Analysis of Biomechanical and Biochemical Markers of Rat Muscle Soleus Fatigue Processes Development during Long-Term Use of C60 Fullerene and N-Acetylcysteine
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of C60FAS
2.2. AFM and STM Analysis
2.3. Animals
2.4. Biomechanical and Biochemical Analysis
2.5. Statistical Analysis
3. Results and Discussion
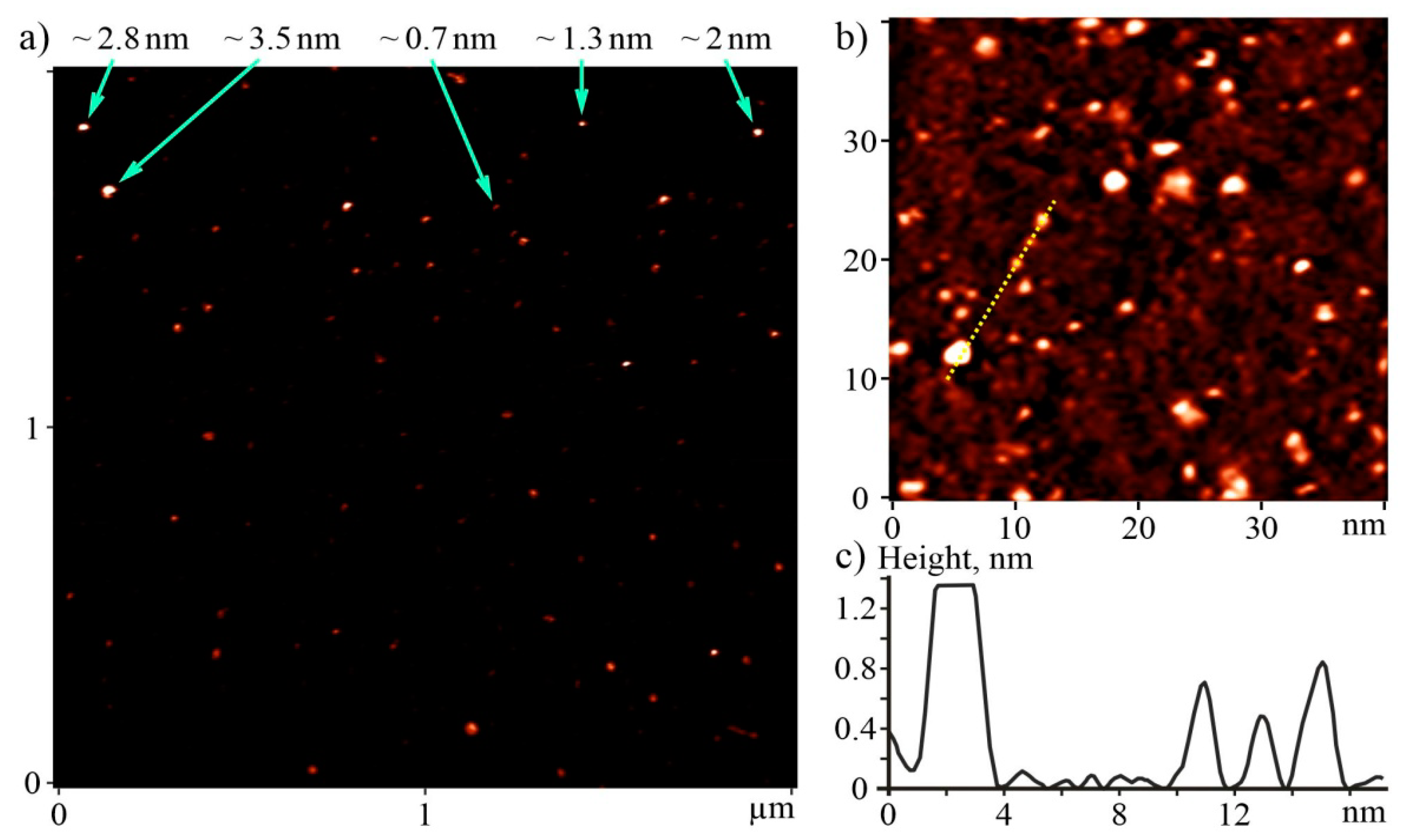
3.1. Characterization of C60FAS
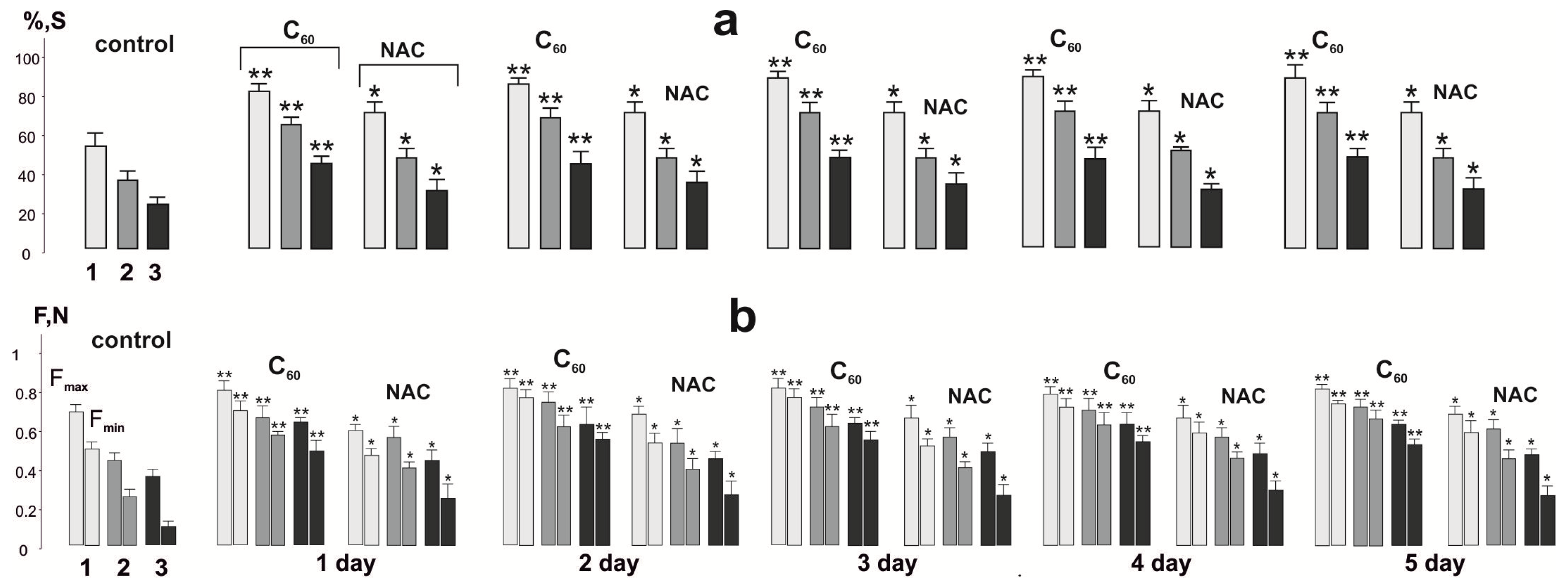
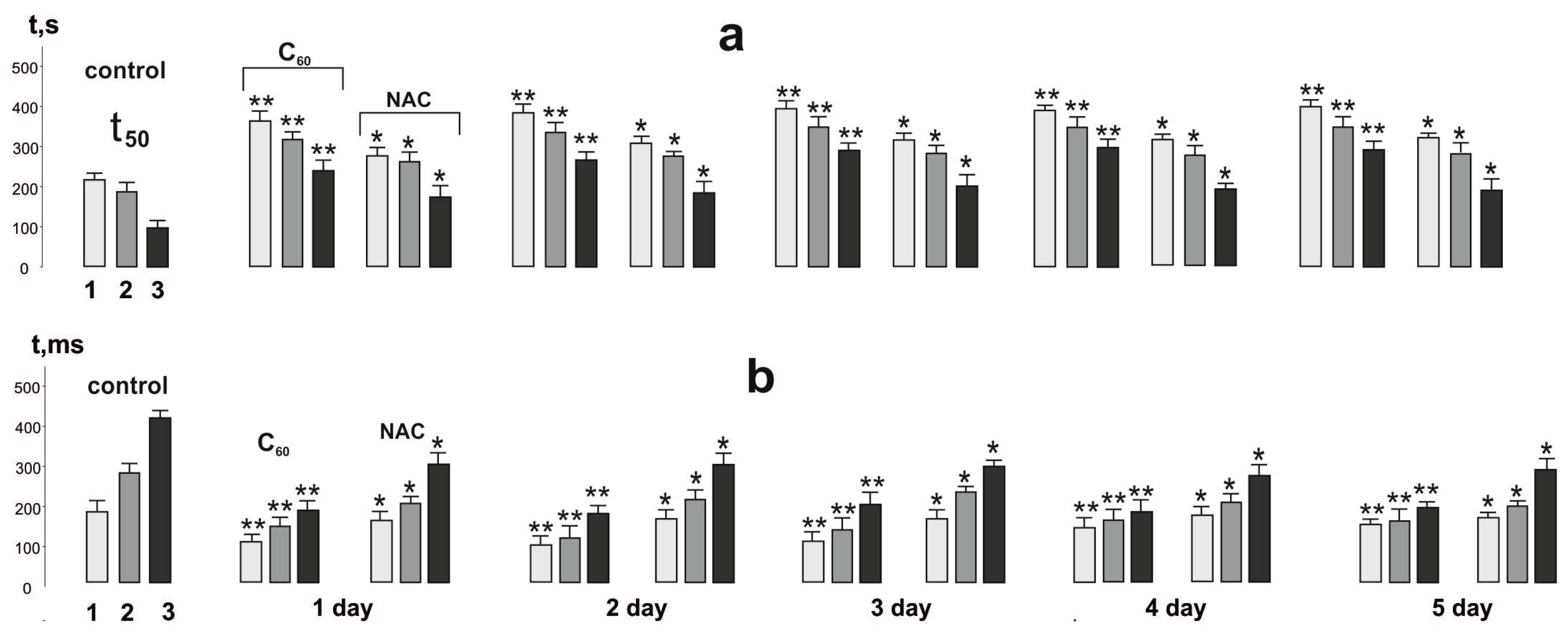
3.2. Biomechanical Analysis
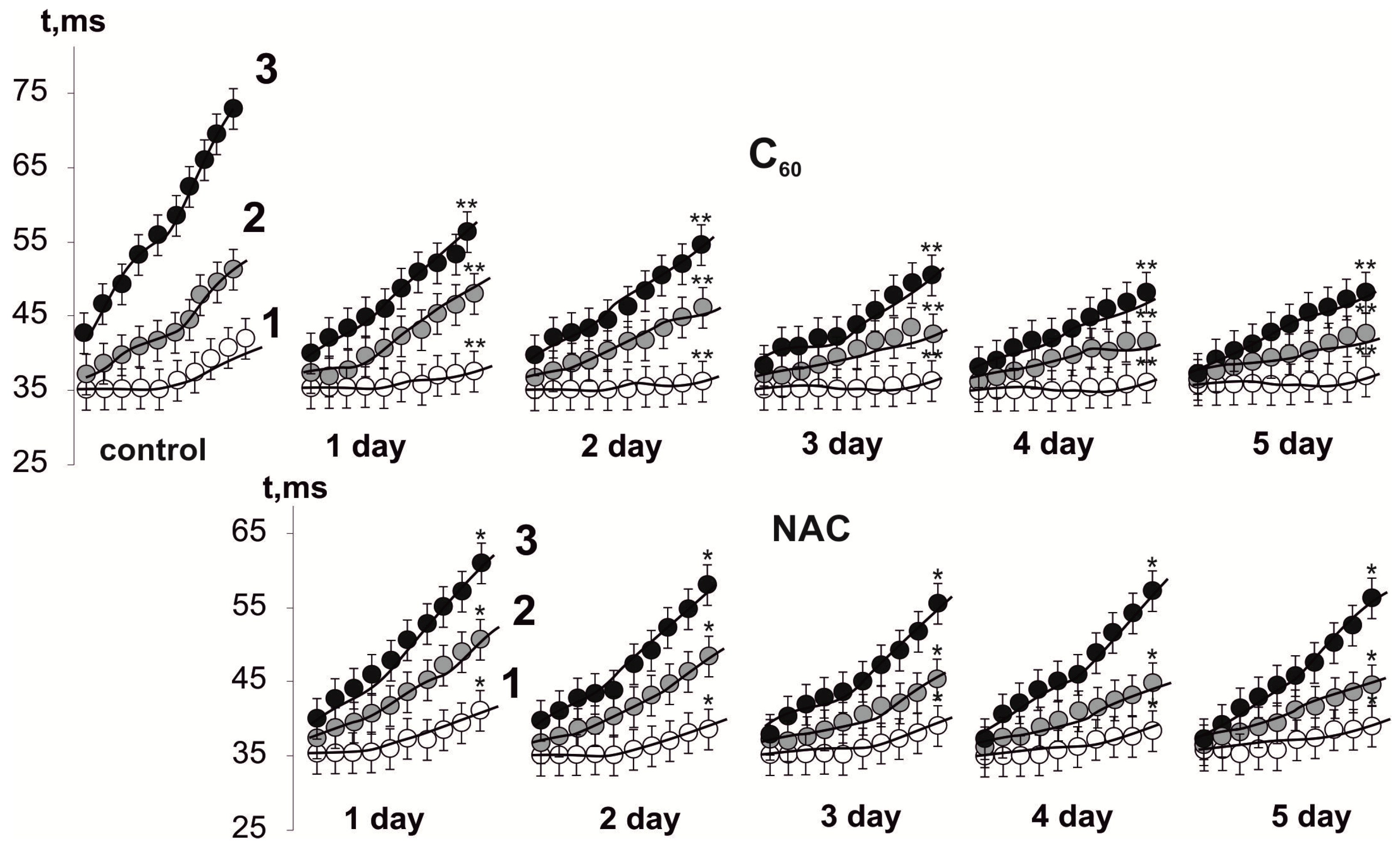
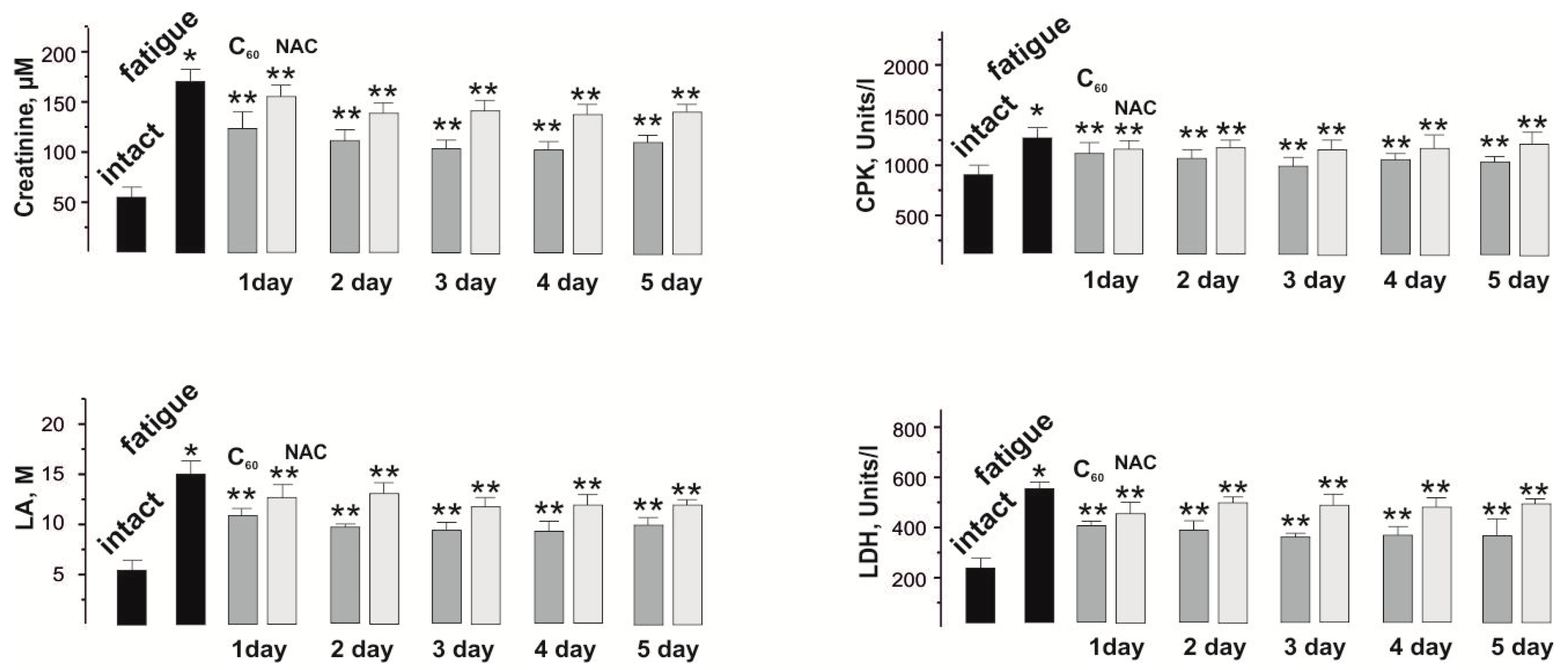
3.3. Biochemical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kenney, W.L.; Wilmore, J.; Costill, D. Physiology of Sport and Exercise, 6th ed.; Human Kinetics: Champaign, IL, USA, 2015; p. 648. [Google Scholar]
- Tipton, M.C. Exercise Physiology: People and Ideas; Elsevier: Amsterdam, The Netherlands, 2003; p. 510. [Google Scholar]
- Schomburg, E.D.; Steffens, H.; Pilyavskii, A.I.; Maisky, V.A.; Brück, W.; Dibaj, P.; Sears, T.A. Long lasting activity of nociceptive muscular afferents facilitates bilateral flexion reflex pattern in the feline spinal cord. Neurosci. Res. 2015, 95, 51–58. [Google Scholar] [CrossRef]
- Kostyukov, A.I.; Day, S.; Hellstrom, F.; Radovanovic, S.; Ljubisavljevic, M.; Windhorst, U.; Johansson, H. Fatigue-related changes in electromyogram activity of cat gastrocnemius during frequency-modulated efferent stimulation. Neuroscience 2000, 97, 801–809. [Google Scholar] [CrossRef]
- Nozdrenko, D.N.; Bogutska, K.I. About molecular mechanisms of fiber muscle contraction at transition to new equilibrium state: Analysis of experimental data using three-componential electrical stimulating signal. Biopolym. Cell 2005, 21, 283–286. [Google Scholar] [CrossRef]
- Place, N.; Bruton, J.D.; Westerblad, H. Mechanisms of fatigue induced by isometric contractions in exercising humans and in mouse isolated single muscle fibres. Clin. Exp. Pharmacol. Physiol. 2009, 36, 334–339. [Google Scholar] [CrossRef]
- Karami, M.; Calvo, B.; Zohoor, H.; Firoozbakhsh, K.; Grasa, J. Assessing the role of Ca2+ in skeletal muscle fatigue using a multi-scale continuum model. J. Theor. Biol. 2019, 461, 76–83. [Google Scholar] [CrossRef]
- Nozdrenko, D.M.; Abramchuk, O.M.; Soroca, V.M.; Miroshnichenko, N.S. Aluminum chloride effect on Ca2+, Mg2+-ATPase activity and dynamic parameters of skeletal muscle contraction. Ukr. Biochem. J. 2015, 87, 38–45. [Google Scholar] [CrossRef]
- Jones, D.A. Changes in the force-velocity relationship of fatigued muscle: Implications for power production and possible causes. J. Physiol. 2010, 588, 2977–2986. [Google Scholar] [CrossRef]
- Khoma, O.M.; Zavodovs’kyĭ, D.A.; Nozdrenko, D.N.; Dolhopolov, O.V.; Miroshnychenko, M.S.; Motuziuk, O.P. Dynamics of ischemic skeletal soleus muscle contraction in rats. Fiziolohichnyi Zhurnal 2014, 60, 34–40. [Google Scholar] [CrossRef]
- Prylutskyy, Y.I.; Vereshchaka, I.V.; Maznychenko, A.V.; Bulgakova, N.V.; Gonchar, O.O.; Kyzyma, O.A.; Ritter, U.; Scharff, P.; Tomiak, T.; Nozdrenko, D.M.; et al. C60 fullerene as promising therapeutic agent for correcting and preventing skeletal muscle fatigue. J. Nanobiotechnol. 2017, 15, 8. [Google Scholar] [CrossRef]
- Cooke, R. Modulation of the actomyosin interaction during fatigue of skeletal muscle. Muscle Nerve 2007, 36, 756–777. [Google Scholar] [CrossRef]
- Nozdrenko, D.N.; Shut, A.N.; Prylutskyy, Y.I. The possible molecular mechanism of the nonlinearity muscle contraction and its experimental substantiation. Biopolym. Cell 2005, 21, 80–83. [Google Scholar] [CrossRef]
- Kalezic, I.; Bugaychenko, L.A.; Kostyukov, A.I.; Pilyavskii, A.I.; Ljubisavljevic, M.; Windhorst, U.; Johansson, H. Fatigue-related depression of the feline monosynaptic gastrocnemius-soleus reflex. J. Physiol. 2004, 556, 283–296. [Google Scholar] [CrossRef]
- Davies, K.J.; Quintanilha, A.T.; Brooks, G.A.; Packer, L. Free radicals and tissue damage produced by exercise. Biochem. Biophys. Res. Commun. 1982, 107, 1198–1205. [Google Scholar] [CrossRef]
- Jackson, M.J.; Edwards, R.H.; Symons, M.C. Electron spin resonance studies of intact mammalian skeletal muscle. Biochem. Biophys. Acta 1985, 847, 185–190. [Google Scholar] [CrossRef]
- Alessio, H.M. Exercise-induced oxidative stress. Med. Sci. Sports Exerc. 1993, 25, 218–224. [Google Scholar] [CrossRef]
- Sen, C.K. Oxidants and antioxidants in exercise. J. Appl. Physiol. 1995, 79, 675–686. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef]
- Krusic, P.J.; Wasserman, E.; Keizer, P.N.; Morton, J.R.; Preston, K.F. Radical reactions of C60. Science 1991, 254, 1183–1185. [Google Scholar] [CrossRef]
- Nozdrenko, D.M.; Zavodovskyi, D.O.; Matvienko, T.Y.; Zay, S.Y.; Bogutska, K.I.; Prylutskyy, Y.I.; Ritter, U.; Scharff, P. C60 Fullerene as Promising Therapeutic Agent for the Prevention and Correction of Skeletal Muscle Functioning at Ischemic Injury. Nanoscale Res. Lett. 2017, 12, 115. [Google Scholar] [CrossRef]
- Nozdrenko, D.; Matvienko, T.; Vygovska, O.; Bogutska, K.; Motuziuk, O.; Nurishchenko, N.; Prylutskyy, Y.; Scharff, P.; Ritter, U. Protective Effect of Water-Soluble C60 Fullerene Nanoparticles on the Ischemia-Reperfusion Injury of the Muscle Soleus in Rats. Int. J. Mol. Sci. 2021, 22, 6812. [Google Scholar] [CrossRef]
- Gharbi, N.; Pressac, M.; Hadchouel, M.; Szwarc, H.; Wilson, S.R.; Moussa, F. [60]Fullerene is a powerful antioxidant in vivo with no acute or subacute toxicity. Nano Lett. 2005, 5, 2578–2585. [Google Scholar] [CrossRef]
- Halenova, T.I.; Vareniuk, I.M.; Roslova, N.M.; Dzerzhynsky, M.E.; Savchuk, O.M.; Ostapchenko, L.I.; Prylutskyy, Y.I.; Ritter, U.; Scharff, P. Hepatoprotective effect of orally applied water-soluble pristine C60 fullerene against CCl4-induced acute liver injury in rats. RSC Adv. 2016, 6, 100046–100055. [Google Scholar] [CrossRef]
- Eswaran, S.V. Water Soluble Nanocarbon Materials: A Panacea for All? Curr. Sci. 2018, 114, 1846–1850. [Google Scholar] [CrossRef]
- Sen, C.K.; Rankinen, T.; Vaisanen, S.; Rauramaa, R. Oxidative stress after human exercise: Effect of N-acetylcysteine supplementation. J. Appl. Physiol. 1994, 76, 2570–2577. [Google Scholar] [CrossRef]
- Didenko, G.; Prylutska, S.; Kichmarenko, Y.; Potebnya, G.; Prylutskyy, Y.; Slobodyanik, N.; Ritter, U.; Scharff, P. Evaluation of the antitumor immune response to C60 fullerene. Mater. Werkst. 2013, 44, 124–128. [Google Scholar] [CrossRef]
- Ritter, U.; Prylutskyy, Y.I.; Evstigneev, M.P.; Davidenko, N.A.; Cherepanov, V.V.; Senenko, A.I.; Marchenko, O.A.; Naumovets, A.G. Structural features of highly stable reproducible C60 fullerene aqueous colloid solution probed by various techniques. Fuller. Nanotub. Carbon Nanostruct. 2015, 23, 530. [Google Scholar] [CrossRef]
- Vereshchaka, I.V.; Bulgakova, N.V.; Maznychenko, A.V.; Gonchar, O.O.; Prylutskyy, Y.I.; Ritter, U.; Moska, W.; Tomiak, T.; Nozdrenko, D.M.; Mishchenko, I.V.; et al. C60 Fullerenes Diminish Muscle Fatigue in Rats Comparable to N-acetylcysteine or β-Alanine. Front. Physiol. 2018, 9, 517. [Google Scholar] [CrossRef]
- Tolkachov, M.; Sokolova, V.; Korolovych, V.; Prylutskyy, Y.; Epple, M.; Ritter, U.; Scharff, P. Study of biocompatibility effect of nanocarbon particles on various cell types in vitro. Mater. Werkst. 2016, 47, 216–221. [Google Scholar] [CrossRef]
- Prylutska, S.V.; Grebinyk, A.G.; Lynchak, O.V.; Byelinska, I.V.; Cherepanov, V.V.; Tauscher, E.; Matyshevska, O.P.; Prylutskyy, Y.I.; Rybalchenko, V.K.; Ritter, U.; et al. In vitro and in vivo toxicity of pristine C60 fullerene aqueous colloid solution. Fuller. Nanotub. Carbon Nanostruct. 2019, 27, 715. [Google Scholar] [CrossRef]
- Nozdrenko, D.N.; Berehovyi, S.M.; Nikitina, N.S.; Stepanova, L.I.; Beregova, T.V.; Ostapchenko, L.I. The influence of complex drug cocarnit on the nerve conduction velocity in nerve tibialis of rats with diabetic polyneuropathy. Biomed. Res. 2018, 29, 3629–3634. [Google Scholar]
- Brancaccio, P.; Lippi, G.; Maffulli, N. Biochemical markers of muscular damage. Clin. Chem. Lab. Med. 2010, 48, 757–767. [Google Scholar] [CrossRef]
- Peudus, D.A.; Mosunov, A.A.; Mykhina, Y.V.; Prylutskyy, Y.I.; Evstigneev, M.P. Fractal C60 fullerene aggregation: Equilibrium thermodynamics approach. Chem. Phys. Lett. 2020, 742, 137161. [Google Scholar] [CrossRef]
- MacIntosh, B.R.; Willis, J.C. Force-frequency relationship and potentiation in mammalian skeletal muscle. J. Appl. Physiol. 2000, 88, 2088–2096. [Google Scholar] [CrossRef]
- Lochyński, D.; Celichowski, J.; Korman, P.; Raglewska, P. Changes of motor unit contractile output during repeated activity. Acta Neurobiol. Exp. 2007, 67, 23–33. [Google Scholar]
- Celichowski, J. Mechanisms underlying the regulation of motor unit contraction in the skeletal muscle. J. Physiol. Pharmacol. 2000, 51, 17–33. [Google Scholar]
- Gordon, T.; Tyreman, N.; Rafuse, V.F.; Munson, J.B. Fast-to-slow conversion following chronic low-frequency activation of medial gastrocnemius muscle in cats. I. Muscle and motor unit properties. J. Neurophysiol. 1997, 77, 2585–2604. [Google Scholar] [CrossRef][Green Version]
- Allen, D.G.; Lamb, G.D.; Westerblad, H. Skeletal muscle fatigue: Cellular mechanisms. Physiol. Rev. 2008, 88, 287–332. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nozdrenko, D.; Prylutska, S.; Bogutska, K.; Cherepanov, V.; Senenko, A.; Vygovska, O.; Khrapatyi, S.; Ritter, U.; Prylutskyy, Y.; Piosik, J. Analysis of Biomechanical and Biochemical Markers of Rat Muscle Soleus Fatigue Processes Development during Long-Term Use of C60 Fullerene and N-Acetylcysteine. Nanomaterials 2022, 12, 1552. https://doi.org/10.3390/nano12091552
Nozdrenko D, Prylutska S, Bogutska K, Cherepanov V, Senenko A, Vygovska O, Khrapatyi S, Ritter U, Prylutskyy Y, Piosik J. Analysis of Biomechanical and Biochemical Markers of Rat Muscle Soleus Fatigue Processes Development during Long-Term Use of C60 Fullerene and N-Acetylcysteine. Nanomaterials. 2022; 12(9):1552. https://doi.org/10.3390/nano12091552
Chicago/Turabian StyleNozdrenko, Dmytro, Svitlana Prylutska, Kateryna Bogutska, Vsevolod Cherepanov, Anton Senenko, Oksana Vygovska, Sergii Khrapatyi, Uwe Ritter, Yuriy Prylutskyy, and Jacek Piosik. 2022. "Analysis of Biomechanical and Biochemical Markers of Rat Muscle Soleus Fatigue Processes Development during Long-Term Use of C60 Fullerene and N-Acetylcysteine" Nanomaterials 12, no. 9: 1552. https://doi.org/10.3390/nano12091552
APA StyleNozdrenko, D., Prylutska, S., Bogutska, K., Cherepanov, V., Senenko, A., Vygovska, O., Khrapatyi, S., Ritter, U., Prylutskyy, Y., & Piosik, J. (2022). Analysis of Biomechanical and Biochemical Markers of Rat Muscle Soleus Fatigue Processes Development during Long-Term Use of C60 Fullerene and N-Acetylcysteine. Nanomaterials, 12(9), 1552. https://doi.org/10.3390/nano12091552








