Continuous Patterning of Silver Nanowire-Polyvinylpyrrolidone Composite Transparent Conductive Film by a Roll-to-Roll Selective Calendering Process
Abstract
1. Introduction
2. Materials and Methods
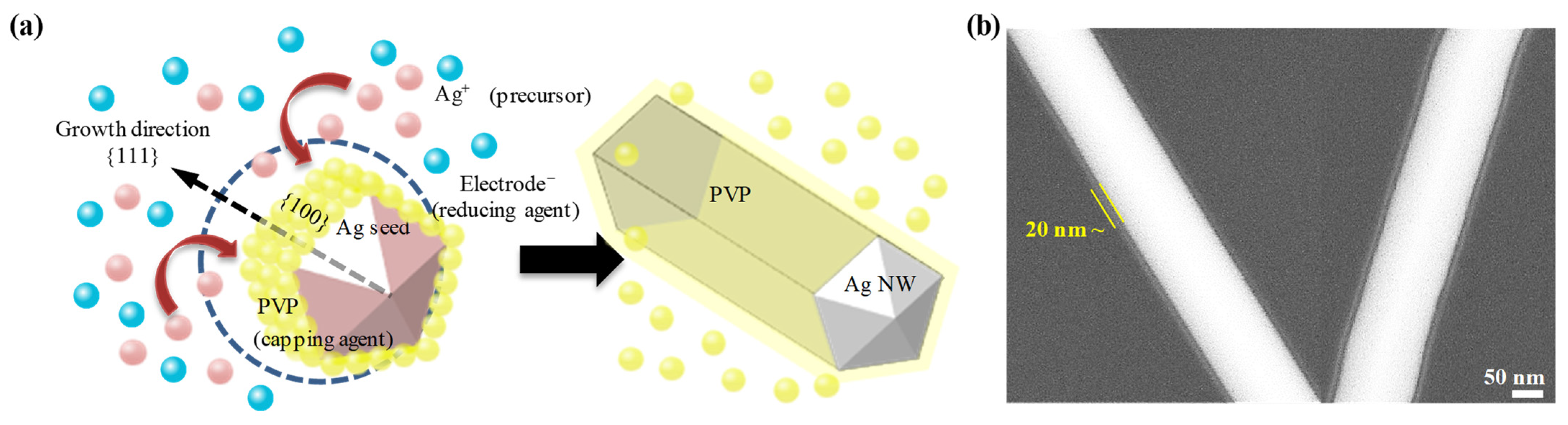
2.1. Synthesis of PVP-Capped Ag NWs
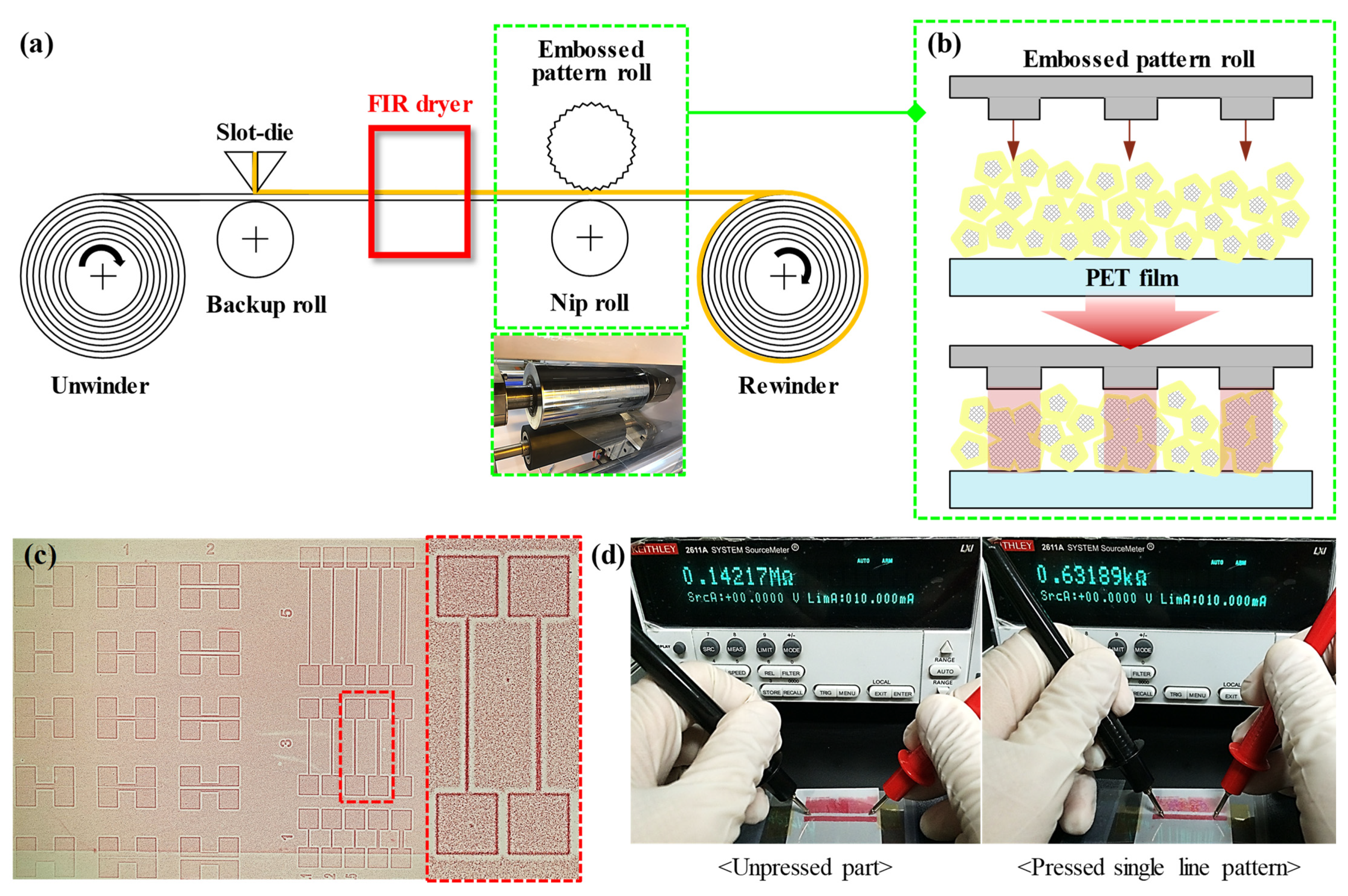
2.2. Fabrication of Patterned Ag NW-PVP Composite TCF
2.3. Measurement and Characterization
2.4. Applications of the Patterned Ag NW-PVP Composite TCF
3. Results and Discussions
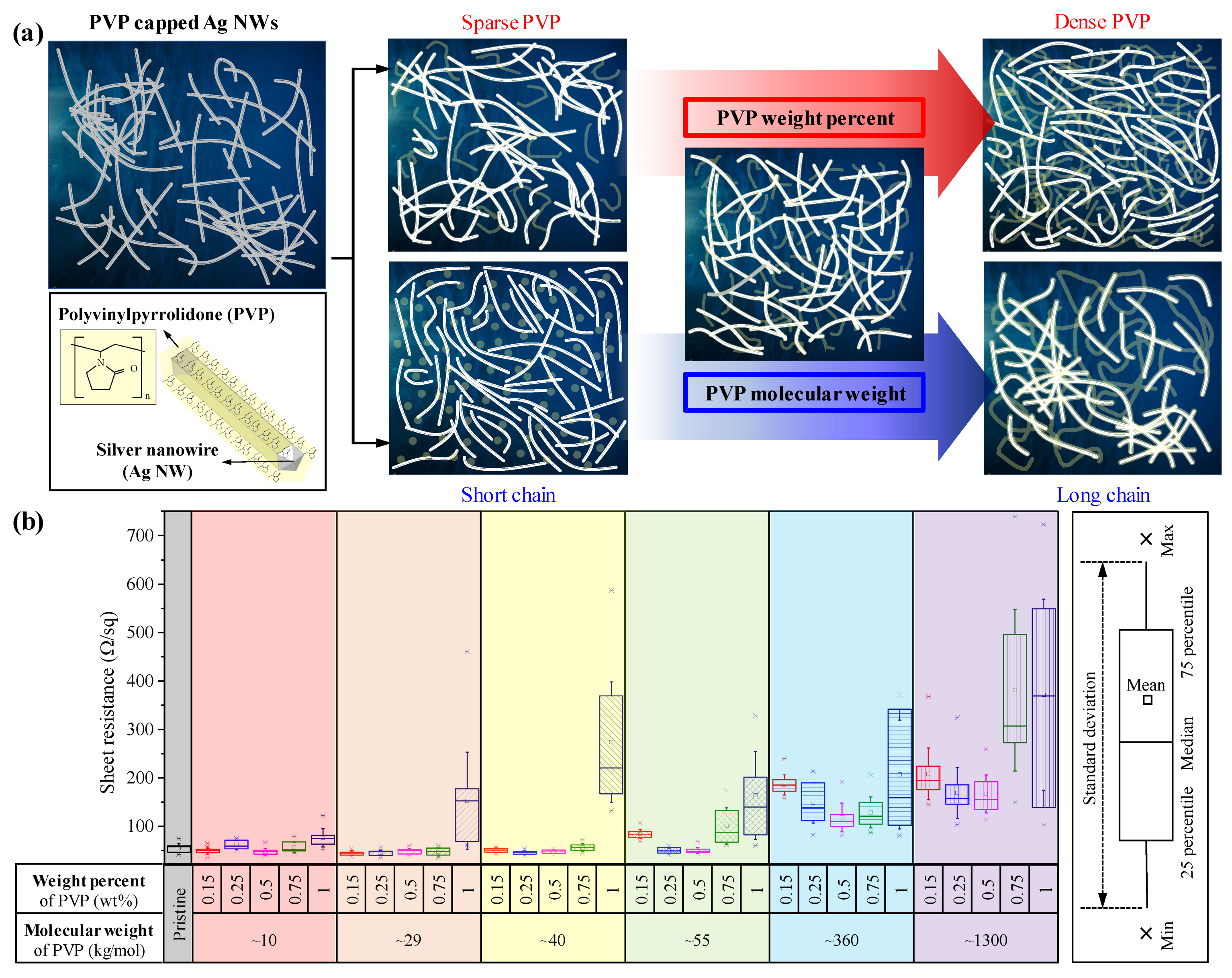
3.1. Ag NW-PVP Composites
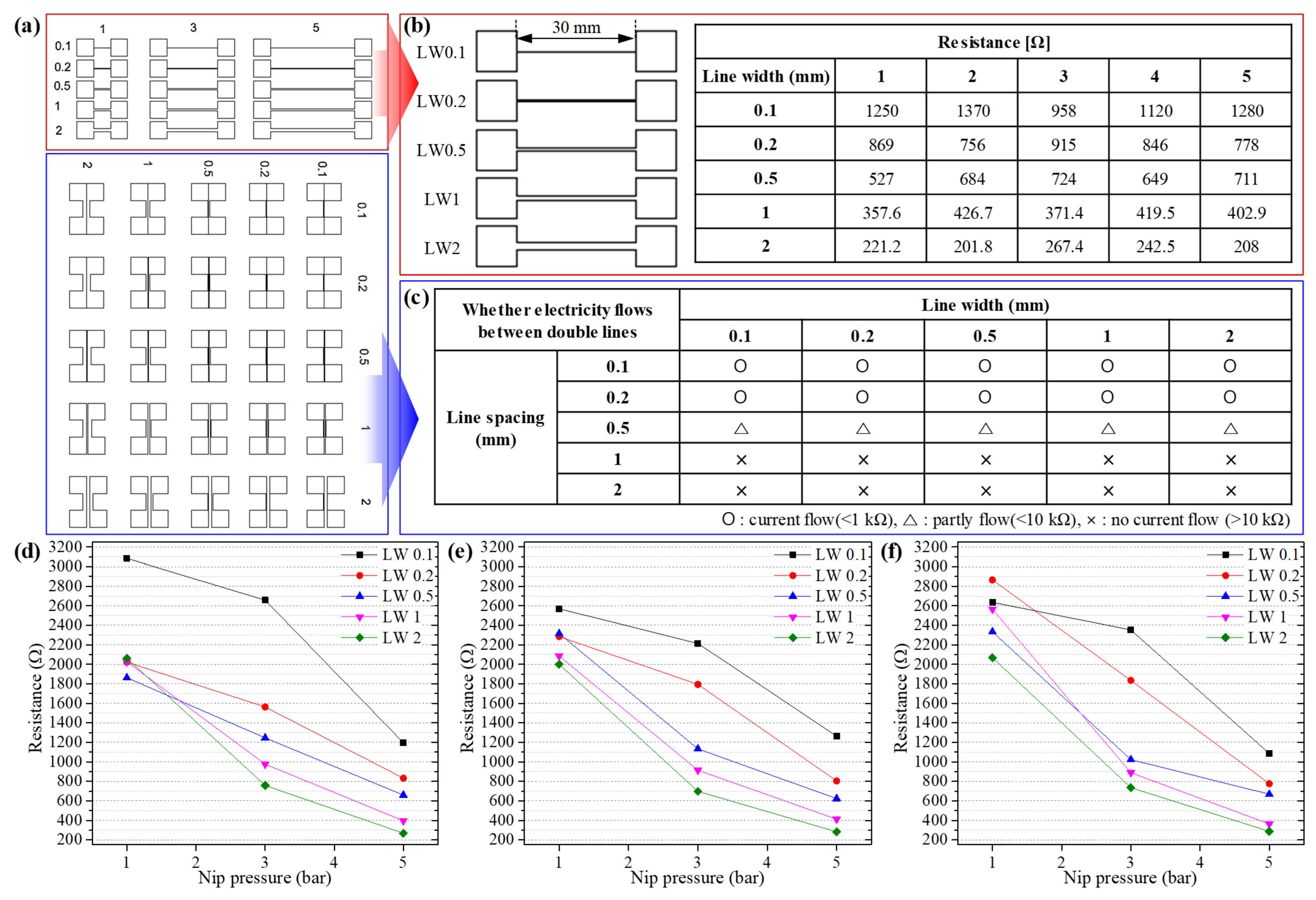
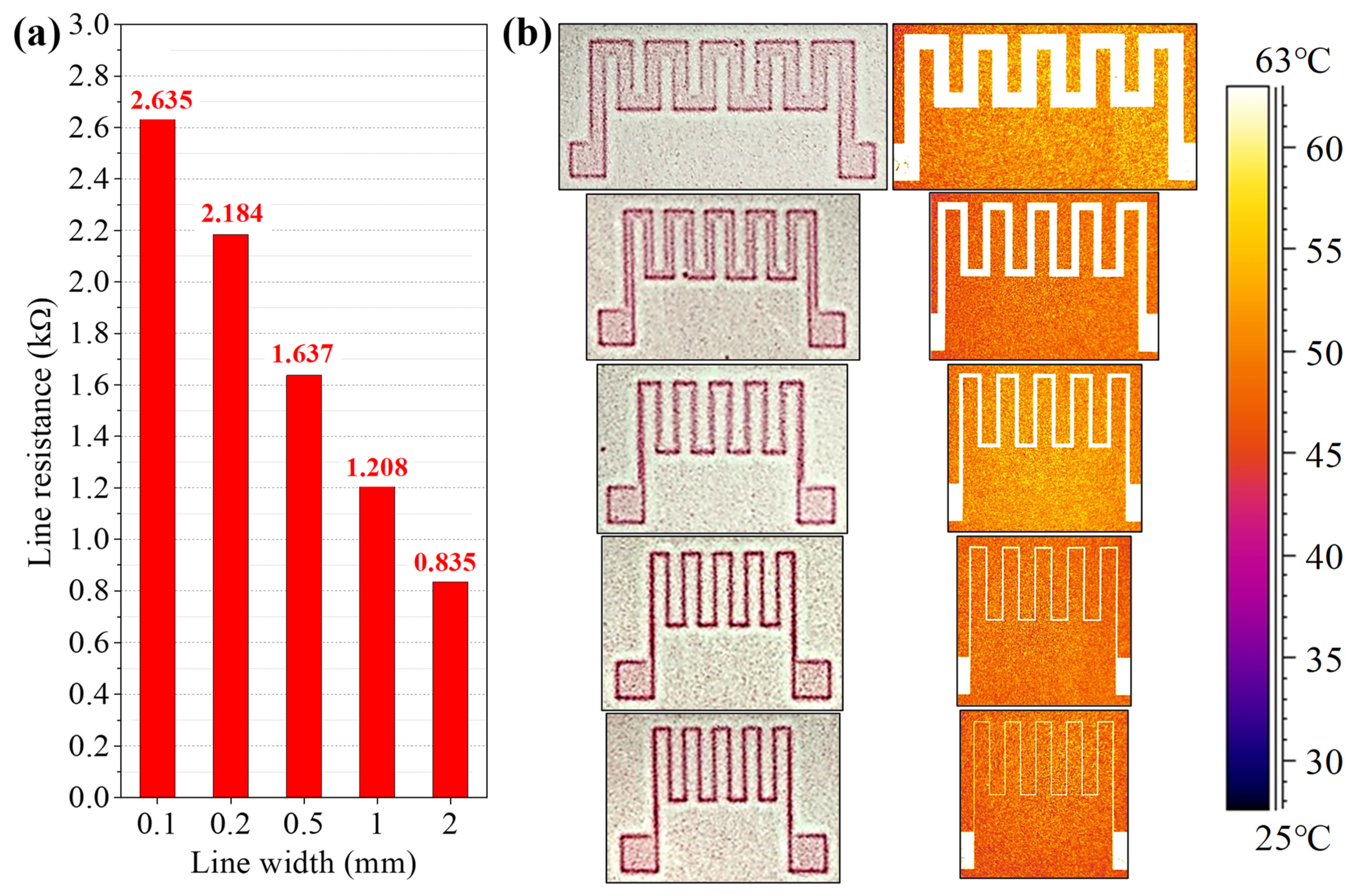
3.2. Roll-to-Roll Continuous Patterning
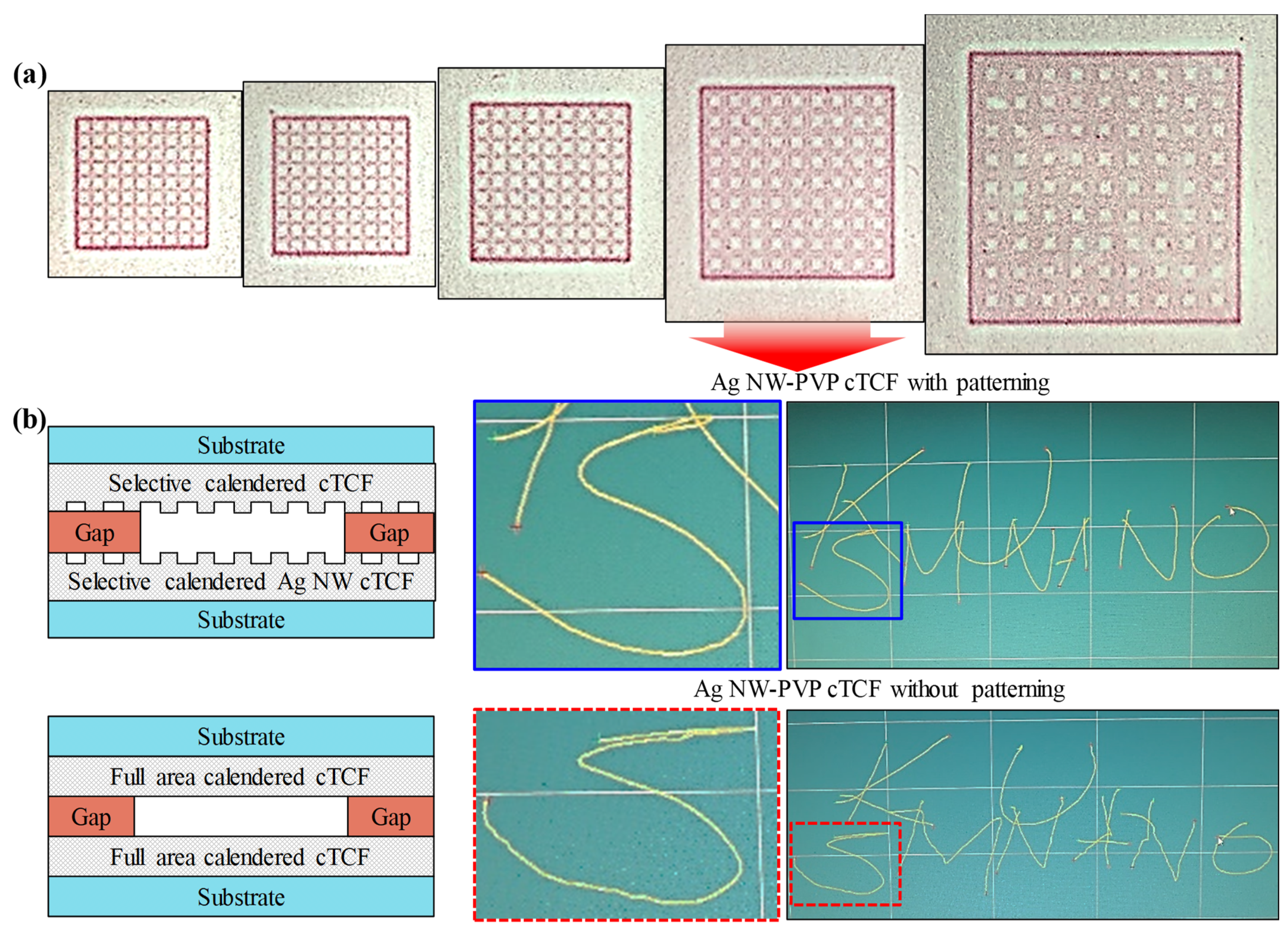
3.3. Application as Functional Devices
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Le, T.S.D.; Phan, H.P.; Kwon, S.; Park, S.; Jung, Y.; Min, J.; Chun, B.J.; Yoon, H.; Ko, S.H.; Kim, S.-W.; et al. Recent Advances in Laser-Induced Graphene: Mechanism, Fabrication, Properties, and Applications in Flexible Electronics. Adv. Funct. Mater. 2022, 32, 2205158. [Google Scholar] [CrossRef]
- Baeg, K.J.; Lee, J. Flexible electronic systems on plastic substrates and textiles for smart wearable technologies. Adv. Mater. Technol. 2020, 5, 2000071. [Google Scholar] [CrossRef]
- Wang, J.L.; Hassan, M.; Liu, J.W.; Yu, S.H. Nanowire assemblies for flexible electronic devices: Recent advances and perspectives. Adv. Mater. 2018, 30, 1803430. [Google Scholar] [CrossRef] [PubMed]
- Kadyrzhanov, K.K.; Shlimas, D.I.; Kozlovskiy, A.L.; Zdorovets, M.V. Research of the shielding effect and radiation resistance of composite CuBi2O4 films as well as their practical applications. J. Mater. Sci. Mater. Electron. 2020, 31, 11729–11740. [Google Scholar] [CrossRef]
- Kozlovskiy, A.L.; Zdorovets, M.V. Synthesis, structural, strength and corrosion properties of thin films of the type CuX (X = Bi, Mg, Ni). J. Mater. Sci. Mater. Electron. 2019, 30, 11819–11832. [Google Scholar] [CrossRef]
- Zubar, T.I.; Usovich, T.I.; Tishkevich, D.I.; Kanafyev, O.D.; Fedkin, V.A.; Kotelnikova, A.N.; Panasyuk, M.I.; Kurochka, A.S.; Nuriev, A.V.; Idris, A.M.; et al. Features of galvanostatic electrodeposition of NiFe films with composition gradient: Influence of substrate characteristics. Nanomaterials 2022, 12, 2926. [Google Scholar] [CrossRef]
- Sharko, S.A.; Serokurova, A.I.; Novitskii, N.N.; Ketsko, V.A.; Smirnova, M.N.; Almuqrin, A.H.; Sayyed, M.I.; Trukhanov, S.V.; Trukhanov, A.V. A new approach to the formation of nanosized gold and beryllium films by ion–beam sputtering deposition. Nanomaterials 2022, 12, 470. [Google Scholar] [CrossRef]
- Tan, D.; Jiang, C.; Li, Q.; Bi, S.; Song, J. Silver nanowire networks with preparations and applications: A review. J. Mater. Sci. Mater. Electron. 2020, 31, 15669–15696. [Google Scholar] [CrossRef]
- Jeong, H.; Park, S.; Lee, J.; Won, P.; Ko, S.H.; Lee, D. Fabrication of transparent conductive film with flexible silver nanowires using roll-to-roll slot-die coating and calendering and its application to resistive touch panel. Adv. Electron. Mater. 2018, 4, 1800243. [Google Scholar] [CrossRef]
- Glier, T.E.; Betker, M.; Witte, M.; Matsuyama, T.; Westphal, L.; Grimm-Lebsanft, B.; Biebl, F.; Akinsinde, L.O.; Fischer, F.; Rübhausen, M. Electrical and network properties of flexible silver–nanowire composite electrodes under mechanical strain. Nanoscale 2020, 12, 23831–23837. [Google Scholar] [CrossRef]
- Feng, Y.; Zou, H.; Tian, M.; Zhang, L.; Mi, J. Relationship between dispersion and conductivity of polymer nanocomposites: A molecular dynamics study. J. Phys. Chem. B 2012, 116, 13081–13088. [Google Scholar] [CrossRef] [PubMed]
- Kiraly, A.; Ronkay, F. Effect of filler dispersion on the electrical conductivity and mechanical properties of carbon/polypropylene composites. Polym. Compos. 2013, 34, 1195–1203. [Google Scholar] [CrossRef]
- Coelho, P.H.; Morales, A.R. Electrical conductivity, percolation threshold and dispersion properties of PMMA nanocomposites of hybrid conducting fillers. In Proceedings of the 14th IEEE International Conference on Nanotechnology, New York, NY, USA, 18–21 August 2014. [Google Scholar]
- Li, J.; Inukai, K.; Takahashi, Y.; Tsuruta, A.; Shin, W. Effect of PVP on the synthesis of high–dispersion core–shell barium–titanate–polyvinylpyrrolidone nanoparticles. J. Asian Ceram. Soc. 2017, 5, 216–225. [Google Scholar] [CrossRef]
- Bae, S.; Jang, M.-H.; Lee, W.C.; Park, J.-W.; Hwang, Y.S. Transport of PVP–coated Silver Nanoparticles in Saturated Porous Media. J. Soil Groundw. Environ. 2016, 21, 104–110. [Google Scholar] [CrossRef]
- Nair, N.M.; Jahanara, M.M.; Ray, D.; Swaminathan, P. Photoresponse of a printed transparent silver nanowire–zinc oxide nanocomposite. Flex. Print. Electron. 2021, 6, 045004. [Google Scholar] [CrossRef]
- Zhang, X.; Shan, J.; Liu, C.; Li, Z.; Guo, X.; Zhao, X.; Yang, H. High corrosion–resistant silver nanowire/poly (3, 4–ethylene dioxythiophene)/poly (styrene sulfonate)@nickel electrode for transparent electromagnetic shielding film. J. Mater. 2022, 8, 1191–1198. [Google Scholar] [CrossRef]
- Park, J.I.; Kim, D.K.; Jang, J.; Kang, I.M.; Kim, H.; Park, J.; Nam, I.W.; Lang, P.; Bae, J.H. Control of silver nanowire–elastomer nanocomposite networks through elaborate direct printing for ultrathin and stretchable strain sensors. Compos. Sci. Technol. 2020, 200, 108471. [Google Scholar] [CrossRef]
- Kumar, A.; Shaikh, M.O.; Kumar, R.R.; Dutt, K.; Pan, C.T.; Chuang, C.H. Highly sensitive, flexible and biocompatible temperature sensor utilizing ultra–long Au@AgNW–based polymeric nanocomposites. Nanoscale 2022, 14, 1742–1754. [Google Scholar] [CrossRef]
- Wang, B.; Zhao, X.; Liang, J.; Liu, J.; Yang, Y.; Zhang, M.; Yu, H.; Li, J.; Tong, Y.; Tang, Q.; et al. Microwave–Welded and Photopolymer–Embedded Silver Nanowire Electrodes for Skin–like Supercapacitors. ACS Appl. Energy Mater. 2022, 5, 10490–10500. [Google Scholar] [CrossRef]
- Yuksel, R.; Coskun, S.; Kalay, Y.E.; Unalan, H.E. Flexible, silver nanowire network nickel hydroxide core–shell electrodes for supercapacitors. J. Power Sources 2016, 328, 167–173. [Google Scholar] [CrossRef]
- Chung, C.H.; Song, T.B.; Bob, B.; Zhu, R.; Duan, H.S.; Yang, Y. Silver nanowire composite window layers for fully solution-deposited thin-film photovoltaic devices. Adv. Mater. 2012, 24, 5499–5504. [Google Scholar] [CrossRef] [PubMed]
- He, Y.J.; Shao, Y.W.; Xiao, Y.Y.; Yang, J.H.; Qi, X.D.; Wang, Y. Multifunctional phase change composites based on elastic MXene/silver nanowire sponges for excellent thermal/solar/electric energy storage, shape memory, and adjustable electromagnetic interference shielding functions. ACS Appl. Mater. Interf. 2022, 14, 6057–6070. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jiu, J.; Sugahara, T.; Nagao, S.; Nogi, M.; Koga, H.; He, P.; Suganuma, K.; Uchida, H. Highly reliable silver nanowire transparent electrode employing selectively patterned barrier shaped by self–masked photolithography. ACS Appl. Mater. Interfaces 2015, 7, 23297–23304. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.; Kim, J.; Kim, D.; Yamauchi, Y.; Kim, J.H.; You, J. A simple silver nanowire patterning method based on poly (ethylene glycol) photolithography and its application for soft electronics. Sci. Rep. 2017, 7, 2282. [Google Scholar] [CrossRef] [PubMed]
- Um, D.S.; Lee, Y.; Kim, T.; Lim, S.; Lee, H.; Ha, M.; Khan, Z.; Kang, S.; Kim, M.P.; Kim, J.Y.; et al. High–resolution filtration patterning of silver nanowire electrodes for flexible and transparent optoelectronic devices. ACS Appl. Mater. Interfaces 2020, 12, 32154–32162. [Google Scholar] [CrossRef]
- Ko, D.; Gu, B.; Kang, S.J.; Jo, S.; Hyun, D.C.; Kim, C.S.; Kim, J. Critical work of adhesion for economical patterning of silver nanowire–based transparent electrodes. J. Mater. Chem. A 2019, 7, 14536–14544. [Google Scholar] [CrossRef]
- Madaria, A.R.; Kumar, A.; Zhou, C. Large scale, highly conductive and patterned transparent films of silver nanowires on arbitrary substrates and their application in touch screens. Nanotechnology 2011, 22, 245201. [Google Scholar] [CrossRef]
- Yoo, D.; Won, D.J.; Cho, W.; Kim, S.; Kim, J. High-Resolution and Facile Patterning of Silver Nanowire Electrodes by Solvent-Free Photolithographic Technique Using UV-Curable Pressure Sensitive Adhesive Film. Small Methods 2021, 5, 2101049. [Google Scholar] [CrossRef]
- Hong, S.; Yeo, J.; Lee, J.; Lee, H.; Lee, P.; Lee, S.S.; Ko, S.H. Selective laser direct patterning of silver nanowire percolation network transparent conductor for capacitive touch panel. J. Nanosci. Nanotechnol. 2015, 15, 2317–2323. [Google Scholar] [CrossRef]
- Chen, Y.; Carmichael, R.S.; Carmichael, T.B. Patterned, flexible, and stretchable silver nanowire/polymer composite films as transparent conductive electrodes. ACS Appl. Mater. Interfaces 2019, 11, 31210–31219. [Google Scholar] [CrossRef]
- Liu, S.; Ho, S.; So, F. Novel patterning method for silver nanowire electrodes for thermal–evaporated organic light emitting diodes. ACS Appl. Mater. Interfaces 2016, 8, 9268–9274. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.R.; Cao, W.; Liu, G.S.; Chen, H.J.; Noh, Y.Y.; Minari, T.; Hsiao, H.-C.; Lee, C.-Y.; Shieh, H.-P.; Liu, C. Microchannel wetting for controllable patterning and alignment of silver nanowire with high resolution. ACS Appl. Mater. Interfaces 2015, 7, 21433–21441. [Google Scholar] [CrossRef] [PubMed]
- Yoo, B.; Kim, Y.; Han, C.J.; Oh, M.S.; Kim, J.W. Recyclable patterning of silver nanowire percolated network for fabrication of flexible transparent electrode. Appl. Surf. Sci. 2018, 429, 151–157. [Google Scholar] [CrossRef]
- Liu, G.S.; Zheng, H.; Zeng, Z.; Wang, Y.; Guo, W.; Wang, T.; Chen, H.; Chen, Y.; Hu, S.; Chen, L.; et al. Self–assembled monolayer modulated Plateau–Rayleigh instability and enhanced chemical stability of silver nanowire for invisibly patterned, stable transparent electrodes. Nano Res. 2022, 15, 4552–4562. [Google Scholar] [CrossRef]
- Liu, G.S.; Wang, T.; Wang, Y.; Zheng, H.; Chen, Y.; Zeng, Z.; Chen, L.; Chen, Y.; Yang, B.; Luo, Y.; et al. One–step plasmonic welding and photolithographic patterning of silver nanowire network by UV–programable surface atom diffusion. Nano Res. 2022, 15, 2582–2591. [Google Scholar] [CrossRef]
- Song, J.Y.; Oh, J.H.; Choi, D.; Park, S.M. Highly efficient patterning technique for silver nanowire electrodes by electrospray deposition and its application to self–powered triboelectric tactile sensor. Sci. Rep. 2021, 11, 21437. [Google Scholar] [CrossRef]
- Park, J.S.; Park, T.G.; Park, J.S. Characterization of silver nanowire flexible transparent electrode with grid pattern formed via thermocompression. Electron. Mater. Lett. 2021, 17, 260–267. [Google Scholar] [CrossRef]
- Park, J.; Kim, G.; Lee, B.; Lee, S.; Won, P.; Yoon, H.; Cho, H.; Ko, S.H.; Hong, Y. Highly Customizable Transparent Silver Nanowire Patterning via Inkjet-Printed Conductive Polymer Templates Formed on Various Surfaces. Adv. Mater. Technol. 2020, 5, 2000042. [Google Scholar] [CrossRef]
- Scheideler, W.J.; Smith, J.; Deckman, I.; Chung, S.; Arias, A.C.; Subramanian, V. A robust, gravure–printed, silver nanowire/metal oxide hybrid electrode for high–throughput patterned transparent conductors. J. Mater. Chem. C 2016, 4, 3248–3255. [Google Scholar] [CrossRef]
- Patil, J.J.; Reese, M.L.; Lee, E.; Grossman, J.C. Oxynitride–Encapsulated Silver Nanowire Transparent Electrode with Enhanced Thermal, Electrical, and Chemical Stability. ACS Appl. Mater. Interfaces 2022, 14, 4423–4433. [Google Scholar] [CrossRef]
- Chae, W.H.; Patil, J.J.; Grossman, J.C. Conformal Encapsulation of Silver Nanowire Transparent Electrodes by Nanosized Reduced Graphene Oxide Leading to Improved All–Round Stability. ACS Appl. Mater. Interfaces 2022, 14, 34997–35009. [Google Scholar] [CrossRef]
- Oh, J.Y.; Lee, D. Core–shell structured graphene sphere–silver nanowire hybrid filler embedded polydimethylsiloxane nanocomposites for stretchable conductor. Nanotechnology 2019, 30, 445706. [Google Scholar] [CrossRef] [PubMed]
- Jiu, J.; Nogi, M.; Sugahara, T.; Tokuno, T.; Araki, T.; Komoda, N.; Suganuma, K.; Uchida, H.; Shinozaki, K. Strongly adhesive and flexible transparent silver nanowire conductive films fabricated with a high–intensity pulsed light technique. J. Mater. Chem. 2012, 22, 23561–23567. [Google Scholar] [CrossRef]
- Tang, Q.; Shen, H.; Yao, H.; Jiang, Y.; Zheng, C.; Gao, K. Preparation of silver nanowire/AZO composite film as a transparent conductive material. Ceram. Int. 2017, 43, 1106–1113. [Google Scholar] [CrossRef]
- Jeong, H.; Noh, Y.; Kim, J.; Ko, S.; Lee, D. Sequential manufacturing via intra–additive hybrid materials approach for fully roll–to–roll processed flexible organic thin film transistors. J. Manuf. Process. 2021, 72, 138–147. [Google Scholar] [CrossRef]
- Xiao, G.; Makeiff, D.; Tao, Y.; Lu, J.; Zhang, Z. Improving The Adhesion Between Silver Nanowire Transparent Electrode and PET Film Using a Crosslinkable Polymer. In Proceedings of the 2018 International Flexible Electronics Technology Conference, Ottawa, ON, Canada, 7–9 August 2018. [Google Scholar]
- Choi, D.Y.; Kang, H.W.; Sung, H.J.; Kim, S.S. Annealing–free, flexible silver nanowire–polymer composite electrodes via a continuous two–step spray–coating method. Nanoscale 2013, 5, 977–983. [Google Scholar] [CrossRef]
- Jeong, H.; Noh, Y.; Ko, S.H.; Lee, D. Flexible resistive pressure sensor with silver nanowire networks embedded in polymer using natural formation of air gap. Compos. Sci. Technol. 2019, 174, 50–57. [Google Scholar] [CrossRef]
- Kumar, A.; Shaikh, M.O.; Chuang, C.H. Silver nanowire synthesis and strategies for fabricating transparent conducting electrodes. Nanomaterials 2021, 11, 693. [Google Scholar] [CrossRef]
- Zhu, J.J.; Kan, C.X.; Wan, J.G.; Han, M.; Wang, G.H. High–yield synthesis of uniform Ag nanowires with high aspect ratios by introducing the long–chain PVP in an improved polyol process. J. Nanomater. 2011, 2011, 40. [Google Scholar] [CrossRef]
- Li, B.; Ye, S.; Stewart, I.E.; Alvarez, S.; Wiley, B.J. Synthesis and purification of silver nanowires to make conducting films with a transmittance of 99%. Nano Lett. 2015, 15, 6722–6726. [Google Scholar] [CrossRef]
- Hwang, J.; Shim, Y.; Yoon, S.M.; Lee, S.H.; Park, S.H. Influence of polyvinylpyrrolidone (PVP) capping layer on silver nanowire networks: Theoretical and experimental studies. RSC Adv. 2016, 6, 30972–30977. [Google Scholar] [CrossRef]
- Ge, Y.; Duan, X.; Zhang, M.; Mei, L.; Hu, J.; Hu, W.; Duan, X. Direct room temperature welding and chemical protection of silver nanowire thin films for high performance transparent conductors. J. Am. Chem. Soc. 2018, 140, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Rathmell, A.R.; Nguyen, M.; Chi, M.; Wiley, B.J. Synthesis of oxidation–resistant cupronickel nanowires for transparent conducting nanowire networks. Nano Lett. 2012, 12, 3193–3199. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.C.; Sung, D.I.; Shin, Y.J.; Yeom, G.Y. Highly oxidation–resistant silver nanowires by CxFy polymers using plasma treatment. Nanotechnology 2019, 30, 285702. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.T.; Huang, S.X. Transparent conductive silver nanowire electrodes with high resistance to oxidation and thermal shock. RSC Adv. 2014, 4, 59226–59232. [Google Scholar] [CrossRef]
- Deignan, G.; Goldthorpe, I.A. The dependence of silver nanowire stability on network composition and processing parameters. RSC Adv. 2017, 7, 35590–35597. [Google Scholar] [CrossRef]
- Khaligh, H.H.; Goldthorpe, I.A. Failure of silver nanowire transparent electrodes under current flow. Nanoscale Res. Lett. 2013, 8, 235. [Google Scholar] [CrossRef]
- Jiu, J.; Wang, J.; Sugahara, T.; Nagao, S.; Nogi, M.; Koga, H.; Suganuma, K.; Hara, M.; Nakazawa, E.; Uchida, H. The effect of light and humidity on the stability of silver nanowire transparent electrodes. RSC Adv. 2015, 5, 27657–27664. [Google Scholar] [CrossRef]
- Trukhanov, S.V.; Trukhanov, A.V.; Vasiliev, A.N.; Balagurov, A.M.; Szymczak, H. Magnetic state of the structural separated anion-deficient La0.70Sr0.30MnO2.85 manganite. J. Exp. Theor. Phys. 2011, 113, 819–825. [Google Scholar] [CrossRef]
- Kozlovskiy, A.; Egizbek, K.; Zdorovets, M.V.; Ibragimova, M.; Shumskaya, A.; Rogachev, A.A.; Ignatovich, Z.V.; Kadyrzhanov, K. Evaluation of the efficiency of detection and capture of manganese in aqueous solutions of FeCeOx nanocomposites doped with Nb2O5. Sensors 2020, 20, 4851. [Google Scholar] [CrossRef]
- Kumar, G.; Soni, R.K. Silver nanocube–and nanowire–based SERS substrates for ultra–low detection of PATP and thiram molecules. Plasmonics 2020, 15, 1577–1589. [Google Scholar] [CrossRef]
- Gebeyehu, M.B.; Chala, T.F.; Chang, S.Y.; Wu, C.M.; Lee, J.Y. Synthesis and highly effective purification of silver nanowires to enhance transmittance at low sheet resistance with simple polyol and scalable selective precipitation method. RSC Adv. 2017, 7, 16139–16148. [Google Scholar] [CrossRef]
- Heidari, B.; Salmani, S.; Sasani Ghamsari, M.; Ahmadi, M.; Majles-Ara, M.H. Ag/PVP nanocomposite thin film with giant optical nonlinearity. Opt. Quantum Electron. 2020, 52, 86. [Google Scholar] [CrossRef]
- Timin, A.S.; Solomonov, A.V.; Musabirov, I.I.; Sergeev, S.N.; Ivanov, S.P.; Rumyantsev, E.V.; Goncharenko, A. Immobilization of bovine serum albumin onto porous poly (vinylpyrrolidone)–modified silicas. Ind. Eng. Chem. Res. 2014, 53, 13699–13710. [Google Scholar] [CrossRef]
- Bashiri Rezaie, A.; Montazer, M.; Mahmoudi Rad, M. Scalable, eco–friendly and simple strategy for nano–functionalization of textiles using immobilized copper–based nanoparticles. Clean Technol. Environ. Policy 2018, 20, 2119–2133. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Y.; Zhang, C.; Wang, X.; Chen, Z.; Qi, H. Highly stable silver nanowire dispersion assisted by sulfated holocellulose nanofibers for functional materials. Compos. Sci. Technol. 2022, 219, 109211. [Google Scholar] [CrossRef]
- Tao, A.; Kim, F.; Hess, C.; Goldberger, J.; He, R.; Sun, Y.; Xia, Y.; Yang, P. Langmuir− Blodgett silver nanowire monolayers for molecular sensing using surface–enhanced Raman spectroscopy. Nano Lett. 2003, 3, 1229–1233. [Google Scholar] [CrossRef]
- Nam, S.; Cho, H.W.; Lim, S.; Kim, D.; Kim, H.; Sung, B.J. Enhancement of electrical and thermomechanical properties of silver nanowire composites by the introduction of nonconductive nanoparticles: Experiment and simulation. ACS Nano 2013, 7, 851–856. [Google Scholar] [CrossRef]
- Wang, H.; Qiao, X.; Chen, J.; Wang, X.; Ding, S. Mechanisms of PVP in the preparation of silver nanoparticles. Mater. Chem. Phys. 2005, 94, 449–453. [Google Scholar] [CrossRef]
- Li, S.F.; Zhang, H.Y. Effect of polyvinylpyrrolidone on the preparation of silver nanowires. Adv. Mater. Res. 2014, 881, 940–943. [Google Scholar] [CrossRef]
- Behrens, S.; Wu, J.; Habicht, W.; Unger, E. Silver nanoparticle and nanowire formation by microtubule templates. Chem. Mater. 2004, 16, 3085–3090. [Google Scholar] [CrossRef]
- Chen, Z.; Li, W.; Li, R.; Zhang, Y.; Xu, G.; Cheng, H. Fabrication of highly transparent and conductive indium–tin oxide thin films with a high figure of merit via solution processing. Langmuir 2013, 29, 13836–13842. [Google Scholar] [CrossRef] [PubMed]
- Noh, Y.; Jeong, H.; Park, J.; Lee, D. Charge–assisted Coating of Silver Nanowire Transparent Conductive Layer and Application to Flexible Film Heater. Surf. Interf. 2022, 32, 102105. [Google Scholar] [CrossRef]
- Jeong, H.; Noh, Y.; Kim, G.Y.; Lee, H.; Lee, D. Roll–to–roll processed silver nanowire/silicon dioxide microsphere composite for high–accuracy flexible touch sensing application. Surf. Interf. 2022, 30, 101976. [Google Scholar] [CrossRef]
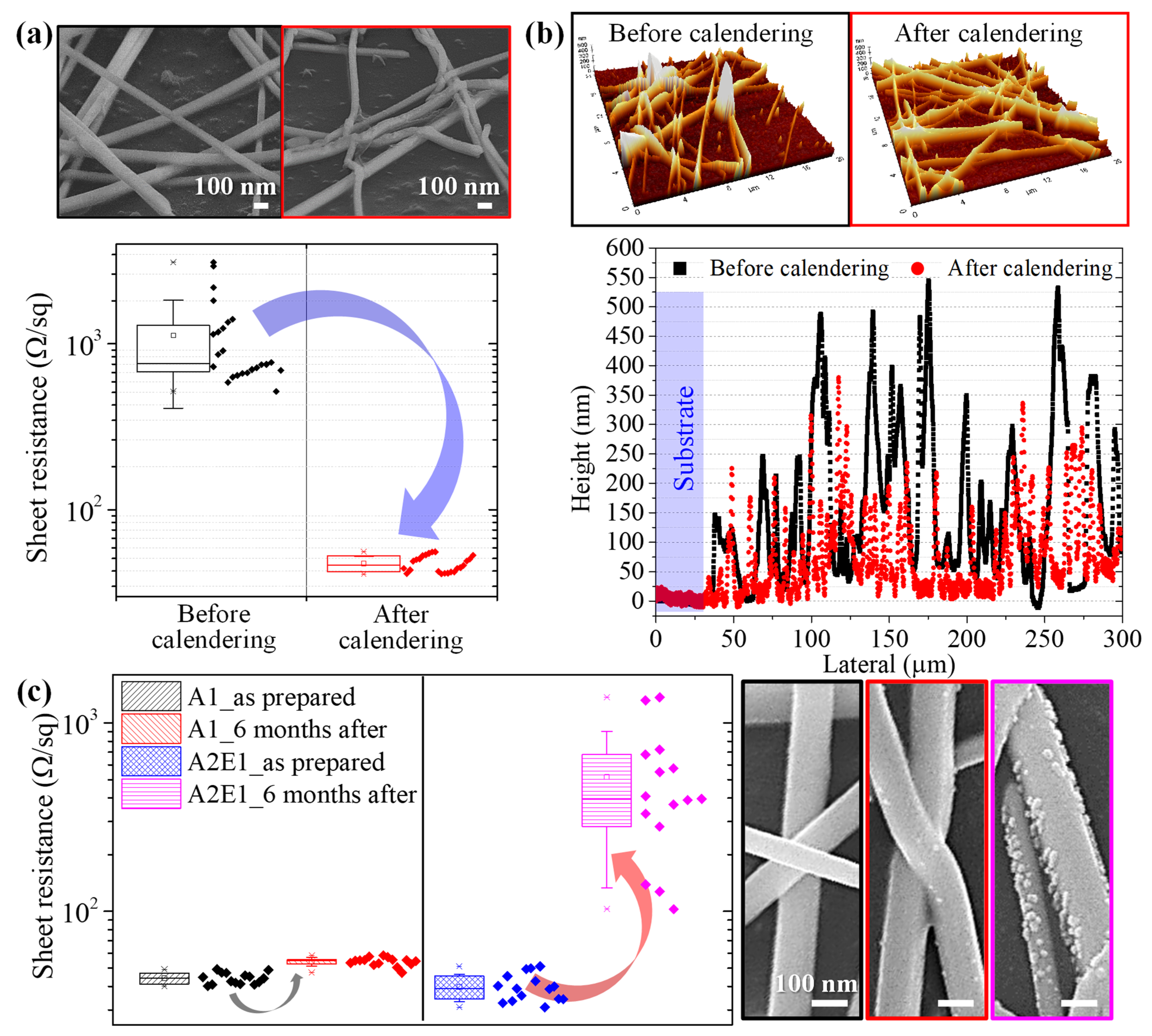

| Elements | Ag, at.w.% | C, at.w.% | O, at.w.% | N, at.w.% | S, at.w.% |
|---|---|---|---|---|---|
| A1_as prepared | 4.32 | 74.0 | 9.31 | 12.30 | - |
| A1_6 months after | 4.13 | 76.05 | 10.07 | 9.76 | - |
| A2E1_as prepared | 8.79 | 61.08 | 17.90 | 12.24 | - |
| A2E1_6 months after | 1.89 | 86.21 | 11.67 | - | 0.22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, H.; Lee, J.H.; Song, J.-Y.; Ghani, F.; Lee, D. Continuous Patterning of Silver Nanowire-Polyvinylpyrrolidone Composite Transparent Conductive Film by a Roll-to-Roll Selective Calendering Process. Nanomaterials 2023, 13, 32. https://doi.org/10.3390/nano13010032
Jeong H, Lee JH, Song J-Y, Ghani F, Lee D. Continuous Patterning of Silver Nanowire-Polyvinylpyrrolidone Composite Transparent Conductive Film by a Roll-to-Roll Selective Calendering Process. Nanomaterials. 2023; 13(1):32. https://doi.org/10.3390/nano13010032
Chicago/Turabian StyleJeong, Hakyung, Jae Hak Lee, Jun-Yeob Song, Faizan Ghani, and Dongjin Lee. 2023. "Continuous Patterning of Silver Nanowire-Polyvinylpyrrolidone Composite Transparent Conductive Film by a Roll-to-Roll Selective Calendering Process" Nanomaterials 13, no. 1: 32. https://doi.org/10.3390/nano13010032
APA StyleJeong, H., Lee, J. H., Song, J.-Y., Ghani, F., & Lee, D. (2023). Continuous Patterning of Silver Nanowire-Polyvinylpyrrolidone Composite Transparent Conductive Film by a Roll-to-Roll Selective Calendering Process. Nanomaterials, 13(1), 32. https://doi.org/10.3390/nano13010032







