Immobilization of Streptavidin on a Plasmonic Au-TiO2 Thin Film towards an LSPR Biosensing Platform
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation and Characterization of the LSPR Biosensor Platform
2.1.1. Deposition of Au-TiO2 by Reactive DC Magnetron Sputtering
2.1.2. Post-Deposition Annealing Treatment to Promote the Growth of the Au Nanoparticles
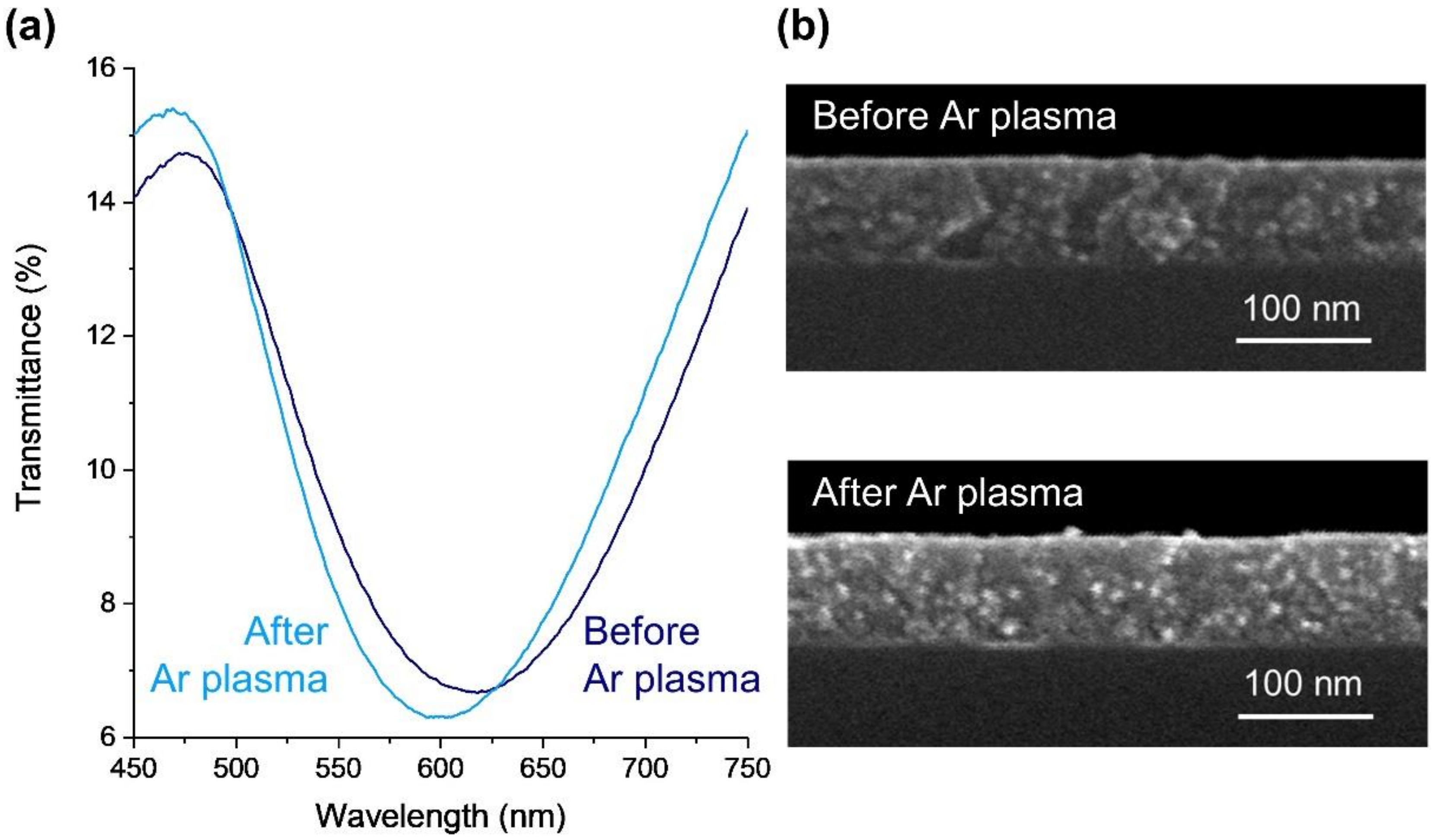
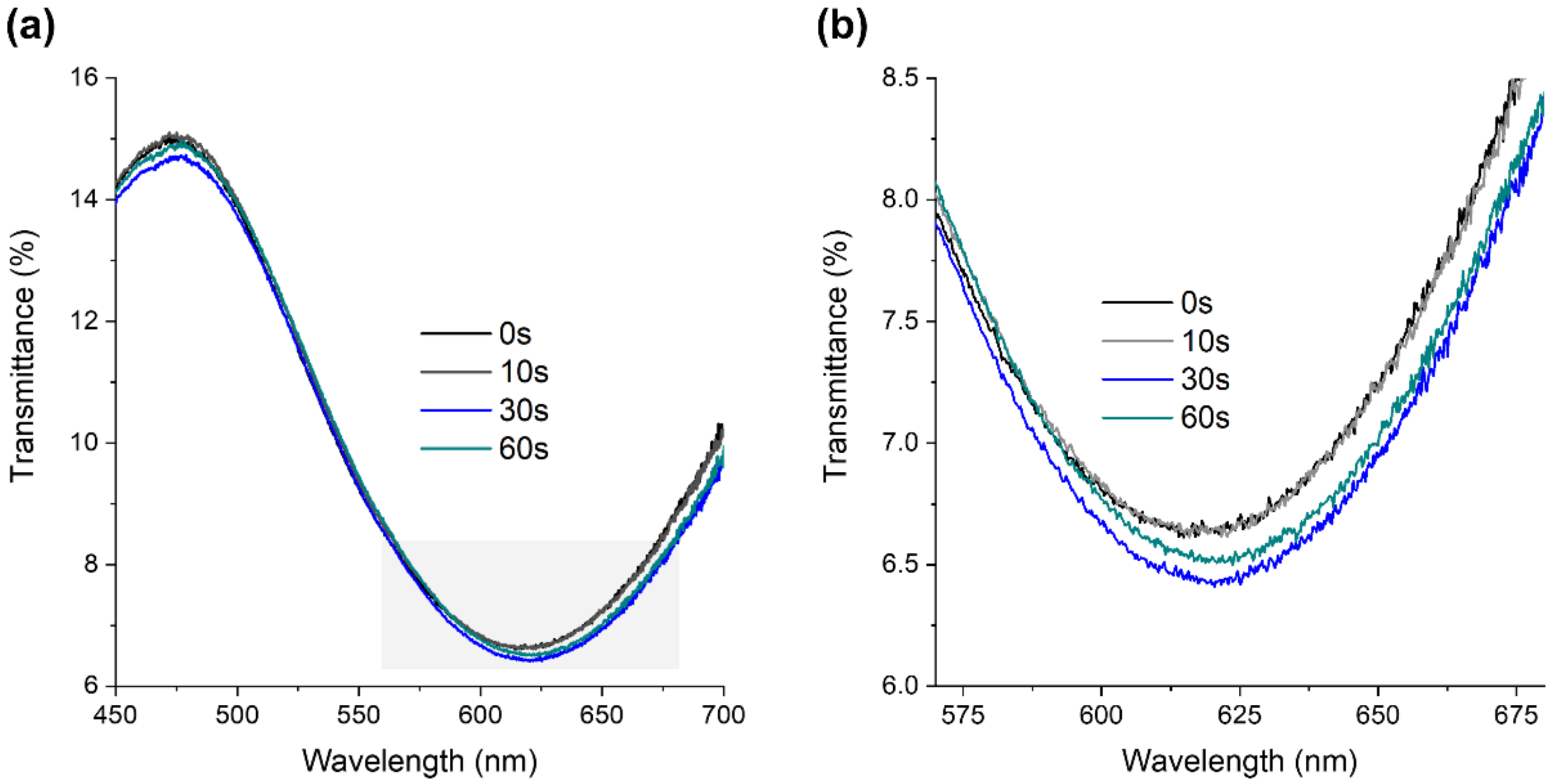
2.1.3. Surface Modification through Plasma Etching
2.1.4. Characterization of Au-TiO2 Thin Films
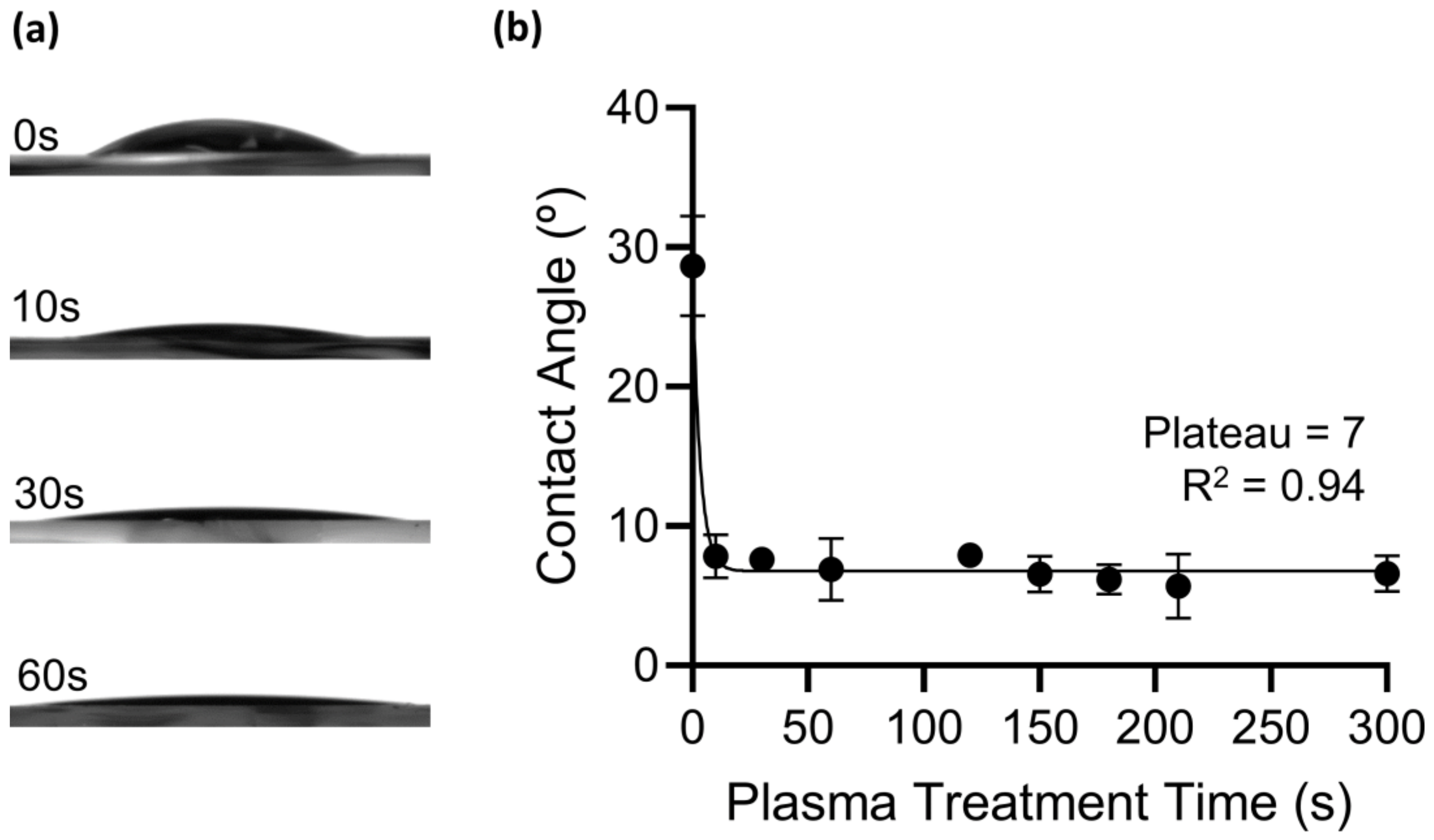
2.1.5. Modification and Measurement of the Thin Films’ Hydrophilic Property
2.2. Protein Immobilization and Binding Assay
2.2.1. Concentration Optimization
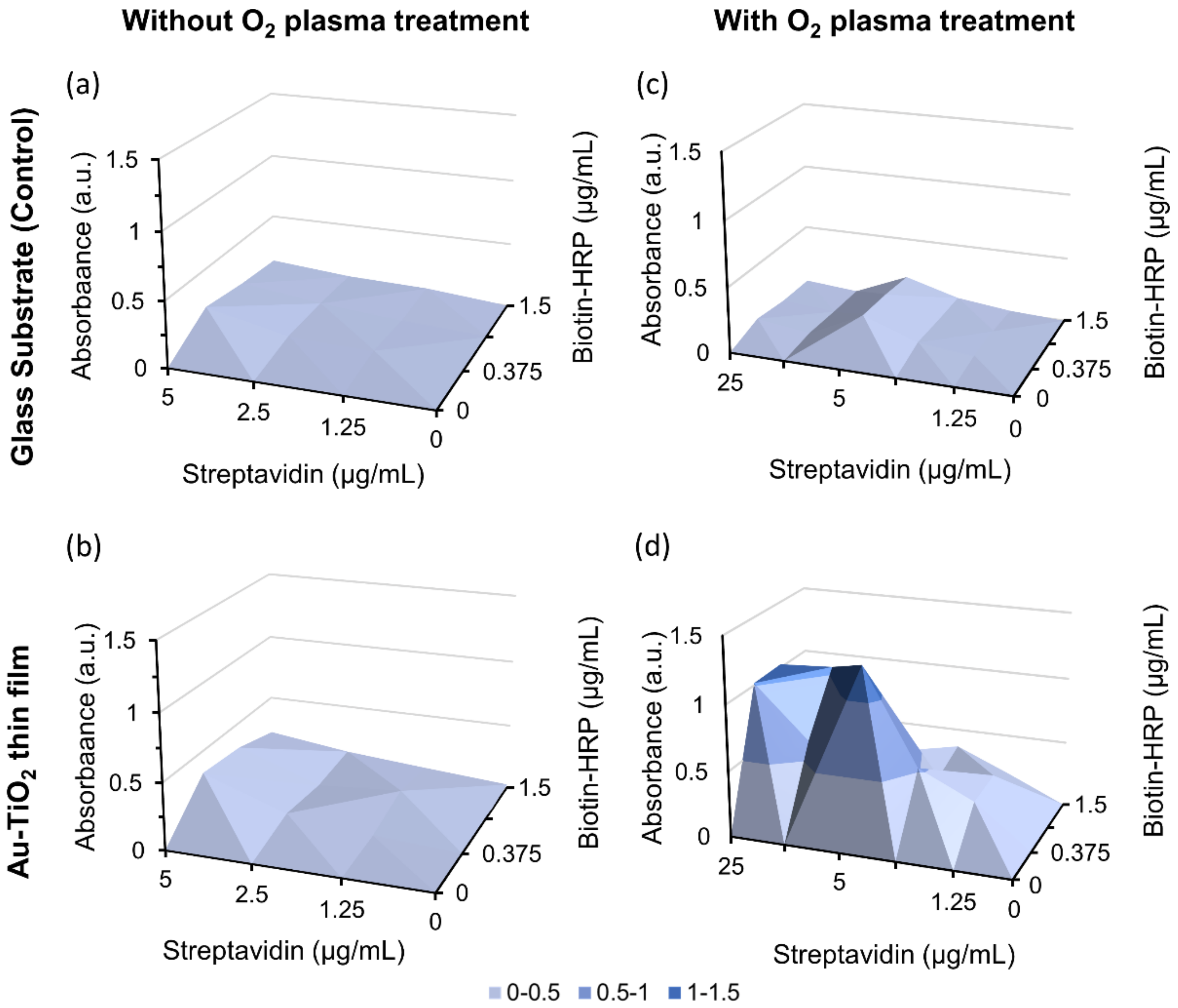
2.2.2. Influence of O2 Plasma on Streptavidin Immobilization
2.2.3. Streptavidin Immobilization on Thin Film’s Surface
2.3. Optical Response of the LSPR Biosensing Platform
2.3.1. Refractive Index Sensitivity of Au-TiO2 Thin Films
2.3.2. LSPR Response of the Streptavidin-Immobilized Thin Films to Biotin–HRP
3. Results and Discussion
3.1. Characterization of Au-TiO2 Thin Films
3.1.1. Chemical, Optical, and Morphological Properties of Au-TiO2 Thin Films
3.1.2. Au-TiO2 Thin Films’ Hydrophilic Properties
3.2. Streptavidin Immobilization and Binding Assay
3.3. LSPR Biosensing Platform Sensitivity
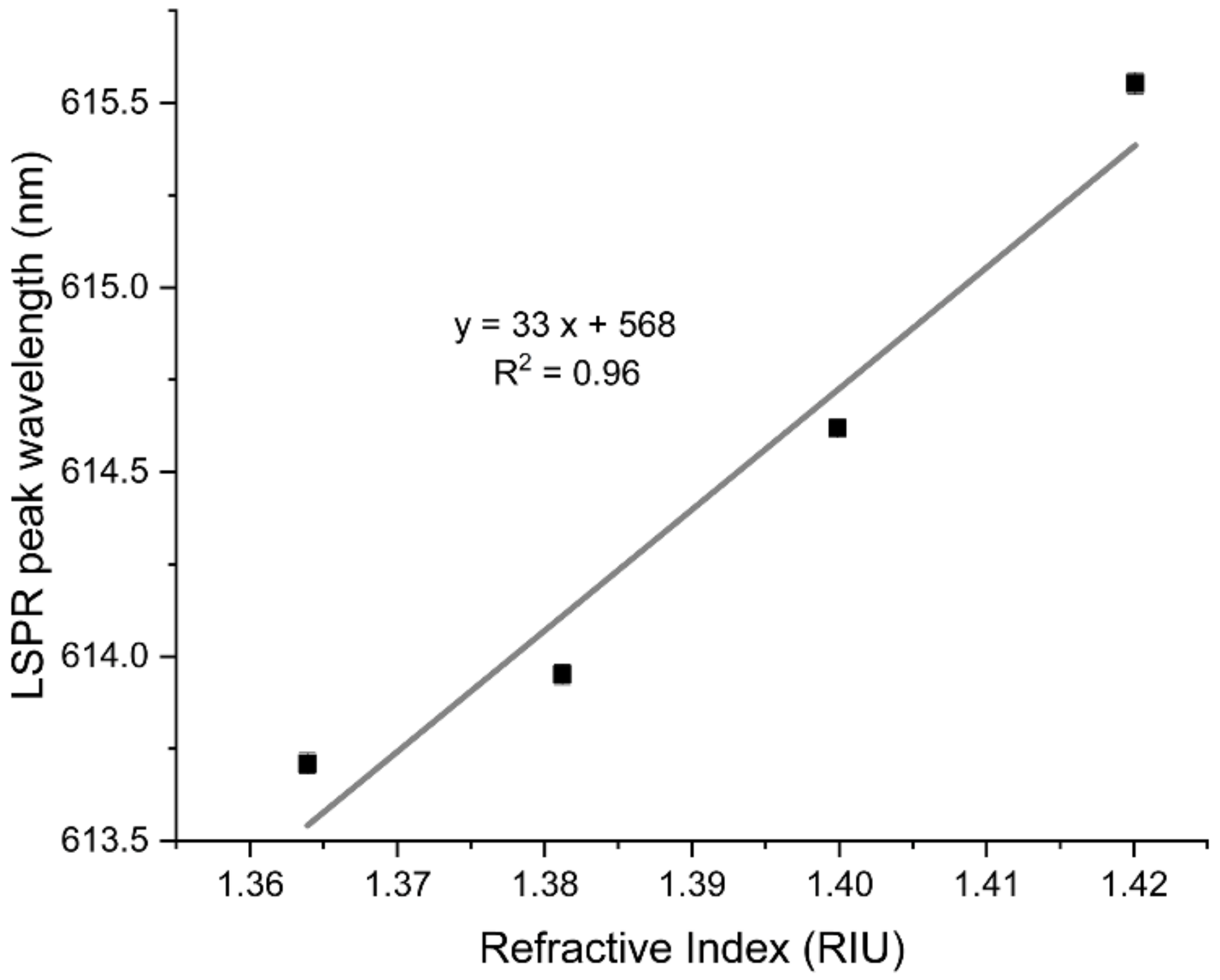
3.3.1. Refractive Index Sensitivity of the Au-TiO2 Thin Films
3.3.2. LSPR Response of the Streptavidin-Immobilized Thin Films to Biotin–HRP
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Justino, C.I.L.; Duarte, A.C.; Rocha-Santos, T.A.P. Recent Progress in Biosensors for Environmental Monitoring: A Review. Sensors 2017, 17, 2918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thakur, M.S.; Ragavan, K.V. Biosensors in Food Processing. J. Food Sci. Technol. 2013, 50, 625–641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Campbell, A.S.; de Ávila, B.E.F.; Wang, J. Wearable Biosensors for Healthcare Monitoring. Nat. Biotechnol. 2019, 37, 389–406. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, A.; Miyata, T. Biosensors. In Biomaterials Nanoarchitectonics; Elsevier: Amsterdam, The Netherlands, 2016; pp. 157–176. [Google Scholar] [CrossRef]
- Bhalla, N.; Jolly, P.; Formisano, N.; Estrela, P. Introduction to Biosensors. Essays Biochem. 2016, 60, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Damborský, P.; Švitel, J.; Katrlík, J. Optical Biosensors. Essays Biochem. 2016, 60, 91–100. [Google Scholar] [CrossRef] [Green Version]
- Pitarke, J.M.; Silkin, V.M.; Chulkov, E.V.; Echenique, P.M. Theory of Surface Plasmons and Surface-Plasmon Polaritons. Rep. Prog. Phys. 2007, 70, 1. [Google Scholar] [CrossRef]
- Hutter, E.; Fendler, J.H. Exploitation of Localized Surface Plasmon Resonance. Adv. Mater. 2004, 16, 1685–1706. [Google Scholar] [CrossRef]
- Xiao, L.; Youji, L.; Feitai, C.; Peng, X.; Ming, L. Facile Synthesis of Mesoporous Titanium Dioxide Doped by Ag-Coated Graphene with Enhanced Visible-Light Photocatalytic Performance for Methylene Blue Degradation. RSC Adv. 2017, 7, 25314–25324. [Google Scholar] [CrossRef] [Green Version]
- Toudert, J.; Simonot, L.; Camelio, S.; Babonneau, D. Advanced Optical Effective Medium Modeling for a Single Layer of Polydisperse Ellipsoidal Nanoparticles Embedded in a Homogeneous Dielectric Medium: Surface Plasmon Resonances. Phys. Rev. B—Condens. Matter Mater. Phys. 2012, 86, 045415. [Google Scholar] [CrossRef] [Green Version]
- Deng, Y.; Cao, G.; Wu, Y.; Zhou, X.; Liao, W. Theoretical Description of Dynamic Transmission Characteristics in MDM Waveguide Aperture-Side-Coupled with Ring Cavity. Plasmonics 2015, 10, 1537–1543. [Google Scholar] [CrossRef]
- Chen, F.; Yang, W. Plasmonic Induced Reflection Based on Al2O3 Nanoslit Side Coupled with Silicon Nanodisk Resonator. Results Opt. 2021, 5, 100126. [Google Scholar] [CrossRef]
- Pereira, R.M.S.; Borges, J.; Peres, F.C.R.; Pereira, P.A.S.; Smirnov, G.V.; Vaz, F.; Cavaleiro, A.; Vasilevskiy, M.I. Effect of Clustering on the Surface Plasmon Band in Thin Films of Metallic Nanoparticles. J. Nanophotonics 2014, 9, 093796. [Google Scholar] [CrossRef]
- Borges, J.; Pereira, R.M.S.; Rodrigues, M.S.; Kubart, T.; Kumar, S.; Leifer, K.; Cavaleiro, A.; Polcar, T.; Vasilevskiy, M.I.; Vaz, F. Broadband Optical Absorption Caused by the Plasmonic Response of Coalesced Au Nanoparticles Embedded in a TiO2 Matrix. J. Phys. Chem. C 2016, 120, 16931–16945. [Google Scholar] [CrossRef]
- Petryayeva, E.; Krull, U.J. Localized Surface Plasmon Resonance: Nanostructures, Bioassays and Biosensing—A Review. Anal. Chim. Acta 2011, 706, 8–24. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Kou, X.; Yang, Z.; Ni, W.; Wang, J. Shape- and Size-Dependent Refractive Index Sensitivity of Gold Nanoparticles. Langmuir 2008, 24, 5233–5237. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Chen, Z.; Yang, H.; Wen, L.; Yi, Z.; Zhou, Z.; Dai, B.; Zhang, J.; Wu, X.; Wu, P. Multi-Mode Surface Plasmon Resonance Absorber Based on Dart-Type Single-Layer Graphene. RSC Adv. 2022, 12, 7821–7829. [Google Scholar] [CrossRef]
- Wu, X.; Zheng, Y.; Luo, Y.; Zhang, J.; Yi, Z.; Wu, X.; Cheng, S.; Yang, W.; Yu, Y.; Wu, P. A Four-Band and Polarization-Independent BDS-Based Tunable Absorber with High Refractive Index Sensitivity. Phys. Chem. Chem. Phys. 2021, 23, 26864–26873. [Google Scholar] [CrossRef]
- Jain, S.; Paliwal, A.; Gupta, V.; Tomar, M. Refractive Index Tuning of SiO2 for Long Range Surface Plasmon Resonance Based Biosensor. Biosens. Bioelectron. 2020, 168, 112508. [Google Scholar] [CrossRef]
- Jia, S.; Bian, C.; Sun, J.; Tong, J.; Xia, S. A Wavelength-Modulated Localized Surface Plasmon Resonance (LSPR) Optical Fiber Sensor for Sensitive Detection of Mercury(II) Ion by Gold Nanoparticles-DNA Conjugates. Biosens. Bioelectron. 2018, 114, 15–21. [Google Scholar] [CrossRef]
- Mayer, K.M.; Hafner, J.H. Localized Surface Plasmon Resonance Sensors. Chem. Rev. 2011, 111, 3828–3857. [Google Scholar] [CrossRef]
- Haes, A.J.; Van Duyne, R.P. Preliminary Studies and Potential Applications of Localized Surface Plasmon Resonance Spectroscopy in Medical Diagnostics. Expert Rev. Mol. Diagn. 2004, 4, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.M.; Park, J.S.; Jung, S.W.; Yeom, J.; Yoo, S.M. Biosensing Applications Using Nanostructure-Based Localized Surface Plasmon Resonance Sensors. Sensors 2021, 21, 3191. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.S.; Costa, D.; Domingues, R.P.; Apreutesei, M.; Pedrosa, P.; Martin, N.; Correlo, V.M.; Reis, R.L.; Alves, E.; Barradas, N.P.; et al. Optimization of Nanocomposite Au/TiO2 Thin Films towards LSPR Optical-Sensing. Appl. Surf. Sci. 2018, 438, 74–83. [Google Scholar] [CrossRef]
- Proença, M.; Borges, J.; Rodrigues, M.S.; Domingues, R.P.; Dias, J.P.; Trigueiro, J.; Bundaleski, N.; Teodoro, O.M.N.D.; Vaz, F. Development of Au/CuO Nanoplasmonic Thin Films for Sensing Applications. Surf. Coat. Technol. 2018, 343, 178–185. [Google Scholar] [CrossRef]
- Barbosa, A.I.; Borges, J.; Meira, D.I.; Costa, D.; Rodrigues, M.S.; Rebelo, R.; Correlo, V.M.; Vaz, F.; Reis, R.L. Development of Label-Free Plasmonic Au-TiO2 Thin Film Immunosensor Devices. Mater. Sci. Eng. C 2019, 100, 424–432. [Google Scholar] [CrossRef]
- Kedem, O.; Vaskevich, A.; Rubinstein, I. Critical Issues in Localized Plasmon Sensing. J. Phys. Chem. C 2014, 118, 8227–8244. [Google Scholar] [CrossRef]
- Lopez, G.A.; Estevez, M.C.; Soler, M.; Lechuga, L.M. Recent Advances in Nanoplasmonic Biosensors: Applications and Lab-on-a-Chip Integration. Nanophotonics 2017, 6, 123–136. [Google Scholar] [CrossRef]
- Aćimović, S.S.; Ortega, M.A.; Sanz, V.; Berthelot, J.; Garcia-Cordero, J.L.; Renger, J.; Maerkl, S.J.; Kreuzer, M.P.; Quidant, R. LSPR Chip for Parallel, Rapid, and Sensitive Detection of Cancer Markers in Serum. Nano Lett. 2014, 14, 2636–2641. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.; Shaban, S.M.; Pyun, D.G.; Kim, D.H. Solid-Phase Colorimetric Apta-Biosensor for Thrombin Detection. Thin Solid Film. 2019, 686, 137428. [Google Scholar] [CrossRef]
- Kim, J.; Moon, B.S.; Hwang, E.; Shaban, S.; Lee, W.; Pyun, D.G.; Lee, D.H.; Kim, D.H. Solid-State Colorimetric Polydiacetylene Liposome Biosensor Sensitized by Gold Nanoparticles. Analyst 2021, 146, 1682–1688. [Google Scholar] [CrossRef]
- Costa, D.; Rodrigues, M.S.; Roiban, L.; Aouine, M.; Epicier, T.; Steyer, P.; Alves, E.; Barradas, N.P.; Borges, J.; Vaz, F. In-Situ Annealing Transmission Electron Microscopy of Plasmonic Thin Films Composed of Bimetallic Au–Ag Nanoparticles Dispersed in a TiO2 Matrix. Vacuum 2021, 193, 110511. [Google Scholar] [CrossRef]
- Pereira-Silva, P.; Borges, J.; Rodrigues, M.S.M.S.; Oliveira, J.C.J.C.; Alves, E.; Barradas, N.P.N.P.; Dias, J.P.J.P.; Cavaleiro, A.; Vaz, F. Nanocomposite Au-ZnO Thin Films: Influence of Gold Concentration and Thermal Annealing on the Microstructure and Plasmonic Response. Surf. Coat. Technol. 2020, 385, 125379. [Google Scholar] [CrossRef]
- Meira, D.I.; Domingues, R.P.; Rodrigues, M.S.; Alves, E.; Barradas, N.P.; Borges, J.; Vaz, F. Thin Films of Au-Al2O3 for Plasmonic Sensing. Appl. Surf. Sci. 2020, 500, 144035. [Google Scholar] [CrossRef]
- Guo, L.; Jackman, J.A.; Yang, H.H.; Chen, P.; Cho, N.J.; Kim, D.H. Strategies for Enhancing the Sensitivity of Plasmonic Nanosensors. Nano Today 2015, 10, 213–239. [Google Scholar] [CrossRef] [Green Version]
- Howarth, M.; Chinnapen, D.J.F.; Gerrow, K.; Dorrestein, P.C.; Grandy, M.R.; Kelleher, N.L.; El-Husseini, A.; Ting, A.Y. A Monovalent Streptavidin with a Single Femtomolar Biotin Binding Site. Nat. Methods 2006, 3, 267–273. [Google Scholar] [CrossRef] [Green Version]
- Dundas, C.M.; Demonte, D.; Park, S. Streptavidin-Biotin Technology: Improvements and Innovations in Chemical and Biological Applications. Appl. Microbiol. Biotechnol. 2013, 97, 9343–9353. [Google Scholar] [CrossRef]
- Wilchek, M.; Bayer, E.A.; Livnah, O. Essentials of Biorecognition: The (Strept)Avidin-Biotin System as a Model for Protein-Protein and Protein-Ligand Interaction. Immunol. Lett. 2006, 103, 27–32. [Google Scholar] [CrossRef]
- Kim, H.M.; Jin, S.M.; Lee, S.K.; Kim, M.G.; Shin, Y.B. Detection of Biomolecular Binding Through Enhancement of Localized Surface Plasmon Resonance (LSPR) by Gold Nanoparticles. Sensors 2009, 9, 2334–2344. [Google Scholar] [CrossRef]
- Pol, L.; Eckstein, C.; Acosta, L.K.; Xifré-Pérez, E.; Ferré-Borrull, J.; Marsal, L.F. Real-Time Monitoring of Biotinylated Molecules Detection Dynamics in Nanoporous Anodic Alumina for Bio-Sensing. Nanomaterials 2019, 9, 478. [Google Scholar] [CrossRef] [Green Version]
- Heidari, A.; Yoon, Y.J.; Park, W.T.; Su, P.C.; Miao, J.; Ming Lin, J.T.; Park, M.K. Biotin-Streptavidin Binding Interactions of Dielectric Filled Silicon Bulk Acoustic Resonators for Smart Label-Free Biochemical Sensor Applications. Sensors 2014, 14, 4585–4598. [Google Scholar] [CrossRef] [Green Version]
- Hermanson, G.T. Enzyme Modification and Conjugation. In Bioconjugate Techniques; Elsevier: Amsterdam, The Netherlands, 2013; pp. 951–957. [Google Scholar]
- Mouse TNF Alpha Uncoated ELISA - Invitrogen by Thermo Fischer Scientific. Available online: https://bit.ly/3MGt0Zu (accessed on 3 May 2021).
- Borges, J.; Rodrigues, M.S.; Lopes, C.; Costa, D.; Couto, F.M.; Kubart, T.; Martins, B.; Duarte, N.; Dias, J.P.; Cavaleiro, A.; et al. Thin Films Composed of Ag Nanoclusters Dispersed in TiO2: Influence of Composition and Thermal Annealing on the Microstructure and Physical Responses. Appl. Surf. Sci. 2015, 358, 595–604. [Google Scholar] [CrossRef] [Green Version]
- Domingues, R.P.; Rodrigues, M.S.; Proença, M.; Costa, D.; Alves, E.; Barradas, N.P.; Oliveira, F.J.; Silva, R.F.; Borges, J.; Vaz, F. Thin Films Composed of Au Nanoparticles Embedded in AlN: Influence of Metal Concentration and Thermal Annealing on the LSPR Band. Vacuum 2018, 157, 414–421. [Google Scholar] [CrossRef]
- Barradas, N.P.; Marriott, P.K.; Jeynes, C.; Webb, R.P. The RBS Data Furnace: Simulated Annealing. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 1998, 136–138, 1157–1162. [Google Scholar] [CrossRef]
- Barradas, N.P.; Pascual-Izarra, C. Double Scattering in RBS Analysis of PtSi Thin Films on Si. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2005, 228, 378–382. [Google Scholar] [CrossRef]
- Barradas, N.P.; Reis, M.A. Accurate Calculation of Pileup Effects in PIXE Spectra from First Principles. X-Ray Spectrom. 2006, 35, 232–237. [Google Scholar] [CrossRef]
- Kim, W.J.; Kim, S.; Lee, B.S.; Kim, A.; Ah, C.S.; Huh, C.; Sung, G.Y.; Yun, W.S. Enhanced Protein Immobilization Efficiency on a TiO2 Surface Modified with a Hydroxyl Functional Group. Langmuir 2009, 25, 11692–11697. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, A.; Liu, Y.H.; Cha, P.; Woodward, R.; Allara, D.; Vogler, E.A. An Evaluation of Methods for Contact Angle Measurement. Colloids Surf. B Biointerfaces 2005, 43, 95–98. [Google Scholar] [CrossRef]
- Rodrigues, M.S.; Borges, J.; Proença, M.; Pedrosa, P.; Martin, N.; Romanyuk, K.; Kholkin, A.L.; Vaz, F. Nanoplasmonic Response of Porous Au-TiO2 Thin Films Prepared by Oblique Angle Deposition. Nanotechnology 2019, 30, 225701. [Google Scholar] [CrossRef]
- Rodrigues, M.S.; Pereira, R.M.S.; Vasilevskiy, M.I.; Borges, J.; Vaz, F. NANOPTICS: In-Depth Analysis of NANomaterials for OPTICal Localized Surface Plasmon Resonance Sensing. SoftwareX 2020, 12, 100522. [Google Scholar] [CrossRef]
- Koneti, S.; Borges, J.; Roiban, L.; Rodrigues, M.S.; Martin, N.; Epicier, T.; Vaz, F.; Steyer, P. Electron Tomography of Plasmonic Au Nanoparticles Dispersed in a TiO2 Dielectric Matrix. ACS Appl. Mater. Interfaces 2018, 10, 42882–42890. [Google Scholar] [CrossRef]
- Borges, J.; Rodrigues, M.S.; Kubart, T.; Kumar, S.; Leifer, K.; Evaristo, M.; Cavaleiro, A.; Apreutesei, M.; Pereira, R.M.S.; Vasilevskiy, M.I.; et al. Thin Films Composed of Gold Nanoparticles Dispersed in a Dielectric Matrix: The Influence of the Host Matrix on the Optical and Mechanical Responses. Thin Solid Film. 2015, 596, 8–17. [Google Scholar] [CrossRef]
- Costa, D.; Oliveira, J.; Rodrigues, M.S.; Borges, J.; Moura, C.; Sampaio, P.; Vaz, F. Development of Biocompatible Plasmonic Thin Films Composed of Noble Metal Nanoparticles Embedded in a Dielectric Matrix to Enhance Raman Signals. Appl. Surf. Sci. 2019, 496, 143701. [Google Scholar] [CrossRef] [Green Version]
- Borges, J.; Costa, D.; Antunes, E.; Lopes, C.; Rodrigues, M.S.; Apreutesei, M.; Alves, E.; Barradas, N.P.; Pedrosa, P.; Moura, C.; et al. Biological Behaviour of Thin Films Consisting of Au Nanoparticles Dispersed in a TiO2 Dielectric Matrix. Vacuum 2015, 122, 360–368. [Google Scholar] [CrossRef]
- Rodrigues, M.S.; Borges, J.; Lopes, C.; Pereira, R.M.S.; Vasilevskiy, M.I.; Vaz, F. Gas Sensors Based on Localized Surface Plasmon Resonances: Synthesis of Oxide Films with Embedded Metal Nanoparticles, Theory and Simulation, and Sensitivity Enhancement Strategies. Appl. Sci. 2021, 11, 5388. [Google Scholar] [CrossRef]
- Sotnikov, D.V.; Berlina, A.N.; Ivanov, V.S.; Zherdev, A.V.; Dzantiev, B.B. Adsorption of Proteins on Gold Nanoparticles: One or More Layers? Colloids Surf. B: Biointerfaces 2019, 173, 557–563. [Google Scholar] [CrossRef]
- Vesel, A.; Mozetic, M. New Developments in Surface Functionalization of Polymers Using Controlled Plasma Treatments. J. Phys. D Appl. Phys. 2017, 50, 293001. [Google Scholar] [CrossRef]
- Huhtamäki, T.; Tian, X.; Korhonen, J.T.; Ras, R.H.A. Surface-Wetting Characterization Using Contact-Angle Measurements. Nat. Protoc. 2018, 13, 1521–1538. [Google Scholar] [CrossRef] [Green Version]
- Vogler, E.A. Structure and Reactivity of Water at Biomaterial Surfaces. Adv. Colloid Interface Sci. 1998, 74, 69–117. [Google Scholar] [CrossRef]
- Lakshmipriya, T.; Gopinath, S.C.B.; Tang, T.H. Biotin-Streptavidin Competition Mediates Sensitive Detection of Biomolecules in Enzyme Linked Immunosorbent Assay. PLoS ONE 2016, 11, e0151153. [Google Scholar] [CrossRef] [Green Version]
- Haes, A.J.; Van Duyne, R.P. A Unified View of Propagating and Localized Surface Plasmon Resonance Biosensors. Anal. Bioanal. Chem. 2004, 379, 920–930. [Google Scholar] [CrossRef]
- Hegde, H.R.; Chidangil, S.; Sinha, R.K. Refractive Index Sensitivity of Au Nanostructures in Solution and on the Substrate. J. Mater. Sci. Mater. Electron. 2022, 33, 4011–4024. [Google Scholar] [CrossRef]
- Martinsson, E.; Sepulveda, B.; Chen, P.; Elfwing, A.; Liedberg, B.; Aili, D. Optimizing the Refractive Index Sensitivity of Plasmonically Coupled Gold Nanoparticles. Plasmonics 2014, 9, 773–780. [Google Scholar] [CrossRef]
- Focsan, M.; Campu, A.; Craciun, A.M.; Potara, M.; Leordean, C.; Maniu, D.; Astilean, S. A Simple and Efficient Design to Improve the Detection of Biotin-Streptavidin Interaction with Plasmonic Nanobiosensors. Biosens. Bioelectron. 2016, 86, 728–735. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira-Silva, P.; Meira, D.I.; Costa-Barbosa, A.; Costa, D.; Rodrigues, M.S.; Borges, J.; Machado, A.V.; Cavaleiro, A.; Sampaio, P.; Vaz, F. Immobilization of Streptavidin on a Plasmonic Au-TiO2 Thin Film towards an LSPR Biosensing Platform. Nanomaterials 2022, 12, 1526. https://doi.org/10.3390/nano12091526
Pereira-Silva P, Meira DI, Costa-Barbosa A, Costa D, Rodrigues MS, Borges J, Machado AV, Cavaleiro A, Sampaio P, Vaz F. Immobilization of Streptavidin on a Plasmonic Au-TiO2 Thin Film towards an LSPR Biosensing Platform. Nanomaterials. 2022; 12(9):1526. https://doi.org/10.3390/nano12091526
Chicago/Turabian StylePereira-Silva, Patrícia, Diana I. Meira, Augusto Costa-Barbosa, Diogo Costa, Marco S. Rodrigues, Joel Borges, Ana V. Machado, Albano Cavaleiro, Paula Sampaio, and Filipe Vaz. 2022. "Immobilization of Streptavidin on a Plasmonic Au-TiO2 Thin Film towards an LSPR Biosensing Platform" Nanomaterials 12, no. 9: 1526. https://doi.org/10.3390/nano12091526
APA StylePereira-Silva, P., Meira, D. I., Costa-Barbosa, A., Costa, D., Rodrigues, M. S., Borges, J., Machado, A. V., Cavaleiro, A., Sampaio, P., & Vaz, F. (2022). Immobilization of Streptavidin on a Plasmonic Au-TiO2 Thin Film towards an LSPR Biosensing Platform. Nanomaterials, 12(9), 1526. https://doi.org/10.3390/nano12091526











