Study on the Characteristics of the Dispersion and Conductivity of Surfactants for the Nanofluids
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.3. Preparation of Samples
3. Results and Discussion
3.1. Structures of CNC
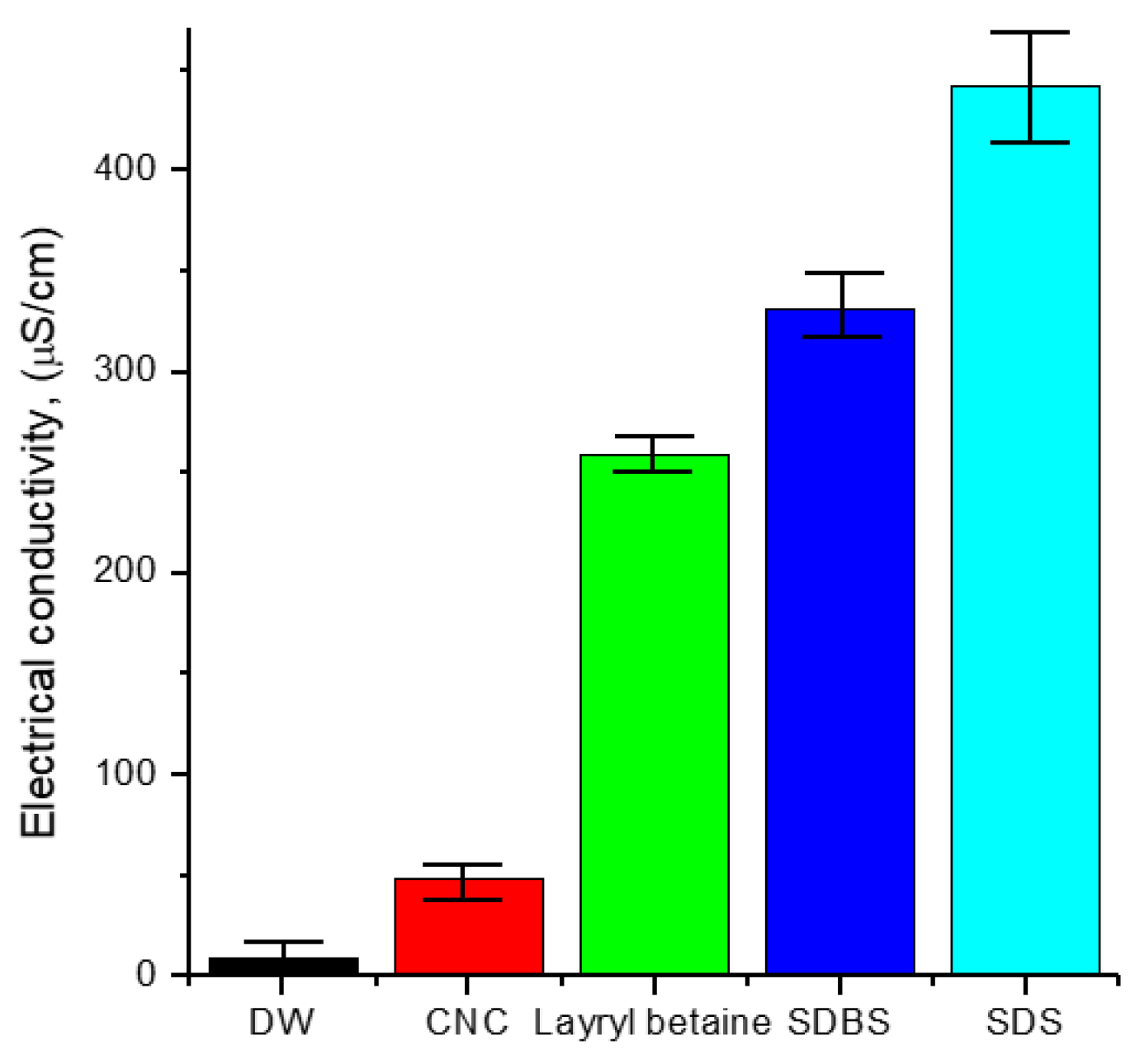
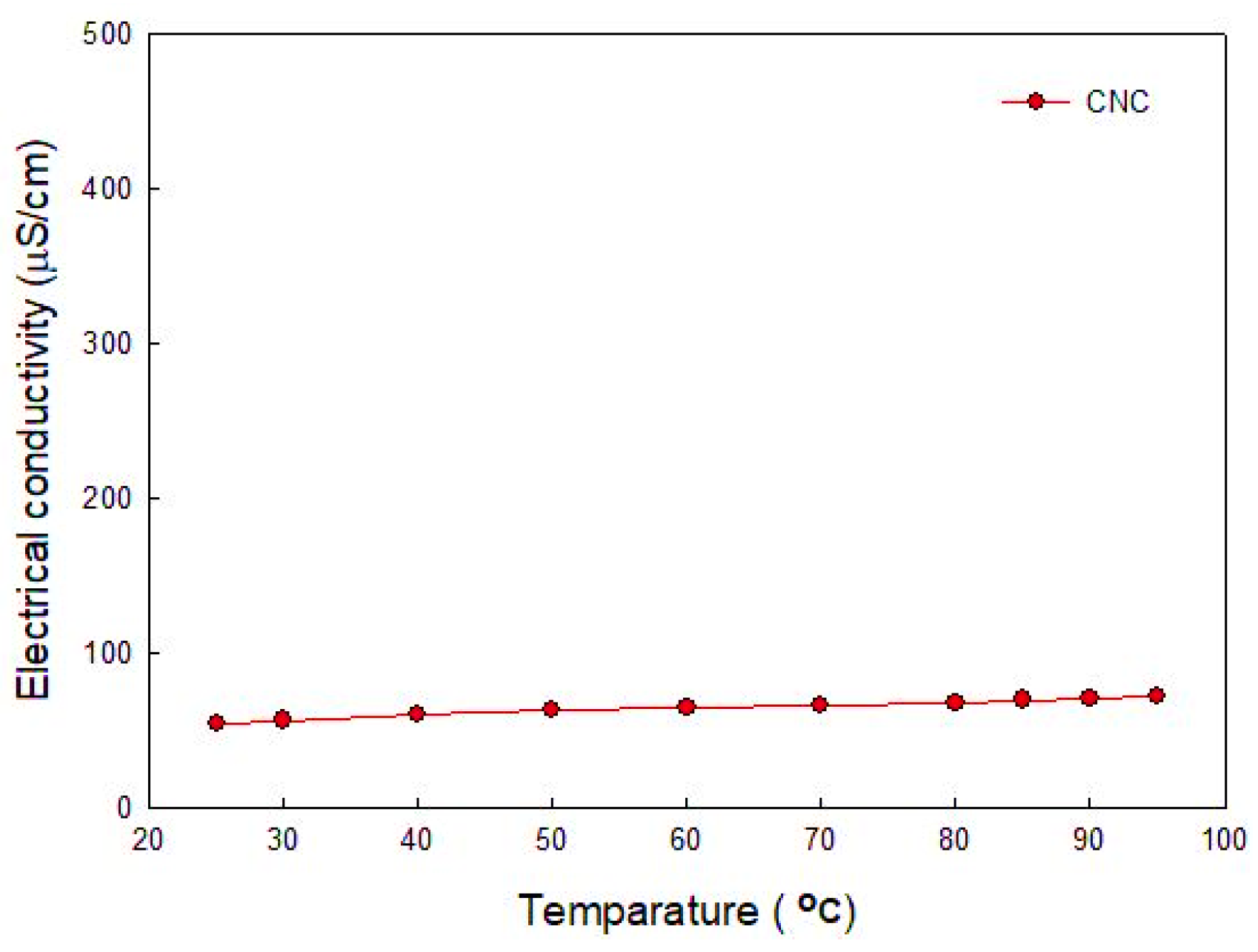
3.2. Electrical Conductivity of Solution
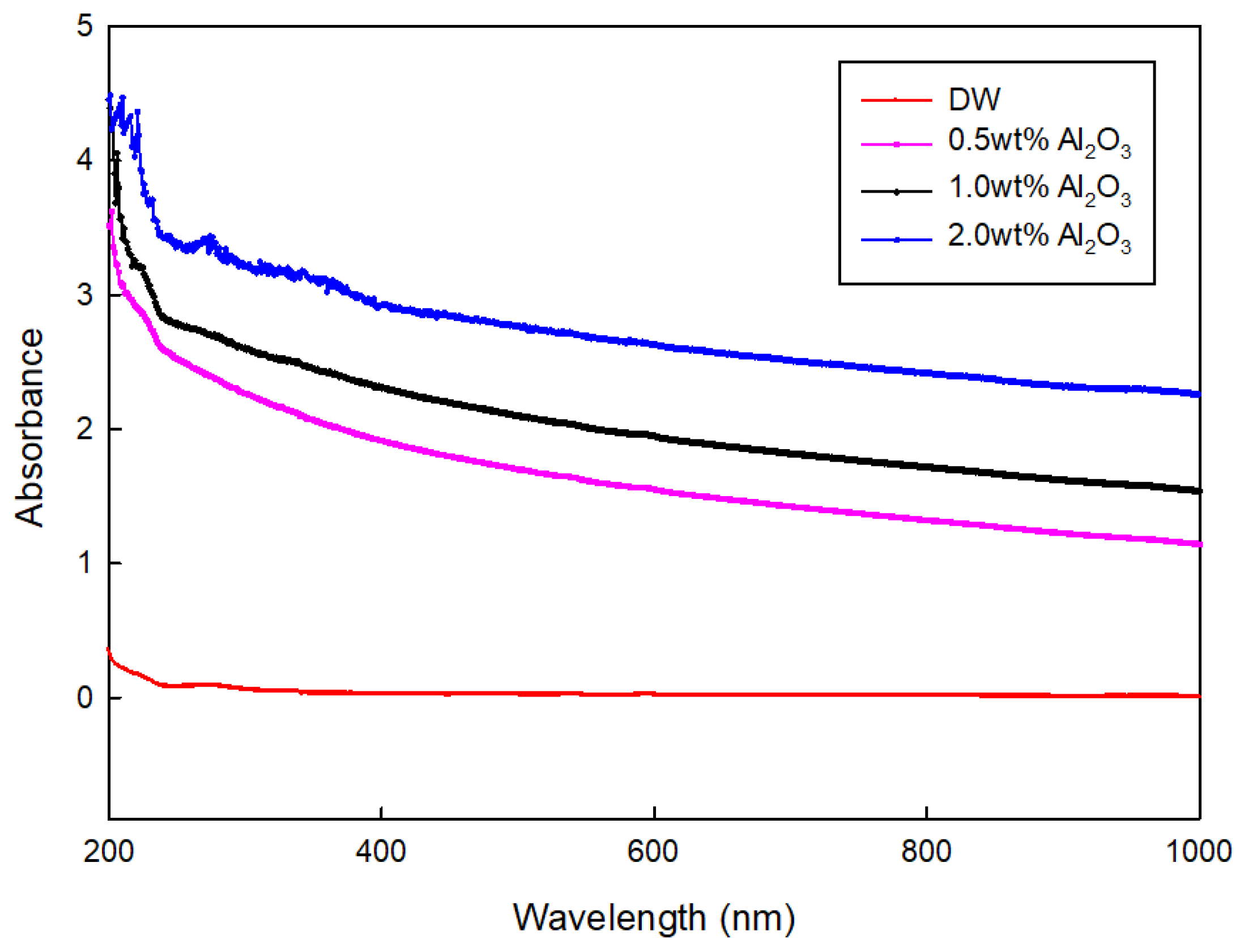
3.3. Dispersibility of Solution
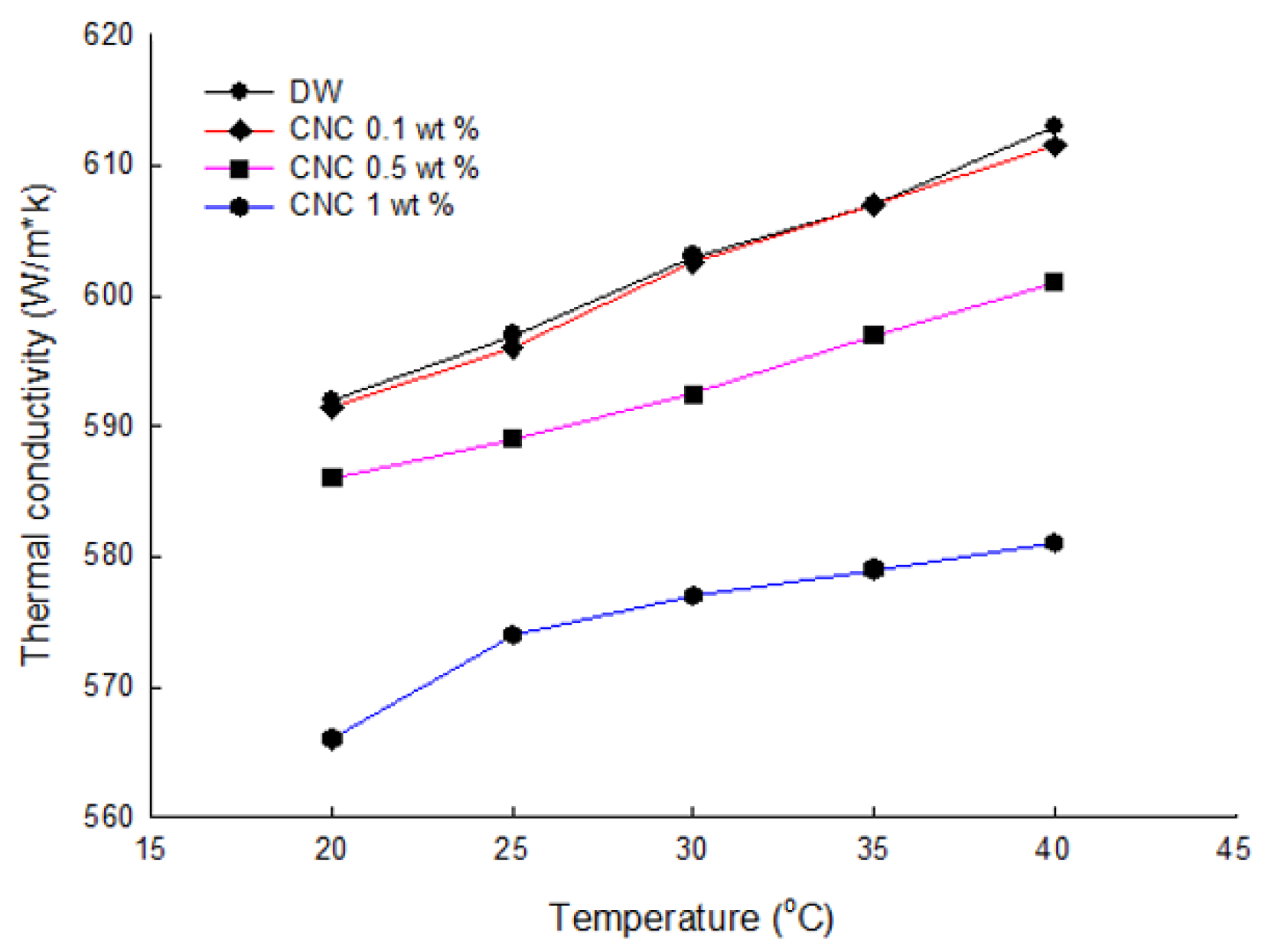
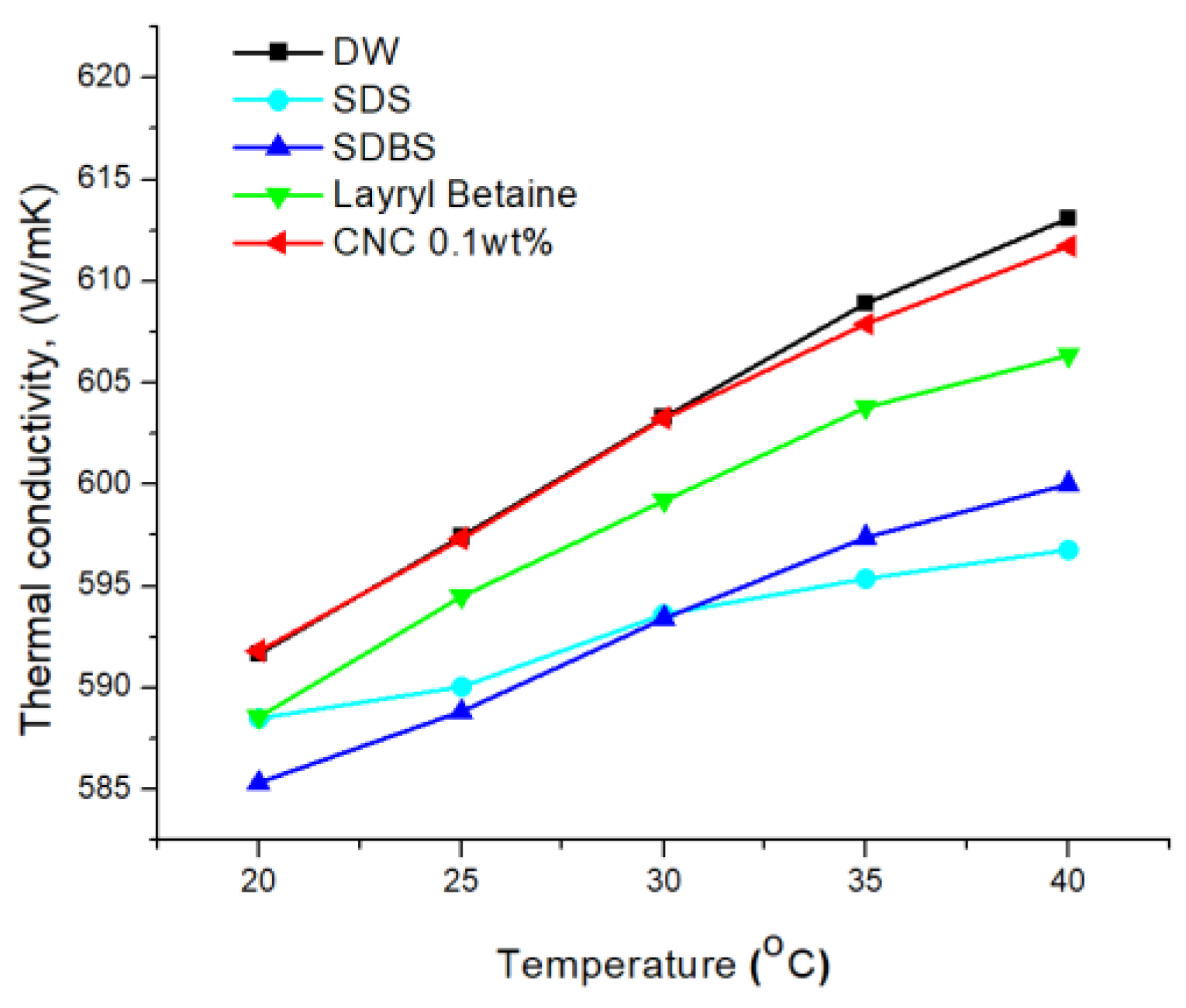
3.4. Thermal Conductivity of Solution
3.5. Heat Capacity of Solution
4. Conclusions
- (1)
- The structure analysis of nano callouses was conducted by the TEM method. It showed rod-shaped nanoparticles acquired from the acid hydrolysis of callouses. The size of the CNC was about 100 nm in diameter and 1000 nm in length. It is shown in Figure 1 that CNC can interact with water strongly by hydrogen bonding because of the hydroxyl group on the molecule.
- (2)
- The electrical conductivity of solutions was studied to figure out the electrical interference in the application of base fluid in industry. CNC had the lowest value of electrical conductivity compared with other surfactants. Furthermore, it was found that, unlike other surfactants, the electrical conductivity of CNC did not change with temperature.
- (3)
- The absorbance of samples was investigated using a UV/VIS spectrophotometer. When other surfactants were used for dispersion, it was hard to use the light absorbance in the ultraviolet range for the evaluation criteria of dispersion and concentration. However, it was revealed that CNC has a stable value across different wavelengths, which indicates that CNC could provide more detailed information on the dispersion and concentration.
- (4)
- The thermal conductivities were examined by the LAMBDA system. First, it was found that the low concentration had high thermal conductivity by comparing the thermal conductivity according to the CNC concentration. As the cellulose content increased, the thermal conductivity of the CNC nanofluid decreased. Overall, CNC had the highest thermal conductivity, followed by LB, and SDS and SDBS had similar thermal conductivity. Moreover, the heat capacity also had a similar value to thermal conductivity, as shown by acquiring the data on differences in temperature when the same quantity of heat was applied.
Funding
Conflicts of Interest
References
- Khanafer, K.; Vafai, K.; Lightstone, M. Buoyancy-driven heat transfer enhancement in a two-dimensional enclosure utilizing nanofluids. Int. J. Heat Mass Transf. 2003, 46, 3639–3653. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Z.; Yang, J.; Yue, Y.; Zhang, H. A Review of Recent Advances in Superhydrophobic Surfaces and Their Applications in Drag Reduction and Heat Transfer. Nanomaterials 2021, 12, 44. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.; Bahman, A.M.; Aljuwayhel, N.F.; Ebrahim, S.A.; Mukherjee, S.; Alsayegh, A. Carbon-Based Nanofluids and Their Advances towards Heat Transfer Applications—A Review. Nanomaterials 2021, 11, 1628. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Song, H.; Yu, K.; Tserengombo, B.; Choi, S.-H.; Chung, H.; Kim, J.; Jeong, H. Comparison of CFD simulations to experiment for heat transfer characteristics with aqueous Al2O3 nanofluid in heat exchanger tube. Int. Commun. Heat Mass Transf. 2018, 95, 123–131. [Google Scholar] [CrossRef]
- Kim, S.; Tserengombo, B.; Choi, S.-H.; Noh, J.; Huh, S.; Choi, B.; Jeong, H. Experimental investigation of heat transfer coefficient with Al2O3 nanofluid in small diameter tubes. Appl. Therm. Eng. 2019, 146, 346–355. [Google Scholar] [CrossRef]
- Brandão, A.T.; Rosoiu, S.; Costa, R.; Silva, A.F.; Anicai, L.; Enachescu, M.; Pereira, C.M. Characterization of Carbon Nanomaterials Dispersions: Can Metal Decoration of MWCNTs Improve Their Physicochemical Properties? Nanomaterials 2021, 12, 99. [Google Scholar] [CrossRef]
- Subrahmanyam, K.S.; Ghosh, A.; Gomathi, A.; Govindaraj, A.; Rao, C.N.R. Covalent and Noncovalent Functionalization and Solubilization of Graphene. Nanosci. Nanotechnol. Lett. 2009, 1, 28–31. [Google Scholar] [CrossRef]
- Hu, C.-Y.; Xu, Y.-J.; Duo, S.-W.; Zhang, R.-F.; Li, M.-S. Non-Covalent Functionalization of Carbon Nanotubes with Surfactants and Polymers. J. Chin. Chem. Soc. 2009, 56, 234–239. [Google Scholar] [CrossRef]
- Vázquez-Velázquez, A.R.; Velasco-Soto, M.A.; Pérez-García, S.A.; Licea-Jiménez, L. Functionalization Effect on Polymer Nanocomposite Coatings Based on TiO2–SiO2 Nanoparticles with Superhydrophilic Properties. Nanomaterials 2018, 8, 369. [Google Scholar] [CrossRef] [Green Version]
- Rastogi, R.; Kaushal, R.; Tripathi, S.; Sharma, A.L.; Kaur, I.; Bharadwaj, L.M. Comparative study of carbon nanotube dispersion using surfactants. J. Colloid Interface Sci. 2008, 328, 421–428. [Google Scholar] [CrossRef]
- Kalia, S.; Dufresne, A.; Cherian, B.M.; Kaith, B.S.; Avérous, L.; Njuguna, J.; Nassiopoulos, E. Cellulose-Based Bio- and Nanocomposites: A Review. Int. J. Polym. Sci. 2011, 2011, 837875. [Google Scholar] [CrossRef]
- Lin, N.; Huang, J.; Dufresne, A. Preparation, properties and applications of polysaccharide nanocrystals in advanced functional nanomaterials: A review. Nanoscale 2012, 4, 3274–3294. [Google Scholar] [CrossRef] [PubMed]
- Wardhono, E.; Pinem, M.; Kustiningsih, I.; Agustina, S.; Oudet, F.; Lefebvre, C.; Guénin, E. Cellulose Nanocrystals to Improve Stability and Functional Properties of Emulsified Film Based on Chitosan Nanoparticles and Beeswax. Nanomaterials 2019, 9, 1707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siqueira, G.; Bras, J.; Dufresne, A. Cellulosic Bionanocomposites: A Review of Preparation, Properties and Applications. Polymers 2010, 2, 728–765. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Tserengombo, B.; Choi, S.-H.; Noh, J.; Huh, S.; Choi, B.; Jeong, H. Experimental investigation of dispersion characteristics and thermal conductivity of various surfactants on carbon based nanomaterial. Int. Commun. Heat Mass Transf. 2018, 91, 95–102. [Google Scholar] [CrossRef]
- Mateos, R.; Vera, S.; Valiente, M.; Díez-Pascual, A.M.; Andrés, M.P.S. Comparison of Anionic, Cationic and Nonionic Surfactants as Dispersing Agents for Graphene Based on the Fluorescence of Riboflavin. Nanomaterials 2017, 7, 403. [Google Scholar] [CrossRef] [Green Version]
- Seelenmeyer, S.; Ballauff, M. Analysis of Surfactants Adsorbed onto the Surface of Latex Particles by Small-Angle X-ray Scattering. Langmuir 2000, 16, 4094–4099. [Google Scholar] [CrossRef]
- Clarke, J.G.; Wicks, S.R.; Farr, S.J. Surfactant mediated effects in pressurized metered dose inhalers formulated as suspensions. I. Drug/surfactant interactions in a model propellant system. Int. J. Pharm. 1993, 93, 221–231. [Google Scholar] [CrossRef]
- Guardia, L.; Fernández-Merino, M.; Paredes, J.I.; Fernández, P.S.; Villar-Rodil, S.; Martinez-Alonso, A.; Tascon, J.M.D. High-throughput production of pristine graphene in an aqueous dispersion assisted by non-ionic surfactants. Carbon 2011, 49, 1653–1662. [Google Scholar] [CrossRef]
- Singh, B.P.; Menchavez, R.; Takai, C.; Fuji, M.; Takahashi, M. Stability of dispersions of colloidal alumina particles in aqueous suspensions. J. Colloid Interface Sci. 2005, 291, 181–186. [Google Scholar] [CrossRef]
- Cushing, B.L.; Kolesnichenko, V.L.; O’Connor, C.J. Recent Advances in the Liquid-Phase Syntheses of Inorganic Nanoparticles. Chem. Rev. 2004, 104, 3893–3946. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.F.; Rojas, E.; Bergey, D.M.; Johnson, A.T.; Yodh, A.G. High Weight Fraction Surfactant Solubilization of Single-Wall Carbon Nanotubes in Water. Nano Lett. 2003, 3, 269–273. [Google Scholar] [CrossRef]
- Yu, J.; Grossiord, N.; Koning, C.E.; Loos, J. Controlling the dispersion of multi-wall carbon nanotubes in aqueous surfactant solution. Carbon 2007, 45, 618–623. [Google Scholar] [CrossRef]
- Li, X.; Zhu, D.; Wang, X.; Wang, N.; Gao, J.; Li, H. Thermal conductivity enhancement dependent pH and chemical surfactant for Cu-H2O nanofluids. Thermochim. Acta 2008, 469, 98–103. [Google Scholar] [CrossRef]
- Kim, J.-K.; Jung, J.Y.; Kang, Y.T. Absorption performance enhancement by nano-particles and chemical surfactants in binary nanofluids. Int. J. Refrig. 2007, 30, 50–57. [Google Scholar] [CrossRef]
- Peng, H.; Ding, G.; Hu, H. Effect of surfactant additives on nucleate pool boiling heat transfer of refrigerant-based nanofluid. Exp. Therm. Fluid Sci. 2011, 35, 960–970. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Han, L.; Zhang, Z.; Fang, X.; Shi, J.; Ma, W. Surfactant-free ionic liquid-based nanofluids with remarkable thermal conductivity enhancement at very low loading of graphene. Nanoscale Res. Lett. 2012, 7, 314. [Google Scholar] [CrossRef] [Green Version]
- Munkhbayar, B.; Tanshen, R.; Jeoun, J.; Chung, H.; Jeong, H. Surfactant-free dispersion of silver nanoparticles into MWCNT-aqueous nanofluids prepared by one-step technique and their thermal characteristics. Ceram. Int. 2013, 39, 6415–6425. [Google Scholar] [CrossRef]
- Yurekli, K.; Mitchell, A.C.A.; Krishnamoorti, R. Small-Angle Neutron Scattering from Surfactant-Assisted Aqueous Dispersions of Carbon Nanotubes. J. Am. Chem. Soc. 2004, 126, 9902–9903. [Google Scholar] [CrossRef]
- Hertel, T.; Hagen, A.; Talalaev, V.; Arnold, K.; Hennrich, F.; Kappes, M.; Flahaut, E. Spectroscopy of Single- and Double-Wall Carbon Nanotubes in Different Environments. Nano Lett. 2005, 5, 511–514. [Google Scholar] [CrossRef]
- Ilyas, R.; Sapuan, S.; Ishak, M.; Zainudin, E. Sugar Palm Nanofibrillated Cellulose Fibre Reinforced Sugar Palm Starch Nanocomposite. Part 1: Morphological, Mechanical and Physical Properties. 2018. Available online: https://europepmc.org/article/ppr/ppr55333 (accessed on 26 April 2022).
- Ilyas, R.A.; Sapuan, S.M.; Ishak, M.R. Isolation and characterization of nanocrystalline cellulose from sugar palm fibres (Arenga Pinnata). Carbohydr. Polym. 2018, 181, 1038–1051. [Google Scholar] [CrossRef]
- Ilyas, R.A.; Sapuan, S.M.; Ishak, M.R.; Zainudin, E.S. Sugar palm nanofibrillated cellulose (Arenga pinnata (Wurmb.) Merr): Effect of cycles on their yield, physic-chemical, morphological and thermal behavior. Int. J. Biol. Macromol. 2019, 123, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Sway, K.; Hovey, J.K.; Tremaine, P.R. Apparent molar heat capacities and volumes of alkylbenzenesulfonate salts in water: Substituent group additivity. Can. J. Chem. 1986, 64, 394–398. [Google Scholar] [CrossRef] [Green Version]
- Bakshi, M.S.; Crisantino, R.; De Lisi, R.; Milioto, S. Volume and heat capacity of sodium dodecyl sulfate-dodecyldimethylamine oxide mixed micelles. J. Phys. Chem. 1993, 97, 6914–6919. [Google Scholar] [CrossRef]
- Garg, P.; Alvarado, J.L.; Marsh, C.; Carlson, T.A.; Kessler, D.A.; Annamalai, K. An experimental study on the effect of ultrasonication on viscosity and heat transfer performance of multi-wall carbon nanotube-based aqueous nanofluids. Int. J. Heat Mass Transf. 2009, 52, 5090–5101. [Google Scholar] [CrossRef]
- Tserengombo, B.; Jeong, H.; Dolgor, E.; Delgado, A.; Kim, S. Effects of Functionalization in Different Conditions and Ball Milling on the Dispersion and Thermal and Electrical Conductivity of MWCNTs in Aqueous Solution. Nanomaterials 2021, 11, 1323. [Google Scholar] [CrossRef]
- Molnes, S.N.; Paso, K.G.; Strand, S.; Syverud, K. The effects of pH, time and temperature on the stability and viscosity of cellulose nanocrystal (CNC) dispersions: Implications for use in enhanced oil recovery. Cellulose 2017, 24, 4479–4491. [Google Scholar] [CrossRef] [Green Version]
- Bentley, J.P. Temperature sensor characteristics and measurement system design. J. Phys. E Sci. Instrum. 1984, 17, 430. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S. Study on the Characteristics of the Dispersion and Conductivity of Surfactants for the Nanofluids. Nanomaterials 2022, 12, 1537. https://doi.org/10.3390/nano12091537
Kim S. Study on the Characteristics of the Dispersion and Conductivity of Surfactants for the Nanofluids. Nanomaterials. 2022; 12(9):1537. https://doi.org/10.3390/nano12091537
Chicago/Turabian StyleKim, Sedong. 2022. "Study on the Characteristics of the Dispersion and Conductivity of Surfactants for the Nanofluids" Nanomaterials 12, no. 9: 1537. https://doi.org/10.3390/nano12091537
APA StyleKim, S. (2022). Study on the Characteristics of the Dispersion and Conductivity of Surfactants for the Nanofluids. Nanomaterials, 12(9), 1537. https://doi.org/10.3390/nano12091537




