Ultrasensitive Luteolin Electrochemical Sensor Based on Novel Lamellar CuZn@ Nitrogen-Containing Carbon Nanosheets
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Equipment
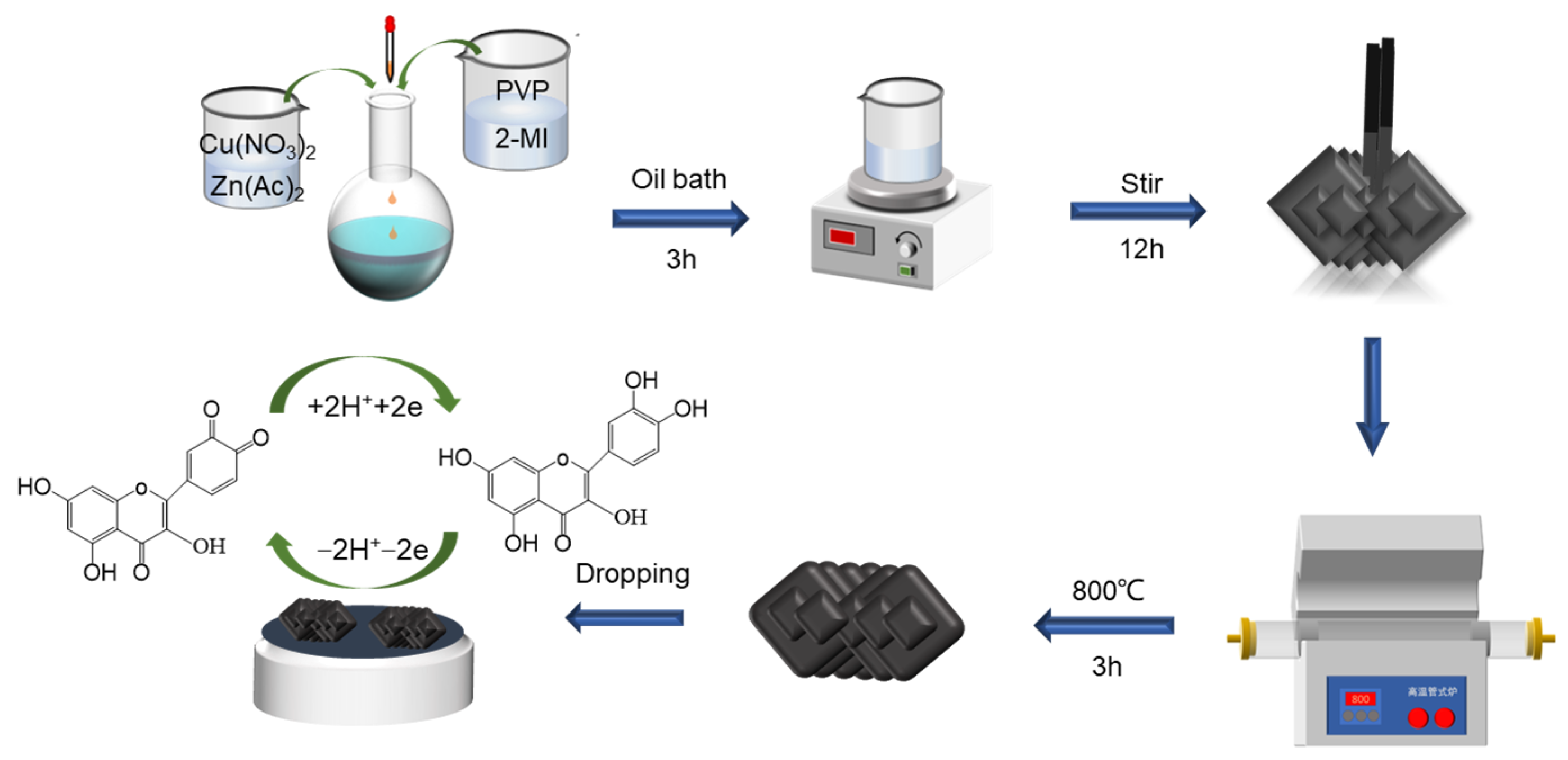
2.2. Synthesis of Cu/Zn-ZIF and CuZn@NC
2.3. Working Electrode Preparation of CuZn@NC/GCE
2.4. Preparation of Actual Samples
2.5. Electrochemical Test Parameters
3. Results and Discussion
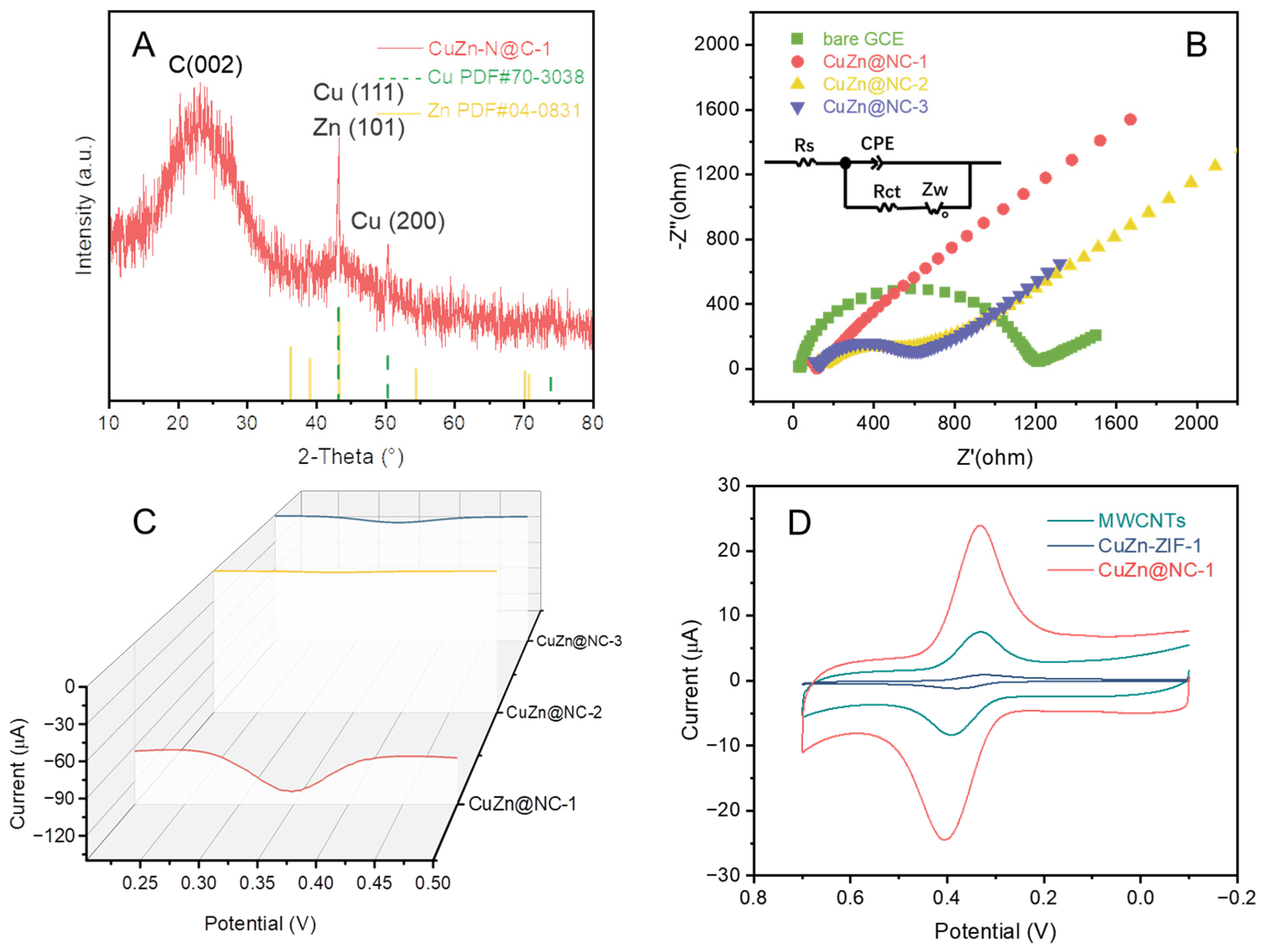
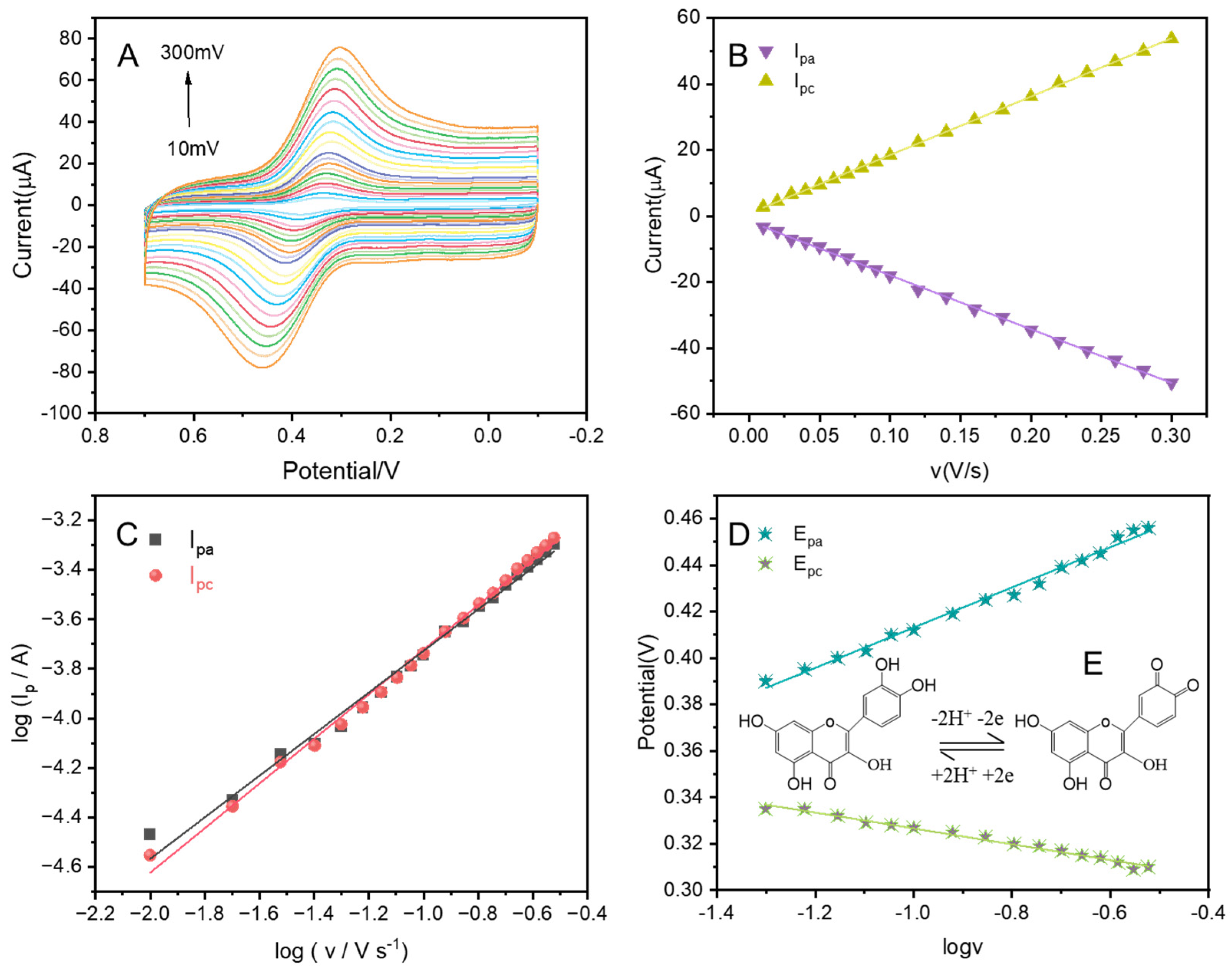
3.1. Physical and Electrochemical Characterization
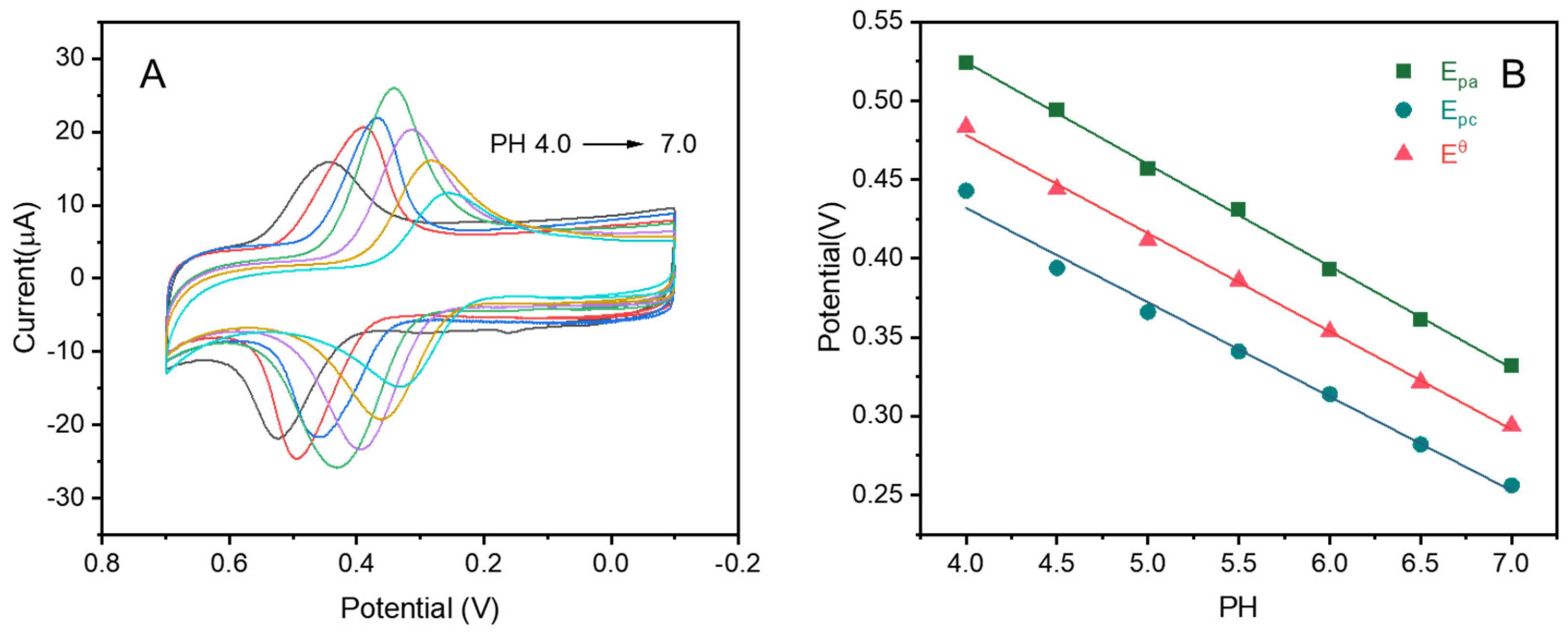
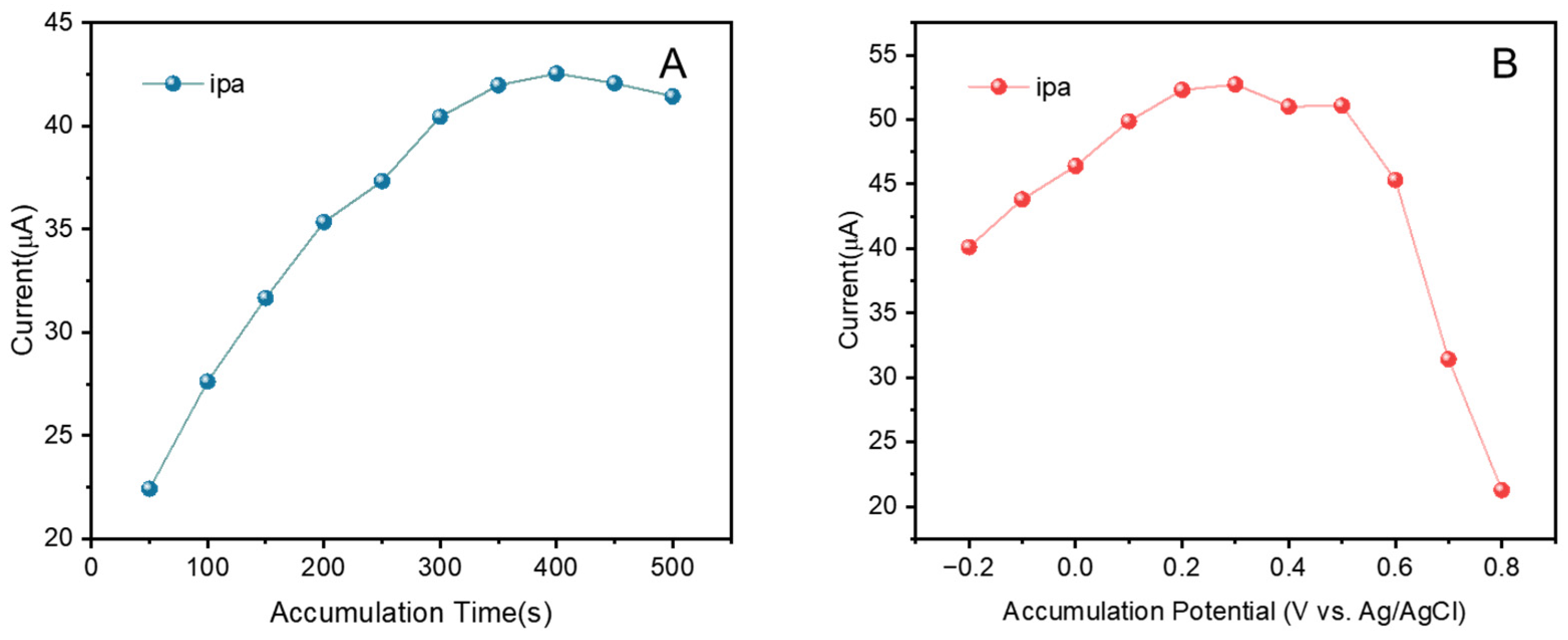
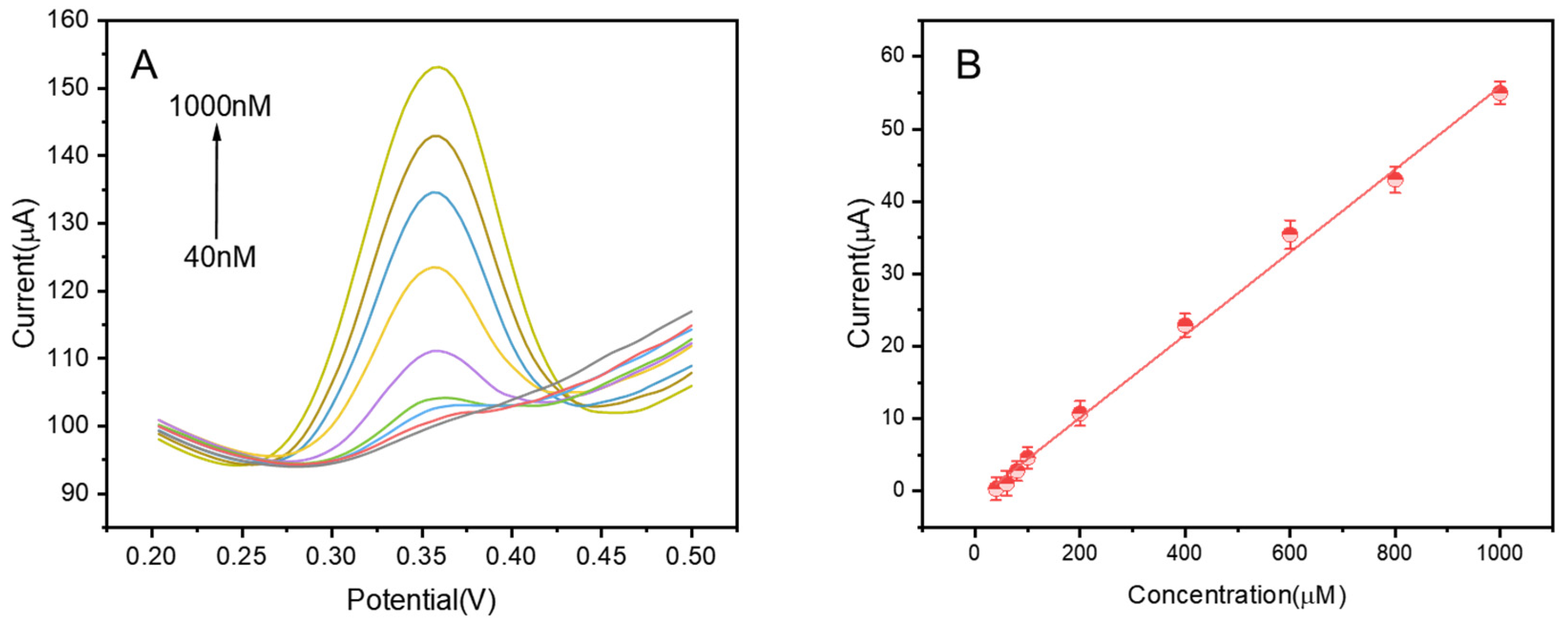
3.2. Quantitative Analysis of Luteolin on CuZn@NC-1/GCE
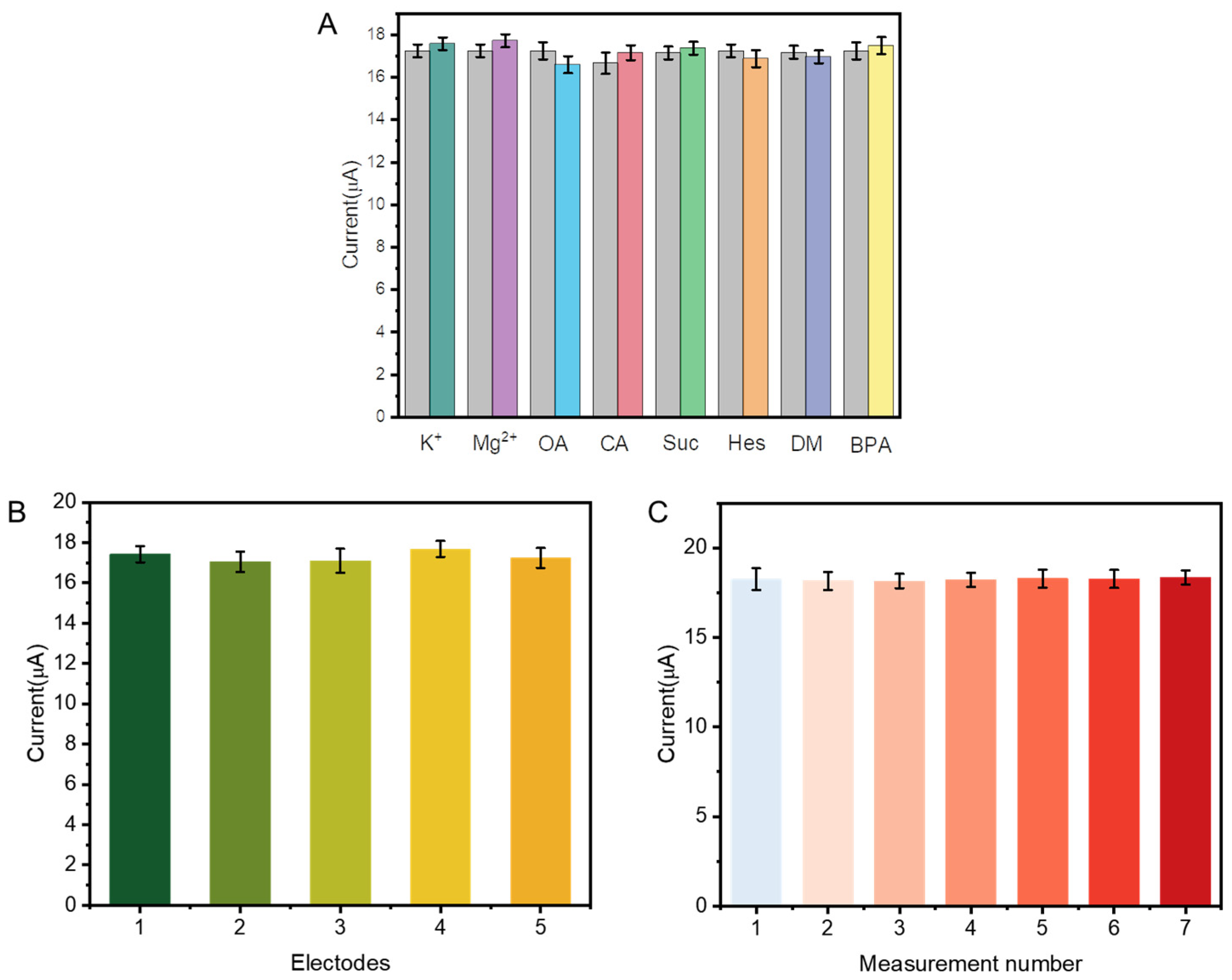
3.3. Interference Resistance, Reproducibility, and Stability Studies
3.4. Testing of Actual Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hu, Y.; Zhang, L.; Zhao, P.; Wang, C.; Fei, J.; Xie, Y. Ultrasensitive luteolin electrochemical sensor based on zeolitic imidazolate frameworks-derived cobalt trioxide @ nitrogen doped carbon nanotube/amino-functionalized graphene quantum dots composites modified glass carbon electrode. Sens. Actuators B Chem. 2022, 351, 130938. [Google Scholar] [CrossRef]
- Sariga; George, A.; Rajeev, R.; Thadathil, D.A.; Varghese, A. A Comprehensive Review on the Electrochemical Sensing of Flavonoids. Crit. Rev. Anal. Chem. 2022, 1–41. [Google Scholar] [CrossRef]
- You, Z.; Fu, Y.; Xiao, A.; Liu, L.; Huang, S. Magnetic molecularly imprinting polymers and reduced graphene oxide modified electrochemical sensor for the selective and sensitive determination of luteolin in natural extract. Arab. J. Chem. 2021, 14, 102990. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, Z.; Mu, X.; Liu, Y.; Shi, Z.; Zheng, Y.; Huang, W. The electrochemical sensor based on Cu/Co binuclear MOFs and PVP cross-linked derivative materials for the sensitive detection of luteolin and rutin. Microchem. J. 2022, 175, 107131. [Google Scholar] [CrossRef]
- Hou, X.; Wu, W.; Zhao, F.; Xie, W.; Yang, Q. Construction of an electrochemical sensor with graphene aerogel doped with ZrO2 nanoparticles and chitosan for the selective detection of luteolin. Mikrochim. Acta 2021, 188, 86. [Google Scholar] [CrossRef]
- Sharif, S.; Nabais, P.; Melo, M.J.; Oliveira, M.C. Traditional Yellow Dyes Used in the 21st Century in Central Iran: The Knowledge of Master Dyers Revealed by HPLC-DAD and UHPLC-HRMS/MS. Molecules 2020, 25, 908. [Google Scholar] [CrossRef]
- Meirinhos, J.; Silva, B.M.; Valentao, P.; Seabra, R.M.; Pereira, J.A.; Dias, A.; Andrade, P.B.; Ferreres, F. Analysis and quantification of flavonoidic compounds from Portuguese olive (Olea europaea L.) leaf cultivars. Nat. Prod. Res. 2005, 19, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Bajoub, A.; Pacchiarotta, T.; Hurtado-Fernandez, E.; Olmo-Garcia, L.; Garcia-Villalba, R.; Fernandez-Gutierrez, A.; Mayboroda, O.A.; Carrasco-Pancorbo, A. Comparing two metabolic profiling approaches (liquid chromatography and gas chromatography coupled to mass spectrometry) for extra-virgin olive oil phenolic compounds analysis: A botanical classification perspective. J. Chromatogr. A 2016, 1428, 267–279. [Google Scholar] [CrossRef]
- Juszczak, A.M.; Zovko-Koncic, M.; Tomczyk, M. Recent Trends in the Application of Chromatographic Techniques in the Analysis of Luteolin and Its Derivatives. Biomolecules 2019, 9, 731. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Y.; Zhang, Q.F.; Sun, H.; Cheung, N.K.; Cheung, H.Y. Simultaneous determination of flavonoid analogs in Scutellariae Barbatae Herba by beta-cyclodextrin and acetonitrile modified capillary zone electrophoresis. Talanta 2013, 105, 393–402. [Google Scholar] [CrossRef]
- Tang, J.; Huang, R.; Zheng, S.; Jiang, S.; Yu, H.; Li, Z.; Wang, J. A sensitive and selective electrochemical sensor based on graphene quantum dots/gold nanoparticles nanocomposite modified electrode for the determination of luteolin in peanut hulls. Microchem. J. 2019, 145, 899–907. [Google Scholar] [CrossRef]
- Kan, X.; Zhang, T.; Zhong, M.; Lu, X. CD/AuNPs/MWCNTs based electrochemical sensor for quercetin dual-signal detection. Biosens. Bioelectron. 2016, 77, 638–643. [Google Scholar] [CrossRef]
- Niu, X.; Huang, Y.; Zhang, W.; Yan, L.; Wang, L.; Li, Z.; Sun, W. Synthesis of gold nanoflakes decorated biomass-derived porous carbon and its application in electrochemical sensing of luteolin. J. Electroanal. Chem. 2021, 880, 114832. [Google Scholar] [CrossRef]
- Wang, Y.; Ni, M.; Chen, J.; Wang, C.; Yang, Y.; Xie, Y.; Zhao, P.; Fei, J. An ultra-sensitive luteolin sensor based on Co-doped nitrogen-containing carbon frameworkMoS2−MWCNTs composite for natural sample detection. Electrochim. Acta 2023, 438, 141534. [Google Scholar] [CrossRef]
- Chen, X.; Li, J.; Li, J.; Zhang, L.; Zhao, P.; Wang, C.; Fei, J.; Xie, Y. Determination of luteolin in Chrysanthemum tea with a ultra-sensitive electrochemical sensor based on MoO3/poly(3,4-ethylene dioxythiophene)/gama-cyclodextrin metal-organic framework composites. Food Chem. 2022, 397, 133723. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhao, P.; Wang, C.; Wang, Y.; Yang, Y.; Xie, Y.; Fei, J. An ultrasensitive luteolin electrochemical sensor based on a glass carbon electrode modified using multi-walled carbon nanotube-supported hollow cobalt sulfide (CoSx) polyhedron/graphene quantum dot composites. Analyst 2022, 147, 2739–2748. [Google Scholar] [CrossRef]
- Lu, X.; Li, Y.; Duan, X.; Zhu, Y.; Xue, T.; Rao, L.; Wen, Y.; Tian, Q.; Cai, Y.; Xu, Q.; et al. A novel nanozyme comprised of electro-synthesized molecularly imprinted conducting PEDOT nanocomposite with graphene-like MoS2 for electrochemical sensing of luteolin. Microchem. J. 2021, 168, 106418. [Google Scholar] [CrossRef]
- Feng, X.; Yin, X.; Bo, X.; Guo, L. An ultrasensitive luteolin sensor based on MOFs derived CuCo coated nitrogen-doped porous carbon polyhedron. Sens. Actuators B Chem. 2019, 281, 730–738. [Google Scholar] [CrossRef]
- Tahmasebi, Z.; Davarani, S.S.H.; Asgharinezhad, A.A. Highly efficient electrochemical determination of propylthiouracil in urine samples after selective electromembrane extraction by copper nanoparticles-decorated hollow fibers. Biosens. Bioelectron. 2018, 114, 66–71. [Google Scholar] [CrossRef]
- Dai, H.; Lu, W.; Zuo, X.; Zhu, Q.; Pan, C.; Niu, X.; Liu, J.; Chen, H.; Chen, X. A novel biosensor based on boronic acid functionalized metal-organic frameworks for the determination of hydrogen peroxide released from living cells. Biosens. Bioelectron. 2017, 95, 131–137. [Google Scholar] [CrossRef]
- Fu, Y.; You, Z.; Xiao, A.; Liu, L. Magnetic molecularly imprinting polymers, reduced graphene oxide, and zeolitic imidazolate frameworks modified electrochemical sensor for the selective and sensitive detection of catechin. Mikrochim. Acta 2021, 188, 71. [Google Scholar] [CrossRef] [PubMed]
- Tu, X.; Gao, F.; Ma, X.; Zou, J.; Yu, Y.; Li, M.; Qu, F.; Huang, X.; Lu, L. Mxene/carbon nanohorn/beta-cyclodextrin-Metal-organic frameworks as high-performance electrochemical sensing platform for sensitive detection of carbendazim pesticide. J. Hazard. Mater. 2020, 396, 122776. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Qian, W.; Li, Y.; Yu, Q.; Yu, Y.; Chen, S.; Qu, F.; Gao, Y.; Lu, L. Multilayer activated biochar/UiO-66-NH2 film as intelligent sensing platform for ultra-sensitive electrochemical detection of Pb2+ and Hg2+. Appl. Surf. Sci. 2021, 569, 151006. [Google Scholar] [CrossRef]
- Chen, B.; He, X.; Yin, F.; Wang, H.; Liu, D.-J.; Shi, R.; Chen, J.; Yin, H. MO-Co@N-Doped Carbon (M = Zn or Co): Vital Roles of Inactive Zn and Highly Efficient Activity toward Oxygen Reduction/Evolution Reactions for Rechargeable Zn-Air Battery. Adv. Funct. Mater. 2017, 27, 1700795. [Google Scholar] [CrossRef]
- Mahmood, A.; Guo, W.; Tabassum, H.; Zou, R. Metal-Organic Framework-Based Nanomaterials for Electrocatalysis. Adv. Energy Mater. 2016, 6, 1600423. [Google Scholar] [CrossRef]
- Tang, J.; Hu, T.; Li, N.; Zhu, Y.; Li, J.; Zheng, S.; Guo, J. Ag doped Co/Ni bimetallic organic framework for determination of luteolin. Microchem. J. 2022, 179, 107461. [Google Scholar] [CrossRef]
- Wang, Z.; Yan, T.; Fang, J.; Shi, L.; Zhang, D. Nitrogen-doped porous carbon derived from a bimetallic metal–organic framework as highly efficient electrodes for flow-through deionization capacitors. J. Mater. Chem. A 2016, 4, 10858–10868. [Google Scholar] [CrossRef]
- Liu, L.; Liu, L.; Wang, Y.; Ye, B.C. A novel electrochemical sensor based on bimetallic metal-organic framework-derived porous carbon for detection of uric acid. Talanta 2019, 199, 478–484. [Google Scholar] [CrossRef]
- Guan, Y.; Li, N.; Li, Y.; Sun, L.; Gao, Y.; Zhang, Q.; He, C.; Liu, J.; Ren, X. Two dimensional ZIF-derived ultra-thin Cu-N/C nanosheets as high performance oxygen reduction electrocatalysts for high-performance Zn-air batteries. Nanoscale 2020, 12, 14259–14266. [Google Scholar] [CrossRef]
- Li, Y.; Deng, H.; Zhou, Z.; Yang, P.; Fei, J.; Xie, Y. Pd12Ag1 nanoalloy on dendritic CNFs catalyst for boosting formic acid oxidation. Appl. Surf. Sci. 2023, 608, 155131. [Google Scholar] [CrossRef]
- Song, Y.; Gu, F.; Wang, Z.; Han, D. ZnFe2O4 Nanoparticles on ZIF-8-Derived ZnO for Enhanced Acetone Sensing. ACS Appl. Nano Mater. 2022, 5, 15298–15309. [Google Scholar] [CrossRef]
- Cao, E.; Guo, Z.; Song, G.; Zhang, Y.; Hao, W.; Sun, L.; Nie, Z. MOF-derived ZnFe2O4/(Fe-ZnO) nanocomposites with enhanced acetone sensing performance. Sens. Actuators B Chem. 2020, 325, 128783. [Google Scholar] [CrossRef]
- Gao, F.; Yan, Z.; Cai, Y.; Yang, J.; Zhong, W.; Gao, Y.; Liu, S.; Li, M.; Lu, L. 2D leaf-like ZIF-L decorated with multi-walled carbon nanotubes as electrochemical sensing platform for sensitively detecting thiabendazole pesticide residues in fruit samples. Anal. Bioanal. Chem. 2021, 413, 7485–7494. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Zhang, H.; Zheng, K.; Zhang, Z.; Jiang, Q.; Li, J. Two-dimensional hydrophilic ZIF-L as a highly-selective adsorbent for rapid phosphate removal from wastewater. Sci. Total Env. 2021, 785, 147382. [Google Scholar] [CrossRef]
- Samuel, E.; Park, C.; Kim, T.; Joshi, B.; Aldalbahi, A.; Alanzi, H.S.; Swihart, M.T.; Yoon, W.Y.; Yoon, S.S. Dodecahedral ZnO/C framework on reduced graphene oxide sheets for high-performance Li-ion battery anodes. J. Alloy. Compd. 2020, 834, 155208. [Google Scholar] [CrossRef]
- Xing, G.Z.; Yi, J.B.; Tao, J.G.; Liu, T.; Wong, L.M.; Zhang, Z.; Li, G.P.; Wang, S.J.; Ding, J.; Sum, T.C.; et al. Comparative Study of Room-Temperature Ferromagnetism in Cu-Doped ZnO Nanowires Enhanced by Structural Inhomogeneity. Adv. Mater. 2008, 20, 3521–3527. [Google Scholar] [CrossRef]
- Dong, Y.; Chui, Y.-S.; Ma, R.; Cao, C.; Cheng, H.; Li, Y.Y.; Zapien, J.A. One-pot scalable synthesis of Cu–CuFe2O4/graphene composites as anode materials for lithium-ion batteries with enhanced lithium storage properties. J. Mater. Chem. A 2014, 2, 13783–13794. [Google Scholar] [CrossRef]
- Gong, J.; Yue, H.; Zhao, Y.; Zhao, S.; Zhao, L.; Lv, J.; Wang, S.; Ma, X. Synthesis of ethanol via syngas on Cu/SiO2 catalysts with balanced Cu0-Cu+ sites. J. Am. Chem. Soc. 2012, 134, 13922–13925. [Google Scholar] [CrossRef]
- Lu, Z.; Du, X.; Sun, M.; Zhang, Y.; Li, Y.; Wang, X.; Wang, Y.; Du, H.; Yin, H.; Rao, H. Novel dual-template molecular imprinted electrochemical sensor for simultaneous detection of CA and TPH based on peanut twin-like NiFe2O4/CoFe2O4/NCDs nanospheres: Fabrication, application and DFT theoretical study. Biosens. Bioelectron. 2021, 190, 113408. [Google Scholar] [CrossRef]
- Zhou, M.; Tang, T.; Qin, D.; Cheng, H.; Wang, X.; Chen, J.; Wågberg, T.; Hu, G. Hematite nanoparticle decorated MIL-100 for the highly selective and sensitive electrochemical detection of trace-level paraquat in milk and honey. Sens. Actuators B Chem. 2023, 376, 132931. [Google Scholar] [CrossRef]
- Liu, Z.; Xue, Q.; Guo, Y. Sensitive electrochemical detection of rutin and isoquercitrin based on SH-beta-cyclodextrin functionalized graphene-palladium nanoparticles. Biosens. Bioelectron. 2017, 89 Pt 1, 444–452. [Google Scholar] [CrossRef]
- Zhong, W.; Gao, F.; Zou, J.; Liu, S.; Li, M.; Gao, Y.; Yu, Y.; Wang, X.; Lu, L. MXene@Ag-based ratiometric electrochemical sensing strategy for effective detection of carbendazim in vegetable samples. Food Chem. 2021, 360, 130006. [Google Scholar] [CrossRef] [PubMed]
- Zamarchi, F.; Silva, T.R.; Winiarski, J.P.; Santana, E.R.; Vieira, I.C. Polyethylenimine-Based Electrochemical Sensor for the Determination of Caffeic Acid in Aromatic Herbs. Chemosensors 2022, 10, 357. [Google Scholar] [CrossRef]
- Shih, Y. Flow injection analysis of zinc pyrithione in hair care products on a cobalt phthalocyanine modified screen-printed carbon electrode. Talanta 2004, 62, 912–917. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tang, J.; Lin, Y.; Li, J.; Yang, Y.; Zhao, P.; Fei, J.; Xie, Y. Ultrasensitive Determination of Natural Flavonoid Rutin Using an Electrochemical Sensor Based on Metal-Organic Framework CAU-1/Acidified Carbon Nanotubes Composites. Molecules 2022, 27, 7761. [Google Scholar] [CrossRef] [PubMed]
- Ezhil Vilian, A.T.; Hwang, S.-K.; Bhaskaran, G.; Alhammadi, M.; Kim, S.; Tiwari, J.N.; Suk Huh, Y.; Han, Y.-K. Polypyrrole-MXene supported gold nanoparticles for the trace-level detection of nitrofurantoin. Chem. Eng. J. 2023, 454, 139980. [Google Scholar] [CrossRef]
- Huang, Q.; Lin, X.; Lin, C.; Zhang, Y.; Zhang, H.; Hu, S.; Wei, C.; Tong, Q.X. Ultrasensitive-electrochemical sensor for the detection of luteolin in Chrysanthemums and Peanut shells using an Au/Pd/reduced graphene oxide nanofilm. Anal. Methods 2016, 8, 6347–6352. [Google Scholar] [CrossRef]
- Li, X.; Zou, R.; Niu, Y.; Sun, W.; Shao, T.; Chen, X. Gold Nanocage-Based Electrochemical Sensing Platform for Sensitive Detection of Luteolin. Sensors 2018, 18, 2309. [Google Scholar] [CrossRef] [PubMed]
- Si, X.; Deng, L.; Wang, Y.; Han, M.; Ding, Y. An electrochemical sensor for the determination of Luteolin using an alizarin red/carboxylic acid group functionalized carbon nanotube. Microchem. J. 2022, 174, 106864. [Google Scholar] [CrossRef]
- Wang, Q.; Gu, C.; Fu, Y.; Liu, L.; Xie, Y. Ultrasensitive Electrochemical Sensor for Luteolin Based on Zirconium Metal-Organic Framework UiO-66/Reduced Graphene Oxide Composite Modified Glass Carbon Electrode. Molecules 2020, 25, 4557. [Google Scholar] [CrossRef]
- Cheng, W.; Zeng, P.; Ma, C.; Peng, H.; Yang, J.; Huang, J.; Zhang, M.; Cheng, F. Electrochemical sensor for sensitive detection of luteolin based on multi-walled carbon nanotubes/poly(3,4-ethylenedioxythiophene)–gold nanocomposites. New J. Chem. 2020, 44, 1953–1961. [Google Scholar] [CrossRef]
- Cheng, H.; Li, T.; Li, X.; Feng, J.; Tang, T.; Qin, D. Facile Synthesis of Co9S8 Nanocages as an Electrochemical Sensor for Luteolin Detection. J. Electrochem. Soc. 2021, 168, 087504. [Google Scholar] [CrossRef]
- Liao, Y.; Wang, N.; Ni, Y.; Xu, J.; Shao, S. Electrochemical sensor based on Nbim/CNT composite for selective determination of luteolin in the flavonoids. J. Electroanal. Chem. 2015, 754, 94–99. [Google Scholar] [CrossRef]
- Liu, A.; Zhang, S.; Huang, L.; Cao, Y.; Yao, H.; Chen, W.; Lin, X. Electrochemical Oxidation of Luteolin at a Glassy Carbon Electrode and Its Application in Pharmaceutical Analysis. Chem. Pharm. Bull. 2008, 56, 745–748. [Google Scholar] [CrossRef] [PubMed]


| Name | Start BE | Peak BE | End BE | FWHM eV | Atomic % |
|---|---|---|---|---|---|
| C1s | 297.98 | 284.68 | 279.18 | 1.64 | 86.28 |
| Cu2p | 964.98 | 932.98 | 925.18 | 2.57 | 0.36 |
| N1s | 409.98 | 399.14 | 392.18 | 4.02 | 5.01 |
| O1s | 544.98 | 532.33 | 525.18 | 3.57 | 7.51 |
| Zn2p | 1051.98 | 1021.91 | 1015.18 | 1.82 | 0.83 |
| Luteolin Sensors | Detection Measures | Linear Range (μM) | LOD (nM) | Reference |
|---|---|---|---|---|
| Au/Pd/rGO/GCE | DPV | 0.01–80 | 0.98 | [47] |
| AuNCs/CILE | DPV | 0.001–1 | 0.4 | [48] |
| AR/f-MWCNT/GCE | i-T | 0.5–45 | 170 | [49] |
| UiO-66/ErGO/GCE | DPV | 0.001–20 | 0.75 | [50] |
| MWCNTs/PEDT-Au/GCE | SWV | 0.1–15; 0.001–0.1 μM dm−3 | 0.22 μM dm−3 | [51] |
| ZrO2/CS/rGOA/GCE | DPV | 0.005–1 | 1 | [5] |
| Co9S8/GCE | DPV | 0.01–20 | 0.8 | [52] |
| Nbim/CNT/GCE | DPV | 0.005–0.32 | 0.6 | [53] |
| Bare GCE | DPV | 0.01–1 | 5 | [54] |
| CuZn@NC/GCE | DPV | 0.04–1 | 15 | This work |
| Add (nM) | Detected (nM) | Recovery (%) | RSD (%) |
|---|---|---|---|
| 0 | 60.0 | --- | --- |
| 30 | 88.9 | 96.3 | 0.32 |
| 60 | 116.8 | 94.7 | 0.46 |
| 90 | 146.3 | 95.9 | 0.28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Yang, Y.; Li, J.; Zhang, L.; Zhao, P.; Fei, J.; Xie, Y. Ultrasensitive Luteolin Electrochemical Sensor Based on Novel Lamellar CuZn@ Nitrogen-Containing Carbon Nanosheets. Nanomaterials 2023, 13, 171. https://doi.org/10.3390/nano13010171
Li Y, Yang Y, Li J, Zhang L, Zhao P, Fei J, Xie Y. Ultrasensitive Luteolin Electrochemical Sensor Based on Novel Lamellar CuZn@ Nitrogen-Containing Carbon Nanosheets. Nanomaterials. 2023; 13(1):171. https://doi.org/10.3390/nano13010171
Chicago/Turabian StyleLi, Yuhong, Yaqi Yang, Jiejun Li, Li Zhang, Pengcheng Zhao, Junjie Fei, and Yixi Xie. 2023. "Ultrasensitive Luteolin Electrochemical Sensor Based on Novel Lamellar CuZn@ Nitrogen-Containing Carbon Nanosheets" Nanomaterials 13, no. 1: 171. https://doi.org/10.3390/nano13010171
APA StyleLi, Y., Yang, Y., Li, J., Zhang, L., Zhao, P., Fei, J., & Xie, Y. (2023). Ultrasensitive Luteolin Electrochemical Sensor Based on Novel Lamellar CuZn@ Nitrogen-Containing Carbon Nanosheets. Nanomaterials, 13(1), 171. https://doi.org/10.3390/nano13010171







