CdS-Modified TiO2 Nanotubes with Heterojunction Structure: A Photoelectrochemical Sensor for Glutathione
Abstract
:1. Introduction
2. Experiment
2.1. Synthesis of CdS-TiO2NTs
2.2. Preparation of CdS-TiO2NTs Electrode
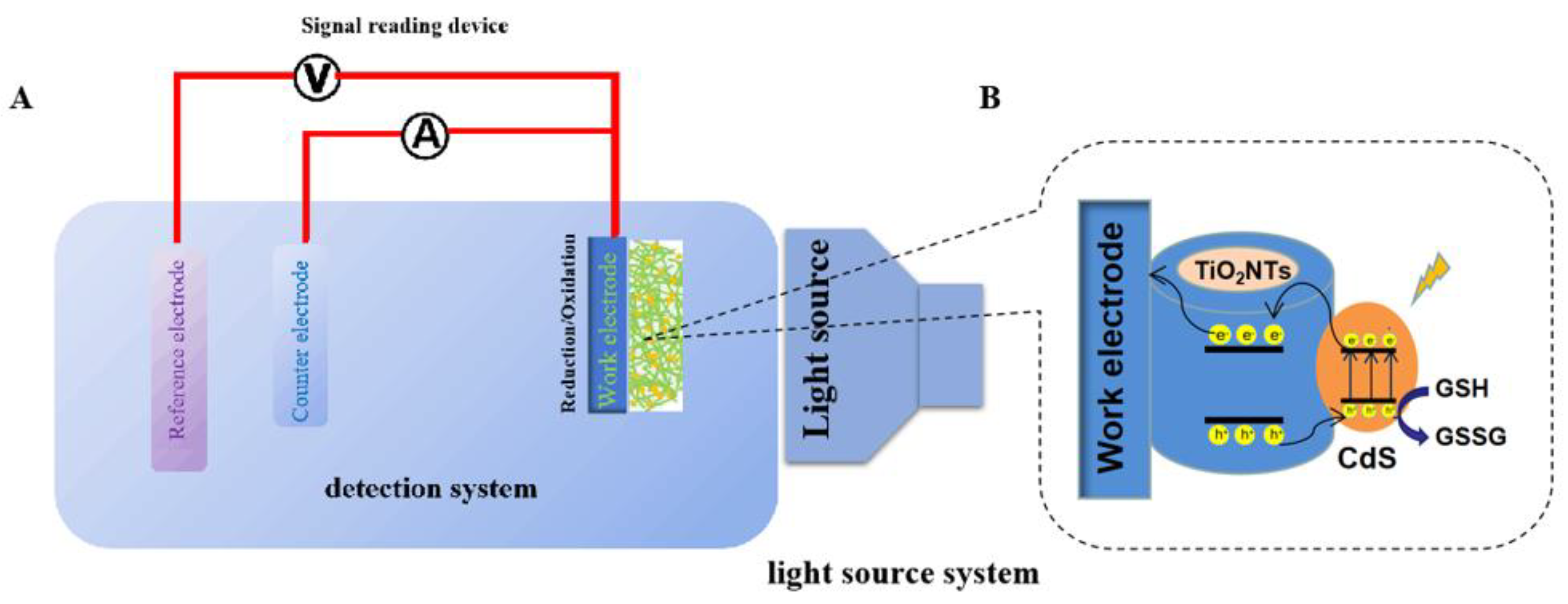
2.3. Photoelectrochemical Testing
2.4. Instrument Model
3. Results and Discussion
3.1. Surface Morphology Characterization
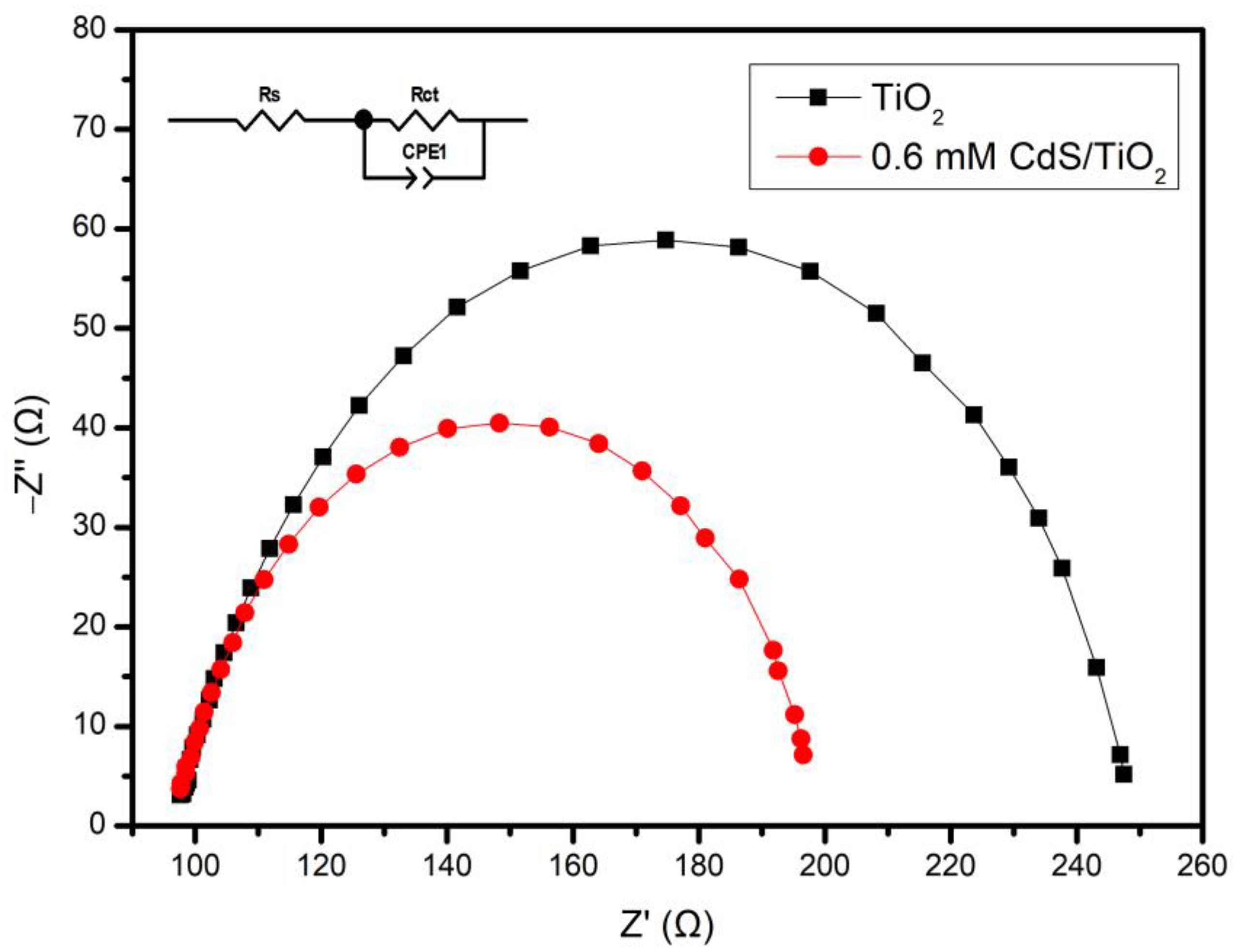
3.2. Electrochemical Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Z.; Zhang, J.; Li, Y.; Zhao, S.; Zhang, P.; Zhang, Y.; Bi, J.; Liu, G.; Yue, Z. Carbon dots based photoelectrochemical sensors for ultrasensitive detection of glutathione and its applications in probing of myocardial infarction. Biosens. Bioelectron. 2018, 99, 251–258. [Google Scholar] [CrossRef]
- Sui, M.R.; Zhao, Y.; Ni, Z.H.; Gu, X.Q. Photoelectrochemical performance and biosensor application for glutathione (GSH) of W-doped BiVO4 thin films. J. Mater. Sci. Mater. Electron. 2018, 12, 10109–10116. [Google Scholar] [CrossRef]
- Shu, J.; Qiu, Z.L.; Lv, S.Z.; Zhang, K.Y.; Tang, D.P. Plasmonic enhancement coupling with defect-engineered TiO2-x: A mode for sensitive photoelectrochemical biosensing. Anal. Chem. 2018, 4, 2425–2429. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Tu, L.; Zhao, S.; Liu, G.; Wang, Y.; Wang, Y.; Yue, Z. Fluorescent gold nanoclusters based photoelectrochemical sensors for detection of H2O2 and glucose. Biosens. Bioelectron. 2015, 67, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Tu, L.; Liu, G.; Zhang, W.; Qin, J.; Yue, Z. Doped QDs based photoelectrochemical sensors for detection of H2O2 and Glucose. IEEE J. Sel. Top. Quantum. 2014, 20, 175–183. [Google Scholar]
- Wu, S.; Song, H.; Song, J.; He, C.; Ni, J.; Zhao, Y.; Wang, X. Development of triphenylamine functional dye for selective photoelectrochemical sensing of cysteine. Anal. Chem. 2014, 86, 5922–5928. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, X.C.; Yan, J.Y.; Guan, Q.L.; Xing, Y.H.; Song, W.B. Split-type cascaded silver etching for “on-super off” PEC-EC dual-mode immunosensing of α-SynO. Sensor. Actuat. B-Chem. 2022, 358, 131449. [Google Scholar] [CrossRef]
- Tao, J.; Gong, Z.; Yao, G.; Cheng, Y.; Zhang, M.; Lv, J.; Shi, S.; He, G.; Chen, X.; Sun, Z. Enhanced photocatalytic and photoelectrochemical properties of TiO2 nanorod arrays sensitized with CdS nanoplates. Ceram. Int. 2016, 42, 11716–11723. [Google Scholar] [CrossRef]
- Okoth, O.K.; Yan, K.; Feng, J.; Zhang, J. Label-free photoelectrochemical aptasensing of diclofenac based on gold nanoparticles and graphene-doped CdS. Sens. Actuators B 2018, 256, 334–341. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, L.; Liu, X.; Li, X.A.; Huang, W. High-performance CdS–ZnS core–shell nanorod array photoelectrode for photoelectrochemical hydrogen generation. J. Mater. Chem. A 2015, 3, 535–541. [Google Scholar] [CrossRef]
- Wang, T.; Jin, B.; Jiao, Z.; Lu, G.; Ye, J.; Bi, Y. Electricfield-directed growth and photoelectro chemical properties of cross-linked Au-ZnO hetero-nanowire arrays. Chem. Commun. 2015, 51, 2103–2106. [Google Scholar] [CrossRef] [PubMed]
- Lu, N.; Shao, C.L.; Li, X.H.; Shen, T.; Zhang, M.Y.; Miao, F.J.; Zhang, P.; Zhang, X.; Wang, K.X.; Zhang, Y.; et al. CuO/Cu2O nanofibers as electrode materials for non-enzymatic glucose sensors with improved sensitivity. RSC Adv. 2014, 4, 31056–31061. [Google Scholar] [CrossRef]
- Tu, W.; Dong, Y.; Lei, J.; Ju, H. Low-potential photoelectrochemical biosensing using porphyrin-functionalized TiO2 nanoparticles. Anal. Chem. 2010, 82, 8711–8716. [Google Scholar] [CrossRef] [PubMed]
- Simonetta, P.; Laura, M.; Michele, M.; Annalisa, V. Trend in using TiO2 nanotubes as photoelectrodes in PEC processes for wastewater treatment. Curr. Opin. Electrochem. 2021, 28, 100699. [Google Scholar]
- Zhu, J.; Huo, X.; Liu, X.; Ju, H. Gold nanoparticles deposited polyaniline-TiO2 nanotube for surface plasmon resonance enhanced photoelectro chemical biosensing. ACS Appl. Mater. Interfaces 2016, 8, 341–349. [Google Scholar] [CrossRef]
- Atchudan, R.; Muthuchamy, N.; Edison, T.N.J.I.; Perumal, S.; Vinodh, R.; Park, K.H.; Lee, Y.R. An ultrasensitive photoelectrochemical biosensor for glucose based on bio-derived nitrogen-doped carbon sheets wrapped titanium dioxide nanoparticles. Biosens. Bioelectron. 2019, 126, 160–169. [Google Scholar] [CrossRef]
- Singh, S.C.; Swarnkar, R.K.; Gopal, R. Synthesis of Titanium Dioxide Nanomaterial by Pulsed Laser Ablation in Water. J. Nanosci. Nanotechnol. 2009, 9, 5367–5371. [Google Scholar] [CrossRef]
- Huo, G.N.; Ma, L.L.; Liu, X.T.; Zhou, K.H.; Suo, Z.C.; Liu, X.T.; Suo, Z.C.; Zhang, F.F.; Zhu, B.L.; Zhang, S.M.; et al. Fabrication and Photoelectrochemical Sensitivity of N, F-TiO2NTs/Ti with 3D structure. Microchem. J. 2022, 172, 106957. [Google Scholar] [CrossRef]
- Tang, J.; Kong, B.; Wang, Y.; Xu, M.; Wang, Y.; Wu, H.; Zheng, G. Photoelectrochemical detection of glutathione by IrO2-hemin-TiO2 nanowire arrays. Nano Lett. 2013, 13, 5350–5354. [Google Scholar] [CrossRef]
- Guo, C.Y.; Huo, H.H.; Han, X.; Xu, C.L.; Li, H.L. Ni/CdS Bifunctional Ti@TiO2 Core−Shell Nanowire Electrode for High-Performance Nonenzymatic Glucose Sensing. Anal. Chem. 2014, 86, 876–883. [Google Scholar] [CrossRef]
- Ruan, Y.F.; Zhang, N.; Zhu, Y.C.; Zhao, W.W.; Xu, J.J.; Chen, H.Y. Photoelectrochemical bioanalysis platform of gold nanoparticles equipped perovskite Bi4NbO8Cl. Anal. Chem. 2017, 89, 7869–7875. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Li, K.; Feng, Y.; Zhang, S.; Wu, S.; Huang, W. Synthesis and catalytic performance of gold-loaded TiO2 nanofibers. Catal. Lett. 2007, 118, 55–58. [Google Scholar] [CrossRef]
- Tian, J.; Zhao, P.; Zhang, S.S.; Huo, G.N.; Suo, Z.C.; Yue, Z.; Zhang, S.M.; Huang, W.P.; Zhu, B.L. Platinum and Iridium Oxide Co-modified TiO2 Nanotubes Array Based Photoelectrochemical Sensors for Glutathione. Nanomaterials 2020, 10, 522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, P.; Jin, S.Y.; Xu, Q.Z.; Yu, S.H. Decorating Pt Co bimetallic alloy nanoparticles on graphene as sensors for glucose detection by catalyzing luminol chemiluminescence. Small 2013, 9, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Wang, Y.; Su, L.; Zhang, H.; Lei, Y. Preparation and characterization of NiO–Ag nanofibers, NiO nanofibers, and porous Ag: Towards the development of a highly sensitive and selective non-enzymatic glucose sensor. J. Mater. Chem. 2010, 20, 9918–9926. [Google Scholar] [CrossRef]
- Bergamonti, L.; Predieri, G.; Paz, Y.; Fornasini, L.; Lottici, P.P.; Bondioli, F. Enhanced self-cleaning properties of N-doped TiO2 coating for Cultural Heritage. Microchem. J. 2017, 133, 1–12. [Google Scholar] [CrossRef]
- Shetti, N.P.; Malode, S.J.; Nayak, D.S.; Aminabhavi, T.M.; Reddy, K.R. Nanostructured silver doped TiO2/CNTs hybrid as an efficient electrochemical sensor for detection of anti-inflammatory drug, cetirizine. Microchem. J. 2019, 150, 104–124. [Google Scholar] [CrossRef]
- Zhu, Y.H.; Xu, Z.W.; Yan, K.; Zhao, H.B.; Zhang, J.D. One-Step Synthesis of CuO-Cu2O Heterojunction by Flame Spray Pyrolysis for Cathodic Photoelectrochemical Sensing of l-Cysteine. ACS Appl. Mater. Interfaces 2017, 9, 40452–40460. [Google Scholar] [CrossRef]
- Li, Y.Y.; Wang, J.H.; Luo, Z.J.; Chen, K.; Cheng, Z.Q.; Ma, L.; Ding, S.J.; Zhou, L.; Wang, Q.Q. Plasmon-Enhanced Photoelectrochemical Current and Hydrogen Production of (MoS2-TiO2)/Au Hybrids. Sci. Rep. 2015, 7, 7178. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.B.; Fei, X.G.; Zhang, L.Y.; Yu, J.G.; Cheng, B.; Ma, Y.H. Solar fuel generation over nature-inspired recyclable TiO2/g-C3N4 S-scheme hierarchical thin-film photocatalyst. J. Mater. Sci. Technol. 2022, 112, 1–10. [Google Scholar] [CrossRef]
- Yao, P.; Yu, S.; Shen, H.; Yang, J.; Min, L.; Yang, Z.; Zhu, X. A TiO2-SnS2 nanocomposite as a novel matrixfor the development of an enzymatic electrochemical glucose biosensor. New J. Chem. 2019, 43, 16748–16752. [Google Scholar] [CrossRef]
- Li, H.; Zhu, B.; Feng, Y.; Wang, S.; Zhang, S.; Huang, W. Synthesis, characterization of TiO2 nanotubes-supported MS (TiO2NTs@MS, M = Cd, Zn) and their photocatalytic activity. J. Solid State Chem. 2007, 180, 2136–2142. [Google Scholar] [CrossRef]
- Low, J.X.; Yu, J.G.; Jaroniec, M.; Wageh, S.; Al-Ghamdi, A.A. Heterojunction photocatalysts. Adv. Mater. 2017, 29, 1601694. [Google Scholar] [CrossRef] [PubMed]
- Huo, H.H.; Xu, Z.D.; Zhang, T.; Xu, C.L. Ni/CdS/TiO2 nanotube array heterostructures for high performance photoelectrochemical biosensing. J. Mater. Chem. A 2015, 3, 5882–5888. [Google Scholar] [CrossRef]
- Cai, J.; Sheng, P.T.; Zhou, L.P.; Shi, L.; Wang, N.Y.; Cai, Q.Y. Label-free photoelectrochemical immunosensor based on CdTe/CdS co-sensitized TiO2 nanotube array structure for octachlorostyrene detection. Biosens. Bioelectron. 2013, 50, 66–71. [Google Scholar] [CrossRef]
- Fan, D.W.; Wu, D.; Cui, J.L.; Chen, Y.C.; Ma, H.M.; Liu, Y.X.; Wei, Q.; Du, B. An ultrasensitive label-free immunosensor based on CdS sensitized Fe-TiO2 with high visible-light photoelectrochemical activity. Biosens. Bioelectron. 2015, 74, 843–848. [Google Scholar] [CrossRef]
- An, H.Q.; Zhu, B.L.; Li, J.X.; Zhou, J.; Wang, S.R.; Zhang, S.M.; Wu, S.H.; Huang, W.P. Synthesis and Characterization of Thermally Stable Nanotubular TiO2 and Its Photocatalytic Activity. J. Phys. Chem. C 2008, 112, 18772–18775. [Google Scholar] [CrossRef]
- Alberto, A.M.; Teresa, M.S.; Liliane, G.D.; Maria, F.M. Application of the Mott-Schottky model to select potentials for EIS studies on electrodes for electrochemical charge storage. Electrochim. Acta 2018, 289, 47–55. [Google Scholar]
- Ma, Q.L.; Wang, H.T.; Zhang, H.X.; Cheng, X.W.; Xie, M.Z.; Cheng, Q.F. Fabrication of MnO2/TiO2 nano-tube arrays photoelectrode and its enhanced visible light photoelectrocatalytic performance and mechanism. Sep. Purif. Technol. 2017, 189, 193–203. [Google Scholar] [CrossRef]
- Zhang, S.S.; Tian, J.; Yue, Z.; Huo, G.N.; Hu, Z.X.; Zhang, S.M.; Huang, W.P.; Zhu, B.L. Heterostructure-based 3D-CdS/TiO2 nanotubes/Ti: Photoelectrochemical performances and interface simulation investigation. Ceram. Int. 2022, 48, 36731–36738. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huo, G.-N.; Zhang, S.-S.; Li, Y.-L.; Li, J.-X.; Yue, Z.; Huang, W.-P.; Zhang, S.-M.; Zhu, B.-L. CdS-Modified TiO2 Nanotubes with Heterojunction Structure: A Photoelectrochemical Sensor for Glutathione. Nanomaterials 2023, 13, 13. https://doi.org/10.3390/nano13010013
Huo G-N, Zhang S-S, Li Y-L, Li J-X, Yue Z, Huang W-P, Zhang S-M, Zhu B-L. CdS-Modified TiO2 Nanotubes with Heterojunction Structure: A Photoelectrochemical Sensor for Glutathione. Nanomaterials. 2023; 13(1):13. https://doi.org/10.3390/nano13010013
Chicago/Turabian StyleHuo, Guo-Na, Sha-Sha Zhang, Yue-Liu Li, Jia-Xing Li, Zhao Yue, Wei-Ping Huang, Shou-Min Zhang, and Bao-Lin Zhu. 2023. "CdS-Modified TiO2 Nanotubes with Heterojunction Structure: A Photoelectrochemical Sensor for Glutathione" Nanomaterials 13, no. 1: 13. https://doi.org/10.3390/nano13010013
APA StyleHuo, G.-N., Zhang, S.-S., Li, Y.-L., Li, J.-X., Yue, Z., Huang, W.-P., Zhang, S.-M., & Zhu, B.-L. (2023). CdS-Modified TiO2 Nanotubes with Heterojunction Structure: A Photoelectrochemical Sensor for Glutathione. Nanomaterials, 13(1), 13. https://doi.org/10.3390/nano13010013





