Aerosol–Cell Exposure System Applied to Semi-Adherent Cells for Aerosolization of Lung Surfactant and Nanoparticles Followed by High Quality RNA Extraction
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Cell Culture
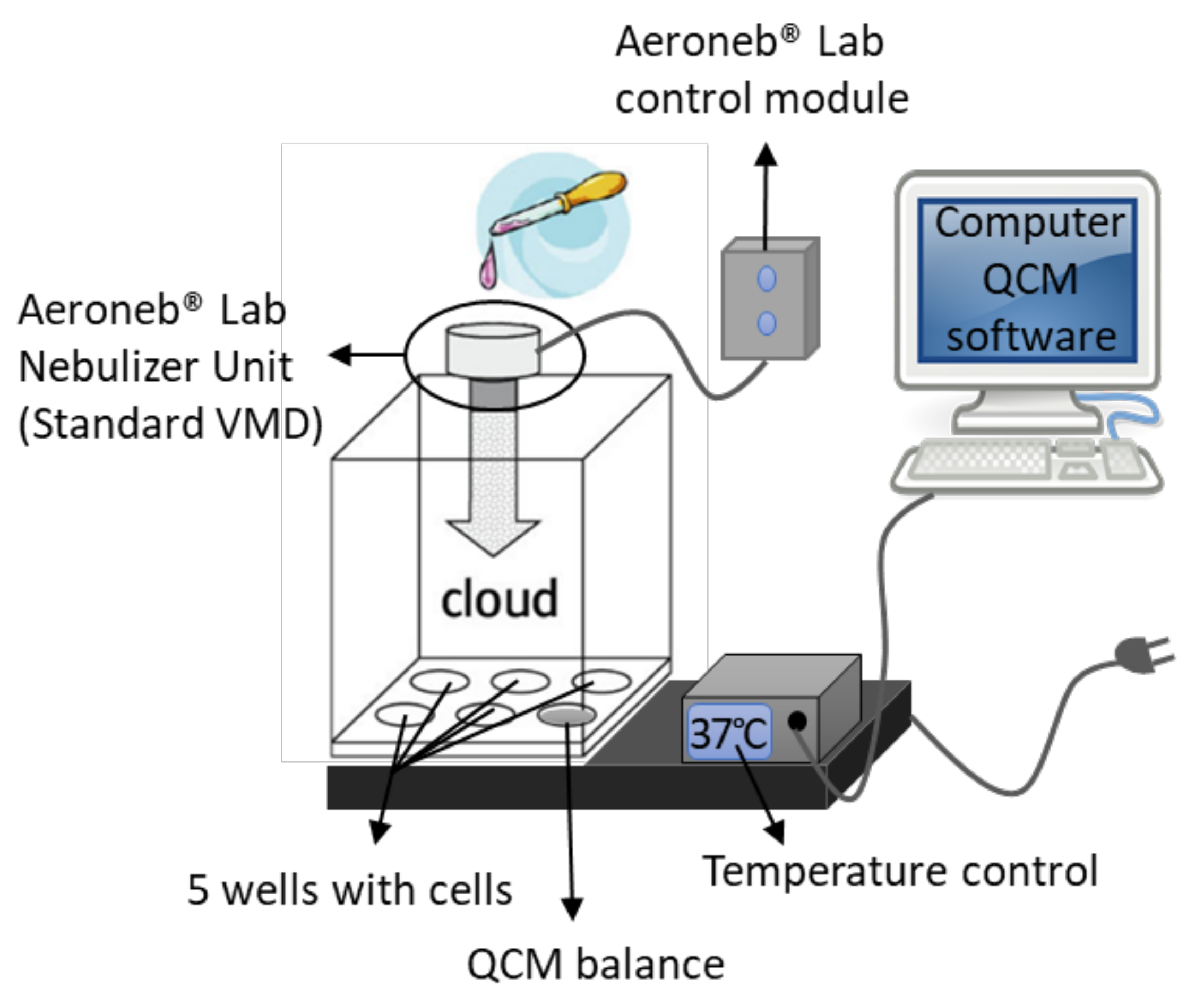
2.3. VITROCELL® Cloud 6 System
2.4. Fluorescent Microscopy for the Assessment of the Homogeneous Deposition of Surfactant
2.5. Pre-Coating of ALI Cells with Surfactant
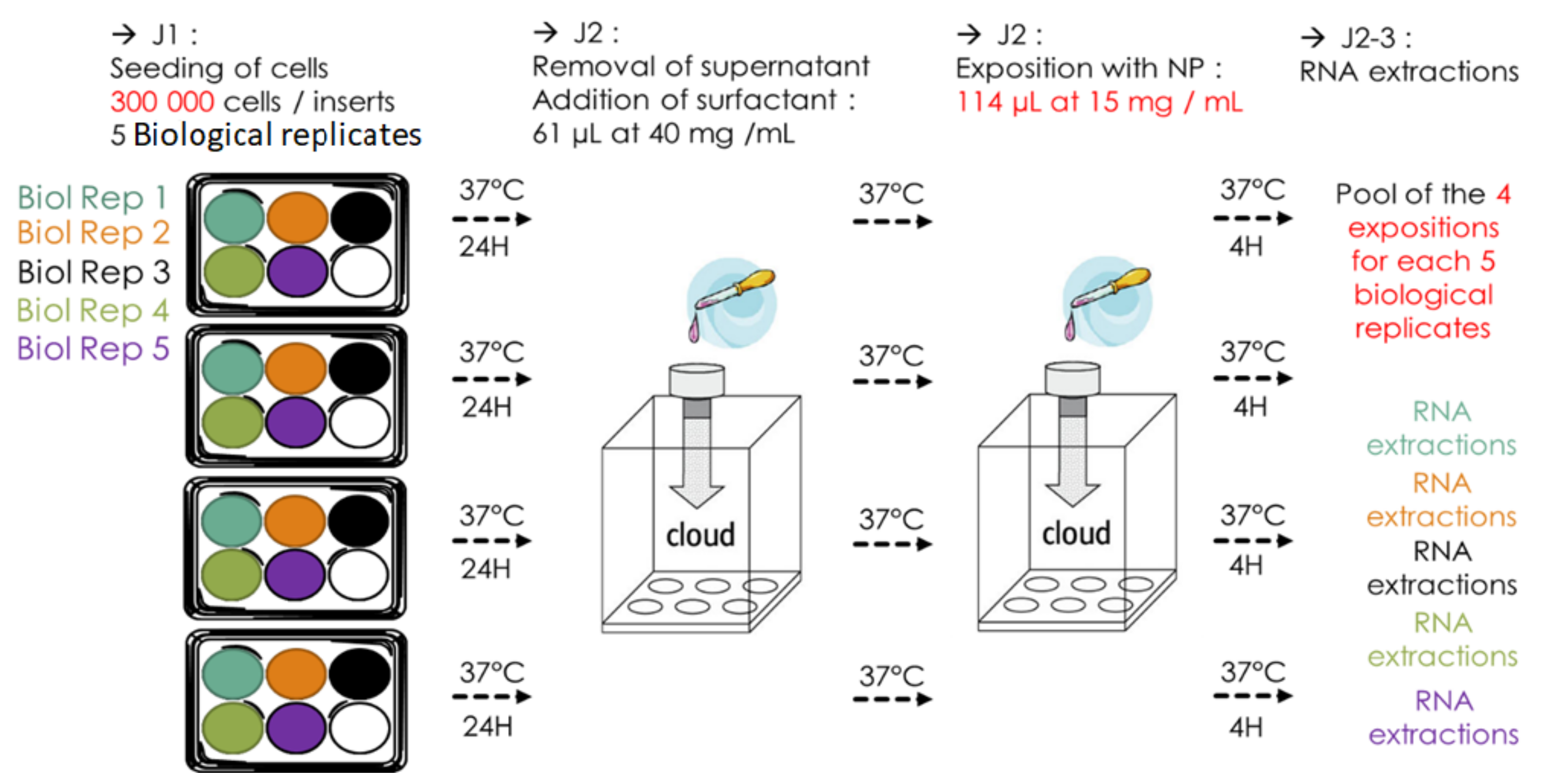
2.6. NP Exposure Protocol
2.7. RNA Extraction
3. Results
3.1. A Uniform Deposition of Surfactant by Aerosolization with the Nebulizer
3.1.1. Reproducible Deposition Assessment by QCM Measurements
3.1.2. Homogeneous Distribution Assessment by Fluorescence Microscopy
3.2. Improvement of the Standard Initial Protocol
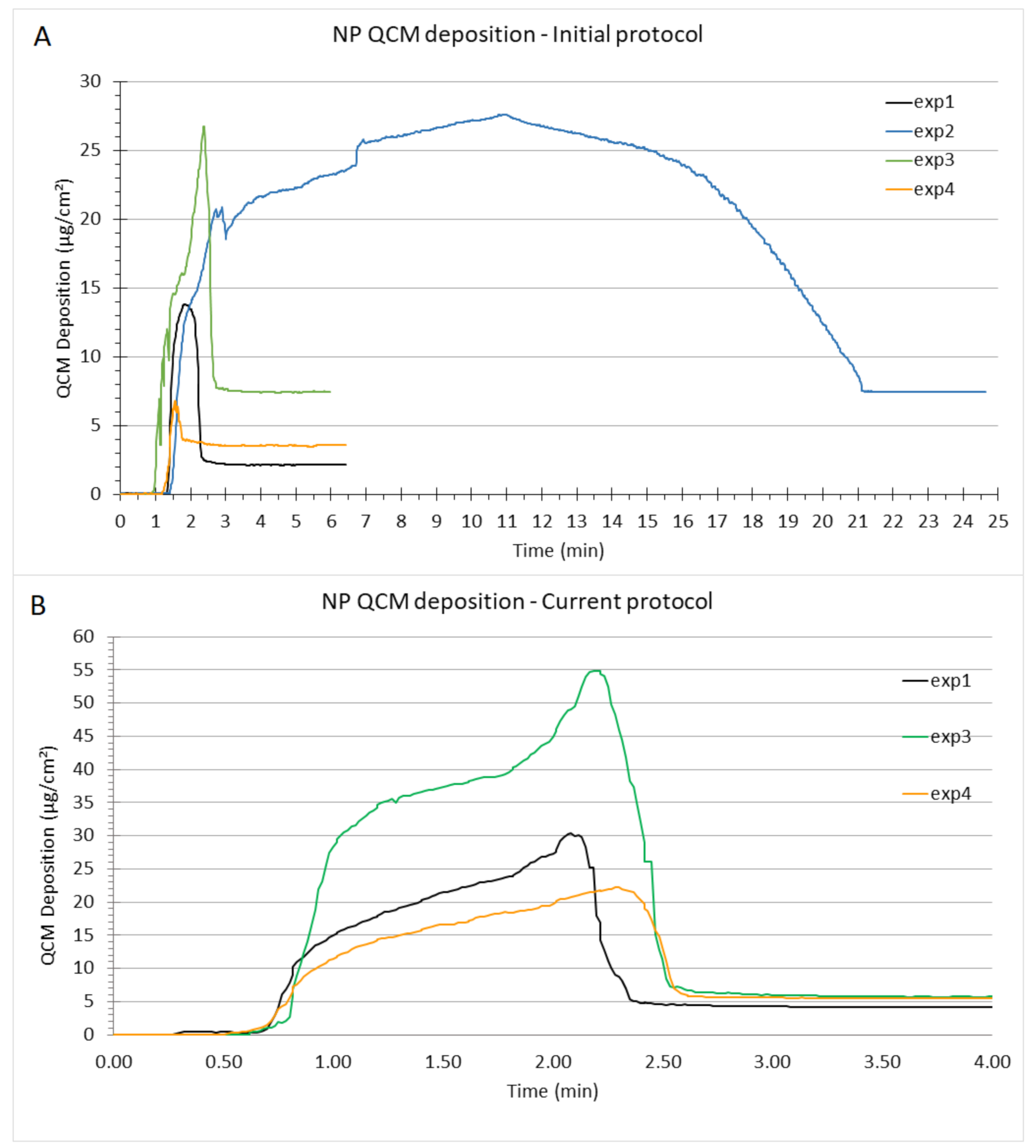
3.2.1. Standard Initial Protocol for NP Nebulization in VITROCELL® Cloud 6 System
3.2.2. Current Improved Protocol for NP Nebulization in VITROCELL® Cloud 6 System
3.3. Troubleshooting Flaws of the Initial Protocol
3.3.1. Preventing Rapid Drying of NPs on the Nebulizer Mesh by Modifying the Volume of Nebulized Liquid
3.3.2. Loss of Cells and Poor RNA Quality
3.3.3. Increasing the Reproducibility and Repeatability
4. Discussion
5. Conclusions
- (1)
- When using semi adherent cells, the number of cells seeded on the insert has to be increased (as compared to submerged culture conditions) to ensure that enough cells stay on the membrane after discarding the apical medium to reach a satisfying RNA yield.
- (2)
- To avoid any clogging of the mesh of the nebulizer and to obtain a reproducible cell-delivered dose, the manufacturer’s recommendations for cleaning of the nebulizer should be adhered to and the TiO NP concentration has to be decreased by increasing the nebulized suspension volume (from 57 L of 30 mg/mL to 114 L of 15 mg/mL in this case for TiO NM105 NP). This will prevent the NP suspension from both drying out on the nebulizer mesh and alteration of the NP deposition on the cells due to low cloud density in the VITROCELL® Cloud exposure chamber.
- (3)
- When two sequential aerosol exposures are performed back-to-back, when applicable, we suggest using a different nebulizer for each liquid nebulized. If the same nebulizer is used, we suggest nebulizing all replicas with the first type of aerosol and then with the second, and cleaning the nebulizer between each sequence of nebulization thoroughly as recommended by the manufacturer.
- (4)
- If it is necessary to proceed to several expositions, we recommend including each BR in each exposure session (divided into technical replicates (TR), one TR per exposition). By doing this and subsequent pooling of the TRs of each BR, the variability in deposited dose amongst expositions will be spread over every TR in the same manner for each BR.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NP | Nanoparticles |
| ALI | Air–liquid interface |
| QCM | Quartz crystal microbalance |
| BAL | Bronchoalveolar lavage |
| BR | Biological replicate |
| TR | Technical replicate |
References
- Omlor, A.J.; Nguyen, J.; Bals, R.; Dinh, Q.T. Nanotechnology in respiratory medicine. Respir. Res. 2015, 16, 64. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, F. UNESCO Science Report: Towards 2030; Number 2015 in UNESCO Science Report; UNESCO Publication: Paris, France, 2015. [Google Scholar]
- Vance, M.E.; Kuiken, T.; Vejerano, E.P.; McGinnis, S.P.; Hochella, M.F.; Rejeski, D.; Hull, M.S. Nanotechnology in the real world: Redeveloping the nanomaterial consumer products inventory. Beilstein J. Nanotechnol. 2015, 6, 1769–1780. [Google Scholar] [CrossRef] [PubMed]
- Er, S.; Laraib, U.; Arshad, R.; Sargazi, S.; Rahdar, A.; Pandey, S.; Thakur, V.K.; Díez-Pascual, A.M. Amino Acids, Peptides, and Proteins: Implications for Nanotechnological Applications in Biosensing and Drug/Gene Delivery. Nanomaterials 2021, 11, 3002. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Saadullah, M.; Alvi, F.; Sherin, L.; Ali, A.; Shad, N.A.; Javed, Y.; Sajid, M.M.; Yasin, G.; Abbas, W. Synergistic effect of silver doped ZnO nanomaterials enhances the anticancer potential against A459 lung cancer cells. J. King Saud Univ.-Sci. 2022, 34, 101724. [Google Scholar] [CrossRef]
- Sabir, F.; Qindeel, M.; Zeeshan, M.; Ul Ain, Q.; Rahdar, A.; Barani, M.; González, E.; Aboudzadeh, M.A. Onco-Receptors Targeting in Lung Cancer via Application of Surface-Modified and Hybrid Nanoparticles: A Cross-Disciplinary Review. Processes 2021, 9, 621. [Google Scholar] [CrossRef]
- Gholami, L.; Ivari, J.R.; Nasab, N.K.; Oskuee, R.K.; Sathyapalan, T.; Sahebkar, A. Recent advances in lung cancer therapy based on nanomaterials: A review. Curr. Med. Chem. 2021; 28, Online ahead of print. [Google Scholar] [CrossRef]
- Durand, M.; Lelievre, E.; Chateau, A.; Berquand, A.; Laurent, G.; Carl, P.; Roux, S.; Chazee, L.; Bazzi, R.; Eghiaian, F.; et al. The detrimental invasiveness of glioma cells controlled by gadolinium chelate-coated gold nanoparticles. Nanoscale 2021, 13, 9236–9251. [Google Scholar] [CrossRef]
- Moussaron, A.; Jouan-Hureaux, V.; Collet, C.; Pierson, J.; Thomas, N.; Choulier, L.; Veran, N.; Doyen, M.; Arnoux, P.; Maskali, F.; et al. Preliminary Study of New Gallium-68 Radiolabeled Peptide Targeting NRP-1 to Detect Brain Metastases by Positron Emission Tomography. Molecules 2021, 26, 7273. [Google Scholar] [CrossRef]
- Vavere, L.A.; Rossin, R. Molecular Imaging of Cancer with Radiolabeled Peptides and PET. Anti-Cancer Agents Med. Chem. 2012, 12, 462–475. [Google Scholar] [CrossRef]
- Niloy, M.S.; Shakil, M.S.; Hossen, M.S.; Alam, M.; Rosengren, R.J. Promise of gold nanomaterials as a lung cancer theranostic agent: A systematic review. Int. Nano Lett. 2021, 11, 93–111. [Google Scholar] [CrossRef]
- Freitas, L.F.; Ferreira, A.H.; Thipe, V.C.; Varca, G.H.C.; Lima, C.S.A.; Batista, J.G.S.; Riello, F.N.; Nogueira, K.; Cruz, C.P.C.; Mendes, G.O.A.; et al. The State of the Art of Theranostic Nanomaterials for Lung, Breast, and Prostate Cancers. Nanomaterials 2021, 11, 2579. [Google Scholar] [CrossRef] [PubMed]
- Bahadar, H.; Maqbool, F.; Niaz, K.; Abdollahi, M. Toxicity of Nanoparticles and an Overview of Current Experimental Models. Iran. Biomed. J. 2016, 20, 1–11. [Google Scholar] [PubMed]
- Crisponi, G.; Nurchi, V.M.; Lachowicz, J.I.; Peana, M.; Medici, S.; Zoroddu, M.A. Toxicity of Nanoparticles: Etiology and Mechanisms. In Antimicrobial Nanoarchitectonics; Elsevier: Amsterdam, The Netherlands, 2017; pp. 511–546. [Google Scholar] [CrossRef]
- Huang, Y.W.; Cambre, M.; Lee, H.J. The Toxicity of Nanoparticles Depends on Multiple Molecular and Physicochemical Mechanisms. Int. J. Mol. Sci. 2017, 18, 2702. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Nanotechnology and Human Health Scientific Evidence and Risk Governance, Report of the WHO Expert Meeting, 10–11 December 2012, Bonn, Germany, No. WHO/EURO: 2013-4491-44254-62511. Copenhagen, WHO Regional Office for Europe, 2013. Available online: http://apps.who.int/iris/bitstream/handle/10665/108626/e96927.pdf (accessed on 3 February 2022).
- Geraets, L.; Oomen, A.G.; Schroeter, J.D.; Coleman, V.A.; Cassee, F.R. Tissue Distribution of Inhaled Micro- and Nano-sized Cerium Oxide Particles in Rats: Results From a 28-Day Exposure Study. Toxicol. Sci. 2012, 127, 463–473. [Google Scholar] [CrossRef]
- Oberdörster, G.; Sharp, Z.; Atudorei, V.; Elder, A.; Gelein, R.; Kreyling, W.; Cox, C. Translocation of Inhaled Ultrafine Particles to the Brain. Inhal. Toxicol. 2004, 16, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Kreyling, W.G.; Hirn, S.; Möller, W.; Schleh, C.; Wenk, A.; Celik, G.; Lipka, J.; Schäffler, M.; Haberl, N.; Johnston, B.D.; et al. Air–Blood Barrier Translocation of Tracheally Instilled Gold Nanoparticles Inversely Depends on Particle Size. ACS Nano 2014, 8, 222–233. [Google Scholar] [CrossRef]
- Braakhuis, H.M.; Gosens, I.; Krystek, P.; Boere, J.A.; Cassee, F.R.; Fokkens, P.H.; Post, J.A.; van Loveren, H.; Park, M.V. Particle size dependent deposition and pulmonary inflammation after short-term inhalation of silver nanoparticles. Part. Fibre Toxicol. 2014, 11, 49. [Google Scholar] [CrossRef]
- Upadhyay, S.; Palmberg, L. Air-Liquid Interface: Relevant In Vitro Models for Investigating Air Pollutant-Induced Pulmonary Toxicity. Toxicol. Sci. 2018, 164, 21–30. [Google Scholar] [CrossRef]
- Aufderheide, M.; Mohr, U. CULTEX—An alternative technique for cultivation and exposure of cells of the respiratory tract to airborne pollutants at the air/liquid interface. Exp. Toxicol. Pathol. 2000, 52, 265–270. [Google Scholar] [CrossRef]
- Ghio, A.J.; Dailey, L.A.; Soukup, J.M.; Stonehuerner, J.; Richards, J.H.; Devlin, R.B. Growth of human bronchial epithelial cells at an air-liquid interface alters the response to particle exposure. Part. Fibre Toxicol. 2013, 10, 25. [Google Scholar] [CrossRef]
- Oberdörster, G.; Oberdörster, E.; Oberdörster, J. Nanotoxicology: An Emerging Discipline Evolving from Studies of Ultrafine Particles. Environ. Health Perspect. 2005, 113, 823–839. [Google Scholar] [CrossRef] [PubMed]
- Gostner, J.M.; Zeisler, J.; Alam, M.T.; Gruber, P.; Fuchs, D.; Becker, K.; Neubert, K.; Kleinhappl, M.; Martini, S.; Überall, F. Cellular reactions to long-term volatile organic compound (VOC) exposures. Sci. Rep. 2016, 6, 37842. [Google Scholar] [CrossRef] [PubMed]
- Schmid, O.; Cassee, F.R. On the pivotal role of dose for particle toxicology and risk assessment: Exposure is a poor surrogate for delivered dose. Part. Fibre Toxicol. 2017, 14, 52. [Google Scholar] [CrossRef] [PubMed]
- Aufderheide, M. Direct exposure methods for testing native atmospheres. Exp. Toxicol. Pathol. 2005, 57, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Tu, B.; Wallin, A.; Moldéus, P.; Cotgreave, I.A. Cytotoxicity of NO2 gas to cultured human and murine cells in an inverted monolayer exposure system. Toxicology 1995, 96, 7–18. [Google Scholar] [CrossRef]
- Kim, S.; Jaques, P.A.; Chang, M.; Froines, J.R.; Sioutas, C. Versatile aerosol concentration enrichment system (VACES) for simultaneous in vivo and in vitro evaluation of toxic effects of ultrafine, fine and coarse ambient particles Part I: Development and laboratory characterization. J. Aerosol Sci. 2001, 32, 1281–1297. [Google Scholar] [CrossRef]
- De Bruijne, K.; Ebersviller, S.; Sexton, K.G.; Lake, S.; Leith, D.; Goodman, R.; Jetters, J.; Walters, G.W.; Doyle-Eisele, M.; Woodside, R.; et al. Design and testing of Electrostatic Aerosol in Vitro Exposure System (EAVES): An alternative exposure system for particles. Inhal. Toxicol. 2009, 21, 91–101. [Google Scholar] [CrossRef]
- Aufderheide, M.; Knebel, J.W.; Ritter, D. A method for the in vitro exposure of human cells to environmental and complex gaseous mixtures: Application to various types of atmosphere. Altern. Lab. Anim. ATLA 2002, 30, 433–441. [Google Scholar] [CrossRef]
- Olivera, D.S.; Boggs, S.E.; Beenhouwer, C.; Aden, J.; Knall, C. Cellular mechanisms of mainstream cigarette smoke-induced lung epithelial tight junction permeability changes in vitro. Inhal. Toxicol. 2007, 19, 13–22. [Google Scholar] [CrossRef]
- Jeannet, N.; Fierz, M.; Kalberer, M.; Burtscher, H.; Geiser, M. Nano aerosol chamber for in-vitro toxicity (NACIVT) studies. Nanotoxicology 2015, 9, 34–42. [Google Scholar] [CrossRef]
- Ji, J.; Hedelin, A.; Malmlöf, M.; Kessler, V.; Seisenbaeva, G.; Gerde, P.; Palmberg, L. Development of Combining of Human Bronchial Mucosa Models with XposeALI® for Exposure of Air Pollution Nanoparticles. PLoS ONE 2017, 12, e0170428. [Google Scholar] [CrossRef] [PubMed]
- Mülhopt, S.; Dilger, M.; Diabaté, S.; Schlager, C.; Krebs, T.; Zimmermann, R.; Buters, J.; Oeder, S.; Wäscher, T.; Weiss, C.; et al. Toxicity testing of combustion aerosols at the air–liquid interface with a self-contained and easy-to-use exposure system. J. Aerosol Sci. 2016, 96, 38–55. [Google Scholar] [CrossRef]
- Lenz, A.G.; Karg, E.; Lentner, B.; Dittrich, V.; Brandenberger, C.; Rothen-Rutishauser, B.; Schulz, H.; Ferron, G.A.; Schmid, O. A dose-controlled system for air-liquid interface cell exposure and application to zinc oxide nanoparticles. Part. Fibre Toxicol. 2009, 6, 32. [Google Scholar] [CrossRef]
- Lenz, A.G.; Stoeger, T.; Cei, D.; Schmidmeir, M.; Semren, N.; Burgstaller, G.; Lentner, B.; Eickelberg, O.; Meiners, S.; Schmid, O. Efficient bioactive delivery of aerosolized drugs to human pulmonary epithelial cells cultured in air-liquid interface conditions. Am. J. Respir. Cell Mol. Biol. 2014, 51, 526–535. [Google Scholar] [CrossRef] [PubMed]
- Ehrmann, S.; Schmid, O.; Darquenne, C.; Rothen-Rutishauser, B.; Sznitman, J.; Yang, L.; Barosova, H.; Vecellio, L.; Mitchell, J.; Heuze-Vourc’h, N. Innovative preclinical models for pulmonary drug delivery research. Expert Opin. Drug Deliv. 2020, 17, 463–478. [Google Scholar] [CrossRef] [PubMed]
- Paur, H.R.; Cassee, F.R.; Teeguarden, J.; Fissan, H.; Diabate, S.; Aufderheide, M.; Kreyling, W.G.; Hänninen, O.; Kasper, G.; Riediker, M.; et al. In-vitro cell exposure studies for the assessment of nanoparticle toxicity in the lung—A dialog between aerosol science and biology. J. Aerosol Sci. 2011, 42, 668–692. [Google Scholar] [CrossRef]
- Bragazzi, N.L.; Amicizia, D.; Panatto, D.; Tramalloni, D.; Valle, I.; Gasparini, R. Quartz-Crystal Microbalance (QCM) for Public Health. In Advances in Protein Chemistry and Structural Biology; Elsevier: Amsterdam, The Netherlands, 2015; Volume 101, pp. 149–211. [Google Scholar] [CrossRef]
- Stobiecka, M.; Jakiela, S.; Chalupa, A.; Bednarczyk, P.; Dworakowska, B. Mitochondria–based biosensors with piezometric and RELS transduction for potassium uptake and release investigations. Biosens. Bioelectron. 2017, 88, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Perez, J.W.; Adams, N.M.; Zimmerman, G.R.; Haselton, F.R.; Wright, D.W. Detecting Respiratory Syncytial Virus Using Nanoparticle-Amplified Immuno-PCR. In NanoBiotechnology Protocols; Rosenthal, S.J., Wright, D.W., Eds.; Series Title: Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2013; Volume 1026, pp. 93–110. [Google Scholar] [CrossRef]
- Oztuna, A.; Nazir, H.; Baysallar, M. Simultaneous Bacillus anthracis Spores Detection via Aminated-Poly(vinyl chloride) Coated Piezoelectric Crystal Immunosensor. J. Coat. 2014, 2014, 1–8. [Google Scholar] [CrossRef]
- Hao, R.Z.; Song, H.B.; Zuo, G.M.; Yang, R.F.; Wei, H.P.; Wang, D.B.; Cui, Z.Q.; Zhang, Z.; Cheng, Z.X.; Zhang, X.E. DNA probe functionalized QCM biosensor based on gold nanoparticle amplification for Bacillus anthracis detection. Biosens. Bioelectron. 2011, 26, 3398–3404. [Google Scholar] [CrossRef]
- Prakrankamanant, P.; Leelayuwat, C.; Promptmas, C.; Limpaiboon, T.; Wanram, S.; Prasongdee, P.; Pientong, C.; Daduang, J.; Jearanaikoon, P. The development of DNA-based quartz crystal microbalance integrated with isothermal DNA amplification system for human papillomavirus type 58 detection. Biosens. Bioelectron. 2013, 40, 252–257. [Google Scholar] [CrossRef]
- Plikusiene, I.; Maciulis, V.; Ramanavicius, A.; Ramanaviciene, A. Spectroscopic Ellipsometry and Quartz Crystal Microbalance with Dissipation for the Assessment of Polymer Layers and for the Application in Biosensing. Polymers 2022, 14, 1056. [Google Scholar] [CrossRef] [PubMed]
- Fuji, S.; Tanaka, K.; Kishikawa, S.; Morita, S.; Doi, M. Quartz crystal microbalance sensor for the detection of collagen model peptides based on the formation of triple helical structure. J. Biosci. Bioeng. 2022, 133, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Weindl, P.; Lenz, A.G.; Mayer, P.; Krebs, T.; Schmid, O. Quartz crystal microbalances (QCM) are suitable for real-time dosimetry in nanotoxicological studies using VITROCELL®Cloud cell exposure systems. Part. Fibre Toxicol. 2020, 17, 44. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Mouton, C.; Hidalgo, A.; Cruz, A.; Pérez-Gil, J. The Lord of the Lungs: The essential role of pulmonary surfactant upon inhalation of nanoparticles. Eur. J. Pharm. Biopharm. 2019, 144, 230–243. [Google Scholar] [CrossRef]
- Schulze, C.; Schaefer, U.F.; Ruge, C.A.; Wohlleben, W.; Lehr, C.M. Interaction of metal oxide nanoparticles with lung surfactant protein A. Eur. J. Pharm. Biopharm. 2011, 77, 376–383. [Google Scholar] [CrossRef]
- Theodorou, I.G.; Ruenraroengsak, P.; Gow, A.; Schwander, S.; Zhang, J.J.; Chung, K.F.; Tetley, T.D.; Ryan, M.P.; Porter, A.E. Effect of pulmonary surfactant on the dissolution, stability and uptake of zinc oxide nanowires by human respiratory epithelial cells. Nanotoxicology 2016, 10, 1351–1362. [Google Scholar] [CrossRef]
- Joshi, N.; Walter, J.M.; Misharin, A.V. Alveolar Macrophages. Cell. Immunol. 2018, 330, 86–90. [Google Scholar] [CrossRef]
- Lambrecht, B.N. Alveolar Macrophage in the Driver’s Seat. Immunity 2006, 24, 366–368. [Google Scholar] [CrossRef]
- Naeem, A.; Rai, S.N.; Pierre, L. Histology, Alveolar Macrophages. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Blanco, O.; Pérez-Gil, J. Biochemical and pharmacological differences between preparations of exogenous natural surfactant used to treat Respiratory Distress Syndrome: Role of the different components in an efficient pulmonary surfactant. Eur. J. Pharmacol. 2007, 568, 1–15. [Google Scholar] [CrossRef]
- Guagliardo, R.; Pérez-Gil, J.; De Smedt, S.; Raemdonck, K. Pulmonary surfactant and drug delivery: Focusing on the role of surfactant proteins. J. Control. Release 2018, 291, 116–126. [Google Scholar] [CrossRef]
- Autilio, C.; Pérez-Gil, J. Understanding the principle biophysics concepts of pulmonary surfactant in health and disease. Arch. Dis. Child.-Fetal Neonatal Ed. 2019, 104, F443–F451. [Google Scholar] [CrossRef] [PubMed]
- Radiom, M.; Sarkis, M.; Brookes, O.; Oikonomou, E.K.; Baeza-Squiban, A.; Berret, J.F. Pulmonary surfactant inhibition of nanoparticle uptake by alveolar epithelial cells. Sci. Rep. 2020, 10, 19436. [Google Scholar] [CrossRef] [PubMed]
- Kokot, H.; Kokot, B.; Sebastijanović, A.; Voss, C.; Podlipec, R.; Zawilska, P.; Berthing, T.; López, C.B.; Danielsen, P.H.; Contini, C.; et al. Prediction of Chronic Inflammation for Inhaled Particles: The Impact of Material Cycling and Quarantining in the Lung Epithelium. Adv. Mater. 2020, 32, 2003913. [Google Scholar] [CrossRef] [PubMed]
- Urbančič, I.; Garvas, M.; Kokot, B.; Majaron, H.; Umek, P.; Cassidy, H.; Škarabot, M.; Schneider, F.; Galiani, S.; Arsov, Z.; et al. Nanoparticles Can Wrap Epithelial Cell Membranes and Relocate Them Across the Epithelial Cell Layer. Nano Lett. 2018, 18, 5294–5305. [Google Scholar] [CrossRef] [PubMed]
- Dhanani, J.A.; Cohen, J.; Parker, S.L.; Chan, H.K.; Tang, P.; Ahern, B.J.; Khan, A.; Bhatt, M.; Goodman, S.; Diab, S.; et al. A research pathway for the study of the delivery and disposition of nebulised antibiotics: An incremental approach from in vitro to large animal models. Intensive Care Med. Exp. 2018, 6, 17. [Google Scholar] [CrossRef]
- Dubus, J.C.; Vecellio, L.; De Monte, M.; Fink, J.B.; Grimbert, D.; Montharu, J.; Valat, C.; Behan, N.; Diot, P. Aerosol Deposition in Neonatal Ventilation. Pediatr. Res. 2005, 58, 10–14. [Google Scholar] [CrossRef][Green Version]
- Leroux, M.M.; Doumandji, Z.; Chézeau, L.; Gaté, L.; Nahle, S.; Hocquel, R.; Zhernovkov, V.; Migot, S.; Ghanbaja, J.; Bonnet, C.; et al. Toxicity of TiO2 Nanoparticles: Validation of Alternative Models. Int. J. Mol. Sci. 2020, 21, 4855. [Google Scholar] [CrossRef]
- Leroux, M.M.; Doumandji, Z.; Chezeau, L.; Hocquel, R.; Ferrari, L.; Joubert, O.; Rihn, P.; Rihn, B.H. Validation of an air/liquid interface device for TiO2 nanoparticle toxicity assessment on NR8383 cells: Preliminary results. Cell. Mol. Biol. 2020, 66, 112–116. [Google Scholar] [CrossRef]
- Bastacky, J.; Lee, C.Y.; Goerke, J.; Koushafar, H.; Yager, D.; Kenaga, L.; Speed, T.P.; Chen, Y.; Clements, J.A. Alveolar lining layer is thin and continuous: Low-temperature scanning electron microscopy of rat lung. J. Appl. Physiol. 1995, 79, 1615–1628. [Google Scholar] [CrossRef]
- Yang, L.; Feuchtinger, A.; Möller, W.; Ding, Y.; Kutschke, D.; Möller, G.; Schittny, J.C.; Burgstaller, G.; Hofmann, W.; Stoeger, T.; et al. Three-Dimensional Quantitative Co-Mapping of Pulmonary Morphology and Nanoparticle Distribution with Cellular Resolution in Nondissected Murine Lungs. ACS Nano 2019, 1029–1041. [Google Scholar] [CrossRef]
- Di Cristo, L.; Maguire, C.; Mc Quillan, K.; Aleardi, M.; Volkov, Y.; Movia, D.; Prina-Mello, A. Towards the Identification of an In Vitro Tool for Assessing the Biological Behavior of Aerosol Supplied Nanomaterials. Int. J. Environ. Res. Public Health 2018, 15, 563. [Google Scholar] [CrossRef] [PubMed]
- Dhanani, J.; Fraser, J.F.; Chan, H.K.; Rello, J.; Cohen, J.; Roberts, J.A. Fundamentals of aerosol therapy in critical care. Crit. Care 2016, 20, 269. [Google Scholar] [CrossRef] [PubMed]
- Dhanani, J.A.; Diab, S.; Chaudhary, J.; Cohen, J.; Parker, S.L.; Wallis, S.C.; Boidin, C.; Barnett, A.; Chew, M.; Roberts, J.A.; et al. Lung Pharmacokinetics of Tobramycin by Intravenous and Nebulized Dosing in a Mechanically Ventilated Healthy Ovine Model. Anesthesiology 2019, 131, 344–355. [Google Scholar] [CrossRef] [PubMed]
- Geiser, M. Update on Macrophage Clearance of Inhaled Micro- and Nanoparticles. J. Aerosol Med. Pulm. Drug Deliv. 2010, 23, 207–217. [Google Scholar] [CrossRef]
- Lehnert, B.E. Pulmonary and thoracic macrophage subpopulations and clearance of particles from the lung. Environ. Health Perspect. 1992, 97, 17–46. [Google Scholar] [CrossRef]
- Semmler, M.; Seitz, J.; Erbe, F.; Mayer, P.; Heyder, J.; Oberdörster, G.; Kreyling, W.G. Long-Term Clearance Kinetics of Inhaled Ultrafine Insoluble Iridium Particles from the Rat Lung, Including Transient Translocation into Secondary Organs. Inhal. Toxicol. 2004, 16, 453–459. [Google Scholar] [CrossRef]
- Doumandji, Z.; Safar, R.; Lovera-Leroux, M.; Nahle, S.; Cassidy, H.; Matallanas, D.; Rihn, B.; Ferrari, L.; Joubert, O. Protein and lipid homeostasis altered in rat macrophages after exposure to metallic oxide nanoparticles. Cell Biol. Toxicol. 2020, 36, 65–82. [Google Scholar] [CrossRef]
- Eidi, H.; Joubert, O.; Attik, G.; Duval, R.; Bottin, M.; Hamouia, A.; Maincent, P.; Rihn, B. Cytotoxicity assessment of heparin nanoparticles in NR8383 macrophages. Int. J. Pharm. 2010, 396, 156–165. [Google Scholar] [CrossRef]
- Nahle, S.; Cassidy, H.; Leroux, M.M.; Mercier, R.; Ghanbaja, J.; Doumandji, Z.; Matallanas, D.; Rihn, B.H.; Joubert, O.; Ferrari, L. Genes expression profiling of alveolar macrophages exposed to non-functionalized, anionic and cationic multi-walled carbon nanotubes shows three different mechanisms of toxicity. J. Nanobiotechnol. 2020, 18, 36. [Google Scholar] [CrossRef]
- Pruniéras, M.; Régnier, M.; Woodley, D. Methods for Cultivation of Keratinocytes with an Air-Liquid Interface. J. Investig. Dermatol. 1983, 81, S28–S33. [Google Scholar] [CrossRef]
- Antunes, M.B.; Woodworth, B.A.; Bhargave, G.; Xiong, G.; Aguilar, J.L.; Ratner, A.J.; Kreindler, J.L.; Rubenstein, R.C.; Cohen, N.A. Murine nasal septa for respiratory epithelial air-liquid interface cultures. BioTechniques 2007, 43, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Kesimer, M.; Kirkham, S.; Pickles, R.J.; Henderson, A.G.; Alexis, N.E.; DeMaria, G.; Knight, D.; Thornton, D.J.; Sheehan, J.K. Tracheobronchial air-liquid interface cell culture: A model for innate mucosal defense of the upper airways? Am. J. Physiol.-Lung Cell. Mol. Physiol. 2009, 296, L92–L100. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.K.; Yoo, J.W.; Lin, H.; Kim, Y.S.; Kim, D.D.; Choi, Y.M.; Park, S.K.; Lee, C.H.; Roh, H.J. Air-Liquid Interface Culture of Serially Passaged Human Nasal Epithelial Cell Monolayer for In Vitro Drug Transport Studies. Drug Deliv. 2005, 12, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Li, H.; Cho, H.J.; Bian, S.; Roh, H.J.; Lee, M.K.; Kim, J.S.; Chung, S.J.; Shim, C.K.; Kim, D.D. Air-Liquid Interface (ALI) Culture of Human Bronchial Epithelial Cell Monolayers as an In Vitro Model for Airway Drug Transport Studies. J. Pharm. Sci. 2007, 96, 341–350. [Google Scholar] [CrossRef]
- Kletting, S. Co-culture of human alveolar epithelial (hAELVi) and macrophage (THP-1) cell lines. ALTEX 2018, 35, 211–222. [Google Scholar] [CrossRef]
- Klein, S.G.; Serchi, T.; Hoffmann, L.; Blömeke, B.; Gutleb, A.C. An improved 3D tetraculture system mimicking the cellular organisation at the alveolar barrier to study the potential toxic effects of particles on the lung. Part. Fibre Toxicol. 2013, 10, 31. [Google Scholar] [CrossRef]
- Fröhlich, E.; Meindl, C. In Vitro Assessment of Chronic Nanoparticle Effects on Respiratory Cells. Nanomater.-Toxic. Risk Assess. 2015. [Google Scholar] [CrossRef]
- Cei, D.; Doryab, A.; Lenz, A.; Schröppel, A.; Mayer, P.; Burgstaller, G.; Nossa, R.; Ahluwalia, A.; Schmid, O. Development of a dynamic in vitro stretch model of the alveolar interface with aerosol delivery. Biotechnol. Bioeng. 2020, 118, 690–702. [Google Scholar] [CrossRef]
- Doryab, A.; Tas, S.; Taskin, M.B.; Yang, L.; Hilgendorff, A.; Groll, J.; Wagner, D.E.; Schmid, O. Evolution of Bioengineered Lung Models: Recent Advances and Challenges in Tissue Mimicry for Studying the Role of Mechanical Forces in Cell Biology. Adv. Funct. Mater. 2019, 29, 1903114. [Google Scholar] [CrossRef]
- Huh, D.; Matthews, B.D.; Mammoto, A.; Montoya-Zavala, M.; Hsin, H.Y.; Ingber, D.E. Reconstituting Organ-Level Lung Functions on a Chip. Science 2010, 328, 1662–1668. [Google Scholar] [CrossRef]
- Agraval, H.; Sharma, J.R.; Dholia, N.; Yadav, U.C.S. Air-Liquid Interface Culture Model to Study Lung Cancer-Associated Cellular and Molecular Changes. In Cancer Biomarkers; Deep, G., Ed.; Series Title: Methods in Molecular Biology; Springer: New York, NY, USA, 2022; Volume 2413, pp. 133–144. [Google Scholar] [CrossRef]
- Ziegler, P.; Tian, Y.; Bai, Y.; Abrahamsson, S.; Bäckerholm, A.; Reznik, A.S.; Green, A.; Moore, J.A.; Lee, S.E.; Myerburg, M.M.; et al. A primary nasopharyngeal three-dimensional air-liquid interface cell culture model of the pseudostratified epithelium reveals differential donor- and cell type-specific susceptibility to Epstein-Barr virus infection. PLoS Pathog. 2021, 17, e1009041. [Google Scholar] [CrossRef] [PubMed]
- Aydin, M.; Naumova, E.A.; Bellm, A.; Behrendt, A.K.; Giachero, F.; Bahlmann, N.; Zhang, W.; Wirth, S.; Paulsen, F.; Arnold, W.H.; et al. From Submerged Cultures to 3D Cell Culture Models: Evolution of Nasal Epithelial Cells in Asthma Research and Virus Infection. Viruses 2021, 13, 387. [Google Scholar] [CrossRef] [PubMed]
- Xiong, C.Y.; Cheng, X.G.; Dong, P.; Rong, D.Y.; Li, P.L.; Dong, S.J.; Jun, D.Z.; Rong, H.B. Three-dimensional Culture of Human Airway Epithelium in Matrigel for Evaluation of Human Rhinovirus C and Bocavirus Infections. Biomed. Environ. Sci. 2018, 31, 136. [Google Scholar] [CrossRef]
- Braun, R.; Lapshyna, O.; Eckelmann, S.; Honselmann, K.; Bolm, L.; ten Winkel, M.; Deichmann, S.; Schilling, O.; Kruse, C.; Keck, T.; et al. Organotypic Slice Cultures as Preclinical Models of Tumor Microenvironment in Primary Pancreatic Cancer and Metastasis. J. Vis. Exp. 2021, 172, 62541. [Google Scholar] [CrossRef] [PubMed]
- Runft, S.; Färber, I.; Krüger, J.; Krüger, N.; Armando, F.; Rocha, C.; Pöhlmann, S.; Burigk, L.; Leitzen, E.; Ciurkiewicz, M.; et al. Alternatives to animal models and their application in the discovery of species susceptibility to SARS-CoV-2 and other respiratory infectious pathogens: A review. Vet. Pathol. 2022. [Google Scholar] [CrossRef]
- Choi, K.Y.G.; Wu, B.C.; Lee, A.H.Y.; Baquir, B.; Hancock, R.E.W. Utilizing Organoid and Air-Liquid Interface Models as a Screening Method in the Development of New Host Defense Peptides. Front. Cell. Infect. Microbiol. 2020, 10, 228. [Google Scholar] [CrossRef]
- Rajan, A.; Weaver, A.M.; Aloisio, G.M.; Jelinski, J.; Johnson, H.L.; Venable, S.F.; McBride, T.; Aideyan, L.; Piedra, F.A.; Ye, X.; et al. The Human Nose Organoid Respiratory Virus Model: An Ex Vivo Human Challenge Model To Study Respiratory Syncytial Virus (RSV) and Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Pathogenesis and Evaluate Therapeutics. mBio 2022, 13, e03511–e03521. [Google Scholar] [CrossRef]
- Tran, B.M.; Grimley, S.L.; McAuley, J.L.; Hachani, A.; Earnest, L.; Wong, S.L.; Caly, L.; Druce, J.; Purcell, D.F.J.; Jackson, D.C.; et al. Air-Liquid-Interface Differentiated Human Nose Epithelium: A Robust Primary Tissue Culture Model of SARS-CoV-2 Infection. Int. J. Mol. Sci. 2022, 23, 835. [Google Scholar] [CrossRef]
| Initital Protcol (57 L, 30 mg/mL NP) | Current Protcol (114 L, 15 mg/mL NP) | |||
|---|---|---|---|---|
| Exposure | Mass Deposited (g/cm2) | Time (min) | Mass Deposited (g/cm2) | Time (min) |
| Exp 1 | 2.1 | 6 | 4.2 | 5 |
| Exp 2 | 7.4 | 24 | 6.1 | 5 |
| Exp 3 | 7.4 | 6 | 5.7 | 5 |
| Exp 4 | 3.5 | 6 | 5.5 | 5 |
| Mean | 5.1 | 10.5 | 5.4 | 5 |
| SD | 2.7 | 9 | 0.8 | 0 |
| Initial Protocol (57 L, 30 mg/mL NP) | Current Protocol (114 L, 15 mg/mL NP) | ||||||
|---|---|---|---|---|---|---|---|
| Exposure | Biological Replicate | RNA Quantity (g/L) | OD 260/280 | OD 260/230 | RNA Quantity (g/L) | OD 260/280 | OD 260/230 |
| 1 | 89.04 | 1.66 | 1.84 | 32.31 | 2.09 | 2.09 | |
| 2 | 7.99 | 2.25 | 0.46 | 55.30 | 2.01 | 1.80 | |
| HO | 3 | 121.07 | 2.12 | 1.14 | 69.60 | 2.13 | 2.18 |
| 4 | 206.30 | 2.04 | 2.32 | 16.09 | 2.08 | 1.43 | |
| 5 | / | / | / | 48.94 | 2.18 | 1.98 | |
| 1 | 3.30 | 1.86 | 0.26 | 3.61 | 1.83 | 3.72 | |
| 2 | 96.96 | 2.03 | 1.81 | 61.96 | 2.14 | 2.16 | |
| TiO NP | 3 | 8.01 | 2.48 | 51.25 | 91.57 | 1.99 | 2.05 |
| 4 | 134.43 | 2.08 | 1.55 | 58.93 | 2.10 | 2.02 | |
| 5 | / | / | / | 66.30 | 2.06 | 2.17 | |
| Mean | 83.61 | 2.07 | 1.33 | 50.46 | 2.06 | 2.16 | |
| SD | 73.27 | 0.24 | 0.70 | 26.34 | 0.10 | 0.59 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leroux, M.M.; Hocquel, R.; Bourge, K.; Kokot, B.; Kokot, H.; Koklič, T.; Štrancar, J.; Ding, Y.; Kumar, P.; Schmid, O.; et al. Aerosol–Cell Exposure System Applied to Semi-Adherent Cells for Aerosolization of Lung Surfactant and Nanoparticles Followed by High Quality RNA Extraction. Nanomaterials 2022, 12, 1362. https://doi.org/10.3390/nano12081362
Leroux MM, Hocquel R, Bourge K, Kokot B, Kokot H, Koklič T, Štrancar J, Ding Y, Kumar P, Schmid O, et al. Aerosol–Cell Exposure System Applied to Semi-Adherent Cells for Aerosolization of Lung Surfactant and Nanoparticles Followed by High Quality RNA Extraction. Nanomaterials. 2022; 12(8):1362. https://doi.org/10.3390/nano12081362
Chicago/Turabian StyleLeroux, Mélanie M., Romain Hocquel, Kevin Bourge, Boštjan Kokot, Hana Kokot, Tilen Koklič, Janez Štrancar, Yaobo Ding, Pramod Kumar, Otmar Schmid, and et al. 2022. "Aerosol–Cell Exposure System Applied to Semi-Adherent Cells for Aerosolization of Lung Surfactant and Nanoparticles Followed by High Quality RNA Extraction" Nanomaterials 12, no. 8: 1362. https://doi.org/10.3390/nano12081362
APA StyleLeroux, M. M., Hocquel, R., Bourge, K., Kokot, B., Kokot, H., Koklič, T., Štrancar, J., Ding, Y., Kumar, P., Schmid, O., Rihn, B. H., Ferrari, L., & Joubert, O. (2022). Aerosol–Cell Exposure System Applied to Semi-Adherent Cells for Aerosolization of Lung Surfactant and Nanoparticles Followed by High Quality RNA Extraction. Nanomaterials, 12(8), 1362. https://doi.org/10.3390/nano12081362







