Face-to-Face Assembly of Ag Nanoplates on Filter Papers for Pesticide Detection by Surface-Enhanced Raman Spectroscopy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Apparatus
2.2. Synthesis of Silver Nanoplates
2.3. Preparation of Paper-Based SERS Substrates
2.4. SERS Measurements
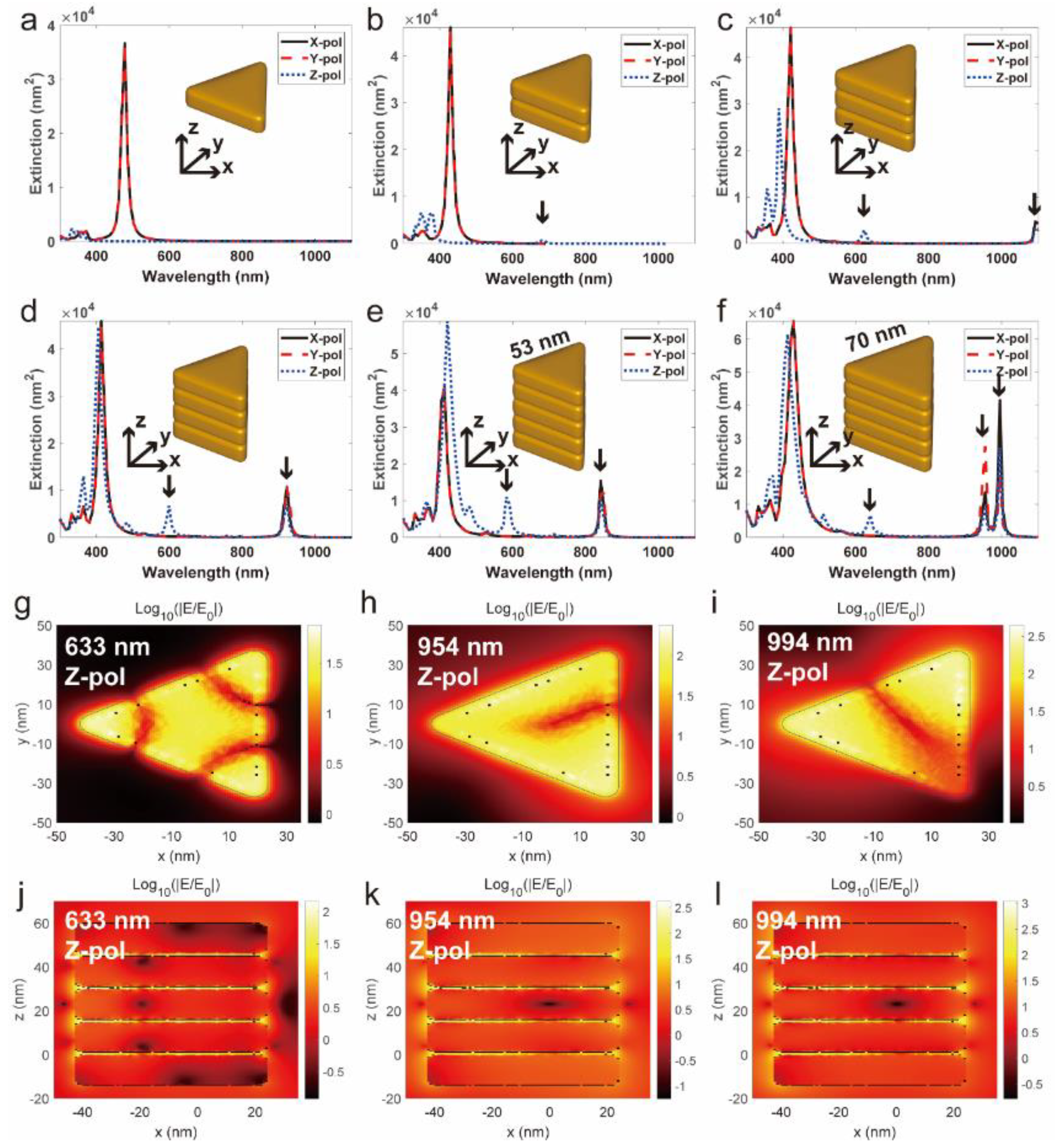
2.5. Theoretical Simulation
3. Results & Discussion
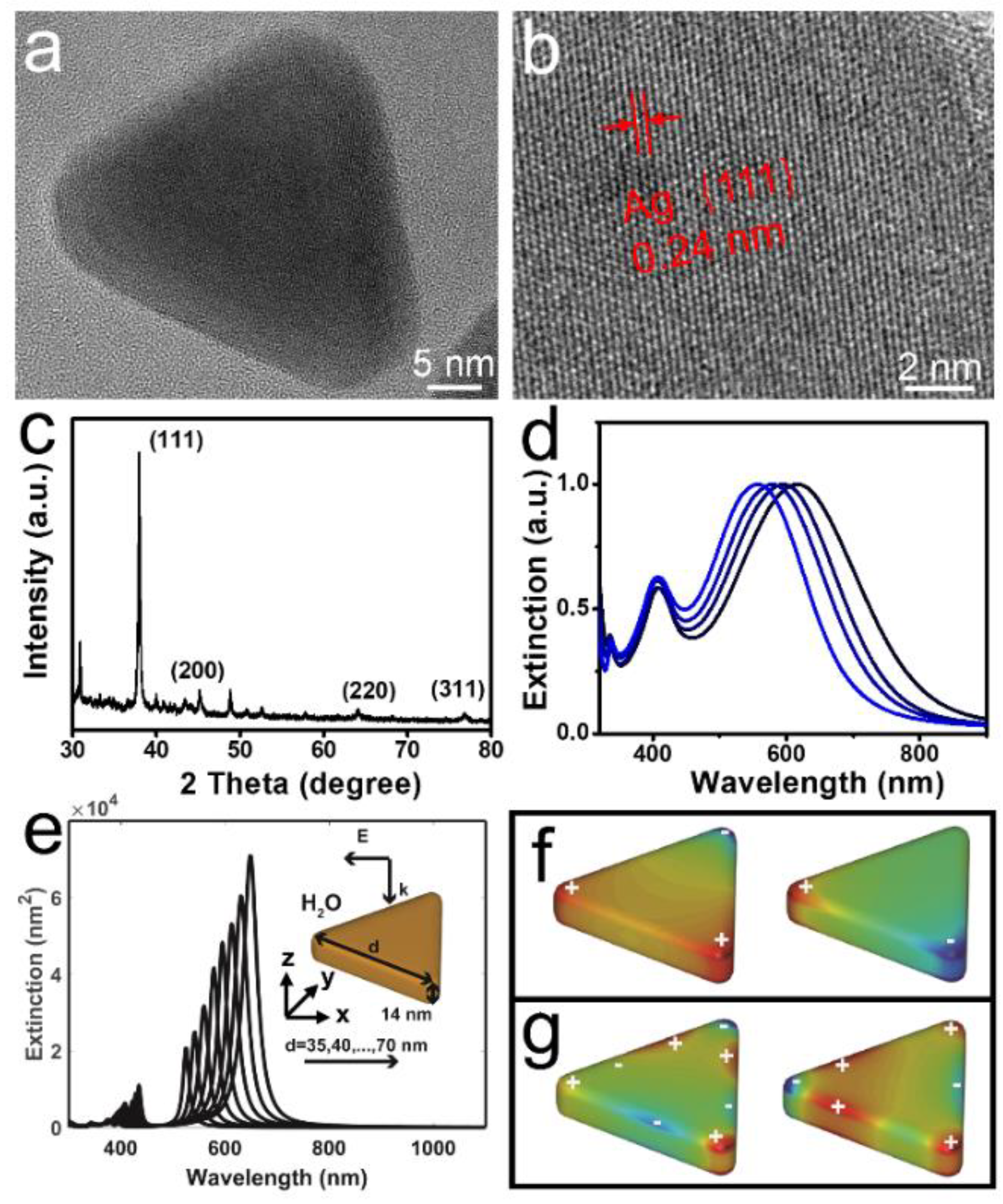
3.1. Morphological and Optical Characterization of Silver Triangle Nanoplates
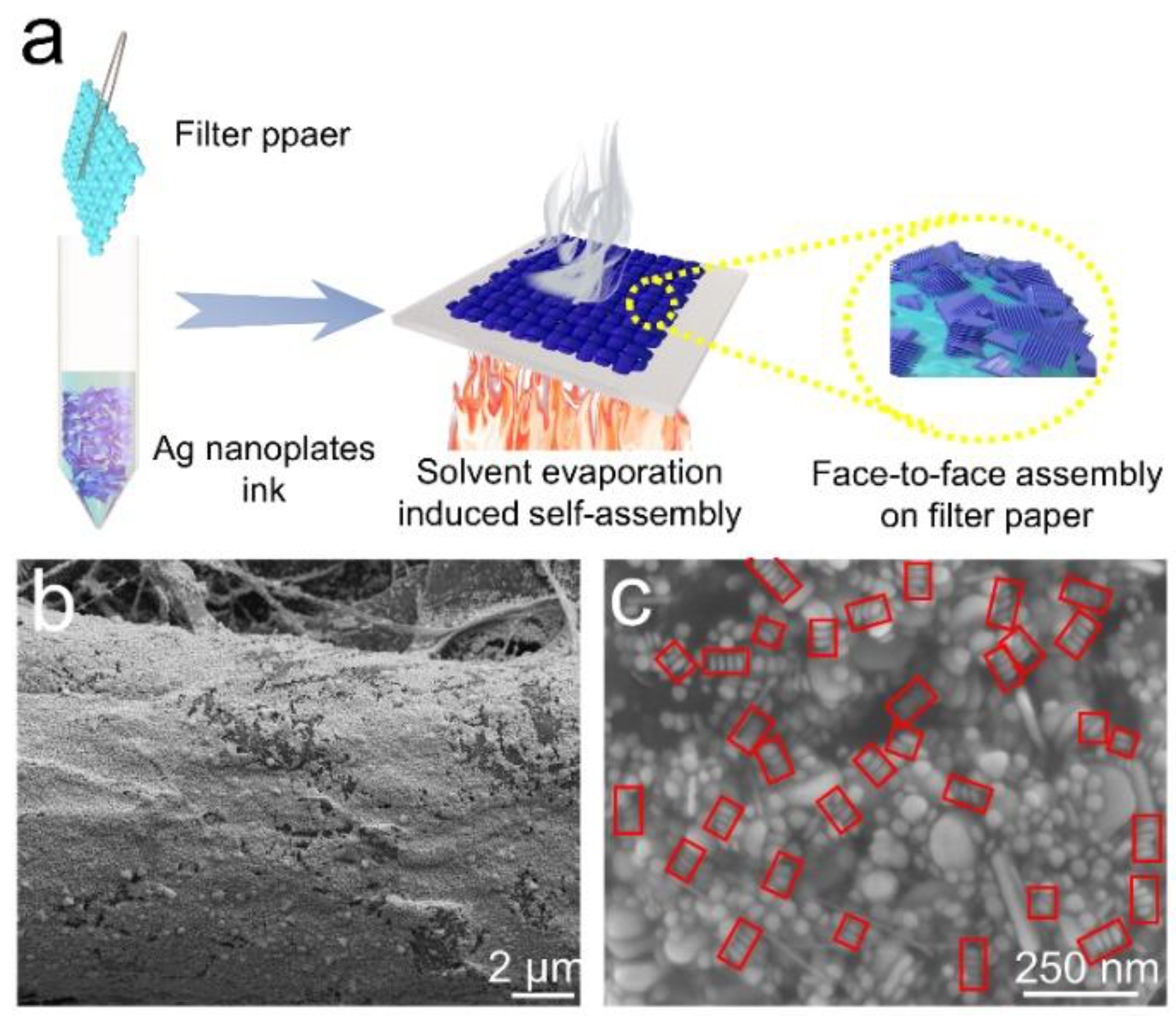
3.2. Self-Assembly of Silver Nanoplates
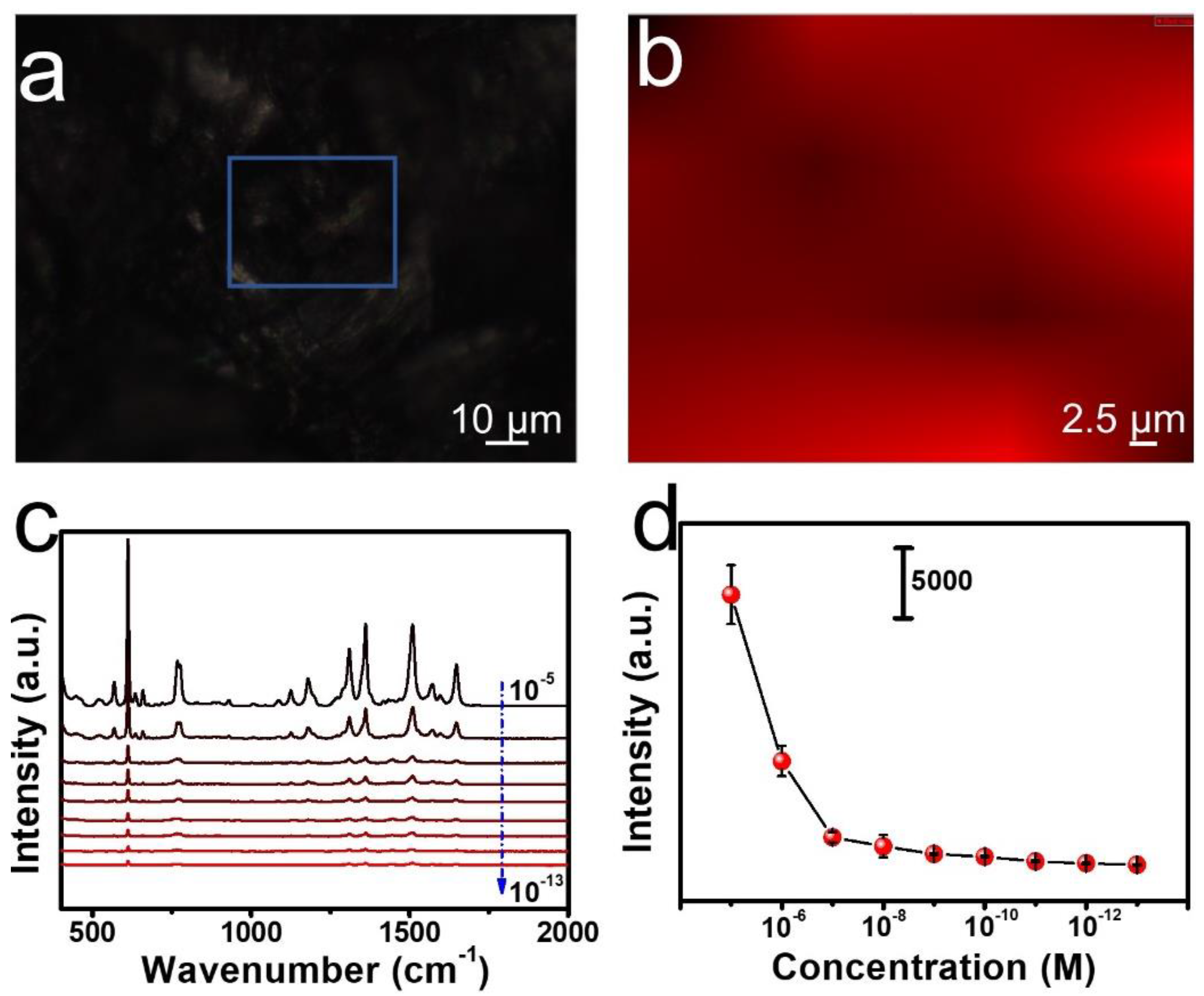
3.3. SERS Measurements of R6G
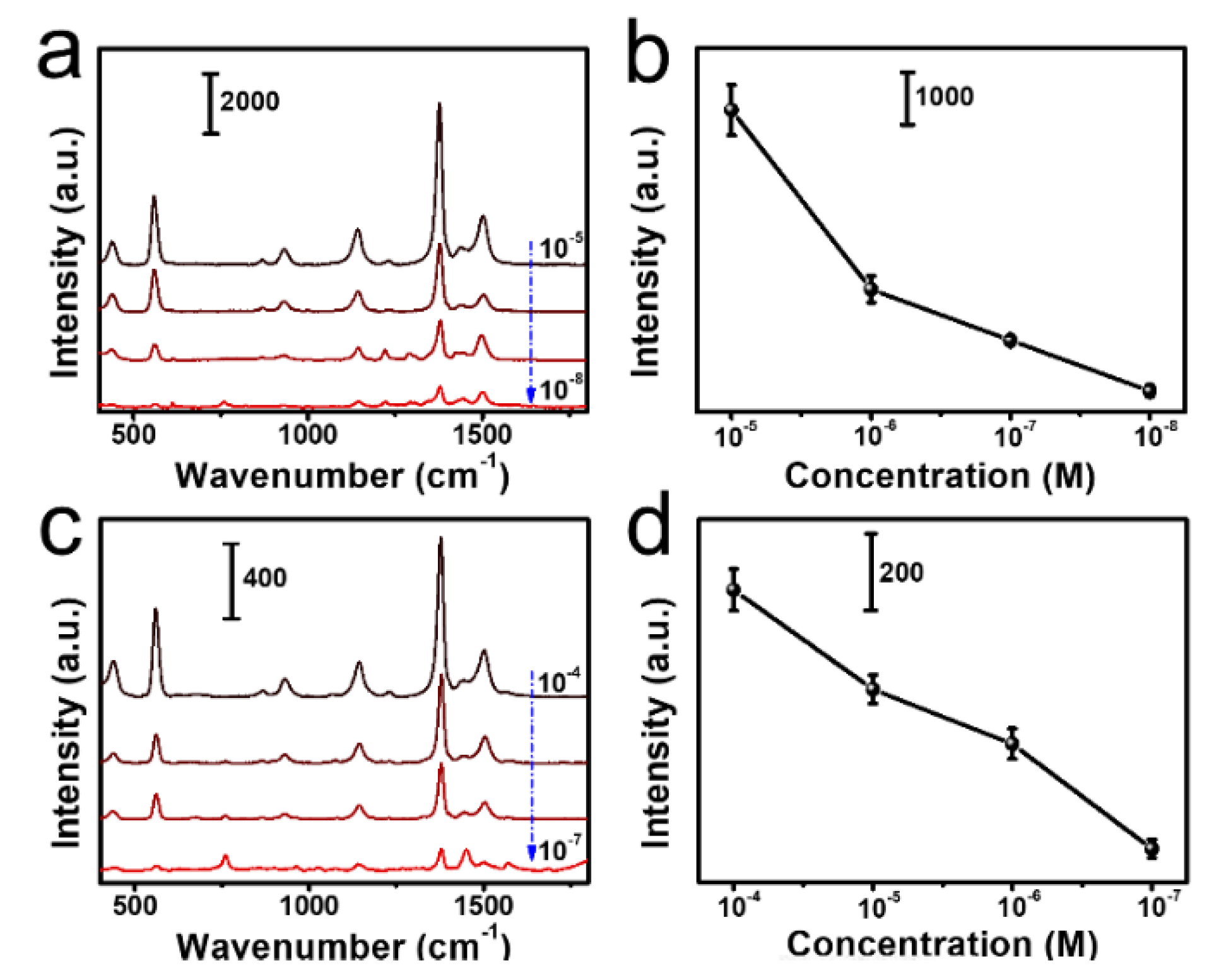
3.4. SERS Application for Thiram Detection
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Langer, J.; Aberasturi, D.J.; Aizpurua, J.; Alvarez-Puebla, R.A.; Auguie, B.; Baumberg, J.J.; Bazan, G.C.; Bell, S.E.; Boisen, A.; Brolo, A.G.; et al. Present and Future of Surface-Enhanced Raman Scattering. Acs Nano 2020, 14, 28–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.J.; Radjenovic, P.M.; Zhou, X.S.; Zhang, H.; Yao, J.L.; Li, J.F. Plasmonic Core-Shell Nanomaterials and their Applications in Spectroscopies. Adv. Mater. 2021, 33, 2005900. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.Q.; Hou, R.N.; Zhu, Y.Z.; Wang, X.T.; Zhang, H.; Zhang, Y.J.; Zhang, L.; Tian, Z.Q.; Li, J.F. Au@ZIF-8 Core-Shell Nanoparticles as a SERS Substrate for Volatile Organic Compound Gas Detection. Anal. Chem. 2021, 93, 7188–7195. [Google Scholar] [CrossRef] [PubMed]
- Murugasenapathi, N.K.; Jebakumari, K.A.E.; Mohamed, S.J.; Giribabu, K.; Palanisamy, T. Pinhole-Free Shell-Isolated Nanoparticle Enhanced Raman Spectroscopy for Interference-Free Probing of Electrochemical Reactions. J. Phys. Chem. Lett. 2021, 12, 7046–7052. [Google Scholar] [CrossRef]
- Zhang, Y.; Esteban, R.; Boto, R.A.; Urbieta, M.; Arrieta, X.; Shan, C.X.; Li, S.Z.; Baumberg, J.J.; Aizpurua, J. Addressing molecular optomechanical effects in nanocavity-enhanced Raman scattering beyond the single plasmonic mode. Nanoscale 2021, 13, 1938–1954. [Google Scholar] [CrossRef]
- Xu, J.Y.; Wang, J.; Kong, L.T.; Zheng, G.C.; Guo, Z.; Liu, J.H. SERS detection of explosive agent by macrocyclic compound functionalized triangular gold nanoprisms. J. Raman. Spectrosc. 2011, 42, 1728–1735. [Google Scholar] [CrossRef]
- Zheng, G.C.; Wang, J.; Kong, L.T.; Cheng, H.F.; Liu, J.H. Cellular-Like Gold Nanofeet: Synthesis, Functionalization, and Surface Enhanced Fluorescence Detection for Mercury Contaminations. Plasmonics 2012, 7, 487–494. [Google Scholar] [CrossRef]
- La Porta, A.; Sanchez-Iglesias, A.; Altantzis, T.; Bals, S.; Grzelczak, M.; Liz-Marzan, L.M. Multifunctional self-assembled composite colloids and their application to SERS detection. Nanoscale 2015, 7, 10377–10381. [Google Scholar] [CrossRef] [Green Version]
- Li, J.M.; Yang, Y.; Qin, D. Hollow nanocubes made of Ag-Au alloys for SERS detection with sensitivity of 10(-8) M for melamine. J. Mater. Chem. C 2014, 2, 9934–9940. [Google Scholar] [CrossRef]
- Celiksoy, S.; Ye, W.X.; Wandner, K.; Kaefer, K.; Sonnichsen, C. Intensity-Based Single Particle Plasmon Sensing. Nano Lett. 2021, 21, 2053–2058. [Google Scholar] [CrossRef]
- Zhu, W.Q.; Crozier, K.B. Quantum mechanical limit to plasmonic enhancement as observed by surface-enhanced Raman scattering. Nat. Commun. 2014, 5, 5228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esteban, R.; Aguirregabiria, G.; Borisov, A.G.; Wang, Y.M.M.; Nordlander, P.; Bryant, G.W.; Aizpurua, J. The Morphology of Narrow Gaps Modifies the Plasmonic Response. Acs Photonics 2015, 2, 295–305. [Google Scholar] [CrossRef] [Green Version]
- Baumberg, J.J.; Aizpurua, J.; Mikkelsen, M.H.; Smith, D.R. Extreme nanophotonics from ultrathin metallic gaps. Nat. Mater. 2019, 18, 668–678. [Google Scholar] [CrossRef] [PubMed]
- Li, D.D.; Wang, J.; Zheng, G.C.; Liu, J.H.; Xu, W.H. A highly active SERS sensing substrate: Core-satellite assembly of gold nanorods/nanoplates. Nanotechnology 2013, 24, 235502. [Google Scholar] [CrossRef]
- Serrano-Montes, A.B.; Aberasturi, D.J.; Langer, J.; Giner-Casares, J.J.; Scarabelli, L.; Herrero, A.; Liz-Marzan, L.M. A General Method for Solvent Exchange of Plasmonic Nanoparticles and Self-Assembly into SERS-Active Monolayers. Langmuir 2015, 31, 9205–9213. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.B.; Yang, D.T.; Ivleva, N.P.; Mircescu, N.E.; Niessner, R.; Haisch, C. SERS Detection of Bacteria in Water by in Situ Coating with Ag Nanoparticles. Anal. Chem. 2014, 86, 1525–1533. [Google Scholar] [CrossRef]
- Wang, X.; Li, M.H.; Meng, L.Y.; Lin, K.Q.; Feng, J.M.; Huang, T.X.; Yang, Z.L.; Ren, B. Probing the Location of Hot Spots by Surface-Enhanced Raman Spectroscopy: Toward Uniform Substrates. Acs Nano 2014, 8, 528–536. [Google Scholar] [CrossRef]
- Kuttner, C.; Holler, R.P.M.; Quintanilla, M.; Schnepf, M.J.; Dulle, M.; Fery, A.; Liz-Marzan, L.M. SERS and plasmonic heating efficiency from anisotropic core/satellite superstructures. Nanoscale 2019, 11, 17655–17663. [Google Scholar] [CrossRef] [Green Version]
- Orendorff, C.J.; Gole, A.; Sau, T.K.; Murphy, C.J. Surface-enhanced Raman spectroscopy of self-assembled monolayers: Sandwich architecture and nanoparticle shape dependence. Anal. Chem. 2005, 77, 3261–3266. [Google Scholar] [CrossRef]
- Gong, J.X.; Li, G.D.; Tang, Z.Y. Self-assembly of noble metal nanocrystals: Fabrication, optical property, and application. Nano Today 2012, 7, 564–585. [Google Scholar] [CrossRef]
- Walker, D.A.; Leitsch, E.K.; Nap, R.J.; Szleifer, I.; Grzybowski, B.A. Geometric curvature controls the chemical patchiness and self-assembly of nanoparticles. Nat. Nanotechnol. 2013, 8, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Hamon, C.; Liz-Marzan, L.M. Colloidal design of plasmonic sensors based on surface enhanced Raman scattering. J. Colloid Interf. Sci. 2018, 512, 834–843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polavarapu, L.; La Porta, A.; Novikov, S.M.; Coronado-Puchau, M.; Liz-Marzan, L.M. Pen-on-Paper Approach Toward the Design of Universal Surface Enhanced Raman Scattering Substrates. Small 2014, 10, 3065–3071. [Google Scholar] [CrossRef] [PubMed]
- Biliuta, G.; Coseri, S. Cellulose: A ubiquitous platform for ecofriendly metal nanoparticles preparation. Coordin. Chem. Rev. 2019, 383, 155–173. [Google Scholar] [CrossRef]
- Das, D.; Senapati, S.; Nanda, K.K. “Rinse, Repeat”: An Efficient and Reusable SERS and Catalytic Platform Fabricated by Controlled Deposition of Silver Nanoparticles on Cellulose Paper. Acs Sustain. Chem. Eng. 2019, 7, 14089–14101. [Google Scholar] [CrossRef] [Green Version]
- Parolo, C.; Merkoci, A. Paper-based nanobiosensors for diagnostics. Chem. Soc. Rev. 2013, 42, 450–457. [Google Scholar] [CrossRef]
- Romo-Herrera, J.M.; Juarez-Moreno, K.; Guerrini, L.; Kang, Y.; Feliu, N.; Parak, W.J.; Alvarez-Puebla, R.A. Paper-based plasmonic substrates as surface-enhanced Raman scattering spectroscopy platforms for cell culture applications. Mater. Today Bio. 2021, 11, 100125. [Google Scholar] [CrossRef]
- Bharati, M.S.S.; Soma, V.R. Flexible SERS substrates for hazardous materials detection: Recent advances. Opto-Electron. Adv. 2021, 4, 210048. [Google Scholar] [CrossRef]
- Wang, K.; Huang, M.; Chen, J.; Lin, L.; Kong, L.; Liu, X.; Wang, H.; Lin, M. A “drop-wipe-test” SERS method for rapid detection of pesticide residues in fruits. J. Raman. Spectrosc. 2018, 49, 493–498. [Google Scholar] [CrossRef]
- Xiong, Z.; Lin, M.; Lin, H.; Huang, M. Facile synthesis of cellulose nanofiber nanocomposite as a SERS substrate for detection of thiram in juice. Carbohydr. Polym. 2018, 189, 79–86. [Google Scholar] [CrossRef]
- Sun, M.; Li, B.; Liu, X.; Chen, J.; Mu, T.; Zhu, L.; Guo, J.; Ma, X. Performance enhancement of paper-based SERS chips by shell-isolated nanoparticle-enhanced Raman spectroscopy. J. Mater. Sci. Technol. 2019, 35, 2207–2212. [Google Scholar] [CrossRef]
- Zheng, G.C.; Polavarapu, L.; Liz-Marzan, L.M.; Pastoriza-Santos, I.; Perez-Juste, J. Gold nanoparticle-loaded filter paper: A recyclable dip-catalyst for real-time reaction monitoring by surface enhanced Raman scattering. Chem. Commun. 2015, 51, 4572–4575. [Google Scholar] [CrossRef]
- Zheng, G.C.; Kaefer, K.; Mourdikoudis, S.; Polavarapu, L.; Vaz, B.; Cartmell, S.E.; Bouleghlimat, A.; Buurma, N.J.; Yate, L.; Lera, A.R.; et al. Palladium Nanoparticle-Loaded Cellulose Paper: A Highly Efficient, Robust, and Recyclable Self-Assembled Composite Catalytic System. J. Phys. Chem. Lett. 2015, 6, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Chen, S.; Jiang, Z.; Shi, Z.; Wang, J.; Du, L. Highly Sensitive and Reproducible SERS Substrates Based on Ordered Micropyramid Array and Silver Nanoparticles. ACS Appl. Mater. Interfaces 2021, 13, 29222–29229. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Zhao, Q.; Wang, X.; Li, Z.; Hu, X. Ag-nanocubes/graphene-oxide/Au-nanoparticles composite film with highly dense plasmonic hotspots for surface-enhanced Raman scattering detection of pesticide. Microchem. J. 2021, 165, 106090. [Google Scholar] [CrossRef]
- Hasi, W.L.J.; Lin, X.; Lou, X.T.; Lin, S.; Yang, F.; Lin, D.Y.; Lu, Z.W. Chloride ion-assisted self-assembly of silver nanoparticles on filter paper as SERS substrate. Appl. Phys. A 2014, 118, 799–807. [Google Scholar] [CrossRef]
- Hanske, C.; Hill, E.H.; Vila-Liarte, D.; Gonzalez-Rubio, G.; Matricardi, C.; Mihi, A.; Liz-Marzan, L.M. Solvent-Assisted Self-Assembly of Gold Nanorods into Hierarchically Organized Plasmonic Mesostructures. Acs Appl. Mater. Inter. 2019, 11, 11763–11771. [Google Scholar] [CrossRef] [Green Version]
- Kong, L.L.; Huang, M.Z.; Chen, J.; Lin, M.S. Fabrication of Sensitive Silver-Decorated Cotton Swabs for SERS Quantitative Detection of Mixed Pesticide Residues in Bitter Gourds. New J. Chem. 2020, 44, 12779–12784. [Google Scholar] [CrossRef]
- Hegde, H.; Santhosh, C.; Sinha, R.K. Seed mediated synthesis of highly stable CTAB capped triangular silver nanoplates for LSPR sensing. Mater. Res. Express. 2019, 6, 105075. [Google Scholar] [CrossRef]
- Abajo, F.J.G.; Howie, A. Retarded field calculation of electron energy loss in inhomogeneous dielectrics. Phys. Rev. B 2002, 65, 115418. [Google Scholar] [CrossRef]
- Abajo, F.J.G. Optical excitations in electron microscopy. Rev. Mod. Phys. 2010, 82, 209–275. [Google Scholar] [CrossRef]
- Hohenester, U.; Trugler, A. MNPBEM-A Matlab toolbox for the simulation of plasmonic nanoparticles. Comput. Phys. Commun. 2012, 183, 370–381. [Google Scholar] [CrossRef] [Green Version]
- Hamon, C.; Novikov, S.; Scarabelli, L.; Basabe-Desmonts, L.; Liz-Marzan, L.M. Hierarchical Self-Assembly of Gold Nanoparticles into Patterned Plasmonic Nanostructures. Acs Nano 2014, 8, 10694–10703. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Lojo, D.; Nunez-Sanchez, S.; Gomez-Grana, S.; Grzelczak, M.; Pastoriza-Santos, I.; Perez-Juste, J.; Liz-Marzan, L.M. Plasmonic Supercrystals. Accounts. Chem. Res. 2019, 52, 1855–1864. [Google Scholar] [CrossRef]
- Rycenga, M.; McLellan, J.M.; Xia, Y. Controlling the Assembly of Silver Nanocubes through Selective Functionalization of Their Faces. Adv. Mater. 2008, 20, 2416–2420. [Google Scholar] [CrossRef]
- Xue, C.; Li, Z.; Mirkin, C.A. Large-scale assembly of single-crystal silver nanoprism monolayers. Small 2005, 1, 513–516. [Google Scholar] [CrossRef]
- Watanabe, H.; Hayazawa, N.; Inouye, Y.; Kawata, S. DFT vibrational calculations of Rhodamine 6G adsorbed on silver: Analysis of tip-enhanced Raman spectroscopy. J. Phys. Chem. B 2005, 109, 5012–5020. [Google Scholar] [CrossRef]
- Ge, K.; Yi, L.; Wu, Q.; Li, Y.; Zhang, H.; Gu, Y. Detection of Formaldehyde by Surface-Enhanced Raman Spectroscopy Based on PbBiO2Br/Au4Ag4 Nanospheres. ACS Appl. Nano Mater. 2021, 4, 10218–10227. [Google Scholar] [CrossRef]
- Verma, A.K.; Soni, R.K. Silver nanodendrites for ultralow detection of thiram based on surface-enhanced Raman spectroscopy. Nanotechnology 2019, 30, 385502. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, L.H.; Ji, W.J.; Song, W.; Zhao, S.Q. Cysteamine-Assisted Highly Sensitive Detection of Bisphenol A in Water Samples by Surface-Enhanced Raman Spectroscopy with Ag Nanoparticle-Modified Filter Paper as Substrate. Food Anal. Methods 2017, 10, 1940–1947. [Google Scholar] [CrossRef]
- Moram, S.S.B.; Byram, C.; Shibu, S.N.; Chiukamarri, B.M.; Soma, V.R. Ag/Au Nanoparticle-Loaded Paper-Based Versatile Surface-Enhanced Raman Spectroscopy Substrates for Multiple Explosives Detection. ACS Omega 2018, 3, 8190–8201. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.J.; Chen, Q.S.; Kutsanedzie, F.Y.H.; Yang, M.X.; Qin, O.Y.; Jiang, H. Highly sensitive and label-free determination of thiram residue using surface-enhanced Raman spectroscopy (SERS) coupled with paper-based microfluidics. Anal. Methods 2017, 9, 6186. [Google Scholar] [CrossRef]
- He, S.; Chua, J.; Tan, E.K.M.; Kah, J.C.Y. Optimizing the SERS enhancement of a facile gold nanostar immobilized paper-based SERS substrate. RSC Adv. 2017, 7, 16264. [Google Scholar] [CrossRef] [Green Version]
- Pagano, R.; Ottolini, M.; Valli, L.; Bettini, S.; Giancane, G. Ag nanodisks decorated filter paper as a SERS platform for nanomolar tetracycline detection. Colloids Surf. A 2021, 624, 126787. [Google Scholar] [CrossRef]
- Tegegnea, W.A.; Sua, W.N.; Tsai, M.C.; Beyenea, A.B.; Hwang, B.J. Ag nanocubes decorated 1T-MoS2 nanosheets SERS substrate for reliable and ultrasensitive detection of pesticides. Appli. Mater. Today 2020, 21, 100871. [Google Scholar] [CrossRef]
- Zhu, C.H.; Wang, X.J.; Shi, X.F.; Yang, F.; Meng, G.W.; Xiong, Q.Z.; Ke, Y.; Wang, H.; Lu, Y.L.; Wu, N.Q. Detection of Dithiocarbamate Pesticides with a Spongelike Surface-Enhanced Raman Scattering Substrate Made of Reduced Graphene Oxide-Wrapped Silver Nanocubes. ACS Appl. Mater. Interfaces 2017, 9, 39618–39625. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.T.; Shang, S.M.; Jiang, S.X. A stable, ultrasensitive and flexible substrate integrated from 1D Ag/α-Fe2O3/SiO2 fibers for practical surface-enhanced Raman scattering detection. Compos. Part B 2019, 177, 107376. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiao, S.; Liu, Y.; Wang, S.; Wang, S.; Ma, F.; Yuan, H.; Zhou, H.; Zheng, G.; Zhang, Y.; Dai, K.; et al. Face-to-Face Assembly of Ag Nanoplates on Filter Papers for Pesticide Detection by Surface-Enhanced Raman Spectroscopy. Nanomaterials 2022, 12, 1398. https://doi.org/10.3390/nano12091398
Jiao S, Liu Y, Wang S, Wang S, Ma F, Yuan H, Zhou H, Zheng G, Zhang Y, Dai K, et al. Face-to-Face Assembly of Ag Nanoplates on Filter Papers for Pesticide Detection by Surface-Enhanced Raman Spectroscopy. Nanomaterials. 2022; 12(9):1398. https://doi.org/10.3390/nano12091398
Chicago/Turabian StyleJiao, Sulin, Yixin Liu, Shenli Wang, Shuo Wang, Fengying Ma, Huiyu Yuan, Haibo Zhou, Guangchao Zheng, Yuan Zhang, Kun Dai, and et al. 2022. "Face-to-Face Assembly of Ag Nanoplates on Filter Papers for Pesticide Detection by Surface-Enhanced Raman Spectroscopy" Nanomaterials 12, no. 9: 1398. https://doi.org/10.3390/nano12091398
APA StyleJiao, S., Liu, Y., Wang, S., Wang, S., Ma, F., Yuan, H., Zhou, H., Zheng, G., Zhang, Y., Dai, K., & Liu, C. (2022). Face-to-Face Assembly of Ag Nanoplates on Filter Papers for Pesticide Detection by Surface-Enhanced Raman Spectroscopy. Nanomaterials, 12(9), 1398. https://doi.org/10.3390/nano12091398







