Activity of Sodium Trimetaphosphate Nanoparticles on Cariogenic-Related Biofilms In Vitro
Abstract
1. Introduction
2. Results
2.1. ECM Composition
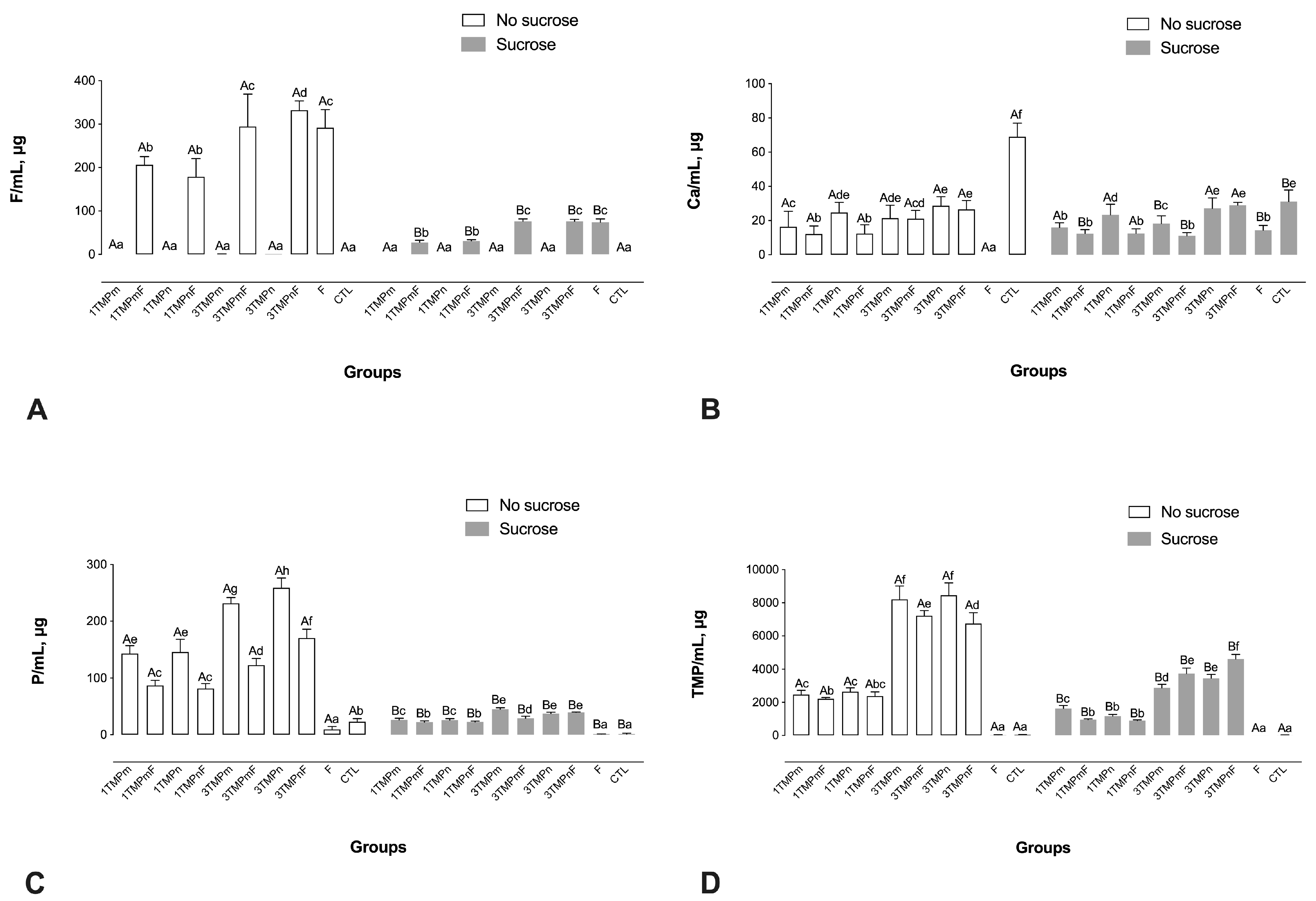
2.2. F, Ca, P, and TMP Concentration in Biofilm Fluids
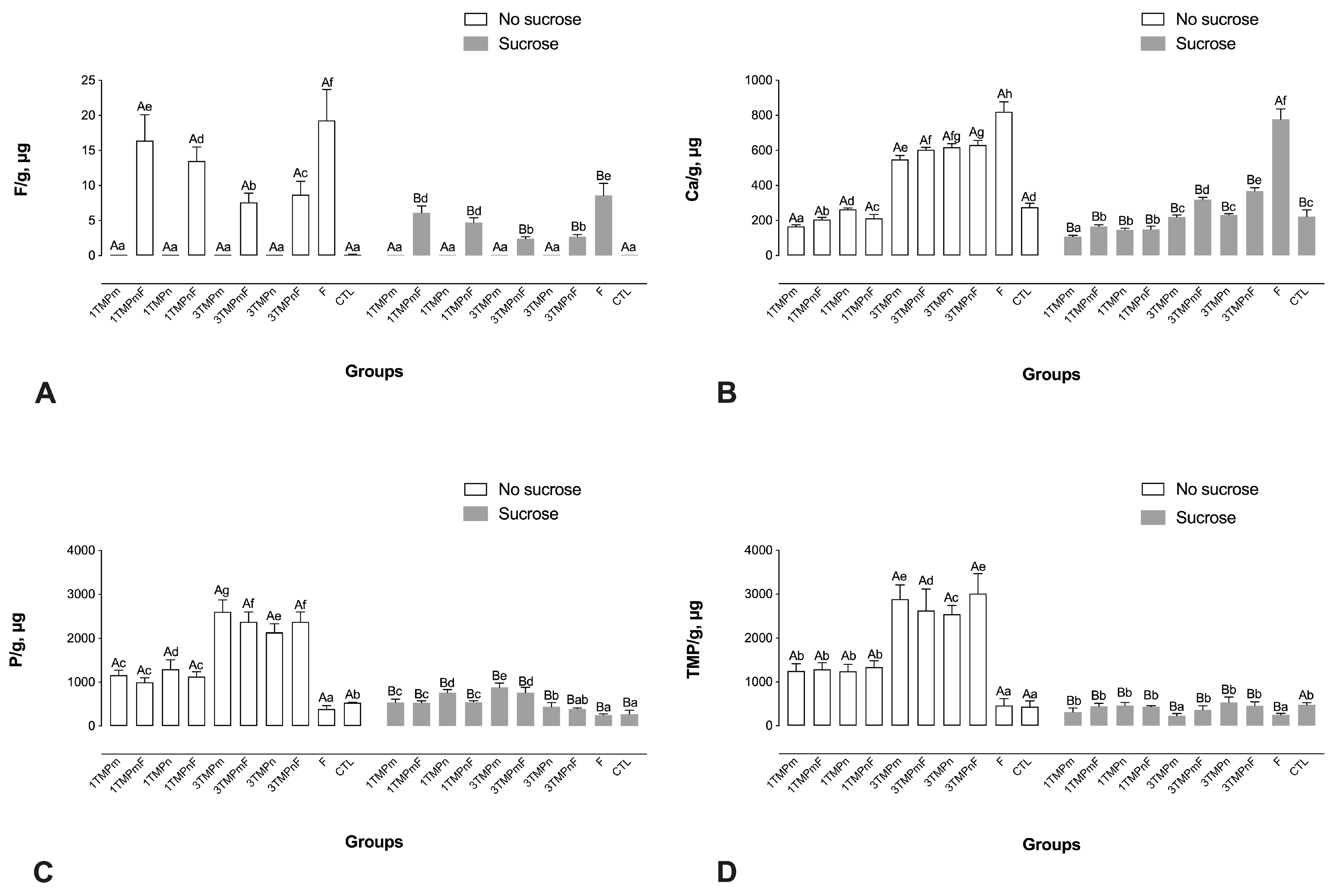
2.3. F, Ca, P, and TMP Concentration in Biofilm Biomass
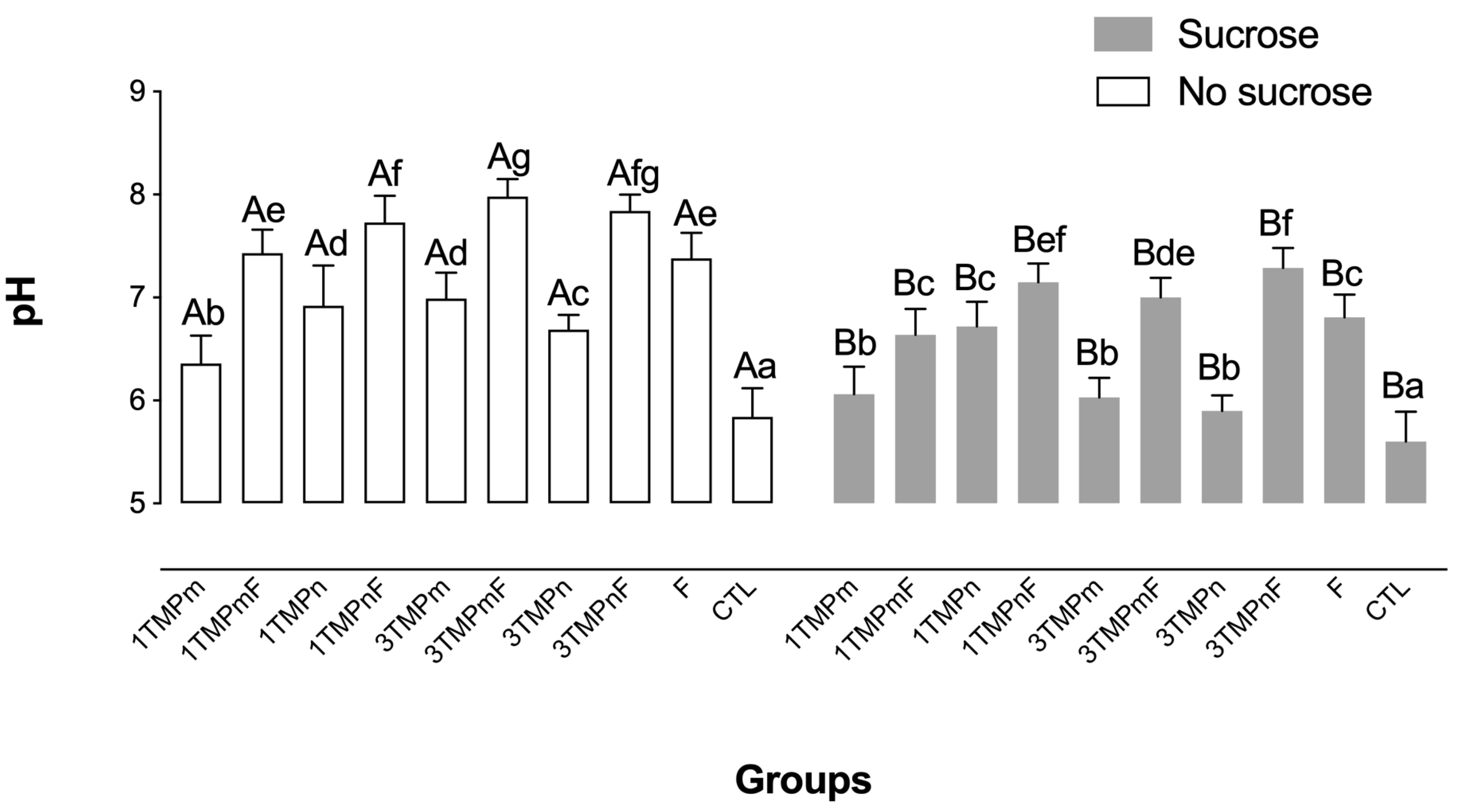
2.4. pH Measurement
3. Discussion
4. Materials and Methods
4.1. Details on the Processing and Characterization of the TMP Nanoparticles
4.2. Microorganisms and Growth Conditions
4.3. Treatment of the Biofilms
4.4. Analysis of the Composition of the Extracellular Matrix of Biofilms
4.5. pH Measurement
4.6. Analysis of F, Ca, P and TMP in the Biofilm Fluid
4.7. Analysis of F, Ca, P and TMP in the Biofilm Biomass
4.8. Statistical Analyzes
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Machiulskiene, V.; Campus, G.; Carvalho, J.C.; Dige, I.; Ekstrand, K.R.; Jablonski-Momeni, A.; Maltz, M.; Manton, D.J. Terminology of Dental Caries and Dental Caries Management: Consensus Report of a Workshop Organized by ORCA and Cariology Research Group of IADR. Caries Res. 2020, 54, 7–14. [Google Scholar] [CrossRef]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial biofilms: A common cause of persistent infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.C.; van Hullebusch, E.D.; Neu, T.R.; Nielsen, P.H.; Seviour, T.; Stoodley, P.; Wingender, J.; Wuertz, S. The biofilm matrix: Multitasking in a shared space. Nat. Rev. Microbiol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Zhou, X.; Li, Y. Strategies for Streptococcus mutans biofilm dispersal through extracellular polymeric substances disruption. Mol. Oral Microbiol. 2022, 37, 1–8. [Google Scholar] [CrossRef]
- Tanaka, M.; Margolis, H.C. Release mineral ions in dental plaque following acid production. Arch. Oral Biol. 1999, 44, 253–258. [Google Scholar] [CrossRef]
- ten Cate, J.M.; Buzalaf, M.A.R. Fluoride Mode of Action: Once There Was an Observant Dentist. J. Dent. Res. 2019, 98, 725–730. [Google Scholar] [CrossRef]
- Shaw, L.; Murray, J.J.; Burchell, K.; Best, J.S. Calcium and phosphorus content of plaque and saliva in relation to dental caries. Caries Res. 1983, 17, 543–548. [Google Scholar] [CrossRef]
- Lemos, J.A.; Palmer, S.R.; Zeng, L.; Wen, Z.T.; Kajfasz, J.K.; Freires, I.A.; Abranches, J.; Brady, L.J. The Biology of Streptococcus mutans. Microbiol. Spectr. 2019, 7. [Google Scholar] [CrossRef]
- Kirthiga, M.; Murugan, M.; Saikia, A.; Kirubakaran, R. Risk Factors for Early Childhood Caries: A Systematic Review and Meta-Analysis of Case Control and Cohort Studies. Pediatr. Dent. 2019, 15, 95–112. [Google Scholar]
- Kim, H.E.; Liu, Y.; Dhall, A.; Bawazir, M.; Koo, H.; Hwang, G. Synergism of Streptococcus mutans and Candida albicans Reinforces Biofilm Maturation and Acidogenicity in Saliva: An In Vitro Study. Front. Cell. Infect. Microbiol. 2021, 10, 623980. [Google Scholar] [CrossRef] [PubMed]
- Bachtiar, E.W.; Bachtiar, B.M. Relationship between Candida albicans and Streptococcus mutans in early childhood caries, evaluated by quantitative PCR. F1000Research 2018, 16, 1645. [Google Scholar] [CrossRef] [PubMed]
- Kassebaum, N.J.; Bernabé, E.; Dahiya, M.; Bhandari, B.; Murray, C.J.; Marcenes, W. Global burden of untreated caries: A systematic review and metaregression. J. Dent. Res. 2015, 94, 650–658. [Google Scholar] [CrossRef] [PubMed]
- Danelon, M.; Pessan, J.P.; Neto, F.N.; de Camargo, E.R.; Delbem, A.C. Effect of toothpaste with nano-sized trimetaphosphate on dental caries: In situ study. J. Dent. 2015, 43, 806–813. [Google Scholar] [CrossRef]
- Takeshita, E.M.; Danelon, M.; Castro, L.P.; Sassaki, K.T.; Delbem, A.C. Effectiveness of a Toothpaste with Low Fluoride Content Combined with Trimetaphosphate on Dental Biofilm and Enamel Demineralization in situ. Caries Res. 2015, 49, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Cavazana, T.P.; Pessan, J.P.; Hosida, T.Y.; Sampaio, C.; Amarante, V.O.Z.; Monteiro, D.R.; Delbem, A.C.B. Effects of Sodium Trimetaphosphate, Associated or Not with Fluoride, on the Composition and pH of Mixed Biofilms, before and after Exposure to Sucrose. Caries Res. 2020, 54, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Cavazana, T.P.; Hosida, T.Y.; Pessan, J.P.; Sampaio, C.; Monteiro, D.R.; Delbem, A.C.B. Activity of sodium trimetaphosphate, associated or not with fluoride, on dual-species biofilms. Biofouling 2019, 35, 710–718. [Google Scholar] [CrossRef] [PubMed]
- Emerenciano, N.G.; Botazzo Delbem, A.C.; Pessan, J.P.; Nunes, G.P.; Souza Neto, F.N.; de Camargo, E.R.; Danelon, M. In situ effect of fluoride toothpaste supplemented with nano-sized sodium trimetaphosphate on enamel demineralization prevention and biofilm composition. Arch. Oral Biol. 2018, 96, 223–229. [Google Scholar] [CrossRef]
- Xiao, J.; Huang, X.; Alkhers, N.; Alzamil, H.; Alzoubi, S.; Wu, T.T.; Castillo, D.A.; Campbell, F.; Davis, J.; Herzog, K.; et al. Candida albicans and Early Childhood Caries: A Systematic Review and Meta-Analysis. Caries Res. 2018, 52, 102–112. [Google Scholar] [CrossRef]
- Bowen, W.H.; Burne, R.A.; Wu, H.; Koo, H. Oral Biofilms: Pathogens, Matrix, and Polymicrobial Interactions in Microenvironments. Trends Microbiol. 2018, 26, 229–242. [Google Scholar] [CrossRef]
- Khoury, Z.H.; Vila, T.; Puthran, T.R.; Sultan, A.S.; Montelongo-Jauregui, D.; Melo, M.A.S.; Jabra-Rizk, M.A. The Role of Candida albicans Secreted Polysaccharides in Augmenting Streptococcus mutans Adherence and Mixed Biofilm Formation: In vitro and in vivo Studies. Front. Microbiol. 2020, 11, 307. [Google Scholar] [CrossRef] [PubMed]
- Jandt, K.D.; Watts, D.C. Nanotechnology in dentistry: Present and future perspectives on dental nanomaterials. Dent. Mater. 2020, 36, 1365–1378. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.H.; Weir, M.D.; Sun, L.; Moreau, J.L.; Takagi, S.; Chow, L.C.; Antonucci, J.M. Strong nanocomposites with Ca, PO(4) and F release for caries inhibition. J. Dent. Res. 2010, 89, 19–28. [Google Scholar] [CrossRef]
- Sampaio, C.; Botazzo Delbem, A.C.; Hosida, T.Y.; de Morais, L.A.; Fernandes, A.V.P.; Souza Neto, F.N.; de Camargo, E.R.; Monteiro, D.R.; Pessan, J.P. Effects of nano-sized sodium hexametaphosphate on the viability, metabolism, matrix composition, and structure of dual-species biofilms of Streptococcus mutans and Candida albicans. Biofouling 2022, 38, 321–330. [Google Scholar] [CrossRef]
- Luo, W.; Huang, Y.; Zhou, X.; Han, Q.; Peng, X.; Ren, B.; Li, J.; Li, M.; Cheng, L. The effect of disaggregated nano-hydroxyapatite on oral biofilm in vitro. Dent. Mater. 2020, 36, e207–e216. [Google Scholar] [CrossRef]
- Boyd, R.F. The effect of some divalent cations on extracellular polysaccharide synthesis in Streptococcus salivarius. J. Dent. Res. 1978, 57, 380–383. [Google Scholar] [CrossRef] [PubMed]
- Hosida, T.Y.; Pessan, J.P.; Cavazana, T.P.; Sampaio, C.; de Morais, L.A.; Monteiro, D.R.; Delbem, A.C.B. Effects of Sodium Hexametaphosphate and Fluoride on the pH and Inorganic Components of Streptococcus mutans and Candida albicans Biofilm after Sucrose Exposure. Antibiotics 2022, 11, 1044. [Google Scholar] [CrossRef] [PubMed]
- Lanigan, R.S. Final report on the safety assessment of sodium metaphosphate, sodium trimetaphosphate, and sodium hexametaphosphate. Int. J. Toxicol. 2001, 20, 75–89. [Google Scholar]
- Sampaio, C.; Delbem, A.C.B.; Hosida, T.Y.; Fernandes, A.V.P.; Alves, G.D.S.G.; Souza, J.A.S.; Monteiro, D.R.; Pessan, J.P. Buffering Capacity and Effects of Sodium Hexametaphosphate Nanoparticles and Fluoride on the Inorganic Components of Cariogenic-Related Biofilms In Vitro. Antibiotics 2022, 30, 1173. [Google Scholar] [CrossRef]
- Cury, J.A.; Rebello, M.A.; Del Bel Cury, A.A. In situ relationship between sucrose exposure and the composition of dental plaque. Caries Res. 1997, 31, 356–360. [Google Scholar] [CrossRef]
- Bayrak, S.; Okte, Z.; Fidanci, U.R. Relationship between caries and dental plaque composition. Am. J. Dent. 2011, 24, 45–48. [Google Scholar] [PubMed]
- Tenuta, L.M.; Del Bel Cury, A.A.; Bortolin, M.C.; Vogel, G.L.; Cury, J.A. Ca, Pi, and F in the fluid of biofilm formed under sucrose. J. Dent. Res. 2006, 85, 834–838. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.M.; Hartman, P.A.; Stahr, H.M.; Olson, D.G.; Williams, F.D. Antibacterial Mechanism of Long-Chain Polyphosphates in Staphylococcus aureus. J. Food Prot. 1994, 57, 289–294. [Google Scholar] [CrossRef]
- Domon-Tawaraya, H.; Nakajo, K.; Washio, J.; Ashizawa, T.; Ichino, T.; Sugawara, H.; Fukumoto, S.; Takahashi, N. Divalent cations enhance fluoride binding to Streptococcus mutans and Streptococcus sanguinis cells and subsequently inhibit bacterial acid production. Caries Res. 2013, 47, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Rose, R.K.; Shellis, R.P.; Lee, A.R. The role of cation bridging in microbial fluoride binding. Caries Res. 1996, 30, 458–464. [Google Scholar] [CrossRef]
- Cochrane, N.J.; Saranathan, S.; Cai, F.; Cross, K.J.; Reynolds, E.C. Enamel subsurface lesion remineralisation with casein phosphopeptide stabilised solutions of calcium, phosphate and fluoride. Caries Res. 2008, 42, 88–97. [Google Scholar] [CrossRef]
- Pessan, J.P.; Alves, K.M.; Ramires, I.; Taga, M.F.; Sampaio, F.C.; Whitford, G.M.; Buzalaf, M.A. Effects of regular and low-fluoride dentifrices on plaque fluoride. J. Dent. Res. 2010, 89, 1106–1110. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.; Wu, C.; Chen, X.; Duan, Z.; Xu, Q.; Jiang, W.; Xu, L.; Wang, T.; Su, L.; et al. Oral Microbiome Alterations Associated with Early Childhood Caries Highlight the Importance of Carbohydrate Metabolic Activities. mSystems 2019, 4, e00450-19. [Google Scholar] [CrossRef]
- Ten Cate, J.M.; Dundon, K.A.; Vernon, P.G.; Damato, F.A.; Huntington, E.; Exterkate, R.A.; Wefel, J.S.; Jordan, T.; Stephen, K.W.; Roberts, A.J. Preparation and measurement of artificial enamel lesion, a four-laboratory ring test. Caries Res. 1996, 30, 400–407. [Google Scholar] [CrossRef]
- Cunha-Cruz, J.; Scott, J.; Rothen, M.; Mancl, L.; Lawhorn, T.; Brossel, K.; Berg, J.; Northwest Practice-based Rsearch Collaborative in Evidence-based DENTistry. Salivary characteristics and dental caries: Evidence from general dental practices. J. Am. Dent. Assoc. 2013, 144, e31–e40. [Google Scholar] [CrossRef]
- Silva, S.; Henriques, M.; Martins, A.; Oliveira, R.; Williams, D.; Azeredo, J. Biofilms of non-Candida albicans Candida species: Quantification, structure and matrix composition. Med. Mycol. 2009, 47, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Vogel, G.L.; Chow, L.C.; Brown, W.E. A microanalytical procedure for the determination of calcium, phosphate and fluoride in enamel biopsy samples. Caries Res. 1983, 17, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Fiske, C.H.; Subbarow, Y. The colorimetric determination of phosphorus. J. Biol. Chem. 1925, 66, 375–400. [Google Scholar] [CrossRef]
- Cury, J.A.; Rebelo, M.A.; Del Bel Cury, A.A.; Derbyshire, M.T.; Tabchoury, C.P. Biochemical composition and cariogenicity of dental plaque formed in the presence of sucrose or glucose and fructose. Caries Res. 2000, 34, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Nobre dos Santos, M.; Melo dos Santos, L.; Francisco, S.B.; Cury, J.A. Relationship among dental plaque composition, daily sugar exposure and caries in the primary dentition. Caries Res. 2002, 36, 347–352. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amarante, V.d.O.Z.; Delbem, A.C.B.; Sampaio, C.; de Morais, L.A.; de Camargo, E.R.; Monteiro, D.R.; Pessan, J.P.; Hosida, T.Y. Activity of Sodium Trimetaphosphate Nanoparticles on Cariogenic-Related Biofilms In Vitro. Nanomaterials 2023, 13, 170. https://doi.org/10.3390/nano13010170
Amarante VdOZ, Delbem ACB, Sampaio C, de Morais LA, de Camargo ER, Monteiro DR, Pessan JP, Hosida TY. Activity of Sodium Trimetaphosphate Nanoparticles on Cariogenic-Related Biofilms In Vitro. Nanomaterials. 2023; 13(1):170. https://doi.org/10.3390/nano13010170
Chicago/Turabian StyleAmarante, Viviane de Oliveira Zequini, Alberto Carlos Botazzo Delbem, Caio Sampaio, Leonardo Antônio de Morais, Emerson Rodrigues de Camargo, Douglas Roberto Monteiro, Juliano Pelim Pessan, and Thayse Yumi Hosida. 2023. "Activity of Sodium Trimetaphosphate Nanoparticles on Cariogenic-Related Biofilms In Vitro" Nanomaterials 13, no. 1: 170. https://doi.org/10.3390/nano13010170
APA StyleAmarante, V. d. O. Z., Delbem, A. C. B., Sampaio, C., de Morais, L. A., de Camargo, E. R., Monteiro, D. R., Pessan, J. P., & Hosida, T. Y. (2023). Activity of Sodium Trimetaphosphate Nanoparticles on Cariogenic-Related Biofilms In Vitro. Nanomaterials, 13(1), 170. https://doi.org/10.3390/nano13010170





