Progress and Recent Trends in the Application of Nanoparticles as Low Carbon Fuel Additives—A State of the Art Review
Abstract
1. Introduction
- (1)
- To the best of our knowledge, studies that holistically review all three biofuels (alcohols, biodiesel, and vegetable oil) in the context of nanoparticles and engine characteristics are scarce; most of these studies typically consider only one type of the biofuels, especially biodiesel, with limited review specifically dedicated for alcohols or vegetable oils in the broader spectrum.
- (2)
- When doing a literature review on the evolution of any theory or concept over time, it is critical to include the development component by posing questions such as, “What are the evolutionary trends in the research field?”, “What future research areas have been emphasized in significant research articles?”, and “What are the major research areas?” [32]. The existing reviews clearly lack these aspects, and it is very imperative to systematically analyse the broad literature body, which could help structure the existing knowledge and identify future research gaps [33].
- (3)
- Energy-based indicators of ICE such as brake thermal efficiency (BTE), brake specific fuel consumption (BSFC), and emission characteristics are usually the most used assessment criteria for nanofuels [34]. However, an assessment based on these energetic indicators alone is not enough to describe an all-round performance of the diesel engine [35]. In addition, it is difficult to examine the renewability and sustainability of an energy resource using energy analysis since this indicator fails to consider the effects of the second law’s limitation on energy conversion [36]. Exergy analysis bridges this gap as it is a combination of both first and second law of thermodynamics and is closely linked to the renewability and sustainability nexus. In order to achieve a better understanding of the irreversibility or resource destruction, one could employ exergy analysis as it is a powerful technique for investigating the imperfections in an energy conversion system [36,37]. Despite its tremendous ability to optimize energy systems, conventional exergy analysis is often criticized for overlooking the economics and environmental aspects of the thermal system being considered. In nutshell, for an overall performance of any fuel in a thermal system, the energy and exergy indicators are very important, but the addition of the economic and environmental analysis is also key in determining the profitability and sustainability of an improvement in process through exergo-economic and exergo-environmental analysis [35]. A number of studies on the aforementioned aspects related to nano-low carbon fuels in diesel engines have been conducted [34,35,36,38,39,40,41,42]—however, these generalized discussions are missing in the extant literature review papers on the current subject.
- (4)
- It is worth noting that, besides the engine emissions, performance, and combustion characteristics, most of the existing reviews have only focused on the dispersion stability, wear and friction loss, corrosion, and cost-related issues with nanoparticles, with limited discussion on a very important aspect of these nano-additives, which is their toxicity and health impacts when they come into contact with humans and animals over a period of exposure. There is numerous evidence supporting how toxic these nanoparticles are and how detrimental they could be to an individual’s health [43,44,45,46,47,48]. It will therefore be prudent to augment the existing literature with these findings.
2. Discussion on Zero Carbon Ecology and Circular Economy
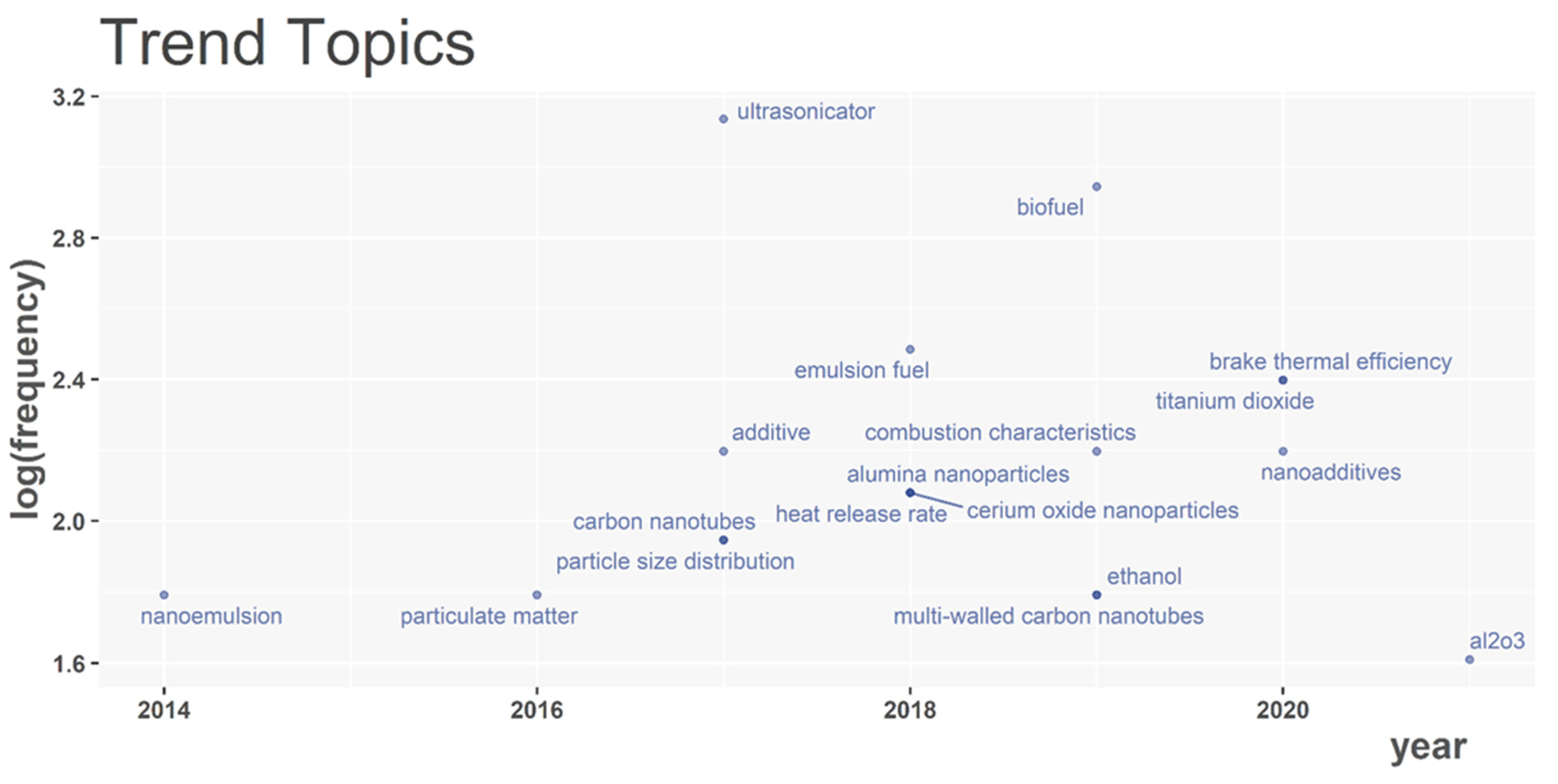
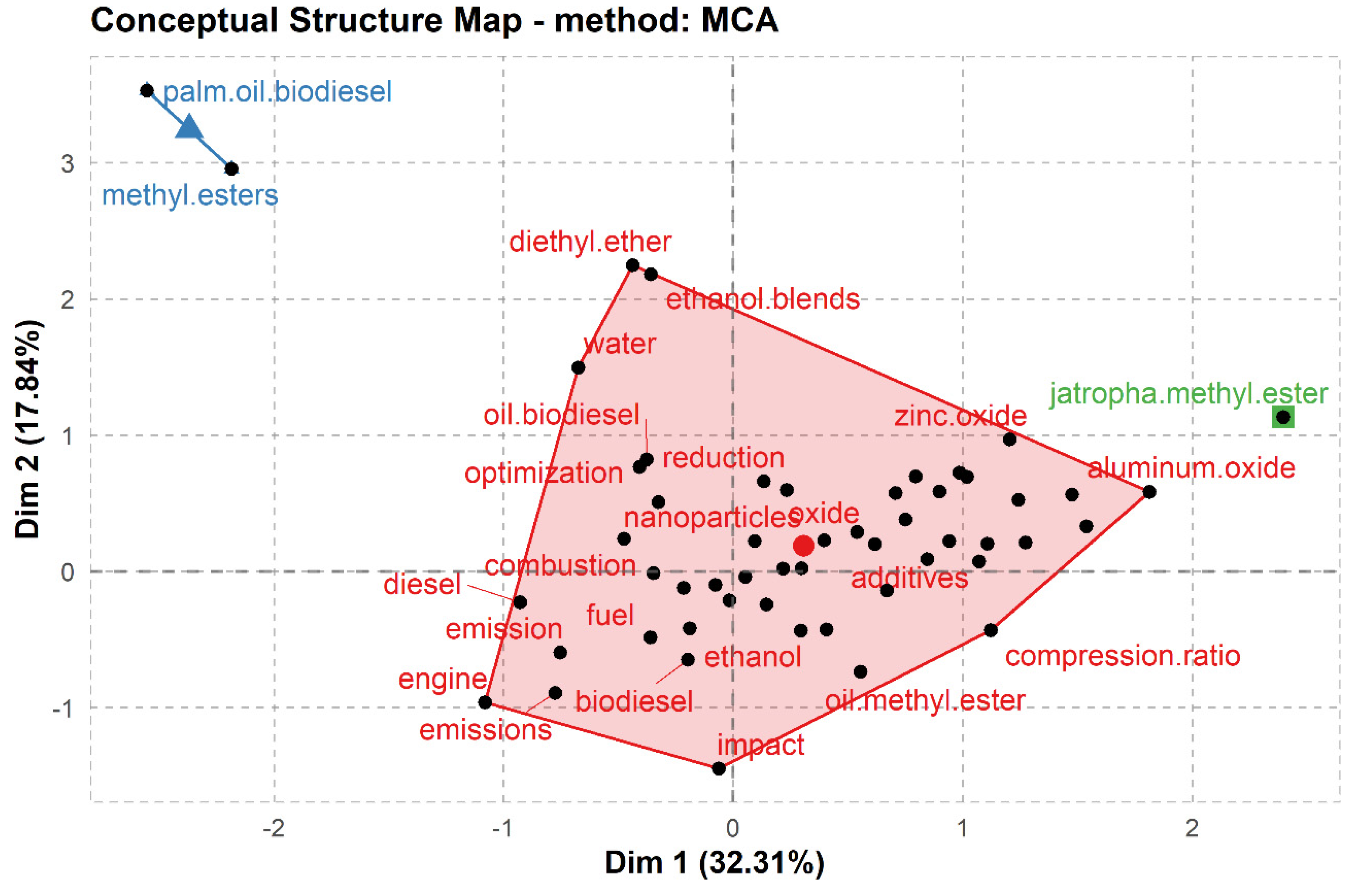
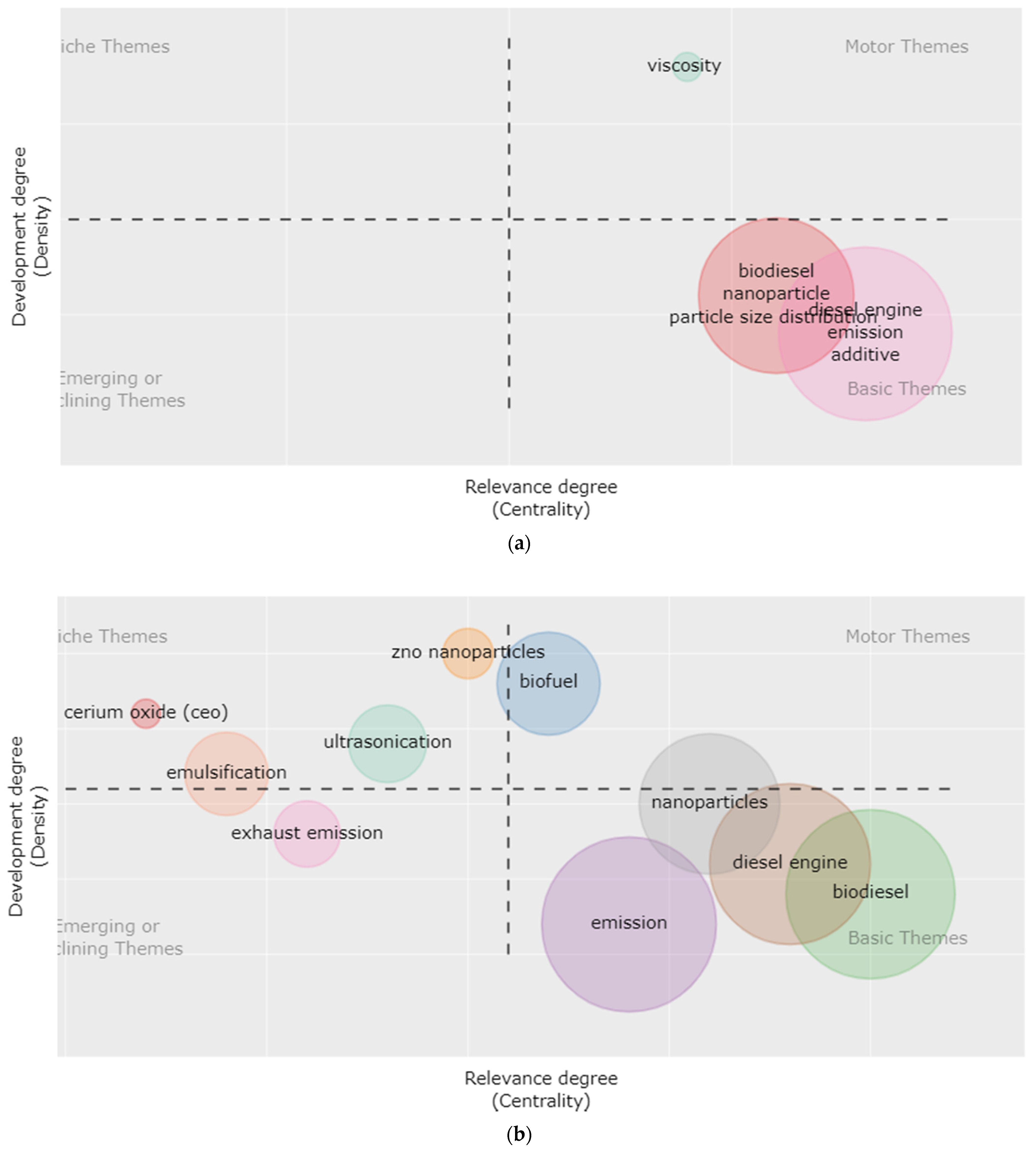
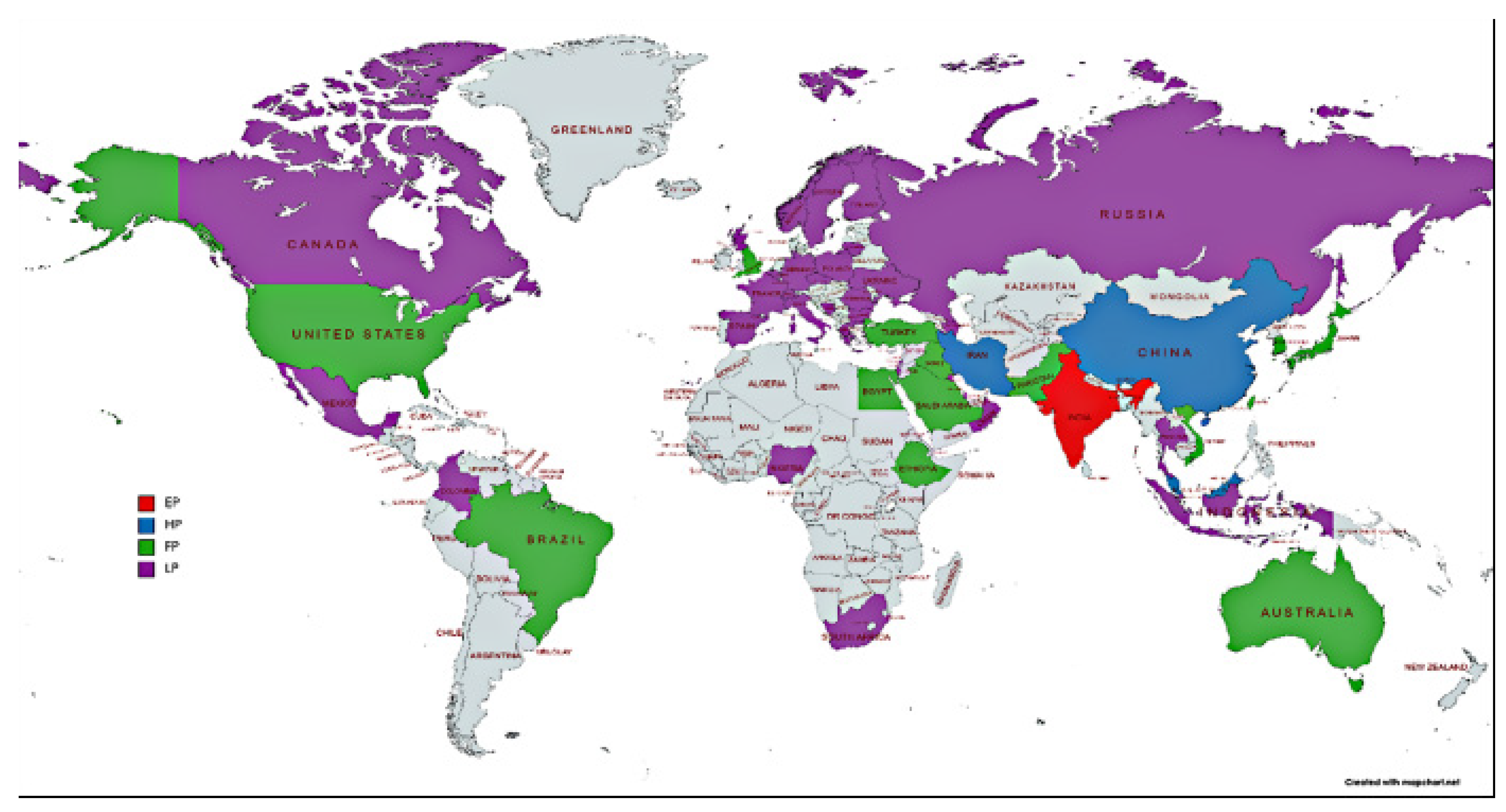
3. Research Hotspots and Evolutionary Trends
4. Fuel Properties, Emissions, Performance, and Combustion Characteristics
4.1. Effect of Nanoparticles on Fuel Properties of Low-Carbon Fuels
4.1.1. Alcohol-Based Fuels
4.1.2. Vegetable Oil-Based Fuels
4.1.3. Biodiesel-Based Fuels
4.2. Effect of Nanoparticles on Engine Performance/Emission/Combustion Characteristics of Low Carbon Fuels
4.2.1. Engine Performance Characteristics of Nanoparticles in Alcohol-Based Fuels
Brake Thermal Efficiency
Brake Specific Fuel Consumption
Brake Power and Brake Torque
4.2.2. Engine Emission Characteristics of Nanoparticles in Alcohol-Based Fuels
Carbon Monoxide
Hydrocarbons
Nitrogen Oxides
4.2.3. Effect of Nano-Additives and Diesel–Alcohol Fuels on Engine Combustion
4.2.4. Engine Performance Characteristics of Nanoparticles in Vegetable Oil-Based Fuels
Brake Thermal Efficiency
Brake Specific Fuel Consumption
4.2.5. Engine Emission Characteristics of Nanoparticles in Vegetable Oil-Based Fuel
Carbon Monoxide
Hydrocarbon
Nitrogen Oxides
4.2.6. Effect of Nano-Additives and Diesel–Vegetable Oil Blend Fuel on Engine Combustion
4.2.7. Engine Performance Characteristics of Nanoparticles in Biodiesel-Based Fuels
Brake Thermal Efficiency
Brake Specific Fuel Consumption
Brake Power and Brake Torque
4.2.8. Engine Emission Characteristics of Nanoparticles in Biodiesel–Based Fuels
Carbon Monoxide
Hydrocarbons
Nitrogen Oxides
4.2.9. Effect of Nano-Additives and Diesel–Biodiesel Blends on Engine Combustion
5. Comparative Strengths of Different Nanoparticles in Same Base Fuel
6. Similarities and Differences in Engine Characteristics of the Same Nanoparticle in Low-Carbon Fuels
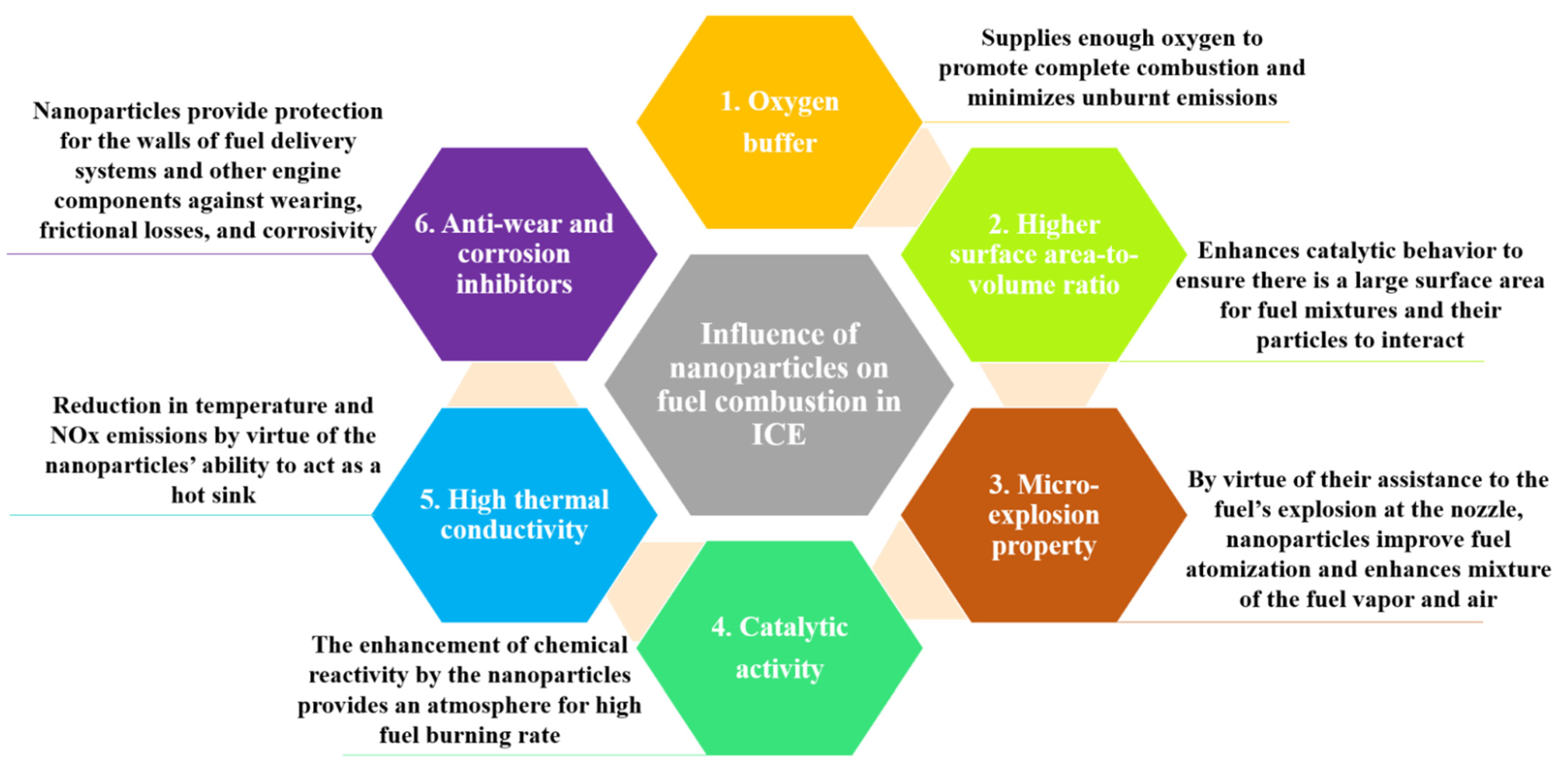
7. Summary of the Mechanism Involved with Nanoparticle’s Role during Low Carbon Fuel Combustion in ICE
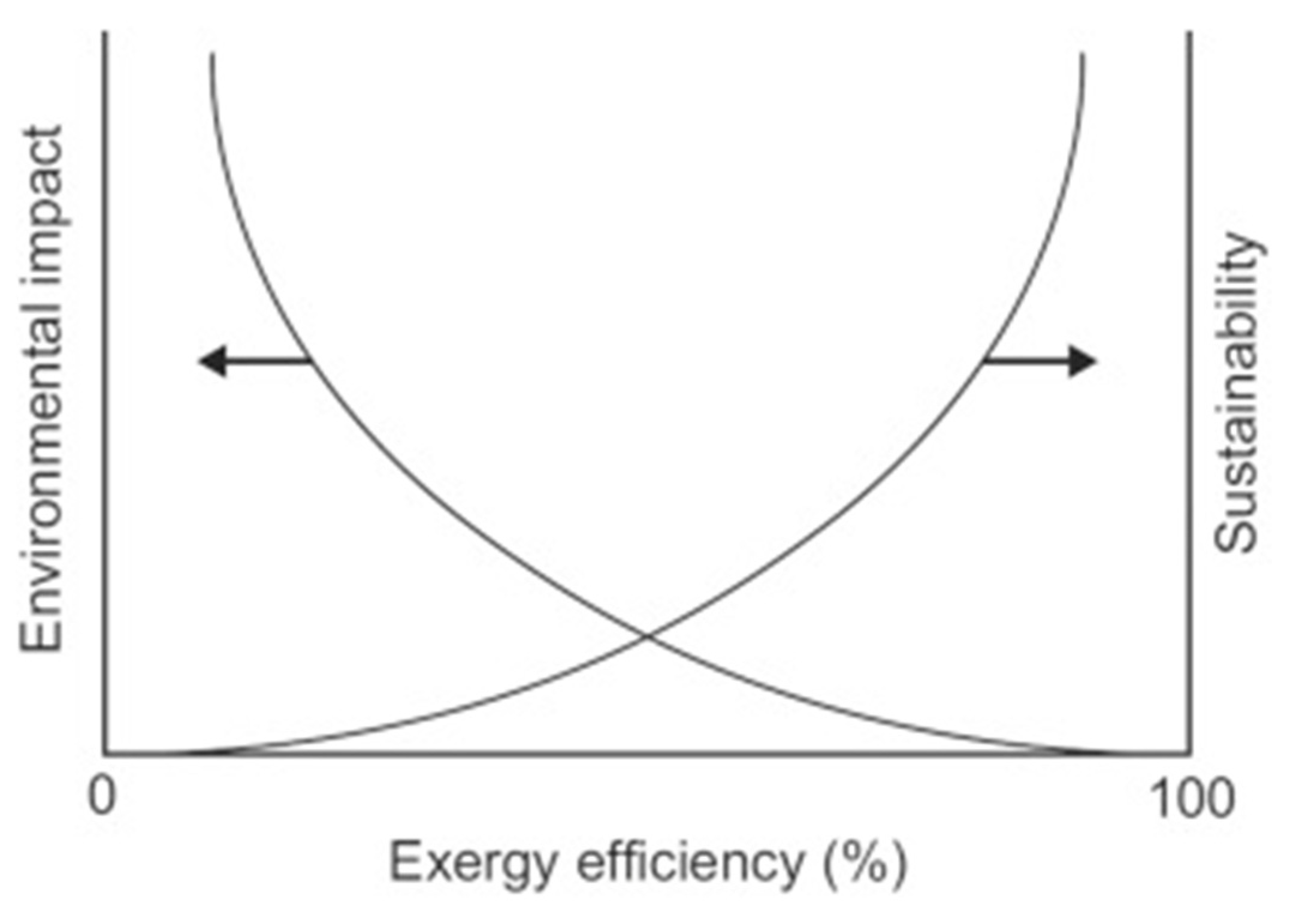
8. Exergy, Exergoeconomic, Exergoenvironmental, and Sustainability of Nano-Additives and Low Carbon Fuels in ICE
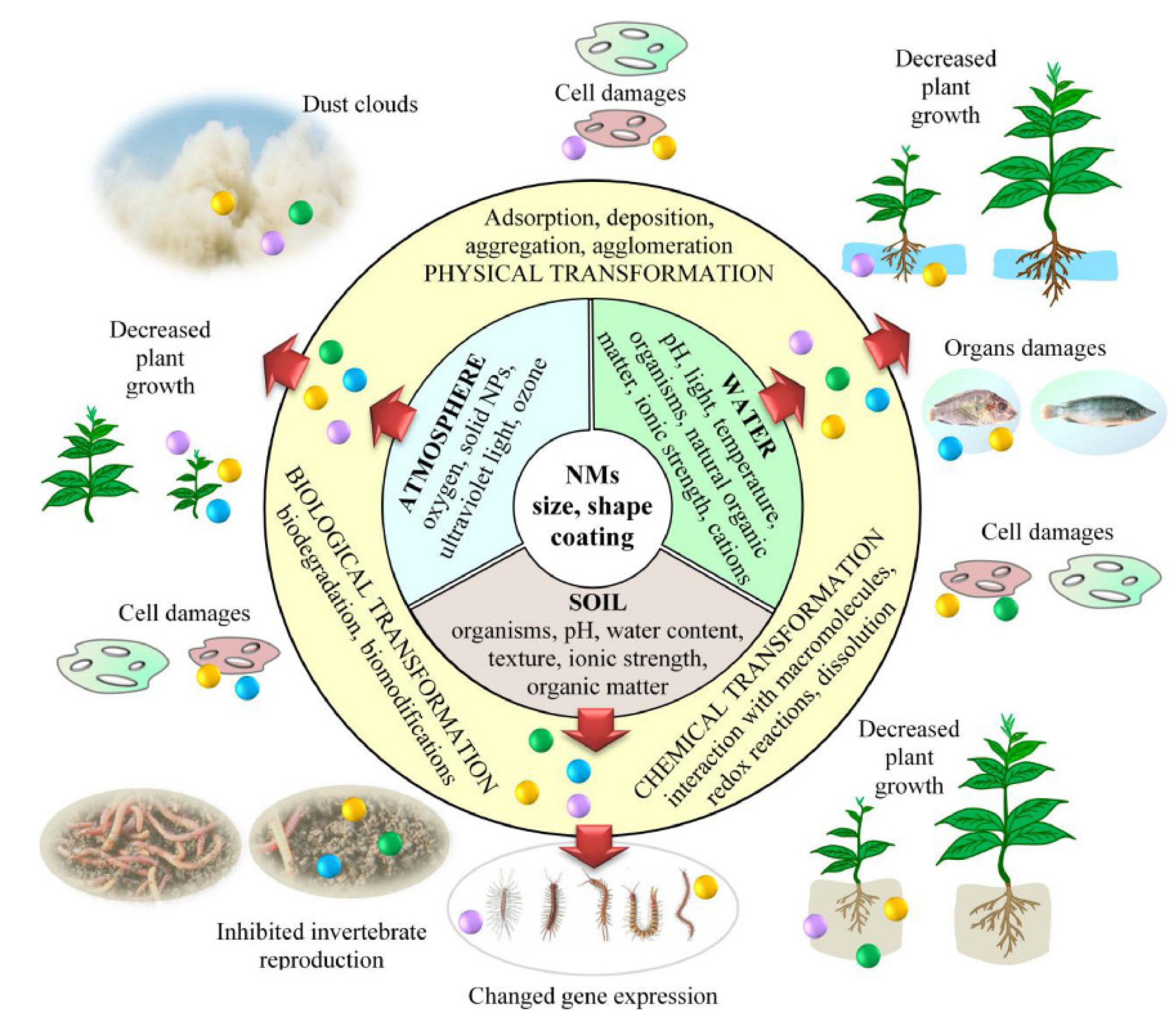
9. Toxicity and Health Impacts of Nanoparticles
10. Conclusions and Future Research Direction
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Joshi, G.; Pandey, J.K.; Rana, S.; Rawat, D.S. Challenges and opportunities for the application of biofuel. Renew. Sustain. Energy Rev. 2017, 79, 850–866. [Google Scholar] [CrossRef]
- Atabani, A.E.; Silitonga, A.S.; Badruddin, I.A.; Mahlia, T.M.I.; Masjuki, H.H.; Mekhilef, S. A comprehensive review on biodiesel as an alternative energy resource and its characteristics. Renew. Sustain. Energy Rev. 2012, 16, 2070–2093. [Google Scholar] [CrossRef]
- Garg, P. Energy Scenario and Vision 2020 in India. J. Sustain. Energy Environ. 2012, 3, 7–17. [Google Scholar]
- EIA. International Energy Outlook 2010. Available online: http://www.eia.doe.gov/oiaf/ieo/pdf/0484%282010%29.pdf (accessed on 18 August 2021).
- EIA. Annual Energy Review (AER), International Energy. Available online: http://www.eia.gov/emeu/aer/inter.html (accessed on 18 August 2021).
- Kumar, N. Oxidative stability of biodiesel: Causes, effects and prevention. Fuel 2017, 190, 328–350. [Google Scholar] [CrossRef]
- Rawat, D.S.; Joshi, G.; Lamba, B.Y.; Tiwari, A.K.; Mallick, S. Impact of additives on storage stability of Karanja (Pongamia Pinnata) biodiesel blends with conventional diesel sold at retail outlets. Fuel 2014, 120, 30–37. [Google Scholar] [CrossRef]
- Peer, M.S.; Kasimani, R.; Rajamohan, S.; Ramakrishnan, P. Experimental evaluation on oxidation stability of biodiesel/diesel blends with alcohol addition by rancimat instrument and FTIR spectroscopy. J. Mech. Sci. Technol. 2017, 31, 455–463. [Google Scholar] [CrossRef]
- Shahir, S.A.; Masjuki, H.H.; Kalam, M.A.; Imran, A.; Ashraful, A.M. Performance and emission assessment of diesel–biodiesel–ethanol/bioethanol blend as a fuel in diesel engines: A review. Renew. Sustain. Energy Rev. 2015, 48, 62–78. [Google Scholar] [CrossRef]
- Balat, M. Modeling Vegetable Oil Viscosity. Energy Sources Part A Recovery Util. Environ. Eff. 2008, 30, 1856–1869. [Google Scholar] [CrossRef]
- Atabani, A.E.; Silitonga, A.S.; Ong, H.C.; Mahlia, T.M.I.; Masjuki, H.H.; Badruddin, I.A.; Fayaz, H. Non-edible vegetable oils: A critical evaluation of oil extraction, fatty acid compositions, biodiesel production, characteristics, engine performance and emissions production. Renew. Sustain. Energy Rev. 2013, 18, 211–245. [Google Scholar] [CrossRef]
- Kuszewski, H. Experimental investigation of the effect of ambient gas temperature on the autoignition properties of ethanol–diesel fuel blends. Fuel 2018, 214, 26–38. [Google Scholar] [CrossRef]
- Abu-Qudais, M.; Haddad, O.; Qudaisat, M. The effect of alcohol fumigation on diesel engine performance and emissions. Energy Convers. Manag. 2000, 41, 389–399. [Google Scholar] [CrossRef]
- Ampah, J.D.; Liu, X.; Sun, X.; Pan, X.; Xu, L.; Jin, C.; Sun, T.; Geng, Z.; Afrane, S.; Liu, H. Study on characteristics of marine heavy fuel oil and low carbon alcohol blended fuels at different temperatures. Fuel 2022, 310, 122307. [Google Scholar] [CrossRef]
- Jin, C.; Liu, X.; Sun, T.; Ampah, J.D.; Geng, Z.; Ikram, M.; Ji, J.; Wang, G.; Liu, H. Preparation of ethanol and palm oil/palm kernel oil alternative biofuels based on property improvement and particle size analysis. Fuel 2021, 305, 121569. [Google Scholar] [CrossRef]
- Jin, C.; Zhang, X.; Han, W.; Geng, Z.; Tessa Margaret Thomas, M.; Ampah, J.D.; Wang, G.; Ji, J.; Liu, H. Macro and micro solubility between low-carbon alcohols and rapeseed oil using different co-solvents. Fuel 2020, 270, 117511. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, X.; Liu, H.; Wu, Y.; Zaman, M.; Geng, Z.; Jin, C.; Zheng, Z.; Yue, Z.; Yao, M. Effect of soybean oil/PODE/ethanol blends on combustion and emissions on a heavy-duty diesel engine. Fuel 2021, 288, 119625. [Google Scholar] [CrossRef]
- Kumar, S.; Dinesha, P.; Bran, I. Experimental investigation of the effects of nanoparticles as an additive in diesel and biodiesel fuelled engines: A review. Biofuels 2019, 10, 615–622. [Google Scholar] [CrossRef]
- Singh, G.; Sharma, S. Performance, combustion and emission characteristics of compression ignition engine using nano-fuel: A review. Int. J. Eng. Sci. Res. Technol. 2015, 4, 1034–1039. [Google Scholar]
- Khond, V.W.; Kriplani, V.M. Effect of nanofluid additives on performances and emissions of emulsified diesel and biodiesel fueled stationary CI engine: A comprehensive review. Renew. Sustain. Energy Rev. 2016, 59, 1338–1348. [Google Scholar] [CrossRef]
- Saxena, V.; Kumar, N.; Saxena, V.K. A comprehensive review on combustion and stability aspects of metal nanoparticles and its additive effect on diesel and biodiesel fuelled C.I. engine. Renew. Sustain. Energy Rev. 2017, 70, 563–588. [Google Scholar] [CrossRef]
- Razzaq, L.; Mujtaba, M.A.; Soudagar, M.E.M.; Ahmed, W.; Fayaz, H.; Bashir, S.; Fattah, I.M.R.; Ong, H.C.; Shahapurkar, K.; Afzal, A.; et al. Engine performance and emission characteristics of palm biodiesel blends with graphene oxide nanoplatelets and dimethyl carbonate additives. J. Environ. Manag. 2021, 282, 111917. [Google Scholar] [CrossRef]
- Hussain, F.; Soudagar, M.E.M.; Afzal, A.; Mujtaba, M.A.; Fattah, I.M.R.; Naik, B.; Mulla, M.H.; Badruddin, I.A.; Khan, T.M.Y.; Raju, V.D.; et al. Enhancement in Combustion, Performance, and Emission Characteristics of a Diesel Engine Fueled with Ce-ZnO Nanoparticle Additive Added to Soybean Biodiesel Blends. Energies 2020, 13, 4578. [Google Scholar] [CrossRef]
- Ribeiro, N.M.; Pinto, A.C.; Quintella, C.M.; da Rocha, G.O.; Teixeira, L.S.G.; Guarieiro, L.L.N.; do Carmo Rangel, M.; Veloso, M.C.C.; Rezende, M.J.C.; Serpa da Cruz, R.; et al. The Role of Additives for Diesel and Diesel Blended (Ethanol or Biodiesel) Fuels: A Review. Energy Fuels 2007, 21, 2433–2445. [Google Scholar] [CrossRef]
- Kegl, T.; Kovač Kralj, A.; Kegl, B.; Kegl, M. Nanomaterials as fuel additives in diesel engines: A review of current state, opportunities, and challenges. Prog. Energy Combust. Sci. 2021, 83, 100897. [Google Scholar] [CrossRef]
- Venkatesan, H.; Sivamani, S.; Sampath, S.; Gopi, V.; Kumar M, D. A Comprehensive Review on the Effect of Nano Metallic Additives on Fuel Properties, Engine Performance and Emission Characteristics. Int. J. Renew. Energy Res. 2017, 7, 825–843. [Google Scholar]
- Hoang, A.T. Combustion behavior, performance and emission characteristics of diesel engine fuelled with biodiesel containing cerium oxide nanoparticles: A review. Fuel Process. Technol. 2021, 218, 106840. [Google Scholar] [CrossRef]
- Shaafi, T.; Sairam, K.; Gopinath, A.; Kumaresan, G.; Velraj, R. Effect of dispersion of various nanoadditives on the performance and emission characteristics of a CI engine fuelled with diesel, biodiesel and blends—A review. Renew. Sustain. Energy Rev. 2015, 49, 563–573. [Google Scholar] [CrossRef]
- Dewangan, A.; Mallick, A.; Yadav, A.K.; Richhariya, A.K. Effect of metal oxide nanoparticles and engine parameters on the performance of a diesel engine: A review. Mater. Today Proc. 2020, 21, 1722–1727. [Google Scholar] [CrossRef]
- Nanthagopal, K.; Kishna, R.S.; Atabani, A.E.; Al-Muhtaseb, A.a.H.; Kumar, G.; Ashok, B. A compressive review on the effects of alcohols and nanoparticles as an oxygenated enhancer in compression ignition engine. Energy Convers. Manag. 2020, 203, 112244. [Google Scholar] [CrossRef]
- Soudagar, M.E.M.; Nik-Ghazali, N.-N.; Abul Kalam, M.; Badruddin, I.A.; Banapurmath, N.R.; Akram, N. The effect of nano-additives in diesel-biodiesel fuel blends: A comprehensive review on stability, engine performance and emission characteristics. Energy Convers. Manag. 2018, 178, 146–177. [Google Scholar] [CrossRef]
- Goyal, S.; Chauhan, S.; Mishra, P. Circular economy research: A bibliometric analysis (2000–2019) and future research insights. J. Clean. Prod. 2021, 287, 125011. [Google Scholar] [CrossRef]
- Meyer, T. Decarbonizing road freight transportation–A bibliometric and network analysis. Transp. Res. Part D Transp. Environ. 2020, 89, 102619. [Google Scholar] [CrossRef]
- Ağbulut, Ü.; Uysal, C.; Cavalcanti, E.J.C.; Carvalho, M.; Karagöz, M.; Saridemir, S. Exergy, exergoeconomic, life cycle, and exergoenvironmental assessments for an engine fueled by diesel–ethanol blends with aluminum oxide and titanium dioxide additive nanoparticles. Fuel 2022, 320, 123861. [Google Scholar] [CrossRef]
- Aghbashlo, M.; Tabatabaei, M.; Mohammadi, P.; Khoshnevisan, B.; Rajaeifar, M.A.; Pakzad, M. Neat diesel beats waste-oriented biodiesel from the exergoeconomic and exergoenvironmental point of views. Energy Convers. Manag. 2017, 148, 1–15. [Google Scholar] [CrossRef]
- Aghbashlo, M.; Tabatabaei, M.; Mohammadi, P.; Mirzajanzadeh, M.; Ardjmand, M.; Rashidi, A. Effect of an emission-reducing soluble hybrid nanocatalyst in diesel/biodiesel blends on exergetic performance of a DI diesel engine. Renew. Energy 2016, 93, 353–368. [Google Scholar] [CrossRef]
- Mojarab Soufiyan, M.; Aghbashlo, M.; Mobli, H. Exergetic performance assessment of a long-life milk processing plant: A comprehensive survey. J. Clean. Prod. 2017, 140, 590–607. [Google Scholar] [CrossRef]
- Aghbashlo, M.; Tabatabaei, M.; Khalife, E.; Najafi, B.; Mirsalim, S.M.; Gharehghani, A.; Mohammadi, P.; Dadak, A.; Roodbar Shojaei, T.; Khounani, Z. A novel emulsion fuel containing aqueous nano cerium oxide additive in diesel–biodiesel blends to improve diesel engines performance and reduce exhaust emissions: Part II–Exergetic analysis. Fuel 2017, 205, 262–271. [Google Scholar] [CrossRef]
- Ağbulut, Ü. Understanding the role of nanoparticle size on energy, exergy, thermoeconomic, exergoeconomic, and sustainability analyses of an IC engine: A thermodynamic approach. Fuel Process. Technol. 2022, 225, 107060. [Google Scholar] [CrossRef]
- Karagoz, M.; Uysal, C.; Agbulut, U.; Saridemir, S. Exergetic and exergoeconomic analyses of a CI engine fueled with diesel-biodiesel blends containing various metal-oxide nanoparticles. Energy 2021, 214, 118830. [Google Scholar] [CrossRef]
- Özcan, H. Energy and exergy analyses of Al2O3-diesel-biodiesel blends in a diesel engine. Int. J. Exergy 2019, 28, 29–45. [Google Scholar] [CrossRef]
- Shadidi, B.; Alizade, H.H.A.; Najafi, G. Performance and exergy analysis of a diesel engine run on petrodiesel and biodiesel blends containing mixed CeO2 and MoO3 nanocatalyst. Biofuels 2022, 13, 1–7. [Google Scholar] [CrossRef]
- Doshi, R.; Braida, W.; Christodoulatos, C.; Wazne, M.; O’Connor, G. Nano-aluminum: Transport through sand columns and environmental effects on plants and soil communities. Environ. Res. 2008, 106, 296–303. [Google Scholar] [CrossRef]
- Karlsson, H.L.; Cronholm, P.; Gustafsson, J.; Möller, L. Copper Oxide Nanoparticles Are Highly Toxic: A Comparison between Metal Oxide Nanoparticles and Carbon Nanotubes. Chem. Res. Toxicol. 2008, 21, 1726–1732. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Xing, B. Phytotoxicity of nanoparticles: Inhibition of seed germination and root growth. Environ. Pollut. 2007, 150, 243–250. [Google Scholar] [CrossRef]
- Long, T.C.; Saleh, N.; Tilton, R.D.; Lowry, G.V.; Veronesi, B. Titanium dioxide (P25) produces reactive oxygen species in immortalized brain microglia (BV2): Implications for nanoparticle neurotoxicity. Environ. Sci Technol. 2006, 40, 4346–4352. [Google Scholar] [CrossRef]
- Long, T.C.; Tajuba, J.; Sama, P.; Saleh, N.; Swartz, C.; Parker, J.; Hester, S.; Lowry, G.V.; Veronesi, B. Nanosize titanium dioxide stimulates reactive oxygen species in brain microglia and damages neurons in vitro. Environ. Health Perspect. 2007, 115, 1631–1637. [Google Scholar] [CrossRef]
- Soutter, W. Nanoparticles as Fuel Additives. Available online: https://www.azonano.com/article.aspx?ArticleID=3085 (accessed on 25 August 2021).
- World Bank. World Bank Open Data. Available online: https://data.worldbank.org/ (accessed on 7 May 2020).
- British Petroleum(BP). BP Energy Outlook. Available online: https://www.bp.com/content/dam/bp/business-sites/en/global/corporate/pdfs/energy-economics/energy-outlook/bp-energy-outlook-2019.pdf (accessed on 25 September 2021).
- International Energy Agency (IEA). Tracking Transport International Energy Agency and Organization for Economic Cooperation and Development, Paris. Available online: https://www.iea.org/reports/tracking-transport-2019 (accessed on 25 September 2021).
- Umar, M.; Ji, X.; Kirikkaleli, D.; Xu, Q. COP21 Roadmap: Do innovation, financial development, and transportation infrastructure matter for environmental sustainability in China? J. Environ. Manag. 2020, 271, 111026. [Google Scholar] [CrossRef]
- Umar, M.; Ji, X.; Mirza, N.; Naqvi, B. Carbon neutrality, bank lending, and credit risk: Evidence from the Eurozone. J. Environ. Manag. 2021, 296, 113156. [Google Scholar] [CrossRef]
- Jain, A.; Sarsaiya, S.; Kumar Awasthi, M.; Singh, R.; Rajput, R.; Mishra, U.C.; Chen, J.; Shi, J. Bioenergy and bio-products from bio-waste and its associated modern circular economy: Current research trends, challenges, and future outlooks. Fuel 2022, 307, 121859. [Google Scholar] [CrossRef]
- KAPSARC. Guide to the Circular Carbon Economy. Available online: https://www.cceguide.org/guide/ (accessed on 3 December 2021).
- Pritchard, A. Statistical bibliography or bibliometrics. J. Doc. 1969, 25, 348–349. [Google Scholar]
- Schneider, J.W.; Borlund, P. Introduction to bibliometrics for construction and maintenance of thesauri. J. Doc. 2004, 60, 524–549. [Google Scholar] [CrossRef]
- Aria, M.; Cuccurullo, C. bibliometrix: An R-tool for comprehensive science mapping analysis. J. Informetr. 2017, 11, 959–975. [Google Scholar] [CrossRef]
- Mao, G.; Liu, X.; Du, H.; Zuo, J.; Wang, L. Way forward for alternative energy research: A bibliometric analysis during 1994–2013. Renew. Sustain. Energy Rev. 2015, 48, 276–286. [Google Scholar] [CrossRef]
- Min, Z.; Hao, M. The Scientometric Evaluation on the Research of Biofuels Based on CiteSpace. Adv. Eng. Res. 2019, 184, 6–11. [Google Scholar]
- Jin, C.; Ampah, J.D.; Afrane, S.; Yin, Z.; Liu, X.; Sun, T.; Geng, Z.; Ikram, M.; Liu, H. Low-carbon alcohol fuels for decarbonizing the road transportation industry: A bibliometric analysis 2000–2021. Environ. Sci. Pollut. Res. 2021, 29, 5577–5604. [Google Scholar] [CrossRef]
- Zhang, M.; Gao, Z.; Zheng, T.; Ma, Y.; Wang, Q.; Gao, M.; Sun, X.J.J.o.M.C.; Management, W. A bibliometric analysis of biodiesel research during 1991–2015. J. Mater. Cycles Waste Manag. 2018, 20, 10–18. [Google Scholar] [CrossRef]
- Hosseini, S.H.; Taghizadeh-Alisaraei, A.; Ghobadian, B.; Abbaszadeh-Mayvan, A. Performance and emission characteristics of a CI engine fuelled with carbon nanotubes and diesel-biodiesel blends. Renew. Energy 2017, 111, 201–213. [Google Scholar] [CrossRef]
- Sadhik Basha, J.; Anand, R.B. Performance, emission and combustion characteristics of a diesel engine using Carbon Nanotubes blended Jatropha Methyl Ester Emulsions. Alex. Eng. J. 2014, 53, 259–273. [Google Scholar] [CrossRef]
- Heydari-Maleney, K.; Taghizadeh-Alisaraei, A.; Ghobadian, B.; Abbaszadeh-Mayvan, A. Analyzing and evaluation of carbon nanotubes additives to diesohol-B2 fuels on performance and emission of diesel engines. Fuel 2017, 196, 110–123. [Google Scholar] [CrossRef]
- Cobo, M.J.; López-Herrera, A.G.; Herrera-Viedma, E.; Herrera, F. An approach for detecting, quantifying, and visualizing the evolution of a research field: A practical application to the Fuzzy Sets Theory field. J. Informetr. 2011, 5, 146–166. [Google Scholar] [CrossRef]
- LCPTi. Key to a Sustainable Economy-Low Carbon Transport Fuels. Available online: http://docs.wbcsd.org/2015/11/LCTPi-LCTF-FinalReport.pdf (accessed on 20 April 2022).
- El-Seesy, A.I.; Kosaka, H.; Hassan, H.; Sato, S. Combustion and emission characteristics of a common rail diesel engine and RCEM fueled by n-heptanol-diesel blends and carbon nanomaterial additives. Energy Convers. Manag. 2019, 196, 370–394. [Google Scholar] [CrossRef]
- Nutakki, P.K.; Gugulothu, S.K.; Ramachander, J.; Sivasurya, M. Effect Of n-amyl alcohol/biodiesel blended nano additives on the performance, combustion and emission characteristics of CRDi diesel engine. Environ. Sci. Pollut. Res. 2021, 29, 82–97. [Google Scholar] [CrossRef]
- Ağbulut, Ü.; Polat, F.; Sarıdemir, S. A comprehensive study on the influences of different types of nano-sized particles usage in diesel-bioethanol blends on combustion, performance, and environmental aspects. Energy 2021, 229, 120548. [Google Scholar] [CrossRef]
- Venu, H.; Raju, V.D.; Lingesan, S.; Elahi M Soudagar, M. Influence of Al2O3 nano additives in ternary fuel (diesel-biodiesel-ethanol) blends operated in a single cylinder diesel engine: Performance, combustion and emission characteristics. Energy 2021, 215, 119091. [Google Scholar] [CrossRef]
- El-Seesy, A.I.; Hassan, H. Combustion Characteristics of a Diesel Engine Fueled by Biodiesel-Diesel-N-Butanol Blend and Titanium Oxide Additives. Energy Procedia 2019, 162, 48–56. [Google Scholar] [CrossRef]
- Prabakaran, B.; Udhoji, A. Experimental investigation into effects of addition of zinc oxide on performance, combustion and emission characteristics of diesel-biodiesel-ethanol blends in CI engine. Alex. Eng. J. 2016, 55, 3355–3362. [Google Scholar] [CrossRef]
- Wei, J.; He, C.; Lv, G.; Zhuang, Y.; Qian, Y.; Pan, S. The combustion, performance and emissions investigation of a dual-fuel diesel engine using silicon dioxide nanoparticle additives to methanol. Energy 2021, 230, 120734. [Google Scholar] [CrossRef]
- Pan, S.; Wei, J.; Tao, C.; Lv, G.; Qian, Y.; Liu, Q.; Han, W. Discussion on the combustion, performance and emissions of a dual fuel diesel engine fuelled with methanol-based CeO2 nanofluids. Fuel 2021, 302, 121096. [Google Scholar] [CrossRef]
- Ramachander, J.; Gugulothu, S.K.; Sastry, G.R.K. Performance and Emission Reduction Characteristics of Metal Based Sio2 Nanoparticle Additives Blended with Ternary Fuel (Diesel-MME-Pentanol) on CRDI Diesel Engine. Silicon 2022, 14, 2249–2263. [Google Scholar] [CrossRef]
- El-Seesy, A.I.; Hassan, H. Investigation of the effect of adding graphene oxide, graphene nanoplatelet, and multiwalled carbon nanotube additives with n-butanol-Jatropha methyl ester on a diesel engine performance. Renew. Energy 2019, 132, 558–574. [Google Scholar] [CrossRef]
- Heidari-Maleni, A.; Mesri Gundoshmian, T.; Jahanbakhshi, A.; Ghobadian, B. Performance improvement and exhaust emissions reduction in diesel engine through the use of graphene quantum dot (GQD) nanoparticles and ethanol-biodiesel blends. Fuel 2020, 267, 117116. [Google Scholar] [CrossRef]
- Shaafi, T.; Velraj, R. Influence of alumina nanoparticles, ethanol and isopropanol blend as additive with diesel–soybean biodiesel blend fuel: Combustion, engine performance and emissions. Renew. Energy 2015, 80, 655–663. [Google Scholar] [CrossRef]
- Venu, H.; Madhavan, V. Effect of Al2O3 nanoparticles in biodiesel-diesel-ethanol blends at various injection strategies: Performance, combustion and emission characteristics. Fuel 2016, 186, 176–189. [Google Scholar] [CrossRef]
- Örs, I.; Sarıkoç, S.; Atabani, A.E.; Ünalan, S.; Akansu, S.O. The effects on performance, combustion and emission characteristics of DICI engine fuelled with TiO2 nanoparticles addition in diesel/biodiesel/n-butanol blends. Fuel 2018, 234, 177–188. [Google Scholar] [CrossRef]
- Annamalai, M.; Dhinesh, B.; Nanthagopal, K.; SivaramaKrishnan, P.; Isaac JoshuaRamesh Lalvani, J.; Parthasarathy, M.; Annamalai, K. An assessment on performance, combustion and emission behavior of a diesel engine powered by ceria nanoparticle blended emulsified biofuel. Energy Convers. Manag. 2016, 123, 372–380. [Google Scholar] [CrossRef]
- Dhinesh, B.; Niruban Bharathi, R.; Isaac JoshuaRamesh Lalvani, J.; Parthasarathy, M.; Annamalai, K. An experimental analysis on the influence of fuel borne additives on the single cylinder diesel engine powered by Cymbopogon flexuosus biofuel. J. Energy Inst. 2017, 90, 634–645. [Google Scholar] [CrossRef]
- Kumaravel, S.T.; Murugesan, A.; Vijayakumar, C.; Thenmozhi, M. Enhancing the fuel properties of tyre oil diesel blends by doping nano additives for green environments. J. Clean. Prod. 2019, 240, 118128. [Google Scholar] [CrossRef]
- Sathiyamoorthi, R.; Sankaranarayanan, G.; Pitchandi, K. Combined effect of nanoemulsion and EGR on combustion and emission characteristics of neat lemongrass oil (LGO)-DEE-diesel blend fuelled diesel engine. Appl. Therm. Eng. 2017, 112, 1421–1432. [Google Scholar] [CrossRef]
- Sheriff, S.A.; Kumar, I.K.; Mandhatha, P.S.; Jambal, S.S.; Sellappan, R.; Ashok, B.; Nanthagopal, K. Emission reduction in CI engine using biofuel reformulation strategies through nano additives for atmospheric air quality improvement. Renew. Energy 2020, 147, 2295–2308. [Google Scholar] [CrossRef]
- Dhinesh, B.; Annamalai, M. A study on performance, combustion and emission behaviour of diesel engine powered by novel nano nerium oleander biofuel. J. Clean. Prod. 2018, 196, 74–83. [Google Scholar] [CrossRef]
- Chinnasamy, C.; Tamilselvam, P.; Ranjith, R. Environmental effects of adding aluminum oxide nano-additives on diesel engine fueled with pyrolyzed biomass oil–diesel blends. Energy Sources Part A Recovery Util. Environ. Eff. 2018, 40, 2735–2744. [Google Scholar] [CrossRef]
- Dobrzyńska, E.; Szewczyńska, M.; Pośniak, M.; Szczotka, A.; Puchałka, B.; Woodburn, J. Exhaust emissions from diesel engines fueled by different blends with the addition of nanomodifiers and hydrotreated vegetable oil HVO. Environ. Pollut. 2020, 259, 113772. [Google Scholar] [CrossRef]
- Sharma, S.K.; Das, R.K.; Sharma, A. Improvement in the performance and emission characteristics of diesel engine fueled with jatropha methyl ester and tyre pyrolysis oil by addition of nano additives. J. Braz. Soc. Mech. Sci. Eng. 2016, 38, 1907–1920. [Google Scholar] [CrossRef]
- Santhanamuthu, M.; Chittibabu, S.; Tamizharasan, T.; Mani, T.P. Evaluation of CI engine performance fuelled by Diesel-Polanga oil blends doped with iron oxide nanoparticles. Int. J. ChemTech Res. 2014, 6, 1299–1308. [Google Scholar]
- El-Seesy, A.I.; Abdel-Rahman, A.K.; Bady, M.; Ookawara, S. Performance, combustion, and emission characteristics of a diesel engine fueled by biodiesel-diesel mixtures with multi-walled carbon nanotubes additives. Energy Convers. Manag. 2017, 135, 373–393. [Google Scholar] [CrossRef]
- Alenezi, R.A.; Norkhizan, A.M.; Mamat, R.; Erdiwansyah; Najafi, G.; Mazlan, M. Investigating the contribution of carbon nanotubes and diesel-biodiesel blends to emission and combustion characteristics of diesel engine. Fuel 2021, 285, 119046. [Google Scholar] [CrossRef]
- Rastogi, P.M.; Sharma, A.; Kumar, N. Effect of CuO nanoparticles concentration on the performance and emission characteristics of the diesel engine running on jojoba (Simmondsia Chinensis) biodiesel. Fuel 2021, 286, 119358. [Google Scholar] [CrossRef]
- Rastogi, P.M.; Kumar, N.; Sharma, A.; Vyas, D.; Gajbhiye, A. Sustainability of Aluminium Oxide Nanoparticles Blended Mahua Biodiesel to the Direct Injection Diesel Engine Performance and Emission Analysis. Pollution 2020, 6, 25–33. [Google Scholar] [CrossRef]
- Nithya, S.; Manigandan, S.; Gunasekar, P.; Devipriya, J.; Saravanan, W.S.R. The effect of engine emission on canola biodiesel blends with TiO2. Int. J. Ambient Energy 2019, 40, 838–841. [Google Scholar] [CrossRef]
- Janakiraman, S.; Lakshmanan, T.; Chandran, V.; Subramani, L. Comparative behavior of various nano additives in a DIESEL engine powered by novel Garcinia gummi-gutta biodiesel. J. Clean. Prod. 2020, 245, 118940. [Google Scholar] [CrossRef]
- Khalife, E.; Tabatabaei, M.; Najafi, B.; Mirsalim, S.M.; Gharehghani, A.; Mohammadi, P.; Aghbashlo, M.; Ghaffari, A.; Khounani, Z.; Roodbar Shojaei, T.; et al. A novel emulsion fuel containing aqueous nano cerium oxide additive in diesel–biodiesel blends to improve diesel engines performance and reduce exhaust emissions: Part I–Experimental analysis. Fuel 2017, 207, 741–750. [Google Scholar] [CrossRef]
- Karthikeyan, S.; Elango, A.; Prathima, A. The effect of cerium oxide additive on the performance and emission characteristics of a CI engine operated with rice bran biodiesel and its blends. Int. J. Green Energy 2016, 13, 267–273. [Google Scholar] [CrossRef]
- Hajjari, M.; Ardjmand, M.; Tabatabaei, M. Experimental investigation of the effect of cerium oxide nanoparticles as a combustion-improving additive on biodiesel oxidative stability: Mechanism. RSC Adv. 2014, 4, 14352–14356. [Google Scholar] [CrossRef]
- Anbarasu, A.; Karthikeyan, A. Performance and Emission Characteristics of a Diesel Engine Using Cerium Oxide Nanoparticle Blended Biodiesel Emulsion Fuel. J. Energy Eng. 2016, 142, 04015009. [Google Scholar] [CrossRef]
- Ramakrishnan, G.; Krishnan, P.; Rathinam, S.; Thiyagu, R.; Devarajan, Y. Role of nano-additive blended biodiesel on emission characteristics of the research diesel engine. Int. J. Green Energy 2019, 16, 435–441. [Google Scholar] [CrossRef]
- Baluchamy, A.; Karuppusamy, M. The combined effects of cobalt chromite nanoparticles and variable injection timing of preheated biodiesel and diesel on performance, combustion and emission characteristics of CI engine. Heat Mass Transf. 2021, 57, 1565–1582. [Google Scholar] [CrossRef]
- Solmaz, H.; Ardebili, S.M.S.; Calam, A.; Yılmaz, E.; İpci, D. Prediction of performance and exhaust emissions of a CI engine fueled with multi-wall carbon nanotube doped biodiesel-diesel blends using response surface method. Energy 2021, 227, 120518. [Google Scholar] [CrossRef]
- Chacko, N.; Jeyaseelan, T. Comparative evaluation of graphene oxide and graphene nanoplatelets as fuel additives on the combustion and emission characteristics of a diesel engine fuelled with diesel and biodiesel blend. Fuel Process. Technol. 2020, 204, 106406. [Google Scholar] [CrossRef]
- Kumar, A.R.M.; Kannan, M.; Nataraj, G. A study on performance, emission and combustion characteristics of diesel engine powered by nano-emulsion of waste orange peel oil biodiesel. Renew. Energy 2020, 146, 1781–1795. [Google Scholar] [CrossRef]
- Sulochana, G.; Bhatti, S.K. Performance, Emission and Combustion Characteristics of a Twin Cylinder 4 Stroke Diesel Engine Using Nano-tubes Blended Waste Fry Oil Methyl Ester. Mater. Today Proc. 2019, 18, 75–84. [Google Scholar] [CrossRef]
- Hoseini, S.S.; Najafi, G.; Ghobadian, B.; Ebadi, M.T.; Mamat, R.; Yusaf, T. Biodiesels from three feedstock: The effect of graphene oxide (GO) nanoparticles diesel engine parameters fuelled with biodiesel. Renew. Energy 2020, 145, 190–201. [Google Scholar] [CrossRef]
- Tewari, P.; Doijode, E.; Banapurmath, N.R.; Yaliwal, V.S. Experimental investigations on a diesel engine fuelled with multiwalled carbon nanotubes blended biodiesel fuels. Int. J. Emerg. Technol. Adv. Eng. 2013, 3, 72–76. [Google Scholar]
- Venkatraman, N.; Suppandipillai, J.; Muthu, M. Experimental investigation of DI diesel engine performance with oxygenated additive and SOME Biodiesel. J. Therm. Sci. Technol. 2014, 10, JTST0014. [Google Scholar]
- Anchupogu, P.; Rao, L.N.; Banavathu, B. Effect of alumina nano additives into biodiesel-diesel blends on the combustion performance and emission characteristics of a diesel engine with exhaust gas recirculation. Environ. Sci. Pollut. Res. 2018, 25, 23294–23306. [Google Scholar] [CrossRef]
- Zenghui, Y.; Jing, H.; Wei, J. Study on the influence of alumina nanomethanol fluid on the performance, combustion and emission of DMDF diesel engine. E3S Web Conf. 2021, 268, 01004. [Google Scholar]
- Mirbagheri, S.A.; Safieddin Ardebili, S.M.; Kiani Deh Kiani, M. Modeling of the engine performance and exhaust emissions characteristics of a single-cylinder diesel using nano-biochar added into ethanol-biodiesel-diesel blends. Fuel 2020, 278, 118238. [Google Scholar] [CrossRef]
- Safieddin Ardebili, S.M.; Taghipoor, A.; Solmaz, H.; Mostafaei, M. The effect of nano-biochar on the performance and emissions of a diesel engine fueled with fusel oil-diesel fuel. Fuel 2020, 268, 117356. [Google Scholar] [CrossRef]
- Mardi K, M.; Antoshkiv, O.; Berg, H.P. Experimental analysis of the effect of nano-metals and novel organic additives on performance and emissions of a diesel engine. Fuel Process. Technol. 2019, 196, 106166. [Google Scholar] [CrossRef]
- Soudagar, M.E.M.; Afzal, A.; Safaei, M.R.; Manokar, A.M.; El-Seesy, A.I.; Mujtaba, M.A.; Samuel, O.D.; Badruddin, I.A.; Ahmed, W.; Shahapurkar, K.; et al. Investigation on the effect of cottonseed oil blended with different percentages of octanol and suspended MWCNT nanoparticles on diesel engine characteristics. J. Therm. Anal. Calorim. 2020, 147, 525–542. [Google Scholar] [CrossRef]
- Khan, H.; Soudagar, M.E.M.; Kumar, R.H.; Safaei, M.R.; Farooq, M.; Khidmatgar, A.; Banapurmath, N.R.; Farade, R.A.; Abbas, M.M.; Afzal, A.; et al. Effect of Nano-Graphene Oxide and n-Butanol Fuel Additives Blended with Diesel—Nigella sativa Biodiesel Fuel Emulsion on Diesel Engine Characteristics. Symmetry 2020, 12, 961. [Google Scholar] [CrossRef]
- Mehregan, M.; Moghiman, M. Numerical Investigation of Effect of Nano-Aluminum Addition on NOx and CO Pollutants Emission in Liquid Fuels Combustion. Int. J. Mater. Mech. Manuf. 2014, 2, 60–63. [Google Scholar] [CrossRef]
- Nour, M.; El-Seesy, A.I.; Abdel-Rahman, A.K.; Bady, M. Influence of adding aluminum oxide nanoparticles to diesterol blends on the combustion and exhaust emission characteristics of a diesel engine. Exp. Therm. Fluid Sci. 2018, 98, 634–644. [Google Scholar] [CrossRef]
- Yusuf, A.A.; Inambao, F.L.; Ampah, J.D. Evaluation of biodiesel on speciated PM2.5, organic compound, ultrafine particle and gaseous emissions from a low-speed EPA Tier II marine diesel engine coupled with DPF, DEP and SCR filter at various loads. Energy 2022, 239, 121837. [Google Scholar] [CrossRef]
- Wei, J.; Yin, Z.; Wang, C.; Lv, G.; Zhuang, Y.; Li, X.; Wu, H. Impact of aluminium oxide nanoparticles as an additive in diesel-methanol blends on a modern DI diesel engine. Appl. Therm. Eng. 2021, 185, 116372. [Google Scholar] [CrossRef]
- Yaşar, A.; Keskin, A.; Yıldızhan, Ş.; Uludamar, E.; Ocakoğlu, K. Effects of titanium-based additive with blends of butanol and diesel fuel on engine characteristics. Int. J. Glob. Warm. 2018, 15, 38. [Google Scholar] [CrossRef]
- Wei, J.; He, C.; Fan, C.; Pan, S.; Wei, M.; Wang, C. Comparison in the effects of alumina, ceria and silica nanoparticle additives on the combustion and emission characteristics of a modern methanol-diesel dual-fuel CI engine. Energy Convers. Manag. 2021, 238, 114121. [Google Scholar] [CrossRef]
- Somasundaram, D.; Elango, A.; Karthikeyan, S. Estimation of carbon credits of fishing boat diesel engine running on diesel-ethanol-bio-diesel blends with nano alumina doped ceria-zirconia. Mater. Today Proc. 2020, 33, 2923–2928. [Google Scholar] [CrossRef]
- Purushothaman, P.; Masimalai, S.; Subramani, V. Effective utilization of mahua oil blended with optimum amount of Al2O3 and TiO2 nanoparticles for better performance in CI engine. Environ. Sci. Pollut. Res. 2021, 28, 11893–11903. [Google Scholar] [CrossRef]
- Ramesh, P.; Vivekanandan, S.; Sivaramakrishnan; Prakash, D. Performance optimization of an engine for canola oil blended diesel with Al2O3 nanoparticles through single and multi-objective optimization techniques. Fuel 2021, 288, 119617. [Google Scholar] [CrossRef]
- Balasubramanian, D.; Venugopal, I.P.; Viswanathan, K. Characteristics Investigation on Di Diesel Engine with Nano-Particles as an Additive in Lemon Grass Oil; SAE Technical Paper 2 019-28-0081; SAE Mobilus: Warrendale, PL, USA, 2019. [Google Scholar]
- Panithasan, M.S.; Gopalakichenin, D.; Venkadesan, G.; Veeraraagavan, S. Impact of rice husk nanoparticle on the performance and emission aspects of a diesel engine running on blends of pine oil-diesel. Environ. Sci. Pollut. Res. 2019, 26, 282–291. [Google Scholar] [CrossRef]
- Elumalai, P.V.; Balasubramanian, D.; Parthasarathy, M.; Pradeepkumar, A.R.; Mohamed Iqbal, S.; Jayakar, J.; Nambiraj, M. An experimental study on harmful pollution reduction technique in low heat rejection engine fuelled with blends of pre-heated linseed oil and nano additive. J. Clean. Prod. 2021, 283, 124617. [Google Scholar] [CrossRef]
- Wamankar, A.K.; Murugan, S. Combustion, performance and emission of a diesel engine fuelled with diesel doped with carbon black. Energy 2015, 86, 467–475. [Google Scholar] [CrossRef]
- Ampah, J.D.; Yusuf, A.A.; Afrane, S.; Jin, C.; Liu, H. Reviewing two decades of cleaner alternative marine fuels: Towards IMO’s decarbonization of the maritime transport sector. J. Clean. Prod. 2021, 320, 128871. [Google Scholar] [CrossRef]
- Rashedul, H.K.; Masjuki, H.H.; Kalam, M.A.; Ashraful, A.M.; Ashrafur Rahman, S.M.; Shahir, S.A. The effect of additives on properties, performance and emission of biodiesel fuelled compression ignition engine. Energy Convers. Manag. 2014, 88, 348–364. [Google Scholar] [CrossRef]
- Chinnasamy, C.; Tamilselvam, P.; Ranjith, R. Influence of aluminum oxide nanoparticle with different particle sizes on the working attributes of diesel engine fueled with blends of diesel and waste plastic oil. Environ. Sci Pollut. Res. Int. 2019, 26, 29962–29977. [Google Scholar] [CrossRef]
- Lu, T.; Cheung, C.S.; Huang, Z. Effects of engine operating conditions on the size and nanostructure of diesel particles. J. Aerosol. Sci. 2012, 47, 27–38. [Google Scholar] [CrossRef]
- Wei, J.; Lu, W.; Pan, M.; Liu, Y.; Cheng, X.; Wang, C. Physical properties of exhaust soot from dimethyl carbonate-diesel blends: Characterizations and impact on soot oxidation behavior. Fuel 2020, 279, 118441. [Google Scholar] [CrossRef]
- Wei, J.; Wang, Y. Effects of biodiesels on the physicochemical properties and oxidative reactivity of diesel particulates: A review. Sci. Total Environ. 2021, 788, 147753. [Google Scholar] [CrossRef]
- Thangavelu S, K.; Arthanarisamy, M. Experimental investigation on engine performance, emission, and combustion characteristics of a DI CI engine using tyre pyrolysis oil and diesel blends doped with nanoparticles. Environ. Prog. Sustain. Energy 2020, 39, e13321. [Google Scholar] [CrossRef]
- Fu, P.; Bai, X.; Yi, W.; Li, Z.; Li, Y.; Wang, L. Assessment on performance, combustion and emission characteristics of diesel engine fuelled with corn stalk pyrolysis bio-oil/diesel emulsions with Ce0.7Zr0.3O2 nanoadditive. Fuel Process. Technol. 2017, 167, 474–483. [Google Scholar] [CrossRef]
- Jeyakumar, N.; Narayanasamy, B. Investigation of performance, emission, combustion characteristics of municipal waste plastic oil fueled diesel engine with nano fluids. Energy Sources Part A: Recovery Util. Environ. Eff. 2020, 1–22. [Google Scholar] [CrossRef]
- Karisathan Sundararajan, N.; Ammal, A.R.B. Improvement studies on emission and combustion characteristics of DICI engine fuelled with colloidal emulsion of diesel distillate of plastic oil, TiO2 nanoparticles and water. Environ. Sci. Pollut. Res. Int. 2018, 25, 11595–11613. [Google Scholar] [CrossRef]
- Sivathanu, N.; Valai Anantham, N. Impact of multi-walled carbon nanotubes with waste fishing net oil on performance, emission and combustion characteristics of a diesel engine. Environ. Technol. 2020, 41, 3670–3681. [Google Scholar] [CrossRef]
- Vellaiyan, S. Enhancement in combustion, performance, and emission characteristics of a biodiesel-fueled diesel engine by using water emulsion and nanoadditive. Renew. Energy 2020, 145, 2108–2120. [Google Scholar] [CrossRef]
- Soukht Saraee, H.; Taghavifar, H.; Jafarmadar, S. Experimental and numerical consideration of the effect of CeO2 nanoparticles on diesel engine performance and exhaust emission with the aid of artificial neural network. Appl. Therm. Eng. 2017, 113, 663–672. [Google Scholar] [CrossRef]
- Perumal Venkatesan, E.; Kandhasamy, A.; Sivalingam, A.; Kumar, A.S.; Ramalingam, K.; Joshua, P.j.t.; Balasubramanian, D. Performance and emission reduction characteristics of cerium oxide nanoparticle-water emulsion biofuel in diesel engine with modified coated piston. Environ. Sci. Pollut. Res. 2019, 26, 27362–27371. [Google Scholar] [CrossRef]
- Senthil Kumar, J.; Ramesh Bapu, B.R. Cerium oxide nano additive impact of VCR diesel engine characteristics by using Ginger grass oil blended with diesel. Int. J. Ambient Energy 2019, 43, 578–583. [Google Scholar] [CrossRef]
- Sam Sukumar, R.; Maddula, M.R.; Gopala Krishna, A. Experimental investigations with zinc oxide and silver doped zinc oxide nanoparticles for performance and emissions study of biodiesel blends in diesel engine. Int. J. Ambient Energy 2020, 1–9. [Google Scholar] [CrossRef]
- Kumaran, P.; Joel Godwin, A.; Amirthaganesan, S. Effect of microwave synthesized hydroxyapatite nanorods using Hibiscus rosa-sinensis added waste cooking oil (WCO) methyl ester biodiesel blends on the performance characteristics and emission of a diesel engine. Mater. Today: Proc. 2020, 22, 1047–1053. [Google Scholar] [CrossRef]
- Debbarma, S.; Misra, R.D. Effects of Iron Nanoparticles Blended Biodiesel on the Performance and Emission Characteristics of a Diesel Engine. J. Energy Resour. Technol. 2017, 139, 042212. [Google Scholar] [CrossRef]
- Tamilvanan, A.; Balamurugan, K.; Vijayakumar, M. Effects of nano-copper additive on performance, combustion and emission characteristics of Calophyllum inophyllum biodiesel in CI engine. J. Therm. Anal. Calorim. 2019, 136, 317–330. [Google Scholar] [CrossRef]
- Sivasaravanan, S.; Booma Devi, P.; Nagaraj, M.; Jeya Jeevahan, J.; Britto Joseph, G. Influence of Rice Husk Nanoparticles on Engine Performance and Emission Characteristics of Diesel and Neem Oil Biodiesel Blends in a Single Cylinder Diesel Engine. Energy Sources Part A Recovery Util. Environ. Eff. 2019, 1–16. [Google Scholar] [CrossRef]
- Karthikeyan, S.; Prathima, A. Environmental effect of CI engine using microalgae methyl ester with doped nano additives. Transp. Res. Part D Transp. Environ. 2017, 50, 385–396. [Google Scholar] [CrossRef]
- Ghanbari, M.; Mozafari-Vanani, L.; Dehghani-Soufi, M.; Jahanbakhshi, A. Effect of alumina nanoparticles as additive with diesel–biodiesel blends on performance and emission characteristic of a six-cylinder diesel engine using response surface methodology (RSM). Energy Convers. Manag. X 2021, 11, 100091. [Google Scholar] [CrossRef]
- Mujtaba, M.A.; Kalam, M.A.; Masjuki, H.H.; Gul, M.; Soudagar, M.E.M.; Ong, H.C.; Ahmed, W.; Atabani, A.E.; Razzaq, L.; Yusoff, M. Comparative study of nanoparticles and alcoholic fuel additives-biodiesel-diesel blend for performance and emission improvements. Fuel 2020, 279, 118434. [Google Scholar] [CrossRef]
- Shekofteh, M.; Gundoshmian, T.M.; Jahanbakhshi, A.; Heidari-Maleni, A. Performance and emission characteristics of a diesel engine fueled with functionalized multi-wall carbon nanotubes (MWCNTs-OH) and diesel–biodiesel–bioethanol blends. Energy Rep. 2020, 6, 1438–1447. [Google Scholar] [CrossRef]
- Srinivasan, S.K.; Kuppusamy, R.; Krishnan, P. Effect of nanoparticle-blended biodiesel mixtures on diesel engine performance, emission, and combustion characteristics. Environ. Sci. Pollut. Res. 2021, 28, 39210–39226. [Google Scholar] [CrossRef]
- Dhanasekar, K.; Sridaran, M.; Arivanandhan, M.; Jayavel, R. A facile preparation, performance and emission analysis of pongamia oil based novel biodiesel in diesel engine with CeO2:Gd nanoparticles. Fuel 2019, 255, 115756. [Google Scholar] [CrossRef]
- Gad, M.S.; Jayaraj, S. A comparative study on the effect of nano-additives on the performance and emissions of a diesel engine run on Jatropha biodiesel. Fuel 2020, 267, 117168. [Google Scholar] [CrossRef]
- Deepak Kumar, T.; Sameer Hussain, S.; Ramesha, D.K. Effect of a zinc oxide nanoparticle fuel additive on the performance and emission characteristics of a CI engine fuelled with cotton seed biodiesel blends. Mater. Today Proc. 2020, 26, 2374–2378. [Google Scholar] [CrossRef]
- Dinesha, P.; Kumar, S.; Rosen, M.A. Effects of particle size of cerium oxide nanoparticles on the combustion behavior and exhaust emissions of a diesel engine powered by biodiesel/diesel blend. Biofuel Res. J. 2021, 8, 1374–1383. [Google Scholar] [CrossRef]
- Yusuf, A.A.; Inambao, F.L.; Farooq, A.A. Impact of n-butanol-gasoline-hydrogen blends on combustion reactivity, performance and tailpipe emissions using TGDI engine parameters variation. Sustain. Energy Technol. Assess. 2020, 40, 100773. [Google Scholar] [CrossRef]
- Subramani, L.; Parthasarathy, M.; Balasubramanian, D.; Ramalingam, K. Novel Garcinia gummi-gutta methyl ester (GGME) as a potential alternative feedstock for existing unmodified DI diesel engine. Renew. Energy 2018, 125, 568–577. [Google Scholar] [CrossRef]
- Srinidhi, C.; Madhusudhan, A.; Channapattana, S.V. Effect of NiO nanoparticles on performance and emission characteristics at various injection timings using biodiesel-diesel blends. Fuel 2019, 235, 185–193. [Google Scholar] [CrossRef]
- Ağbulut, Ü.; Karagöz, M.; Sarıdemir, S.; Öztürk, A. Impact of various metal-oxide based nanoparticles and biodiesel blends on the combustion, performance, emission, vibration and noise characteristics of a CI engine. Fuel 2020, 270, 117521. [Google Scholar] [CrossRef]
- Muruganantham, P.; Pandiyan, P.; Sathyamurthy, R. Analysis on performance and emission characteristics of corn oil methyl ester blended with diesel and cerium oxide nanoparticle. Case Stud. Therm. Eng. 2021, 26, 101077. [Google Scholar]
- Soudagar, M.E.M.; Nik-Ghazali, N.-N.; Kalam, M.A.; Badruddin, I.A.; Banapurmath, N.R.; Bin Ali, M.A.; Kamangar, S.; Cho, H.M.; Akram, N. An investigation on the influence of aluminium oxide nano-additive and honge oil methyl ester on engine performance, combustion and emission characteristics. Renew. Energy 2020, 146, 2291–2307. [Google Scholar] [CrossRef]
- Ranjan, A.; Dawn, S.S.; Jayaprabakar, J.; Nirmala, N.; Saikiran, K.; Sai Sriram, S. Experimental investigation on effect of MgO nanoparticles on cold flow properties, performance, emission and combustion characteristics of waste cooking oil biodiesel. Fuel 2018, 220, 780–791. [Google Scholar] [CrossRef]
- Praveena, V.; Martin, M.L.J.; Geo, V.E. Experimental characterization of CI engine performance, combustion and emission parameters using various metal oxide nanoemulsion of grapeseed oil methyl ester. J. Therm. Anal. Calorim. 2020, 139, 3441–3456. [Google Scholar] [CrossRef]
- Dhana Raju, V.; Kishore, P.S.; Nanthagopal, K.; Ashok, B. An experimental study on the effect of nanoparticles with novel tamarind seed methyl ester for diesel engine applications. Energy Convers. Manag. 2018, 164, 655–666. [Google Scholar] [CrossRef]
- Sivakumar, M.; Shanmuga Sundaram, N.; Ramesh kumar, R.; Syed Thasthagir, M.H. Effect of aluminium oxide nanoparticles blended pongamia methyl ester on performance, combustion and emission characteristics of diesel engine. Renew. Energy 2018, 116, 518–526. [Google Scholar] [CrossRef]
- Jaichandar, S.; Thamaraikannan, M.; Yogaraj, D.; Samuelraj, D. A comprehensive study on the effects of internal air jet piston on the performance of a JOME fueled DI diesel engine. Energy 2019, 185, 1174–1182. [Google Scholar] [CrossRef]
- Kumar, S.; Dinesha, P.; Rosen, M.A. Effect of injection pressure on the combustion, performance and emission characteristics of a biodiesel engine with cerium oxide nanoparticle additive. Energy 2019, 185, 1163–1173. [Google Scholar] [CrossRef]
- Suhel, A.; Abdul Rahim, N.; Abdul Rahman, M.R.; Bin Ahmad, K.A.; Teoh, Y.H.; Zainal Abidin, N. An Experimental Investigation on the Effect of Ferrous Ferric Oxide Nano-Additive and Chicken Fat Methyl Ester on Performance and Emission Characteristics of Compression Ignition Engine. Symmetry 2021, 13, 265. [Google Scholar] [CrossRef]
- Gavhane, R.S.; Kate, A.M.; Soudagar, M.E.M.; Wakchaure, V.D.; Balgude, S.; Rizwanul Fattah, I.M.; Nik-Ghazali, N.-N.; Fayaz, H.; Khan, T.M.Y.; Mujtaba, M.A.; et al. Influence of Silica Nano-Additives on Performance and Emission Characteristics of Soybean Biodiesel Fuelled Diesel Engine. Energies 2021, 14, 1489. [Google Scholar] [CrossRef]
- Rameshbabu, A.; Senthilkumar, G. Emission and performance investigation on the effect of nano-additive on neat biodiesel. Energy Sources Part A Recovery Util. Environ. Eff. 2021, 43, 1315–1328. [Google Scholar] [CrossRef]
- Reddy, S.N.K.; Wani, M.M. An investigation on the performance and emission studies on diesel engine by addition of nanoparticles and antioxidants as additives in biodiesel blends. Int. Rev. Appl. Sci. Eng. 2021, 12, 111–118. [Google Scholar] [CrossRef]
- Oyedepo, S.O.; Fagbenle, R.O.; Adefila, S.S.; Alam, M.M. Thermoeconomic and thermoenvironomic modeling and analysis of selected gas turbine power plants in Nigeria. Energy Sci. Eng. 2015, 3, 423–442. [Google Scholar] [CrossRef]
- Rosen, M.A.; Dincer, I. Exergy as the confluence of energy, environment and sustainable development. Exergy Int. J. 2001, 1, 3–13. [Google Scholar] [CrossRef]
- Aghbashlo, M.; Tabatabaei, M.; Khalife, E.; Roodbar Shojaei, T.; Dadak, A. Exergoeconomic analysis of a DI diesel engine fueled with diesel/biodiesel (B5) emulsions containing aqueous nano cerium oxide. Energy 2018, 149, 967–978. [Google Scholar] [CrossRef]
- Oberdörster, G.; Oberdörster, E.; Oberdörster, J. Nanotoxicology: An Emerging Discipline Evolving from Studies of Ultrafine Particles. Environ. Health Perspect. 2005, 113, 823–839. [Google Scholar] [CrossRef] [PubMed]
- Brohi, R.D.; Wang, L.; Talpur, H.S.; Wu, D.; Khan, F.A.; Bhattarai, D.; Rehman, Z.-U.; Farmanullah, F.; Huo, L.-J. Toxicity of Nanoparticles on the Reproductive System in Animal Models: A Review. Front. Pharmacol. 2017, 8, 606. [Google Scholar] [CrossRef] [PubMed]
- Ray, P.C.; Yu, H.; Fu, P.P. Toxicity and environmental risks of nanomaterials: Challenges and future needs. J. Environ. Sci. Health. Part C Environ. Carcinog. Ecotoxicol. Rev. 2009, 27, 1–35. [Google Scholar] [CrossRef] [PubMed]
- Kreyling, W.G.; Semmler-Behnke, M.; Möller, W. Ultrafine particle-lung interactions: Does size matter? J. Aerosol Med. Off. J. Int. Soc. Aerosols Med. 2006, 19, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Anderson, P.J.; Wilson, J.D.; Hiller, F.C. Respiratory tract deposition of ultrafine particles in subjects with obstructive or restrictive lung disease. Chest 1990, 97, 1115–1120. [Google Scholar] [CrossRef]
- Balasubramanyam, A.; Sailaja, N.; Mahboob, M.; Rahman, M.F.; Hussain, S.M.; Grover, P. In vivo genotoxicity assessment of aluminium oxide nanomaterials in rat peripheral blood cells using the comet assay and micronucleus test. Mutagenesis 2009, 24, 245–251. [Google Scholar] [CrossRef]
- Kim, Y.; Choi, H.; Song, M.; Youk, D.; Kim, J.; Ryu, J. Genotoxicity of Aluminum Oxide Nanoparticle in Mammalian Cell Lines. Mol. Cell. Toxicol. 2009, 5, 172–178. [Google Scholar]
- Trouiller, B.; Reliene, R.; Westbrook, A.; Solaimani, P.; Schiestl, R.H. Titanium dioxide nanoparticles induce DNA damage and genetic instability in vivo in mice. Cancer Res. 2009, 69, 8784–8789. [Google Scholar] [CrossRef]
- Liu, R.; Yin, L.; Pu, Y.; Liang, G.; Zhang, J.; Su, Y.; Xiao, Z.; Ye, B. Pulmonary toxicity induced by three forms of titanium dioxide nanoparticles via intra-tracheal instillation in rats. Prog. Nat. Sci. 2009, 19, 573–579. [Google Scholar] [CrossRef]
- Bhattacharya, K.; Davoren, M.; Boertz, J.; Schins, R.P.; Hoffmann, E.; Dopp, E. Titanium dioxide nanoparticles induce oxidative stress and DNA-adduct formation but not DNA-breakage in human lung cells. Part. Fibre Toxicol. 2009, 6, 17. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, X.; Pu, Y.; Yin, L.; Li, Y.; Zhang, X.; Liang, G.; Li, X.; Zhang, J. Small-sized titanium dioxide nanoparticles mediate immune toxicity in rat pulmonary alveolar macrophages in vivo. J. Nanosci. Nanotechnol. 2010, 10, 5161–5169. [Google Scholar] [CrossRef]
- Liu, H.; Ma, L.; Zhao, J.; Liu, J.; Yan, J.; Ruan, J.; Hong, F. Biochemical toxicity of nano-anatase TiO2 particles in mice. Biol. Trace Elem. Res. 2009, 129, 170–180. [Google Scholar] [CrossRef]
- Liu, G.; Gao, J.; Ai, H.; Chen, X. Applications and potential toxicity of magnetic iron oxide nanoparticles. Small 2013, 9, 1533–1545. [Google Scholar] [CrossRef]
- Naqvi, S.; Samim, M.; Abdin, M.; Ahmed, F.J.; Maitra, A.; Prashant, C.; Dinda, A.K. Concentration-dependent toxicity of iron oxide nanoparticles mediated by increased oxidative stress. Int. J. Nanomed. 2010, 5, 983–989. [Google Scholar] [CrossRef]
- Chen, L.; Yokel, R.A.; Hennig, B.; Toborek, M. Manufactured Aluminum Oxide Nanoparticles Decrease Expression of Tight Junction Proteins in Brain Vasculature. J. Neuroimmune Pharmacol. 2008, 3, 286–295. [Google Scholar] [CrossRef]
- Muller, J.; Huaux, F.; Moreau, N.; Misson, P.; Heilier, J.-F.; Delos, M.; Arras, M.; Fonseca, A.; Nagy, J.B.; Lison, D. Respiratory toxicity of multi-wall carbon nanotubes. Toxicol. Appl. Pharmacol. 2005, 207, 221–231. [Google Scholar] [CrossRef]
- Cha, K.E.; Myung, H. Cytotoxic effects of nanoparticles assessed in vitro and in vivo. J. Microbiol. Biotechnol. 2007, 17, 1573–1578. [Google Scholar]
- Asharani, P.V.; Lian Wu, Y.; Gong, Z.; Valiyaveettil, S. Toxicity of silver nanoparticles in zebrafish models. Nanotechnology 2008, 19, 255102. [Google Scholar] [CrossRef]
- Najahi-Missaoui, W.; Arnold, R.D.; Cummings, B.S. Safe Nanoparticles: Are We There Yet? Int. J. Mol. Sci. 2021, 22, 385. [Google Scholar] [CrossRef]
- George, S.; Pokhrel, S.; Xia, T.; Gilbert, B.; Ji, Z.; Schowalter, M.; Rosenauer, A.; Damoiseaux, R.; Bradley, K.A.; Mädler, L.; et al. Use of a Rapid Cytotoxicity Screening Approach To Engineer a Safer Zinc Oxide Nanoparticle through Iron Doping. ACS Nano 2010, 4, 15–29. [Google Scholar] [CrossRef]
- Khare, V.; Saxena, A.K.; Gupta, P.N. Chapter 15-Toxicology Considerations in Nanomedicine; William Andrew Publishing, Oxford, Institution: Oxford, UK, 2015; pp. 239–261. [Google Scholar]
- Cai, X.; Lee, A.; Ji, Z.; Huang, C.; Chang, C.H.; Wang, X.; Liao, Y.-P.; Xia, T.; Li, R. Reduction of pulmonary toxicity of metal oxide nanoparticles by phosphonate-based surface passivation. Part. Fibre Toxicol. 2017, 14, 13. [Google Scholar] [CrossRef]
- Sukhanova, A.; Bozrova, S.; Sokolov, P.; Berestovoy, M.; Karaulov, A.; Nabiev, I. Dependence of Nanoparticle Toxicity on Their Physical and Chemical Properties. Nanoscale Res. Lett. 2018, 13, 44. [Google Scholar] [CrossRef]

| Alcohol | Case # | Fuel | Nanoparticle (DOSAGE) | Density (kgm−3) | Viscosity (mm2/s) | Flash Point (°C) | Calorific Value (MJ/kg) | Cetane Number |
|---|---|---|---|---|---|---|---|---|
| Ethanol [73] | 1a | D40B30E30 | Absent | 828.5 | 2.42 | 10 | 39.90 | 57 |
| 1b | D40B30E30 | ZnO (250 ppm) | 836.3 | 2.32 | 16 | 36.89 | 55 | |
| Methanol [74] | 2a | M100 | Absent | 790 | 0.59 | - | 20.3 | - |
| 2b | MSN25 | SiO2 (25 ppm) | 793 | 0.62 | - | 21.9 | - | |
| 2c | MSN50 | SiO2 (50 ppm) | 798 | 0.65 | - | 22.4 | - | |
| 2d | MSN100 | SiO2 (100 ppm) | 804 | 0.71 | - | 23.2 | - | |
| Pentanol [76] | 3a | TF | Absent | 841 | 3.3 | 3 | 41.62 | 48 |
| 3b | TF40 | SiO2 (40 ppm) | 839 | 3.37 | 2.8 | 41.73 | 48.5 | |
| 3c | TF80 | SiO2 (80 ppm) | 837 | 3.21 | 4 | 41.96 | 55 | |
| 3d | TF120 | SiO2 (120 ppm) | 830 | 3.01 | 3 | 42.97 | 47.4 | |
| N-amyl [69] | 4a | TF | Absent | 841 | 3.3 | 3 | 41.62 | 48 |
| 4b | TF40 | Fe2O3 (40 ppm) | 839 | 3.37 | 2.8 | 41.73 | 48.5 | |
| 4c | TF80 | Fe2O3 (80 ppm) | 837 | 3.21 | 4 | 41.96 | 55 | |
| 4d | TF120 | Fe2O3 (120 ppm) | 830 | 3.01 | 3 | 42.97 | 47.4 | |
| Ethanol [70] | 5a | DF90E10 | Absent | 821.5 | 2.7 | - | 41.7 | 52.44 |
| 5b | DF90E10 | Al2O3 (100 ppm) | 821.6 | 2.8 | - | 42.5 | 53.68 | |
| 5c | DF90E10 | TiO2 (100 ppm) | 821.6 | 2.8 | - | 42.3 | 53.24 | |
| Ethanol [71] | 6a | TF | Absent | 852 | 3.18 | 59 | 43.18 | 48.4 |
| 6b | TF10 | Al2O3 (10 ppm) | 849 | 3.07 | 60 | 43.41 | 48.6 | |
| 6c | TF20 | Al2O3 (20 ppm) | 848 | 3.02 | 63 | 43.85 | 48.7 | |
| 6d | TF30 | Al2O3 (30 ppm) | 845 | 3.1 | 62 | 43.58 | 48.4 | |
| Ethanol [80] | 7a | BDE | Absent | 840.2 | 2.86 | 20 | 39.98 | 53 |
| 7b | BDE | Al2O3 (25 ppm) | 837.2 | 2.57 | 22 | 39.14 | 54 | |
| Methanol [75] | 8a | M100 | Absent | 790 | 0.59 | - | 20.3 | - |
| 8b | MCN25 | CeO2 (25 ppm) | 800 | 0.62 | - | 20.8 | - | |
| 8c | MCN100 | CeO2 (100 ppm) | 810 | 0.66 | - | 22.1 | - | |
| Isopropanol, Butanol [79] | 9a | B20 | Absent | 847 * | 3.70 | - | 43 | 42 |
| 9b | D80SBD15E4S1 | Al2O3 (100 mg/L) | 840 * | 3.37 | - | 42.59 | 52 | |
| Butanol [72] | 10a | J50D10Bu | Absent | 848 * | 4.49 | - | 44.99 | 52.5 |
| 10b | J50D10Bu25TiO2 | TiO2 (25 mg/L) | 849 * | 4.51 | - | 45.11 | 53.5 | |
| 10c | J50D10Bu50TiO2 | TiO2 (50 mg/L) | 849 * | 4.55 | - | 45.14 | 54.5 | |
| Butanol [81] | 11a | B20But10 | Absent | 840.1 | 2.62 | 46.75 | 39.96 | - |
| 11b | B20But10 | TiO2 (0.01% by mass) | 840.2 | 2.63 | 45 | 39.84 | - | |
| Ethanol [65] | 12a | B2 | Absent | 820.7 | 2.31 | - | 42.66 | - |
| 12b | B2E2C20 | Carbon nanotubes (20 ppm) | 821.8 | 2.39 | - | 42.23 | - | |
| 12c | B2E2C60 | Carbon nanotubes (60 ppm) | 821.8 | 2.38 | - | 42.27 | - | |
| 12d | B2E2C100 | Carbon nanotubes (100 ppm) | 821.9 | 2.39 | - | 42.23 | - | |
| 12e | B2E4C20 | Carbon nanotubes (20 ppm) | 820.7 | 2.31 | - | 42.66 | - | |
| 12f | B2E4C60 | Carbon nanotubes (60 ppm) | 820.8 | 2.31 | - | 42.68 | - | |
| 12g | B2E4C100 | Carbon nanotubes (100 ppm) | 820.9 | 2.31 | - | 42.62 | - | |
| 12h | B2E6C20 | Carbon nanotubes (20 ppm) | 819.6 | 2.24 | - | 43.11 | - | |
| 12i | B2E6C60 | Carbon nanotubes (40 ppm) | 819.7 | 2.24 | - | 43.13 | - | |
| 12j | B2E6C100 | Carbon nanotubes (100 ppm) | 819.9 | 2.25 | - | 43.03 | - | |
| Ethanol [78] | 13a | B10 | Absent | 835 | 3.33 | 70 | - | - |
| 13b | B10E2GQD30 | Graphene quantum dot (30 ppm) | 834 | 3.11 | <28 | - | - | |
| 13c | B10E4GQD30 | Graphene quantum dot (30 ppm) | 834 | 2.99 | <28 | - | - | |
| 13d | B10E6GQD30 | Graphene quantum dot (30 ppm) | 834 | 2.94 | <28 | - | - | |
| 13e | B10E8GQD30 | Graphene quantum dot (30 ppm) | 834 | 2.83 | <28 | - | - | |
| Heptanol [68] | 14a | H20D | Absent | 839.5 * | 3.34 | - | 34.65 | 48.5 |
| 14b | H40D | Absent | 838.1 * | 3.33 | - | 43.11 | 45.5 | |
| 14c | H20DMWCNT | Multi-walled carbon nanotubes (50 mg/L) | 842.2 * | 3.16 | - | 44.79 | 51.5 | |
| 14d | H20DGNP | Graphene nanoplatelets (50 mg/L) | 842.1 * | 3.11 | - | 44.79 | 50.5 | |
| 14e | H20DGO | Graphene oxide (50 mg/L) | 842.3 * | 3.12 | - | 44.80 | 51 | |
| 14f | H40DMWCNT | Multi-walled carbon nanotubes (50 mg/L) | 841 * | 3.16 | - | 43.60 | 49.5 | |
| 14g | H40DGNP | Graphene nanoplatelets (50 mg/L) | 840.5 * | 3.13 | - | 43.59 | 50 | |
| 14h | H40DGO | Graphene oxide (50 mg/L) | 840.7 * | 3.13 | - | 43.60 | 50.5 | |
| Butanol [77] | 15a | JME40B | Absent | 849.9 * | 3.73 | - | 37.53 | 43.53 |
| 15b | JME40B50GO | Graphene oxide (50 mg/L) | 851.0 * | 3.65 | - | 37.55 | 48.10 | |
| 15c | JME40BGNPs | Graphene nanoplatelets (50 mg/L) | 851.1 * | 3.68 | - | 37.56 | 47.95 | |
| 15d | JME40BMWCNTs | Multi-walled nanocarbon nanotubes (50 mg/L) | 851.1 * | 3.69 | - | 37.56 | 47.98 |
| Vegetable Oil | Case # | Fuel | Nanoparticle (Dosage) | Density (kgm−3) | Viscosity (mm2/s) | Flash Point (°C) | Calorific Value (MJ/kg) | Cetane Number |
|---|---|---|---|---|---|---|---|---|
| Polanga seed oil [91] | 1a | Neat polanga | Absent | 937.4 * | 57.8 | - | - | - |
| 1b | Diesel + polanga | Fe2O3 (100 ppm) | 835.3 * | 3.49 | - | 44.08 | - | |
| 1c | Diesel + polanga | Fe2O3 (200 ppm) | 837.3 * | 3.62 | - | 44.03 | - | |
| 1d | Diesel + polanga | Fe2O3 (300 ppm) | 837.5 * | 3.39 | - | 44.00 | - | |
| Tyre oil ** [84] | 2a | B10 | Absent | 820 | 6.59 | 49 | 42.90 | - |
| 2b | B10D85 | CeO2 (50 ppm) | 822 | 6.65 | 50 | 42.94 | - | |
| 2c | B10D80 | CeO2 (100 ppm) | 824 | 6.72 | 51 | 42.98 | - | |
| Lemongrass oil [85] | 3a | LGO25 | Absent | 870 * | 3.48 | 53 | 41.69 | - |
| 3b | LGO25 + WE + CE | CeO2 (50 ppm) | 910 * | 4.16 | 58 | 41.06 | - | |
| Pyrolyzed biomass oil ** [88] | 4a | PBO20 | Absent | 845 | 4.24 | 96 | 41.1 | - |
| 4b | PB020 | Al2O3 (50 ppm) | 839 | 4.08 | 94 | 41.2 | - | |
| 4c | PBO40 | Absent | 862 | 4.86 | 108 | 39.5 | - | |
| 4d | PBO40 | Al2O3 (100 ppm) | 852 | 4.72 | 104 | 41.3 | - | |
| Lemon peel oil [86] | 5a | LPO20 | CeO2 (50 ppm) | 856 | 2.43 | 44 | 41.20 | - |
| 5b | LPO20 | CeO2 (100 ppm) | 856 | 2.56 | 40 | 42.44 | - | |
| 5c | LPO20 | CNT (50 ppm) | 856 | 2.38 | 42 | 42.11 | - | |
| 5d | LPO20 | CNT (100 ppm) | 856 | 2.64 | 44 | 41.88 | - | |
| Orange peel oil [86] | 6a | OPO20 | CeO2 (50 ppm) | 858 | 2.54 | 46 | 42.48 | - |
| 6b | OPO20 | CeO2 (100 ppm) | 858 | 2.80 | 42 | 42.32 | - | |
| 6c | OPO20 | CNT (50 ppm) | 858 | 2.72 | 44 | 42.41 | - | |
| 6d | OPO20 | CNT (100 ppm) | 858 | 3.01 | 43 | 42.17 | - | |
| Nerium olender [87] | 7a | ENOB | Absent | 906 | 4.67 | 74 | 35.8 | - |
| 7b | NENOB | CeO2 (30 ppm) | 916.4 | 4.99 | 67 | 36.2 | - | |
| Lemongrass oil [82] | 8a | Neat LGO | Absent | 905 | 4.60 | 55 | 37 | 48 |
| 8b | LGO emulsion | Absent | 906 | 4.67 | 74 | 35.8 | 46.3 | |
| 8c | LGO nano emulsion | CeO2 (30 ppm) | 916.4 | 4.99 | 67 | 36.2 | 48.8 | |
| Hydrotreated vegetable oil [89] | 9a | B7 + 10%HVO | Absent | 828.5 | 2.73 | 59 | - | 55.2 |
| 9b | B7 + 10%HVO | CeO2 (1:4000) | 828.3 | 2.73 | 60 | - | 53.1 | |
| 9c | B7 + 10%HVO | Nano ferrocen (1:1000) | 828.1 | 2.72 | 59 | - | 57.7 | |
| Tyre pyrolysis oil ** [90] | 10a | JME90TPO10 | Absent | 868.7 | 6.39 | - | 9962.7 *** | - |
| 10b | JME90TPO10 | CeO2 (100 ppm) | 868.3 | 6.39 | - | 9537.5 *** | - | |
| 10c | JME90TPO10 | CNT (100 ppm) | 872.6 | 5.25 | - | 9311.5 *** | - | |
| 10d | JME80TPO20 | Absent | 874.1 | 6.36 | - | 10,001.43 *** | - | |
| 10e | JME80TPO20 | CeO2 (100 ppm) | 873.5 | 6.40 | - | 9630.2 *** | - | |
| 10f | JME80TPO20 | CNT (100 ppm) | 878.1 | 5.35 | - | 9482.6 *** | - | |
| 10g | JME70TPO30 | Absent | 880.4 | 6.48 | - | 10062 *** | - | |
| 10h | JME70TPO30 | CeO2 (100 ppm) | 880.3 | 6.39 | - | 9726.8 *** | - | |
| 10i | JME70TPO30 | CNT (100 ppm) | 881.8 | 5.29 | - | 9656.5 *** | - | |
| Cymbopogon flexuosus biofuel [83] | 11a | C20D80 | Absent | 843 | 3.21 | 49 | 42.19 | - |
| 11b | C20D80 | CeO2 (10 ppm) | 844.1 | 3.28 | 47 | 42.14 | - | |
| 11c | C20D80 | CeO2 (20 ppm) | 844.5 | 3.31 | 46 | 41.88 | - | |
| 11d | C20D80 | CeO2 (30 ppm) | 844.9 | 3.37 | 45 | 41.62 | - |
| Biodiesel | Case # | Fuel | Nanoparticle (Dosage) | Density (kgm−3) | Viscosity (mm2/s) | Flash Point (°C) | Calorific Value (MJ/kg) | Cetane Number |
|---|---|---|---|---|---|---|---|---|
| Jatropha [92] | 1a | JB20D | Absent | 847.1 * | 4.06 | - | 45.43 | 52 |
| 1b | JB20D | MWCNT (10 mg/L) | 847.1 * | 4.1 | - | 45.43 | 52.7 | |
| 1c | JB20D | MWCNT (20 mg/L) | 847.1 * | 4.19 | - | 45.45 | 53.5 | |
| 1d | JB20D | MWCNT (30 mg/L) | 847.1 * | 4.25 | - | 45.45 | 54.2 | |
| 1e | JB20D | MWCNT (40 mg/L) | 847.1 * | 4.31 | - | 45.46 | 55.4 | |
| 1f | JB20D | MWCNT (50 mg/L) | 847.1 * | 4.35 | - | 45.46 | 56 | |
| Canola biodiesel [101] | 2a | Canola biodiesel | Absent | 886.5 | 5.38 | 172 | 38.76 | 48 |
| 2b | Canola emulsion | CeO2 (50 ppm) | 906.8 | 17.2 | 185 | 33.54 | 38 | |
| Jatropha [80] | 3a | BDE | Absent | 840.2 | 2.86 | 20 | 39.98 | 53 |
| 3b | BDE | Al2O3 (25 ppm) | 837.2 | 2.57 | 22 | 39.14 | 54 | |
| Jojoba [94] | 4a | JB20 | Absent | 845.36 | 3.59 | 71 | 41.93 | - |
| 4b | JB20CN25 | CuO (25 ppm) | 858.15 | 3.68 | 66 | 41.22 | - | |
| 4c | JB20CN50 | CuO (50 ppm) | 864.56 | 3.76 | 64 | 41.43 | - | |
| 4d | JB20CN75 | CuO (75 ppm) | 871.17 | 3.87 | 63 | 41.66 | - | |
| Rice bran [99] | 5a | B20 | Absent | 828 | 6.62 | 39 | 38.96 | - |
| 5b | B20 | CeO2 (50 ppm) | 830 | 6.16 | 35 | 39.44 | - | |
| 5c | B20 | CeO2 (100 ppm) | 826 | 5.96 | 45 | 39.25 | - | |
| Madhuca Indica [95] | 6a | B100 | Absent | 889 | 5.21 | 173 | 40.30 | - |
| 6b | B10A0.2 | Al2O3 (0.2 gm) | 848 | 4.38 | 65 | 41.78 | - | |
| 6c | B10A0.4 | Al2O3 (0.4 gm) | 853 | 4.35 | 63 | 41.82 | - | |
| 6d | B20A0.2 | Al2O3 (0.2 gm) | 858 | 4.49 | 59 | 41.91 | - | |
| 6e | B20A0.4 | Al2O3 (0.4 ppm) | 862 | 4.42 | 56 | 41.92 | - | |
| Palm oil [93] | 7a | B100 | Absent | 860 | 4.61 | - | 38.6 | 62.5 |
| 7b | B30C100 | MWCNT (100 ppm) | 852 | 5.12 | - | 40.3 | 52.2 | |
| Waste cooking oil [98] | 8a | B5W3 | Absent | - | 3.6 | 78 | 44.35 | - |
| 8b | B5W5 | Absent | - | 3.57 | 76 | 42.84 | - | |
| 8c | B5W7 | Absent | - | 3.92 | 74 | 42.49 | - | |
| 8d | B5W3m | CeO2 (90 ppm) | - | 3.82 | 80 | 43.48 | - | |
| 8e | B5W5m | CeO2 (90 ppm) | - | 3.82 | 78 | 42.73 | - | |
| 8f | B5W7m | CeO2 (90 ppm) | - | 3.88 | 77 | 42.38 | - | |
| Neem oil [102] | 9a | NBD | Absent | 830 | 4.1 | - | 38.96 | 53 |
| 9b | NBDCNT 50 | Carbon nanotubes (50 ppm) | 820 | 3.8 | - | 39.15 | 54 | |
| 9c | NBDCNT100 | Carbon nanotubes (100 ppm) | 810 | 3.5 | - | 39.56 | 55 | |
| Canola oil [96] | 10a | B20 | Absent | 915 | 4.8 | - | - | 42 |
| 10b | B20 | TiO2 (300 ppm) | 840 | 3.4 | - | - | 56 | |
| Kapok oil [103] | 11a | B100 | Absent | 931 | 4.2 | 170 | 38 | 48 |
| 11b | B20 | Cobalt chromite (50 ppm) | 845 | 3.8 | 145 | 39 | 49 | |
| Used cooking oil [104] | 12a | B20 | Absent | 843.2 | 3.19 | 76 | 43.33 | 52.5 |
| 12b | B20 | MWCNT (25 ppm) | 843.9 | 3.15 | 74 | 43.37 | 52.9 | |
| 12c | B20 | MWCNT (50 ppm) | 845.2 | 3.09 | 71 | 43.4 | 53.4 | |
| 12d | B20 | MWCNT (75 ppm) | 846.9 | 2.97 | 69 | 43.45 | 54.1 | |
| 12e | B20 | MWCNT (100 ppm) | 848.1 | 2.95 | 67 | 43.62 | 55.3 | |
| Garcinia gummi-gutta [97] | 13a | B20 | Absent | 863 | 4.51 | 90.7 | 40.81 | 50.7 |
| 13b | B20 | TiO2 (25 ppm) | 864 | 4.39 | 96.8 | 41.06 | 51.62 | |
| 13c | B20 | CeO2 (25 ppm) | 863 | 4.54 | 90.2 | 40.68 | 50.85 | |
| 13d | B20 | ZrO2 (25 ppm) | 866 | 4.51 | 93.1 | 41.31 | 50.91 | |
| Karanja oil/waste cooking oil [105] | 14a | KBD20 | Graphene oxide (60 ppm) | 839 | 3.66 | 80 | 41.82 | - |
| 14b | KBD20 | Graphene nanoplatelets (60 ppm) | 837 | 3.65 | 81 | 41.8 | - | |
| 14c | WBD20 | Graphene oxide (60 ppm) | 838 | 3.57 | 79 | 41.7 | - | |
| 14d | WBD20 | Graphene nanoplatelets (60 ppm) | 837 | 3.56 | 81 | 41.7 | - | |
| 14e | KBD20 | Absent | 836 | 3.65 | 81 | 41.8 | - | |
| 14f | WBD20 | Absent | 836.6 | 3.55 | 80 | 41.7 | - | |
| Orange peel oil [106] | 15a | OOME | Absent | 850.7 | 4.83 | 94 | 38.1 | 47 |
| 15b | OOMET50 | TiO2 (50 ppm) | 856.5 | 5.17 | 96 | 35.98 | 50 | |
| 15c | OOMET100 | TiO2 (100 ppm) | 861.3 | 5.42 | 99 | 36.1 | 53 | |
| Waste frying oil [107] | 16a | WFOME | Absent | 898 | 4.21 | 160 | 43.85 | - |
| 16b | WFOME | MWCNT (25 ppm) | 830 | 4.75 | 57 | 43.73 | - | |
| 16c | WFOME | MWCNT (50 ppm) | 831.1 | 4.45 | 65 | 43.93 | - | |
| Camelina oil [108] | 17a | B20 | Absent | 836 | 5.67 | - | 44.09 | - |
| 17b | B20G60 | Graphene oxide (60 ppm) | 832 | 5.53 | - | 44.49 | - | |
| Honge oil [109] | 18a | HOME | Absent | - | 5.6 | 170 | 36.02 | - |
| 18b | HOME25CNT | MWCNT (25 ppm) | - | 5.7 | 166 | 34.56 | - | |
| 18c | HOME50CNT | - | 5.8 | 164 | 35.1 | - | ||
| Sardine oil [110] | 19a | SOME | Absent | 890 | 4.5 | 58 | 37.41 | 45 |
| 19b | SOME | CeO2 (25 ppm) | 894 | 5.6 | 191 | 43.37 | 56 | |
| Calophyllum inophyllum [111] | 20a | CIB20 | Absent | 843.3 | 3.56 | 69 | 40.92 | 53.85 |
| 20b | CIB20ANP40 | Al2O3 (40 ppm) | 858 | 3.64 | 64 | 41.44 | 54.58 |
| Type of NPs Used | Alcohol Based Fuel | Blends | Size of NPs/NPs Concentration | Engine Sp. | Combustion | Performance | Gaseous Emission | Observation | Reference | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HRR | ICP | BFSC | BTE | CO | HC | NOx | |||||||
| Al2O3 | Ethanol | DF90E10 + A100, DF90E10 and DF | 48 nm/100 ppm | Lombardini 15 LD 350, CI, CR20.3:1, RP7.5HP, RS3600 rpm, IP 207 bar. | --- | ↓ with DF90E10 + A100compared to DF | ↓ by 2.25% with DF90E10 + A100 compared to DF | ↑ by 3.48% with DF90E10 + A100 compared to DF | ↓ by 25% with DF90E10 + A100 compared to DF | ↓ by 30.15% with DF90E10 + A100 compared to DF | ↓ by 3.02% with DF90E10 + A100 compared to DF |
| [70] |
| Ethanol | TF (TF + 10, TF + 20 and TF + 30) and DF | 28 nm-30 nm/10 ppm, 20 ppm and 30 ppm | Kirloskar TAF 1, 1C, 4S, CI, CR17.5:1, RP4.4 kW, RS1500 rpm, IT 23° bTDC, IP 200 bar. | ↑ highly with DF100 compared to other fuels | ↓ by 2.33% with TF + 20 compared to DF | BSEC ↓ by 4.93% with TF + 20 compared to TF and DF | ↑ by 7.8% with TF + 20 compared to TF and DF | ↓ by 11.25% with TF + 20 compared to TF and DF | ↓ by 5.63% with TF + 20 compared to TF and DF | ↓ by 9.39% with TF + 20 compared to TF |
| [71] | |
| Methanol | MDFs (M10, M30, and M50); MD-NFs (M10A25, M10A50, M10A100, M30A25, M30A50, M30A100, M50A25, M50A50, and M50A100) and DF | 30 nm/25 ppm, 50 ppm and 100 ppm | Kirloskar, 1C, 4S, CI, CR17.5:1, RP11.32 kW, RS2200 rpm, IT 20° bTDC, IP 18 MPa. | ↓ with MDFs-NFs compared to DF and MDF at high load. | ↑ by 30–50% with MD-NFs compared to MDFs and DF | --- | --- | ↓ with MD-NFs highly compared to MDFs and DF | ↓ with MD-NFs highly compared to MDFs and DF | ↑ with MD-NFs significantly compared to MDFs and DF |
| [123] | |
| Methanol | MDFs (M5 and M15); MD-NFs (M5A50, M5A100, M15A50 and M15A100) and DF | 30 nm/50 ppm and 100 ppm | Kirloskar, 1C, 4S, WC, CI, CR17.5:1, RP11.32 kW, RS2200 rpm, IT 20° bTDC, IP 18 MPa. | Improved by 16.1% with ↓ of 6.9% in ID via 100 ppm | Improved by 2.5% with ↓ of 16% in CD via 100 ppm | ↓ by ~3.7% with M5A100 and M15A100 compared to MDFs | ↑ by ~3.6% with M5A100 and M15A100 compared to MDFs | ↓ by 83.3% with MD-NFs compared to MDFs | ↓ by ~40.9% with MD-NFs compared to MDFs | ↑ slightly by 14.4% with MD-NFs compared to MDFs |
| [121] | |
| Ethanol | D45EB10; D45EB10 + Al2O3 (D45EB10A50, D45EB10A75 and D45EB10A100) | 30 nm/50 ppm, 75 ppm and 100 ppm | Kirloskar TVI, 1C, 4S, WC, CI, CR17.5:1, RP7 kW, RS1500 rpm, IT 23° bTDC, IP 220 kgf/cm2. | ↑ highly with D45EB10A100 compared to D45EB10 | ↑ highly with D45EB10A100 | --- | --- | ↓ slightly by 0.02% with D45EB10A100 at 100% load. | --- | ↑ with an ↑ in Al2O3 rate and load. |
| [124] | |
| Methanol and Ethanol | E.M.BioD.Al (5%Eth, 3%Meth, 86%BioD and 50 ppm) | 20 nm/50 ppm | KIPOR KM186FA, 1C, 4S, AC, CI, CR19:1, RP5.7 kW, RS3000 rpms | Significantly improved | ↑ highly with E.M.BioD.Al compared to other fuels | ↓ with E.M.BioD.Al compared to BioD. | ↑ by 6% with E.M.BioD.Al compared to BioD. | ↓ by 12% with E.M.BioD.Al compared to BioD. | ↓ slightly with E.M.BioD.Al compared to BioD. | ↓ by 12.3% with E.M.BioD.Al compared to BioD. |
| [115] | |
| Ethanol | JE20D; JE20D + Al2O3 (JE20D25A, JE20D50A, JE20D75A, JE20D100A) and DF | 20 nm-50 nm/25 ppm, 50 ppm, 75 ppm and 100 ppm | HATZ-1B30-2, 1C, AC, CI, CR21.5:1, RP5.4 kW, RS3600 rpm, IT 20° bTDC, IP 18 MPa. | ↑ highly with JE20D + Al2O3 compared to JE20D and DF | ↑ highly with JE20D + Al2O3 compared to JE20D. | ↓ by 17–25% with JE20D + Al2O3 compared to DF | ↑ highly with JE20D + Al2O3 compared to JE20D | ↓ by 20% with JE20D + Al2O3 | ↓ by 60% with JE20D + Al2O3 | ↓ by 30–50% with JE20D + Al2O3 |
| [119] | |
| CeO2 | Methanol | M10 and M30; MCN (M10C25, M10C100, M30C25 and M30C100) and DF | 25 ppm and 100 ppm | Kirloskar, 1C, 4S, WC, CI, CR17.5:1, RP11.32 kW, RS2200 rpm, IT 20° bTDC, IP 18 MPa. | ↑ by 7.9% slightly with MCN | ↑ with MCN | ↓ by 5.7–8.1% with MCN compared to M10 and M30 | Improved by adding CeO2 to M10 and M30 with 5.2–108% | ↓ by 79.8% with MCN compared to DF, M10 and M30. | ↓ by 56.3% with MCN compared to DF, M10 and M30. | ↓ by 70–90% with MCN compared to DF, M10 and M30. |
| [75] |
| CNTs | Ethanol | D100, B2C20, B2C60, B2C100, B2E2C20, B2E2C60, B2E2C100, B2E4C20, B2E4C60, B2E4C100, B2E6C20, B2E6C60, and B2E6C100 | 4 nm–8 nm/20 ppm, 60 ppm and 100 ppm | DICOM 50.1 15/5, 1C, 4S, AC, DI, CI, RP9kW, RS3000 rpm. | --- | --- | ↓ by 11.73% with an ↑ in CNTs NPs. | ↑ by 13.97% with an ↑ in CNTs NPs. | ↓ by ~5.47% with CNTs NPs | ↓ by 31.72% with CNTs NPs | ↑ by 12.22% with CNTs NPs. |
| [65] |
| Fe2O3 | Pentanol | TF (P10B20D70); TF40, TF80 and TF120 | 40 ppm, 80 ppm and 120 ppm | Kirloskar TVI, 1C, 4S, CRDI, CI, CR18.0:1, RP3.7 kW, RS3000 rpm, IP 250–500 kgf/cm2. | ↑ with an ↑ in Fe2O3 NPs | Significantly improve with the presence of Fe2O3 NPs | ↓ significantly by 4.93% with TF80 and TF120 compared to TF | ↑ by 7.8% with TF120 compared to TF. | ↓ significantly by 5.69% with TF120 compared to other fuels | ↓ significantly by 11.24% with TF120 compared to other fuels | ↓ significantly by 9.39% with TF120 compared to other fuels |
| [69] |
| GQD | Ethanol | BEGQD (B10E2GQD30, B10E4GQD30, B10E6GQD30 and B10E8GQD30) and DF | 30 ppm | DICOM, 1C, 4S, AC, CI. | ↓ by ~14.35% with BEGQD compared to DF. | --- | --- | --- | ↓ by ~29.54% with BEGQD compared to DF. | ↓ by ~31.12% with BEGQD compared to DF. | ↓ with DF compared to BEGQD. |
| [78] |
| SiO2 | Methanol | MSN (M10); M10Si (M10Si25, M10Si50 and M10Si100) and DF | 20 nm–30 nm/25 ppm, 50 ppm and 100 ppm | Kirloskar, 1C, 4S, CI, CR17.5:1, RP11.32 kW, RS2200 rpm, IT 20° bTDC, IP 18 MPa. | ↑ by ~8.6% max with M10Si100. | --- | ↓ by 6.2% with an ↑ in SiO2 NPs. | ↑ by 5.1% with an ↑ in SiO2 NPs. | ↓ by 55.4% with SiO2 NPs. | ↓ by 38.5% with SiO2 NPs. | ↓ by 5.2% with SiO2 NPs. |
| [74] |
| Nano-biochar | Ethanol | DB2E2, DB4E4, DB6E6, DB8E8; DBE (with 25–125 ppm) and DF | 25 ppm–125 ppm | CT-159, 1C, 4S, CI, CR 21:1 | ↓ by ~3% with DBE compared to DF. | --- | --- | --- | ↓ by ~0.03–0.015% with an ↑ in nano-biochar DBE. | ↓ by ~28% with 125 ppm fuels compared to other fuels. | ↓ by ~15% with 100 ppm compared to other fuels. |
| [113] |
| TiO2 | Ethanol | DF90E10 + T100, DF90E10 and DF | 48 nm/100 ppm | Lombardini 15 LD 350, CI, CR20.3:1, RP7.5HP, RS3600 rpm, IP 207 bar. | --- | ↓ with DF90E10 + T100 compared to DF | ↓ by 1.26% with DF90E10 + T100 compared to DF | ↑ by 2.94% with DF90E10 + T100 compared to DF | ↓ by 21.43% with DF90E10 + T100 compared to DF | ↓ by 26.47% with DF90E10 + T100 compared to DF | ↓ by 1.57% with DF90E10 + T100 compared to DF |
| [70] |
| Butanol | J50Bu10; JBu + TiO2 (J50Bu10T25 and J50Bu10T50) and DF | 25 ppm and 50 ppm | HATZ-1B30-2, 1C, 4S, WC, VVA, CI, CR8.39:1, RS1000 rpm, IT 6° bTDC, IP 150 bar. | ↑ with JBu + TiO2 | ↑ with JBu + TiO2 | ↑ by 15% highly with JBu + TiO2 | ↑ by 17% highly with JBu + TiO2 | ↓ by 30% significantly with JBu + TiO2 | ↓ by 50% significantly with JBu + TiO2 | ↑ with an ↑ in TiO2 NPs. |
| [72] | |
| Butanol | B20 and B100; B20Bu20; B + TiO2 (B20 + TiO2 and B20Bu10 + TiO2) and DF | 0.1689 g | 3 LD 510, 1C, 4S, WC, CI, CR17.5:1, RP9 kW, RS3300 rpm, IP 190 bar. | --- | ↑ with B + TiO2 | ↓ by 27.73–28.37% with B + TiO2 compared to all other fuels. | ↑ by 0.34–0.66% with B + TiO2 compared to other fuels. | ↓ by 14–38% with B + TiO2 compared to all other fuels except B100. | ↓ by 22.38–34.39% with B + TiO2 compared to all other fuels except B100. | ↑ by 1.20–3.94% with B + TiO2 compared to other fuels. |
| [81] | |
| Butanol | B5 and B10; BTiO2 (B5T25, B5T50, B10T25 and B10T50) and DF | 25 ppm and 50 ppm | Kirloskar, 1C, 4S, WC, CI, CR18:1, RP3.5 kW, RS1500 rpm, IT 25° bTDC. | --- | ↑ slightly with an ↑ in engine load. | ↓ by 2.87–6.47% with all BTiO2 except B5T25 which ↑ by 7.91% compared toDF. | --- | ↓ by 22.34–36.17% with BTiO2 compared to DF. | --- | ↑ by 0.89–0.7.78% with B5T25, B10T25 and B10T50 while B5T50 ↓ by 2.69%. |
| [122] | |
| ZnO | Ethanol | D40B30E30; D40B30E30Z250; TFu (D40B30E30C6 and D40B30E30Z250C6) and DF | 30 nm/250 ppm | Kirloskar TAF 1, 1C, 4S, CI, CR17.5:1, RP4.41 kW, RS1500 rpm, IT 23° bTDC, IP 200 bar. | ↑ significantly with TFu compared to DF. | ↑ by 8% and 13% with TFu compared to DF. | ↑ by 14–39% with TFu compared to DF. | ↓ by 9–21% with TFu compared to DF. | ↓ by 62–92% with TFu compared to DF. | ↑ by 21% with D40B30E30C6 and ↓ by 9% with D40B30E30Z250C6. | ↓ by 16–35% with TFu. |
| [73] |
| Type of NPs Used | Vegetable Based Fuel | Blends | Size of NPs/NPs Concentration | Engine Sp. | Combustion | Performance | Gaseous Emission | Observation | Reference | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HRR | ICP | BFSC | BTE | CO | HC | NOx | |||||||
| Al2O3 | Mahua oil | MO; EMOA (EMOA25, EMOA50, EMOA75 and EMOA100) and DF | 25 ppm, 50 ppm, 75 ppm and 100 ppm | Kirloskar AVI, 1C, 4S, WC, CI, CR16.5:1, RP3.7 kW, RS1500 rpm, IT 23° bTDC, IP 220 bars. | ↑ with Al2O3 NPs. | ↑ with Al2O3 NPs. | --- | ↑ by ~29.2% with EMOA100 compared to other fuels. | ↓ by 87.4% with EMOA100 and ↓ by 24.2% with MO than DF. | ↓ by 37.3% with EMOA100 compared to DF and MO. | ↓ by 10% with EMOA than MO and ↓ by 51% with MO than DF. |
| [125] |
| Waste plastic oil | WPO20; WPO20A10-I, WPO20A20-I, WPO20A10-II and WPO20A20-II | 20 nm and 100 nm/10 ppm and 20 ppm | Kirloskar 240PE, 1C, 4S, DI, CR17.5:1, RP3.7 kW, RS1500 rpm, IT 23° bTDC, IP 200 bar. | ↑ with WPO20A10-I and WPO20A20-I. | ↑ with WPO20A10-I and WPO20A20-I. | ↓ by 8–11% with WPO NP fuels. | ↓ by 8–12.2% with WPO NP fuels. | ↓ with NP fuels. | ↓ with NP fuels. | ↑ with an ↑ in load. |
| [133] | |
| Lemon grass oil | B20; BA (B20A10, B20A20 and B20A30) | 20 nm–30 nm/10 ppm, 20 ppm and 30 ppm | Kirloskar TV1, 1C, 4S, DI, CI, CR17.5:1, RP5.2 kW, RS1500 rpm, IT 23° bTDC, IP 220 bar | ↑ by 20.4% with B20A20 compared to B20. | ↑ by 4.75% with B20A20 compared to B20. | BSEC ↓ with an ↑ in loads and NPs rate. | ↑ by 2.71% with B20A20 at full load. | ↓ by ~15.15% with NPs fuels compared to B20. | ↓ by ~5.98% with NPs fuels compared to B20. | ↓ by ~2.2% with NPs fuels compared to B20. |
| [127] | |
| CeO2 | Tyre pyrolysis oil | B5, B10, B15 and B20; and B10D85C100 | 50 ppm and 100 ppm | Kirloskar TV1, 1C, 4S, DI, CR17.5:1, RP3.7 kW, RS1500 rpm, IT 23° bTDC, IP 200 bar. | ↑ with CeO2 NP fuel | ↓ slightly with B5 NP fuel compared to DF | --- | Improved by 2.85% with B5D85C100 | ↓ by 13.33% with B5D85C100 compared to DF. | ↓ by 3.0% with B5D85C100 compared to DF. | ↑ slightly by 1.4% with B5D85C100. |
| [137] |
| Orange peel oil | OPO20; OPO20C50 and OPO20C100 | 32 nm/50 ppm and 100 ppm | Kirloskar TV1, 1C, 4S, DI, CI, CR17.5:1, RP5.2 kW, RS1500 rpm, IT 23° bTDC, IP 200 bar | ↑ with an ↑ in loads and ICP. | Improved due to Ä. | ↓ with an ↑ in loads and NPs rate. | ↑ with an ↑ in loads and NPs rate. | ↓ with and without NPs addition compared to DF due to Ã. | ↓ with and without NPs addition compared to DF due to Ã. | ↓ with an ↑ in CeO2 NPs due to Þ. |
| [86] | |
| Lemon grass oil | LGO; LGO emulsion, LGO nano-emulsion and DF. | 10 nm–20 nm/20 ppm–80 ppm | Kirloskar TV1, 1C, 4S, WC, CI, CR17.5:1, RP5.2 kW, RS1500 rpm, IT 23° bTDC, IP 200 bar. | --- | --- | ↓ with an ↑ in EP for all the fuels. | ↑ by 31.25% with LGO nano-emulsion compared to other test fuels.. | ↓ by 15.21% with LGO nano-emulsion compared to other fuels. | ↓ by 16.12% with LGO nano-emulsion compared to other fuels. | ↑ with LGO emulsion compared to other fuels. |
| [144] | |
| Ginger grass oil | G10C30, G20C30, G30C30 and G40C30; and DF | 30 ppm | Kirloskar, 1C, 4S, CI, CR17.5:1, RP5.2 kW, RS1500 rpm, IT 23° bTDC, IP 200 bar. | --- | --- | ↓ slightly with an ↑ in load. | ↓ with an ↑ in load. | ↑ with NP fuels | ↓ with G40C30 compared to DF. | ↓ with DF compared to NP fuels. |
| [145] | |
| Nerium oleander | SFDF, NOB, ENOB and NENOB | 15.01 nm/30 ppm | Kirloskar, 1C, 4S, WC, CI, CR17.5:1, RP5.2 kW, RS1500 rpm, IT 23° bTDC, IP 200 bar. | ↓ with NP fuels compared to DF. | ↓ with NP fuels | BSEC ↑ higher with NENOB compared to other fuels. | ↓ with NOB compared to other fuels | ↓ with NP fuels but ↑ with highest EP. | ↓ significantly with NENOB compared to DF. | ↓ slightly with NENOB compared to DF. |
| [87] | |
| Lemon grass oil | LGO; LGO emulsion, LGO nano-emulsion and DF. | 16.27 nm/30 ppm | Kirloskar, 1C, 4S, WC, CI, CR17.5:1, RP5.2 kW, RS1500 rpm, IT 27° bTDC, IP 200 bar. | ↓ with NP fuels due to § | ↓ with NP fuels due to Ÿ | BSEC ↑ with LGO nano-emulsion compared to other fuels. | ↑ with LGO compared to other fuels. | ↓ with LGO nano-emulsion but ↑ at highest EP. | ↓ with LGO nano-emulsion compared to other fuels. | ↑ with an ↑ in EP across all fuels. |
| [82] | |
| Ce0.7Zr0.3O2 | Corn stalk pyrolysis bio-oil | CB10C50, CB15C50, CB20C50 and CB25C50; and DF | 50 ppm | 1C, 4S, WC, CI, CR17.0:1, RP13.2 kW, RS2200 rpm, IP 190 bar. | --- | ↓ with CBs fuels | ↓ with CBs and ↑ with an ↑ in load. | ↑ by CBs with an ↑ in EP. | ↑ with CB25C50 and ↑ with an ↑ in load. | ↑ with CB25C50 and ↑ with an ↑ in load. | ↓ with CB25C50 compared to other fuels. |
| [138] |
| MgO | Municipal waste plastic oil | MPO20; MPO20M100 and DF | 100 ppm | Kirloskar, 1C, 4S, DI, CI, CR17.5:1, RP3.5 kW, RS1500 rpm, IT 23° bTDC, IP 220 bar | ↓ by 7.04% with MPO20M100 compared to DF and ↓ by 17.5% with MPO20 than DF. | ↓ by 11.96% with MPO20M100 compared to DF and ↓ by 19.52% with MPO20 than DF. | ↓ with MPO20M100 compared to MPO20. | ↑ with MPO20M100 compared to MPO20. | ↓ by 18.18% with MPO20M100 compared to MPO20. | ↓ by 21.87% with MPO20M100 compared to DF. | ↑ by 14.47% with MPO20M100 compared to DF. |
| [139] |
| MWCNT | Lemon peel oil | LPO20; LPO20CNT50 and LPO20CNT100 | 10 nm/50 ppm and 100 ppm | Kirloskar TV1, 1C, 4S, DI, CI, CR17.5:1, RP5.2 kW, RS1500 rpm, IT 23° bTDC, IP 200 bar | ↑ with an ↑ in loads and ICP. | Improved due to Ä. | ↓ with an ↑ in loads and NPs rate. | ↑ with an ↑ in loads and NPs rate. | ↓ with and without NPs addition compared to DF due to Ã. | ↓ with and without NPs addition compared to DF due to Ã. | ↑ with an ↑ in MWCNT NPs due to ß. |
| [86] |
| Waste fishing net oil | WFNO; WM50 and DF | 50 ppm | Kirloskar, 1C, 4S, WC, CI, CR17.5:1, RP3.5 kW, RS1500 rpm, IT 23° bTDC. | ↑ with WFNO compared to other fuels. | ↑ with WFNO compared to other fuels. | ↓ by 3.87% with MWCNT fuel compared to WFNO. | ↑ by 3.83% with MWCNT fuel compared to WFNO. | ↓ by 25% | ↓ by 9.09% | ↓ by 5.25% |
| [141] | |
| Rice husk | Pine oil | B10 and B20; B10RH and B20RH; and DF | <100 nm/0.1% (I g/l) | Kirloskar TV1, 1C, 4S, DI, CI, CR17.5:1, RP5.2 kW, RS1500 rpm, IT 23° bTDC, IP 210 bar | --- | --- | ↑ by 4.1–8.7% with BRH compared to DF. | ↓ by 3.04% with RH NPs compared to DF. | ↓ by 27.27% with B20RH compared to other fuels. | ↓ by 19.64% with B20RH compared to other fuels. | ↑ with an ↑ in RH NPs. |
| [128] |
| TiO2 | Linseed oil | LS100; PLS20; PLS50, PLS100, PLS150 and PLS200 | 25 nm–150 nm/50 ppm, 100 ppm, 150 ppm and 200 ppm | Kirloskar TV1, 1C, 4S, WC, CI, CR17.5:1, RP5.2 kW, RS1500 rpm, IT 23° bTDC, IP 200 bar. | --- | --- | ↓ with an ↑ in load and TiO2 NPs conc. | ↑ slightly by 8.11% with PLS200 compared to LS20. | ↓ by 21.05% with PLS200 compared to LS20. | ↓ by 33.82% with an ↑ in TiO2 NPs conc. | ↑ by ~6.53% with an ↑ in TiO2 NPs conc. |
| [129] |
| Orange oil | OM; OMT50 and OMT100; DF | 20 nm/50 ppm and 100 ppm | Kirloskar TV1, 1C, 4S, WC, CI, CR17.5:1, RP5.2 kW, RS1500 rpm, IT 23° bTDC, IP 200 bar. | ↑ with TiO2 NPs fuels. | Improved with an ↑ in NPs. | ↑ slightly with an ↑ in TiO2 NPs conc. | Improved for OMT50 and OMT100 by 1.6% and 3.0%, resp. compared to DF. | ↓ significantly by 22.4% with OMT100 compared to DF. | ↓ by 18.7% with OMT100 compared to DF. | ↑ slightly by 7.2–10.4% with an ↑ in TiO2 NPs conc. |
| [106] | |
| Plastic oil | CPD 2S 5W; PDO 2S 5W; CWT, PWT and DF | 40 nm–50 nm/20 ppm, 40 ppm and 60 ppm | Kirloskar TAF1, 1C, 4S, AC, CI, CR17.5:1, RP4.4 kW, RS1500 rpm, IT 26° bTDC, IP 215 bar. | ↑ by 4.12% PDO compared to CPD. | Improved with an ↑ in NPs. | ↓ with an ↑ in load and TiO2 NPs conc. | ↓ with an ↑ in load. | ↓ with an ↑ in load and TiO2 NPs conc. | ↓ with an ↑ in load and TiO2 NPs conc. | ↓ with an ↑ in load and TiO2 NPs conc. |
| [140] | |
| Type of NPs Used | Source of Biodiesel Fuel | Blends | Size of NPs/NPs Concentration | Engine Sp. | Combustion | Performance | Gaseous Emission | Observation | Reference | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HRR | ICP | BFSC | BTE | CO | HC | NOx | |||||||
| Al2O3 | Honge oil | HOME20, HOME2020, HOME2040, and HOME2060 | 10 nm/20 ppm, 40 ppm and 60 ppm | Kirloskar TV1, 1C, 4S, WC, CI, CR17.5:1, RP5.5 kW, RS1500 rpm, IT 23° bTDC, IP 205 bar. | ↑ with HOME2040 compared to other fuels. | ↑ with HOME2040 compared to other fuels. | ↓ by 11.6% HOME2040 compared to HOME20. | ↑ by 10.57% with HOME2040 compared to HOME20. | ↓ significantly by 47.43% HOME2040 compared to HOME20. | ↓ by 37.72% HOME2040 compared to HOME20. | ↑ with an ↑ in Al2O3 NPs conc. |
| [165] |
| Tamarind seed oil | TS20; TSA (TS20A30 and TS20A60) and DF | 20 nm/30 ppm and 60 ppm | Kirloskar TVI, 1C, 4S, WC, DI, CI, CR17.5:1, RP5.2 kW, RS1500 rpm, IT 23° bTDC, IP 220 bars. | ↑ with TS20 compared to TSA. | ↑ with DF compared to TSA blend. | ↑ with TSA fuels compared to TS20 blend. | ↑ by 1.56% with TS20A60 compared to DF. | ↓ by 56.6% with NPs fuels compared to DF. | ↓ with an ↑ in Al2O3 NPs. | ↑ higher with TS20A60 but lower than TS20. |
| [168] | |
| Pongamia oil methyl ester | B25; B25A (B25A50 and B25A100) and DF | 50 ppm and 100 ppm | Kirloskar TVI, 1C, 4S, WC, DI, CI, CR16.5:1, RP3.7 kW, RS1500 rpm, IT 23° bTDC, IP 220 bars. | ↑ with an ↑ in Al2O3 NPs rate. | ↑ with an ↑ in Al2O3 NPs rate. | ↓ highly with B25A100 compared to other fuels. | Improved with an ↑ in Al2O3 NPs. | ↓ marginal with B25A compared to B25 and DF. | ↓ marginal with B25A compared to B25 and DF. | ↑ with an ↑ in Al2O3 NPs rate. |
| [169] | |
| CeO2 | WCO | B20; B20C80-10, B20C80-30 and B20C80-80. | 10 nm, 30 nm and 80 nm/80 ppm | Kirloskar TV1, 1C, 4S, WC, CI, CR17.5:1, RP5.2 kW, RS1500 rpm, IP 180 bar. | ↑ slightly with B20C80-30 | ↑ by ~1.7% with B20C80-30 compared to other fuels. | ↓ by 2.5% with B20C80-30 compared to DF. | ↑ with CeO2 NPs conc. | ↓ by 56% with B20C80-30 compared to DF. | ↓ by 27% with B20C80-30 compared to DF. | ↓ by 17% with B20C80-30. |
| [159] |
| Corn oil | CO10; CO10C25, CO10C50 and CO10C75 | 50 nm–70 nm/25 ppm, 50 ppm, and 75 ppm | Kirloskar TV1, 1C, 4S, WC, CI, CR17.5:1, RP5.2 kW, RS1500 rpm, IT 23° bTDC, IP 200 bar. | --- | ↑ with CeO2 NPs conc. | ↓ with an ↑ in CeO2 NPs conc. | ↑ with CO10C50 at max. eff. 34.8% and load. | ↓ with CO10C50 | ↓ with CO10C50 | ↑ with an ↑ in load and CeO2 NPs conc. |
| [164] | |
| Co(Al, Cr)2O4 | Kapok oil | SIT KC1-RET, SIT KC2-RET, SIT KC3-RET, SIT KC4-RET, SIT KC1-ADV, SIT KC2-ADV, SIT KC3-ADV, SIT KC4-ADV | 50 ppm, 100 ppm, 150 ppm, and 200 ppm | Kirloskar SV1, 1C, 4S, WC, CR17.5:1, RP5.9 kW, RS1800 rpm, IT varies. | ↑ by 5.09% with IT of 23CAD bTDC | ↑ by 5.27% with IT of 23CAD bTDC | ↓ by 21.23% with SIT KC4-ADV. | ↑ by 7.2% with KC4-ADV than SIT. | ↓ by 41.66% with SIT KC4-ADV | ↓ by 37.86% with SIT KC4-ADV | ↓ by 16.45% with SIT KC1-RET. |
| [103] |
| FeO.Fe2O3 | Chicken fat oil | B10, B20 and B30; B10F50, B10F100, B10F150, B20F50, B20F100, B20F150, B30F50, B30F100 and B30F150 | 18.21 nm/50 ppm, 100 ppm, and 150 ppm | Kirloskar TV1, 1C, 4S, WC, CI, CR17.5:1, RP5.2 kW, RS1500 rpm, IT 23° bTDC, IP 200 bar. | --- | --- | ↓ highly by 10.64% with B20F100 compared to B20. | Improved by 4.84% for B20F100 compared to B20. | ↓ Max 56.66% with B30F100 compared to B30. | ↓ by 22.72% with B30F100 compared to B30. | ↓ by ~15.39% with FeO.Fe2O3 NP fuels. |
| [172] |
| MgO | WCO | B’s (B10, B20 and B100); MgO NPs fuels (B10W30A, B20W30A and B100W30A) and DF | 30 ppm | Kirloskar TVI, 1C, 4S, WC, CI, CR17.5:1, RP7 kW, RS1500 rpm, IT 23° bTDC, IP 220 kgf/cm2. | ↓ with an ↑ in NPs. | ↓ highly with MgO NPs fuels. | ↑ with MgO NPs fuels compared to B’s. | ↑ with MgO NPs fuels compared to B’s. | ↓ with MgO NPs fuels compared to other fuels. | ↓ with MgO NPs fuels compared to other fuels. | ↑ with MgO NPs fuels and B’s compared to DF. |
| [166] |
| MW-CNTs | WCO | B20; B20M20, B20M50, B20M75 and B20M100. | 2nm–16 nm/20 ppm, 50 ppm, 75 ppm, and 100 ppm | Lombardini, 1C, 4S, WC, CI, CR17.5:1, RP5.77 kW, RS3000 rpm, IP 190 bar. | --- | --- | ↓ by ~6.7% as the load ↑ | Improved by ~7.4% with an ↑ NPs. | ↓ with an ↑ in MWCNTs fuels. | ↓ with an ↑ in MWCNTs fuels. | ↑ significantly |
| [104] |
| Jatropha | J20; J20C25, J20C50 and J20C100 | 20 nm–25 nm/25 ppm, 50 ppm, and 100 ppm | DEUTZ F1L511, 1C, 4S, AC, CI, CR17.5:1, RP5.77 kW, RS1500 rpm, IT 24° bTDC, IP 175 bar. | --- | --- | ↓ with an ↑ in load. | ↑ with an ↑ in load and NPs conc. | ↓ with an ↑ in CNTs NP conc. | ↓ with an ↑ in CNTs NP conc. | ↓ partially with CNTs fuels at high loads.. |
| [157] | |
| NiO | Neem oil | NB25, NB25N25, NB25N50, NB25N75 and NB25N100 | 7 nm–10 nm/25 ppm, 50 ppm, 75 ppm, and 100 ppm | Rocket Engg, VCR, 1C, 4S, WC, CR17.5:1, RP4.8 kW, RS1500 rpm, IT 23–27° bTDC. | --- | --- | ↓ with an ↑ in NP conc. at 27° bTDC. | ↑ by 6.3% with NiO fuels. | ↓ significantly by NiO fuels. | ↓ significantly by NiO fuels. | ↑ with NiO NP fuels by advanced fuel injection. |
| [162] |
| SiO2 | Soybean | SB25; SB25S25, SB25S50 and SB25S75; DF | 5 nm–20 nm/25 ppm, 50 ppm, and 75 ppm | Kirloskar VCR, 1C, 4S, CI, CR21.5:1, RP5HP, RS1800 rpm. | --- | --- | ↓ with an ↑ in load. | ↓ slightly with SiO2 fuels compared to DF. | ↑ significantly by SiO2 NP fuels with an ↑ in load. | ↑ slightly with SiO2 NP fuels compared to DF. | ↑ significantly with an ↑ in load. |
| [173] |
| WCO | B20; B20SiO2 and DF. | 100 ppm | Lombardini 15 LD 350, 1C, 4S, WC, DI, CR20.3:1, RP7.5HP, RS3600 rpm, IP207 bar. | ↑ with an ↑ in load for all fuels. | ↑ with an ↑ in load for all fuels. | ↓ by B10SiO2 with an ↑ in loads. | ↑ with an ↑ in load for all fuels. | ↓ slightly with B10SiO2 compared to DF. | ↓ by 80.98% with B10SiO2 compared to DF. | ↑ significantly with B10SiO2 compared to DF. |
| [163] | |
| TiO2 | Cottonseed oil | CSBD; CSBD50 and CSBD100; and DF | 17 nm–28 nm/50 ppm, and 100 ppm | Kirloskar AV1, 1C, 4S, WC, CI, CR18.5:1, RP3.5 kW, RS1400 rpm, IT 23° bTDC, IP 200 bar. | --- | --- | ↓ with an ↑ in load at all tested fuels. | ↓ with CSBD and TiO2 NP fuels compared to DF. | ↓ with an ↑ in TiO2 NP. | ↓ by 14.7–16.2% with CSBD100 compared to DF. | ↓ with an ↑ in TiO2 NP at all load conditions. |
| [174] |
| Palm oil | B0, B20; B20T60 and B20AOT60; DF | 60 ppm | TECH-ED, 1C, 4S, WC, VRC, CR20:1, RP4 kW, RS1500 rpm. | --- | --- | ↓ with B20AOT60 compared to B20. | ↑ higher with B20AOT60 compared to other fuels. | ↓ with B20AOT60 | ↓ with B20AOT60 | ↑ with B20 but much lesser with B20AOT60 compared to DF. |
| [175] | |
| ZnO | Grapeseed oil | GS; GSZ50 and GSZ100 | 36 nm/50 ppm and 100 ppm | Kirloskar TV1, 1C, 4S, CI, CR17.5:1, RP5.2 kW, RS1500 rpm, IT 27° bTDC, IP 200 bar. | ↓ with GS compared to DF. | ↓ with GS compared to DF. | ↓ with DF compared to other fuels. | Max. ↑ was at GSZ100. | ↓ with an ↑ in EP and NPs conc. | ↓ by ~13% with GSZ100 compared to other fuels. | ↑ with GS compared to DF. |
| [167] |
| ZrO2 | Garcinia gummi-gutta | B100, B20 and B20Z25 | 25 ppm | Kirloskar TAF-1, 1C, 4S, AC, CR17.6:1, RP5.2 kW, RS1500 rpm, IT 23° bTDC. | ↑ with B20 | ↑ with B20 | ↓ with B20Z25 compared to B100. | ↓ with B20Z25 compared to DF. | ↓ with B20Z25 compared to DF and B100. | ↓ with NP fuel compared to DF. | ↓ slightly by B20z25 with an ↑ in EP. |
| [97] |
| Base Fuel | Nanoparticles | Remarks | Ref. | |||
|---|---|---|---|---|---|---|
| Exergy | Economic | Environmental | Sustainability | |||
| Diesel–ethanol (D90E10) | Al2O3 and TiO2 at 100 ppm | The exergy efficiency at all loads followed a decreasing trend in superiority in the order: D90E10Al2O3 > D90E10TiO2 > D100 > D90E10. Clearly the addition of nanoparticles to diesel–ethanol blends improved the exergy. The presence of NPs increased the heating values of the fuels. In addition to this, the combustion efficiency and exergy efficiency of the fuels improved by virtue of the catalytic effect, micro-explosions, oxygen buffering, and large surface area-to-volume ratio of the NPs which causes chemical reactions to accelerate and provides excellent thermal properties. | The presence of NPs to D90E10 led to a reduction in fuel consumption and the specific exergy of the base fuel was increased. This led to a decrease in the fuel cost flow rate. At all loads, the cost of crankshaft work per unit energy is $/GJ followed a decreasing trend in inferiority in the order: D90E10 > D90E10TiO2 > D90E10Al2O3 > D100. The exergoeconomic analysis thus favoured the nanofuels compared to diesel–ethanol blend. | The presence of NPs ensured higher exergy efficiencies and this led to the production of nanofuels with relatively lower environmental impact. At all loads, the environmental impact rate pr unit of break power followed a decreasing trend in inferiority in the order: D90E10 > D100 > D90E10TiO2 > D90E10Al2O3. Nanofuels have thus presented better exergoenvironmental feasibility compared to both pure diesel and diesel–ethanol blend. | The most sustainable test fuel according to their sustainability index was D90E10Al2O3. This is sequentially followed by D90E10TiO2, D100, and D90E10. | [34] |
| Diesel–canola oil biodiesel (C10) | TiO2 at 100 ppm and 3 different sizes (29 nm, 45 nm, and 200 nm) | The exergy loss and exergy destruction increase with increase in NP size. As NP size gets larger, there is a general reduction in surface area-to-volume ratio, catalytic activity while fuel consumption and exergy inlet rate increases. The aggregation of these events at larger NP sizes leads to a lower exergy efficiency. At all loads, the cumulative exergy efficiency followed a decreasing trend in superiority in the order: C10 + 28 nm TiO2 (81.60%) > C10 + 45 nm TiO2 (79.06%) > C10 + 200 nm TiO2 (77.37%) > D100 (74.98%) > C10 (71.50%). | Similarly, the presence of NPs led to a superior thermoeconomic results in nanofuels compared to pure diesel and its blend with biodiesel. The NPs improve energy and exergy efficiencies and this produced optimal thermoeconomic results. The best thermoeconomic results was obtained at the smallest NP size. However, an opposite trend is observed for the unit cost and specific exergy cost. In this context, neat diesel and C10 had an economic advantage over their NP-doped counterparts. Reducing the grain size of the NPs led to the production of a worst fuel from an economic point of view. The heating value of the base fuel increases in the presence of the NPs, causing an increment in specific exergy cost for the nanofuels. Despite this trend, it is worth noting that per their advantage in exergy efficiencies, nanofuels showed beneficial and superior exergoeconomic results against the base fuel. | - | At all engine loads, the highest sustainability index of the diesel engine was recorded for C10 + 28 nm TiO2 test fuel as a result of its superior exergy efficiencies in contrast to other test fuels. This is followed by C10 + 45 nm TiO2 > C10 + 200 nm TiO2 > D100 > C10 in a decreasing order of sustainability. | [39] |
| Diesel–biodiesel (B5) | Al2O3 at 50 and 100 ppm | Averagely, the presence of Al2O3 increased the exergy efficiency by 7.28% compared to B5. Similarly, the Addition of the NP to the base fuel reduced unaccounted losses by 31.8% on an average. Additionally, there was a slight change in exergy loss to the cooling water when the NP was used. It is worth noting that, increase in NP dosage led to superior exergy efficiencies and entropy generation results. The high surface area of the Al2O3 NP led high ignition qualities–shortening the combustion time. Al2O3′s high catalytic activity and surface area-to-volume ratio also ensure that the carbon activation temperature is lowered–leading to the promotion of fuel oxidation and complete combustion. Thermal properties thus increase and causes enhanced exergy efficiencies of the low carbon fuelled-diesel engines under the influence of the NPs. | - | - | - | [41] |
| Diesel–biodiesel (B5 and B10) | Hybrid nano catalysts additives comprising cerium oxide and molybdenum oxide on amide-functionalized MWCNTs at 30, 60, 90 ppm | The nano-additives provided sufficient oxygen to promote complete combustion and decrease the amount of exhaust air pollutants. The occurrence of these mechanisms in the cylinder by virtue of the inclusion of the nano-additives ensured that there is a decrease in the exergy rate of the exhaust gas and the heat transfer exergy rate of the diesel engine. Increasing the concentration of the nano-additives made this observation more obvious. Furthermore, the net exergy work rate of the diesel engine benefits from the presence of the nano-additives compared to the nano-additive-free blends. In addition to their oxygen buffering characteristics, the nano-additives exhibit nanocluster explosiveness which help the decomposition of sediments and deposits, and prevents their reformation. The absence of iron and carbon deposits reduces friction of the engine’s movable parts. These factors contributed to an increase in engine power and causes the net exergy work rate of the engine to increase. The net exergy work is directly proportional to the exergy efficiency. Hence, the exergy efficiency of the diesel engine increases with increase in the amount of nano-additives. | - | - | - | [42] |
| Diesel–biodiesel (B5) emulsified with water at concentrations of 3, 5, and 7 wt%. Tween 80 and Span 80 used as surfactants | Aqueous nano CeO2 at 0 and 90 ppm | The presence of water decreases the exergy efficiency of pure B5, but the situation is greatly improved with the addition of Aqueous nano CeO2. At all loads, B5 with 3 wt% water and 90 ppm of NP (B5W3m) showed the best exergy efficiency amongst all test blended fuels. Similar findings are witnessed for the thermal efficiency. | Despite the excellent results for exergy efficiency, the exergoeconomic analysis revealed that pure diesel was more favourable than B5W5m. | - | Due to its high exergy efficiency, B5W3m in the test engine had the most favourable sustainability index among all test blended fuels. | [38,178] |
| Diesel–waste cooking oil biodiesel (D90B10) | Al2O3, TiO2, SiO2 at 100 ppm | The exergy efficiency of pure diesel degrades after the addition of biodiesel. The trend is significantly reversed with the inclusion of the NPs. D90B10Al2O3 recorded the highest exergy efficiency. This is followed by D90B10SiO2 > D90B10TiO2 > D100 > D90B10. Similarly, the lowest and highest exergy destruction was observed D90B10Al2O3 and D90B10, respectively. In addition, the crankshaft work followed an increasing trend of superiority in the order D90B10 < D100 < D90B10TiO2 < D90B10SiO2 < D90B10Al2O3. | Adding NPs led to a decrease in fuel consumption–hence, at all load conditions, the highest and lowest cost flow rate was recorded by D100 and D90B10SiO2. In the same way, D90B10SiO2 recorded the lowest exhaust cost flow rate and loss cost flow rate, closely followed by D90B10Al2O3. However, for cost flow rate of crankshaft work, D90B10Al2O3 was the most economical ahead of D90B10SiO2. The exergo-economic factor for the nanofuels were superior than the base blend and pure diesel at all engine loads with D90B10SiO2 being the highest of all. | - | At each engine load, the depletion number of the diesel engine followed a decreasing trend in the order D90B10 < D100 < D90B10TiO2 < D90B10SiO2 < D90B10Al2O3. In addition, the sustainability index of the nanofuels were better than the base blend and pure diesel at all engine loads with D90B10Al2O3 being the highest of all. | [40] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ampah, J.D.; Yusuf, A.A.; Agyekum, E.B.; Afrane, S.; Jin, C.; Liu, H.; Fattah, I.M.R.; Show, P.L.; Shouran, M.; Habil, M.; et al. Progress and Recent Trends in the Application of Nanoparticles as Low Carbon Fuel Additives—A State of the Art Review. Nanomaterials 2022, 12, 1515. https://doi.org/10.3390/nano12091515
Ampah JD, Yusuf AA, Agyekum EB, Afrane S, Jin C, Liu H, Fattah IMR, Show PL, Shouran M, Habil M, et al. Progress and Recent Trends in the Application of Nanoparticles as Low Carbon Fuel Additives—A State of the Art Review. Nanomaterials. 2022; 12(9):1515. https://doi.org/10.3390/nano12091515
Chicago/Turabian StyleAmpah, Jeffrey Dankwa, Abdulfatah Abdu Yusuf, Ephraim Bonah Agyekum, Sandylove Afrane, Chao Jin, Haifeng Liu, Islam Md Rizwanul Fattah, Pau Loke Show, Mokhtar Shouran, Monier Habil, and et al. 2022. "Progress and Recent Trends in the Application of Nanoparticles as Low Carbon Fuel Additives—A State of the Art Review" Nanomaterials 12, no. 9: 1515. https://doi.org/10.3390/nano12091515
APA StyleAmpah, J. D., Yusuf, A. A., Agyekum, E. B., Afrane, S., Jin, C., Liu, H., Fattah, I. M. R., Show, P. L., Shouran, M., Habil, M., & Kamel, S. (2022). Progress and Recent Trends in the Application of Nanoparticles as Low Carbon Fuel Additives—A State of the Art Review. Nanomaterials, 12(9), 1515. https://doi.org/10.3390/nano12091515













