Improving the Detectability of Microplastics in River Waters by Single Particle Inductively Coupled Plasma Mass Spectrometry
Abstract
1. Introduction
2. Materials and Methods
2.1. Instrumentation
2.1.1. Inductively Coupled Plasma Mass Spectrometry (ICP-MS)
2.1.2. Raman Microscopy
2.1.3. Field Emission Scanning Electron Microscopy (FESEM)
2.2. Standards
2.3. River Water Samples
2.4. Procedures
2.4.1. SP-ICP-MS Analysis
2.4.2. Acidic Pre-Treatment of River Water Samples for SP-ICP-MS Analysis
2.4.3. Preparation of Ag-Labelled Bacterial Suspension
2.4.4. FESEM Analysis
2.4.5. Raman Microscopy Analysis
3. Results
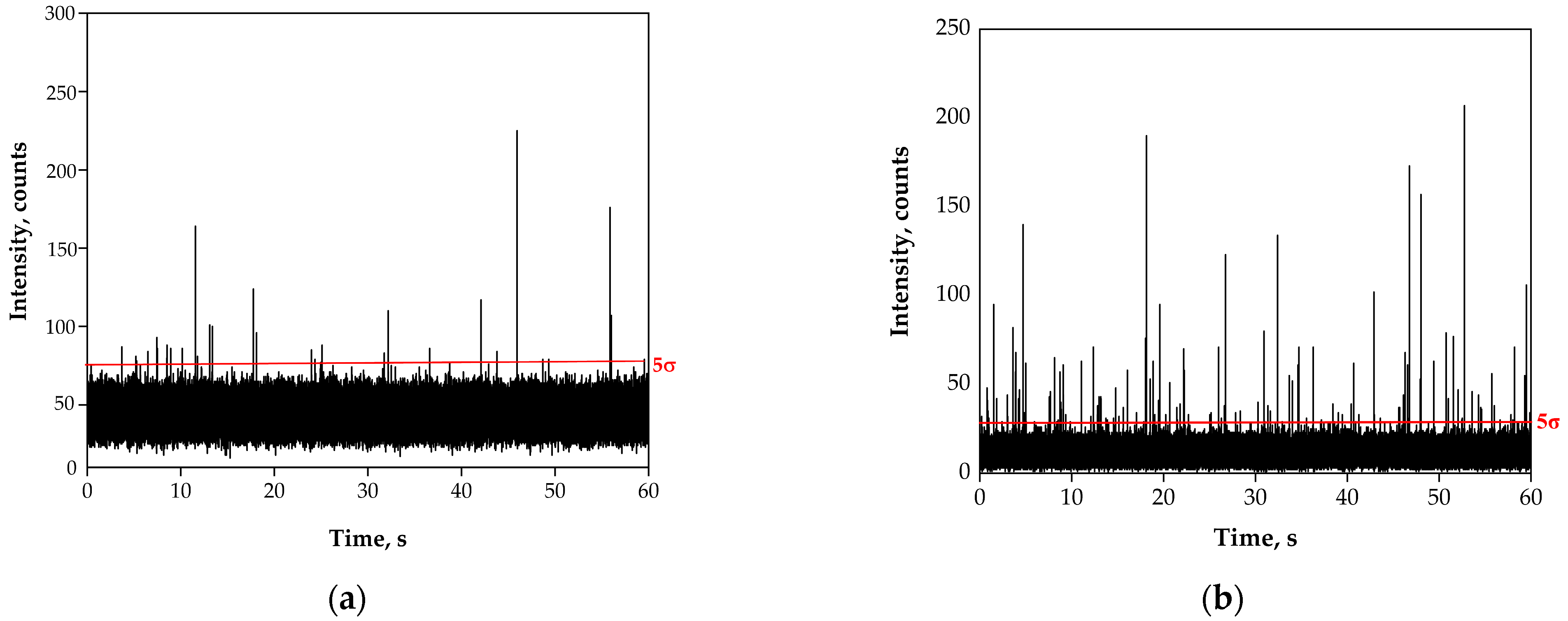
3.1. Preliminary Analysis of River Water Samples by SP-ICP-MS
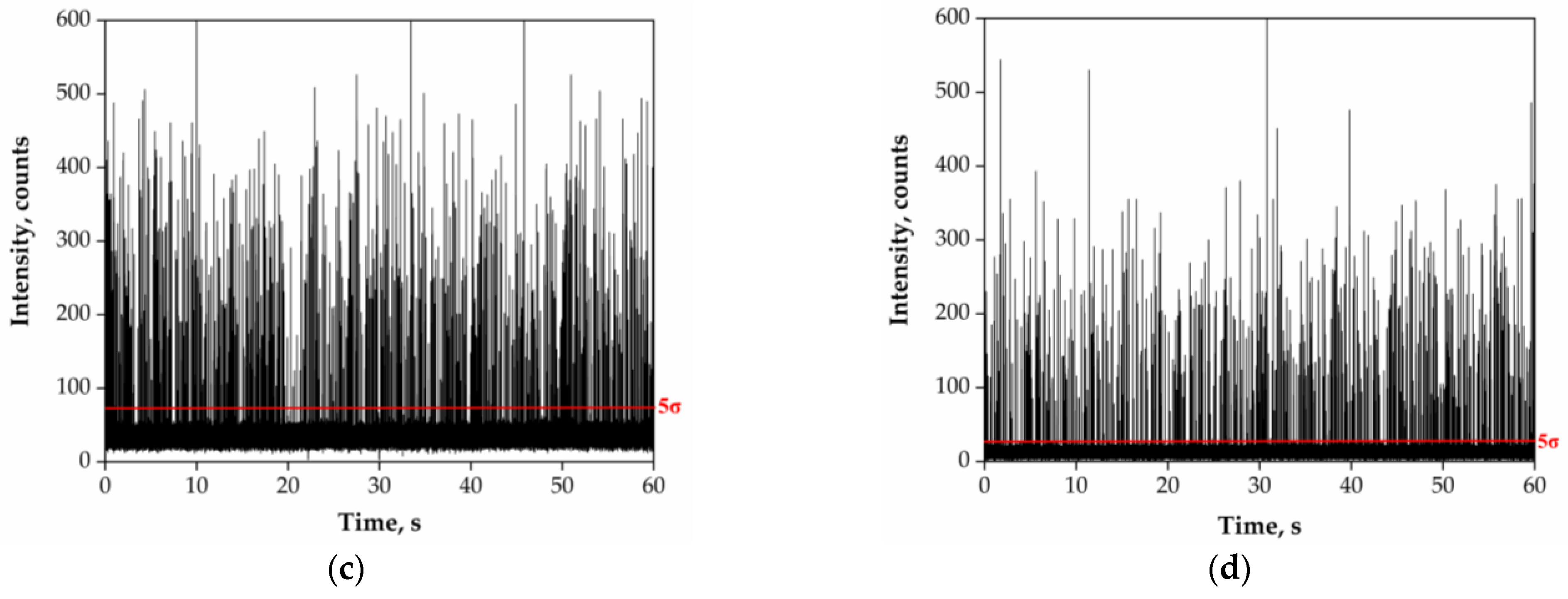
3.2. Acidic Pre-Treatment of River Water Samples
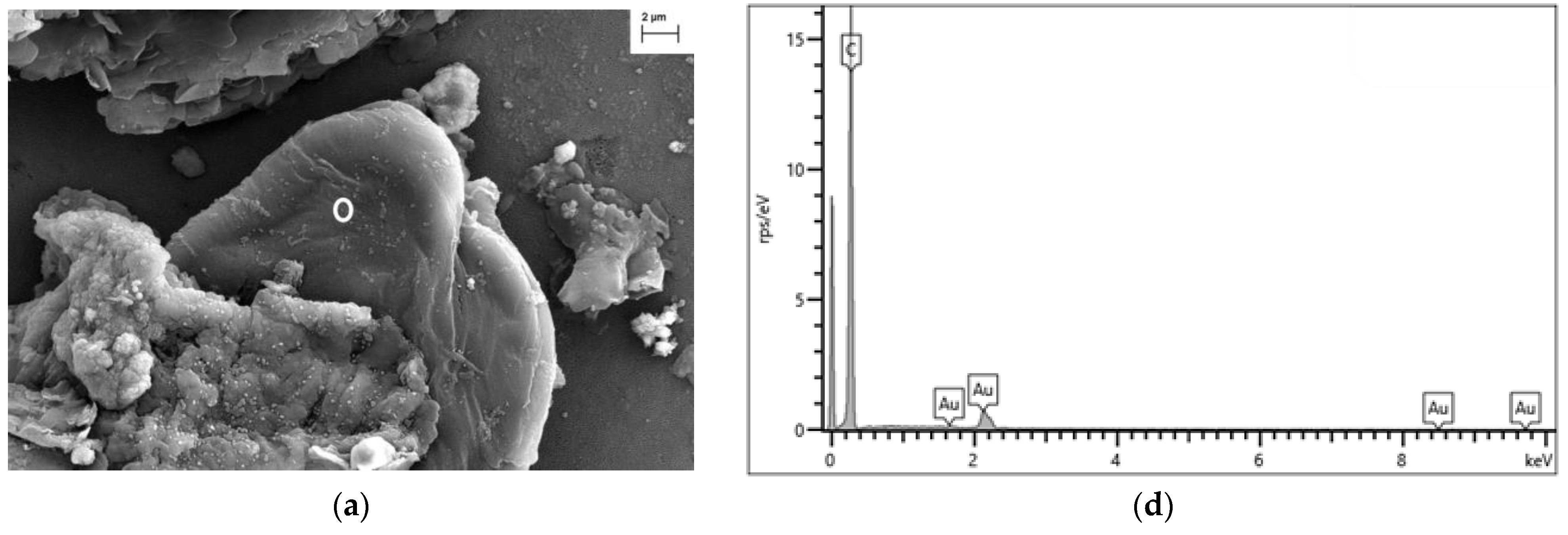
3.3. Analysis of River Water Samples by Scanning Electron and Raman Microscopies
4. Discussion
4.1. Acidic Pre-Treatment of River Water Samples
4.2. Analysis of River Water Samples by SP-ICP-MS
4.3. Analysis of River Water Samples by Electron and Raman Microscopy
4.3.1. FESEM-EDX Analysis
4.3.2. Raman Microscopy
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Alexy, P.; Anklam, E.; Emans, T.; Furfari, A.; Galgani, F.; Hanke, G.; Koelmans, A.; Pant, R.; Saveyn, H.; Sokull Kluettgen, B. Managing the Analytical Challenges Related to Micro- and Nanoplastics in the Environment and Food: Filling the Knowledge Gaps. Food Addit. Contam. Part A 2020, 37, 1–10. [Google Scholar] [CrossRef]
- Hartmann, N.B.; Hüffer, T.; Thompson, R.C.; Hassellöv, M.; Verschoor, A.; Daugaard, A.E.; Rist, S.; Karlsson, T.; Brennholt, N.; Cole, M.; et al. Are We Speaking the Same Language? Recommendations for a Definition and Categorization Framework for Plastic Debris. Environ. Sci. Technol. 2019, 53, 1039–1047. [Google Scholar] [CrossRef]
- Frias, J.P.G.L.; Nash, R. Microplastics: Finding a Consensus on the Definition. Mar. Pollut. Bull. 2019, 138, 145–147. [Google Scholar] [CrossRef]
- Gigault, J.; ter Halle, A.; Baudrimont, M.; Pascal, P.Y.; Gauffre, F.; Phi, T.L.; El Hadri, H.; Grassl, B.; Reynaud, S. Current Opinion: What is a Nanoplastic? Environ. Pollut. 2018, 235, 1030–1034. [Google Scholar] [CrossRef] [PubMed]
- Bianco, A.; Passananti, M. Atmospheric Micro and Nanoplastics: An Enormous Microscopic Problem. Sustainability 2020, 12, 7327. [Google Scholar] [CrossRef]
- Murphy, F.; Ewins, C.; Carbonnier, F.; Quinn, B. Wastewater Treatment Works (WwTW) as a Source of Microplastics in the Aquatic Environment. Environ. Sci. Technol. 2016, 50, 5800–5808. [Google Scholar] [CrossRef]
- Magnusson, K.; Norén, F. Screening of Microplastic Particles in and Down-Stream a Wastewater Treatment Plant; IVL Swedish Environmental Research Institute: Stockholm, Sweden, 2014. [Google Scholar]
- Enfrin, M.; Dumée, L.F.; Lee, J. Nano/Microplastics in Water and Wastewater Treatment Processes—Origin, Impact and Potential Solutions. Water Res. 2019, 161, 621–638. [Google Scholar] [CrossRef] [PubMed]
- Tagg, A.S.; Sapp, M.; Harrison, J.P.; Sinclair, C.J.; Bradley, E.; Ju-Nam, Y.; Ojeda, J.J. Microplastic Monitoring at Different Stages in a Wastewater Treatment Plant Using Reflectance Micro-FTIR Imaging. Front. Environ. Sci. 2020, 8, 145. [Google Scholar] [CrossRef]
- D’Avignon, G.; Gregory-Eaves, I.; Ricciardi, A. Microplastics in Lakes and Rivers: An Issue of Emerging Significance to Limnology. Environ. Rev. 2022, 30, 228–244. [Google Scholar] [CrossRef]
- Cordova, M.R.; Nurhati, I.S.; Shiomoto, A.; Hatanaka, K.; Saville, R.; Riani, E. Spatiotemporal Macro Debris and Microplastic Variations Linked to Domestic Waste and Textile Industry in the Supercritical Citarum River, Indonesia. Mar. Pollut. Bull. 2022, 175, 113338. [Google Scholar] [CrossRef] [PubMed]
- Winkler, A.; Antonioli, D.; Masseroni, A.; Chiarcos, R.; Laus, M.; Tremolada, P. Following the Fate of Microplastic in Four Abiotic and Biotic Matrices along the Ticino River (North Italy). Sci. Total Environ. 2022, 823, 153638. [Google Scholar] [CrossRef] [PubMed]
- Pol, W.; Żmijewska, A.; Stasińska, E.; Zieliński, P. Spatial-Temporal Distribution of Microplastics in Lowland Rivers Flowing through Two Cities (Ne-Poland). Water Air Soil Pollut. 2022, 233, 140. [Google Scholar] [CrossRef]
- Yuan, W.; Christie-Oleza, J.A.; Xu, E.G.; Li, J.; Zhang, H.; Wang, W.; Lin, L.; Zhang, W.; Yang, Y. Environmental Fate of Microplastics in the World’s Third-Largest River: Basin-Wide Investigation and Microplastic Community Analysis. Water Res. 2022, 210, 118002. [Google Scholar] [CrossRef]
- Devereux, R.; Westhead, E.K.; Jayaratne, R.; Newport, D. Microplastic Abundance in the Thames River during the New Year Period. Mar. Pollut. Bull. 2022, 177, 113534. [Google Scholar] [CrossRef]
- Zhdanov, I.; Lokhov, A.; Belesov, A.; Kozhevnikov, A.; Pakhomova, S.; Berezina, A.; Frolova, N.; Kotova, E.; Leshchev, A.; Wang, X.; et al. Assessment of Seasonal Variability of Input of Microplastics from the Northern Dvina River to the Arctic Ocean. Mar. Pollut. Bull. 2022, 175, 113370. [Google Scholar] [CrossRef]
- Treilles, R.; Gasperi, J.; Tramoy, R.; Dris, R.; Gallard, A.; Partibane, C.; Tassin, B. Microplastic and Microfiber Fluxes in the Seine River: Flood Events versus Dry Periods. Sci. Total Environ. 2022, 805, 150123. [Google Scholar] [CrossRef]
- Sá, B.; Pais, J.; Antunes, J.; Pequeno, J.; Pires, A.; Sobral, P. Seasonal Abundance and Distribution Patterns of Microplastics in the Lis River, Portugal. Sustainability 2022, 14, 2255. [Google Scholar] [CrossRef]
- Laermanns, H.; Reifferscheid, G.; Kruse, J.; Földi, C.; Dierkes, G.; Schaefer, D.; Scherer, C.; Bogner, C.; Stock, F. Microplastic in Water and Sediments at the Confluence of the Elbe and Mulde Rivers in Germany. Front. Environ. Sci. 2021, 9, 590. [Google Scholar] [CrossRef]
- de Carvalho, A.R.; Garcia, F.; Riem-Galliano, L.; Tudesque, L.; Albignac, M.; ter Halle, A.; Cucherousset, J. Urbanization and Hydrological Conditions Drive the Spatial and Temporal Variability of Microplastic Pollution in the Garonne River. Sci. Total Environ. 2021, 769, 144479. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.; Zhong, X.; Duan, Z.; Yi, X.; Cheng, F.; Xu, W.; Yang, X. Micro- and Nanoplastics Released from Biodegradable and Conventional Plastics during Degradation: Formation, Aging Factors, and Toxicity. Sci. Total Environ. 2022, 833, 155275. [Google Scholar] [CrossRef]
- Laborda, F.; Trujillo, C.; Lobinski, R. Analysis of Microplastics in Consumer Products by Single Particle-Inductively Coupled Plasma Mass Spectrometry Using the Carbon-13 Isotope. Talanta 2021, 221, 121486. [Google Scholar] [CrossRef] [PubMed]
- Bolea-Fernandez, E.; Rua-Ibarz, A.; Velimirovic, M.; Tirez, K.; Vanhaecke, F. Detection of Microplastics Using Inductively Coupled Plasma-Mass Spectrometry (ICP-MS) Operated in Single-Event Mode. J. Anal. At. Spectrom. 2020, 35, 455–460. [Google Scholar] [CrossRef]
- Liu, Z.; Zhu, Y.; Lv, S.; Shi, Y.; Dong, S.; Yan, D.; Zhu, X.; Peng, R.; Keller, A.A.; Huang, Y. Quantifying the Dynamics of Polystyrene Microplastics UV-Aging Process. Environ. Sci. Technol. Lett. 2022, 9, 50–56. [Google Scholar] [CrossRef]
- Li, F.; Armstrong, D.W.; Houk, R. Behavior of Bacteria in the Inductively Coupled Plasma: Atomization and Production of Atomic Ions for Mass Spectrometry. Anal. Chem. 2005, 77, 1407–1413. [Google Scholar] [CrossRef]
- Riisom, M.; Gammelgaard, B.; Lambert, I.H.; Stürup, S. Development and Validation of an ICP-MS Method for Quantification of Total Carbon and Platinum in Cell Samples and Comparison of Open-Vessel and Microwave-Assisted Acid Digestion Methods. J. Pharm. Biomed. Anal. 2018, 158, 144–150. [Google Scholar] [CrossRef]
- Abril, G.; Etcheber, H.; Delille, B.; Frankignoulle, M. Carbonate Dissolution in the Turbid and Eutrophic Loire Estuary. Mar. Ecol. Prog. Ser. 2003, 259, 129–138. [Google Scholar] [CrossRef]
- Laborda, F.; Gimenez-Ingalaturre, A.C.; Bolea, E.; Castillo, J.R. Single Particle Inductively Coupled Plasma Mass Spectrometry as Screening Tool for Detection of Particles. Spectrochim. Acta Part B At. Spectrosc. 2019, 159, 105654. [Google Scholar] [CrossRef]
- Currie, L.A. Limits for Qualitative Detection and Quantitative Determination Application to Radiochemistry. Anal. Chem. 1968, 40, 586–593. [Google Scholar] [CrossRef]
- Pace, H.E.; Rogers, N.J.; Jarolimek, C.; Coleman, V.A.; Higgins, C.P.; Ranville, J.F. Determining Transport Efficiency for the Purpose of Counting and Sizing Nanoparticles via Single Particle Inductively Coupled Plasma Mass Spectrometry. Anal. Chem. 2011, 83, 9361–9369. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.S.; Elsamahy, T.; Koutra, E.; Kornaros, M.; El-Sheekh, M.; Abdelkarim, E.A.; Zhu, D.; Sun, J. Degradation of Conventional Plastic Wastes in the Environment: A Review on Current Status of Knowledge and Future Perspectives of Disposal. Sci. Total Environ. 2021, 771, 144719. [Google Scholar] [CrossRef]
- Li, P.; Lai, Y.; Li, Q.; Dong, L.; Tan, Z.; Yu, S.; Chen, Y.; Sharma, V.K.; Liu, J.; Jiang, G. Total Organic Carbon as a Quantitative Index of Micro- and Nano-Plastic Pollution. Anal. Chem. 2022, 94, 740–747. [Google Scholar] [CrossRef]
- López, A.D.F.; Fabiani, M.; Lassalle, V.L.; Spetter, C.V.; Severini, M.D.F. Critical Review of the Characteristics, Interactions, and Toxicity of Micro/Nanomaterials Pollutants in Aquatic Environments. Mar. Pollut. Bull. 2022, 174, 113276. [Google Scholar] [CrossRef]
- Yu, Y.; Mo, W.Y.; Luukkonen, T. Adsorption Behaviour and Interaction of Organic Micropollutants with Nano and Microplastics—A Review. Sci. Total Environ. 2021, 797, 149140. [Google Scholar] [CrossRef] [PubMed]
- Confederación Hidrográfica del Ebro. Plan Hidrológico Del Río Gállego; CHE: Zaragoza, Spain, 2007. [Google Scholar]
- Instituto Geológico y Minero de España. Geochemical Database. Available online: https://info.igme.es/Geoquimica/ (accessed on 29 March 2023).
- Geochemical Atlas of Europe. Available online: http://weppi.gtk.fi/publ/foregsatlas/index.php (accessed on 29 March 2023).
- Gonzalez de Vega, R.; Goyen, S.; Lockwood, T.E.; Doble, P.A.; Camp, E.F.; Clases, D. Characterisation of Microplastics and Unicellular Algae in Seawater by Targeting Carbon via Single Particle and Single Cell ICP-MS. Anal. Chim. Acta 2021, 1174, 338737. [Google Scholar] [CrossRef]
- Laborda, F.; Gimenez-Ingalaturre, A.C.; Bolea, E.; Castillo, J.R. About Detectability and Limits of Detection in Single Particle Inductively Coupled Plasma Mass Spectrometry. Spectrochim. Acta Part B At. Spectrosc. 2020, 169, 105883. [Google Scholar] [CrossRef]
- Lampman, S. Characterization and Failure Analysis of Plastics; ASM International: Almer, The Netherlands, 2003. [Google Scholar]
- Azevedo, A.G.; Barros, C.; Miranda, S.; Machado, A.V.; Castro, O.; Silva, B.; Saraiva, M.; Silva, A.S.; Pastrana, L.; Carneiro, O.S.; et al. Active Flexible Films for Food Packaging: A Review. Polymers 2022, 14, 2442. [Google Scholar] [CrossRef] [PubMed]
- Ranakoti, L.; Gangil, B.; Mishra, S.K.; Singh, T.; Sharma, S.; Ilyas, R.A.; El-Khatib, S. Critical Review on Polylactic Acid: Properties, Structure, Processing, Biocomposites, and Nanocomposites. Materials 2022, 15, 4312. [Google Scholar] [CrossRef]
- Wanner, P. Plastic in Agricultural Soils—A Global Risk for Groundwater Systems and Drinking Water Supplies?—A Review. Chemosphere 2021, 264, 128453. [Google Scholar] [CrossRef]



| River | Country | Technique | Type of Plastic | Size | Plastic Concentration | Ref. |
|---|---|---|---|---|---|---|
| Citarum | Indonesia | Optical microscopy FTIR Raman microscopy | PET PS Cellophane Nylon PP PE | ˂300 µm - >1000 µm | 3.35 ± 0.54 m−3 | [11] |
| Ticino | Italy | Optical microscopy FTIR microscopy | LDPE PET PP | >20 µm | 33 ± 21 m−3 | [12] |
| Biala Czarna Hancza | Poland | Optical microscopy | - | >0.04–4 µm | 10.83 ± 3.96 L−1 10.29 ± 3.90 L−1 | [13] |
| Yangtze | China | Optical microscopy Raman microscopy | PP PE PA PS PVC PET PC | >0.11 µm | 1.27 ± 0.83 L−1 | [14] |
| Thames | United Kingdom | Optical microscopy FTIR | Rubber PVC PE | >0.5 mm - 5 mm | 51 ± 10 L−1 | [15] |
| Northern Dvina | Russia | FTIR | PE PP EEA | >0.5 mm | 0.6–1.4 × 104 km−2 | [16] |
| Seine | France | Optical microscopy FTIR microscopy | PP PE PES | 32–2528 µm | 15.5 ± 4.9 L−1 | [17] |
| Lis | Portugal | Optical microscopy FTIR FTIR microscopy | PP PVC PC Nylon | 14–4726 µm | 234 ± 398 m−3 | [18] |
| Elbe Mulde | Germany | Optical microscopy Pyr-GC-MS | PE PP PS | >50 µm | 15 ± 2 m−3 0.33–1.19 mg m−3 | [19] |
| Garone | France | Optical microscopy ATR-FTIR | PE PP PS | 700 µm - 5 mm | 0.15 ± 0.46 m−3 | [20] |
| Sample | XCsize (μm) | Number of Particle Events Detected | Particle Concentration (×104 L−1) |
|---|---|---|---|
| UP water | 1.25 | 3 ± 2 | - |
| RW01 | 1.44 | 38 ± 10 | 295 ± 32 |
| RW02 | 1.44 | 40 ± 12 | 310 ± 25 |
| RW03 | 1.53 | 28 ± 8 | 217 ± 21 |
| RW04 | 1.48 | 10 ± 3 | 109 ± 12 |
| RW05 | 1.49 | 28 ± 7 | 217 ± 15 |
| RW06 | 1.55 | 22 ± 5 | 171 ± 20 |
| RW07 | 1.63 | 17 ± 6 | 132 ± 19 |
| RW08 | 1.54 | 15 ± 4 | 116 ± 26 |
| RW09 | 1.41 | 16 ± 4 | 124 ± 15 |
| RW10 | 1.47 | 19 ± 8 | 147 ± 10 |
| RW11 | 1.39 | 13 ± 3 | 101 ± 9 |
| RW12 | 1.45 | 17 ± 4 | 132 ± 9 |
| RW13 | 1.51 | 10 ± 5 | 78 ± 7 |
| RW14 | 1.47 | 27 ± 6 | 209 ± 19 |
| RW15 | 1.46 | 24 ± 4 | 186 ± 13 |
| RW16 | 1.52 | 6 ± 2 | 47 ± 5 |
| RW17 | 1.46 | 20 ± 4 | 155 ± 20 |
| RW18 | 1.48 | 19 ± 3 | 147 ± 27 |
| RW19 | 1.47 | 16 ± 2 | 124 ± 30 |
| RW20 | 1.49 | 12 ± 2 | 93 ± 20 |
| RW21 | 1.35 | 20 ± 3 | 155 ± 32 |
| RW22 | 1.31 | 18 ± 4 | 140 ± 16 |
| RW23 | 1.37 | 42 ± 11 | 326 ± 42 |
| RW24 | 1.37 | 27 ± 10 | 209 ± 21 |
| RW25 | 1.31 | 43 ± 9 | 334 ± 44 |
| RW26 | 1.50 | 11 ± 3 | 85 ± 6 |
| RW27 | 1.35 | 29 ± 6 | 225 ± 30 |
| RW28 | 1.32 | 16 ± 5 | 124 ± 10 |
| Sample | HNO3 (% v/v) | Mean Diameter (μm) | Particle Concentration (×106 L−1) | Particle Recovery (%) |
|---|---|---|---|---|
| 2 µm | - | 2.12 ± 0.02 | 313 ± 10 | - |
| RW07 + 2 µm | - | 2.54 ± 0.03 | 227 ± 11 | 73 |
| RW07 + 2 µm | 10 | 2.23 ± 0.01 | 261 ± 5 | 83 |
| 3 µm | - | 3.41 ± 0.03 | 220 ± 21 | - |
| RW07 + 3 µm | - | 3.41 ± 0.02 | 169 ± 19 | 77 |
| RW07 + 3 µm | 10 | 3.15 ± 0.03 | 130 ± 2 | 60 |
| Sample | Baseline Intensity (Counts) | BEC (mg L−1) | XCsize (μm) | Number of Particle Events Detected | Particle Concentration (×104 L−1) |
|---|---|---|---|---|---|
| direct analysis | |||||
| UP water | 6 ± 2 | 27 | 1.26 | 3 ± 2 | - |
| RW01 | 45 ± 1 | 150 | 1.72 | 88 ± 10 | 188 ± 21 |
| RW02 | 41 ± 2 | 137 | 1.70 | 84 ± 11 | 180 ± 24 |
| RW03 | 57 ± 2 | 190 | 1.84 | 45 ± 15 | 96 ± 31 |
| RW05 | 50 ± 3 | 167 | 1.82 | 41 ± 16 | 88 ± 34 |
| RW14 | 46 ± 2 | 153 | 1.75 | 230 ± 21 | 490 ± 45 |
| RW23 | 30 ± 1 | 100 | 1.62 | 110 ± 4 | 249 ± 9 |
| RW24 | 18 ± 1 | 60 | 1.48 | 119 ± 10 | 253 ± 21 |
| RW25 | 27 ± 1 | 90 | 1.58 | 95 ± 8 | 182 ± 17 |
| RW27 | 20 ± 2 | 67 | 1.50 | 91 ± 18 | 194 ± 39 |
| acidic pre-treament (10% HNO3 24 h) | |||||
| Proc. blank | 7 ± 1 | 23 | 1.25 | 2 ± 2 | - |
| RW01 | 7 ± 1 | 23 | 1.28 | 50 ± 8 | 106 ± 18 |
| RW02 | 7 ± 1 | 23 | 1.26 | 46 ± 5 | 97 ± 11 |
| RW03 | 9 ± 1 | 30 | 1.33 | 256 ± 37 | 554 ± 78 |
| RW05 | 9 ± 1 | 30 | 1.31 | 214 ± 49 | 470 ± 70 |
| RW14 | 8 ± 1 | 26 | 1.32 | 164 ± 40 | 349 ± 84 |
| RW23 | 7 ± 2 | 23 | 1.25 | 107 ± 11 | 227 ± 15 |
| RW24 | 7 ± 1 | 23 | 1.27 | 104 ± 11 | 221 ± 23 |
| RW25 | 6 ± 1 | 20 | 1.24 | 120 ± 19 | 277 ± 37 |
| RW27 | 7 ± 1 | 23 | 1.27 | 93 ± 10 | 197 ± 22 |
| KnowitAllTM | ||
|---|---|---|
| Sample | Composition | HQI |
| RW01 | Polylactic acid | 76.2 |
| PMMA | 73.9 | |
| Polylactic acid | 64.7 | |
| Polylactic acid | 65.3 | |
| PMMA | 65.1 | |
| RW02 | HDPE | 90.6 |
| Polylactic acid | 66.4 | |
| HDPE | 91.4 | |
| RW03 | PVA | 73.1 |
| Polylactic acid | 78.4 | |
| PE | 90.9 | |
| RW23 | PVA | 66.8 |
| PE | 85.2 | |
| PP | 89.5 | |
| PP | 87.7 | |
| RW25 | PMMA | 87.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trujillo, C.; Pérez-Arantegui, J.; Lobinski, R.; Laborda, F. Improving the Detectability of Microplastics in River Waters by Single Particle Inductively Coupled Plasma Mass Spectrometry. Nanomaterials 2023, 13, 1582. https://doi.org/10.3390/nano13101582
Trujillo C, Pérez-Arantegui J, Lobinski R, Laborda F. Improving the Detectability of Microplastics in River Waters by Single Particle Inductively Coupled Plasma Mass Spectrometry. Nanomaterials. 2023; 13(10):1582. https://doi.org/10.3390/nano13101582
Chicago/Turabian StyleTrujillo, Celia, Josefina Pérez-Arantegui, Ryszard Lobinski, and Francisco Laborda. 2023. "Improving the Detectability of Microplastics in River Waters by Single Particle Inductively Coupled Plasma Mass Spectrometry" Nanomaterials 13, no. 10: 1582. https://doi.org/10.3390/nano13101582
APA StyleTrujillo, C., Pérez-Arantegui, J., Lobinski, R., & Laborda, F. (2023). Improving the Detectability of Microplastics in River Waters by Single Particle Inductively Coupled Plasma Mass Spectrometry. Nanomaterials, 13(10), 1582. https://doi.org/10.3390/nano13101582