Interaction of Mg Alloy with PLA Electrospun Nanofibers Coating in Understanding Changes of Corrosion, Wettability, and pH
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Equipment
2.3. Procedures
2.3.1. Preparation of AZ31 Biodegradable Alloy
2.3.2. Preparation of Simulated Body Fluid Solution (SBF)
2.3.3. Deposition of PLA Nanofibers on AZ31 Alloy
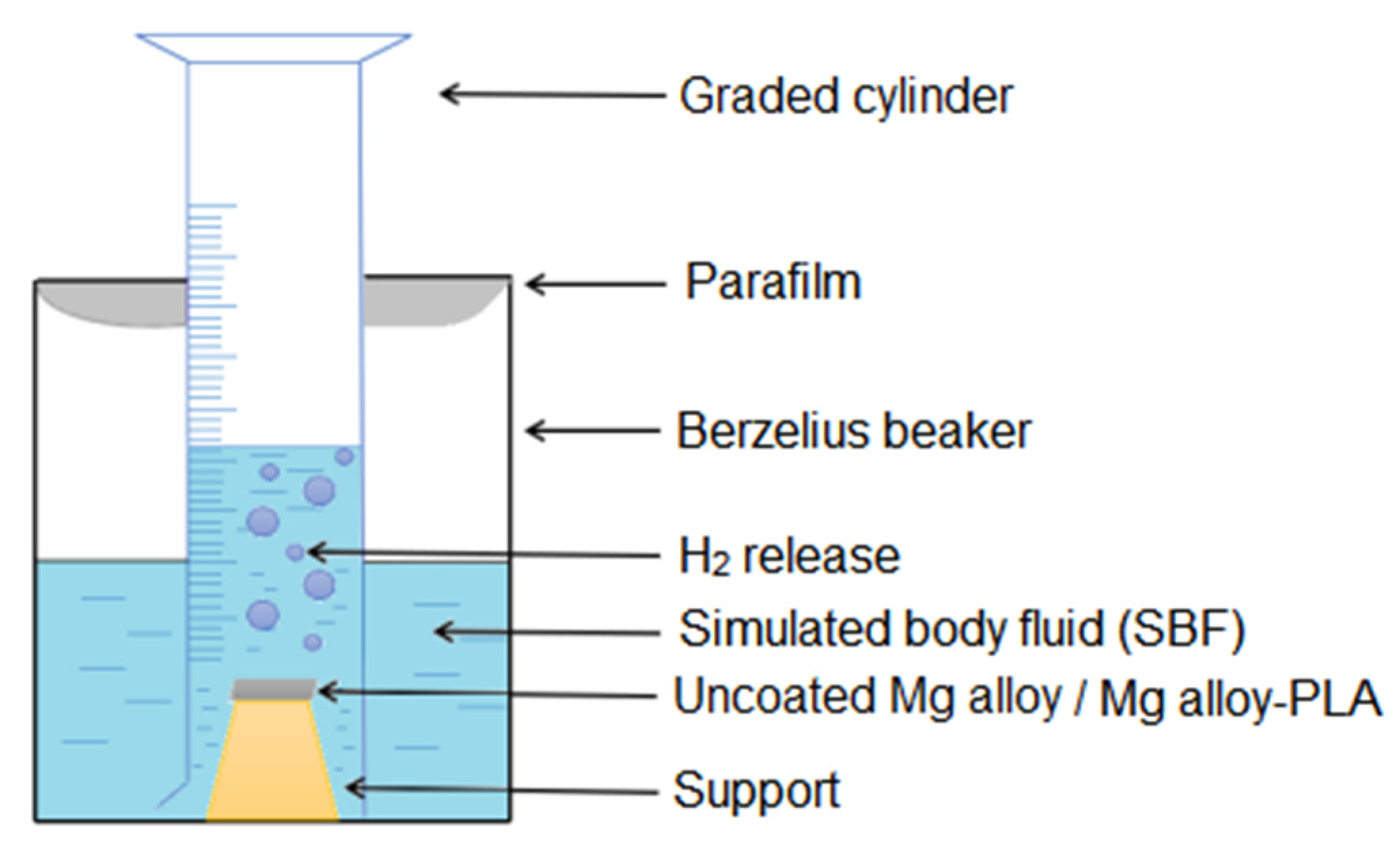
2.3.4. Hydrogen Evolution
3. Results and Discussion
3.1. Polymer Coating Characterization
3.1.1. FT-IR Analysis of Coating
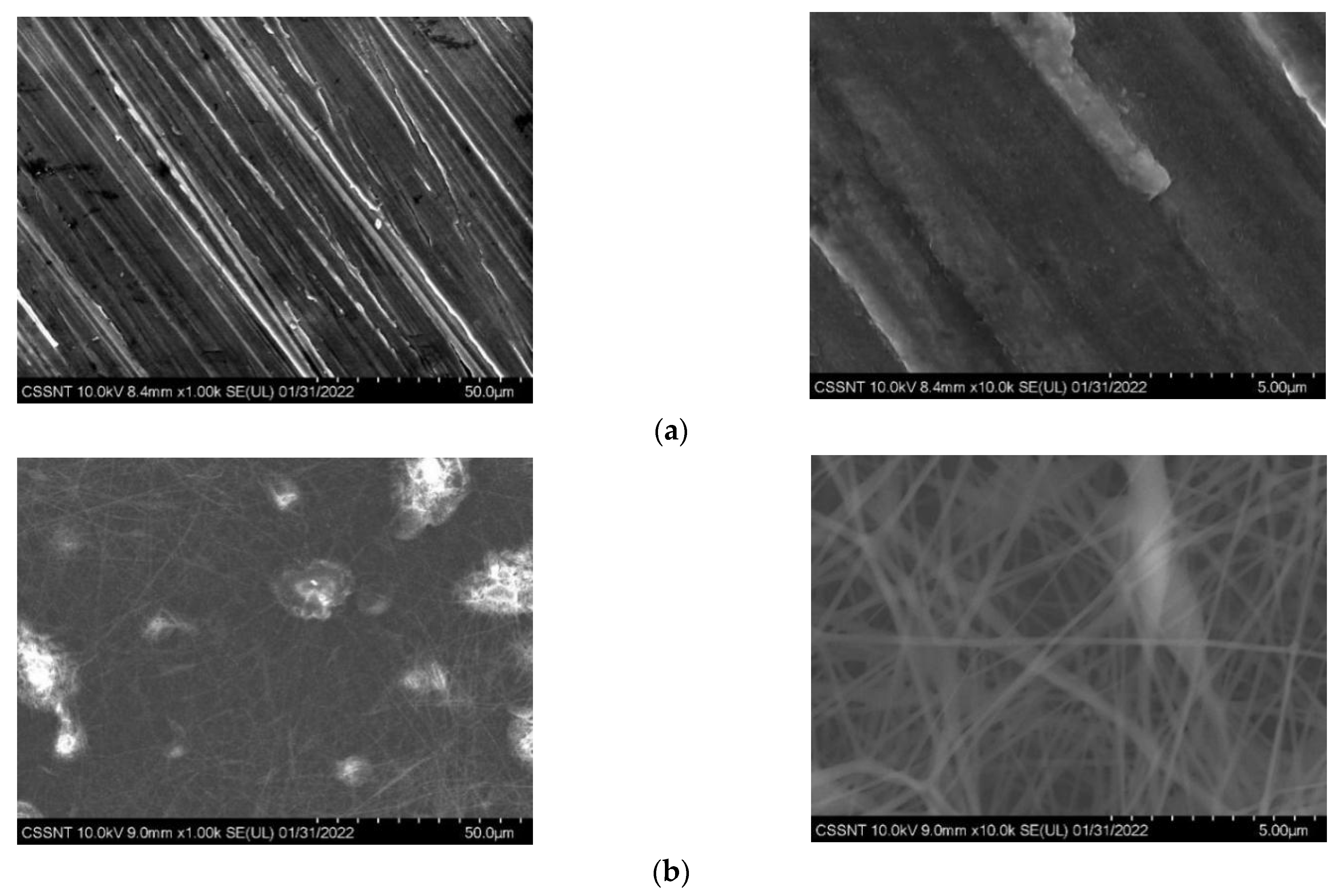
3.1.2. Morphology and Elemental Composition
3.1.3. Hydrophilicity and Hydrophobicity of Mg Alloy
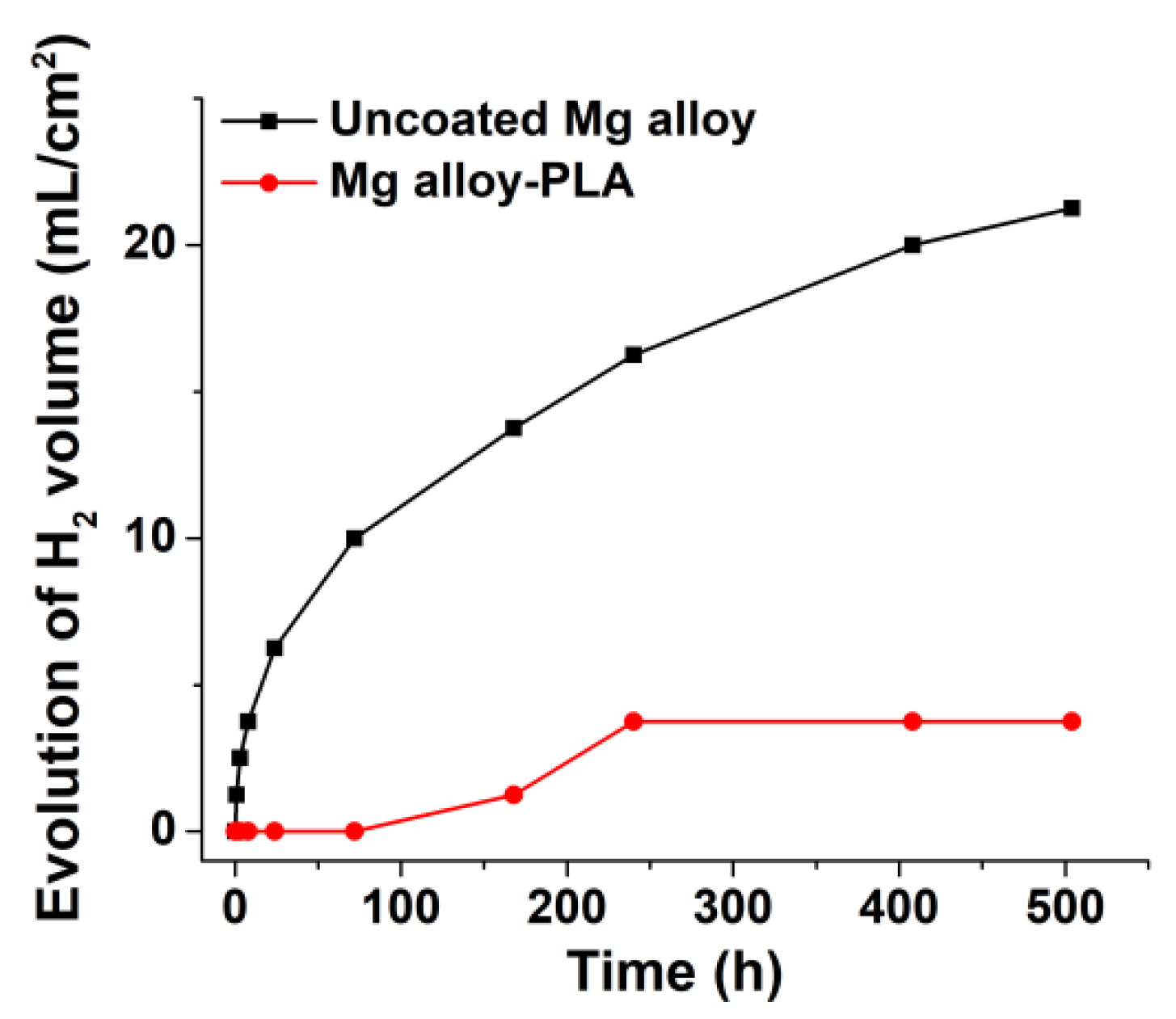
3.1.4. Hydrogen Release in SBF
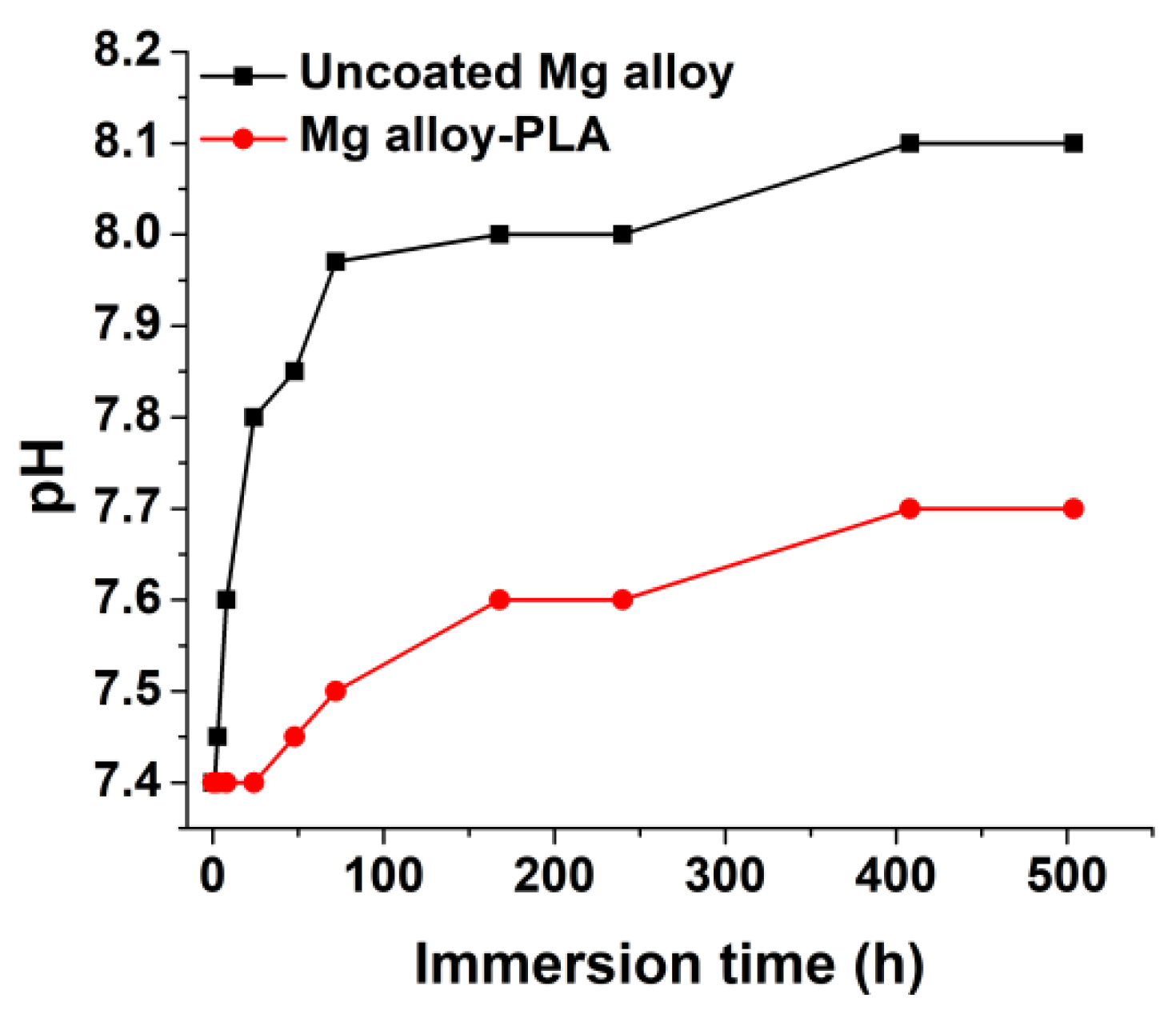
3.1.5. pH Variation
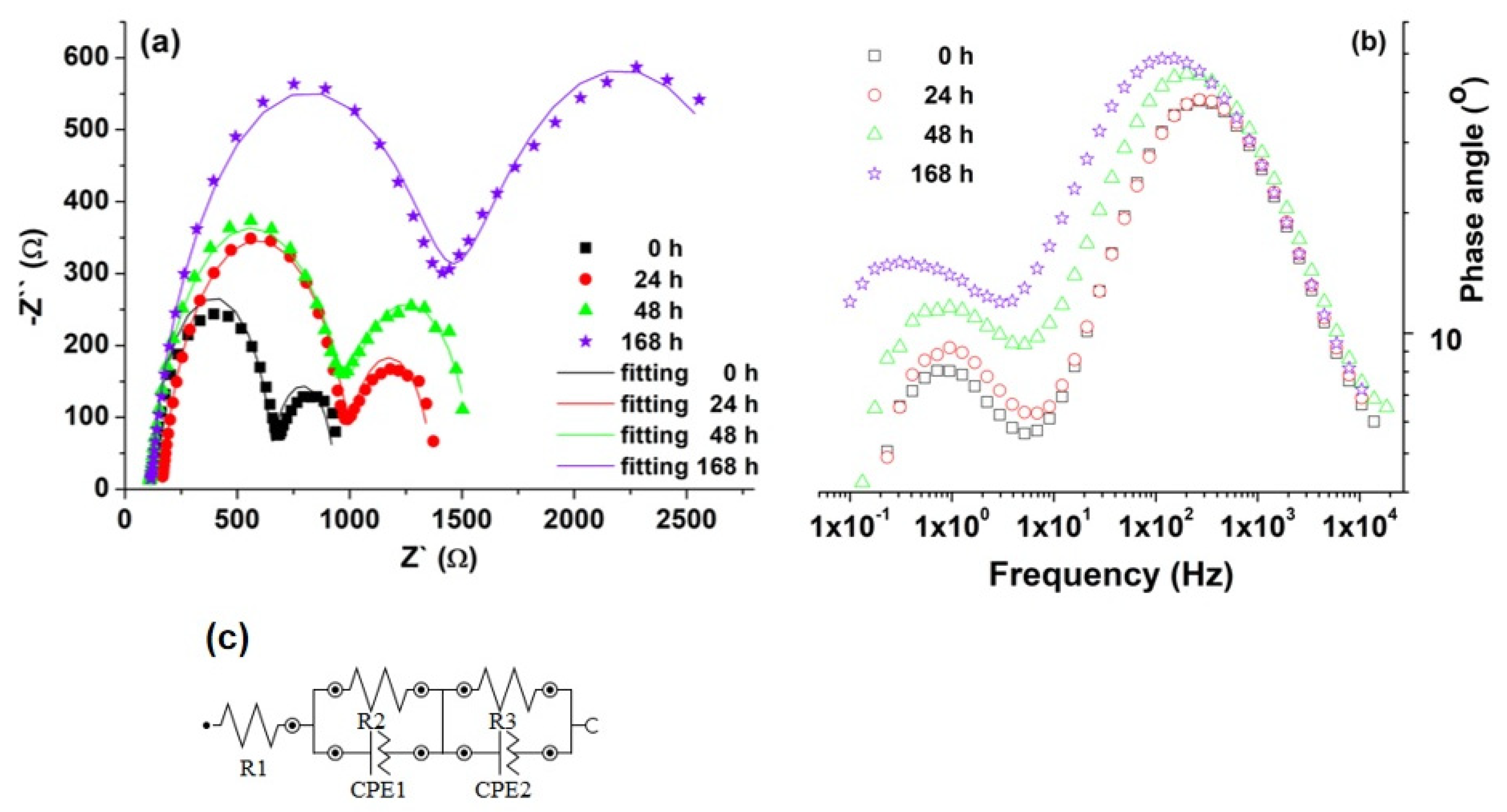
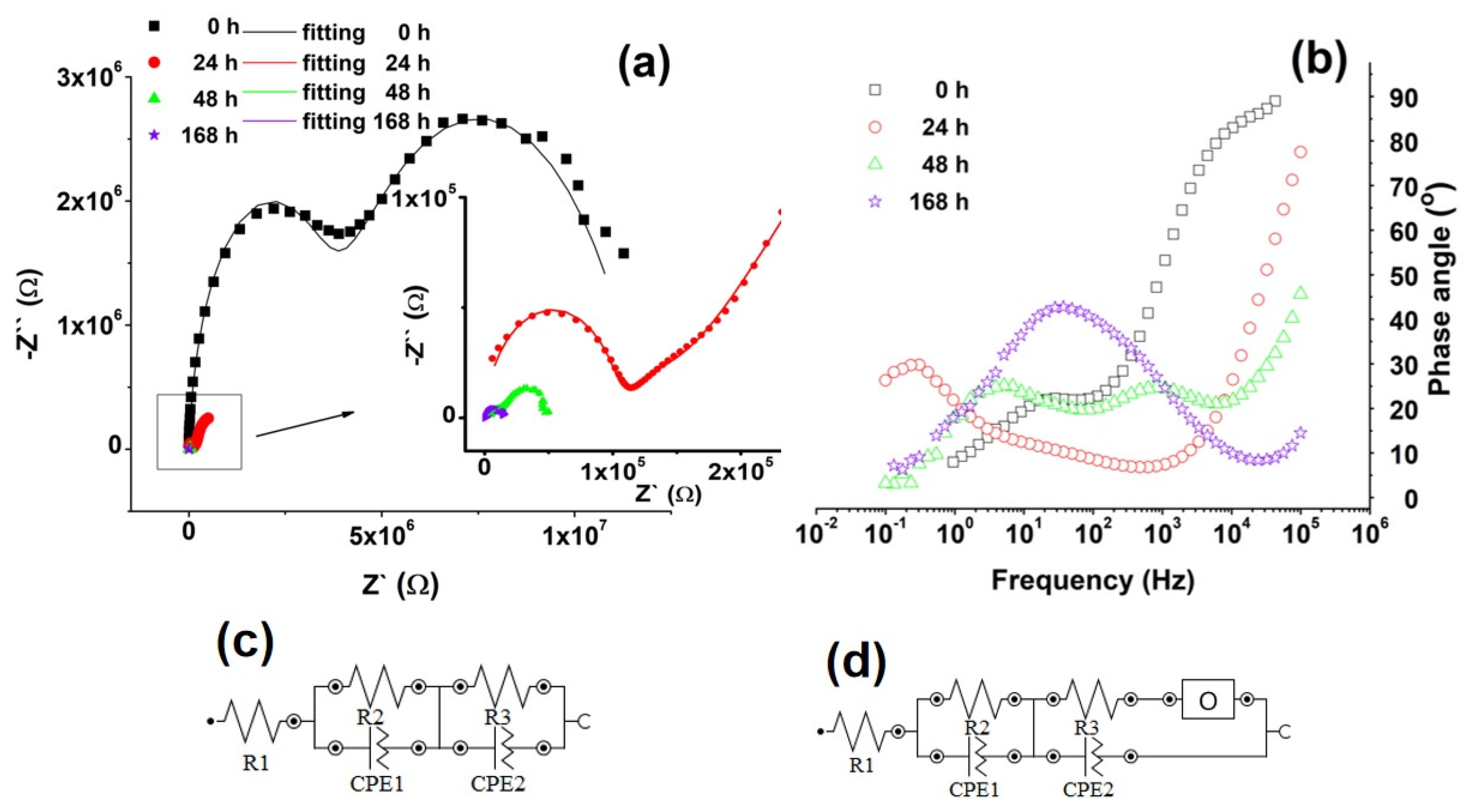
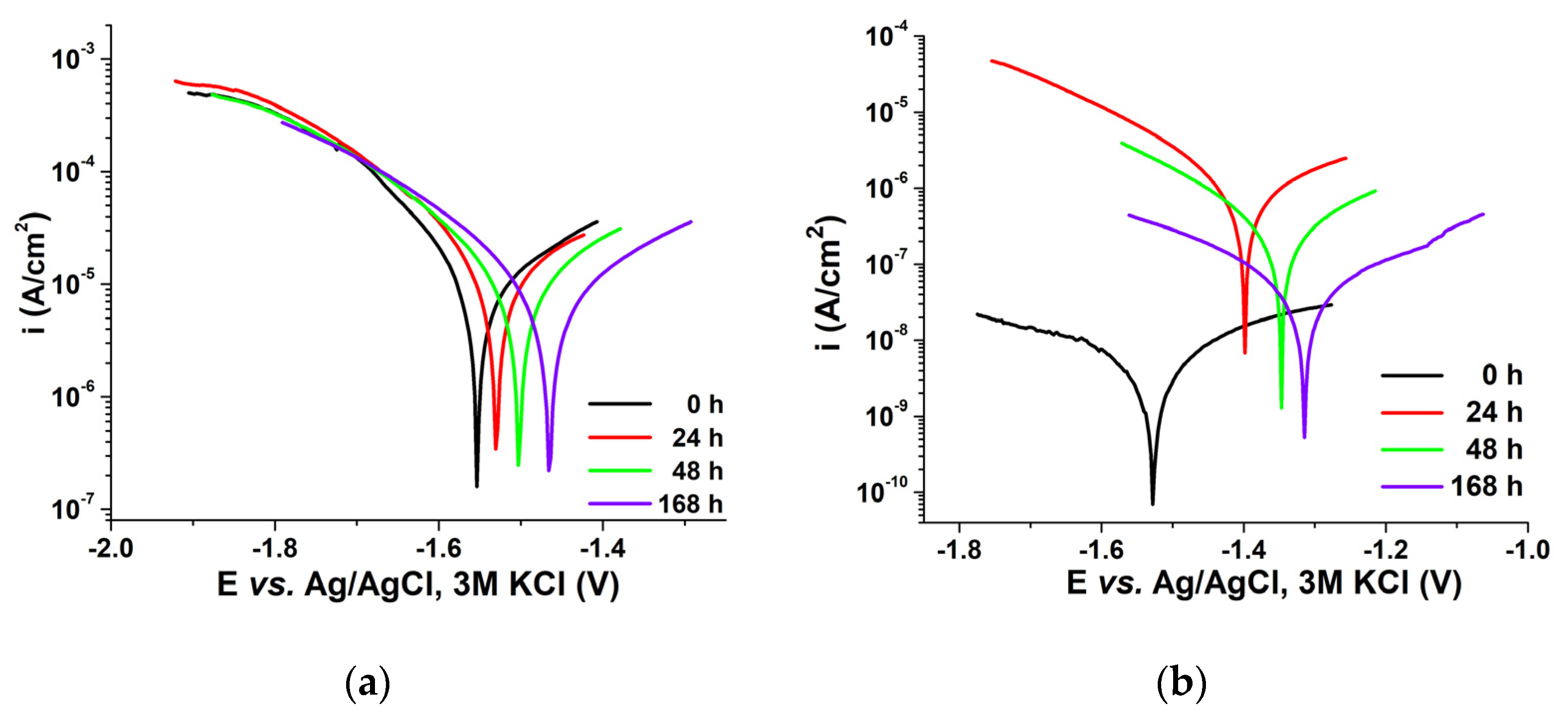
3.1.6. Electrochemical Impedance Spectroscopy (EIS) and Potentiodynamic Voltammetry (Tafel Curves)
- (a)
- Uncoated sample immersion in SBF: the presence of natural Mg oxides formed in the atmosphere.
- (b)
- Mg(OH)2 and H2 formation.
- (c)
- H2 destroyed MgO and Mg(OH)2 films; deposit of salts from SBF.
- (d)
- MgO, Mg(OH)2, and salts covered the Mg surface; due to H2 release the film had cracks.
- (a’)
- Sample coated with PLA nanofibers in SBF: the presence of natural Mg oxides formed in atmosphere under PLA; SBF diffusion through nanofibers.
- (b’)
- Mg(OH)2 and H2 formation.
- (c’)
- PLA nanofibers degradation; (RCOO)2 Mg formation; H2 broke the polymeric film.
- (d’)
- Deposition of salts from SBF will occupy free spaces without PLA.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prasad, K.; Bazaka, O.; Chua, M.; Rochford, M.; Fedrick, L.; Spoor, J.; Symes, R.; Tieppo, M.; Collins, C.; Cao, A.; et al. Metallic Biomaterials: Current Challenges and Opportunities. Materials 2017, 10, 884. [Google Scholar] [CrossRef] [PubMed]
- Ionita, D.; Man, I.; Demetrescu, I. The behaviour of electrochemical deposition ofphosphate coating on CoCr bio alloys. Key Eng. Mater. 2007, 330, 545–548. [Google Scholar] [CrossRef]
- Popa, M.V.; Demetrescu, I.; Vasilescu, E.; Drob, P.; Ionita, D. Stability of some dentalimplant materials in oral biofluids. Rev. Roum. Chim. 2005, 50, 399–406. [Google Scholar]
- Ungureanu, C.; Pirvu, C.; Mindroiu, M.; Demetrescu, I. Antibacterial polymeric coating based on polypyrrole and polyethylene glycol on a new alloy TiAlZr Progress in Organic. Coatings 2012, 75, 349–355. [Google Scholar] [CrossRef]
- Zhang, E.L.; Liu, C. A new antibacterial Co-Cr-Mo-Cu alloy: Preparation, biocorrosion, mechanical and antibacterial property. Mater. Sci. Eng. C 2016, 69, 134–143. [Google Scholar] [CrossRef]
- Zheng, Y.F.; Gu, X.N.; Witte, F. Biodegradable metals. Mater. Sci. Eng. R 2014, 77, 1–34. [Google Scholar] [CrossRef]
- Kabir, H.; Munir, K.; Wen, C.; Li, Y. Recent research and progress of biodegradable zinc alloys and composites for biomedical applications: Biomechanical and biocorrosion perspectives. Bioact. Mater. 2021, 6, 836–879. [Google Scholar] [CrossRef]
- Huafang, L.; Zhenga, Y.; Qin, L. Progress of biodegradable metals. Nat. Sci. Mater. Int. 2014, 24, 414–422. [Google Scholar]
- Yang, J.; Koons, G.L.; Cheng, G.; Zhao, L.; Mikos, A.G.; Cui, F. A review on the exploitation of biodegradable magnesium-based composites for medical applications. Biomed. Mater. 2012, 13, 022001. [Google Scholar] [CrossRef]
- Li, H.; Yang, H.; Zheng, Y.; Zhou, F.; Qiu, K.; Wang, X. Design and characterizations of novel biodegradable ternary Zn-based alloys with IIA nutrient alloying elements Mg, Ca and Sr. Mater. Des. 2015, 83, 95–102. [Google Scholar] [CrossRef]
- Rai, P.; Rai, A.; Kumar, V.; Chaturvedi, R.K.; Singh, V.K. Corrosion study of biodegradable magnesium based 1393 bioactive glass in simulated body fluid. Ceram. Int. 2019, 45, 16893–16903. [Google Scholar] [CrossRef]
- Chen, Y.; Song, Y.; Zhang, S.; Li, J.; Zhao, C.; Zhang, Z. Interaction between a high purity magnesium surface and PCL and PLA coatings during dynamic degradation. Biomed. Mater. 2011, 6, 025005. [Google Scholar] [CrossRef]
- Saberi, A.; Bakhsheshi-Rad, H.R.; Abazari, S.; Ismail, A.F.; Sharif, S.; Ramakrishna, S.; Daroonparvar, M.; Berto, F.A. Comprehensive Review on Surface Modifications of Biodegradable Magnesium-Based Implant Alloy: Polymer Coatings Opportunities and Challenges. Coatings 2021, 11, 747. [Google Scholar] [CrossRef]
- Agarwal, S.; Curtin, J.; Duffy, B.; Jaiswal, S. Biodegradable magnesium alloys for orthopaedic applications: A review on corrosion, biocompatibility and surface modifications. Mater. Sci. Eng. C 2016, 68, 948–963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adel-Gawad, S.; Shoeib, M.A. Corrosion studies and microstructure of Mg−Zn−Ca alloys for biomedical applications. Surf. Interfaces 2019, 14, 108–116. [Google Scholar] [CrossRef]
- Muñoz, M.; Torres, B.; Mohedano, M.; Matykin, E.; Arrabal, R.; López, A.J.; Rams, J. PLA deposition on surface treated magnesium alloy: Adhesion, toughness and corrosion behaviour. Surf. Coat. Technol. 2020, 388, 125593. [Google Scholar] [CrossRef]
- Sezer, N.; Evis, Z.; Kayhan, S.M.; Tahmasebifar, A.; Koç, M. Review of magnesium-based biomaterials and their applications. J. Magnes. Alloys 2018, 6, 23–43. [Google Scholar] [CrossRef]
- Zhou, Y.L.; Li, Y.; Luo, D.M.; Ding, Y.; Hodgson, P. Microstructures, mechanical and corrosion properties and biocompatibility of as extruded Mg–Mn–Zn–Nd alloys for biomedical applications. Mater. Sci. Eng. C 2015, 49, 93–100. [Google Scholar] [CrossRef]
- Zhang, D.; Peng, F.; Liu, X. Protection of magnesium alloys: From physical barrier coating to smart self-healing coating. J. Alloys Compd. 2020, 4, 157010. [Google Scholar] [CrossRef]
- Li, X.; Liu, X.; Wu, S.; Yeung, K.W.; Zheng, Y.; Chu, P.K. Design of magnesium alloys with controllable degradation for biomedical implants: From bulk to surface. Acta Biomater. 2016, 45, 2–30. [Google Scholar] [CrossRef]
- Prajapati, S.K.; Jain, A.; Jain, A.; Jain, S. Biodegradable polymers and constructs: A novel approach in drug delivery. Eur. Polym. J. 2019, 120, 109191. [Google Scholar] [CrossRef]
- Mehdi, R.; Mohammadhossein, F.; Omid, S.; Lobat, T.; Daryoosh, V. Biodegradable Magnesium Bone Implants Coated with a Novel Bioceramic Nanocomposite. Materials 2020, 13, 1315. [Google Scholar]
- Zhu, Y.; Liu, W.; Ngai, T. Polymer coatings on magnesium-based implants for orthopaedic applications-Review. J. Polym. Sci. 2021, 60, 32–51. [Google Scholar] [CrossRef]
- Kokubo, T.; Kushitani, H.; Sakka, S.; Kitsugi, T.; Yamamuro, T. Solutions able to reproduce in vivo surface-structure changes in bioactive glass-ceramic A-W. J. Biomed. Mater. Res. 1990, 24, 721–734. [Google Scholar] [CrossRef]
- ASTM International. Standard Practice for Laboratory Immersion Corrosion Testing of Metals; ASTM-G31-72; ASTM: West Conshohocken, PA, USA, 2004. [Google Scholar]
- Jang, Y.; Tan, Z.; Jurey, C.; Collins, B.; Badve, A.; Dong, Z.; Park, C.; Kim, C.S.; Sankar, J.; Yun, Y. Systematic understanding of corrosion behavior of plasma electrolytic oxidation treated AZ31 magnesium alloy using a mouse model of subcutaneous implant. Mater. Sci. Eng. C 2014, 45, 45–55. [Google Scholar] [CrossRef]
- Dwivedi, C.; Pandey, H.; Pandey, A.C.; Ramteke, P.W. Fabrication and Assessment of Gentamicin Loaded Electrospun Nanofibrous Scaffolds as a Quick Wound Healing Dressing Material. Curr. Nanosci. 2015, 11, 222–228. [Google Scholar] [CrossRef]
- El-Sayyad, G.S.; El-Bastawisy, H.S.; Gobara, G.; El-Batal, A.I. Gentamicin-Assisted Mycogenic Selenium Nanoparticles Synthesized Under Gamma Irradiation for Robust Reluctance of Resistant Urinary Tract Infection-Causing Pathogens. Biol. Trace Elem. Res. 2020, 195, 323–342. [Google Scholar] [CrossRef]
- Zeng, R.; Dietzel, W.; Witte, F.; Hort, N.; Blawert, C. Progress and challenge for magnesium alloys as biomaterials. Adv. Eng. Mater. 2008, 10, B3–B14. [Google Scholar] [CrossRef]
- Rudawska, A.; Jacniacka, E. Analysis for determining surface free energy uncertainty by the Owen–Wendt method. Int. J. Adhes. 2009, 29, 451–457. [Google Scholar] [CrossRef]
- Mahadik, S.A.; Pedraza, F.; Mahadik, S.S.; Relekar, B.P.; Thorat, S.S. Biocompatible superhydrophobic coating material for biomedical applications. J. Sol.-Gel. Sci. Technol. 2017, 81, 791–796. [Google Scholar] [CrossRef]
- Anusha Thampi, V.V.; Chukwuike, V.I.; Shtansky, D.V.; Subramanian, B. Biocompatibility study of nanocomposite titanium boron nitride (TiBN) thin films for orthopedic implant applications. Surf. Coat. Technol. 2021, 410, 126968. [Google Scholar]
- Thanka Rajan, S.; Das, M.; Sasi Kumar, P.; Arockiarajan, A.; Subramanian, B. Biological performance of metal metalloid (TiCuZrPd:B) TFMG fabricated by pulsed laser deposition. Colloids Surf. B Biointerfaces 2021, 202, 111684. [Google Scholar] [CrossRef] [PubMed]
- Szewczyk, P.K.; Ura, D.P.; Metwally, S.; Knapczyk-Korczak, J.; Gajek, M.; Marzec, M.M.; Bernasik, A.; Stachewicz, U. Roughness and Fiber Fraction Dominated Wetting of Electrospun Fiber-Based Porous Meshes. Polymers 2019, 11, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ponsonnet, L.; Reybiera, K.; Jaffrezica, N.; Comteb, V.; Lagneaub, C.; Lissacb, M.; Martelet, C. Relationship between surface properties (roughness, wettability) of titanium and titanium alloys and cell behavior. Mater. Sci. Eng. C 2003, 23, 551–560. [Google Scholar] [CrossRef]
- Van Oss, C.J.; Chaudhury, M.K.; Good, R.C. Interfacial Lifshitz-van der Waals and polar interactions in macroscopic systems. Chem. Rev. 1988, 88, 927–941. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Zeng, R.C.; Liu, C.L.; Gao, J.C. Comparison of calcium phosphate coatings on Mg-Al and Mg-Ca alloys and their corrosion behavior in Hank’s solution. Surf. Coat. Technol. 2010, 204, 3636–3640. [Google Scholar]
- Gray-Munro, J.E.; Strong, M. The mechanism of deposition of calcium phosphate coatings from solution onto magnesium alloy AZ31. J. Biomed. Mater. Res. Part A 2009, 90A, 339–350. [Google Scholar] [CrossRef]
- Makkar, P.; Sarkar, S.K.; Padalhin, A.R.; Moon, N.-G.; Lee, Y.S.; Lee, B.T. In vitro and in vivo assessment of biomedical Mg-Ca alloys for bone implant applications. J. Appl. Biomater. Funct. Mater. 2018, 16, 126–136. [Google Scholar] [CrossRef] [Green Version]
- Yufu, R.; Babaie, E.; Sarit, B. Nanostructured amorphous magnesium phosphate/poly (lactic acid) composite coating for enhanced corrosion resistance and bioactivity of biodegradable AZ31 magnesium alloy. Prog. Org. Coat. 2018, 118, 1–8. [Google Scholar]
- Al-Abbas, F.M.; Williamson, C.; Bhola, S.M.; Spear, J.R.; Olson, D.L.; Mishra, B.; Kakpovbia, A.E. Influence of sulfate reducing bacterial biofilm on corrosion behavior of low-alloy, high-strength steel (API-5L X80). Int. Biodeterior. Biodegrad. 2013, 78, 34–42. [Google Scholar] [CrossRef]
- Araujo, W.S.; Margarit, C.P.; Ferreira, M.; Mattos, O.R.; Lima Neto, P. Undoped polyaniline anticorrosive properties. Electrochim. Acta 2001, 46, 1307–1312. [Google Scholar] [CrossRef]
- Liu, C.; Bi, Q.; Leyland, A.; Matthews, A. An electrochemical impedance spectroscopy study of the corrosion behaviour of PVD coated steels in 0.5 N NaCl aqueous solution: Part I. Establishment of equivalent circuits for EIS data modelling. Corros. Sci. 2003, 45, 1243–1256. [Google Scholar] [CrossRef]
- Wang, H.; Ding, S.; Zhui, J.; Zhang, Z.; Zhang, J.; Cao, C. Corrosion behavior of 907 steel under thin electrolyte layers of artificial seawater. J. Cent. South Univ. 2015, 22, 806–814. [Google Scholar] [CrossRef]
- Ma, W.H.; Liu, Y.J.; Wang, W.; Zhang, Y.Z. Improved biological performance of magnesium by micro-arc oxidation. Braz. J. Med. Biol. Res. 2015, 48, 214–225. [Google Scholar] [CrossRef] [Green Version]
- Yanjin, W.; Wan, P.; Zhang, B.; Tan, L.; Yang, K.; Lin, J. Research on the corrosion resistance and formation of double-layer calcium phosphate coating on AZ31 obtained at varied temperatures. Mater. Sci. Eng. C 2014, 43, 264–271. [Google Scholar]


| Component | Concentration [g/500 mL] |
|---|---|
| NaCl | 3.998 |
| NaHCO3 | 0.175 |
| KCl | 0.112 |
| K2HPO4·3H2O | 0.114 |
| MgCl2·6H2O | 0.152 |
| CaCl2 | 0.139 |
| Na2SO4 | 0.035 |
| (CH2OH)3CNH2 | 3.028 |
| Element | Uncoated Mg Alloy | Mg Alloy-PLA | ||
|---|---|---|---|---|
| Wt% | Std. Dev. | Wt% | Std. Dev. | |
| Mg | 87.83 | 0.47 | 19.81 | 3.15 |
| Al | 2.62 | 0.1 | 0.67 | 0.14 |
| Zn | 1.02 | 0.08 | 0.16 | 0.16 |
| C | 3.83 | 0.30 | 58.54 | 1.82 |
| O | 4.59 | 0.31 | 20.74 | 2.96 |
| Liquids | Surface Energy [mJ/m2] | ||
|---|---|---|---|
| Water | 72.8 | 21.8 | 51 |
| Ethylene glycol | 47.7 | 21.3 | 26.4 |
| Diiodomethane | 50.8 | 48.5 | 2.3 |
| Uncoated Mg Alloy | Mg Alloy-PLA | |
|---|---|---|
| Water, ° | 59 ± 0.5 | 98 ± 0.3 |
| Ethylene glycol, ° | 44 ± 0.2 | 72 ± 0.6 |
| Diiodomethane, ° | 57 ± 0.6 | 62 ± 0.4 |
| (SFE), mJ/m2 | 45.01 | 37.55 |
| , mJ/m2 | 23.74 | 1.59 |
| , mJ/m2 | 21.27 | 35.96 |
| Sample | Time [h] | R1 [kΩ] | R2 [kΩ] | CPE1 | R3 [kΩ] | CPE2 | ||
|---|---|---|---|---|---|---|---|---|
| YO [μMho] | N1 * | YO [μMho] | N2 * | |||||
| Uncoated Mg alloy | 0 | 0.117 | 0.576 | 5.46 | 0.881 | 0.273 | 874 | 0.964 |
| 24 | 0.116 | 0.837 | 4.02 | 0.877 | 0.365 | 672 | 0.967 | |
| 48 | 0.110 | 0.881 | 6.16 | 0.866 | 0.563 | 563 | 0.908 | |
| 168 | 0.115 | 1.32 | 7.71 | 0.855 | 1.61 | 531 | 0.783 | |
| Sample | Time [h] | R1 [kΩ] | R2 [kΩ] | CPE1 | R3 [kΩ] | CPE2 | O | |||
|---|---|---|---|---|---|---|---|---|---|---|
| YO [μMho] | N1 * | YO [μMho] | N2 * | YO [μMho] | B | |||||
| Mg alloy-PLA | 0 | 0.115 | 3390 | 69.6 × 10−5 | 0.982 | 8460 | 6.46 × 10−3 | 0.705 | - | - |
| 24 | 0.113 | 46.6 | 0.659 | 0.566 | 101 | 1.06 × 10−4 | 0.961 | 3.23 | 1.88 | |
| 48 | 0.11 | 2.62 | 3.23 × 10−3 | 0.895 | 14.7 | 1.43 | 0.547 | 12.5 | 0.407 | |
| 168 | 0.119 | 0.324 | 1.61 × 10−3 | 0.9 | 5.88 | 9.86 | 0.633 | 10.4 | 0.1 | |
| Sample | Time [h] | Ecorr [V] | Icorr [μA] | Vcorr [μm/Year] | Ba [V/Decade] | Bc [V/Decade] |
|---|---|---|---|---|---|---|
| Uncoated Mg alloy | 0 | −1.55 | 26.8 | 2114 | 0.19 | 11.33 |
| 24 | −1.53 | 22.3 | 1756 | 0.19 | 0.60 | |
| 48 | −1.50 | 17.05 | 1344 | 0.22 | 0.37 | |
| 168 | −1.47 | 2.38 | 187.58 | 0.08 | 0.07 | |
| Mg alloy-PLA | 0 | −1.52 | 0.0021 | 0.048 | 0.20 | 0.14 |
| 24 | −1.31 | 0.0195 | 0.45 | 0.15 | 0.23 | |
| 48 | −1.35 | 0.126 | 2.91 | 0.25 | 0.34 | |
| 168 | −1.39 | 0.382 | 8.82 | 0.23 | 0.25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Voicu, M.E.; Demetrescu, I.; Dorobantu, A.; Enachescu, M.; Buica, G.-O.; Ionita, D. Interaction of Mg Alloy with PLA Electrospun Nanofibers Coating in Understanding Changes of Corrosion, Wettability, and pH. Nanomaterials 2022, 12, 1369. https://doi.org/10.3390/nano12081369
Voicu ME, Demetrescu I, Dorobantu A, Enachescu M, Buica G-O, Ionita D. Interaction of Mg Alloy with PLA Electrospun Nanofibers Coating in Understanding Changes of Corrosion, Wettability, and pH. Nanomaterials. 2022; 12(8):1369. https://doi.org/10.3390/nano12081369
Chicago/Turabian StyleVoicu, Manuela Elena, Ioana Demetrescu, Andrei Dorobantu, Marius Enachescu, George-Octavian Buica, and Daniela Ionita. 2022. "Interaction of Mg Alloy with PLA Electrospun Nanofibers Coating in Understanding Changes of Corrosion, Wettability, and pH" Nanomaterials 12, no. 8: 1369. https://doi.org/10.3390/nano12081369
APA StyleVoicu, M. E., Demetrescu, I., Dorobantu, A., Enachescu, M., Buica, G.-O., & Ionita, D. (2022). Interaction of Mg Alloy with PLA Electrospun Nanofibers Coating in Understanding Changes of Corrosion, Wettability, and pH. Nanomaterials, 12(8), 1369. https://doi.org/10.3390/nano12081369








