Improved TEA Sensitivity and Selectivity of In2O3 Porous Nanospheres by Modification with Ag Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Characterizations
2.3. Gas sensor Fabrication and Analysis
3. Results and Discussion
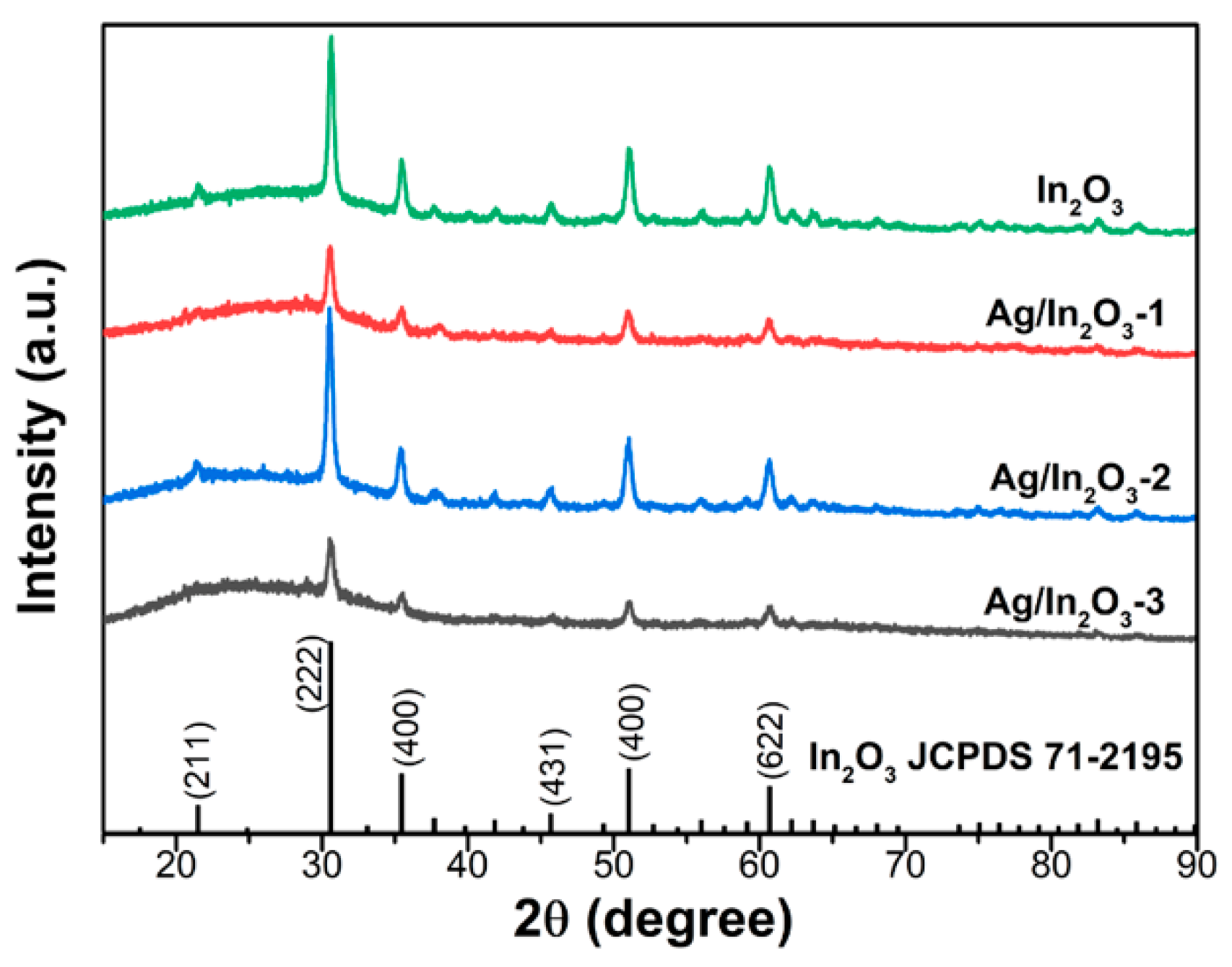
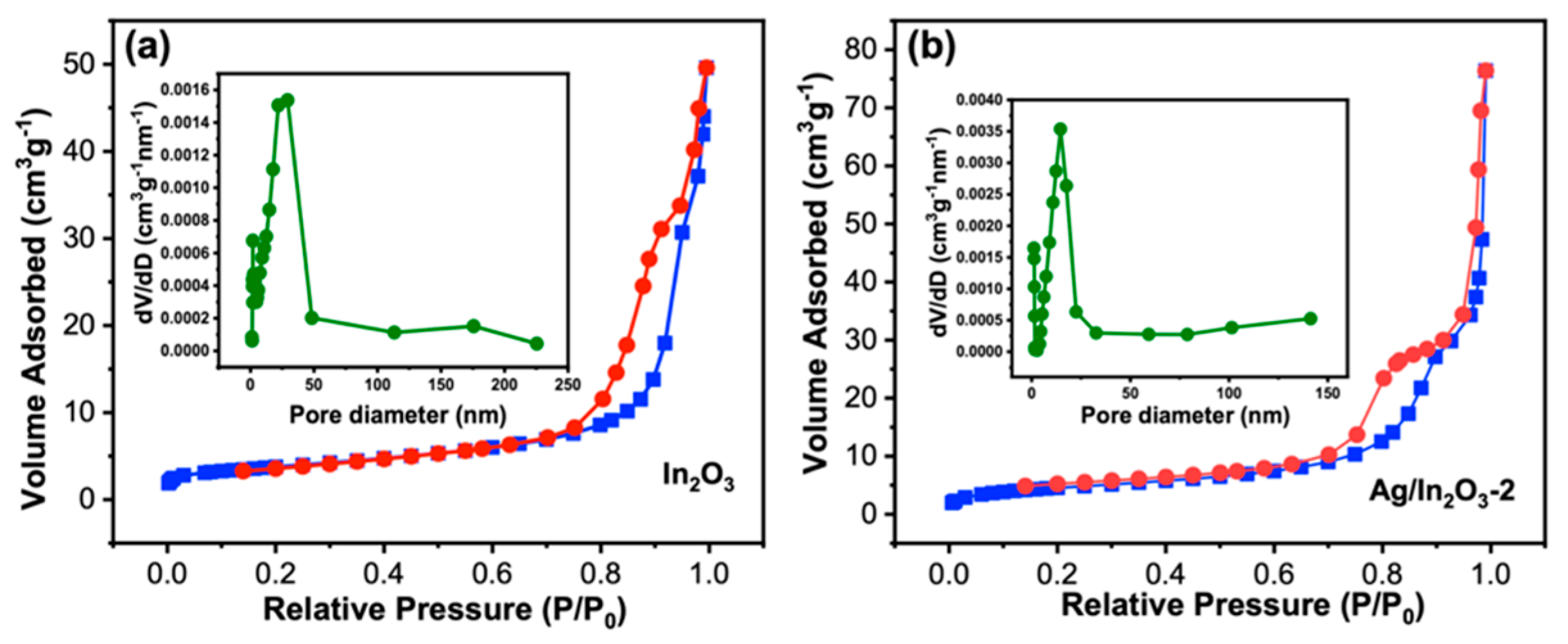
3.1. Sample Characterization
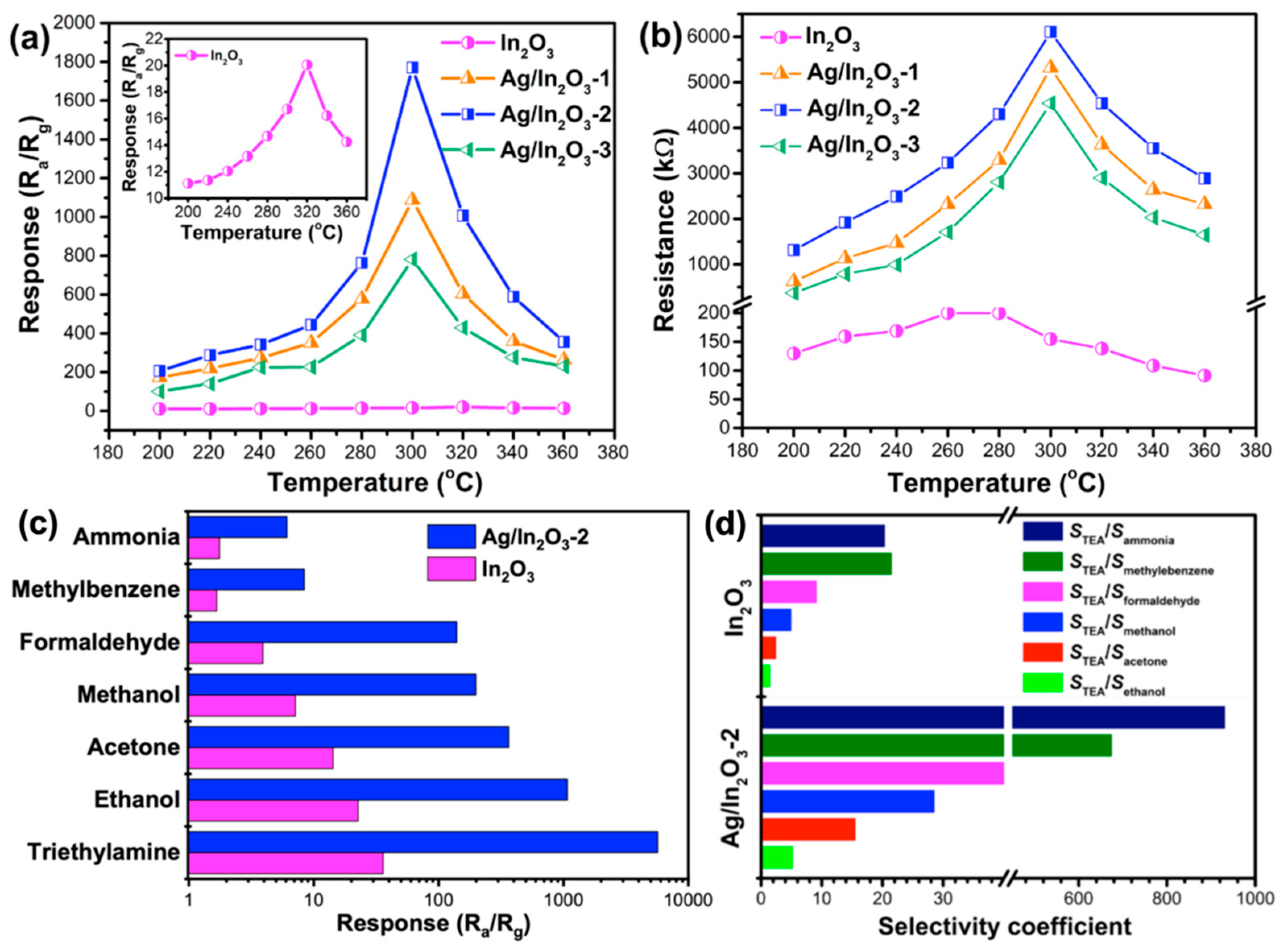
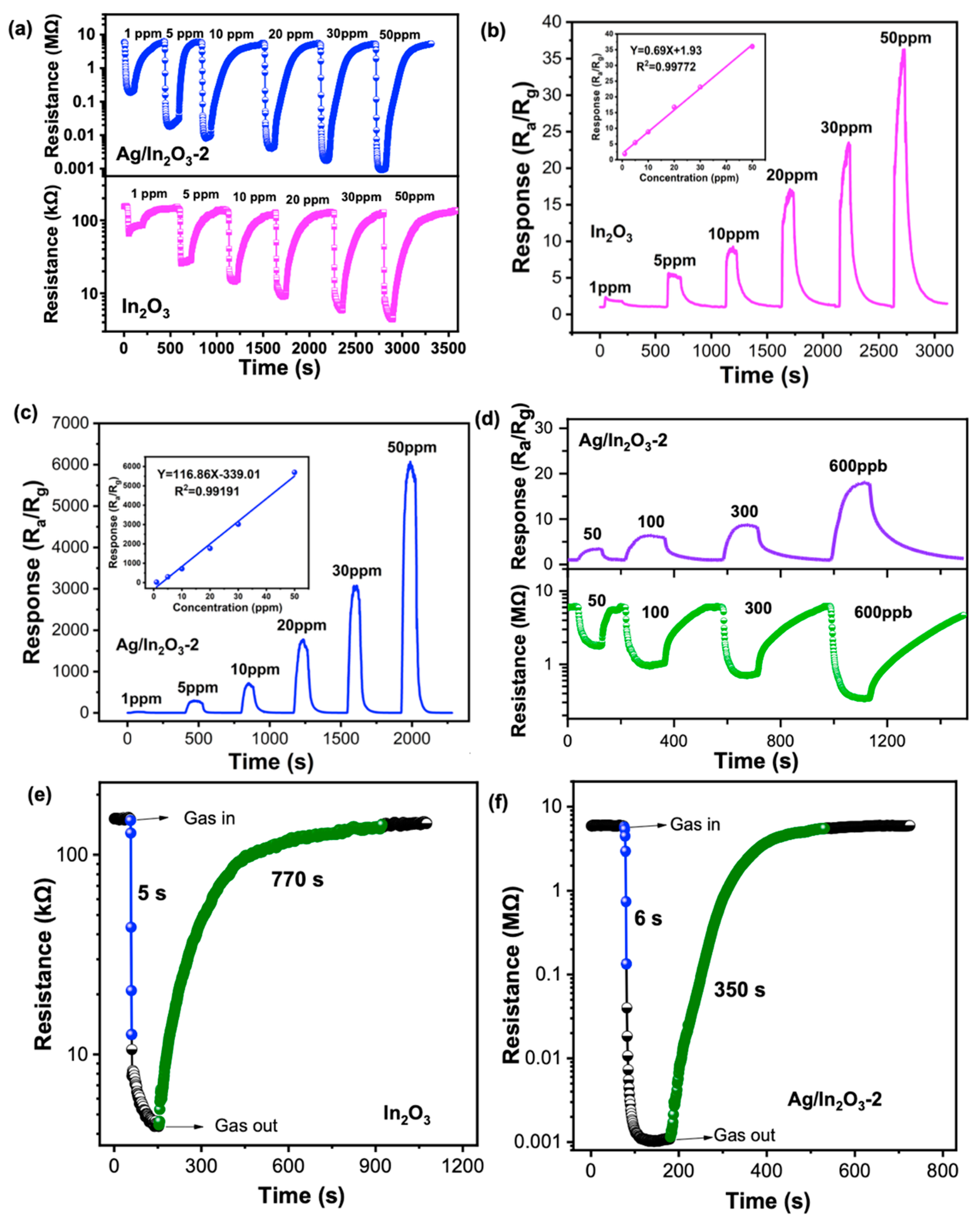
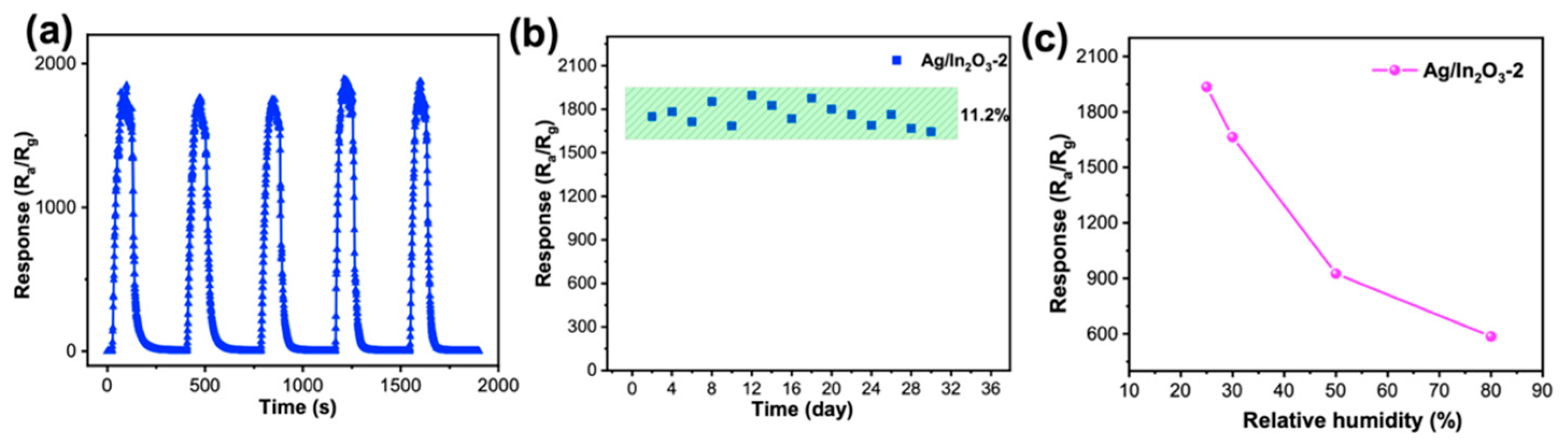
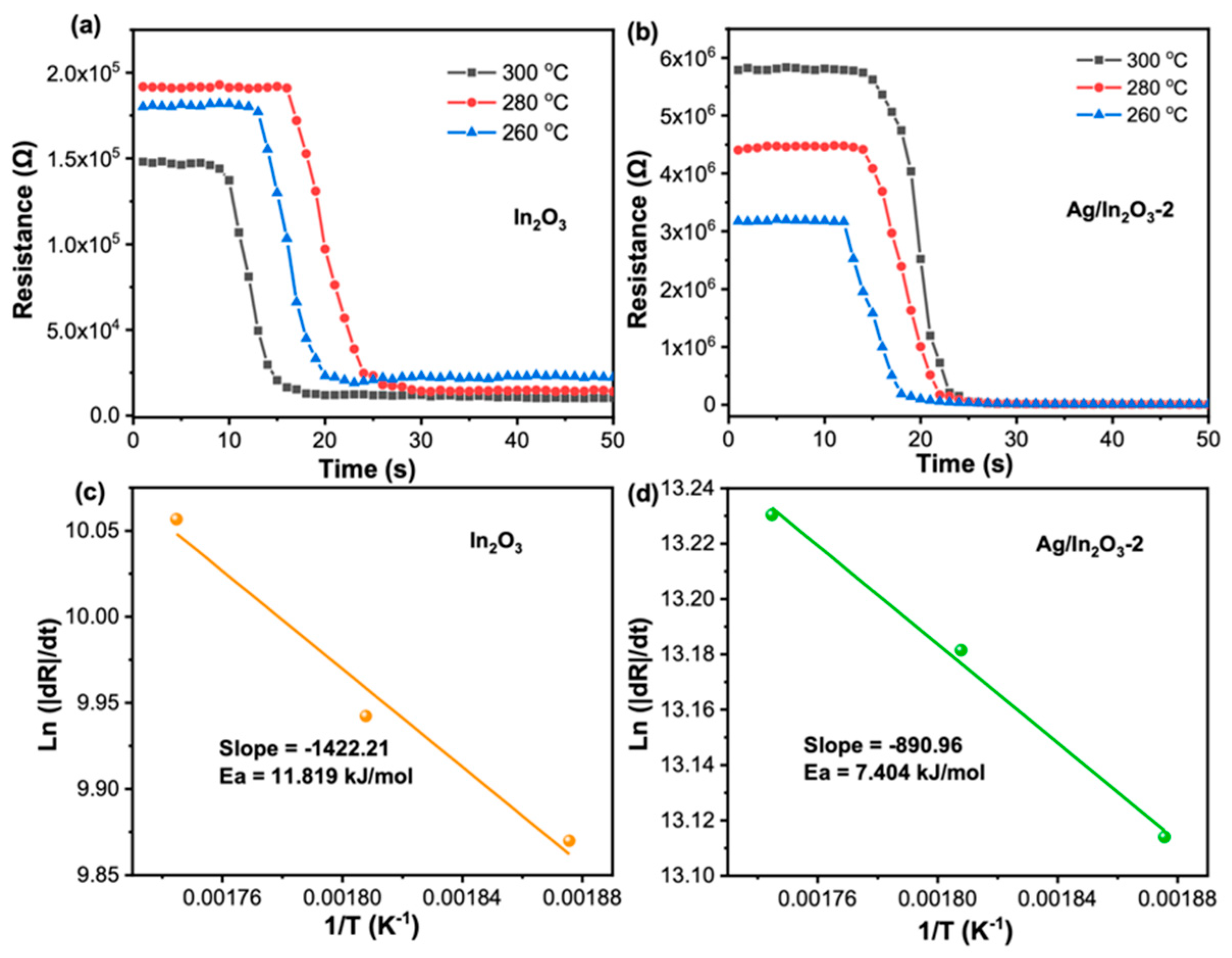
3.2. Gas Sensing Performance
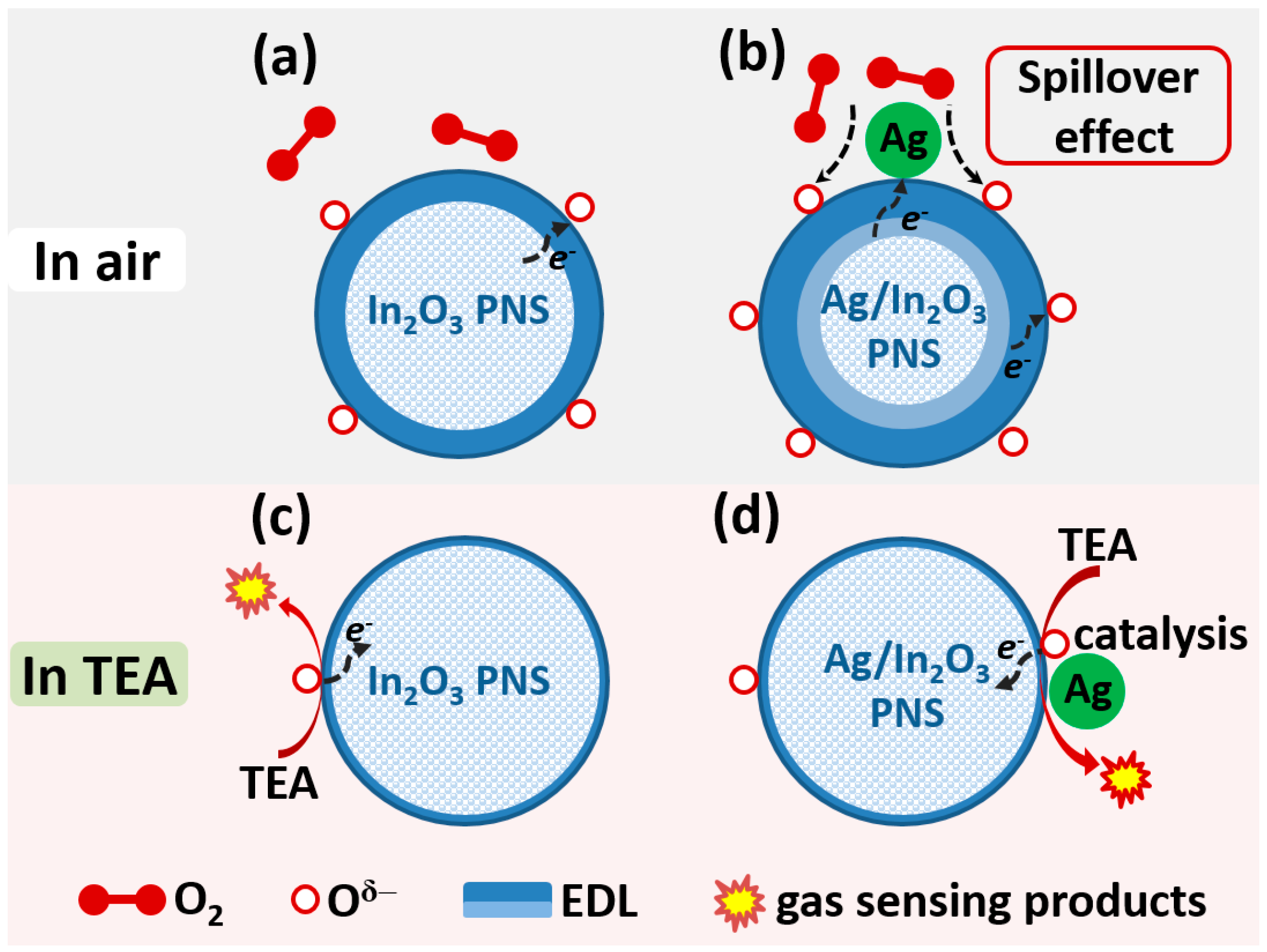
3.3. Gas Sensing Mechanism
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Capelli, L.; Sironi, S.; Del Rosso, R. Electronic noses for environmental monitoring applications. Sensors 2014, 14, 19979–20007. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Moon, H.G.; Lim, C.; Choi, K.; Song, H.S.; Bae, S.; Kim, S.M.; Seo, M.; Lee, T.; Lee, S.; et al. Humidity-tolerant single-stranded DNA-Functionalized graphene probe for medical applications of exhaled breath analysis. Adv. Funct. Mater. 2017, 27, 1700068. [Google Scholar] [CrossRef]
- Lloyd Spetz, A.; Huotari, J.; Bur, C.; Bjorklund, R.; Lappalainen, J.; Jantunen, H.; Schütze, A.; Andersson, M. Chemical sensor systems for emission control from combustions. Sens. Actuators B-Chem. 2013, 187, 184–190. [Google Scholar] [CrossRef] [Green Version]
- Love, C.; Nazemi, H.; El-Masri, E.; Ambrose, K.; Freund, M.S.; Emadi, A. A review on advanced sensing materials for agricultural gas sensors. Sensors 2021, 21, 3423. [Google Scholar] [CrossRef] [PubMed]
- Ju, D.X.; Xu, H.Y.; Qiu, Z.W.; Guo, J.; Zhang, J.; Cao, B.Q. Highly sensitive and selective triethylamine-sensing properties of nanosheets directly grown on ceramic tube by forming NiO/ZnO PN heterojunction. Sens. Actuators B-Chem. 2014, 200, 288–296. [Google Scholar] [CrossRef]
- Liu, S.R.; Guan, M.Y.; Li, X.Z.; Guo, Y. Light irradiation enhanced triethylamine gas sensing materials based on ZnO/ZnFe2O4 composites. Sens. Actuators B-Chem. 2016, 236, 350–357. [Google Scholar] [CrossRef]
- Bai, S.L.; Zhao, Y.H.; Sun, J.H.; Tong, Z.F.; Luo, R.X.; Li, D.Q.; Chen, A.F. Preparation of conducting films based on alpha-MoO3/PANI hybrids and their sensing properties to triethylamine at room temperature. Sens. Actuators B-Chem. 2017, 239, 131–138. [Google Scholar] [CrossRef]
- Zou, Y.H.; Chen, S.; Sun, J.; Liu, J.Q.; Che, Y.K.; Liu, X.H.; Zhang, J.; Yang, D.J. Highly efficient gas sensor using a hollow SnO2 microfiber for triethylamine detection. ACS Sens. 2017, 2, 897–902. [Google Scholar] [CrossRef]
- Shi, S.X.; Zhang, F.; Lin, H.M.; Wang, Q.; Shi, E.B.; Qu, F.Y. Enhanced triethylamine-sensing properties of P-N heterojunction Co3O4/In2O3 hollow microtubes derived from metal-organic frameworks. Sens. Actuators B-Chem. 2018, 262, 739–749. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, T. An overview: Facet-dependent metal oxide semiconductor gas sensors. Sens. Actuators B-Chem. 2018, 277, 604–633. [Google Scholar] [CrossRef]
- Mirzaei, A.; Kim, S.S.; Kim, H.W. Resistance-based H2S gas sensors using metal oxide nanostructures: A review of recent advances. J. Hazard. Mater. 2018, 357, 314–331. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Li, W.; Zhao, X.; Jiang, X.; Lin, S.; Zhen, Z.; Chen, W.; Xie, D.; Zhu, H. High-response room-temperature NO2 Sensor and ultrafast humidity sensor based on SnO2 with rich oxygen vacancy. ACS Appl. Mater. Interfaces 2019, 11, 13441–13449. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Aljneibi, S.A.A.A.; Yuan, J.; Wang, Y.; Liu, H.; Fang, J.; Tang, C.; Yan, X.; Cai, H.; Gu, Y.; et al. ZnO nanosheets abundant in oxygen vacancies derived from metal-organic frameworks for ppb-level gas sensing. Adv. Mater. 2019, 31, 1807161. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zheng, L.; Yang, C.; Zheng, W.; Liu, X.; Zhang, J. Oxygen vacancies enabled porous SnO2 thin films for highly sensitive detection of triethylamine at room temperature. ACS Appl. Mater. 2020, 12, 20704–20713. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Zhang, X.; Mi, R.; Liu, M.; Chen, Y.; Chen, C.; Ruan, S. On the high response towards TEA of gas sensors based on Ag-loaded 3D porous ZnO microspheres. Sens. Actuators B-Chem. 2018, 270, 492–499. [Google Scholar] [CrossRef]
- Zhang, B.; Li, Y.W.; Luo, N.; Xu, X.T.; Sun, G.; Wang, Y.; Cao, J.L. TiO2/ZnCo2O4 porous nanorods: Synthesis and temperature-dependent dual selectivity for sensing HCHO and TEA. Sens. Actuators B-Chem. 2020, 321, 128461. [Google Scholar] [CrossRef]
- Gu, F.; Cui, Y.; Han, D.; Hong, S.; Flytzani-Stephanopoulos, M.; Wang, Z. Atomically dispersed Pt (II) on WO3 for highly selective sensing and catalytic oxidation of triethylamine. Appl. Catal. B Environ. 2019, 256, 117809. [Google Scholar] [CrossRef]
- Sun, X.; Liu, X.; Deng, X.; Xu, X. Synthesis of Zn-doped In2O3 nano sphere architectures as a triethylamine gas sensor and photocatalytic properties. RSC Adv. 2016, 6, 89847–89854. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, D.; Wang, D.; Xu, Z.; Zhang, J. A high-stability weighing paper/polytetrafluoroethylene-based triboelectric nanogenerator for self-powered In2O3 nanocubes/SnS2 nanoflower NO2 gas sensors. J. Mater. Chem. A 2021, 9, 14495–14506. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, Y.T.; Song, P.; Yang, Z.X.; Wang, Q. ppb level triethylamine detection of yolk-shell SnO2/Au/Fe2O3 nanoboxes at low-temperature. Appl. Surf. Sci. 2019, 476, 391–401. [Google Scholar] [CrossRef]
- Yang, S.; Song, Z.; Gao, N.; Hu, Z.; Zhou, L.; Liu, J.; Zhang, B.; Zhang, G.; Jiang, S.; Li, H.-Y.; et al. Near room temperature operable H2S sensors based on In2O3 colloidal quantum dots. Sens. Actuators B-Chem. 2019, 286, 22–31. [Google Scholar] [CrossRef]
- Zhang, C.; Huan, Y.C.; Sun, D.J.; Lu, Y.L. Synthesis and NO2 sensing performances of CuO nanoparticles loaded In2O3 hollow spheres. J. Alloys Compd. 2020, 842, 155857. [Google Scholar] [CrossRef]
- Zhang, D.; Cao, Y.; Yang, Z.; Wu, J. Nanoheterostructure construction and DFT study of Ni-doped In2O3 nanocubes/WS2 hexagon nanosheets for formaldehyde sensing at room temperature. ACS Appl. Mater. Interfaces 2020, 12, 11979–11989. [Google Scholar] [CrossRef] [PubMed]
- Quan, W.; Hu, X.; Min, X.; Qiu, J.; Tian, R.; Ji, P.; Qin, W.; Wang, H.; Pan, T.; Cheng, S.; et al. A highly sensitive and selective ppb-level acetone sensor based on a Pt-doped 3D Porous SnO2 hierarchical structure. Sensors 2020, 20, 1150. [Google Scholar] [CrossRef] [Green Version]
- Gu, F.; Di, M.; Han, D.; Hong, S.; Wang, Z. Atomically dispersed Au on In2O3 Nanosheets for highly sensitive and selective detection of formaldehyde. ACS Sens. 2020, 5, 2611–2619. [Google Scholar] [CrossRef]
- Zhang, D.; Yang, Z.; Yu, S.; Mi, Q.; Pan, Q. Diversiform metal oxide-based hybrid nanostructures for gas sensing with versatile prospects. Coord. Chem. Rev. 2020, 413, 213272. [Google Scholar] [CrossRef]
- Zhang, K.; Qin, S.W.; Tang, P.G.; Feng, Y.J.; Li, D.Q. Ultra-sensitive ethanol gas sensors based on nanosheet-assembled hierarchical ZnO-In2O3 heterostructures. J. Hazard. Mater. 2020, 391, 122191. [Google Scholar] [CrossRef]
- Zhang, D.; Yang, Z.; Li, P.; Zhou, X. Ozone gas sensing properties of metal-organic frameworks-derived In2O3 hollow microtubes decorated with ZnO nanoparticles. Sens. Actuators B-Chem. 2019, 301, 127081. [Google Scholar] [CrossRef]
- Sukee, A.; Alharbi, A.A.; Staerz, A.; Wisitsoraat, A.; Liewhiran, C.; Weimar, U.; Barsan, N. Effect of AgO loading on flame-made LaFeO3 p-type semiconductor nanoparticles to acetylene sensing. Sens. Actuators B-Chem. 2020, 312, 127990. [Google Scholar] [CrossRef]
- Cai, Z.; Park, S. Synthesis of Pd nanoparticle-decorated SnO2 nanowires and determination of the optimum quantity of Pd nanoparticles for highly sensitive and selective hydrogen gas sensor. Sens. Actuators B-Chem. 2020, 322, 128651. [Google Scholar] [CrossRef]
- Yang, X.; Fu, H.; Tian, Y.; Xie, Q.; Xiong, S.; Han, D.; Zhang, H.; An, X. Au decorated In2O3 hollow nanospheres: A novel sensing material toward amine. Sens. Actuators B-Chem. 2019, 296, 126696. [Google Scholar] [CrossRef]
- Zhou, S.; Lu, Q.; Chen, M.; Li, B.; Wei, H.; Zi, B.; Zeng, J.; Zhang, Y.; Zhang, J.; Zhu, Z.; et al. Platinum-supported cerium-doped indium oxide for highly sensitive triethylamine gas sensing with good antihumidity. ACS Appl. Mater. Interfaces 2020, 12, 42962–42970. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zheng, W.; Liu, X.; Zhang, L.; Zheng, L.; Yang, C.; Pinna, N.; Zhang, J. Platinum single atoms on tin oxide ultrathin films for extremely sensitive gas detection. Mater. Horiz. 2020, 7, 1519–1527. [Google Scholar] [CrossRef]
- Pan, Q.N.; Li, T.T.; Zhang, D.Z. Ammonia gas sensing properties and density functional theory investigation of coral-like Au-SnSe2 schottky junction. Sens. Actuators B-Chem. 2021, 332, 129440. [Google Scholar] [CrossRef]
- Majhi, S.M.; Naik, G.K.; Lee, H.J.; Song, H.G.; Lee, C.R.; Lee, I.H.; Yu, Y.T. Au@NiO core-shell nanoparticles as a p-type gas sensor: Novel synthesis, characterization, and their gas sensing properties with sensing mechanism. Sens. Actuators B-Chem. 2018, 268, 223–231. [Google Scholar] [CrossRef]
- Zhang, D.; Sun, Y.E.; Jiang, C.; Yao, Y.; Wang, D.; Zhang, Y. Room-temperature highly sensitive CO gas sensor based on Ag-loaded zinc oxide/molybdenum disulfide ternary nanocomposite and its sensing properties. Sens. Actuators B-Chem. 2017, 253, 1120–1128. [Google Scholar] [CrossRef]
- Ju, D.X.; Xu, H.Y.; Qiu, Z.W.; Zhang, Z.C.; Xu, Q.; Zhang, J.; Wang, J.Q.; Cao, B.Q. Near room temperature, fast-response, and highly sensitive triethylamine sensor assembled with Au-Loaded ZnO/SnO2 Core-Shell nanorods on flat alumina substrates. ACS Appl. Mater. Interfaces 2015, 7, 19163–19171. [Google Scholar] [CrossRef] [PubMed]
- Peng, R.; Li, Y.; Liu, T.; Si, P.; Feng, J.; Suhr, J.; Ci, L. Boron-doped graphene coated Au@SnO2 for high-performance triethylamine gas detection. Mater. Chem. Phys. 2020, 239, 121961. [Google Scholar] [CrossRef]
- Zhang, D.; Yang, Z.; Li, P.; Pang, M.; Xue, Q. Flexible self-powered high-performance ammonia sensor based on Au-decorated MoSe2 nanoflowers driven by single layer MoS2-flake piezoelectric nanogenerator. Nano Energy 2019, 65, 103974. [Google Scholar] [CrossRef]
- Zhao, H.; Yin, H.; Yu, X.X.; Zhang, W.; Li, C.; Zhu, M.Q. In2O3 nanoparticles/carbon fiber hybrid mat as free-standing anode for lithium-ion batteries with enhanced electrochemical performance. J. Alloys Compd. 2018, 735, 319–326. [Google Scholar] [CrossRef]
- Zhu, B.; Wu, X.H.; Liu, W.J.; Lu, H.L.; Zhang, D.W.; Fan, Z.Y.; Ding, S.J. High-performance on-chip supercapacitors based on mesoporous silicon coated with ultrathin atomic layer-deposited In2O3 films. ACS Appl. Mater. Interfaces 2019, 11, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Chao, Y.G.; Zhou, P.; Li, N.; Lai, J.P.; Yang, Y.; Zhang, Y.L.; Tang, Y.H.; Yang, W.X.; Du, Y.P.; Su, D.; et al. Ultrathin visible-light-driven Mo incorporating In2O3-ZnIn2Se4 Z-scheme nanosheet photocatalysts. Adv. Mater. 2019, 31, 1807226. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.H.; Hou, C.L.; De, Q.M.; Gu, F.B.; Han, D.M. One-step synthesis of Co-doped In2O3 nanorods for high response of formaldehyde sensor at low temperature. ACS Sens. 2018, 3, 468–475. [Google Scholar] [CrossRef]
- Hu, J.; Sun, Y.; Xue, Y.; Zhang, M.; Li, P.; Lian, K.; Zhuiykov, S.; Zhang, W.; Chen, Y. Highly sensitive and ultra-fast gas sensor based on CeO2-loaded In2O3 hollow spheres for ppb-level hydrogen detection. Sens. Actuators B-Chem. 2018, 257, 124–135. [Google Scholar] [CrossRef]
- Hwang, J.; Jung, H.; Shin, H.S.; Kim, D.S.; Kim, D.S.; Ju, B.K.; Chun, M. The effect of noble metals on co gas sensing properties of In2O3 nanoparticles. Appl. Sci. 2021, 11, 4903. [Google Scholar] [CrossRef]
- Xue, D.P.; Zhang, S.S.; Zhang, Z.Y. Hydrothermal synthesis of methane sensitive porous In2O3 nanosheets. Mater. Lett. 2019, 252, 169–172. [Google Scholar] [CrossRef]
- Zhang, S.; Song, P.; Zhang, J.; Yan, H.H.; Li, J.; Yang, Z.X.; Wang, Q. Highly sensitive detection of acetone using mesoporous In2O3 nanospher es decorated with Au nanoparticles. Sens. Actuators B-Chem. 2017, 242, 983–993. [Google Scholar] [CrossRef]
- Li, Z.J.; Yan, S.N.; Wu, Z.L.; Li, H.; Wang, J.Q.; Shen, W.Z.; Wang, Z.G.; Fu, Y.Q. Hydrogen gas sensor based on mesoporous In2O3 with fast response/recovery and ppb level detection limit. Int. J. Hydrogen Energy 2018, 43, 22746–22755. [Google Scholar] [CrossRef]
- Qin, Z.J.; Liu, Y.K.; Chen, W.W.; Ai, P.; Wu, Y.M.; Li, S.H.; Yu, D.P. Highly sensitive alcohol sensor based on a single Er-doped In2O3 nanoribbon. Chem. Phys. Lett. 2016, 646, 12–17. [Google Scholar] [CrossRef]
- Han, D.M.; Zhai, L.L.; Gu, F.B.; Wang, Z.H. Highly sensitive NO2 gas sensor of ppb-level detection based on In2O3 nanobricks at low temperature. Sens. Actuators B-Chem. 2018, 262, 655–663. [Google Scholar] [CrossRef]
- Zheng, L.; Ma, T.; Zhao, Y.; Xu, Y.; Sun, L.; Zhang, J.; Liu, X. Synergy between Au and In2O3 microspheres: A superior hybrid structure for the selective and sensitive detection of triethylamine. Sens. Actuators B-Chem. 2019, 290, 155–162. [Google Scholar] [CrossRef]
- Liu, X.J.; Zhao, K.R.; Sun, X.L.; Zhang, C.; Duan, X.P.; Hou, P.Y.; Zhao, G.; Zhang, S.W.; Yang, H.C.; Cao, R.Y.; et al. Rational design of sensitivity enhanced and stability improved TEA gas sensor assembled with Pd nanoparticles-functionalized In2O3 composites. Sens. Actuators B-Chem. 2019, 285, 1–10. [Google Scholar] [CrossRef]
- Ding, M.; Xie, N.; Wang, C.; Kou, X.; Zhang, H.; Guo, L.; Sun, Y.; Chuai, X.; Gao, Y.; Liu, F.; et al. Enhanced NO2 gas sensing properties by Ag-doped hollow urchin-like In2O3 hierarchical nanostructures. Sens. Actuators B-Chem. 2017, 252, 418–427. [Google Scholar] [CrossRef]
- Yan, S.; Li, Z.; Li, H.; Wu, Z.; Wang, J.; Shen, W.; Fu, Y.Q. Ultra-sensitive room-temperature H2S sensor using Ag–In2O3 nanorod composites. J. Mater. Sci. 2018, 53, 16331–16344. [Google Scholar] [CrossRef]
- Jin, X.H.; Li, Y.W.; Zhang, B.; Xu, X.T.; Sun, G.; Wang, Y. Temperature-dependent dual selectivity of hierarchical porous In2O3 nanospheres for sensing ethanol and TEA. Sens. Actuators B-Chem. 2021, 330, 129271. [Google Scholar] [CrossRef]
- Li, Y.W.; Luo, N.; Sun, G.; Zhang, B.; Jin, H.H.; Lin, L.; Bala, H.; Cao, J.L.; Zhang, Z.Y.; Wang, Y. Synthesis of porous nanosheets-assembled ZnO/ZnCo2O4 hierarchical structure for TEA detection. Sens. Actuators B-Chem. 2019, 287, 199–208. [Google Scholar] [CrossRef]
- Liu, X.; Sun, X.; Duan, X.; Zhang, C.; Zhao, K.; Xu, X. Core-shell Ag@In2O3 hollow hetero-nanostructures for selective ethanol detection in air. Sens. Actuators B-Chem. 2020, 305, 127450. [Google Scholar] [CrossRef]
- Han, L.; Yin, D.; Yang, X.; Li, J.; Gao, X.; Liu, H. Incorporation of Ag-In2O3 nanostructures into sodium borosilicate glass: A combined route for high transparency and fluorescence. Mater. Chem. Phys. 2016, 181, 234–240. [Google Scholar] [CrossRef]
- Zeng, J.; Rong, Q.; Xiao, B.; Yu, R.; Zi, B.; Kuang, X.; Deng, X.; Ma, Y.; Zhang, J.; Wu, J.; et al. Single-atom silver loaded on tungsten oxide with oxygen vacancies for high performance triethylamine gas sensors. J. Mater. Chem. A 2021, 9, 8704–8710. [Google Scholar] [CrossRef]
- Navaneethan, M.; Patil, V.L.; Ponnusamy, S.; Muthamizhchelvan, C.; Kawasaki, S.; Patil, P.S.; Hayakawa, Y. Sensitivity enhancement of ammonia gas sensor based on Ag/ZnO flower and nanoellipsoids at low temperature. Sens. Actuators B-Chem. 2018, 255, 672–683. [Google Scholar]
- Zheng, L.; Xie, J.; Liu, X.; Yang, C.; Zheng, W.; Zhang, J. Unveiling the Electronic Interaction in ZnO/PtO/Pt nanoarrays for catalytic detection of triethylamine with ultrahigh sensitivity. ACS Appl. Mater. Interfaces 2020, 12, 46267–46276. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Tian, X.; Liu, P.; Sun, Y.; Du, G. In2O3 nanoplates with different crystallinity and porosity: Controllable synthesis and gas-sensing properties investigation. J. Alloys Compd. 2019, 787, 1063–1073. [Google Scholar] [CrossRef]
- Zhu, M.; Yang, T.; Zhai, C.; Du, L.; Zhang, J.; Zhang, M. Fast triethylamine gas sensing response properties of Ho-doped SnO2 nanoparticles. J. Alloys Compd. 2020, 817, 152724. [Google Scholar] [CrossRef]
- Xu, H.Y.; Li, W.R.; Han, R.; Zhai, T.; Yu, H.Q.; Chen, Z.R.; Wu, X.W.; Wang, J.Q.; Cao, B.Q. Enhanced triethylamine sensing properties by fabricating Au@SnO2/alpha-Fe2O3 core-shell nanoneedles directly on alumina tubes. Sens. Actuators B-Chem. 2018, 262, 70–78. [Google Scholar] [CrossRef]
- Kim, J.S.; Na, C.W.; Kwak, C.H.; Li, H.Y.; Yoon, J.W.; Kim, J.H.; Jeong, S.Y.; Lee, J.H. Humidity-independent gas sensors using Pr-doped In2O3 macroporous spheres: Role of cyclic Pr3+/Pr4+ redox reactions in suppression of water-poisoning effect. ACS Appl. Mater. Interfaces 2019, 11, 25322–25329. [Google Scholar] [CrossRef] [PubMed]
- Gu, F.; Li, C.; Han, D.; Wang, Z. Manipulating the defect structure (VO) of In2O3 nanoparticles for enhancement of formaldehyde detection. ACS Appl. Mater. Interfaces 2018, 10, 933–942. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wang, B.; Wang, Y. High-temperature gas sensor based on novel Pt Single Atoms@SnO2 Nanorods@SiC nanosheets multi-heterojunctions. ACS Appl. Mater. Interfaces 2020, 12, 21808–21817. [Google Scholar] [CrossRef]


| Materials | Con. (ppm) | Response (Ra/Rg) | Temp. (°C) | Tres/Trec (s) | STEA/Sethanol | Ref. |
|---|---|---|---|---|---|---|
| Ag/In2O3 porous nanosphere | 50 | 5697.15 | 300 | 6/350 | 5.30 | This work |
| Single crystalline In2O3 nanoplates | 50 | 7.8 | 320 | 10/19 | 5.20 | [62] |
| SnO2: Ho3+ nanoparticles | 50 | 12 | 175 | 2/120 | 1.88 | [63] |
| Zn-doped In2O3 nanospheres | 50 | 36 | 280 | 9/7 | 3.00 | [18] |
| Yolk-shell SnO2/Au/Fe2O3 nanoboxes | 100 | 126.84 | 240 | 7/10 | 1.41 | [20] |
| Pd NPs-In2O3 | 50 | 47.56 | 220 | 4/17 | 1.85 | [52] |
| Au@SnO2/α-Fe2O3 nanoneedles | 100 | 39 | 300 | 4/203 | 2.57 | [64] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.; Li, Y.; Wang, X.; Sun, G.; Cao, J.; Wang, Y. Improved TEA Sensitivity and Selectivity of In2O3 Porous Nanospheres by Modification with Ag Nanoparticles. Nanomaterials 2022, 12, 1532. https://doi.org/10.3390/nano12091532
Li D, Li Y, Wang X, Sun G, Cao J, Wang Y. Improved TEA Sensitivity and Selectivity of In2O3 Porous Nanospheres by Modification with Ag Nanoparticles. Nanomaterials. 2022; 12(9):1532. https://doi.org/10.3390/nano12091532
Chicago/Turabian StyleLi, Dengke, Yanwei Li, Xiaohua Wang, Guang Sun, Jianliang Cao, and Yan Wang. 2022. "Improved TEA Sensitivity and Selectivity of In2O3 Porous Nanospheres by Modification with Ag Nanoparticles" Nanomaterials 12, no. 9: 1532. https://doi.org/10.3390/nano12091532
APA StyleLi, D., Li, Y., Wang, X., Sun, G., Cao, J., & Wang, Y. (2022). Improved TEA Sensitivity and Selectivity of In2O3 Porous Nanospheres by Modification with Ag Nanoparticles. Nanomaterials, 12(9), 1532. https://doi.org/10.3390/nano12091532








