Effect of Biosynthesized Silver Nanoparticles on the Growth of the Green Microalga Haematococcus pluvialis and Astaxanthin Synthesis
Abstract
1. Introduction
2. Materials and Methods
2.1. Organism and Culturing Conditions
2.2. Experimental Design
2.3. Analytical Procedures
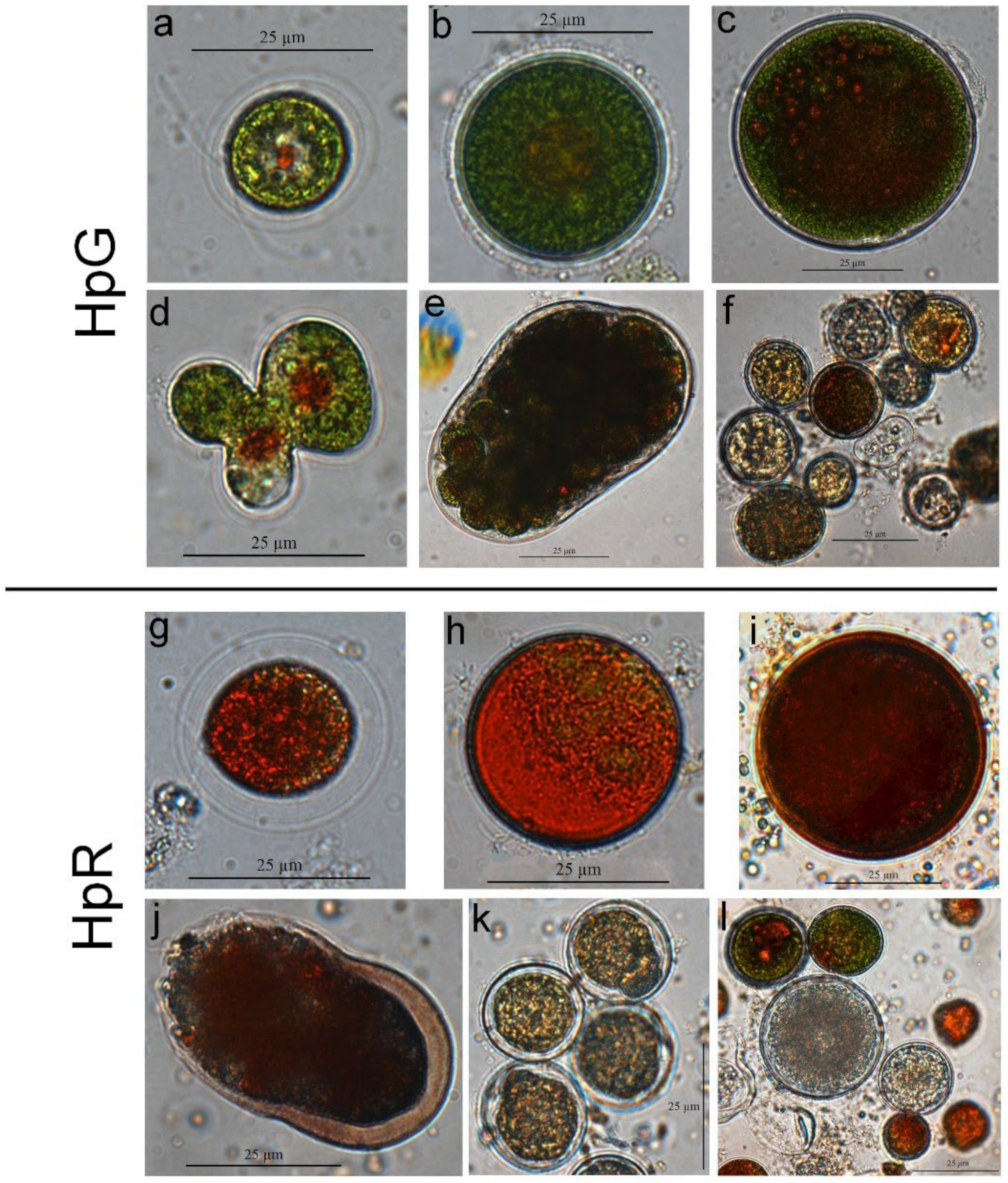
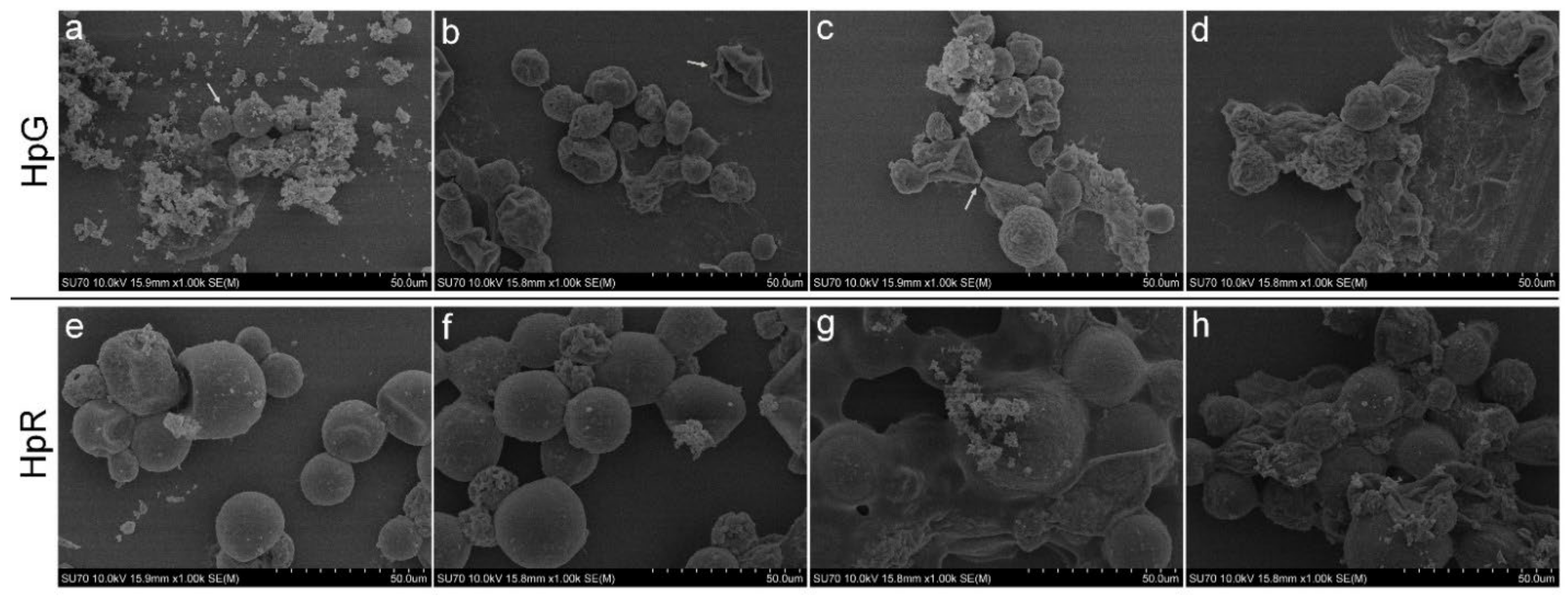
2.4. Microscopic Analysis
2.5. Synthesis and Characterization of Silver Nanoparticles
2.6. Statistical Analysis
3. Results
3.1. Characterization of AgNPs in Algal Culture Medium
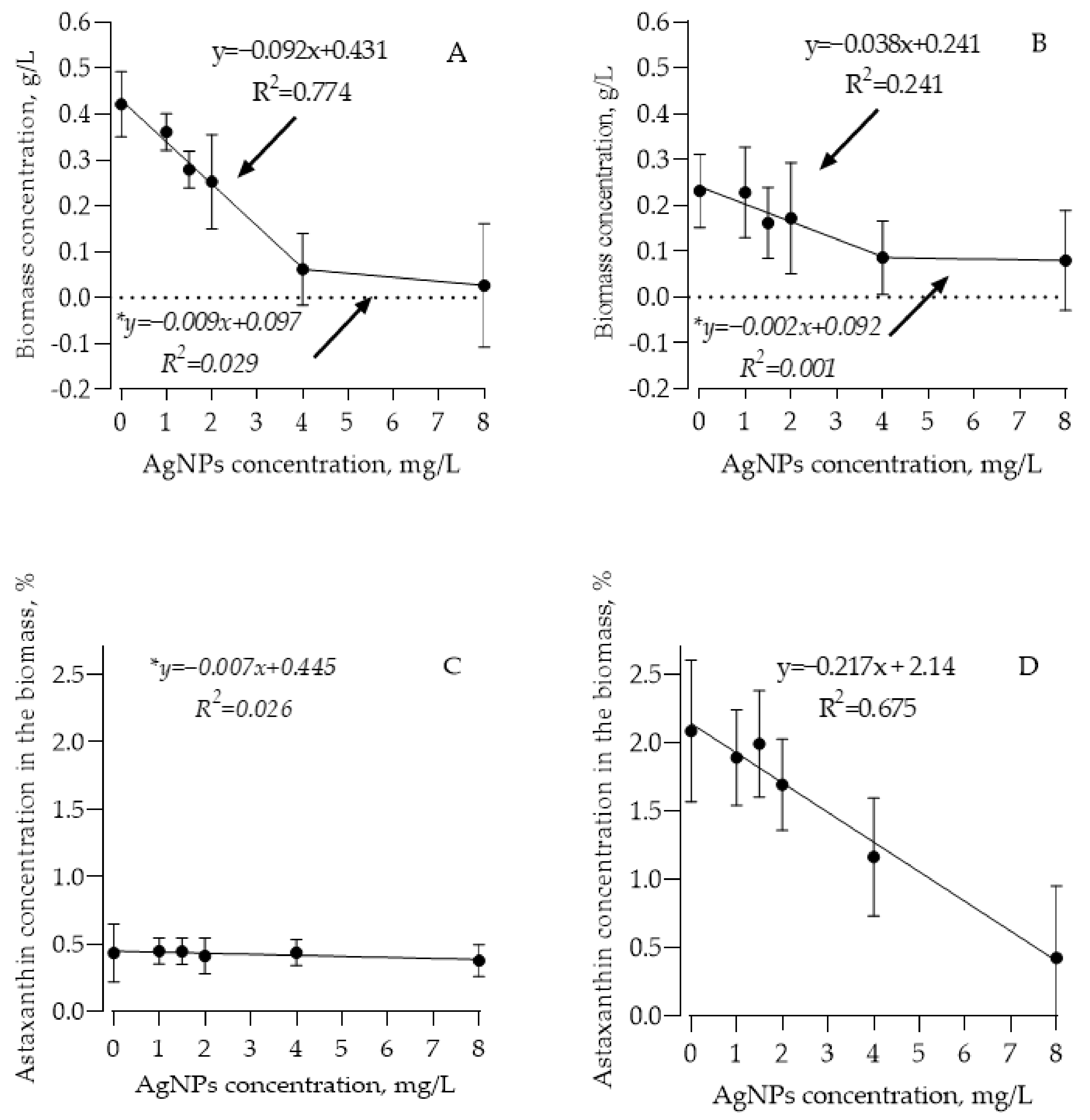
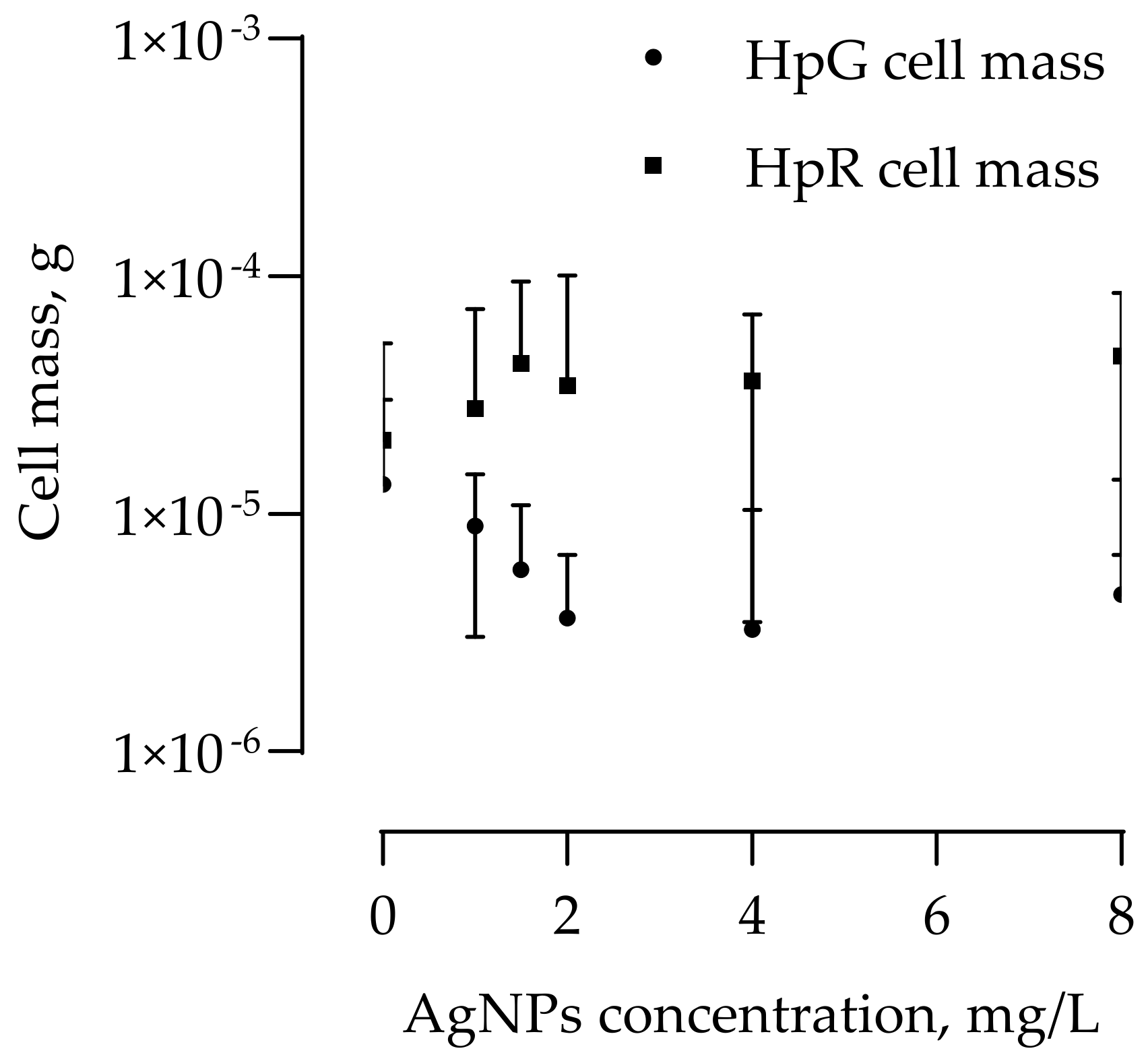
3.2. AgNPs’ Effect on Algae Biomass Growth and Astaxanthin Synthesis
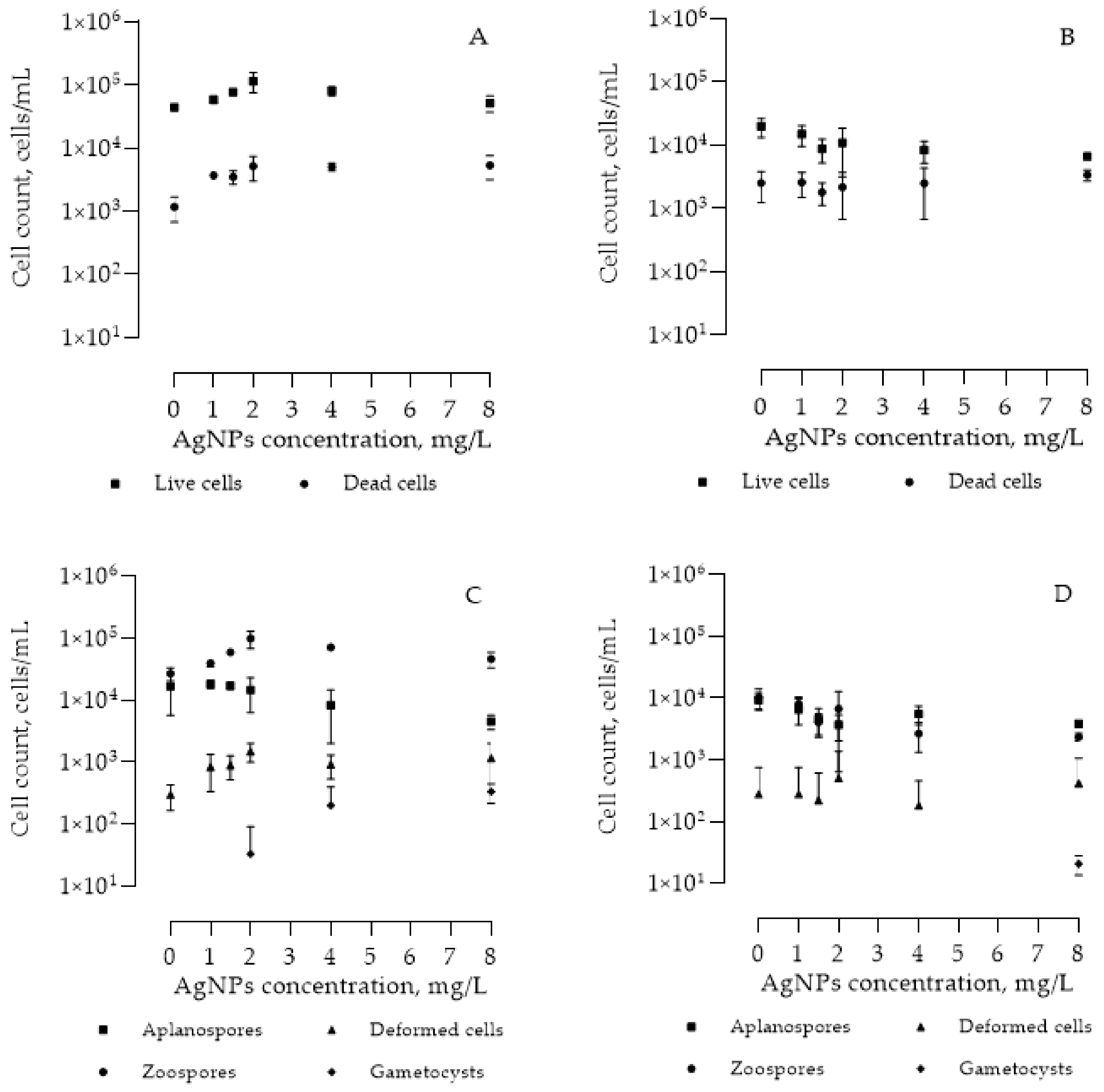
AgNPs’ Effect on Morphology and Count
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beyth, N.; Houri-Haddad, Y.; Domb, A.; Khan, W.; Hazan, R. Alternative Antimicrobial Approach: Nano-Antimicrobial Materials. Evid. Based Complement. Altern. Med. 2015, 2015, 246012. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Guan, W.; Xu, L.; Ding, Z.; Ma, H.; Ma, A.; Terry, N. Effects of Nanoparticles on Algae: Adsorption, Distribution, Ecotoxicity and Fate. Appl. Sci. 2019, 9, 1534. [Google Scholar] [CrossRef]
- Yusuf, M. Silver Nanoparticles: Synthesis and Applications. In Handbook of Ecomaterials; Martínez, L.M.T., Kharissova, O.V., Kharisov, B.I., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 2343–2356. ISBN 978-3-319-68254-9. [Google Scholar]
- Yin, I.X.; Zhang, J.; Zhao, I.S.; Mei, M.L.; Li, Q.; Chu, C.H. The Antibacterial Mechanism of Silver Nanoparticles and Its Application in Dentistry. IJN 2020, 15, 2555–2562. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, Applications and Toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Khodashenas, B.; Ghorbani, H.R. Synthesis of Silver Nanoparticles with Different Shapes. Arab. J. Chem. 2019, 12, 1823–1838. [Google Scholar] [CrossRef]
- Cepoi, L.; Zinicovscaia, I.; Rudi, L.; Chiriac, T.; Rotari, I.; Turchenko, V.; Djur, S. Effects of PEG-Coated Silver and Gold Nanoparticles on Spirulina Platensis Biomass during Its Growth in a Closed System. Coatings 2020, 10, 717. [Google Scholar] [CrossRef]
- Vihodceva, S.; Šutka, A.; Sihtmäe, M.; Rosenberg, M.; Otsus, M.; Kurvet, I.; Smits, K.; Bikse, L.; Kahru, A.; Kasemets, K. Antibacterial Activity of Positively and Negatively Charged Hematite (α-Fe2O3) Nanoparticles to Escherichia coli, Staphylococcus aureus and Vibrio fischeri. Nanomaterials 2021, 11, 652. [Google Scholar] [CrossRef]
- Lara, H.H.; Garza-Treviño, E.N.; Ixtepan-Turrent, L.; Singh, D.K. Silver Nanoparticles Are Broad-Spectrum Bactericidal and Virucidal Compounds. J. Nanobiotechnol. 2011, 9, 30. [Google Scholar] [CrossRef]
- Gupta, S.; Sharma, S.; Singh, S. Hexavalent chromium toxicity to cyanobacterium spirulina platensis. Int. Res. J. Pharm. 2014, 5, 910–914. [Google Scholar] [CrossRef]
- Qing, Y.; Cheng, L.; Li, R.; Liu, G.; Zhang, Y.; Tang, X.; Wang, J.; Liu, H.; Qin, Y. Potential Antibacterial Mechanism of Silver Nanoparticles and the Optimization of Orthopedic Implants by Advanced Modification Technologies. IJN 2018, 13, 3311–3327. [Google Scholar] [CrossRef]
- Mohanpuria, P.; Rana, N.K.; Yadav, S.K. Biosynthesis of Nanoparticles: Technological Concepts and Future Applications. J. Nanopart. Res. 2008, 10, 507–517. [Google Scholar] [CrossRef]
- Ghasemi, S.M.; Dormanesh, B.; Hosseini Abari, A.; Aliasghari, A.; Farahnejad, Z. Comparative Characterization of Silver Nanoparticles Synthesized by Spore Extract of Bacillus Subtilis and Geobacillus Stearothermophilus. Nanomed. J. 2018, 5, 46–51. [Google Scholar] [CrossRef]
- Deljou, A.; Goudarzi, S. Green Extracellular Synthesis of the Silver Nanoparticles Using Thermophilic Bacillus sp. AZ1 and Its Antimicrobial Activity against Several Human Pathogenetic Bacteria. Iran J. Biotechnol. 2016, 14, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Mohammed Fayaz, A.; Girilal, M.; Rahman, M.; Venkatesan, R.; Kalaichelvan, P.T. Biosynthesis of Silver and Gold Nanoparticles Using Thermophilic Bacterium Geobacillus Stearothermophilus. Process Biochem. 2011, 46, 1958–1962. [Google Scholar] [CrossRef]
- Mukherjee, K.; Gupta, R.; Kumar, G.; Kumari, S.; Biswas, S.; Padmanabhan, P. Synthesis of Silver Nanoparticles by Bacillus Clausii and Computational Profiling of Nitrate Reductase Enzyme Involved in Production. J. Genet. Eng. Biotechnol. 2018, 16, 527–536. [Google Scholar] [CrossRef]
- Davinelli, S.; Nielsen, M.; Scapagnini, G. Astaxanthin in Skin Health, Repair, and Disease: A Comprehensive Review. Nutrients 2018, 10, 522. [Google Scholar] [CrossRef]
- Lim, K.C.; Yusoff, F.; Shariff, M.; Kamarudin, M.S. Astaxanthin as Feed Supplement in Aquatic Animals. Rev. Aquac. 2018, 10, 738–773. [Google Scholar] [CrossRef]
- Do, T.-T.; Ong, B.-N.; Nguyen Tran, M.-L.; Nguyen, D.; Melkonian, M.; Tran, H.-D. Biomass and Astaxanthin Productivities of Haematococcus pluvialis in an Angled Twin-Layer Porous Substrate Photobioreactor: Effect of Inoculum Density and Storage Time. Biology 2019, 8, 68. [Google Scholar] [CrossRef]
- Chekanov, K.; Lobakova, E.; Selyakh, I.; Semenova, L.; Sidorov, R.; Solovchenko, A. Accumulation of Astaxanthin by a New Haematococcus pluvialis Strain BM1 from the White Sea Coastal Rocks (Russia). Mar. Drugs 2014, 12, 4504–4520. [Google Scholar] [CrossRef]
- Shah, M.M.R.; Liang, Y.; Cheng, J.J.; Daroch, M. Astaxanthin-Producing Green Microalga Haematococcus pluvialis: From Single Cell to High Value Commercial Products. Front. Plant Sci. 2016, 7, 531. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, J.; Zhang, L. Cell Cycles and Proliferation Patterns in Haematococcus pluvialis. Chin. J. Oceanol. Limnol. 2017, 35, 1205–1211. [Google Scholar] [CrossRef]
- Damiani, M.C.; Leonardi, P.I.; Pieroni, O.I.; Cáceres, E.J. Ultrastructure of the Cyst Wall of Haematococcus pluvialis (Chlorophyceae): Wall Development and Behaviour during Cyst Germination. Phycologia 2006, 45, 616–623. [Google Scholar] [CrossRef]
- Ambati, R.; Phang, S.-M.; Ravi, S.; Aswathanarayana, R. Astaxanthin: Sources, Extraction, Stability, Biological Activities and Its Commercial Applications—A Review. Mar. Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Li, Y.; Hu, Q. Biology and Commercial Aspects of Haematococcus pluvialis. In Handbook of Microalgal Culture; Richmond, A., Hu, Q., Eds.; John Wiley & Sons, Ltd.: Oxford, UK, 2013; pp. 388–405. ISBN 978-1-118-56716-6. [Google Scholar]
- Wang, S.-B.; Hu, Q.; Sommerfeld, M.; Chen, F. Cell Wall Proteomics of the Green Alga Haematococcus pluvialis (Chlorophyceae). Proteomics 2004, 4, 692–708. [Google Scholar] [CrossRef] [PubMed]
- Jannel, S.; Caro, Y.; Bermudes, M.; Petit, T. Novel Insights into the Biotechnological Production of Haematococcus pluvialis-Derived Astaxanthin: Advances and Key Challenges to Allow Its Industrial Use as Novel Food Ingredient. JMSE 2020, 8, 789. [Google Scholar] [CrossRef]
- Oslan, S.N.H.; Shoparwe, N.F.; Yusoff, A.H.; Rahim, A.A.; Chang, C.S.; Tan, J.S.; Oslan, S.N.; Arumugam, K.; Ariff, A.B.; Sulaiman, A.Z.; et al. A Review on Haematococcus pluvialis Bioprocess Optimization of Green and Red Stage Culture Conditions for the Production of Natural Astaxanthin. Biomolecules 2021, 11, 256. [Google Scholar] [CrossRef]
- Wong, L.S.; Batumalai, T.; Cheng, W.H.; Ong, G.H. The Effect of Copper Nanoparticle to Astaxanthin Content in Microalgae. Adv. Sci. Technol. 2019, 6, 81–85. [Google Scholar]
- Bischoff, H.W.; Bold, H.C. Phycological Studies IV. In Some Soil Algae from Enchanted Rock and Related Algal Species; No. 6318; University of Texas Publication: Austin, TX, USA, 1963. [Google Scholar]
- Boussiba, S.; Vonshak, A. Astaxanthin Accumulation in the Green Alga Haematococcus pluvialis. Plant Cell Physiol. 1991, 32, 1077–1082. [Google Scholar] [CrossRef]
- Cekuolyte, K.; Gudiukaite, R.; Klimkevicius, V.; Mazrimaite, V.; Maneikis, A.; Lastauskiene, E. Biosynthesis of Silver Nanoparticles Produced Using Geobacillus spp. Bacteria. Nanomaterials 2023, 13, 702. [Google Scholar] [CrossRef]
- Kim, Y.-E.; Matter, I.A.; Lee, N.; Jung, M.; Lee, Y.-C.; Choi, S.-A.; Lee, S.Y.; Kim, J.R.; Oh, Y.-K. Enhancement of Astaxanthin Production by Haematococcus pluvialis Using Magnesium Aminoclay Nanoparticles. Bioresour. Technol. 2020, 307, 123270. [Google Scholar] [CrossRef]
- Djearamane, S.; Lim, Y.M.; Wong, L.S.; Lee, P.F. Cellular Accumulation and Cytotoxic Effects of Zinc Oxide Nanoparticles in Microalga Haematococcus pluvialis. PeerJ 2019, 7, e7582. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Powell, B.A.; Mortimer, M.; Ke, P.C. Adaptive Interactions between Zinc Oxide Nanoparticles and Chlorella sp. Environ. Sci. Technol. 2012, 46, 12178–12185. [Google Scholar] [CrossRef] [PubMed]
- Oukarroum, A.; Bras, S.; Perreault, F.; Popovic, R. Inhibitory Effects of Silver Nanoparticles in Two Green Algae, Chlorella Vulgaris and Dunaliella Tertiolecta. Ecotoxicol. Environ. Saf. 2012, 78, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Das, R.; Gang, S.; Nath, S.S. Preparation and Antibacterial Activity of Silver Nanoparticles. JBNB 2011, 2, 472–475. [Google Scholar] [CrossRef]
- Vijay Kumar, P.P.N.; Pammi, S.V.N.; Kollu, P.; Satyanarayana, K.V.V.; Shameem, U. Green Synthesis and Characterization of Silver Nanoparticles Using Boerhaavia Diffusa Plant Extract and Their Anti Bacterial Activity. Ind. Crops Prod. 2014, 52, 562–566. [Google Scholar] [CrossRef]
- Baudelet, P.-H.; Ricochon, G.; Linder, M.; Muniglia, L. A New Insight into Cell Walls of Chlorophyta. Algal Res. 2017, 25, 333–371. [Google Scholar] [CrossRef]
- Han, X.; Zeng, H.; Bartocci, P.; Fantozzi, F.; Yan, Y. Phytohormones and Effects on Growth and Metabolites of Microalgae: A Review. Fermentation 2018, 4, 25. [Google Scholar] [CrossRef]
- Blaby-Haas, C.E.; Merchant, S.S. The Ins and Outs of Algal Metal Transport. Biochim. Biophys. Acta (BBA) 2012, 1823, 1531–1552. [Google Scholar] [CrossRef]
- Hagen, C.; Siegmund, S.; Braune, W. Ultrastructural and Chemical Changes in the Cell Wall of Haematococcus pluvialis (Volvocales, Chlorophyta) during Aplanospore Formation. Euro. J. Phycol. 2002, 37, 217–226. [Google Scholar] [CrossRef]
- Desai, R.K.; Streefland, M.; Wijffels, R.H.; Eppink, M.H.M. Novel Astaxanthin Extraction from Haematococcus pluvialis Using Cell Permeabilising Ionic Liquids. Green Chem. 2016, 18, 1261–1267. [Google Scholar] [CrossRef]
- Branyikova, I.; Prochazkova, G.; Potocar, T.; Jezkova, Z.; Branyik, T. Harvesting of Microalgae by Flocculation. Fermentation 2018, 4, 93. [Google Scholar] [CrossRef]
- Chen, F.; Xiao, Z.; Yue, L.; Wang, J.; Feng, Y.; Zhu, X.; Wang, Z.; Xing, B. Algae Response to Engineered Nanoparticles: Current Understanding, Mechanisms and Implications. Environ. Sci. Nano 2019, 6, 1026–1042. [Google Scholar] [CrossRef]
- Salachna, P.; Byczyńska, A.; Zawadzińska, A.; Piechocki, R.; Mizielińska, M. Stimulatory Effect of Silver Nanoparticles on the Growth and Flowering of Potted Oriental Lilies. Agronomy 2019, 9, 610. [Google Scholar] [CrossRef]
| Diameter of AgNPs, nm | % |
|---|---|
| 10–20 | 1 |
| 20–30 | 7 |
| 30–40 | 9 |
| 40–50 | 14 |
| 50–60 | 19 |
| 60–70 | 11 |
| 70–80 | 14 |
| 80–90 | 5 |
| 90–100 | 66 |
| >100 | 14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Venckus, P.; Endriukaitytė, I.; Čekuolytė, K.; Gudiukaitė, R.; Pakalniškis, A.; Lastauskienė, E. Effect of Biosynthesized Silver Nanoparticles on the Growth of the Green Microalga Haematococcus pluvialis and Astaxanthin Synthesis. Nanomaterials 2023, 13, 1618. https://doi.org/10.3390/nano13101618
Venckus P, Endriukaitytė I, Čekuolytė K, Gudiukaitė R, Pakalniškis A, Lastauskienė E. Effect of Biosynthesized Silver Nanoparticles on the Growth of the Green Microalga Haematococcus pluvialis and Astaxanthin Synthesis. Nanomaterials. 2023; 13(10):1618. https://doi.org/10.3390/nano13101618
Chicago/Turabian StyleVenckus, Petras, Ieva Endriukaitytė, Kotryna Čekuolytė, Renata Gudiukaitė, Andrius Pakalniškis, and Eglė Lastauskienė. 2023. "Effect of Biosynthesized Silver Nanoparticles on the Growth of the Green Microalga Haematococcus pluvialis and Astaxanthin Synthesis" Nanomaterials 13, no. 10: 1618. https://doi.org/10.3390/nano13101618
APA StyleVenckus, P., Endriukaitytė, I., Čekuolytė, K., Gudiukaitė, R., Pakalniškis, A., & Lastauskienė, E. (2023). Effect of Biosynthesized Silver Nanoparticles on the Growth of the Green Microalga Haematococcus pluvialis and Astaxanthin Synthesis. Nanomaterials, 13(10), 1618. https://doi.org/10.3390/nano13101618







