Facile Synthesis of Green Fluorescent Carbon Dots and Their Application to Fe3+ Detection in Aqueous Solutions
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Instruments
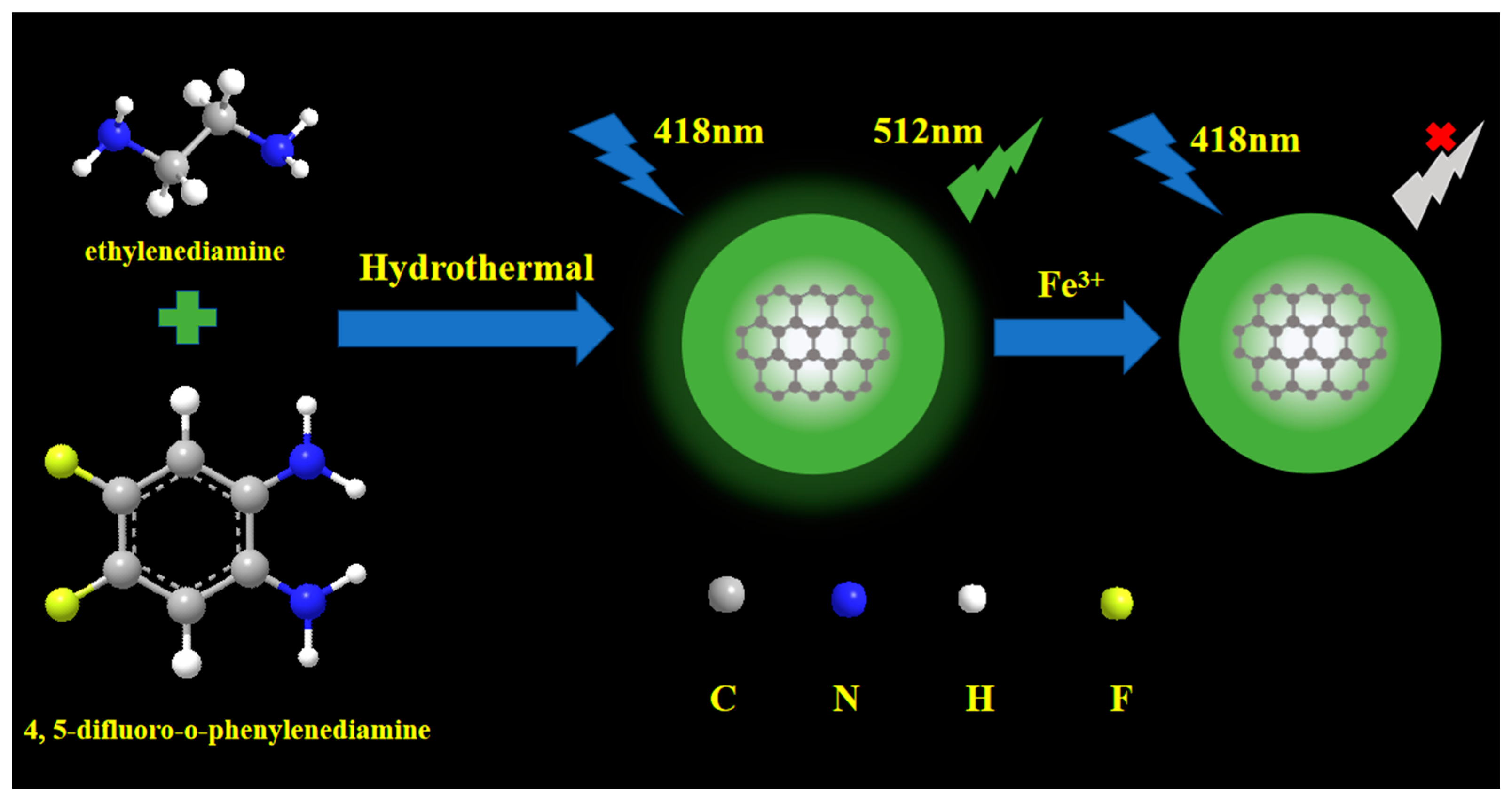
2.3. Synthesis of FNCDs
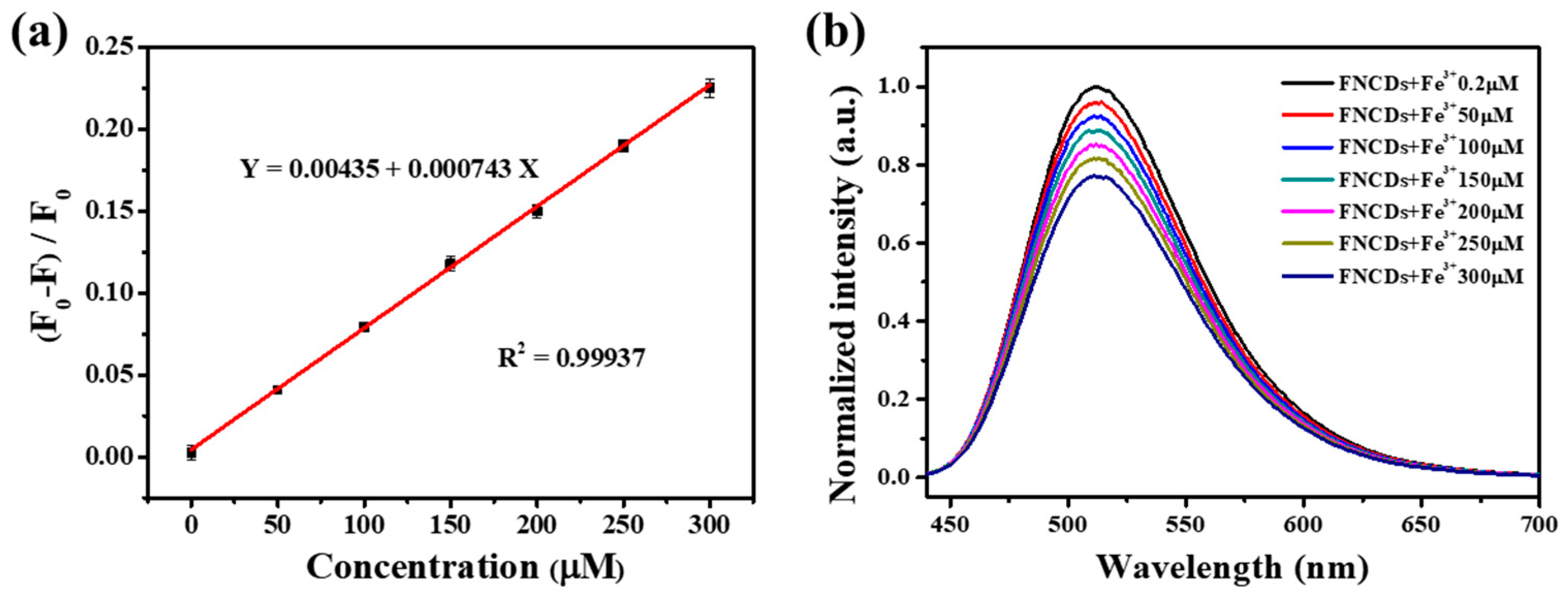
2.4. Quantitative Detection of Fe3+
2.5. Detection of Fe3+ in Different Water Samples
3. Results and Discussion
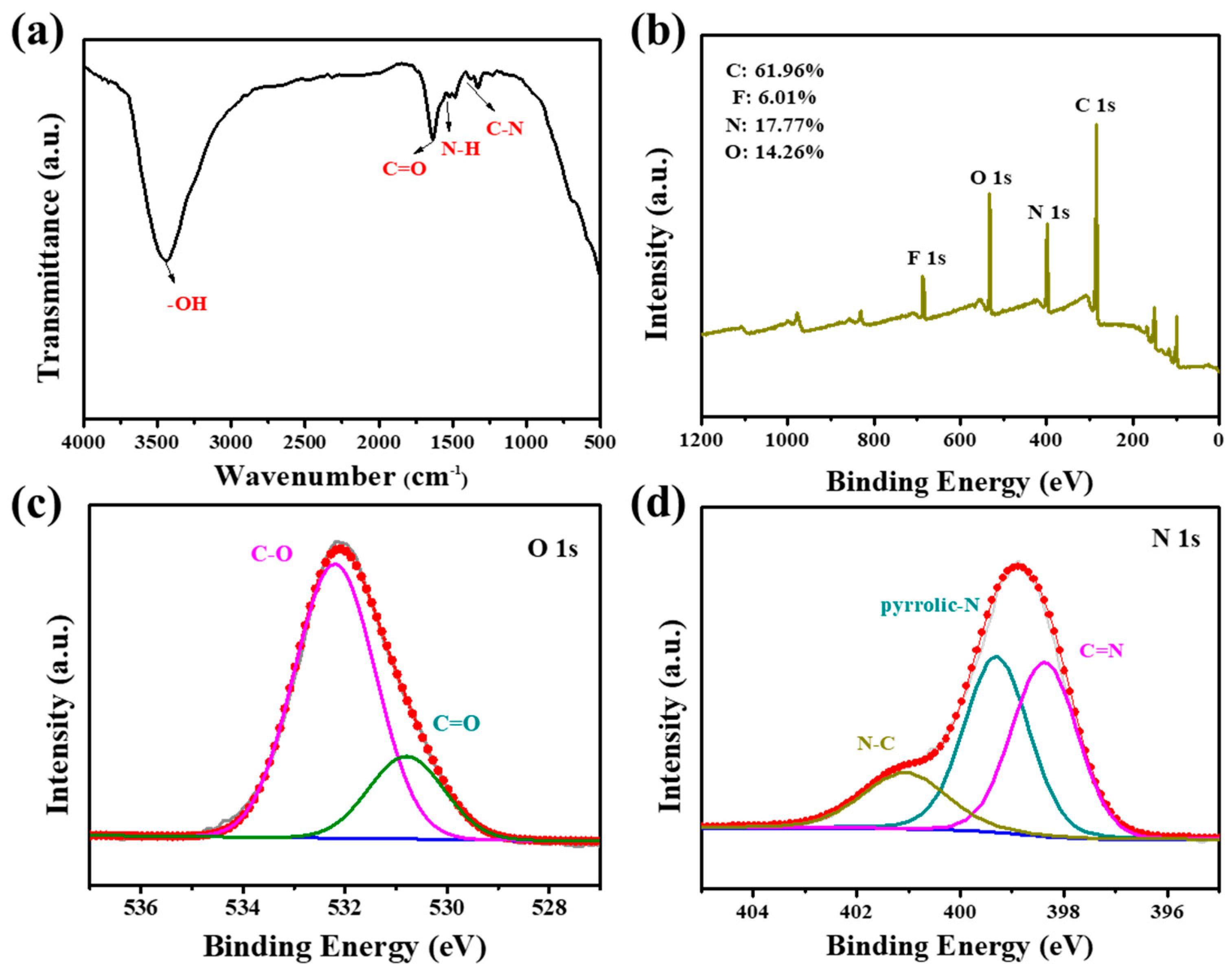
3.1. Characterization of FNCDs
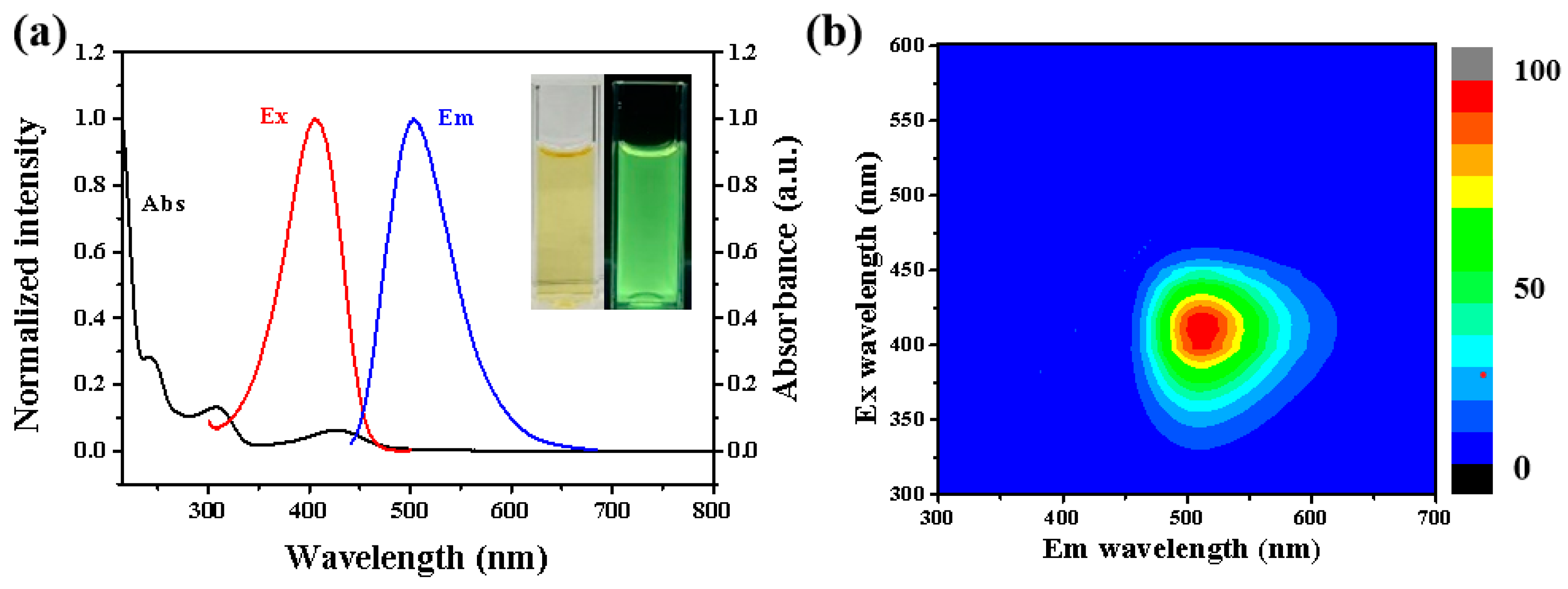
3.2. Optical Characterization
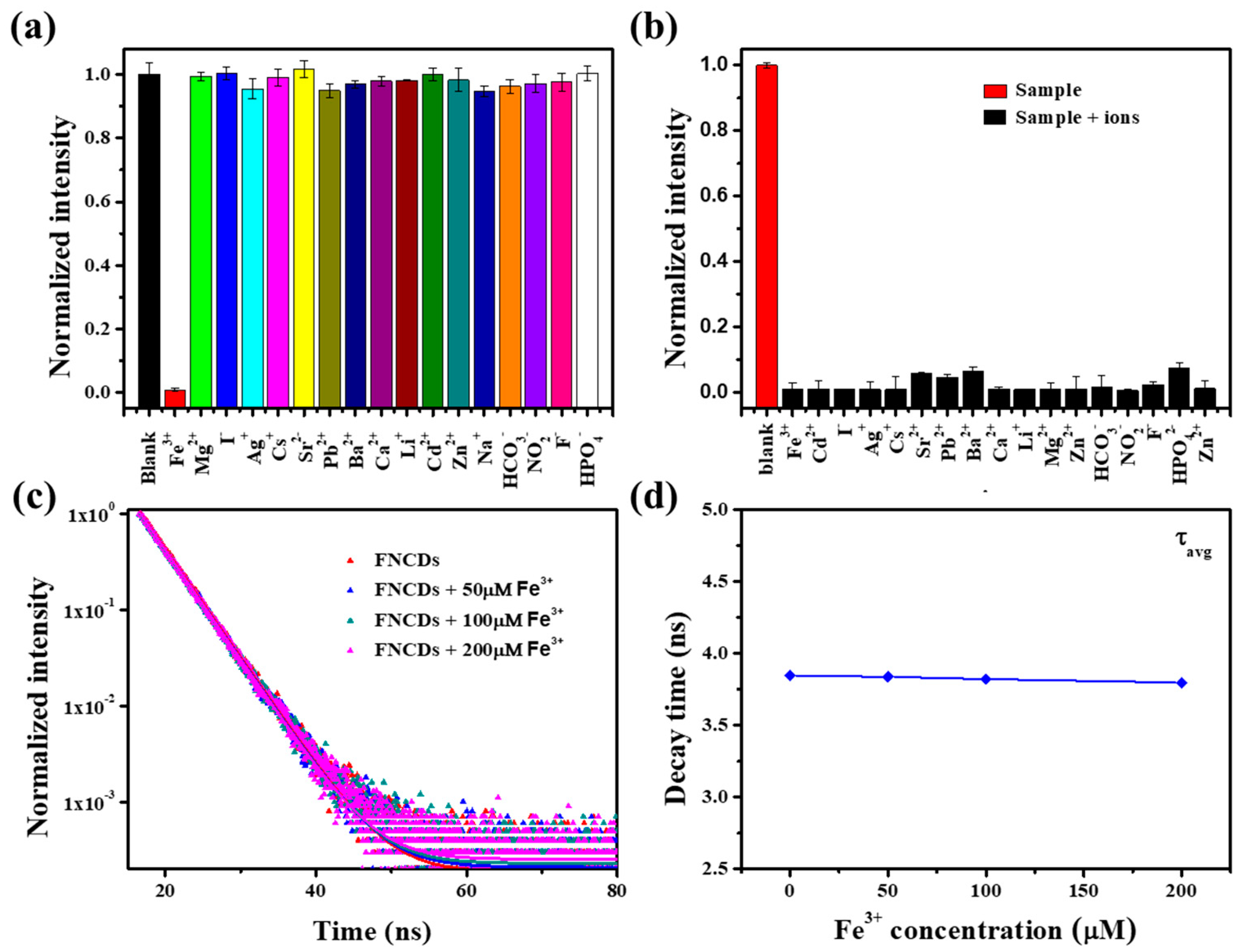
3.3. Selectivity of Fe3+
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, X.; Zhuo, Y.; Zhu, S.; Luo, Y.; Feng, Y.; Dou, Y. Novel and green synthesis of high-fluorescent carbon dots originated from honey for sensing and imaging. Biosens. Bioelectron. 2014, 60, 292–298. [Google Scholar] [CrossRef]
- Xu, J.; Miao, Y.; Zheng, J.; Yang, Y.; Liu, X. Ultrahigh Brightness Carbon Dot–Based Blue Electroluminescent LEDs by Host–Guest Energy Transfer Emission Mechanism. Adv. Opt. Mater. 2018, 6, 1800181. [Google Scholar] [CrossRef]
- Bai, Y.; Zhao, J.; Wang, S.; Lin, T.; Ye, F.; Zhao, S. Carbon Dots with Absorption Red-Shifting for Two-Photon Fluorescence Imaging of Tumor Tissue pH and Synergistic Phototherapy. ACS Appl. Mater. Interfaces 2021, 13, 35365–35375. [Google Scholar] [CrossRef]
- Wang, B.-B.; Wang, Y.-Y.; Zhang, X.-Y.; Xu, Z.-Q.; Jiang, P.; Jiang, F.-L.; Liu, Y. Bifunctional carbon dots for cell imaging and inhibition of human insulin fibrillation in the whole aggregation process. Int. J. Biol. Macromol. 2020, 147, 453–462. [Google Scholar] [CrossRef]
- Das, P.; Ganguly, S.; Mondal, S.; Ghorai, U.K.; Maity, P.P.; Choudhary, S.; Gangopadhyay, S.; Dhara, S.; Banerjee, S.; Das, N.C. Dual doped biocompatible multicolor luminescent carbon dots for bio labeling, UV-active marker and fluorescent polymer composite. Luminescence 2018, 33, 1136–1145. [Google Scholar] [CrossRef]
- Kang, Z.; Lee, S.-T. Carbon dots: Advances in nanocarbon applications. Nanoscale 2019, 11, 19214–19224. [Google Scholar] [CrossRef]
- Castro, R.C.; Soares, J.X.; Ribeiro, D.S.M.; Santos, J.L.M. Dual-emission ratiometric probe combining carbon dots and CdTe quantum dots for fluorometric and visual determination of H2O2. Sens. Actuators B Chem. 2019, 296, 126665. [Google Scholar] [CrossRef]
- Wang, Y.; Lao, S.; Ding, W.; Zhang, Z.; Liu, S. A novel ratiometric fluorescent probe for detection of iron ions and zinc ions based on dual-emission carbon dots. Sens. Actuators B Chem. 2019, 284, 186–192. [Google Scholar] [CrossRef]
- Deng, Y.; Zhou, Y.; Li, Q.; Qian, J. One-step hydrothermal synthesis of nitrogen-doped carbon dots for high-sensitivity visual detection of nitrite and ascorbic acid. Anal. Methods 2021, 13, 3685–3692. [Google Scholar] [CrossRef]
- Das, P.; Ganguly, S.; Margel, S.; Gedanken, A. Tailor made magnetic nanolights: Fabrication to cancer theranostics applications. Nanoscale Adv. 2021, 3, 6762–6796. [Google Scholar] [CrossRef]
- Halliwell, B. Reactive Oxygen Species and the Central Nervous System. J. Neurochem. 1992, 59, 1609–1623. [Google Scholar] [CrossRef] [PubMed]
- Swaminathan, S.; Fonseca, V.A.; Alam, M.G.; Shah, S.V. The Role of Iron in Diabetes and Its Complications. Diabetes Care 2007, 30, 1926–1933. [Google Scholar] [CrossRef] [Green Version]
- Galaris, D.; Skiada, V.; Barbouti, A. Redox signaling and cancer: The role of “labile” iron. Cancer Lett. 2008, 266, 21–29. [Google Scholar] [CrossRef]
- Witte, D.L.; Crosby, W.H.; Edwards, C.Q.; Fairbanks, V.F.; Mitros, F.A. Hereditary hemochromatosis. Clin. Chim. Acta 1996, 245, 139–200. [Google Scholar] [CrossRef]
- Kehrer, J.P. The Haber–Weiss reaction and mechanisms of toxicity. Toxicology 2000, 149, 43–50. [Google Scholar] [CrossRef]
- Ge, G.; Li, L.; Chen, M.; Wu, X.; Yang, Y.; Wang, D.; Zuo, S.; Zeng, Z.; Xiong, W.; Guo, C. Green Synthesis of Nitrogen–Doped Carbon Dots from Fresh Tea Leaves for Selective Fe3+ Ions Detection and Cellular Imaging. Nanomaterials 2022, 12, 986. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, X.; Pan, W.; Yu, G.; Wang, J. Fe3+-Sensitive Carbon Dots for Detection of Fe3+ in Aqueous Solution and Intracellular Imaging of Fe3+ Inside Fungal Cells. Front. Chem. 2020, 7, 911. [Google Scholar] [CrossRef]
- Zulfajri, M.; Gedda, G.; Chang, C.-J.; Chang, Y.-P.; Huang, G.G. Cranberry Beans Derived Carbon Dots as a Potential Fluorescence Sensor for Selective Detection of Fe3+ Ions in Aqueous Solution. ACS Omega 2019, 4, 15382–15392. [Google Scholar] [CrossRef] [Green Version]
- Phan, L.M.; Hoang, T.X.; Cho, S. Fluorescent Carbon Dots for Sensitive and Rapid Monitoring of Intracellular Ferrous Ion. Biosensors 2022, 12, 41. [Google Scholar] [CrossRef]
- Yan, F.; Zhang, H.; Yu, N.; Sun, Z.; Chen, L. Conjugate area-controlled synthesis of multiple-color carbon dots and application in sensors and optoelectronic devices. Sens. Actuators B Chem. 2021, 329, 129263. [Google Scholar] [CrossRef]
- Guo, J.; Ye, S.; Li, H.; Song, J.; Qu, J. Novel fluorescence probe based on bright emitted carbon dots for ClO− detection in real water samples and living cells. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 240, 118592. [Google Scholar] [CrossRef]
- Luo, J.; Wang, K.; Hua, X.; Wang, W.; Li, J.; Zhang, S.; Chen, S. Pyridinic-N Protected Synthesis of 3D Nitrogen-Doped Porous Carbon with Increased Mesoporous Defects for Oxygen Reduction. Small 2019, 15, 1805325. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Ding, H.; Lu, S.; Geng, T.; Xiao, G.; Zou, B.; Bi, H. Photoactivated Fluorescence Enhancement in F,N-Doped Carbon Dots with Piezochromic Behavior. Angew. Chem. Int. Ed. 2020, 59, 9986–9991. [Google Scholar] [CrossRef] [PubMed]
- Long, P.; Feng, Y.; Cao, C.; Li, Y.; Han, J.; Li, S.; Peng, C.; Li, Z.; Feng, W. Self-Protective Room-Temperature Phosphorescence of Fluorine and Nitrogen Codoped Carbon Dots. Adv. Funct. Mater. 2018, 28, 1800791. [Google Scholar] [CrossRef]
- Li, C.-L.; Ou, C.-M.; Huang, C.-C.; Wu, W.-C.; Chen, Y.-P.; Lin, T.-E.; Ho, L.-C.; Wang, C.-W.; Shih, C.-C.; Zhou, H.-C.; et al. Carbon dots prepared from ginger exhibiting efficient inhibition of human hepatocellular carcinoma cells. J. Mater. Chem. B 2014, 2, 4564–4571. [Google Scholar] [CrossRef] [PubMed]
- Pang, S.; Liu, S. Dual-emission carbon dots for ratiometric detection of Fe3+ ions and acid phosphatase. Anal. Chim. Acta 2020, 1105, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Senol, A.M.; Bozkurt, E. Facile green and one-pot synthesis of seville orange derived carbon dots as a fluorescent sensor for Fe3+ ions. Microchem. J. 2020, 159, 105357. [Google Scholar] [CrossRef]
- Desai, M.L.; Basu, H.; Saha, S.; Singhal, R.K.; Kailasa, S.K. Investigation of silicon doping into carbon dots for improved fluorescence properties for selective detection of Fe3+ ion. Opt. Mater. 2019, 96, 109374. [Google Scholar] [CrossRef]
- Wang, C.; Shi, H.; Yang, M.; Yan, Y.; Liu, E.; Ji, Z.; Fan, J. Facile synthesis of novel carbon quantum dots from biomass waste for highly sensitive detection of iron ions. Mater. Res. Bull. 2020, 124, 110730. [Google Scholar] [CrossRef]
- Wu, H.; Pang, L.-F.; Fu, M.-J.; Guo, X.-F.; Wang, H. Boron and nitrogen codoped carbon dots as fluorescence sensor for Fe3+ with improved selectivity. J. Pharm. Biomed. Anal. 2020, 180, 113052. [Google Scholar] [CrossRef]

| Samples | Detection Amount (μM) | Adding Fe3+ (μM) | Found Fe3+ (μM) | Recovery (%) | RSD (n = 3, %) |
|---|---|---|---|---|---|
| Laboratory water | - | 40 | 39.08 | 97.7 | 1.0 |
| 80 | 77.85 | 97.3 | 0.7 | ||
| 120 | 114.96 | 95.8 | 0.6 | ||
| Dormitory water | - | 40 | 37.43 | 93.6 | 0.3 |
| 80 | 86.00 | 107.5 | 0.6 | ||
| 120 | 125.69 | 104.7 | 1.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, S.; Zhang, M.; Guo, J.; Song, J.; Zeng, P.; Qu, J.; Chen, Y.; Li, H. Facile Synthesis of Green Fluorescent Carbon Dots and Their Application to Fe3+ Detection in Aqueous Solutions. Nanomaterials 2022, 12, 1487. https://doi.org/10.3390/nano12091487
Ye S, Zhang M, Guo J, Song J, Zeng P, Qu J, Chen Y, Li H. Facile Synthesis of Green Fluorescent Carbon Dots and Their Application to Fe3+ Detection in Aqueous Solutions. Nanomaterials. 2022; 12(9):1487. https://doi.org/10.3390/nano12091487
Chicago/Turabian StyleYe, Shuai, Mingming Zhang, Jiaqing Guo, Jun Song, Pengju Zeng, Junle Qu, Yue Chen, and Hao Li. 2022. "Facile Synthesis of Green Fluorescent Carbon Dots and Their Application to Fe3+ Detection in Aqueous Solutions" Nanomaterials 12, no. 9: 1487. https://doi.org/10.3390/nano12091487
APA StyleYe, S., Zhang, M., Guo, J., Song, J., Zeng, P., Qu, J., Chen, Y., & Li, H. (2022). Facile Synthesis of Green Fluorescent Carbon Dots and Their Application to Fe3+ Detection in Aqueous Solutions. Nanomaterials, 12(9), 1487. https://doi.org/10.3390/nano12091487








