeposition, the FE of CH3OH can reach 94–96% on the sample CuO-Cu2O nanorod. Under continuous illumination, the photoexcited electrons can transfer from Cu2O to CuO, or directly to CO2 adsorbed on the surface of the catalyst, improving the photoelectrochemical performance (Figure 9b). Later, Li et al. prepared Fe2O3 nanotubes modified with double-layer Cu2O spheres (Cu2O/Fe2O3 NTs) by electrodeposition method for PEC CO2RR (Figure 9c) [89]. Cu2O/Fe2O3 NTs-30 showed a three-layer structure: the bottom layer was Fe2O3 nanotubes, the middle layer was Cu2O spheres of 200–500 nm, and the top layer was Cu2O spheres with a larger diameter (Figure 9d,e). This novel three-layer structure successfully converted CO2 to CH3OH in 6 h and the FE of CH3OH was as high as 93% at −1.3 V vs. SCE, which was higher than Cu2O/Fe and Fe2O3 NTs (Figure 9f). The efficient conversion of CO2 into CH3OH is mainly because the novel three-layer structure has a conduction band position suitable for CO2 reduction and the synergy between PC and EC can promote electron transfer (Figure 9g). Further focus on the effect of Fe:Cu ratio on the product distribution over Cu2O/Fe2O3 will be more helpful in exploring its reaction kinetics and product selectivity.
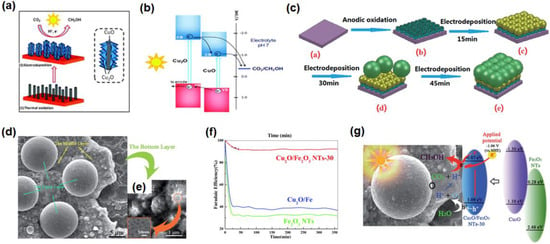
Figure 9.
(a) Schematic diagram of CuO/Cu2O nanorod synthesis and the CO2RR; (b) energy band diagram and electron transfer route of CuO/Cu2O nanorod array for the synthesis of CH3OH. Adapted with permission from Ref. [64]. Copyright 2013, Royal Society of Chemistry. (c) Growth mechanism of Cu2O/Fe2O3NTs-30 composite; (d,e) SEM of Cu2O/Fe2O3 NTs-30; (f) FE of CH3OH on Cu2O/Fe2O3 NTs-30, Cu2O/Fe, and Fe2O3 NTs; (g) mechanism of the PEC CO2RR on Cu2O/Fe2O3 NTs-30 nanotubes (30: electrodeposition times). Adapted with permission from Ref. [89]. Copyright 2014, Royal Society of Chemistry.
Recently, Kang et al. [90] prepared a hierarchically structured photocathode material Cu2O/TiO2 by controlling the synthesis temperature, atmosphere and pressure (Figure 10a). The single-phase Cu2O could be obtained by analyzing the chemical potential and accurately calculating the Gibbs free energy of CuO, Cu2O, and Cu (Figure 10b,c). In PEC CO2RR, TiO2 can protect Cu2O from corrosion, but the photogenerated electrons, on the CB of Cu2O, directly participate in CO2RR through the TiO2 layer (Figure 10d,e). Interestingly, when the thickness of the TiO2 layer is higher than 5 nm, TiO2 and Cu2O mainly form a p-n heterojunction and the photogenerated electrons on Cu2O can transfer to TiO2. Therefore, whether TiO2 participates in the transfer of electrons mainly depends on its thickness. Through the ingenious design of fewer than 5 nm thick TiO2 and pure phase cuprous oxide composite material, the stability and photoactivity have been greatly improved compared with pure Cu2O or only deposition TiO2 on the pure Cu2O but not thermodynamically programmed calcination (Figure 10f). The selectivity of CH3OH exceeds 90% on the Cu2O/TiO2 with 5 nm TiO2 layers (Figure 10g), effectively demonstrating that the products based on Cu-based photocathodes achieve high selectivity. Nevertheless, this synthesis method is relatively complex, which is not conducive to large-scale promotion.

Figure 10.
(a) The preparation process of hierarchically structured Cu2O/TiO2 photocathode; (b) diagram of the chemical potential of oxygen at different temperatures; (c) calculation of the Gibbs free energy of O2 at different pressures; (d) schematic of the hierarchical structure of Cu2O/TiO2; (e) band diagram of the Cu2O/TiO2 system with different thickness of the TiO2 passivation layer (blue line: CB, green line: VB, red dashed line: forbidden ban); (f) the effect of TiO2 passivation and the post-annealing process on photostability; (g) FE of CO2RR for different samples. Adapted with permission from Ref. [90]. Copyright 2018, American Chemical Society.
4.3.6. g-C3N4-Based Photoelectrodes
g-C3N4 is a polymer semiconductor with a bandwidth of 2.7 eV, which has high chemical stability under visible light irradiation, high reduction capacity, and sensitivity to visible light [91,92]. Recently, Nobuhiro Sagara et al. [57] prepared B-doped g-C3N4 (BCNx) for PEC CO2RR to CH3CH2OH. Although g-C3N4 is an n-type semiconductor, B-doped g-C3N4 is a proven p-type semiconductor. Compared with the pure g-C3N4, the potentials of the CB and VB of the sample BCNx are significantly increased, but the potential of the CB is still sufficient to reduce the CO2 (Figure 11a). It has been found that the photocurrent response of the sample BCNx could be improved after doping B (Figure 11b). Moreover, the photocurrent response on the different co-catalyst (Au, Ag, Rh)-loaded BCN3.0 could be further improved (Figure 11c). Rh or Au-loaded BCN3.0 show a higher number of products than BCN3.0 (Figure 11d), but the FEs of CH3CH2OH on Rh-loaded BCN3.0 (36%) and Au-loaded BCN3.0 (47%) are lower than Ag-loaded, B-doped BCN3.0 (73%) and BCN3.0 (78%).
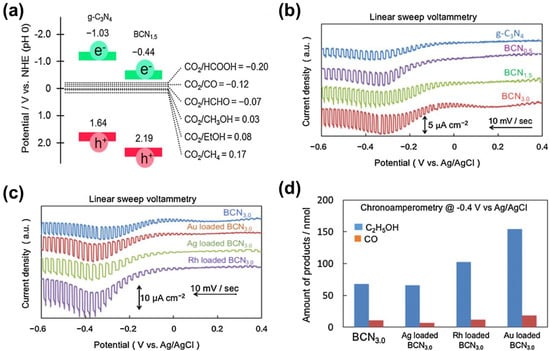
Figure 11.
(a) Band potential diagram for g-C3N4 and BCN1.5 together with the thermodynamic potentials for CO2 reduction to various reduction products vs. NHE at pH 0; (b) linear sweep voltammetry of the g-C3N4 and BCNx electrodes; (c) linear sweep voltammetry of the BCN3.0 electrodes loaded with or not loaded with co-catalysts; (d) product analyses of photoelectrochemical reduction of CO2 over co-catalyst loaded BCN3.0 electrodes. Adapted with permission from Ref. [57]. Copyright 2016, Elsevier Ltd.
The composite g-C3N4 and ZnTe, as a photocathode, can form a type II heterojunction and convert CO2 to CH3CH2OH [93]. Through the analysis of the UV-vis absorption spectra, the composite g-C3N4/ZnTe presents similar visible light adsorption performance and bandwidth to pure ZnTe (Figure 12a). According to PL spectra, it has been found that the peak intensity of the sample g-C3N4/ZnTe is significantly lower than ZnTe, indicating that the g-C3N4/ZnTe has a lower electron-hole recombination rate (Figure 12b). The yield of CH3CH2OH on the heterojunction g-C3N4/ZnTe is 17.1 μmol cm−2·h−1 at −1.1 V vs. Ag/AgCl, and the competitive hydrogen evolution is efficiently suppressed (Figure 12c), which is ascribed to the ZnTe with high CO2 adsorption capacity, and g-C3N4 rich in pyridine N contributes to C-C coupling. Moreover, a pipeline mechanism has been proposed to explain the PEC CO2RR process (Figure 12d), consisting of three steps: the adsorption and activation of CO2, the formation of CO intermediate, and the adsorption of CO intermediate on pyridine N. DFT theoretical calculations indicated that pyridine N sites were very important for the adsorption of intermediates and the formation of ethanol. The results of the studies show that polymer semiconductors, and metal loads used for the construction of heterojunctions, are also effective for PEC CO2RR. It is expected that advanced film preparation methods will further improve the film quality of g-C3N4/ZnTe photocathodes to achieve effective charge separation, which will enhance the activity of PEC CH3CH2OH production.
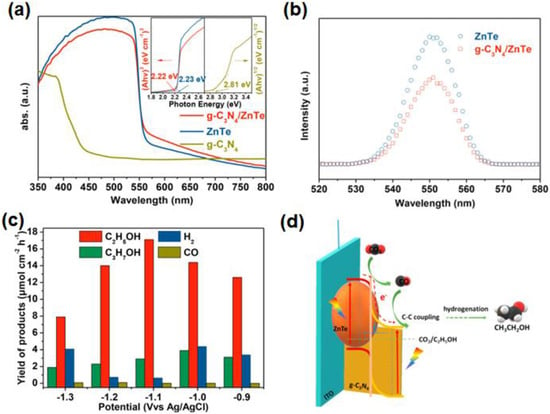
Figure 12.
(a) UV–vis diffuse reflectance spectra of three photocathodes g-C3N4/ZnTe, g-C3N4 and ZnTe; (b) PL spectra for g-C3N4/ZnTe and ZnTe; (c) products of g-C3N4/ZnTe for PEC CO2RR at different applied potentials; (d) schematic diagram of formed heterojunction and the charge separation process. Adapted with permission from Ref. [93]. Copyright 2019, Elsevier Ltd.
4.3.7. Titanium (Ti)-Based Photoelectrodes
The main structures of TiO2 are rutile and anatase, with band gaps of 3.2 eV and 3.0 eV, respectively [94]. TiO2, as the most popular semiconductor, is very stable under ultraviolet light and can be used as a photoanode or a photocathode. However, TiO2 shows a weak adsorption performance in the visible range. Cardoso et al. [95] reported that Ti/TiO2 nanotubes (TiO2NT) modified ZIF-8 as a photocathode for the PEC CO2RR (Figure 13a). It has been found that pure ZIF-8 absorbs very little visible light, while the Ti/TiO2NT-ZIF-8 shows an enhanced visible light absorption performance and reduced bandwidth, compared with Ti/TiO2NT, implying an interaction between nanotubes of Ti/TiO2NT and ZIF-8 (Figure 13b,c). In the PEC CO2RR, the selectivity to CH3CH2OH of the sample Ti/TiO2NT-ZIF-8 increased significantly, and its conversion rate increased by nearly 430 times in 1 h at −0.7 V vs. Ag/AgCl (Figure 13d). Compared with PC CO2RR, the conversion of CO2 significantly improved in the PEC CO2RR, owing to the synergy of light and electricity (Figure 13e). Moreover, ascorbic acid (AA) as a sacrificial agent was also studied in the PEC CO2RR [96]. Sacrificial agents, as donors of electrons, can promote the conversion of CO2 and increase CH3CH2OH conversion, and the FE of CH3CH2OH reached 86% on the sample Ti/TiO2NT and ZIF-8 in the presence of AA (Figure 13f). The reduced N sites of imidazolate groups in ZIF-8 can enhance the adsorption of CO2 and form carbamate to promote CO2 conversion. Meanwhile, imidazole compounds can act as electron acceptors to enhance light absorption, and thus improve photocurrent response. Such a combination of titania with MOF materials shows great potential in PEC CO2RR. Additionally, the results show that adding a sacrificial agent to the reaction is also an effective way to improve the selectivity of the product.
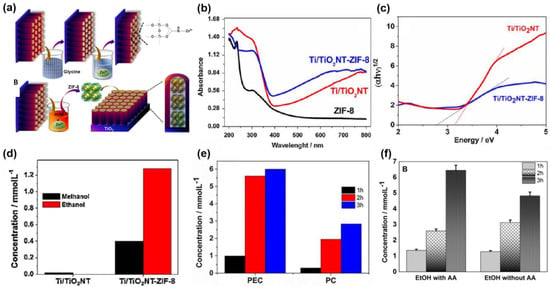
Figure 13.
(a) Synthetic diagram of Ti/TiO2NT-ZIF-8; (b) UV–vis absorption spectra of ZIF-8, TiO2NT, and TiO2NT-ZIF-8; (c) Tauc plot of Ti/TiO2NT and Ti/TiO2NT-ZIF-8; (d) concentrations of methanol and ethanol generated in the PEC processes at −0.7 V vs. Ag/AgCl, in 0.1 mol L−1 Na2SO4 (pH 4.5), using the Ti/TiO2NT and Ti/TiO2NT-ZIF-8 electrodes for 1 h; (e) the concentration of ethanol generated by using sample Ti/TiO2NT-ZIF-8 in the PEC and PC CO2RR. Adapted with permission from Ref. [95]. Copyright 2017, Elsevier B.V. (f) The concentration of ethanol on the sample Ti/TiO2NT-ZIF-8 with and without ascorbic acid (AA) in the PEC CO2RR. Adapted with permission from Ref. [96]. Copyright 2019, Elsevier B.V.
The products and performance of different representative catalysts mentioned above were summarized in Table 2.

Table 2.
Summary of products and PEC CO2 reduction performance of different representative photocathodes.
5. Possible PEC CO2RR Mechanisms
CO2 has stable chemical properties and low reactivity; thus, high energy is required to realize the conversion of CO2.. Moreover, CO2RR is also a complex process involving the coupling of many protons and the electron transfer processes. Using different catalysts and controlling the reaction conditions in the PEC CO2RR, different products could be generated. Generally, the conversion of CO2 to C1 and C2 products is easier than other complex hydrocarbon products. Up to now, though the mechanism of CO2RR is not particularly clear, many possible reaction pathways have been proposed [21].
Table 3 provides the possible half-reactions of electrochemical CO2 reduction and the electrochemical potential of thermodynamically stable CO2 molecules into C1, C2 and C3 hydrocarbon molecules. Wherein, the CO2 molecule combines with an electron to form anion radical intermediate CO2·−, which is generally regarded as the rate-limiting step, since this step needs to overcome a large redox potential, which is much higher than that required by other processes.

Table 3.
The CO2 reduction potential in aqueous solutions for the production of different hydrocarbon fuels [97].
At present, researchers have explored CO2 reduction pathways using elemental catalysts, especially metals, and proposed possible mechanisms of CO2 reduction in the aqueous solution, as shown in Figure 14 [97]. In the activation process, the straight CO2 molecule can bend as it gains electrons. Additionally, different catalysts have different adsorption strengths for the intermediate and can stabilize different intermediates, thus producing various products, which is essential for the efficient conversion of CO2. The main products of CO2RR on the transition metals are CO and HCOOH, and the binding energy of the first intermediate and the catalyst influences the final product. When the intermediate CO2·− is weakly adsorbed on the catalyst surface, HCOOH or HCOO− is the main product, and the metal catalysts applied in this reaction are mainly Sn, In, Hg, Pb, etc.

Figure 14.
Schematic mechanism of CO2 reduction on metal electrodes in aqueous solution. Reprinted with permission from Ref. [97]. Copyright 2018, Wiley.
Furthermore, some researchers also believe that the intermediate CO2·−, that produces HCOOH or HCOO−, may also bind to the catalyst by one or two oxygen atoms, but the source of hydrogen for this mechanism is unclear. It is generally believed that hydrogen can include at least two sources: (1) a metallic hydrogen bond; and (2) hydrogen protons in solution (Figure 15a–c) [98]. With regard to the metal catalysts Au, Ag, and Zn, etc., they can be closely combined with CO2·− and *COOH, but it is difficult to combine with the generated *CO intermediate, thus the reduction product is mainly CO. Based on previous research, it is known that Cu is a special metal, which can produce more complex carbon products. Firstly, the *CO intermediate is generated, then the formation of subsequent production products can be roughly divided into three pathways: (1) the combination of electrons and hydrogen protons to form the CO and H2O; (2) the conversion of intermediate *CO to *CHO or *COH and the formation of CH3OH or CH4; (3) dimerizing itself to obtain different products, such as CH3CH2OH, HCHO, etc. by getting different numbers of electrons and protons (Figure 14 and Figure 15b).
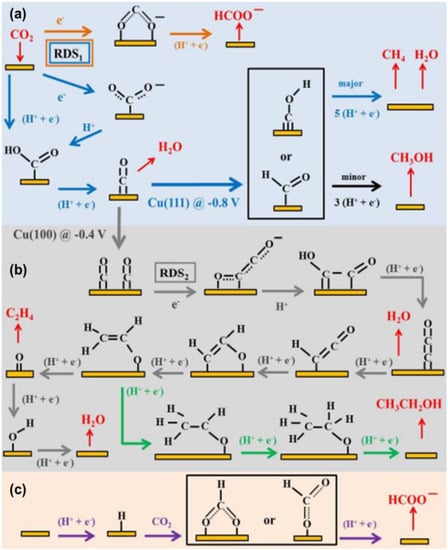
Figure 15.
(a–c) Schematic mechanisms of CO2 reduction on Cu electrodes in aqueous solution. Adapted with permission from Ref. [98]. Copyright 2015, American Chemical Society.
In addition, another mechanism is to avoid the formation of CO2·− intermediate by proton-coupled electron transfer (PCET) [99]. The activation barrier caused by high recombination energy and unstable intermediates is avoided by the transfer of a proton with an electron, but the proton concentration, pH, and temperature and other synthesis conditions have a greater impact on the PECT process [100,101].
In summary, there are still many unclear factors and processes in the PEC CO2RR, and the research on its catalytic mechanism is still in its infancy. Therefore, we need to further explore the related mechanisms of CO2RR, based on the experiment dates, advanced characteristic techniques, and DFT calculations.
6. Conclusions, Remarks, and Outlook
In the face of the global energy crisis and increasingly serious environmental issues, the reduction of CO2 and the utilization of natural resources (light) have attracted significant attention. The current research, including the design of photocathodes to achieve effective CO2 reduction by doping metal and non-metal; construction of heterojunctions such as Z-type heterojunctions, type II heterojunctions and even p-p type heterojunctions; as well as modification of co-catalysts, etc., has made great progress. Nevertheless, there are still great challenges in the practical applications; for example, the problems of low photocurrent density, low FE of reduction products, poor yield of products, and serious photo-corrosion of photocathode materials, etc.
The design of photocathode materials is crucial to the PEC CO2RR process. How to improve the light absorption of the designed photocathode material, as well as how to promote the separation of charge carriers and improve the selectivity of the product, etc., will be the emphases of the research. To this end, the following issues should also be taken into consideration:
Firstly, it is crucial to develop a new p-type semiconductor photocathode material with good stability, ultra-visible light adsorption, and CO2 conversion performance. Although some p-type semiconductor materials have been developed as photocathodes to achieve the application of PEC CO2RR, most of them have serious issues such as photo-corrosion and the complexity of reduction products. Therefore, the developed new p-type photocathode material should have photo corrosion resistance and good selectivity for CO2 reduction products. However, the development of new photocathode materials is relatively difficult and requires a long-term process, which requires deeper theoretical foundations and the assistance of DFT calculations.
Secondly, a highly efficient catalyst can be designed by reasonably optimizing the structure and composition, which is currently one of the most universally applicable measures to achieve highly efficient CO2 reduction. For example, the combination of photocatalysts with good light absorption and electrocatalysts with super selectivity in CO2RR not only preserves the advantages of each component but also regulates the selectivity of the product. More importantly, by loading co-catalysts, visible light can be effectively utilized and photogenerated electrons can be effectively separated. In addition, metals have stronger conductivity, and metal co-catalysts can thus increase current density, making them closer to industrial applications. At the same time, in the design of photocathode materials, combining semiconductor materials with appropriate conduction band positions is also an effective mean of promoting CO2 reduction.
Thirdly, improving or optimizing the catalyst preparation process is also an effective way to improve its performance, such as surface coating, surface modification, or a combination of surface technology. Different preparation processes can expose different crystal planes or generate different surface states and configurations, which are conducive to the adsorption of CO2 and the desorption of intermediates and products, improving light utilization efficiency, and enhancing the activity and stability of catalytic reactions. Moreover, it is also crucial to control and regulate the contact interface of photocathode materials. Additionally, the contact interface generally serves as a barrier for electron transfer, so precise control of the contact interface can effectively promote the separation of photo-generated charge carriers. For example, more active sites or defects can be exposed at the interface contact with electrolytes, to promote CO2 activation.
Last but not least, it is necessary to further strengthen the exploration of CO2 reduction mechanisms. A better understanding of the mechanism helps to design the structure of the target catalyst and achieve effective CO2 conversion, especially through theoretical calculations, to strengthen the study of the internal relationship between kinetics and thermodynamics.
Overall, PEC CO2RR is a promising approach for addressing fossil fuel depletion and mitigating greenhouse effects. Based on the existing technologies, combined with the development of new materials, reasonable design of catalyst structure and components, and optimization or improvement of preparation methods, it is expected to achieve more efficient CO2 conversion.
Author Contributions
K.X. and Q.Z. have equally contributed to the manuscript. Investigation, K.X., Q.Z., M.Z. and X.Z.; writing—original draft preparation, K.X. and Q.Z.; writing, review and editing, K.X., X.Z. and H.C.; supervision, H.C.; project administration, H.C.; funding acquisition, H.C. All authors have read and agreed to the published version of the manuscript.
Funding
This review was funded by the National Natural Science Foundation of China (51961165107), and the project of the Shanghai Science and Technology Commission (19520761000).
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| PEC | Photoelectrochemical |
| CO2RR | Carbon dioxide reduction reaction |
| PC | Photocatalysis |
| EC | lectrochemical |
| FE | Faraday efficiency |
| STF | Solar-to-fuel efficiency |
| IPCE | Incident photon-to-current efficiency |
| APCE | Absorbed photon-current conversion efficiency |
| TON | Turnover number |
| TOF | Turnover frequency |
| RHE | Reversible hydrogen electrode |
| NHE | Normal hydrogen electrode |
| SCE | Saturated calomel electrode |
| CB | Conduction band |
| VB | Valence band |
| AA | Ascorbic acid |
| PCET | Proton-coupled electron transfer |
References
- Chen, Z.; Zhang, G.; Chen, H.; Prakash, J.; Zheng, Y.; Sun, S. Multi-metallic catalysts for the electroreduction of carbon dioxide: Recent advances and perspectives. Renew. Sustain. Energy Rev. 2022, 155, 111922. [Google Scholar] [CrossRef]
- Jinmo, K.; Woong, C.; Woo, P.J.; Cheonghee, K.; Minjun, K.; Hyunjoon, S. Branched Copper Oxide Nanoparticles Induce Highly Selective Ethylene Production by Electrochemical Carbon Dioxide Reduction. J. Am. Chem. Soc. 2019, 141, 6986–6994. [Google Scholar]
- Michele, A. Catalysis for the valorization of exhaust carbon: From CO2 to chemicals, materials, and fuels. Technological use of CO2. Chem. Rev. 2014, 3, 1709–1742. [Google Scholar]
- Liu, Y.; Yang, Y.; Sun, Q.; Wang, Z.; Huang, B.; Dai, Y.; Qin, X.; Zhang, X. Chemical Adsorption Enhanced CO2 Capture and Photoreduction over a Copper Porphyrin Based Metal Organic Framework. ACS Appl. Mater. Interfaces 2013, 5, 7654–7658. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Liu, Y.; Quan, X.; Chen, S.; Yu, H. CO2 Electroreduction at Low Overpotential on Oxide-Derived Cu/Carbons Fabricated from Metal Organic Framework. ACS Appl. Mater. Interfaces 2017, 9, 5302–5311. [Google Scholar] [CrossRef] [PubMed]
- Diau, E.W.-G. Next-Generation Solar Cells and Conversion of Solar Energy. ACS Energy Lett. 2017, 2, 334–335. [Google Scholar] [CrossRef]
- Feng, X.; Zou, H.; Zheng, R.; Wei, W.; Wang, R.; Zou, W.; Lim, G.; Hong, J.; Duan, L.; Chen, H. Bi2O3/BiO2 Nanoheterojunction for Highly Efficient Electrocatalytic CO2 Reduction to Formate. Nano Lett. 2022, 22, 1656–1664. [Google Scholar] [CrossRef]
- Yoon, T.P.; Ischay, M.A.; Du, J. Visible light photocatalysis as a greener approach to photochemical synthesis. Nat. Chem. 2010, 2, 527–532. [Google Scholar] [CrossRef]
- Kondratenko, E.V.; Mul, G.; Baltrusaitis, J.; Larrazábal, G.O.; Pérez-Ramírez, J. Status and perspectives of CO2 conversion into fuels and chemicals by catalytic, photocatalytic and electrocatalytic processes. Energy Environ. Sci. 2013, 6, 3112–3135. [Google Scholar] [CrossRef]
- Gao, J.; Bahmanpour, A.; Kröcher, O.; Zakeeruddin, S.M.; Ren, D.; Grätzel, M. Electrochemical synthesis of propylene from carbon dioxide on copper nanocrystals. Nat. Chem. 2023, 15, 705–713. [Google Scholar] [CrossRef]
- Li, H.; Yue, X.; Che, J.; Xiao, Z.; Yu, X.; Sun, F.; Xue, C.; Xiang, J. High Performance 3D Self-Supporting Cu-Bi Aerogels for Electrocatalytic Reduction of CO2 to Formate. ChemSusChem 2022, 15, e202200226. [Google Scholar] [PubMed]
- Kecsenovity, E.; Endrődi, B.; Pápa, Z.; Hernádi, K.; Rajeshwar, K.; Janáky, C. Decoration of ultra-long carbon nanotubes with Cu2O nanocrystals: A hybrid platform for enhanced photoelectrochemical CO2 reduction. J. Mater. Chem. A 2016, 4, 3139–3147. [Google Scholar] [CrossRef]
- Sivula, K.; Roel, V.D.K. Semiconducting materials for photoelectrochemical energy conversion. Nat. Rev. Mater. 2016, 1, 15010. [Google Scholar] [CrossRef]
- Albero, J.; Peng, Y.; García, H. Photocatalytic CO2 Reduction to C2+ Products. ACS Catal. 2020, 10, 5734–5749. [Google Scholar] [CrossRef]
- Kočí, K.; Van, H.D.; Edelmannová, M.; Reli, M.; Wu, J.C. Photocatalytic reduction of CO2 using Pt/C3N4 photocatalyts. Appl. Surf. Sci. 2020, 503, 144426. [Google Scholar] [CrossRef]
- Lee, W.; Kim, Y.E.; Youn, M.H.; Jeong, S.K.; Park, K.T. Catholyte-Free Electrocatalytic CO2 Reduction to Formate. Angew. Chem. Int. Ed. 2018, 57, 6883–6887. [Google Scholar] [CrossRef] [PubMed]
- Mezzavilla, S.; Horch, S.; Stephens, I.E.L.; Seger, B.; Chorkendorff, I. Structure Sensitivity in the Electrocatalytic Reduction of CO2 with Gold Catalysts. Angew. Chem. Int. Ed. 2019, 58, 3774–3778. [Google Scholar] [CrossRef]
- Roger, I.; Shipman, M.A.; Symes, M.D. Earth-abundant catalysts for electrochemical and photoelectrochemical water splitting. Nat. Rev. Chem. 2017, 1, 3. [Google Scholar] [CrossRef]
- Zhang, N.; Long, R.; Gao, C.; Xiong, Y. Recent progress on advanced design for photoelectrochemical reduction of CO2 to fuels. Sci. China Mater. 2018, 61, 771–805. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhou, X.; Kuang, Z.; Xue, Y.; Li, C.; Zhu, M.; Mou, C.; Chen, H. A Bismuth Species-Decorated ZnO/p-Si Photocathode for High Selectivity of Formate in CO2 Photoelectrochemical Reduction. ACS Sustain. Chem. Eng. 2022, 10, 2380–2387. [Google Scholar] [CrossRef]
- White, J.L.; Baruch, M.F.; Pander, J.E., III; Hu, Y.; Fortmeyer, I.C.; Park, J.E.; Zhang, T.; Liao, K.; Gu, J.; Yan, Y.; et al. Light-Driven Heterogeneous Reduction of Carbon Dioxide: Photocatalysts and Photoelectrodes. Chem. Rev. 2015, 115, 12888–12935. [Google Scholar] [CrossRef] [PubMed]
- Xiaoxia, C.; Tuo, W.; Piaoping, Y.; Gong, Z.; Gong, J. The Development of Cocatalysts for Photoelectrochemical CO2 Reduction. Adv. Mater. 2019, 31, 1804710. [Google Scholar]
- Yuan, J. Photoelectrochemical Reduction of Carbon Dioxide to Ethanol at Cu2O Foam Cathode. Int. J. Electrochem. Sci. 2017, 12, 8288–8294. [Google Scholar] [CrossRef]
- Schreier, M.; Luo, J.; Gao, P.; Moehl, T.; Mayer, M.T.; Grätzel, M. Covalent Immobilization of a Molecular Catalyst on Cu2O Photocathodes for CO2 Reduction. J. Am. Chem. Soc. 2016, 138, 1938–1946. [Google Scholar] [CrossRef]
- Chertkova, V.P.; Iskortseva, A.N.; Pazhetnov, E.M.; Arkharova, N.A.; Ryazantsev, S.V.; Levin, E.E.; Nikitina, V.A. Evaluation of the Efficiency of Photoelectrochemical Activity Enhancement for the Nanostructured LaFeO3 Photocathode by Surface Passivation and Co-Catalyst Deposition. Nanomaterials 2022, 12, 4327. [Google Scholar] [CrossRef]
- Barton, E.E.; Rampulla, D.M.; Bocarsly, A.B. Selective Solar-Driven Reduction of CO2 to Methanol Using a Catalyzed p-GaP Based Photoelectrochemical Cell. J. Am. Chem. Soc. 2008, 130, 6342–6344. [Google Scholar] [CrossRef]
- Flaisher, H.; Tenne, R.; Halmann, M. Photoelectrochemical reduction of carbon dioxide in aqueous solutions on p-GaP electrodes: An a.c. impedance study with phase-sensitive detection. J. Electroanal. Chem. 1996, 402, 97–105. [Google Scholar] [CrossRef]
- Noh, S.; Shin, J.; Yu, Y.-T.; Ryu, M.-Y.; Kim, J.S. Manipulation of Photoelectrochemical Water Splitting by Controlling Direction of Carrier Movement Using InGaN/GaN Hetero-Structure Nanowires. Nanomaterials 2023, 13, 358. [Google Scholar] [CrossRef]
- Kobayashi, K.; Lou, S.N.; Takatsuji, Y.; Haruyama, T.; Shimizu, Y.; Ohno, T. Photoelectrochemical reduction of CO2 using a TiO2 photoanode and a gas diffusion electrode modified with a metal phthalocyanine catalyst. Electrochim. Acta 2020, 338, 135805. [Google Scholar] [CrossRef]
- Xu, Y.; Jia, Y.; Zhang, Y.; Nie, R.; Zhu, Z.; Wang, J.; Jing, H. Photoelectrocatalytic reduction of CO2 to methanol over the multi-functionalized TiO2 photocathodes. Appl. Catal. B Environ. 2017, 205, 254–261. [Google Scholar] [CrossRef]
- Hong, J.; Zhang, W.; Ren, J.; Xu, R. Photocatalytic reduction of CO2: A brief review on product analysis and systematic methods. Anal. Methods 2013, 5, 1086–1097. [Google Scholar] [CrossRef]
- Austin, N.; Ye, J.; Mpourmpakis, G. CO2 activation on Cu-based Zr-decorated nanoparticles. Catal. Sci. Technol. 2017, 7, 2245–2251. [Google Scholar] [CrossRef]
- Liu, W.; Zhai, P.; Li, A.; Wei, B.; Si, K.; Wei, Y.; Wang, X.; Zhu, G.; Chen, Q.; Gu, X.; et al. Electrochemical CO2 reduction to ethylene by ultrathin CuO nanoplate arrays. Nat. Commun. 2022, 13, 1877. [Google Scholar] [CrossRef]
- Ba, X.; Yan, L.-L.; Huang, S.; Yu, J.; Xia, X.-J.; Yu, Y. New Way for CO2 Reduction under Visible Light by a Combination of a Cu Electrode and Semiconductor Thin Film: Cu2O Conduction Type and Morphology Effect. J. Phys. Chem. C 2014, 118, 24467–24478. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, J.; Wang, Y.; Wang, Y.; Zheng, G. Efficient solar-driven electrocatalytic CO2 reduction in a redox-medium-assisted system. Nat. Commun. 2018, 9, 5003. [Google Scholar] [CrossRef]
- Kumar, B.; Atla, V.; Brian, J.P.; Kumari, S.; Nguyen, T.Q.; Sunkara, M.; Spurgeon, J.M. Reduced SnO2 Porous Nanowires with a High Density of Grain Boundaries as Catalysts for Efficient Electrochemical CO2-into-HCOOH Conversion. Angew. Chem. 2017, 129, 3699–3703. [Google Scholar] [CrossRef]
- Hu, X.; Yang, H.; Guo, M.; Gao, M.; Zhang, E.; Tian, H.; Liang, Z.; Liu, X. Synthesis and Characterization of (Cu, S) Co-doped SnO2 for Electrocatalytic Reduction of CO2 to Formate at Low Overpotential. Chemelectrochem 2018, 5, 1330–1335. [Google Scholar] [CrossRef]
- Kalamaras, E.; Maroto-Valer, M.M.; Shao, M.; Xuan, J.; Wang, H. Solar carbon fuel via photoelectrochemistry. Catal. Today 2018, 317, 56–75. [Google Scholar] [CrossRef]
- Chen, Z.; Jaramillo, T.F.; Deutsch, T.G.; Kleiman-Shwarsctein, A.; Forman, A.J.; Gaillard, N.; Garland, R.; Takanabe, K.; Heske, C.; Sunkara, M.; et al. Accelerating materials development for photoelectrochemical hydrogen production: Standards for methods, definitions, and reporting protocols. J. Mater. Res. 2011, 25, 3–16. [Google Scholar] [CrossRef]
- Pawar, A.U.; Kim, C.W.; Nguyen-Le, M.-T.; Kang, Y.S. General Review on the Components and Parameters of Photoelectrochemical System for CO2 Reduction with in Situ Analysis. ACS Sustain. Chem. Eng. 2019, 7, 7431–7455. [Google Scholar] [CrossRef]
- Lianos, P. Review of recent trends in photoelectrocatalytic conversion of solar energy to electricity and hydrogen. Appl. Catal. B Environ. 2017, 210, 235–254. [Google Scholar] [CrossRef]
- Varghese, O.K.; Grimes, C.A. Appropriate strategies for determining the photoconversion efficiency of water photoelectrolysis cells: A review with examples using titania nanotube array photoanodes. Sol. Energy Mater. Sol. Cells 2008, 92, 374–384. [Google Scholar] [CrossRef]
- Jiang, C.; Moniz, S.J.A.; Wang, A.; Zhang, T.; Tang, J. Photoelectrochemical devices for solar water splitting—Materials and challenges. Chem. Soc. Rev. 2017, 46, 4645–4660. [Google Scholar] [CrossRef] [PubMed]
- Boudart, M. Turnover Rates in Heterogeneous Catalysis. Chem. Rev. 2002, 95, 661–666. [Google Scholar] [CrossRef]
- Dunwell, M.; Luc, W.; Yan, Y.; Jiao, F.; Xu, B. Understanding Surface-Mediated Electrochemical Reactions: CO2 Reduction and Beyond. ACS Catal. 2018, 8, 8121–8129. [Google Scholar] [CrossRef]
- Arán-Ais, R.M.; Gao, D.; Cuenya, B.R. Structure- and Electrolyte-Sensitivity in CO2 Electroreduction. Acc. Chem. Res. 2018, 51, 2906–2917. [Google Scholar] [CrossRef]
- Li, C.; Zhou, X.; Zhang, Q.; Xue, Y.; Kuang, Z.; Zhao, H.; Mou, C.-Y.; Chen, H. Construction of Heterostructured Sn/TiO2/Si Photocathode for Efficient Photoelectrochemical CO2 Reduction. ChemSusChem 2022, 15, e202200188. [Google Scholar]
- Xia, D.; Yu, H.; Xie, H.; Huang, P.; Menzel, R.; Titirici, M.M.; Chai, G. Recent progress of Bi-based electrocatalysts for electrocatalytic CO2 reduction. Nanoscale 2022, 14, 7957–7973. [Google Scholar] [CrossRef]
- Li, D.; Yang, K.; Lian, J.; Yan, J.; Liu, S. Powering the World with Solar Fuels from Photoelectrochemical CO2 Reduction: Basic Principles and Recent Advances. Adv. Energy Mater. 2022, 12, 2201070. [Google Scholar] [CrossRef]
- Ji, S.; Jiang, B.; Hao, H.; Chen, Y.; Dong, J.; Mao, Y.; Zhang, Z.; Gao, R.; Chen, W.; Zhang, R.; et al. Matching the kinetics of natural enzymes with a single-atom iron nanozyme. Nat. Catal. 2021, 4, 407–417. [Google Scholar] [CrossRef]
- Ju, W.; Bagger, A.; Hao, G.-P.; Varela, A.S.; Sinev, I.; Bon, V.; Cuenya, B.R.; Kaskel, S.; Rossmeisl, J.; Strasser, P. Understanding activity and selectivity of metal-nitrogen-doped carbon catalysts for electrochemical reduction of CO2. Nat. Commun. 2017, 8, 944. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Li, L.; Ji, X.; Zhang, Y.; Mou, S.; Wu, T.; Liu, Q.; Li, B.; Zhu, X.; Luo, Y.; et al. Highly Selective Electrochemical Reduction of CO 2 to Alcohols on an FeP Nanoarray. Angew. Chem. Int. Ed. 2020, 59, 758–762. [Google Scholar] [CrossRef]
- Chen, P.; Zhang, P.; Kang, X.; Zheng, L.; Mo, G.; Wu, R.; Tai, J.; Han, B. Efficient Electrocatalytic Reduction of CO2 to Ethane over Nitrogen-Doped Fe2O3. J. Am. Chem. Soc. 2022, 144, 14769–14777. [Google Scholar] [CrossRef] [PubMed]
- Kuk, S.K.; Singh, R.K.; Nam, D.H.; Singh, R.; Lee, J.-K.; Park, C.B. Photoelectrochemical Reduction of Carbon Dioxide to Methanol through a Highly Efficient Enzyme Cascade. Angew. Chem. Int. Ed. 2017, 56, 3827–3832. [Google Scholar] [CrossRef]
- Qiu, J.; Zeng, G.; Ha, M.-A.; Ge, M.; Lin, Y.; Hettick, M.; Hou, B.; Alexandrova, A.N.; Javey, A.; Cronin, S.B. Artificial Photosynthesis on TiO2-Passivated InP Nanopillars. Nano Lett. 2015, 15, 6177–6181. [Google Scholar] [CrossRef]
- Gu, J.; Wuttig, A.; Krizan, J.W.; Hu, Y.; Detweiler, Z.M.; Cava, R.J.; Bocarsly, A.B. Mg-Doped CuFeO2 Photocathodes for Photoelectrochemical Reduction of Carbon Dioxide. J. Phys. Chem. C 2013, 117, 12415–12422. [Google Scholar] [CrossRef]
- Sagara, N.; Kamimura, S.; Tsubota, T.; Ohno, T. Photoelectrochemical CO2 reduction by a p-type boron-doped g-C3N4 electrode under visible light. Appl. Catal. B Environ. 2016, 192, 193–198. [Google Scholar] [CrossRef]
- Jang, Y.J.; Bhatt, M.D.; Lee, J.; Choi, S.H.; Lee, B.J.; Lee, J.S. Metal-Free Artificial Photosynthesis of Carbon Monoxide Using N-Doped ZnTe Nanorod Photocathode Decorated with N-Doped Carbon Electrocatalyst Layer. Adv. Energy Mater. 2018, 8, 1702636. [Google Scholar] [CrossRef]
- Li, W.; Bandosz, T.J. Role of Heteroatoms in S,N-Codoped Nanoporous Carbon Materials in CO2 (Photo)electrochemical Reduction. ChemSusChem 2018, 11, 2987–2999. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Creel, E.B.; Corson, E.R.; McCloskey, B.D.; Urban, J.J.; Kostecki, R. Surface-Plasmon-Assisted Photoelectrochemical Reduction of CO2 and NO3−on Nanostructured Silver Electrodes. Adv. Energy Mater. 2018, 8, 1800363. [Google Scholar] [CrossRef]
- Zhou, W.; Guo, J.-K.; Shen, S.; Pan, J.; Tang, J.; Chen, L.; Au, C.-T.; Yin, S.-F. Progress in Photoelectrocatalytic Reduction of Carbon Dioxide. Acta Phys. Chim. Sin. 2020, 36, 1906048. [Google Scholar] [CrossRef]
- Jang, Y.J.; Jang, J.-W.; Lee, J.; Kim, J.H.; Kumagai, H.; Lee, J.; Minegishi, T.; Kubota, J.; Domen, K.; Lee, J.S. Selective CO production by Au coupled ZnTe/ZnO in the photoelectrochemical CO2 reduction system. Energy Environ. Sci. 2015, 8, 3597–3604. [Google Scholar] [CrossRef]
- Jang, Y.J.; Jeong, I.; Lee, J.; Lee, J.; Ko, M.J.; Lee, J.S. Unbiased Sunlight-Driven Artificial Photosynthesis of Carbon Monoxide from CO2 Using a ZnTe-Based Photocathode and a Perovskite Solar Cell in Tandem. ACS Nano 2016, 10, 6980–6987. [Google Scholar] [CrossRef] [PubMed]
- Ghadimkhani, G.; de Tacconi, N.R.; Chanmanee, W.; Janaky, C.; Rajeshwar, K. Efficient solar photoelectrosynthesis of methanol from carbon dioxide using hybrid CuO-Cu2O semiconductor nanorod arrays. Chem. Commun. 2013, 49, 1297–1299. [Google Scholar] [CrossRef] [PubMed]
- Kang, U.; Choi, S.K.; Ham, D.J.; Ji, S.M.; Choi, W.; Han, D.S.; Abdel-Wahab, A.; Park, H. Photosynthesis of formate from CO2 and water at 1% energy efficiency via copper iron oxide catalysis. Energy Environ. Sci. 2015, 8, 2638–2643. [Google Scholar] [CrossRef]
- Deng, X.; Li, R.; Wu, S.; Wang, L.; Hu, J.; Ma, J.; Jiang, W.; Zhang, N.; Zheng, X.; Gao, C.; et al. Metal-Organic Framework Coating Enhances the Performance of Cu2O in Photoelectrochemical CO2 Reduction. J. Am. Chem. Soc. 2019, 141, 10924–10929. [Google Scholar] [CrossRef]
- Roy, S.; Miller, M.; Warnan, J.; Leung, J.J.; Sahm, C.D.; Reisner, E. Electrocatalytic and Solar-Driven Reduction of Aqueous CO2 with Molecular Cobalt Phthalocyanine–Metal Oxide Hybrid Materials. ACS Catal. 2021, 11, 1868–1876. [Google Scholar] [CrossRef]
- Hasan, R.; Hamid, S.B.A.; Basirun, W.J. Charge transfer behavior of graphene-titania photoanode in CO2 photoelectrocatalysis process. Appl. Surf. Sci. 2015, 339, 22–27. [Google Scholar] [CrossRef]
- Llorente, M.; Froehlich, J.; Dang, T.; Sathrum, A.; Kubiak, C.P. Photochemical and Photoelectrochemical Reduction of CO2. Annu. Rev. Phys. Chem. 2012, 63, 541–569. [Google Scholar] [CrossRef]
- Kaniyankandy, S.; Rawalekar, S.; Verma, S.; Ghosh, H.N. Ultrafast Hole Transfer in CdSe/ZnTe Type II Core−Shell Nanostructure. J. Phys. Chem. C 2011, 115, 1428–1435. [Google Scholar] [CrossRef]
- Jang, J.-W.; Cho, S.; Magesh, G.; Jang, Y.J.; Kim, J.Y.; Kim, W.Y.; Seo, J.K.; Kim, S.; Lee, K.-H.; Lee, J.S. Aqueous-Solution Route to Zinc Telluride Films for Application to CO2 Reduction. Angew. Chem. Int. Ed. 2014, 53, 5852–5857. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Shen, Q.; Liu, J.; Yang, N.; Zhao, G. A CO2 adsorption-enhanced semiconductor/metal-complex hybrid photoelectrocatalytic interface for efficient formate production. Energy Environ. Sci. 2016, 9, 3161–3171. [Google Scholar] [CrossRef]
- Gasparotto, A.; Barreca, D.; Bekermann, D.; Devi, A.; Fischer, R.A.; Fornasiero, P.; Gombac, V.; Lebedev, O.I.; Maccato, C.; Montini, T.; et al. F-Doped Co3O4 Photocatalysts for Sustainable H2 Generation from Water/Ethanol. J. Am. Chem. Soc. 2011, 133, 19362–19365. [Google Scholar] [CrossRef]
- Huang, X.; Cao, T.; Liu, M.; Zhao, G. Synergistic Photoelectrochemical Synthesis of Formate from CO2 on {12(1)over-bar} Hierarchical Co3O4. J. Phys. Chem. C 2013, 117, 26432–26440. [Google Scholar] [CrossRef]
- Shen, Q.; Chen, Z.; Huang, X.; Liu, M.; Zhao, G. High-Yield and Selective Photoelectrocatalytic Reduction of CO2 to Formate by Metallic Copper Decorated Co3O4 Nanotube Arrays. Environ. Sci. Technol. 2015, 49, 5828–5835. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Huang, X.; Liu, J.; Guo, C.; Zhao, G. Biomimetic photoelectrocatalytic conversion of greenhouse gas carbon dioxide: Two-electron reduction for efficient formate production. Appl. Catal. B Environ. 2017, 201, 70–76. [Google Scholar] [CrossRef]
- Song, J.T.; Ryoo, H.; Cho, M.; Kim, J.; Kim, J.-G.; Chung, S.-Y.; Oh, J. Nanoporous Au Thin Films on Si Photoelectrodes for Selective and Efficient Photoelectrochemical CO2 Reduction. Adv. Energy Mater. 2017, 7, 1601103. [Google Scholar] [CrossRef]
- Bradley, M.; Tysak, T. p-Type silicon based photoelectrochemical cells for optical energy conversion: Electrochemistry of tetra-azomacrocyclic metal complexes at illuminated. J. Electroanal. Chem. Interfacial Electrochem. 1982, 135, 153–157. [Google Scholar] [CrossRef]
- Choi, S.; Kang, U.; Lee, S.; Ham, D.; Ji, S.; Park, H. Sn-Coupled p-Si Nanowire Arrays for Solar Formate Production from CO2. Adv. Energy Mater. 2014, 4, 1301614. [Google Scholar] [CrossRef]
- Chu, S.; Fan, S.; Wang, Y.; Rossouw, D.; Wang, Y.; Botton, G.A.; Mi, Z. Tunable Syngas Production from CO2 and H2O in an Aqueous Photoelectrochemical Cell. Angew. Chem. Int. Edit. 2016, 55, 14262–14266. [Google Scholar] [CrossRef]
- Read, C.G.; Park, Y.; Choi, K.-S. Electrochemical Synthesis of p-Type CuFeO2 Electrodes for Use in a Photoelectrochemical Cell. J. Phys. Chem. Lett. 2012, 3, 1872–1876. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Xu, J.; Wu, C.; Jing, H.; Li, P.; Yin, H. New insight into photoelectric converting CO2 to CH3OH on the one-dimensional ribbon CoPc enhanced Fe2O3 NTs. Appl. Catal. B Environ. 2014, 156–157, 249–256. [Google Scholar] [CrossRef]
- Jiang, M.; Wu, H.; Li, Z.; Ji, D.; Li, W.; Liu, Y.; Yuan, D.; Wang, B.; Zhang, Z. Highly Selective Photoelectrochemical Conversion of Carbon Dioxide to Formic Acid. ACS Sustain. Chem. Eng. 2017, 6, 82–87. [Google Scholar] [CrossRef]
- Luo, J.; Steier, L.; Son, M.-K.; Schreier, M.; Mayer, M.T.; Grätzel, M. Cu2O Nanowire Photocathodes for Efficient and Durable Solar Water Splitting. Nano Lett. 2016, 16, 1848–1857. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Wang, Y.; Dong, C.; Nga, T.T.T.; Wei, D.; Wang, J.; Zhao, X.; Wang, M.; Zhang, K.; Li, M.; et al. Localized Geometry Determined Selectivity of Iodide-Derived Copper for Electrochemical CO2 Reduction. Adv. Energy Mater. 2023, 13, 2203896. [Google Scholar] [CrossRef]
- Dubale, A.A.; Su, W.-N.; Tamirat, A.G.; Pan, C.-J.; Aragaw, B.A.; Chen, H.-M.; Chen, C.-H.; Hwang, B.-J. The synergetic effect of graphene on Cu2O nanowire arrays as a highly efficient hydrogen evolution photocathode in water splitting. J. Mater. Chem. A 2014, 2, 18383–18397. [Google Scholar] [CrossRef]
- Paracchino, A.; Laporte, V.; Sivula, K.; Grätzel, M.; Thimsen, E. Highly active oxide photocathode for photoelectrochemical water reduction. Nat. Mater. 2011, 10, 456–461. [Google Scholar] [CrossRef]
- Rajeshwar, K.; De Tacconi, N.R.; Ghadimkhani, G.; Chanmanee, W.; Janáky, C. Tailoring Copper Oxide Semiconductor Nanorod Arrays for Photoelectrochemical Reduction of Carbon Dioxide to Methanol. ChemPhysChem 2013, 14, 2251–2259. [Google Scholar] [CrossRef]
- Li, P.; Jing, H.; Xu, J.; Wu, C.; Peng, H.; Lu, J.; Lu, F. High-efficiency synergistic conversion of CO2 to methanol using Fe2O3 nanotubes modified with double-layer Cu2O spheres. Nanoscale 2014, 6, 11380–11386. [Google Scholar] [CrossRef]
- Kang, H.-Y.; Nam, D.-H.; Yang, K.D.; Joo, W.; Kwak, H.; Kim, H.-H.; Hong, S.-H.; Nam, K.T.; Joo, Y.-C. Synthetic Mechanism Discovery of Monophase Cuprous Oxide for Record High Photoelectrochemical Conversion of CO2 to Methanol in Water. ACS Nano 2018, 12, 8187–8196. [Google Scholar] [CrossRef]
- Dong, F.; Wu, L.; Sun, Y.; Fu, M.; Wu, Z.; Lee, S.C. Efficient synthesis of polymeric g-C3N4 layered materials as novel efficient visible light driven photocatalysts. J. Mater. Chem. 2011, 21, 15171–15174. [Google Scholar] [CrossRef]
- Hui, X.; Song, Y.; Song, Y.; Zhu, J.; Zhu, T.; Liu, C.; Zhao, D.; Zhang, Q.; Li, H. Synthesis and characterization of g-C3N4/Ag2CO3 with enhanced visible-light photocatalytic activity for the degradation of organic pollutants. RSC Adv. 2014, 4, 34539–34547. [Google Scholar]
- Wang, Q.; Wang, X.; Yu, Z.; Jiang, X.; Chen, J.; Tao, L.; Wang, M.; Shen, Y. Artificial photosynthesis of ethanol using type-II g-C3N4/ZnTe heterojunction in photoelectrochemical CO2 reduction system. Nano Energy 2019, 60, 827–835. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, H.; Andino, J.M.; Li, Y. Photocatalytic CO2 Reduction with H2O on TiO2 Nanocrystals: Comparison of Anatase, Rutile, and Brookite Polymorphs and Exploration of Surface Chemistry. ACS Catal. 2012, 2, 1817–1828. [Google Scholar] [CrossRef]
- Cardoso, J.C.; Stulp, S.; De Brito, J.F.; Flor, J.B.S.; Frem, R.C.G.; Zanoni, M.V.B. MOFs based on ZIF-8 deposited on TiO2 nanotubes increase the surface adsorption of CO2 and its photoelectrocatalytic reduction to alcohols in aqueous media. Appl. Catal. B Environ. 2018, 225, 563–573. [Google Scholar] [CrossRef]
- Cardoso, J.C.; Stulp, S.; de Souza, M.K.; Hudari, F.F.; Gubiani, J.R.; Frem, R.C.G.; Zanoni, M.V.B. The effective role of ascorbic acid in the photoelectrocatalytic reduction of CO2 preconcentrated on TiO2 nanotubes modified by ZIF-8. J. Electroanal. Chem. 2020, 856, 113384. [Google Scholar] [CrossRef]
- Zhang, W.; Hu, Y.; Ma, L.; Zhu, G.; Wang, Y.; Xue, X.; Chen, R.; Yang, S.; Jin, Z. Progress and Perspective of Electrocatalytic CO2 Reduction for Renewable Carbonaceous Fuels and Chemicals. Adv. Sci. 2018, 5, 1700275. [Google Scholar] [CrossRef]
- Kortlever, R.; Shen, J.; Schouten, K.J.P.; Calle-Vallejo, F.; Koper, M.T.M. Catalysts and Reaction Pathways for the Electrochemical Reduction of Carbon Dioxide. J. Phys. Chem. Lett. 2015, 6, 4073–4082. [Google Scholar] [CrossRef]
- Goyal, P.; Hammes-Schiffer, S. Tuning the Ultrafast Dynamics of Photoinduced Proton-Coupled Electron Transfer in Energy Conversion Processes. ACS Energy Lett. 2017, 2, 512–519. [Google Scholar] [CrossRef]
- Costentin, C.; Robert, M.; Savéant, J.-M. Catalysis of the electrochemical reduction of carbon dioxide. Chem. Soc. Rev. 2013, 42, 2423–2436. [Google Scholar] [CrossRef]
- Hammes-Schiffer, S. Theory of Proton-Coupled Electron Transfer in Energy Conversion Processes. Acc. Chem. Res. 2009, 42, 1881–1889. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).