Electrospun Donor/Acceptor Nanofibers for Efficient Photocatalytic Hydrogen Evolution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Solution Preparation
2.2. Electrospinning Experiments
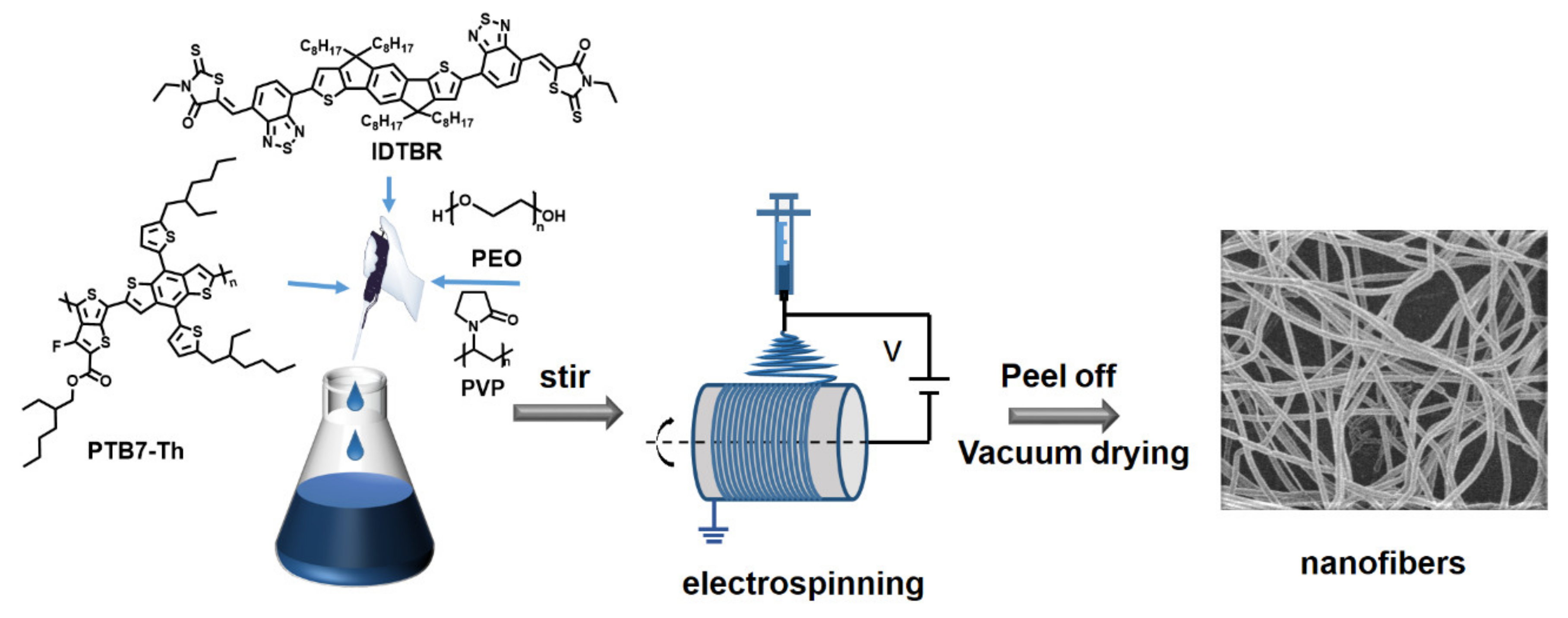
2.3. Characterization
2.4. Photocatalytic Processes
3. Results and Discussion
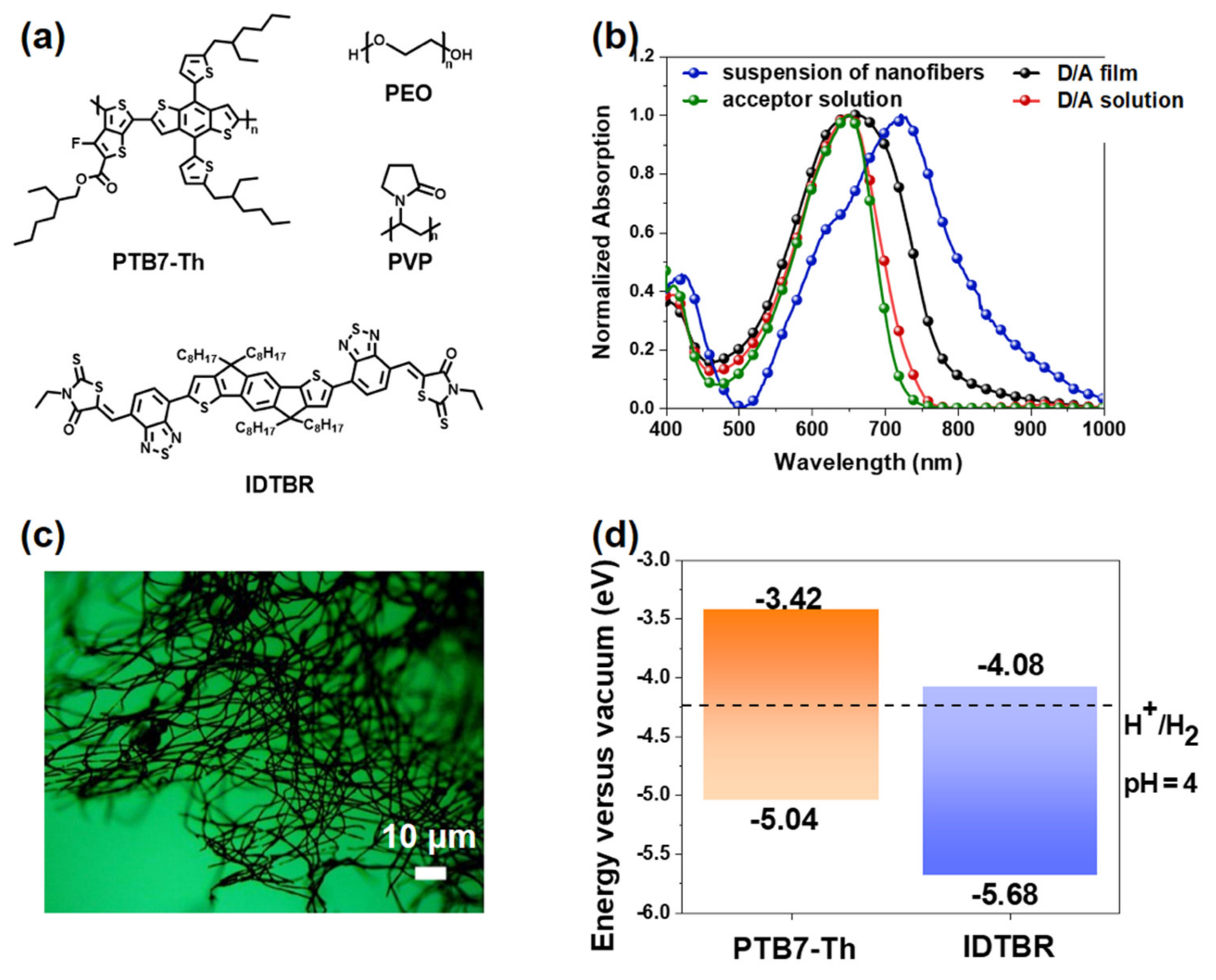
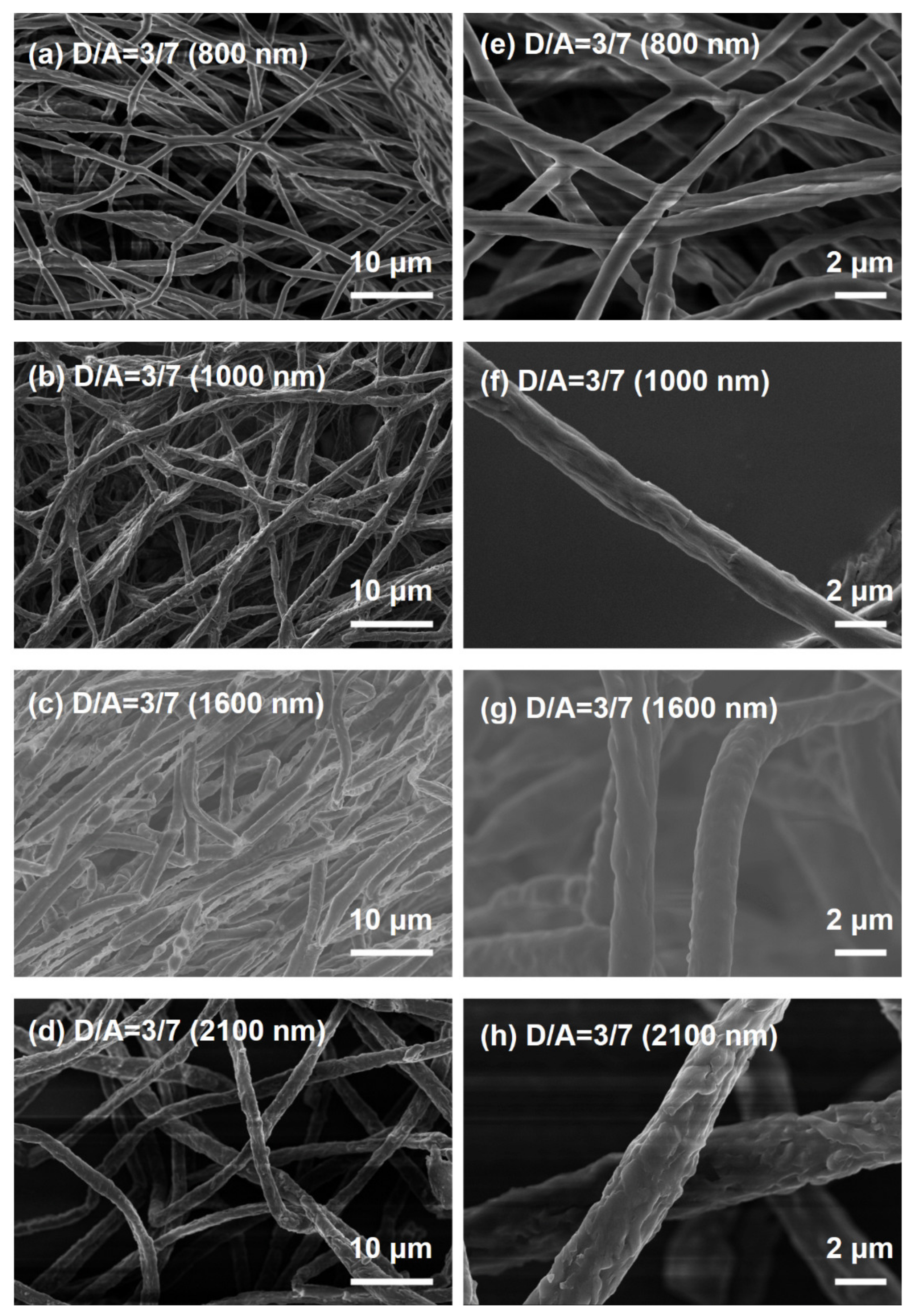
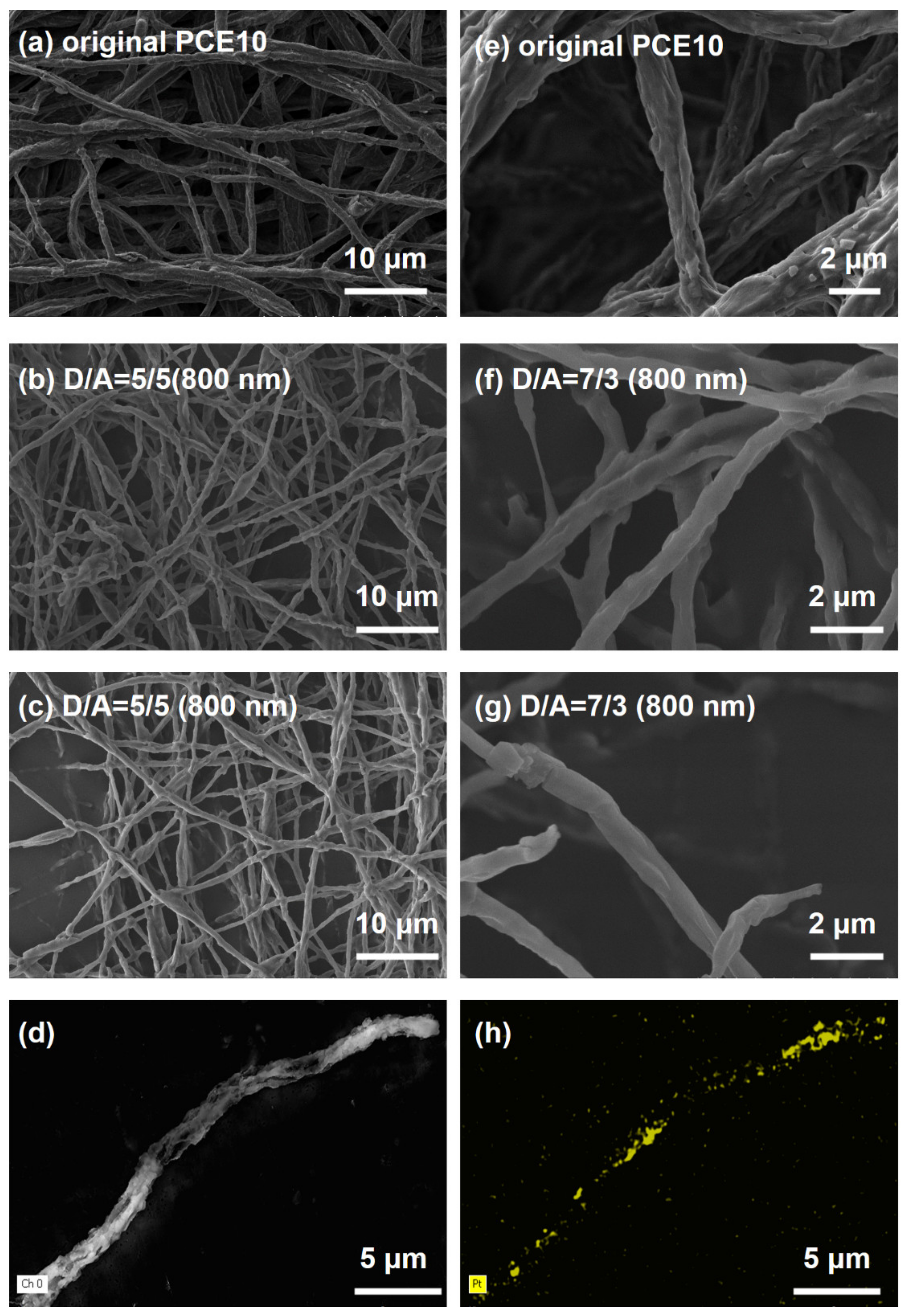
3.1. Absorption Spectroscopy Properties and Morphology of Electrospun Nanofibers
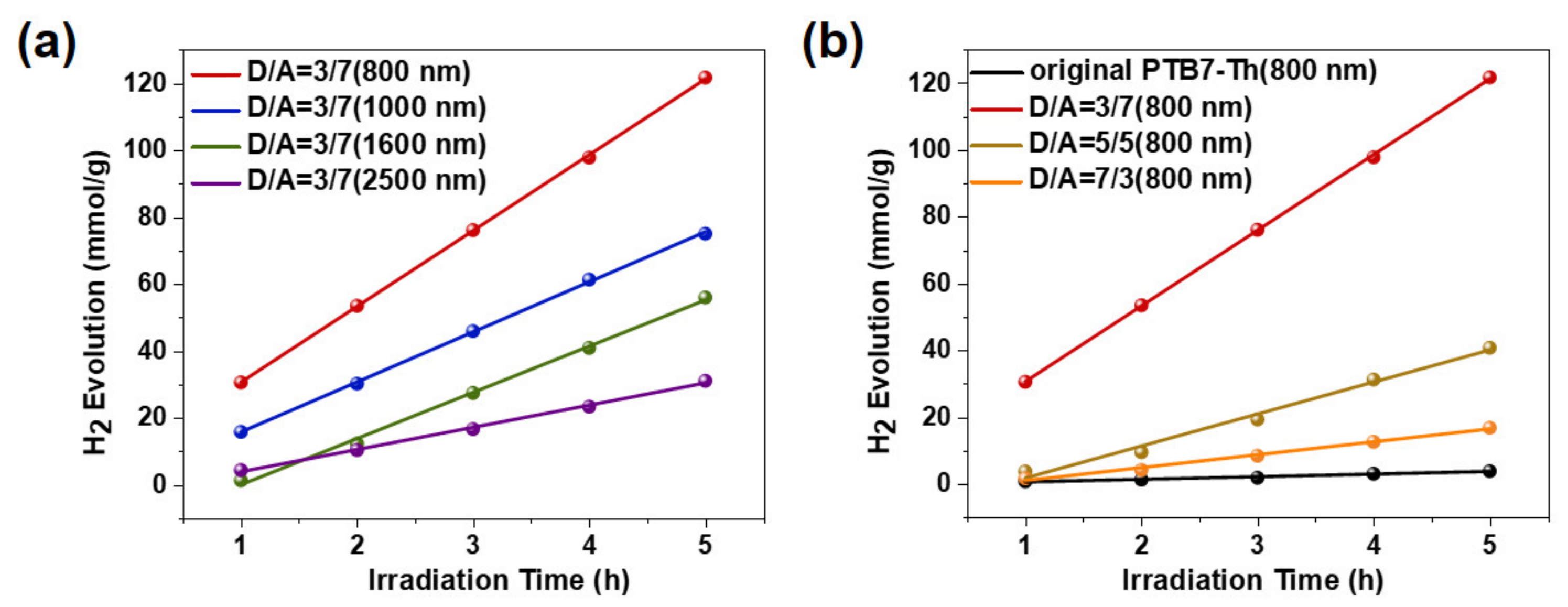
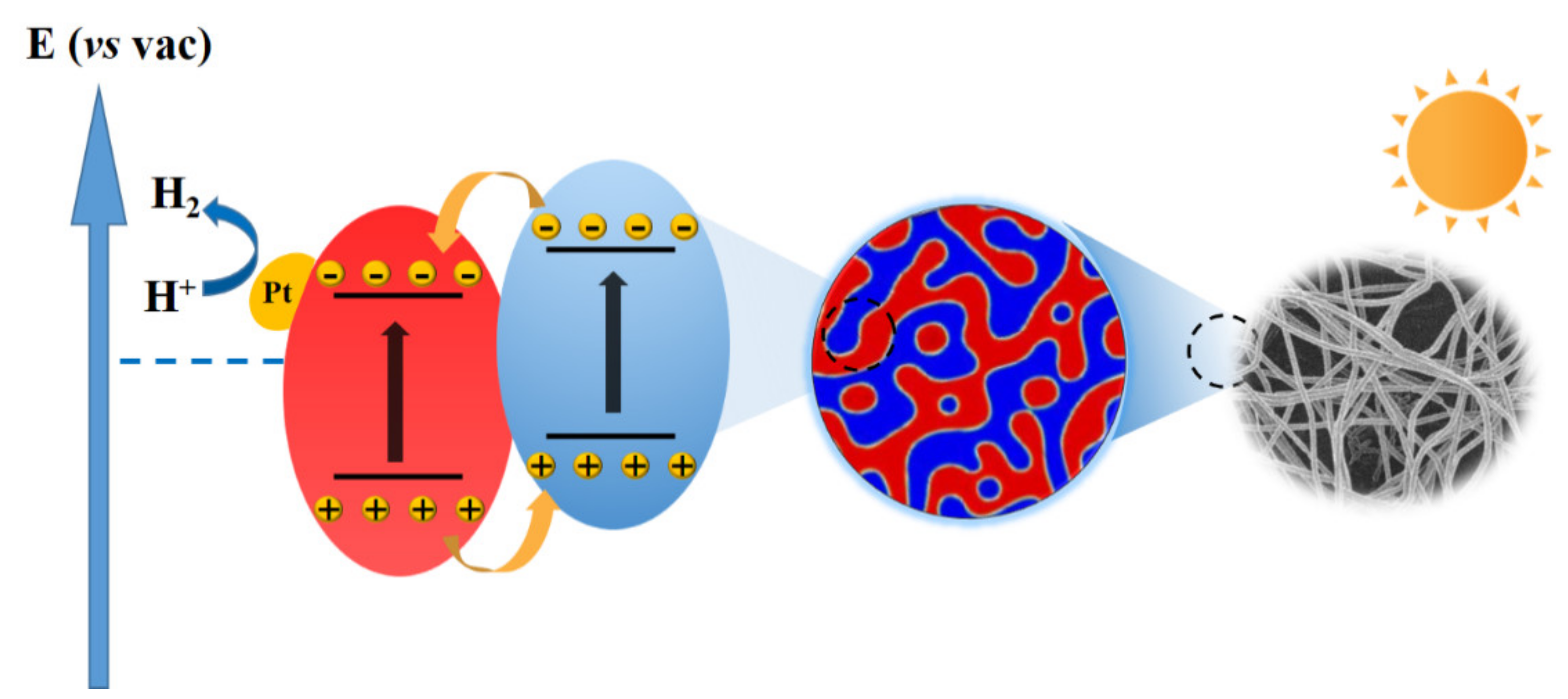
3.2. Photocatalytic Hydrogen Evolution
3.3. Stability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Armaroli, N.; Balzani, V. Towards an electricity-powered world. Energy Environ. Sci. 2011, 4, 3193–3222. [Google Scholar] [CrossRef]
- Ronge, J.; Bosserez, T.; Martel, D.; Nervi, C.; Boarino, L.; Taulelle, F.; Decher, G.; Bordiga, S.; Martens, J.A. Monolithic cells for solar fuels. Chem. Soc. Rev. 2014, 43, 7963–7981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, S.; Jain, S.; Ps, V.; Tiwari, A.K.; Nouni, M.R.; Pandey, J.K.; Goel, S. Hydrogen: A sustainable fuel for future of the transport sector. Renew. Sustain. Energy Rev. 2015, 51, 623–633. [Google Scholar] [CrossRef]
- Zou, Z.G.; Ye, J.H.; Sayama, K.; Arakawa, H. Direct splitting of water under visible light irradiation with an oxide semiconductor photocatalyst. Nature 2001, 414, 625–627. [Google Scholar] [CrossRef] [PubMed]
- Tee, S.Y.; Win, K.Y.; Teo, W.S.; Koh, L.-D.; Liu, S.; Teng, C.P.; Han, M.-Y. Recent Progress in Energy-Driven Water Splitting. Adv. Sci. 2017, 4, 1600337. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Vogel, A.; Sachs, M.; Sprick, R.S.; Wilbraham, L.; Moniz, S.J.A.; Godin, R.; Zwijnenburg, M.A.; Durrant, J.R.; Cooper, A.I.; et al. Current understanding and challenges of solar-driven hydrogen generation using polymeric photocatalysts. Nat. Energy 2019, 4, 746–760. [Google Scholar] [CrossRef]
- Li, Z.; Chueh, C.-C.; Jen, A.K.Y. Recent advances in molecular design of functional conjugated polymers for high-performance polymer solar cells. Prog. Polym. Sci. 2019, 99, 101175. [Google Scholar] [CrossRef]
- Genene, Z.; Mammo, W.; Wang, E.; Andersson, M.R. Recent Advances in n-Type Polymers for All-Polymer Solar Cells. Adv. Mater. 2019, 31, 1807275. [Google Scholar] [CrossRef]
- Jayakumar, J.; Chou, H.-H. Recent Advances in Visible-Light-Driven Hydrogen Evolution from Water using Polymer Photocatalysts. ChemCatChem 2020, 12, 689–704. [Google Scholar] [CrossRef]
- Zhao, C.; Chen, Z.; Shi, R.; Yang, X.; Zhang, T. Recent Advances in Conjugated Polymers for Visible-Light-Driven Water Splitting. Adv. Mater. 2020, 32, e1907296. [Google Scholar] [CrossRef]
- Dai, C.; Pan, Y.; Liu, B. Conjugated Polymer Nanomaterials for Solar Water Splitting. Adv. Energy Mater. 2020, 10, 2002474. [Google Scholar] [CrossRef]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Wang, Z.; Zhang, S.; Ye, H.; Kong, K.; Gong, X.; Hua, J.; Tian, H. Molecular Engineering of Donor–Acceptor Conjugated Polymer/g-C3N4 Heterostructures for Significantly Enhanced Hydrogen Evolution Under Visible-Light Irradiation. Adv. Funct. Mater. 2018, 28, 1804512. [Google Scholar] [CrossRef]
- Dong, J.; Zhang, Y.; Hussain, M.I.; Zhou, W.; Chen, Y.; Wang, L.-N. g-C3N4: Properties, Pore Modifications, and Photocatalytic Applications. Nanomaterials 2022, 12, 121. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.-Y.; Wang, W. Covalent organic frameworks (COFs): From design to applications. Chem. Soc. Rev. 2013, 42, 548–568. [Google Scholar] [CrossRef]
- Pachfule, P.; Acharjya, A.; Roeser, J.; Langenhahn, T.; Schwarze, M.; Schomäcker, R.; Thomas, A.; Schmidt, J. Diacetylene Functionalized Covalent Organic Framework (COF) for Photocatalytic Hydrogen Generation. J. Am. Chem. Soc. 2018, 140, 1423–1427. [Google Scholar] [CrossRef]
- Banerjee, T.; Gottschling, K.; Savasci, G.; Ochsenfeld, C.; Lotsch, B.V. H2 Evolution with Covalent Organic Framework Photocatalysts. ACS Energy Lett. 2018, 3, 400–409. [Google Scholar] [CrossRef] [Green Version]
- Zhi, Y.; Wang, Z.; Zhang, H.-L.; Zhang, Q. Recent Progress in Metal-Free Covalent Organic Frameworks as Heterogeneous Catalysts. Small 2020, 16, 2001070. [Google Scholar] [CrossRef]
- Huang, W.-Y.; Shen, Z.-Q.; Cheng, J.-Z.; Liu, L.-L.; Yang, K.; Chen, X.; Wen, H.-R.; Liu, S.-Y. C–H activation derived CPPs for photocatalytic hydrogen production excellently accelerated by a DMF cosolvent. J. Mater. Chem. A 2019, 7, 24222–24230. [Google Scholar] [CrossRef]
- Lee, J.-S.M.; Cooper, A.I. Advances in Conjugated Microporous Polymers. Chem. Rev. 2020, 120, 2171–2214. [Google Scholar] [CrossRef] [Green Version]
- Bai, Y.; Hu, Z.; Jiang, J.-X.; Huang, F. Hydrophilic Conjugated Materials for Photocatalytic Hydrogen Evolution. Chem.–Asian J. 2020, 15, 1780–1790. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Gao, J.; Hummelen, J.C.; Wudl, F.; Heeger, A.J. Polymer Photovoltaic Cells: Enhanced Efficiencies via a Network of Internal Donor-Acceptor Heterojunctions. Science 1995, 270, 1789–1791. [Google Scholar] [CrossRef] [Green Version]
- Knupfer, M. Exciton binding energies in organic semiconductors. Appl. Phys. A 2003, 77, 623–626. [Google Scholar] [CrossRef]
- Kim, F.S.; Ren, G.; Jenekhe, S.A. One-Dimensional Nanostructures of π-Conjugated Molecular Systems: Assembly, Properties, and Applications from Photovoltaics, Sensors, and Nanophotonics to Nanoelectronics. Chem. Mater. 2011, 23, 682–732. [Google Scholar] [CrossRef]
- Zhang, G.; Zhao, J.; Chow, P.C.Y.; Jiang, K.; Zhang, J.; Zhu, Z.; Zhang, J.; Huang, F.; Yan, H. Nonfullerene Acceptor Molecules for Bulk Heterojunction Organic Solar Cells. Chem. Rev. 2018, 118, 3447–3507. [Google Scholar] [CrossRef] [PubMed]
- Moniz, S.J.A.; Shevlin, S.A.; Martin, D.J.; Guo, Z.-X.; Tang, J. Visible-light driven heterojunction photocatalysts for water splitting—A critical review. Energy Environ. Sci. 2015, 8, 731–759. [Google Scholar] [CrossRef]
- Kosco, J.; Bidwell, M.; Cha, H.; Martin, T.; Howells, C.T.; Sachs, M.; Anjum, D.H.; Gonzalez Lopez, S.; Zou, L.; Wadsworth, A.; et al. Enhanced photocatalytic hydrogen evolution from organic semiconductor heterojunction nanoparticles. Nat. Mater. 2020, 19, 559–565. [Google Scholar] [CrossRef]
- Yang, H.; Li, X.; Sprick, R.S.; Cooper, A.I. Conjugated polymer donor–molecular acceptor nanohybrids for photocatalytic hydrogen evolution. Chem. Commun. 2020, 56, 6790–6793. [Google Scholar] [CrossRef]
- Zhang, X.; Shen, F.; Hu, Z.; Wu, Y.; Tang, H.; Jia, J.; Wang, X.; Huang, F.; Cao, Y. Biomass Nanomicelles Assist Conjugated Polymers/Pt Cocatalysts To Achieve High Photocatalytic Hydrogen Evolution. ACS Sustain. Chem. Eng. 2019, 7, 4128–4135. [Google Scholar] [CrossRef]
- Hu, Z.; Wang, Z.; Zhang, X.; Tang, H.; Liu, X.; Huang, F.; Cao, Y. Conjugated Polymers with Oligoethylene Glycol Side Chains for Improved Photocatalytic Hydrogen Evolution. iScience 2019, 13, 33–42. [Google Scholar] [CrossRef] [Green Version]
- Hu, Z.; Zhang, X.; Yin, Q.; Liu, X.; Jiang, X.-f.; Chen, Z.; Yang, X.; Huang, F.; Cao, Y. Highly efficient photocatalytic hydrogen evolution from water-soluble conjugated polyelectrolytes. Nano Energy 2019, 60, 775–783. [Google Scholar] [CrossRef]
- Dai, C.; Liu, B. Conjugated polymers for visible-light-driven photocatalysis. Energy Environ. Sci. 2020, 13, 24–52. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, X.; Liang, Y.; Hu, Z.; Huang, F. Porphyrin-Based Conjugated Polyelectrolytes for Efficient Photocatalytic Hydrogen Evolution. Macromolecules 2021, 54, 4902–4909. [Google Scholar] [CrossRef]
- Rafiq, M.; Jing, J.; Liang, Y.; Hu, Z.; Zhang, X.; Tang, H.; Tian, L.; Li, Y.; Huang, F. A pyridinium-pended conjugated polyelectrolyte for efficient photocatalytic hydrogen evolution and organic solar cells. Polym. Chem. 2021, 12, 1498–1506. [Google Scholar] [CrossRef]
- Li, L.; Hadt, R.G.; Yao, S.; Lo, W.-Y.; Cai, Z.; Wu, Q.; Pandit, B.; Chen, L.X.; Yu, L. Photocatalysts Based on Cobalt-Chelating Conjugated Polymers for Hydrogen Evolution from Water. Chem. Mater. 2016, 28, 5394–5399. [Google Scholar] [CrossRef]
- Diao, R.; Ye, H.; Yang, Z.; Zhang, S.; Kong, K.; Hua, J. Significant improvement of photocatalytic hydrogen evolution of diketopyrrolopyrrole-based donor–acceptor conjugated polymers through side-chain engineering. Polym. Chem. 2019, 10, 6473–6480. [Google Scholar] [CrossRef]
- Kumar, E.N.; Jose, R.; Archana, P.S.; Vijila, C.; Yusoff, M.M.; Ramakrishna, S. High performance dye-sensitized solar cells with record open circuit voltage using tin oxide nanoflowers developed by electrospinning. Energy Environ. Sci. 2012, 5, 5401–5407. [Google Scholar] [CrossRef]
- Liu, D.; Guo, Q.; Hou, H.; Niwa, O.; You, T. PdxCoy Nanoparticle/Carbon Nanofiber Composites with Enhanced Electrocatalytic Properties. ACS Catal. 2014, 4, 1825–1829. [Google Scholar] [CrossRef]
- Peng, S.; Jin, G.; Li, L.; Li, K.; Srinivasan, M.; Ramakrishna, S.; Chen, J. Multi-functional electrospun nanofibres for advances in tissue regeneration, energy conversion & storage, and water treatment. Chem. Soc. Rev. 2016, 45, 1225–1241. [Google Scholar]
- Chinnappan, A.; Baskar, C.; Baskar, S.; Ratheesh, G.; Ramakrishna, S. An overview of electrospun nanofibers and their application in energy storage, sensors and wearable/flexible electronics. J. Mater. Chem. C 2017, 5, 12657–12673. [Google Scholar] [CrossRef]
- Selvan, R.T.; Jia, C.Y.; Jayathilaka, W.A.D.M.; Chinnappan, A.; Alam, H.; Ramakrishna, S. Enhanced Piezoelectric Performance of Electrospun PVDF-MWCNT-Cu Nanocomposites for Energy Harvesting Application. Nano 2020, 15, 2050049. [Google Scholar] [CrossRef]
- Jiang, J.; Zheng, G.; Wang, X.; Li, W.; Kang, G.; Chen, H.; Guo, S.; Liu, J. Arced Multi-Nozzle Electrospinning Spinneret for High-Throughput Production of Nanofibers. Micromachines 2020, 11, 27. [Google Scholar] [CrossRef] [Green Version]
- Lang, X.; Gopalan, S.; Fu, W.; Ramakrishna, S. Photocatalytic Water Splitting Utilizing Electrospun Semiconductors for Solar Hydrogen Generation: Fabrication, Modification and Performance. Bull. Chem. Soc. Jpn. 2021, 94, 8–20. [Google Scholar] [CrossRef]
- Liao, M.; Wang, C.; Hong, Y.; Zhang, Y.; Cheng, X.; Sun, H.; Huang, X.; Ye, L.; Wu, J.; Shi, X.; et al. Industrial scale production of fibre batteries by a solution-extrusion method. Nat. Nanotechnol. 2022, 17, 372–377. [Google Scholar] [CrossRef]
- Ren, X.-N.; Hu, Z.-Y.; Jin, J.; Wu, L.; Wang, C.; Liu, J.; Liu, F.; Wu, M.; Li, Y.; Van Tendeloo, G.; et al. Cocatalyzing Pt/PtO Phase-Junction Nanodots on Hierarchically Porous TiO2 for Highly Enhanced Photocatalytic Hydrogen Production. ACS Appl. Mater. Interfaces 2017, 9, 29687–29698. [Google Scholar] [CrossRef]
- Hasan, M.M.; Tolba, S.A.; Allam, N.K. In Situ Formation of Graphene Stabilizes Zero-Valent Copper Nanoparticles and Significantly Enhances the Efficiency of Photocatalytic Water Splitting. ACS Sustain. Chem. Eng. 2018, 6, 16876–16885. [Google Scholar] [CrossRef]
- Brooks, R.M.; Maafa, I.M.; Al-Enizi, A.M.; El-Halwany, M.M.; Ubaidullah, M.; Yousef, A. Electrospun Bimetallic NiCr Nanoparticles@Carbon Nanofibers as an Efficient Catalyst for Hydrogen Generation from Ammonia Borane. Nanomaterials 2019, 9, 1082. [Google Scholar] [CrossRef] [Green Version]
- Hieu, H.N.; Nghia, N.V.; Vuong, N.M.; Van Bui, H. Omnidirectional Au-embedded ZnO/CdS core/shell nanorods for enhanced photoelectrochemical water-splitting efficiency. Chem. Commun. 2020, 56, 3975–3978. [Google Scholar] [CrossRef]
- Liu, S.; Shang, S.; Lv, R.; Wang, Y.; Wang, J.; Ren, W.; Wang, Y. Molybdenum Carbide Buried in D-Shaped Fibers as a Novel Saturable Absorber Device for Ultrafast Photonics Applications. ACS Appl. Mater. Interfaces 2021, 13, 19128–19137. [Google Scholar] [CrossRef]
- Bedford, N.M.; Dickerson, M.B.; Drummy, L.F.; Koerner, H.; Singh, K.M.; Vasudev, M.C.; Durstock, M.F.; Naik, R.R.; Steckl, A.J. Nanofiber-Based Bulk-Heterojunction Organic Solar Cells Using Coaxial Electrospinning. Adv. Energy Mater. 2012, 2, 1136–1144. [Google Scholar] [CrossRef]
- Taylor, G.I.S. Disintegration of water drops in an electric field. Proc. Math. Phys. Eng. Sci. 1964, 280, 383–397. [Google Scholar]
- Lu, L.; Zheng, T.; Wu, Q.; Schneider, A.M.; Zhao, D.; Yu, L. Recent Advances in Bulk Heterojunction Polymer Solar Cells. Chem. Rev. 2015, 115, 12666–12731. [Google Scholar] [CrossRef]
- Mok, J.W.; Hu, Z.; Sun, C.; Barth, I.; Muñoz, R.; Jackson, J.; Terlier, T.; Yager, K.G.; Verduzco, R. Network-Stabilized Bulk Heterojunction Organic Photovoltaics. Chem. Mater. 2018, 30, 8314–8321. [Google Scholar] [CrossRef]
- Son, W.K.; Youk, J.H.; Lee, T.S.; Park, W.H. The effects of solution properties and polyelectrolyte on electrospinning of ultrafine poly(ethylene oxide) fibers. Polymer 2004, 45, 2959–2966. [Google Scholar] [CrossRef]
- Luo, C.J.; Stride, E.; Edirisinghe, M. Mapping the Influence of Solubility and Dielectric Constant on Electrospinning Polycaprolactone Solutions. Macromolecules 2012, 45, 4669–4680. [Google Scholar] [CrossRef]
- Kuo, C.-C.; Lin, C.-H.; Chen, W.-C. Morphology and Photophysical Properties of Light-Emitting Electrospun Nanofibers Prepared from Poly(fluorene) Derivative/PMMA Blends. Macromolecules 2007, 40, 6959–6966. [Google Scholar] [CrossRef]
- Laforgue, A.; Robitaille, L. Fabrication of poly-3-hexylthiophene/polyethylene oxide nanofibers using electrospinning. Synth. Met. 2008, 158, 577–584. [Google Scholar] [CrossRef] [Green Version]
- Müller, C.; Ferenczi, T.A.M.; Campoy-Quiles, M.; Frost, J.M.; Bradley, D.D.C.; Smith, P.; Stingelin-Stutzmann, N.; Nelson, J. Binary Organic Photovoltaic Blends: A Simple Rationale for Optimum Compositions. Adv. Mater. 2008, 20, 3510–3515. [Google Scholar] [CrossRef]
- Ho, C.H.Y.; Cheung, S.H.; Li, H.-W.; Chiu, K.L.; Cheng, Y.; Yin, H.; Chan, M.H.; So, F.; Tsang, S.-W.; So, S.K. Using Ultralow Dosages of Electron Acceptor to Reveal the Early Stage Donor–Acceptor Electronic Interactions in Bulk Heterojunction Blends. Adv. Energy Mater. 2017, 7, 1602360. [Google Scholar] [CrossRef]
- Wang, C.-T.; Kuo, C.-C.; Chen, H.-C.; Chen, W.-C. Non-woven and aligned electrospun multicomponent luminescent polymer nanofibers: Effects of aggregated morphology on the photophysical properties. Nanotechnology 2009, 20, 375604. [Google Scholar] [CrossRef]
- Liu, H.A.; Zepeda, D.; Ferraris, J.P.; Balkus, K.J., Jr. Electrospinning of Poly(alkoxyphenylenevinylene) and Methanofullerene Nanofiber Blends. ACS Appl. Mater. Interfaces 2009, 1, 1958–1965. [Google Scholar] [CrossRef]
- Liang, F.-C.; Kuo, C.-C.; Chen, B.-Y.; Cho, C.-J.; Hung, C.-C.; Chen, W.-C.; Borsali, R. RGB-Switchable Porous Electrospun Nanofiber Chemoprobe-Filter Prepared from Multifunctional Copolymers for Versatile Sensing of pH and Heavy Metals. ACS Appl. Mater. Interfaces 2017, 9, 16381–16396. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, X.; Liang, Y.; Hu, Z.; Zhang, X.; Liang, Y.; Hu, Z.; Huang, F.; Cao, Y. Electrospun Donor/Acceptor Nanofibers for Efficient Photocatalytic Hydrogen Evolution. Nanomaterials 2022, 12, 1535. https://doi.org/10.3390/nano12091535
Lin X, Liang Y, Hu Z, Zhang X, Liang Y, Hu Z, Huang F, Cao Y. Electrospun Donor/Acceptor Nanofibers for Efficient Photocatalytic Hydrogen Evolution. Nanomaterials. 2022; 12(9):1535. https://doi.org/10.3390/nano12091535
Chicago/Turabian StyleLin, Xiaoyu, Yuanying Liang, Zhicheng Hu, Xi Zhang, Youcai Liang, Zhengwei Hu, Fei Huang, and Yong Cao. 2022. "Electrospun Donor/Acceptor Nanofibers for Efficient Photocatalytic Hydrogen Evolution" Nanomaterials 12, no. 9: 1535. https://doi.org/10.3390/nano12091535
APA StyleLin, X., Liang, Y., Hu, Z., Zhang, X., Liang, Y., Hu, Z., Huang, F., & Cao, Y. (2022). Electrospun Donor/Acceptor Nanofibers for Efficient Photocatalytic Hydrogen Evolution. Nanomaterials, 12(9), 1535. https://doi.org/10.3390/nano12091535





