Ferromagnetic Behavior and Magneto-Optical Properties of Semiconducting Co-Doped ZnO
Abstract
1. Introduction
2. Growth, Structural and Magnetic/MO Characterization of ZCO Thin Films
2.1. ZCO Growth and Microstructure
2.2. Magnetic and MO Properties
3. Results
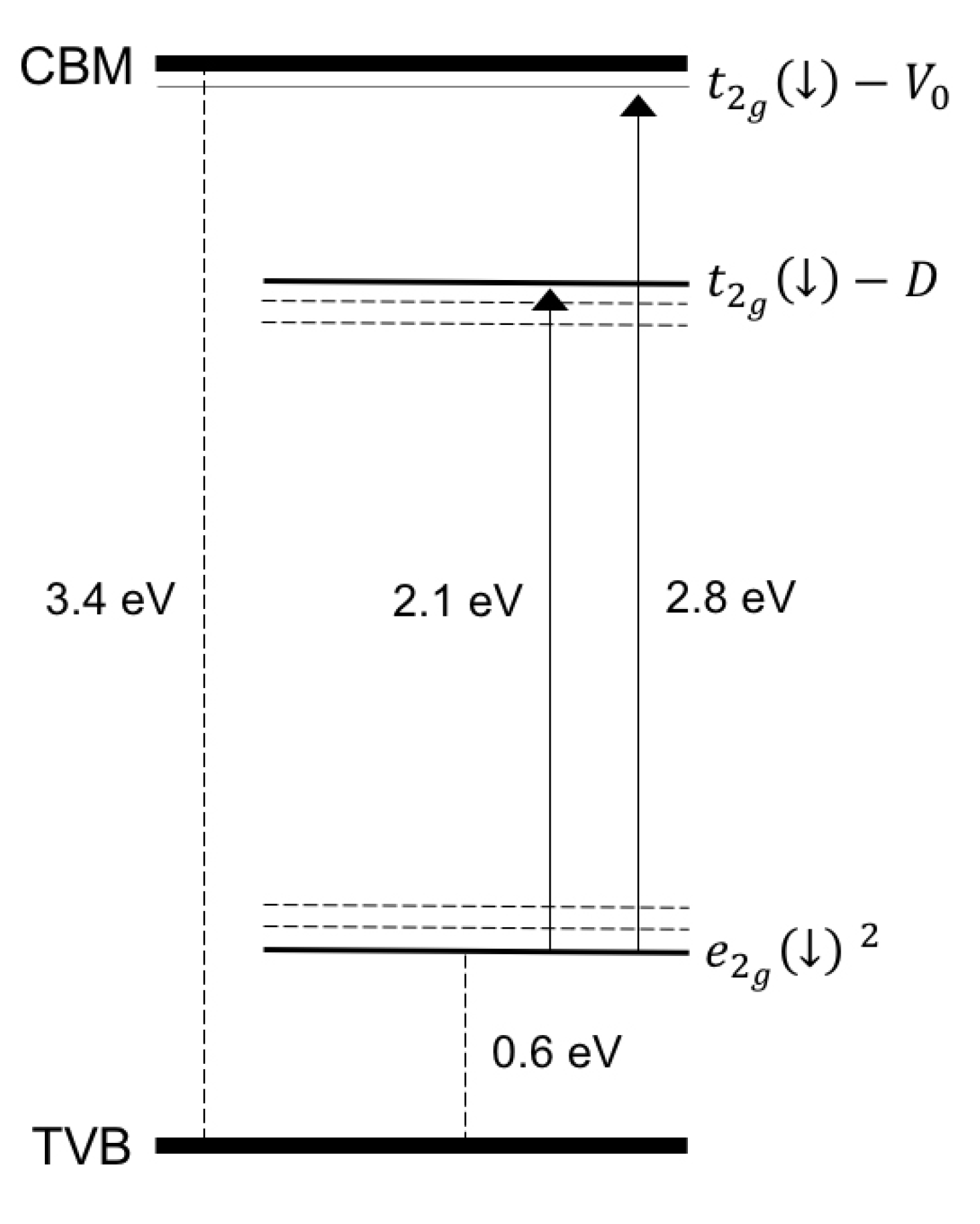
3.1. Role of Defects and n-Carriers in the ZCO Ferromagnetic Behavior
3.2. Effects of Hydrogenation Treatments on the ZCO Magnetic Properties
3.2.1. Increase in ZCO Ferromagnetism
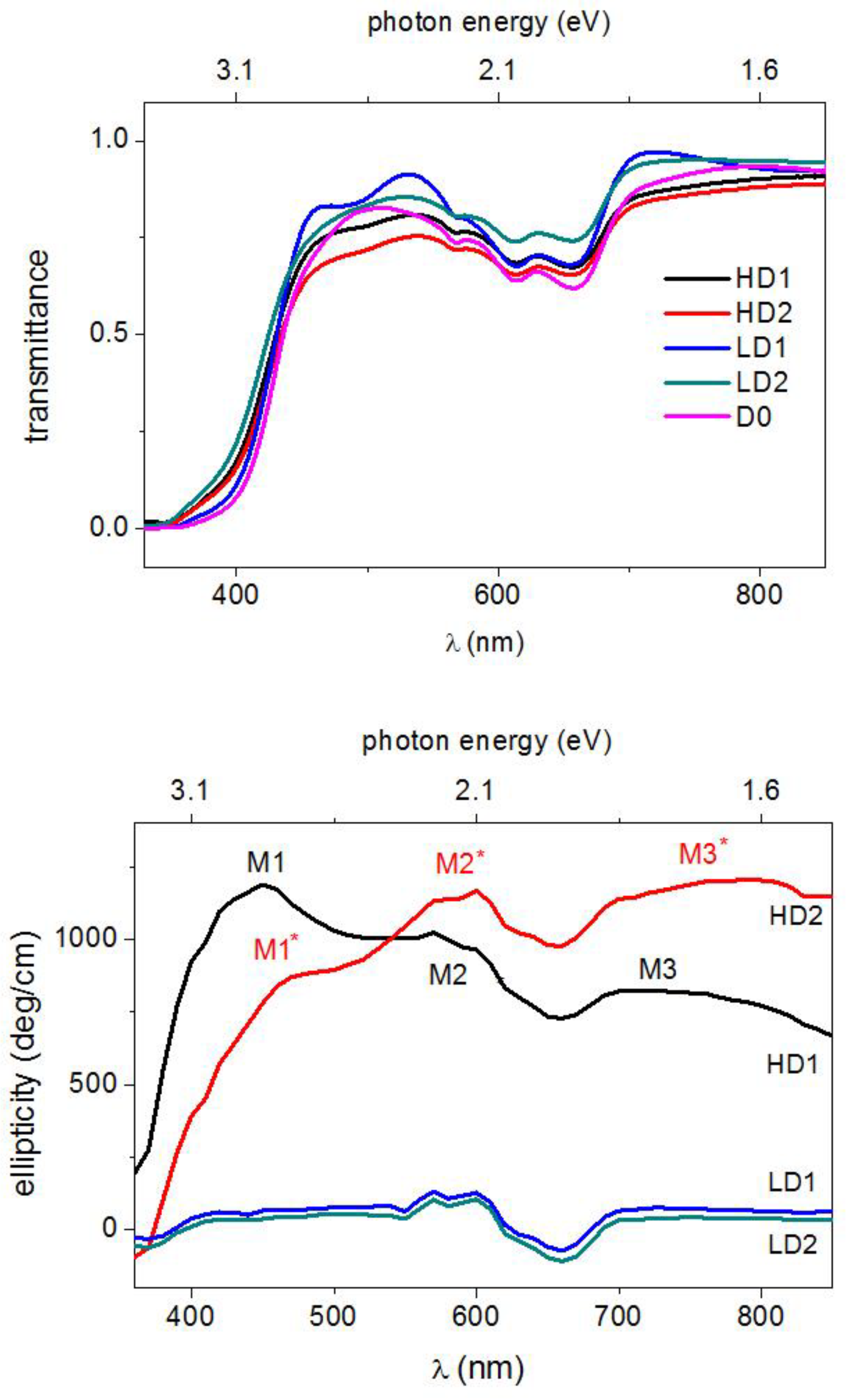
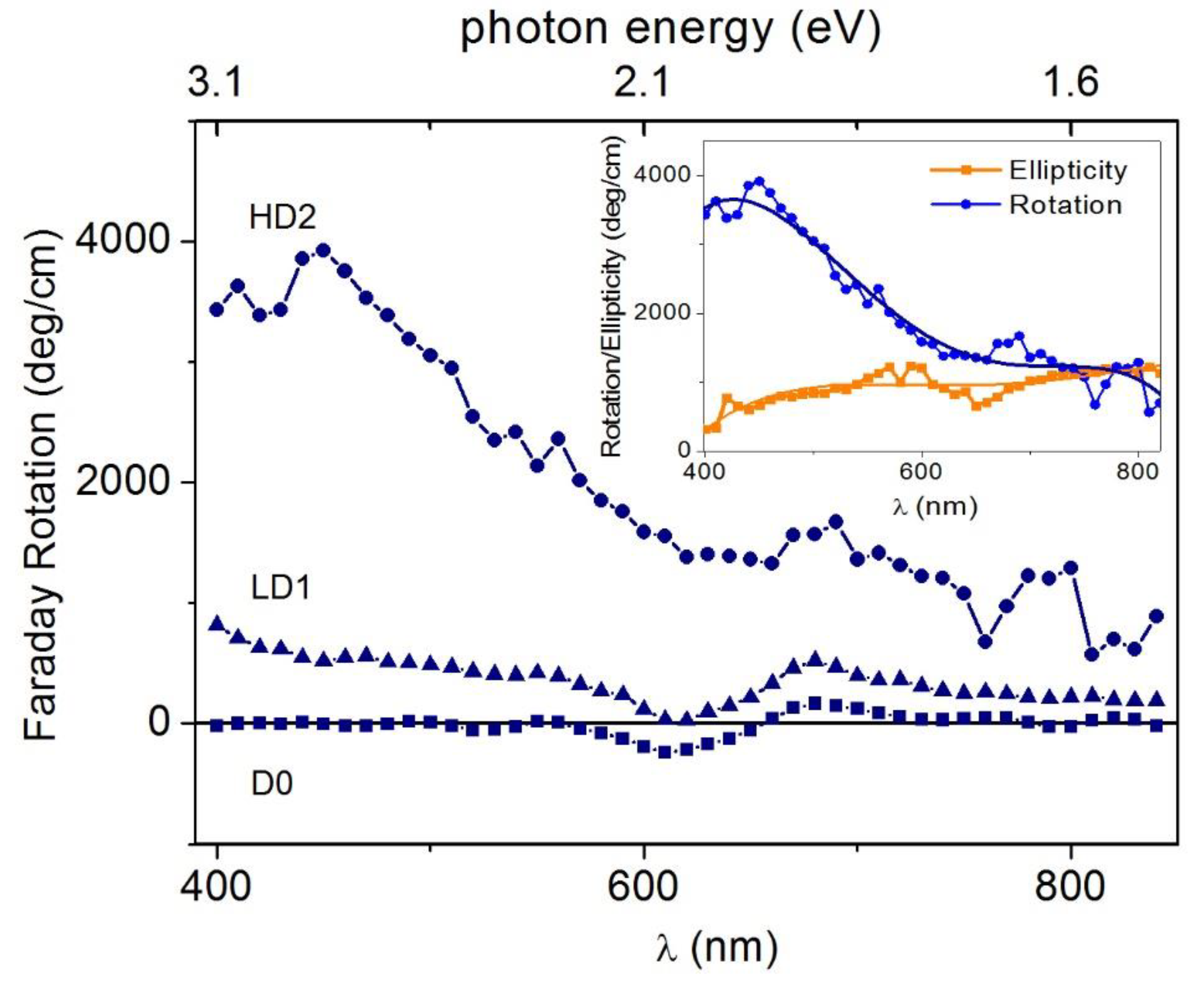
3.2.2. Improvements to ZCO Magneto-Optical Properties
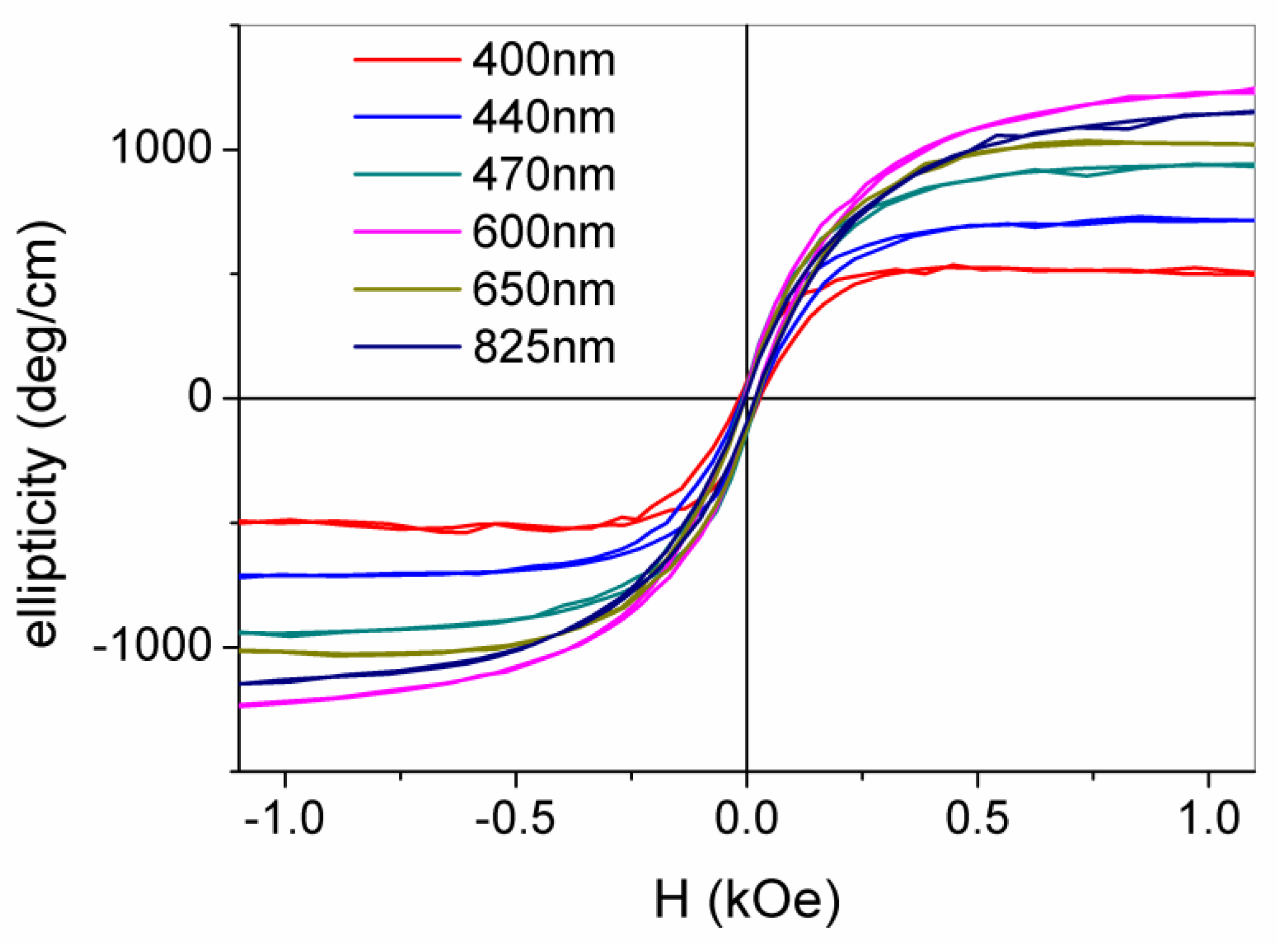
3.2.3. Local MO Response
3.3. Other Effects of Co Doping and H Co Doping on the ZnO Properties
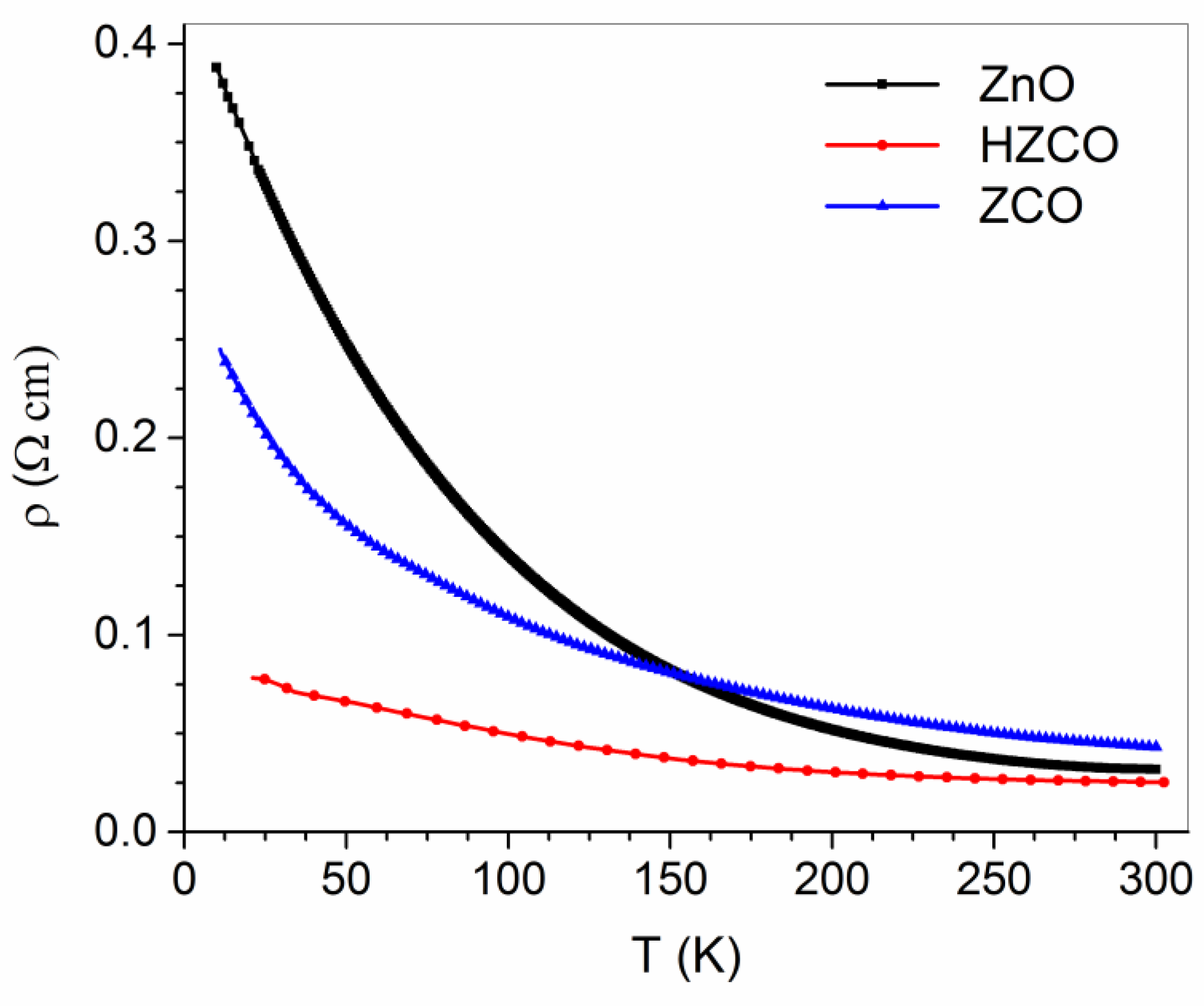
3.3.1. Transport Properties in ZCO and Hydrogenated ZCO
3.3.2. Effects of Co Doping on the ZnO Surface Reactivity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Klingshirn, C.F.; Waag, A.; Hoffmann, A.; Geurts, J. Zinc Oxide from Fundamental Properties towards Novel Application; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar] [CrossRef]
- Litton, C.W.; Reynolds, D.C.; Collins, T.C. (Eds.) Zinc Oxide Materials for Electronic and Optoelectronic Device Applications; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2011. [Google Scholar] [CrossRef]
- D’Agostino, D.; Di Giorgio, C.; Di Trolio, A.; Guarino, A.; Cucolo, A.M.; Vecchione, A.; Bobba, F. Piezoelectricity and Charge Trapping in ZnO and Co-Doped ZnO Thin Films. AIP Adv. 2017, 7, 055010. [Google Scholar] [CrossRef]
- Kittilstved, K.R.; Norberg, N.S.; Gamelin, D.R. Chemical Manipulation of Tc Ferromagnetism in ZnO Diluted Magnetic Semiconductors. Phys. Rev. Lett. 2005, 94, 147209. [Google Scholar] [CrossRef] [PubMed]
- Ueda, K.; Tabata, H.; Kawai, T. Magnetic and Electric Properties of Transition-Metal-Doped ZnO Films. Appl. Phys. Lett. 2001, 79, 988. [Google Scholar] [CrossRef]
- Ramachandran, S.; Tiwari, A.; Narayan, J. Zn0.9Co0.1O-Based Diluted Magnetic Semiconducting Thin Films. Appl. Phys. Lett. 2004, 84, 5255. [Google Scholar] [CrossRef]
- Tuan, A.C.; Bryan, J.D.; Pakhomov, A.B.; Shutthanandan, V.; Thevuthasan, S.; McCready, D.E.; Gaspar, D.; Engelhard, M.H.; Rogers, J.W.; Krishnan, K.; et al. Epitaxial Growth and Properties of Cobalt-Doped ZnO on α-Al2O3 Single-Crystal Substrates. Phys. Rev. B-Condens. Matter Mater. Phys. 2004, 70, 054424. [Google Scholar] [CrossRef]
- Venkatesan, M.; Fitzgerald, C.B.; Lunney, J.G.; Coey, J.M.D. Anisotropic Ferromagnetism in Substituted Zinc Oxide. Phys. Rev. Lett. 2004, 93, 177206. [Google Scholar] [CrossRef]
- Di Trolio, A.; Veroli, C.; Testa, A.M.; Fiorani, D. Ferromagnetism above Room Temp erature in Mn-Doped ZnO Thin Films. Superlattices Microstruct. 2009, 46, 101–106. [Google Scholar] [CrossRef]
- Di Trolio, A.; Larciprete, R.; Turchini, S.; Zema, N. Bulk Sensitive X-Ray Absorption and Magnetic Circular Dichroism Investigation of Mn- and Co-Doped ZnO Thin Films. Appl. Phys. Lett. 2010, 97, 052505. [Google Scholar] [CrossRef]
- Kossut, J.; Gaj, J.A. Introduction to the Physics of Diluted Magnetic Semiconductors; Series in Materials Science; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar] [CrossRef]
- Rode, K.; Anane, A.; Mattana, R.; Contour, J.-P.; Durand, O.; LeBourgeois, R. Magnetic Semiconductors Based on Cobalt Substituted ZnO. J. Appl. Phys. 2003, 93, 7676–7678. [Google Scholar] [CrossRef]
- Prellier, W.; Fouchet, A.; Mercey, B.; Simon, C.; Raveau, B. Laser Ablation of Co:ZnO Films Deposited from Zn and Co Metal Targets on (0001) Al2O3 Substrates. Appl. Phys. Lett. 2003, 82, 3490–3492. [Google Scholar] [CrossRef]
- Dorneles, L.S.; Venkatesan, M.; Gunning, R.; Stamenov, P.; Alaria, J.; Rooney, M.; Lunney, J.G.; Coey, J.M.D. Magnetic and Structural Properties of Co-Doped ZnO Thin Films. J. Magn. Magn. Mater. 2007, 310 Pt 3, 2087–2088. [Google Scholar] [CrossRef]
- Kobayashi, M.; Ishida, Y.; Hwang, J.L.; Mizokawa, T.; Fujimori, A.; Mamiya, K.; Okamoto, J.; Takeda, Y.; Okane, T.; Saitoh, Y.; et al. Characterization of Magnetic Components in the Diluted Magnetic Semiconductor Zn1−X CoxO by X-ray Magnetic Circular Dichroism. Phys. Rev. B-Condens. Matter Mater. Phys. 2005, 72, 201201. [Google Scholar] [CrossRef]
- Gacic, M.; Jakob, G.; Herbort, C.; Adrian, H.; Tietze, T.; Brück, S.; Goering, E. Magnetism of Co-Doped ZnO Thin Films. Phys. Rev. B 2007, 75, 205206. [Google Scholar] [CrossRef]
- Ivill, M.; Pearton, S.J.; Rawal, S.; Leu, L.; Sadik, P.; Das, R.; Hebard, A.F.; Chisholm, M.; Budai, J.D.; Norton, D.P. Structure and Magnetism of Cobalt-Doped ZnO Thin Films. New J. Phys. 2008, 10, 065002. [Google Scholar] [CrossRef]
- Lee, H.-J.; Jeong, S.-Y.; Cho, C.R.; Park, C.H. Study of Diluted Magnetic Semiconductor: Co-Doped ZnO. Appl. Phys. Lett. 2002, 81, 4020–4022. [Google Scholar] [CrossRef]
- Kittilstved, K.R.; Schwartz, D.A.; Tuan, A.C.; Heald, S.M.; Chambers, S.A.; Gamelin, D.R. Direct Kinetic Correlation of Carriers and Ferromagnetism in Co2+:ZnO. Phys. Rev. Lett. 2006, 97, 037203. [Google Scholar] [CrossRef]
- Schwartz, D.A.; Gamelin, D.R. Reversible 300 K Ferromagnetic Ordering in a Diluted Magnetic Semiconductor. Adv. Mater. 2004, 16, 2115–2119. [Google Scholar] [CrossRef]
- Kittilstved, K.R.; Liu, W.K.; Gamelin, D.R. Electronic Structure Origins of Polarity-Dependent High-TC Ferromagnetism in Oxide-Diluted Magnetic Semiconductors. Nat. Mater. 2006, 5, 291–297. [Google Scholar] [CrossRef]
- Lee, H.-J.; Park, C.H.; Jeong, S.-Y.; Yee, K.-J.; Cho, C.R.; Jung, M.-H.; Chadi, D.J. Hydrogen-Induced Ferromagnetism in ZnCoO. Appl. Phys. Lett. 2006, 88, 062504. [Google Scholar] [CrossRef]
- Cho, Y.C.; Kim, S.-J.; Lee, S.; Kim, S.J.; Cho, C.R.; Nahm, H.-H.; Park, C.H.; Jeong, I.K.; Park, S.; Hong, T.E.; et al. Reversible Ferromagnetic Spin Ordering Governed by Hydrogen in Co-Doped ZnO Semiconductor. Appl. Phys. Lett. 2009, 95, 172514. [Google Scholar] [CrossRef]
- Di Trolio, A.; Alippi, P.; Ciatto, G.; Scavia, G.; Valentini, M.; Amore Bonapasta, A. The Effect of Co Doping on the Conductive Properties of Ferromagnetic Zn1−XCoxO Films. J. Mater. Chem. C 2015, 3, 10188–10194. [Google Scholar] [CrossRef]
- Di Trolio, A.; Alippi, P.; Bauer, E.M.; Ciatto, G.; Chu, M.H.; Varvaro, G.; Polimeni, A.; Capizzi, M.; Valentini, M.; Bobba, F.; et al. Ferromagnetism and Conductivity in Hydrogen Irradiated Co-Doped ZnO Thin Films. ACS Appl. Mater. Interfaces 2016, 8, 12925. [Google Scholar] [CrossRef]
- Liu, G.L.; Cao, Q.; Deng, J.X.; Xing, P.F.; Tian, Y.F.; Chen, Y.X.; Yan, S.S.; Mei, L.M. High TC Ferromagnetism of Zn1−XCoxO Diluted Magnetic Semiconductors Grown by Oxygen Plasma-Assisted Molecular Beam Epitaxy. Appl. Phys. Lett. 2007, 90, 052504. [Google Scholar] [CrossRef]
- Cui, J.; Gibson, U. Thermal Modification of Magnetism in Cobalt-Doped ZnO Nanowires Grown at Low Temperatures. Phys. Rev. B 2006, 74, 045416. [Google Scholar] [CrossRef]
- Hsu, H.S.; Huang, J.C.A.; Huang, Y.H.; Liao, Y.F.; Lin, M.Z.; Lee, C.H.; Lee, J.F.; Chen, S.F.; Lai, L.Y.; Liu, C.P. Evidence of Oxygen Vacancy Enhanced Room-Temperature Ferromagnetism in Co-Doped ZnO. Appl. Phys. Lett. 2006, 88, 242507. [Google Scholar] [CrossRef]
- Dinia, A.; Schmerber, G.; Mény, C.; Pierron-Bohnes, V.; Beaurepaire, E. Room-Temperature Ferromagnetism in Zn1-XCoxO Magnetic Semiconductors Prepared by Sputtering. J. Appl. Phys. 2005, 97, 123908. [Google Scholar] [CrossRef]
- Han, X.; Wang, G.; Jie, J.; Zhu, X.; Hou, J.G. Properties of Zn1−XCoxO Thin Films Grown on Silicon Substrates Prepared by Pulsed Laser Deposition. Thin Solid Films 2005, 491, 249–252. [Google Scholar] [CrossRef]
- Van de Walle, C.G. Computational Studies of Conductivity in Wide-Band-Gap Semiconductors and Oxides. J. Phys. Condens. Matter 2008, 20, 064230. [Google Scholar] [CrossRef]
- Pemmaraju, C.D.; Hanafin, R.; Archer, T.; Braun, H.B.; Sanvito, S. Impurity-Ion Pair Induced High-Temperature Ferromagnetism in Co-Doped ZnO. Phys. Rev. B-Condens. Matter Mater. Phys. 2008, 78, 054428. [Google Scholar] [CrossRef]
- Behan, A.J.; Mokhtari, A.; Blythe, H.J.; Score, D.; Xu, X.-H.; Neal, J.R.; Fox, A.M.; Gehring, G.A. Two Magnetic Regimes in Doped ZnO Corresponding to a Dilute Magnetic Semiconductor and a Dilute Magnetic Insulator. Phys. Rev. Lett. 2008, 100, 047206. [Google Scholar] [CrossRef]
- Lee, H.-J.; Helgren, E.; Hellman, F. Gate-Controlled Magnetic Properties of the Magnetic Semiconductor (Zn,Co)O. Appl. Phys. Lett. 2009, 94, 212106. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yun, F.; Morkoç, H. Ferromagnetism of ZnO and GaN: A Review. J. Mater. Sci. Mater. Electron. 2005, 16, 555. [Google Scholar] [CrossRef]
- Ogale, S.B. Dilute Doping, Defects, and Ferromagnetism in Metal Oxide Systems. Adv. Mater. 2010, 22, 3125–3155. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Zhang, W.; Xu, Y.; Yan, M. Effect of Oxygen Deficiency on Room Temperature Ferromagnetism in Co Doped ZnO. Appl. Phys. Lett. 2012, 100, 202401. [Google Scholar] [CrossRef]
- Kim, S.J.; Cha, S.Y.; Kim, J.Y.; Shin, J.M.; Cho, Y.C.; Lee, S.; Kim, W.-K.; Jeong, S.-Y.; Yang, Y.S.; Cho, C.R.; et al. Ferromagnetism in ZnCoO Due to Hydrogen-Mediated Co–H–Co Complexes: How to Avoid the Formation of Co Metal Clusters? J. Phys. Chem. C 2012, 116, 12196–12202. [Google Scholar] [CrossRef]
- Wang, C.C.; Liu, M.; Man, B.Y.; Chen, C.S.; Jiang, S.Z.; Yang, S.Y.; Gao, X.G.; Xu, S.C.; Hu, B.; Sun, Z.C.; et al. Role of Cobalt in Room-Temperature Ferromagnetic Co-Doped ZnO Thin Films. AIP Adv. 2012, 2, 012182. [Google Scholar] [CrossRef]
- Wang, C.C.; Man, B.Y.; Liu, M.; Chen, C.S.; Jiang, S.Z.; Yang, S.Y.; Xu, S.C.; Gao, X.G.; Hu, B. The Intrinsic Room-Temperature Ferromagnetism in ZnO:Co Thin Films Deposited by PLD. Adv. Condens. Matter Phys. 2012, 2012, 363981. [Google Scholar] [CrossRef]
- Zhang, H.; Cao, Y.; Yang, Z.; Si, L.; Zhong, W.; Wu, D.; Xu, M.; Xu, Q. Enhanced Room Temperature Ferromagnetism in Co-Doped ZnO Mediated by Interstitial H. Mater. Lett. 2012, 89, 209–211. [Google Scholar] [CrossRef]
- de Godoy, M.P.F.; Mesquita, A.; Avansi, W.; Neves, P.P.; Chitta, V.A.; Ferraz, W.B.; Boselli, M.A.; Sabioni, A.C.S.; de Carvalho, H.B. Evidence of Defect-Mediated Magnetic Coupling on Hydrogenated Co-Doped ZnO. J. Alloy. Compd. 2013, 555, 315–319. [Google Scholar] [CrossRef]
- Lardjane, S.; Merad, G.; Fenineche, N.; Billard, A.; Faraoun, H.I. Ab Initio Study of ZnCoO Diluted Magnetic Semiconductor and Its Magnetic Properties. J. Alloy. Compd. 2013, 551, 306–311. [Google Scholar] [CrossRef]
- Cho, Y.C.; Lee, S.; Park, J.H.; Kim, W.K.; Nahm, H.-H.; Park, C.H.; Jeong, S.-Y. Hydrogen-Induced Anomalous Hall Effect in Co-Doped ZnO. New J. Phys. 2014, 16, 073030. [Google Scholar] [CrossRef]
- Opel, M.; Goennenwein, S.T.B.; Althammer, M.; Nielsen, K.-W.; Karrer-Müller, E.-M.; Bauer, S.; Senn, K.; Schwark, C.; Weier, C.; Güntherodt, G.; et al. Zinc Oxide –From Dilute Magnetic Doping to Spin Transport. Phys. Status Solidi B 2014, 251, 1700–1709. [Google Scholar] [CrossRef]
- Vijayaprasath, G.; Murugan, R.; Ravi, G.; Mahalingam, T.; Hayakawa, Y. Characterization of Dilute Magnetic Semiconducting Transition Metal Doped ZnO Thin Films by Sol–Gel Spin Coating Method. Appl. Surf. Sci. 2014, 313, 870–876. [Google Scholar] [CrossRef]
- Henne, B.; Ney, V.; Ollefs, K.; Wilhelm, F.; Rogalev, A.; Ney, A. Magnetic Interactions in the Zn-Co-O System: Tuning Local Structure, Valence and Carrier Type from Extremely Co Doped ZnO to ZnCo2O4. Sci. Rep. 2015, 5, 16863. [Google Scholar] [CrossRef]
- Lee, S.; Kim, B.-S.; Park, J.H.; Lee, T.-W.; Chan Cho, Y.; Jeong, S.-Y. Study on the Formation of Magnetic Nanoclusters and Change in Spin Ordering in Co-Doped ZnO Using Magnetic Susceptibility. RSC Adv. 2015, 5, 65840–65846. [Google Scholar] [CrossRef]
- Li, G.; Wang, H.; Wang, Q.; Zhao, Y.; Wang, Z.; Du, J.; Ma, Y. Structure and Properties of Co-Doped ZnO Films Prepared by Thermal Oxidization under a High Magnetic Field. Nanoscale Res. Lett. 2015, 10, 112. [Google Scholar] [CrossRef]
- Tseng, L.T.; Suter, A.; Wang, Y.R.; Xiang, F.X.; Bian, P.; Ding, X.; Tseng, A.; Hu, H.L.; Fan, H.M.; Zheng, R.K.; et al. Intrinsic and Spatially Nonuniform Ferromagnetism in Co-Doped ZnO Films. Phys. Rev. B 2017, 96, 104423. [Google Scholar] [CrossRef]
- Wang, D.D.; Zhao, B.; Qi, N.; Chen, Z.Q.; Kawasuso, A. Vacancy-Mediated Ferromagnetism in Co-Implanted ZnO Studied Using a Slow Positron Beam. J. Mater. Sci. 2017, 52, 7067–7076. [Google Scholar] [CrossRef]
- Yeh, C.-C.; Colis, S.; Fioux, P.; Zan, H.-W.; Berling, D.; Soppera, O. Nanoscale Ferromagnetic Cobalt-Doped ZnO Structures Formed by Deep-UV Direct-Patterning. Adv. Mater. Interfaces 2017, 4, 1700738. [Google Scholar] [CrossRef]
- de Godoy, M.P.F.; Gratens, X.; Chitta, V.A.; Mesquita, A.; de Lima, M.M.; Cantarero, A.; Rahman, G.; Morbec, J.M.; de Carvalho, H.B. Defect Induced Room Temperature Ferromagnetism in High Quality Co-Doped ZnO Bulk Samples. J. Alloy. Compd. 2021, 859, 157772. [Google Scholar] [CrossRef]
- Saeedi, A.M.A.; Alshammari, M.S.; Peng, N.; Adachi, Y.; Heald, S.M.; Alanazi, A.F.; Gehring, G.A. Magnetism from Co and Eu Implanted into ZnO. J. Magn. Magn. Mater. 2021, 527, 167741. [Google Scholar] [CrossRef]
- Verma, K.C. Diluted Magnetic Semiconductor ZnO: Magnetic Ordering with Transition Metal and Rare Earth Ions. In Magnetic Materials and Magnetic Levitation; Sahu, D.R., Stavrou, V.N., Eds.; IntechOpen: Rijeka, Croatia, 2021. [Google Scholar] [CrossRef]
- Thakur, V.; Verma, U. Growth of Co-Doped ZnO Thin Films Exhibiting Room Temperature Ferromagnetism Using a Low-Cost Spray Pyrolysis Technique. Bull. Mater. Sci. 2022, 45, 32. [Google Scholar] [CrossRef]
- Park, J.H.; Kim, M.G.; Jang, H.M.; Ryu, S.; Kim, Y.M. Co-Metal Clustering as the Origin of Ferromagnetism in Co-Doped ZnO Thin Films. Appl. Phys. Lett. 2004, 84, 1338–1340. [Google Scholar] [CrossRef]
- Fukuma, Y.; Asada, H.; Yamamoto, J.; Odawara, F.; Koyanagi, T. Large Magnetic Circular Dichroism of Co Clusters in Co-Doped ZnO. Appl. Phys. Lett. 2008, 93, 142510. [Google Scholar] [CrossRef]
- Deka, S.; Joy, P.A. Ferromagnetism Induced by Hydrogen in Polycrystalline Nonmagnetic Zn0.95Co0.05O. Appl. Phys. Lett. 2006, 89, 032508. [Google Scholar] [CrossRef]
- Zhang, L.-J.; Wang, J.-Q.; Li, J.; Zhou, J.; Cai, W.-P.; Cheng, J.; Xu, W.; Yin, G.; Wu, X.; Jiang, Z.; et al. High-Tc Ferromagnetism in a Co-Doped ZnO System Dominated by the Formation of a Zinc-Blende Type Co-Rich ZnCoO Phase. Chem. Commun. 2012, 48, 91–93. [Google Scholar] [CrossRef]
- Lardé, R.; Talbot, E.; Pareige, P.; Bieber, H.; Schmerber, G.; Colis, S.; Pierron-Bohnes, V.; Dinia, A. Evidence of Superparamagnetic Co Clusters in Pulsed Laser Deposition-Grown Zn0.9Co0.1O Thin Films Using Atom Probe Tomography. J. Am. Chem. Soc. 2011, 133, 1451–1458. [Google Scholar] [CrossRef][Green Version]
- Sasikala Devi, A.A.; Roqan, I.S. The Origin of Room Temperature Ferromagnetism Mediated by Co–VZn Complexes in the ZnO Grain Boundary. RSC Adv. 2016, 6, 50818–50824. [Google Scholar] [CrossRef]
- Chanda, A.; Gupta, S.; Vasundhara, M.; Joshi, S.R.; Mutta, G.R.; Singh, J. Study of Structural, Optical and Magnetic Properties of Cobalt Doped ZnO Nanorods. RSC Adv. 2017, 7, 50527–50536. [Google Scholar] [CrossRef]
- Rubi, D.; Fontcuberta, J.; Calleja, A.; Aragonès, L.; Capdevila, X.G.; Segarra, M. Reversible Ferromagnetic Switching in ZnO:(Co, Mn) Powders. Phys. Rev. B 2007, 75, 155322. [Google Scholar] [CrossRef]
- Walsh, A.; Da Silva, J.L.F.; Wei, S.-H. Theoretical Description of Carrier Mediated Magnetism in Cobalt Doped ZnO. Phys. Rev. Lett. 2008, 100, 256401. [Google Scholar] [CrossRef] [PubMed]
- Hou, Q.Y.; Liu, Y.J. Effects of Co Doping and Point Defect on the Ferromagnetism of ZnO. J. Supercond. Nov. Magn. 2019, 32, 1135–1142. [Google Scholar] [CrossRef]
- Janotti, A.; Van de Walle, C.G. Hydrogen Multicentre Bonds. Nat. Mater. 2007, 6, 44–47. [Google Scholar] [CrossRef] [PubMed]
- Khare, N.; Kappers, M.J.; Wei, M.; Blamire, M.G.; MacManus-Driscoll, J.L. Defect-Induced Ferromagnetism in Co-Doped ZnO. Adv. Mater. 2006, 18, 1449–1452. [Google Scholar] [CrossRef]
- Lu, Z.L.; Hsu, H.S.; Tzeng, Y.H.; Zhang, F.M.; Du, Y.W.; Huang, J.C.A. The Origins of Ferromagnetism in Co-Doped ZnO Single Crystalline Films: From Bound Magnetic Polaron to Free Carrier-Mediated Exchange Interaction. Appl. Phys. Lett. 2009, 95, 102501. [Google Scholar] [CrossRef]
- Lee, S.; Park, J.H.; Kim, B.-S.; Cho, D.-Y.; Choi, Y.N.; Lee, T.-W.; Kim, W.-K.; Kim, D.; Cho, C.R.; Moriyoshi, C.; et al. Formation of Ferromagnetic Co–H–Co Complex and Spin-Polarized Conduction Band in Co-Doped ZnO. Sci. Rep. 2017, 7, 11101. [Google Scholar] [CrossRef]
- Coey, J.M.D.; Venkatesan, M.; Fitzgerald, C.B. Donor Impurity Band Exchange in Dilute Ferromagnetic Oxides. Nat. Mater. 2005, 4, 173–179. [Google Scholar] [CrossRef]
- Norton, D.P.; Overberg, M.E.; Pearton, S.J.; Pruessner, K.; Budai, J.D.; Boatner, L.A.; Chisholm, M.F.; Lee, J.S.; Khim, Z.G.; Park, Y.D.; et al. Ferromagnetism in Cobalt-Implanted ZnO. Appl. Phys. Lett. 2003, 83, 5488–5490. [Google Scholar] [CrossRef]
- Ciatto, G.; Di Trolio, A.; Fonda, E.; Alippi, P.; Testa, A.M.; Bonapasta, A.A. Evidence of Cobalt-Vacancy Complexes in Zn1−XCoxO Dilute Magnetic Semiconductors. Phys. Rev. Lett. 2011, 107, 127206. [Google Scholar] [CrossRef]
- Varvaro, G.; Di Trolio, A.D.; Polimeni, A.; Gabbani, A.; Pineider, F.; Fernández, C.D.J.; Barucca, G.; Mengucci, P.; Bonapasta, A.A.; Testa, A.M. Giant Magneto-Optical Response in H+ Irradiated Zn1−XCoxO Thin Films. J. Mater. Chem. C 2019, 7, 78–85. [Google Scholar] [CrossRef]
- Esquinazi, P.D.; Hergert, W.; Stiller, M.; Botsch, L.; Ohldag, H.; Spemann, D.; Hoffmann, M.; Adeagbo, W.A.; Chassé, A.; Nayak, S.K.; et al. Defect-Induced Magnetism in Nonmagnetic Oxides: Basic Principles, Experimental Evidence, and Possible Devices with ZnO and TiO2. Phys. Status Solidi B 2020, 257, 1900623. [Google Scholar] [CrossRef]
- Di Trolio, A.; Testa, A.M.; Amore Bonapasta, A. Role of the Carrier Density in the Transport Mechanisms of Polycrystalline ZnO Films. Phys. Chem. Chem. Phys. 2021, 23, 13918–13925. [Google Scholar] [CrossRef]
- D’Agostino, D.; Di Giorgio, C.; Bobba, F.; Di Trolio, A.; Alippi, P.; Cucolo, A.M.; Amore Bonapasta, A. Effects of Cobalt Substitution on ZnO Surface Reactivity and Electronic Structure. J. Mater. Chem. C 2019, 7, 8364–8373. [Google Scholar] [CrossRef]
- Abdolhosseini Sarsari, I.; Pemmaraju, C.D.; Salamati, H.; Sanvito, S. Many-Body Quasiparticle Spectrum of Co-Doped ZnO: A GW Perspective. Phys. Rev. B 2013, 87, 245118. [Google Scholar] [CrossRef]
- Raebiger, H.; Lany, S.; Zunger, A. Electronic Structure, Donor and Acceptor Transitions, and Magnetism of 3d Impurities in In2O3 and ZnO. Phys. Rev. B 2009, 79, 165202. [Google Scholar] [CrossRef]
- Pearton, S.J.; Corbett, J.W.; Stavola, M. Hydrogen in Crystalline Semiconductors; Materials Science; Hans-Joachim Queisser; Springer: Berlin/Heidelberg, Germany, 1992; ISBN 978-3-540-55491-2. [Google Scholar]
- Sluiter, M.H.F.; Kawazoe, Y.; Sharma, P.; Inoue, A.; Raju, A.R.; Rout, C.; Waghmare, U.V. First Principles Based Design and Experimental Evidence for a ZnO-Based Ferromagnet at Room Temperature. Phys. Rev. Lett. 2005, 94, 187204. [Google Scholar] [CrossRef]
- Dong, J.J.; Zhang, X.W.; You, J.B.; Cai, P.F.; Yin, Z.G.; An, Q.; Ma, X.B.; Jin, P.; Wang, Z.G.; Chu, P.K. Effects of Hydrogen Plasma Treatment on the Electrical and Optical Properties of ZnO Films: Identification of Hydrogen Donors in ZnO. ACS Appl. Mater. Interfaces 2010, 2, 1780–1784. [Google Scholar] [CrossRef]
- Chen, W.; Zhu, L.; Li, Y.; Hu, L.; Guo, Y.; Xu, H.; Ye, Z. Origin of Highly Stable Conductivity of H Plasma Exposed ZnO Films. Phys. Chem. Chem. Phys. 2013, 15, 17763–17766. [Google Scholar] [CrossRef]
- Van de Walle, C.G. Hydrogen as a Cause of Doping in Zinc Oxide. Phys. Rev. Lett. 2000, 85, 1012–1015. [Google Scholar] [CrossRef]
- Gaspar, D.; Pereira, L.; Gehrke, K.; Galler, B.; Fortunato, E.; Martins, R. High Mobility Hydrogenated Zinc Oxide Thin Films. Sol. Energy Mater. Sol. Cells 2017, 163, 255–262. [Google Scholar] [CrossRef]
- Stephens, P.J. Magnetic Circular Dichroism. Annu. Rev. Phys. Chem. 1974, 25, 201–232. [Google Scholar] [CrossRef]
- Schwartz, D.A.; Norberg, N.S.; Nguyen, Q.P.; Parker, J.M.; Gamelin, D.R. Magnetic Quantum Dots: Synthesis, Spectroscopy, and Magnetism of Co2+ and Ni2+ Doped ZnO Nanocrystals. J. Am. Chem. Soc. 2003, 125, 13205–13218. [Google Scholar] [CrossRef]
- Vlaskin, V.A.; Beaulac, R.; Gamelin, D.R. Dopant−Carrier Magnetic Exchange Coupling in Colloidal Inverted Core/Shell Semiconductor Nanocrystals. Nano Lett. 2009, 9, 4376–4382. [Google Scholar] [CrossRef]
- Barrows, C.J.; Vlaskin, V.A.; Gamelin, D.R. Absorption and Magnetic Circular Dichroism Analyses of Giant Zeeman Splittings in Diffusion-Doped Colloidal Cd1–XMnxSe Quantum Dots. J. Phys. Chem. Lett. 2015, 6, 3076–3081. [Google Scholar] [CrossRef]
- Schimpf, A.M.; Gamelin, D.R. Thermal Tuning and Inversion of Excitonic Zeeman Splittings in Colloidal Doped CdSe Quantum Dots. J. Phys. Chem. Lett. 2012, 3, 1264–1268. [Google Scholar] [CrossRef]
- Chakraborty, P.; Jin, Y.; Barrows, C.J.; Dunham, S.T.; Gamelin, D.R. Kinetics of Isovalent (Cd2+) and Aliovalent (In3+) Cation Exchange in Cd1–XMnxSe Nanocrystals. J. Am. Chem. Soc. 2016, 138, 12885–12893. [Google Scholar] [CrossRef]
- Pinchetti, V.; Di, Q.; Lorenzon, M.; Camellini, A.; Fasoli, M.; Zavelani-Rossi, M.; Meinardi, F.; Zhang, J.; Crooker, S.A.; Brovelli, S. Excitonic Pathway to Photoinduced Magnetism in Colloidal Nanocrystals with Nonmagnetic Dopants. Nat. Nanotechnol. 2018, 13, 145–151. [Google Scholar] [CrossRef]
- Muckel, F.; Yang, J.; Lorenz, S.; Baek, W.; Chang, H.; Hyeon, T.; Bacher, G.; Fainblat, R. Digital Doping in Magic-Sized CdSe Clusters. ACS Nano 2016, 10, 7135–7141. [Google Scholar] [CrossRef]
- Faraday, M.I. Experimental Researches in Electricity.Nineteenth Series. Philos. Trans. R. Soc. Lond. 1846, 136, 1–20. [Google Scholar] [CrossRef]
- Ando, K.; Saito, H.; Jin, Z.; Fukumura, T.; Kawasaki, M.; Matsumoto, Y.; Koinuma, H. Large Magneto-Optical Effect in an Oxide Diluted Magnetic Semiconductor Zn1−xCoxO. Appl. Phys. Lett. 2001, 78, 2700–2702. [Google Scholar] [CrossRef]
- Jin, Z.-W.; Fukumura, T.; Hasegawa, K.; Yoo, Y.-Z.; Ando, K.; Sekiguchi, T.; Ahmet, P.; Chikyow, T.; Hasegawa, T.; Koinuma, H.; et al. Optical and Electrical Properties of Co-Doped Epitaxial ZnO Films. J. Cryst. Growth 2002, 237–239, 548–552. [Google Scholar] [CrossRef]
- Neal, J.R.; Behan, A.J.; Ibrahim, R.M.; Blythe, H.J.; Ziese, M.; Fox, A.M.; Gehring, G.A. Room-Temperature Magneto-Optics of FerromagneticTransition-Metal-Doped ZnThin Films. Phys. Rev. Lett. 2006, 96, 197208. [Google Scholar] [CrossRef] [PubMed]
- Kittilstved, K.R.; Zhao, J.; Liu, W.K.; Bryan, J.D.; Schwartz, D.A.; Gamelin, D.R. Magnetic Circular Dichroism of Ferromagnetic Co2+-Doped ZnO. Appl. Phys. Lett. 2006, 89, 062510. [Google Scholar] [CrossRef]
- Koidl, P. Optical Absorption of Co2+ in ZnO. Phys. Rev. B 1977, 15, 2493–2499. [Google Scholar] [CrossRef]
- Gong, S.-H.; Alpeggiani, F.; Sciacca, B.; Garnett, E.C.; Kuipers, L. Nanoscale Chiral Valley-Photon Interface through Optical Spin-Orbit Coupling. Science 2018, 359, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Di Trolio, A.; Polichetti, M.; Polimeni, A.; Testa, A.M. Local Magneto-Optical Response of H+ Irradiated Zn1−XCoxO Thin Films. Eur. Phys. J. Spec. Top. 2019, 228, 683–687. [Google Scholar] [CrossRef]

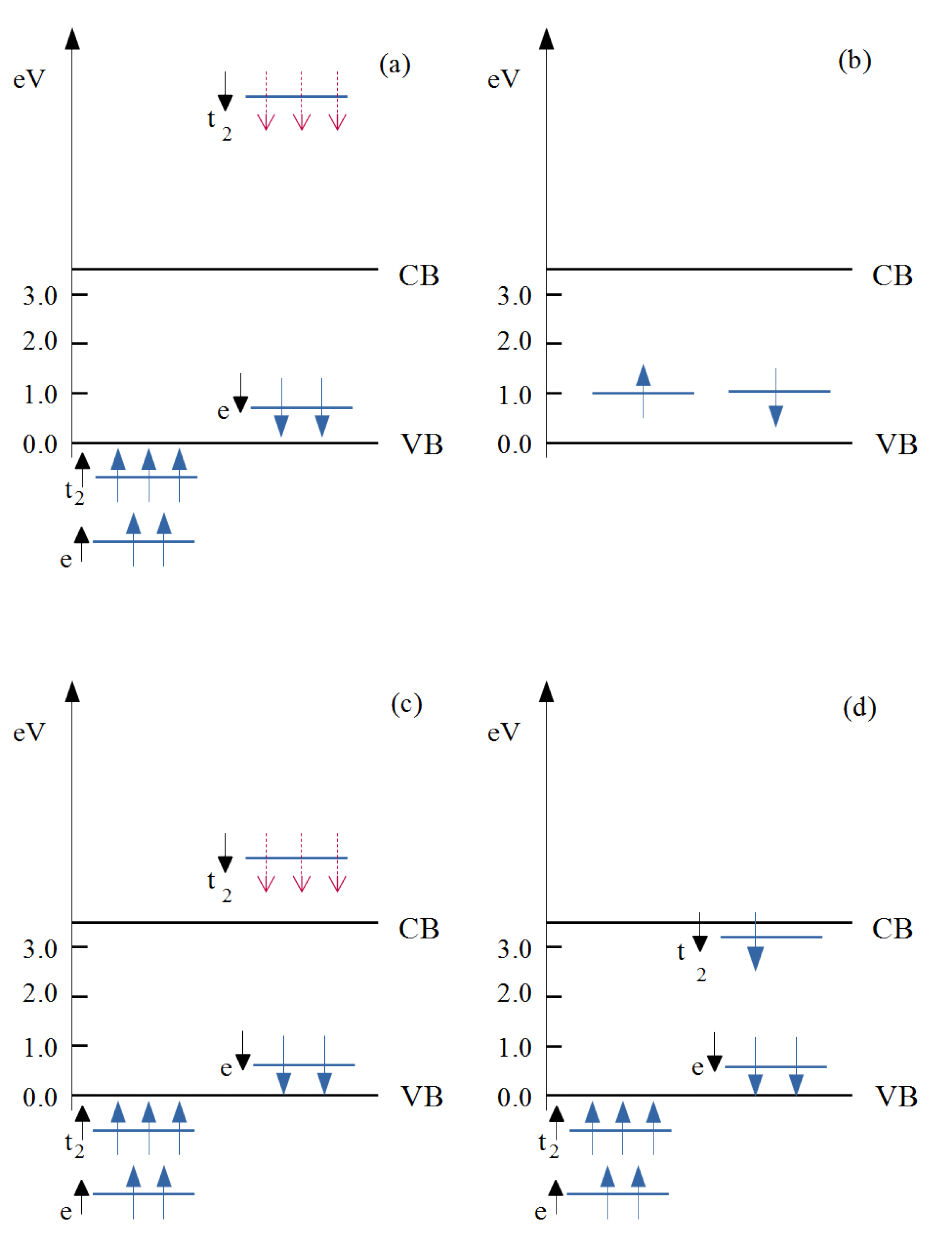

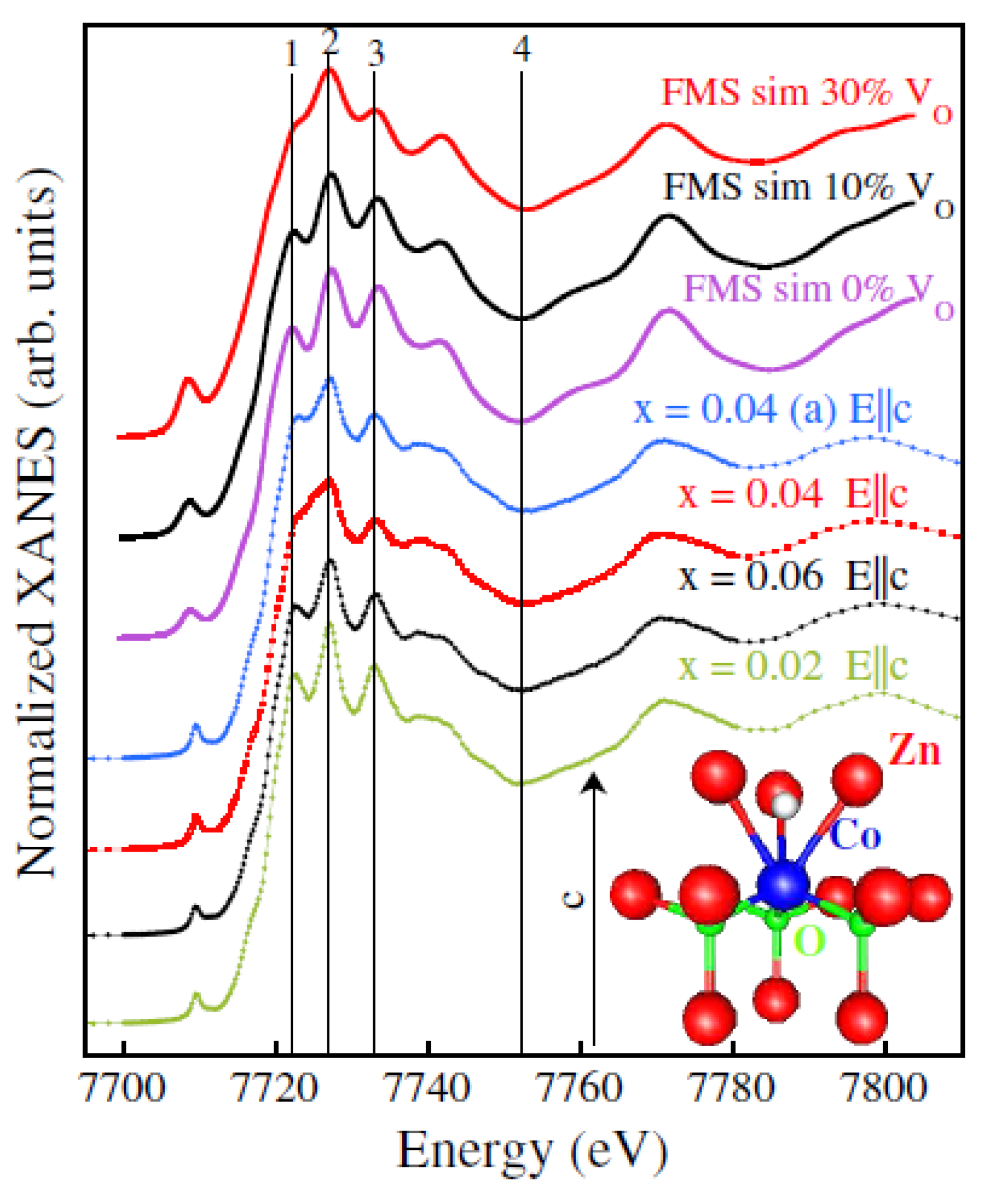
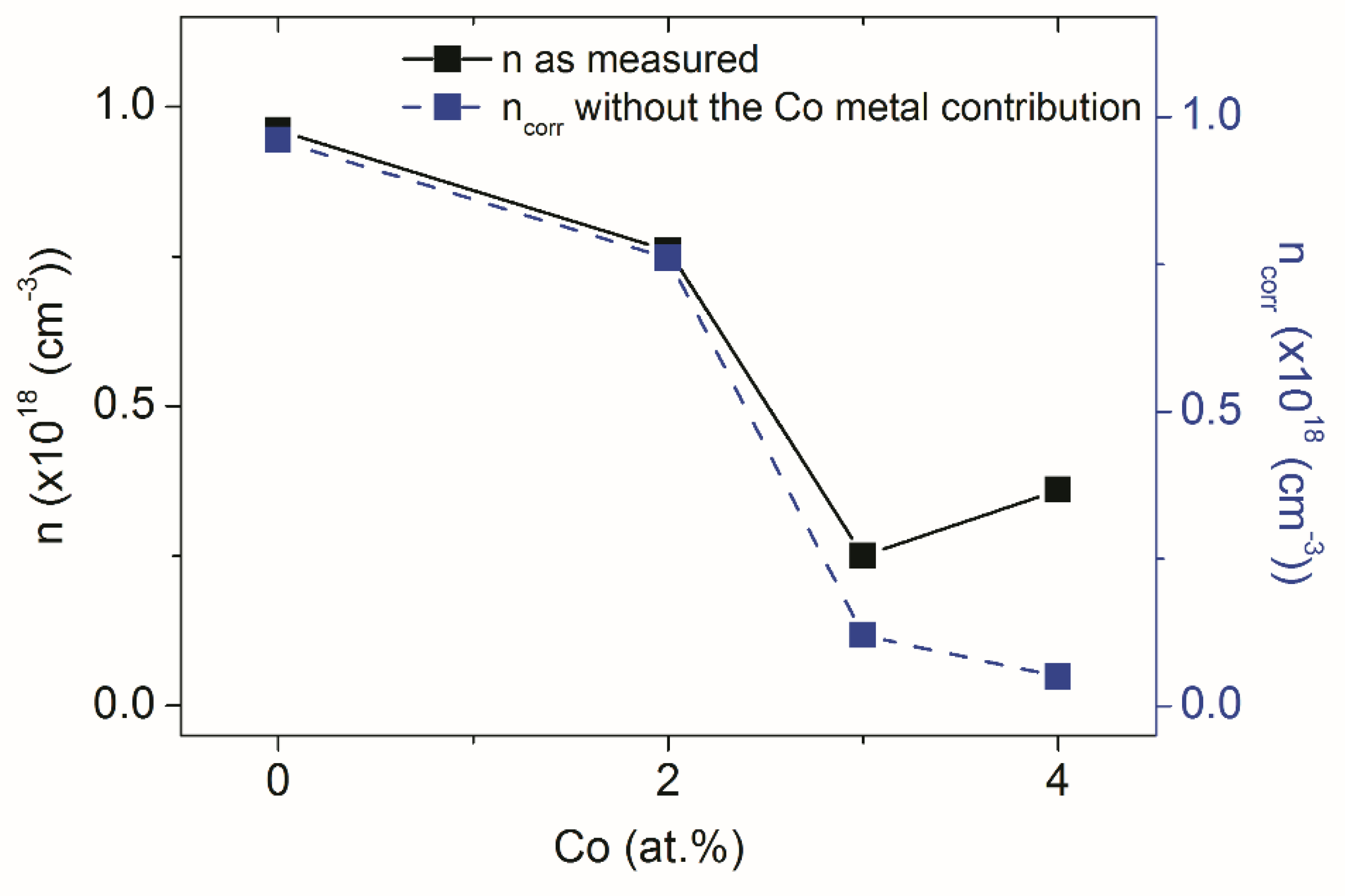
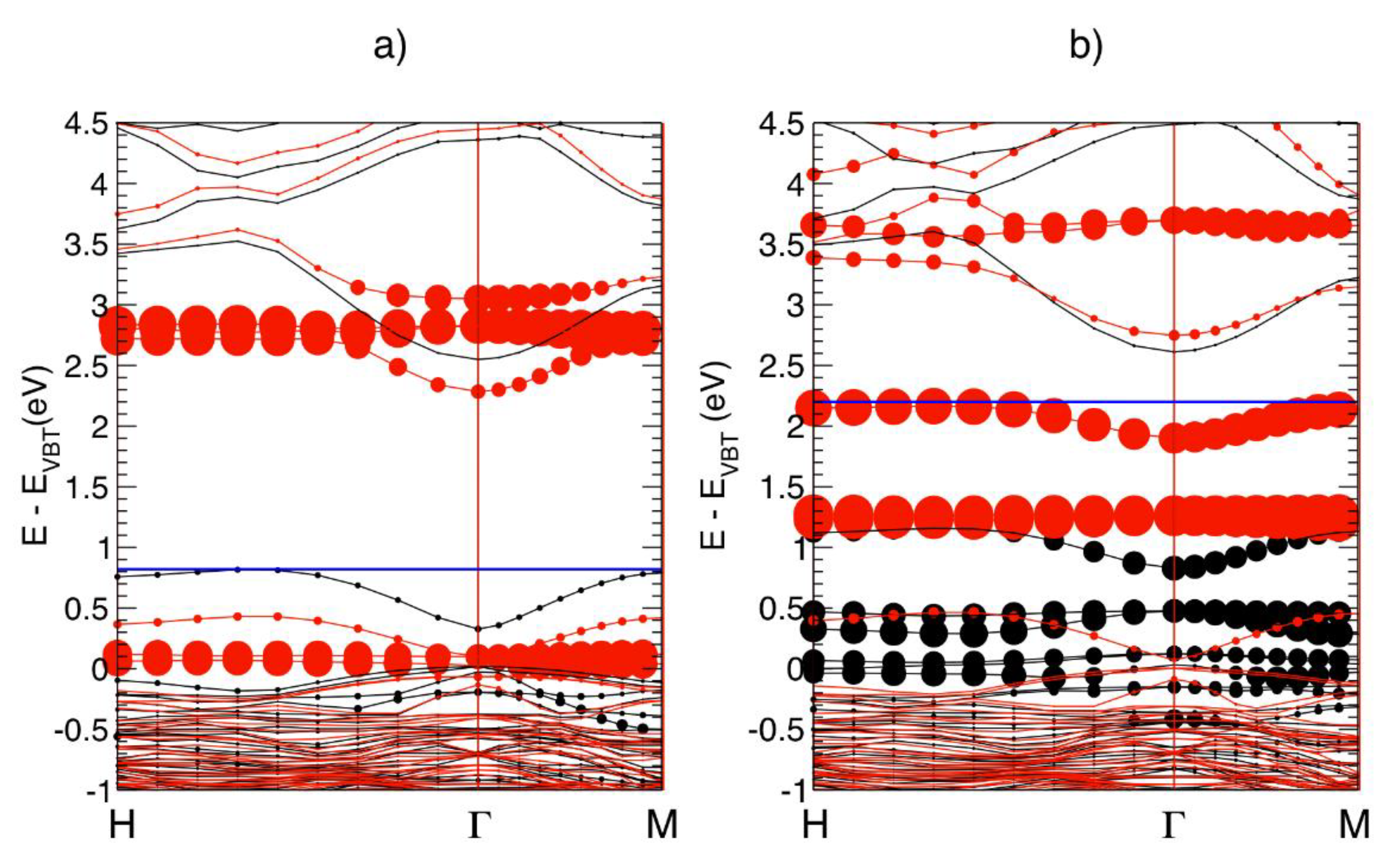


| C0 | C2 | C3 | C4 | |
|---|---|---|---|---|
| Ms (emu/cm3) (mean error ± 0.45) | 0.0 | 0.80 | 2.22 | 5.08 |
| Ms (μB/cm3) (mean error ± 0.45 × 1020) | 0.0 | 0.86 × 1020 | 2.40 × 1020 | 5.48 × 1020 |
| Co-VO (% of Zn ions) (mean error ± 0.2) | n.d. | 0.0 | 0.6 | 1.2 |
| Co-VO (cm−3) mean error ± 0.9 × 1020 | n.d. | 0.0 | 2.0 × 1020 | 4.0 × 1020 |
| Co metal (% of Co ions) (mean error ± 2) | n.d. | 0.1 | 2.1 | 3.7 |
| Samples | N (cm−3) as grown | Ms (μB/Co) as grown | N (cm−3) H-treated | Ms (μB/Co) H-treated | N (cm−3) Annealed @250 °C | Ms (μB/Co) Annealed @250 °C | N (cm−3) Annealed @500 °C | Ms (μB/Co) Annealed @500 °C |
|---|---|---|---|---|---|---|---|---|
| C1 dH = 1.5C | 2.8 × 1018 | 0.22 | 1.2 × 1019 | 0.37 | 6.2 × 1018 | 0.41 | <1016 | 0.26 |
| C2 dH = 3C | 2.0 × 1018 | 0.23 | 3.4 × 1018 | 0.85 | --- | --- | --- | --- |
| C3 dH = 4.5C | 2.7 × 1018 | 0.23 | 5.6 × 1018 | 1.10 | 9.2 × 1018 | 0.78 | <1016 | 0.24 |
| C4 dH = 6C | 2.0 × 1018 | 0.23 | 5.2 × 1018 | 1.50 | --- | --- | --- | --- |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Trolio, A.; Testa, A.M.; Amore Bonapasta, A. Ferromagnetic Behavior and Magneto-Optical Properties of Semiconducting Co-Doped ZnO. Nanomaterials 2022, 12, 1525. https://doi.org/10.3390/nano12091525
Di Trolio A, Testa AM, Amore Bonapasta A. Ferromagnetic Behavior and Magneto-Optical Properties of Semiconducting Co-Doped ZnO. Nanomaterials. 2022; 12(9):1525. https://doi.org/10.3390/nano12091525
Chicago/Turabian StyleDi Trolio, Antonio, Alberto M. Testa, and Aldo Amore Bonapasta. 2022. "Ferromagnetic Behavior and Magneto-Optical Properties of Semiconducting Co-Doped ZnO" Nanomaterials 12, no. 9: 1525. https://doi.org/10.3390/nano12091525
APA StyleDi Trolio, A., Testa, A. M., & Amore Bonapasta, A. (2022). Ferromagnetic Behavior and Magneto-Optical Properties of Semiconducting Co-Doped ZnO. Nanomaterials, 12(9), 1525. https://doi.org/10.3390/nano12091525






