A Study of Ta2O5 Nanopillars with Ni Tips Prepared by Porous Anodic Alumina Through-Mask Anodization
Abstract
1. Introduction
2. Experimental
3. Results and Discussion
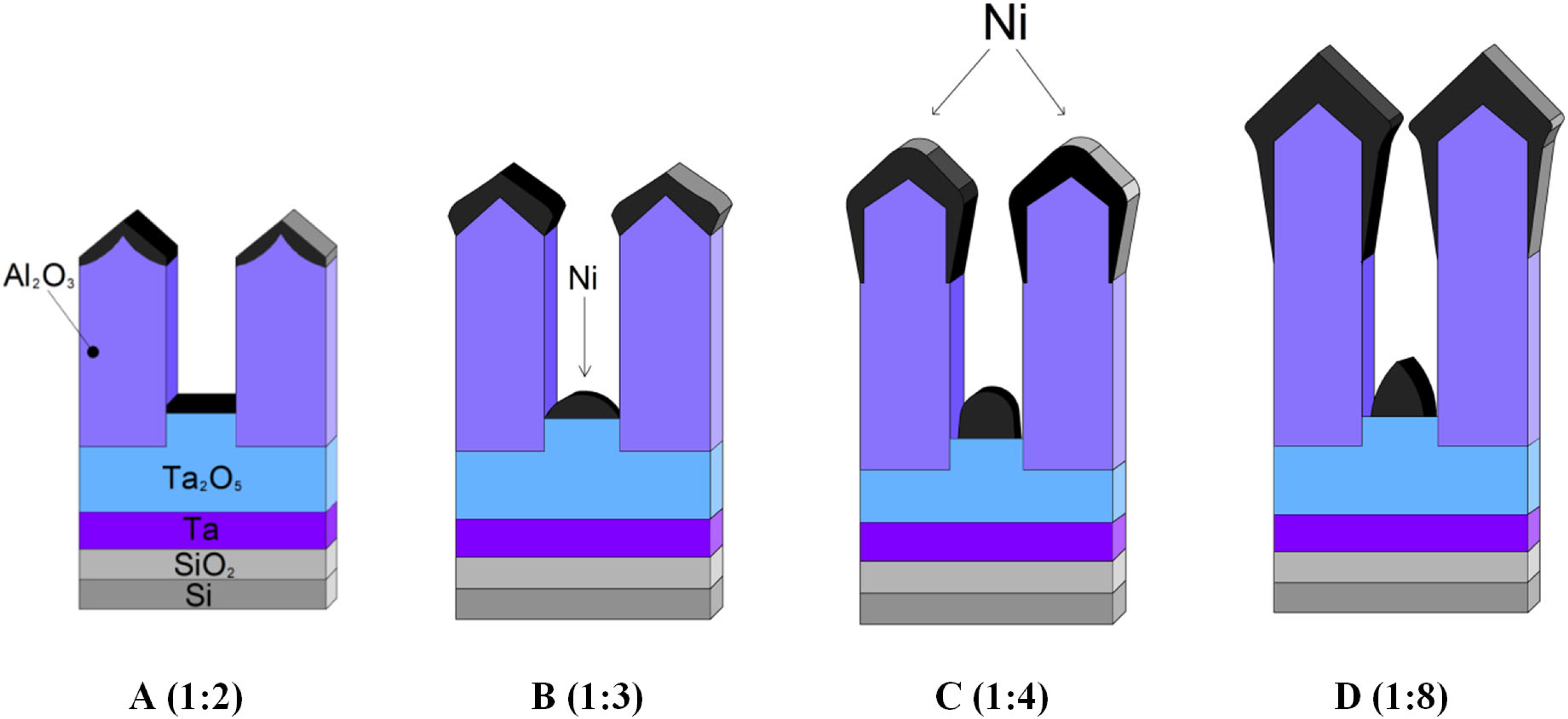
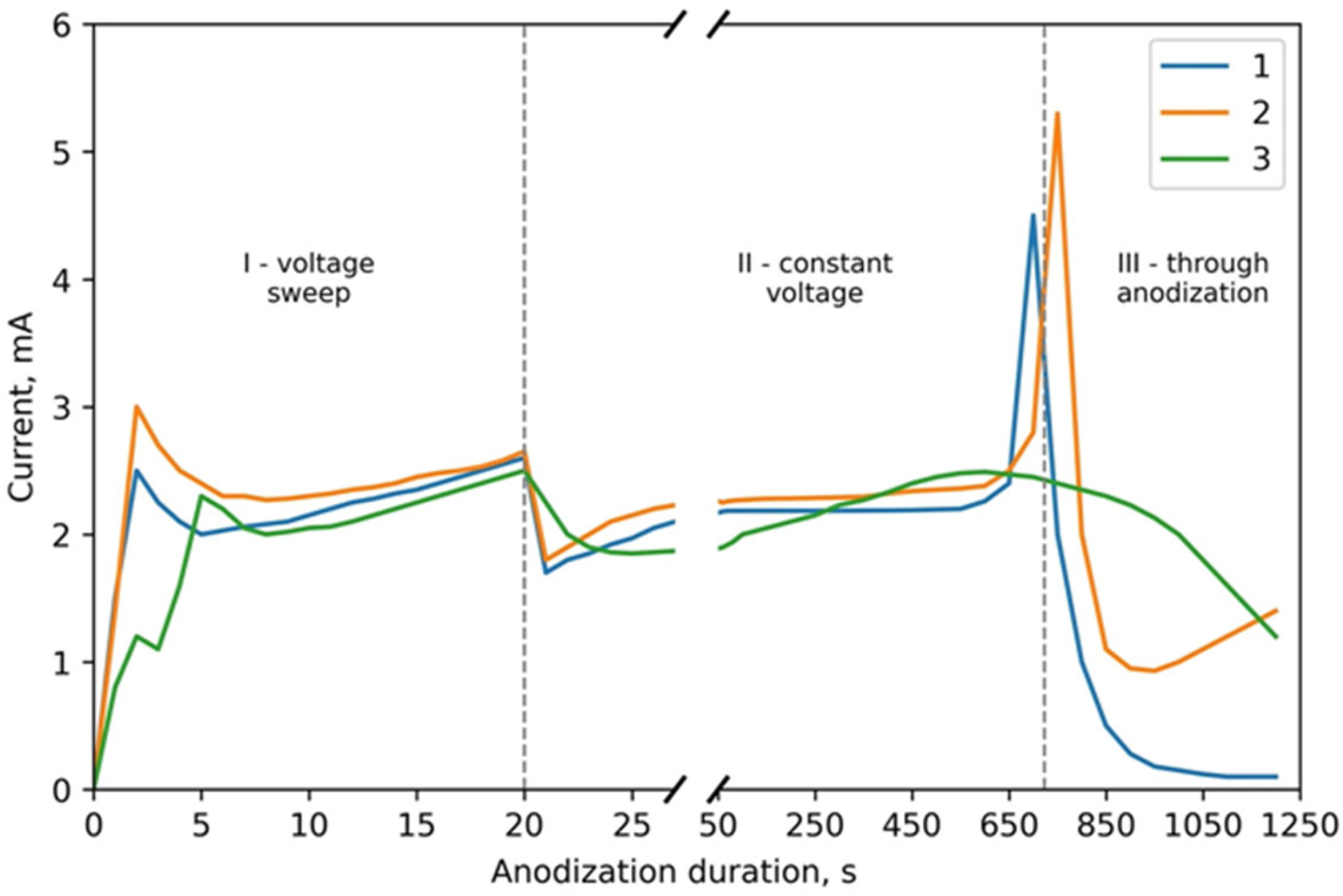
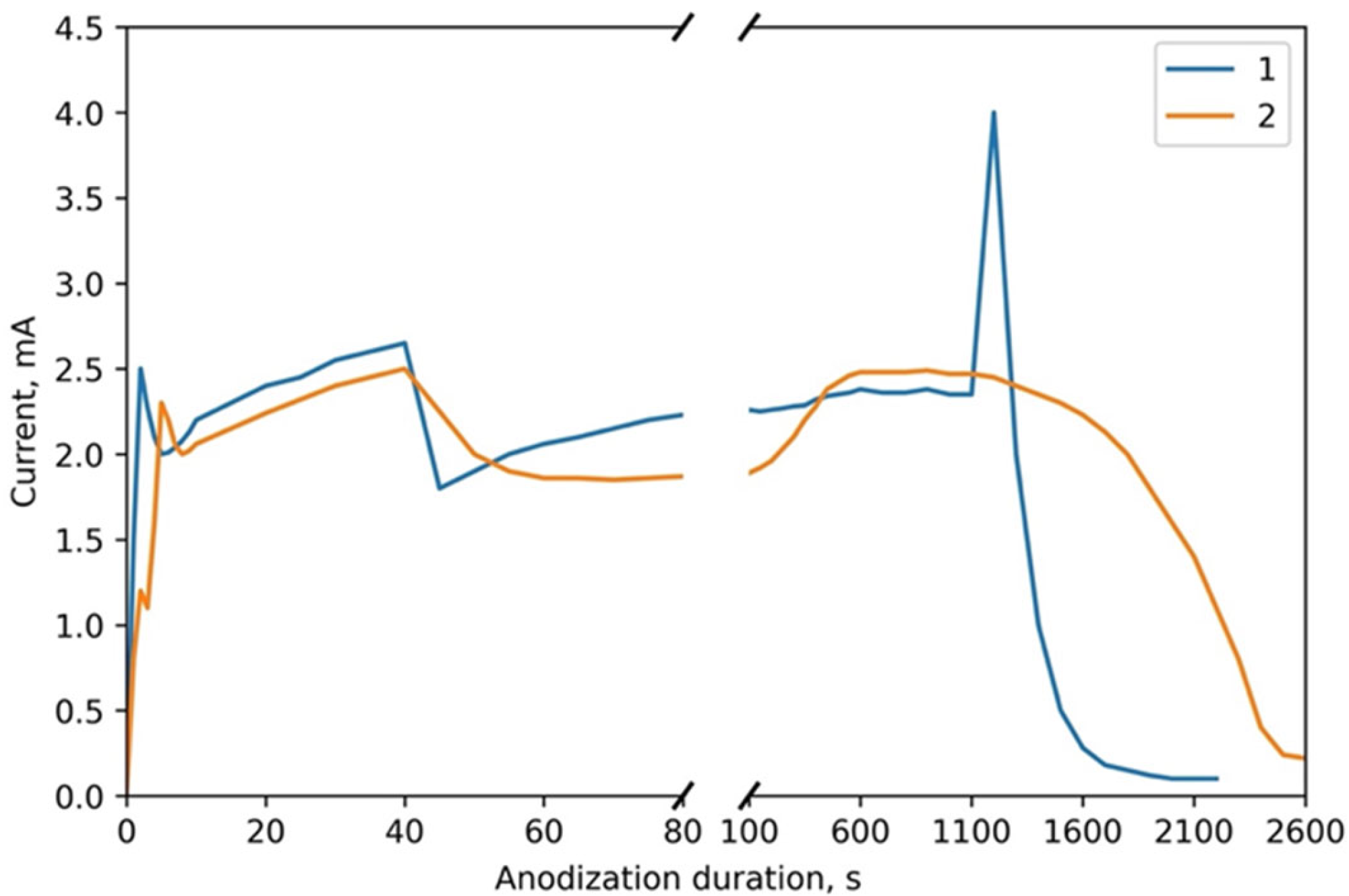
3.1. Mechanism of the Ta2O5 Pillar Growth
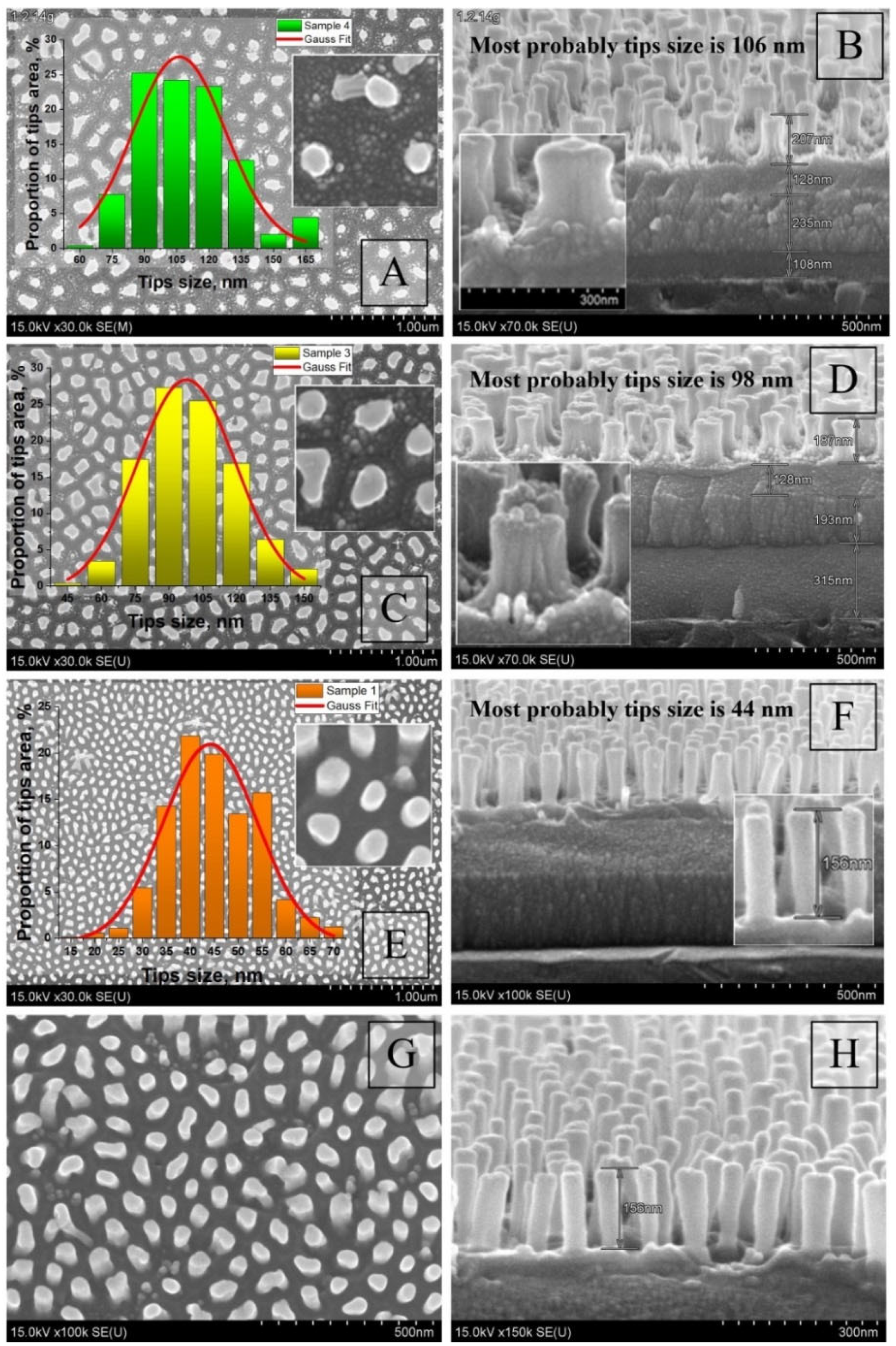
3.2. Morphological and Microstructural Characterization
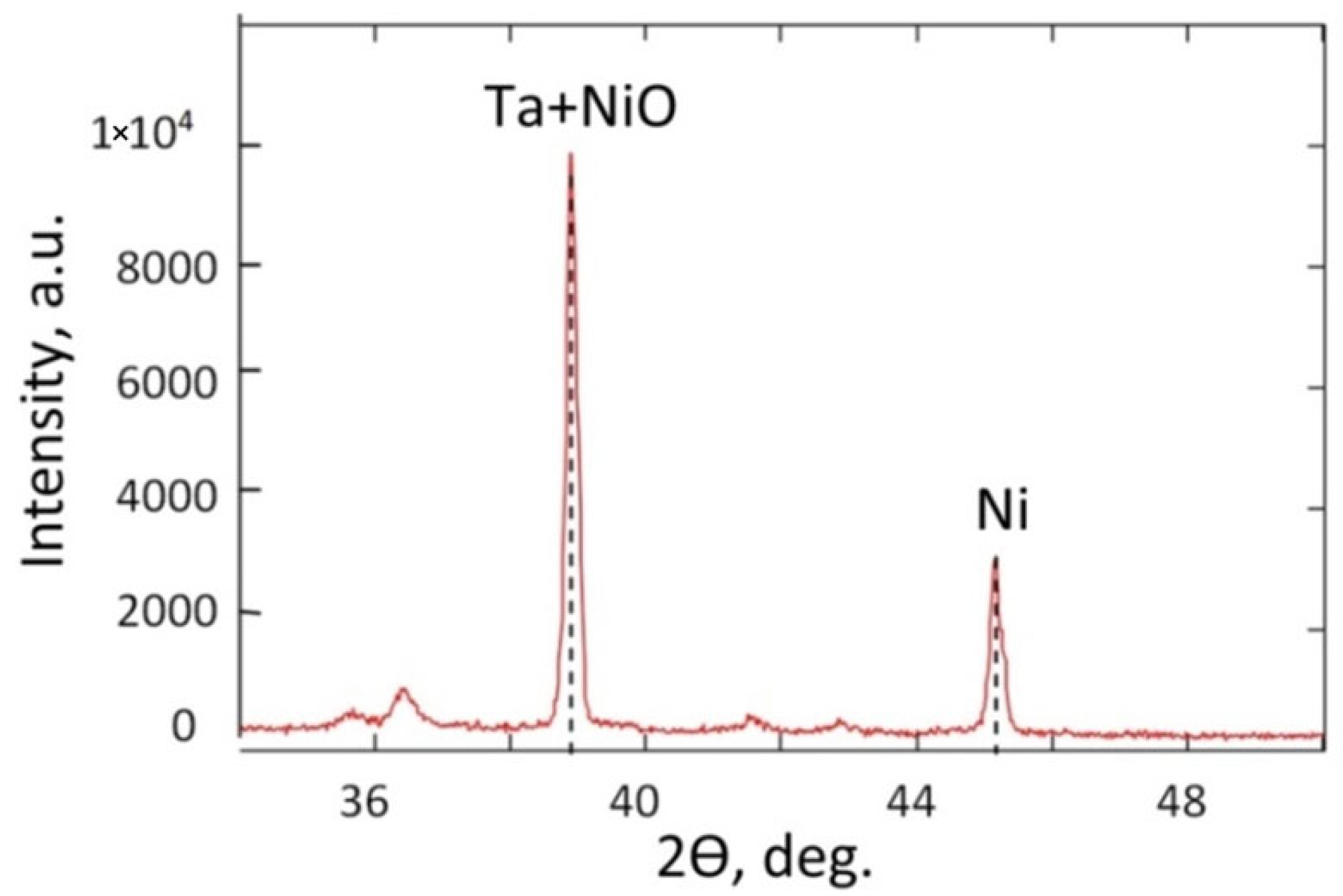
3.3. Crystal Structure Analysis
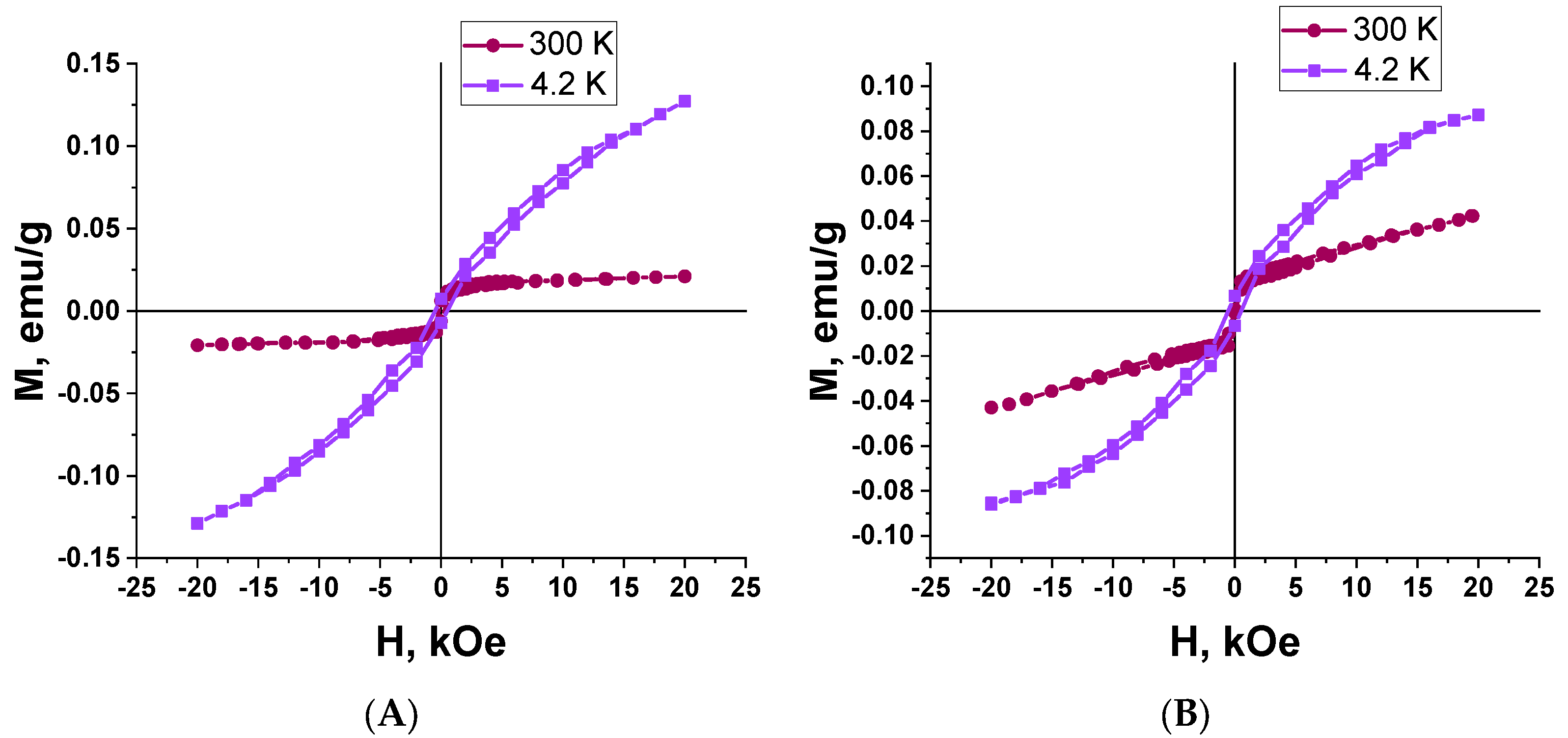
3.4. Magnetic Properties Study
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, C.; Akakuru, O.U.; Zheng, J.; Wu, A. Applications of Iron Oxide-Based Magnetic Nanoparticles in the Diagnosis and Treatment of Bacterial Infections. Front. Bioeng. Biotechnol. 2019, 7, 141. [Google Scholar] [CrossRef] [PubMed]
- Darton, N.J.; Ionescu, A.; Llandro, J. (Eds.) Magnetic Nanoparticles in Biosensing and Medicine; Cambridge University Press: Cambridge, UK, 2019. [Google Scholar] [CrossRef]
- Shen, W.-Z.; Cetinel, S.; Montemagno, C. Application of biomolecular recognition via magnetic nanoparticle in nanobiotechnology. J. Nanopart. Res. 2018, 20, 130. [Google Scholar] [CrossRef]
- Tsurin, V.A.; Yermakov, A.Y.; Uimin, M.A.; Mysik, A.A.; Shchegoleva, N.N.; Gaviko, V.S.; Maikov, V.V. Synthesis, structure, and magnetic properties of iron and nickel nanoparticles encapsulated into carbon. Phys. Solid State 2014, 56, 287–301. [Google Scholar] [CrossRef]
- Wu, K.; Su, D.; Liu, J.; Saha, R.; Wang, J.-P. Magnetic nanoparticles in nanomedicine: A review of recent advances. Nanotechnology 2019, 30, 502003. [Google Scholar] [CrossRef] [PubMed]
- Abd Elrahman, A.A.; Mansour, F.R. Targeted magnetic iron oxide nanoparticles: Preparation, functionalization and biomedical application. J. Drug Deliv. Sci. Technol. 2019, 52, 702–712. [Google Scholar] [CrossRef]
- Vorobjova, A.; Tishkevich, D.; Shimanovich, D.; Zdorovets, M.; Kozlovskiy, A.; Zubar, T.; Vinnik, D.; Dong, M.; Trukhanov, S.; Trukhanov, A.; et al. Electrochemical Behaviour of Ti/Al2O3/Ni Nanocomposite Material in Artificial Physiological Solution: Prospects for Biomedical Application. Nanomaterials 2020, 10, 173. [Google Scholar] [CrossRef]
- Mohammadi Ziarani, G.; Malmir, M.; Lashgari, N.; Badiei, A. The role of hollow magnetic nanoparticles in drug delivery. RSC Adv. 2019, 9, 25094–25106. [Google Scholar] [CrossRef]
- Vangijzegem, T.; Stanicki, D.; Laurent, S. Magnetic iron oxide nanoparticles for drug delivery: Applications and characteristics. Expert Opin. Drug Deliv. 2019, 16, 69–78. [Google Scholar] [CrossRef]
- Domagalski, J.T.; Xifre-Perez, E.; Tabrizi, M.A.; Ferre-Borrull, J.; Marsal, L.F. Magnetic nanoparticle decorated anodic alumina nanotubes for fluorescent detection of cathepsin B. J. Colloid Interface Sci. 2021, 584, 236–245. [Google Scholar] [CrossRef]
- Lee, J.S.; Gu, G.H.; Kim, H.; Jeong, K.S.; Bae, J.; Suh, J.S. Growth of Carbon Nanotubes on Anodic Aluminum Oxide Templates: Fabrication of a Tube-in-Tube and Linearly Joined Tube. Chem. Mater. 2001, 13, 2387–2391. [Google Scholar] [CrossRef]
- Vorobjova, A.; Prudnikava, A.; Shaman, Y.; Shulitski, B.; Labunov, V.; Gavrilo, S.; Belov, A.; Basaev, A. Specific Features of the Carbon Nanotubes Nucleation and Growth in the Porous Alumina Membrane. Adv. Mater. Sci. Appl. 2014, 3, 46–52. [Google Scholar] [CrossRef]
- Choi, J.; Sauer, G.; Nielsch, K.; Wehrspohn, R.B.; Gösele, U. Hexagonally Arranged Monodisperse Silver Nanowires with Adjustable Diameter and High Aspect Ratio. Chem. Mater. 2003, 15, 776–779. [Google Scholar] [CrossRef]
- Sander, M.S.; Tan, L.-S. Nanoparticle Arrays on Surfaces Fabricated Using Anodic Alumina Films as Templates. Adv. Funct. Mater. 2003, 13, 393–397. [Google Scholar] [CrossRef]
- Yin, A.J.; Li, J.; Jian, W.; Bennett, A.J.; Xu, J.M. Fabrication of highly ordered metallic nanowire arrays by electrodeposition. Appl. Phys. Lett. 2001, 79, 1039–1041. [Google Scholar] [CrossRef]
- Selvakumar, N.; Barshilia, H.C. Review of physical vapor deposited (PVD) spectrally selective coatings for mid- and high-temperature solar thermal applications. Sol. Energy Mater. Sol. Cells 2012, 98, 1–23. [Google Scholar] [CrossRef]
- Ahmadzadeh, M.; Almasi-Kashi, M. Investigations of Magnetic Properties Through Electrodeposition Current and Controlled Cu Content in Pulse Electrodeposited CoFeCu Nanowires. J. Nanostruct. 2015, 5, 257–261. [Google Scholar]
- Pathak, S.; Verma, R.; Kumar, P.; Singh, A.; Singhal, S.; Sharma, P.; Jain, K.; Pant, R.P.; Wang, X. Facile Synthesis, Static, and Dynamic Magnetic Characteristics of Varying Size Double-Surfactant-Coated Mesoscopic Magnetic Nanoparticles Dispersed Stable Aqueous Magnetic Fluids. Nanomaterials 2021, 11, 3009. [Google Scholar] [CrossRef]
- Kumar, P.; Pathak, S.; Singh, A.; Khanduri, H.; Kuldeep; Jain, K.; Tawale, J.; Wang, L.; Basheed, G.A.; Pant, R.P. Enhanced static and dynamic magnetic properties of PEG-400 coated CoFe2−xErxO4 (0.7 × 0) nanoferrites. J. Alloys Compd. 2021, 887, 161418. [Google Scholar] [CrossRef]
- Acharya, S.; Hill, J.P.; Ariga, K. Soft Langmuir-Blodgett Technique for Hard Nanomaterials. Adv. Mater. 2009, 21, 2959–2981. [Google Scholar] [CrossRef]
- Moehwald, H.; Brezesinski, G. From Langmuir Monolayers to Multilayer Films. Langmuir 2016, 32, 10445–10458. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Samiei, M.; Davaran, S. Magnetic nanoparticles: Preparation, physical properties, and applications in biomedicine. Nanoscale Res. Lett. 2012, 7, 144. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Park, S.-J. Porous Anodic Aluminum Oxide: Anodization and Templated Synthesis of Functional Nanostructures. Chem. Rev. 2014, 114, 7487–7556. [Google Scholar] [CrossRef] [PubMed]
- Tishkevich, D.I.; Vorobjova, A.I.; Vinnik, D.A. Template Assisted Ni Nanowires Fabrication. Mater. Sci. Forum 2019, 946, 235–241. [Google Scholar] [CrossRef]
- Vorobjova, A.I.; Shimanovich, D.L.; Sycheva, O.A.; Ezovitova, T.I.; Tishkevich, D.I.; Trykhanov, A.V. Studying the Thermodynamic Properties of Composite Magnetic Material Based on Anodic Alumina. Russ. Microelectron. 2019, 48, 107–118. [Google Scholar] [CrossRef]
- Tishkevich, D.I.; Grabchikov, S.S.; Grabchikova, E.A.; Vasin, D.S.; Lastovskiy, S.B.; Yakushevich, A.S.; Vinnik, D.A.; Zubar, T.I.; Kalagin, I.V.; Mitrofanov, S.V.; et al. Modeling of paths and energy losses of high-energy ions in single-layered and multilayered materials. IOP Conf. Ser. Mater. Sci. Eng. 2020, 848, 012089. [Google Scholar] [CrossRef]
- Sousa, C.T.; Leitao, D.C.; Proenca, M.P.; Ventura, J.; Pereira, A.M.; Araujo, J.P. Nanoporous alumina as templates for multifunctional applications. Appl. Phys. Rev. 2014, 1, 031102. [Google Scholar] [CrossRef]
- Vorobjova, A.I.; Shimanovich, D.L.; Yanushkevich, K.I.; Prischepa, S.L.; Outkina, E.A. Properties of Ni and Ni–Fe nanowires electrochemically deposited into a porous alumina template. Beilstein J. Nanotechnol. 2016, 7, 1709–1717. [Google Scholar] [CrossRef]
- Zhou, Z.; Nonnenmann, S.S. Progress in Nanoporous Templates: Beyond Anodic Aluminum Oxide and Towards Functional Complex Materials. Materials 2019, 12, 2535. [Google Scholar] [CrossRef]
- Vorobjova, A.I.; Shimanovich, D.L.; Outkina, E.A.; Khodin, A.A. Highly ordered porous alumina membranes for Ni–Fe nanowires fabrication. Appl. Phys. A 2018, 124, 23. [Google Scholar] [CrossRef]
- Vorobjova, A.; Tishkevich, D.; Shimanovich, D.; Zubar, T.; Astapovich, K.; Kozlovskiy, A.; Zdorovets, M.; Zhaludkevich, A.; Lyakhov, D.; Michels, D.; et al. The influence of the synthesis conditions on the magnetic behaviour of the densely packed arrays of Ni nanowires in porous anodic alumina membranes. RSC Adv. 2021, 11, 3952. [Google Scholar] [CrossRef]
- Tishkevich, D.I.; Vorobjova, A.I.; Shimanovich, D.L.; Vinnik, D.A.; Zubar, T.I.; Kozlovskiy, A.L.; Zdorovets, M.V.; Yakimchuk, D.V.; Trukhanov, S.V.; Trukhanov, A.V. Formation and corrosion properties of Ni-based composite material in the anodic alumina porous matrix. J. Alloys Compd. 2019, 804, 139–146. [Google Scholar] [CrossRef]
- Tishkevich, D.I.; Vorobjova, A.I.; Vinnik, D.A. Formation and corrosion behavior of Nickel/Alumina nanocomposites. Solid State Phenom. 2020, 299, 100–106. [Google Scholar] [CrossRef]
- Roslyakov, I.V.; Napolskii, K.S.; Evdokimov, P.V.; Napolskiy, F.S.; Dunaev, A.V.; Eliseev, A.A.; Lukashin, A.V.; Tretyakov, Y.D. Thermal properties of anodic alumina membranes. Nanosyst. Phys. Chem. Math. 2013, 4, 120–129. [Google Scholar]
- Vorobjova, A.; Prudnikava, A.; Shaman, Y.; Shulitski, B.; Labunov, V.; Gavrilov, S. Carbon Nanotube-Based Composites Synthesized Using Porous Aluminum Oxide. Adv. Mater. Sci. Appl. 2014, 3, 46–52. [Google Scholar]
- Trukhanov, A.V.; Kozlovskiy, A.L.; Ryskulov, A.E.; Uglov, V.V.; Kislitsin, S.B.; Zdorovets, M.V.; Trukhanov, S.V.; Zubar, T.I.; Astapovich, K.A.; Tishkevich, D.I. Control of structural parameters and thermal conductivity of BeO ceramics using heavy ion irradiation and post-radiation annealing. Ceram. Int. 2019, 45, 15412–15416. [Google Scholar] [CrossRef]
- Poinern, G.E.J.; Le, X.T.; Hager, M.; Becker, T.; Fawcett, D. Electrochemical Synthesis, Characterisation, and Preliminary Biological Evaluation of an Anodic Aluminium Oxide Membrane with a pore size of 100 nanometres for a Potential Cell Culture Substrate. Am. J. Biomed. Eng. 2013, 3, 119–131. [Google Scholar]
- Vorobyova, A.I.; Sokol, V.A.; Outkina, E.A. SEM investigation of pillared microstructures formed by electrochemical anodization. Appl. Phys. A Mater. Sci. Process. 1998, 67, 487–492. [Google Scholar] [CrossRef]
- Vorobyova, A.I.; Outkina, E.A.; Komar, O.M. Study of metal pillar nanostructure formation with thin porous alumina template. Thin Solid Films 2013, 548, 109–117. [Google Scholar] [CrossRef]
- Alborzi, Z.; Hassanzadeh, A.; Golzan, M.M. Superparamagnetic Behavior of the Magnetic Hysteresis Loop in the Fe2O3@Pt Core-Shell Nanoparticles. Int. J. Nanosci. Nanotechnol. 2012, 8, 93–98. [Google Scholar]
- Liu, H.; Wu, J.; Min, J.H.; Hou, P.; Song, A.-Y.; Kim, Y.K. Non-aqueous synthesis of water-dispersible Fe3O4–Ca3(PO4)2 core–shell nanoparticles. Nanotechnology 2011, 22, 055701. [Google Scholar] [CrossRef]
- Hirotaka, S.; Takayuki, H. Fabrication of Magnetic Nanodot Arrays for Patterned Magnetic Recording Media. J. Nanosci. Nanotechnol. 2007, 7, 225–231. [Google Scholar]
- Yasui, K.; Nishio, K.; Masuda, H. Fabrication of Nanocomposites by Filling Nanoholes in Highly Ordered Anodic Porous Alumina by Vacuum Deposition of Metal. Jpn. J. Appl. Phys. 2005, 44, L1181–L1183. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, Y.W.; Lee, J.S.; Yoo, J.-B.; Park, C.Y. Magnetic Properties of Ni Nanostructures Fabricated Using Anodic Aluminum Oxide Templates. J. Korean Phys. Soc. 2005, 47, 313–317. [Google Scholar]
- Liu, K.; Nogue, J.; Leighton, C.; Masuda, H.; Nishio, K.; Roshchin, I.V.; Schuller, I.K. Fabrication and thermal stability of arrays of Fe nanodots. Appl. Phys. Lett. 2002, 81, 4434–4436. [Google Scholar] [CrossRef]
- Xu, Q.; Meng, G.; Han, F. Porous AAO template-assisted rational synthesis of large-scale 1D hybrid and hierarchically branched nanoarchitectures. Prog. Mater. Sci. 2018, 95, 243–285. [Google Scholar] [CrossRef]
- Ivanov, Y.P.; Alfadhel, A.; Alnassar, M.; Perez, J.E.; Vazquez, M.; Chuvilin, A.; Kosel, J. Tunable magnetic nanowires for biomedical and harsh environment applications. Sci. Rep. 2016, 6, 24189. [Google Scholar] [CrossRef]
- Moreno, J.A.; Bran, C.; Vazquez, M.; Kosel, J. Cylindrical Magnetic Nanowires Applications. IEEE Trans. Magn. 2021, 57, 1–17. [Google Scholar] [CrossRef]
- Tishkevich, D.I.; Vorobjova, A.I.; Trukhanov, A.V. Thermal stability of nano-crystalline nickel electrodeposited into porous alumina. Solid State Phenom. 2020, 299, 281–286. [Google Scholar] [CrossRef]
- Lei, Y.; Cai, W.; Wilde, G. Highly ordered nanostructures with tunable size, shape and properties: A new way to surface nano-patterning using ultra-thin alumina masks. Prog. Mater. Sci. 2007, 52, 465–539. [Google Scholar] [CrossRef]
- Tishkevich, D.; Grabchikov, S.; Zubar, T.; Vasin, D.; Trukhanov, S.; Vorobjova, A.; Yakimchuk, D.; Kozlovskiy, A.; Zdorovets, M.; Giniyatova, S.; et al. Early-Stage Growth Mechanism and Synthesis Conditions-Dependent Morphology of Nanocrystalline Bi Films Electrodeposited from Perchlorate Electrolyte. Nanomaterials 2020, 10, 1245. [Google Scholar] [CrossRef]
- Slimani, Y.; Algarou, N.A.; Almessiere, M.A.; Sadaqat, A.; Vakhitov, M.G.; Klygach, D.S.; Tishkevich, D.I.; Trukhanov, A.V.; Guner, S.; Hakeem, A.S.; et al. Fabrication of exchange coupled hard/soft magnetic nanocomposites: Correlation between composition, magnetic, optical and microwave properties. Arab. J. Chem. 2021, 14, 102992. [Google Scholar] [CrossRef]
- Pringle, J.P.S. The anodic oxidation of superimposed metallic layers: Theory. Electrochim. Acta 1980, 25, 1423–1437. [Google Scholar] [CrossRef]
- Pringle, J.P.S. Transport Numbers of Metal and Oxygen during the Anodic Oxidation of Tantalum. J. Electrochem. Soc. 1973, 120, 398. [Google Scholar] [CrossRef]
- Vorobyova, A.I.; Outkina, E.A. Study of pillar microstructure formation with anodic oxides. Thin Solid Films 1998, 324, 1–10. [Google Scholar] [CrossRef]
- Mozalev, A.; Sakairi, M.; Saeki, I.; Takahashi, H. Nucleation and growth of the nanostructured anodic oxides on tantalum and niobium under the porous alumina film. Electrochim. Acta 2003, 48, 3155–3170. [Google Scholar] [CrossRef]
- Sjöström, T.; Fox, N.; Su, B. A study on the formation of titania nanopillars during porous anodic alumina through-mask anodization of Ti substrates. Electrochim. Acta 2010, 56, 203–210. [Google Scholar] [CrossRef]
- Chu, S.-Z.; Inoue, S.; Wada, K.; Hishita, S.; Kurashima, K. Self-Organized Nanoporous Anodic Titania Films and Ordered Titania Nanodots/Nanorods on Glass. Adv. Func. Mater. 2005, 15, 1343–1349. [Google Scholar] [CrossRef]
- Chen, P.-L.; Kuo, C.-T.; Tsai, T.-G.; Wu, B.-W.; Hsu, C.-C.; Pan, F.-M. Self-organized titanium oxide nanodot arrays by electrochemical anodization. Appl. Phys. Lett. 2003, 82, 2796–2798. [Google Scholar] [CrossRef]
- Li, A.P.; Müller, F.; Birner, A.; Nielsch, K.; Gösele, U. Hexagonal pore arrays with a 50–420 nm interpore distance formed by self-organization in anodic alumina. J. Appl. Phys. 1998, 84, 6023–6026. [Google Scholar] [CrossRef]
- Nielsch, K.; Choi, J.; Schwirn, K.; Wehrspohn, R.B.; Gösele, U. Self-ordering Regimes of Porous Alumina: The 10 Porosity Rule. Nano Lett. 2002, 2, 677–680. [Google Scholar] [CrossRef]
- Lu, Q.; Mato, S.; Skeldon, P.; Thompson, G.E.; Masheder, D.; Habazaki, H.; Shimizu, K. Anodic film growth on tantalum in dilute phosphoric acid solution at 20 and 85 °C. Electrochim. Acta 2002, 47, 2761–2767. [Google Scholar] [CrossRef]
- Ren, W.; Yang, G.-D.; Feng, A.-L.; Miao, R.-X.; Xia, J.-B.; Wang, Y.-G. Annealing effects on the optical and electrochemical properties of tantalum pentoxide films. J. Adv. Ceram. 2021, 10, 704–713. [Google Scholar] [CrossRef]
- Bright, T.J.; Watjen, J.I.; Zhang, Z.M.; Muratore, C.; Voevodin, A.A.; Koukis, D.I.; Tanner, D.B.; Arenas, D.J. Infrared optical properties of amorphous and nanocrystalline Ta2O5 thin films. J. Appl. Phys. 2013, 114, 083515. [Google Scholar] [CrossRef]
- Zubar, T.I.; Panina, L.V.; Kovaleva, N.N.; Sharko, S.A.; Tishkevich, D.I.; Vinnik, D.A.; Gudkova, S.A.; Trukhanova, E.L.; Trofimov, E.A.; Chizhik, S.A.; et al. Anomalies in growth of electrodeposited Ni-Fe nanogranular films. CrystEngComm 2019, 21, 2464. [Google Scholar] [CrossRef]
- Tochitskii, T.A.; Jones, G.A.; Blythe, H.J.; Fedosyuk, V.M.; Castro, J. Fine structure and possible growth mechanisms of some electrodeposited CuCo granular films. J. Magn. Magn. Mater. 2001, 224, 221–232. [Google Scholar] [CrossRef]
- Sorensen, C.M. Magnetism. In Nanoscale Materials in Chemistry; Klabunde, K.J., Ed.; John Wiley & Sons, Inc.: New York, NY, USA, 2001; pp. 169–221. [Google Scholar] [CrossRef]
- Fedosyuk, V.M. Nanogranular electrodeposited magnetic cobalt alloys. In Encyclopedia of Nanoscience and Nanotechnology; Nalwa, H.S., Ed.; American Scientific Publishers: Valencia, CA, USA, 2003; pp. 1–24. [Google Scholar]
- Prida, V.M.; Pirota, K.R.; Navas, D.; Asenjo, A.; Hernández-Vélez, M.; Vázquez, M. Self-Organized Magnetic Nanowire Arrays Based on Alumina and Titania Templates. J. Nanosci. Nanotechnol. 2007, 7, 272–285. [Google Scholar] [CrossRef]
- Tishkevich, D.; Vorobjova, A.; Shimanovich, D.; Kaniukov, E.; Kozlovskiy, A.; Zdorovets, M.; Vinnik, D.; Turutin, A.; Kubasov, I.; Kislyuk, A.; et al. Magnetic Properties of the Densely Packed Ultra-Long Ni Nanowires Encapsulated in Alumina Membrane. Nanomaterials 2021, 11, 1775. [Google Scholar] [CrossRef]
- Banerjee, P.; Perez, I.; Henn-Lecordier, L.; Lee, S.B.; Rubloff, G.W. Nanotubular metal–insulator–metal capacitor arrays for energy storage. Nat. Nanotechnol. 2009, 4, 292–296. [Google Scholar] [CrossRef]
- Yogeswaran, U.; Chen, S.-M. A Review on the Electrochemical Sensors and Biosensors Composed of Nanowires as Sensing Material. Sensors 2008, 8, 290–313. [Google Scholar] [CrossRef]
- Santos, A.; Vojkuvka, L.; Pallarés, J.; Ferré-Borrull, J.; Marsal, L. Cobalt and Nickel Nanopillars on Aluminium Substrates by Direct Current Electrodeposition Process. Nanoscale Res. Lett. 2009, 4, 1021. [Google Scholar] [CrossRef]
- Donkov, N.; Zykova, A.; Safonov, V.; Matveev, E. Modern Methods of Ta2O5 coatings deposition for biomedical applications. Probl. At. Sci. Technol. 2009, 1, 153–155. [Google Scholar]
- Um, J.; Park, J.J.; Flatau, A.; Zhou, W.; Zhang, Y.; Franklin, R.; Reddy, K.S.M.; Tan, L.; Sharma, A.; Sung, S.-Y.; et al. Template-assisted electrodeposited magnetic nanowires and their properties for applications. In Magnetic Nano-Microwires, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 675–695. [Google Scholar] [CrossRef]
- Shoso, S.; Takafumi, Y.; Hironori, M.; Seiji, A.; Yasuhiko, A.; Sumio, N.; Sumio, N.; Tomomi, Y.; Kennichi, S. Fabrication of ultra high density magnetic recording media using self-organized porous alumina nanohole array. Sci. Technol. Rep. Kansai Univ. 2009, 51, 1–7. [Google Scholar]
- Kazarkin, B.A.; Stsiapanau, A.A.; Belyaev, V.V.; Chausov, D.N. New applications of nanostructured materials in the prospect electronic devices. Int. Sci. J. Ind. 4.0 2017, 2, 272–274. [Google Scholar]
- Wang, S.K.; Chen, M.; Wang, H.; Li, G.; Chen, J.; Liu, L.; Xu, Y.; Jian, C.; Meng, X.; Zheng, S.; et al. Controllable synthesis of nickel nanowires and its application in high sensitivity, stretchable strain sensor for body motion sensing. J. Mater. Chem. C. 2018, 6, 4737–4745. [Google Scholar] [CrossRef]




| Deposition Process Parameters | Tantalum | Aluminum |
|---|---|---|
| Residual gas pressure, Pa | 1.3 × 10−3 | 1.4 × 10−4 |
| Substrate temperature, K | 523 | 423 |
| Accelerating voltage, kV | 8 | 8 |
| Beam current, A | 0.4 | 1.2 |
| Deposition rate, nm·s−1 | 1.0 ± 0.2 | 5.0 ± 0.5 |
| Thickness, nm | 450 ± 50 | (1000–2000) ± 50 |
| Sample Type | Sample No. | Electrolyte | Voltage, UAl, V | Voltage, UTa, V | Pillar (Pore) Diameter, nm | Interpore Distance, nm | Pillar Height, nm | Pillar Density, per cm2 |
|---|---|---|---|---|---|---|---|---|
| type I | 1 | C2H2O4 | 40 | 40 | 40 ± 5 | 100 ± 5 | 150 ± 10 | 10 × 109 |
| 2 | 70 | 40 ± 5 | 100 ± 5 | 160 ± 10 | 10 × 109 | |||
| type II | 3 | H3PO4 | 80 | 80 | 100 ± 5 | 200 ± 5 | 180 ± 10 | 3 × 109 |
| 4 | 90 | 100 ± 5 | 210 ± 5 | 220 ± 10 | 2 × 109 |
| Material | Crystallographic Direction | 2θ, deg. | I, % | Coherence Region Size L, nm |
|---|---|---|---|---|
| β-Ta | (002) | 36.4 (35.61) JCPDS (25-1280) | ||
| α-Ta | (110) | 38.37 (38.4) JCPDS (25-1280) | 100 | - |
| Ni | (111) face-centered cubic (fcc) structure | 44.15 (44.51) JCPDS PDF-card (270-989) | 32 | 13 |
| NiO | (111) | 37.8 (38.33) JCPDS (47-1049) | - | - |
| Type | Sample | Temperature, K | Hc, kOe | Mr, emu/g | Ms, emu/g | Mr/Ms |
|---|---|---|---|---|---|---|
| I | 2 | 4.2 | 511 | 0.007 | 0.127 | 0.06 |
| 300 | - | - | - | - | ||
| II | 3 | 4.2 | 525 | 0.007 | 0.087 | 0.08 |
| 300 | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vorobjova, A.I.; Tishkevich, D.I.; Outkina, E.A.; Shimanovich, D.L.; Razanau, I.U.; Zubar, T.I.; Bondaruk, A.A.; Zheleznova, E.K.; Dong, M.; Aloraini, D.A.; et al. A Study of Ta2O5 Nanopillars with Ni Tips Prepared by Porous Anodic Alumina Through-Mask Anodization. Nanomaterials 2022, 12, 1344. https://doi.org/10.3390/nano12081344
Vorobjova AI, Tishkevich DI, Outkina EA, Shimanovich DL, Razanau IU, Zubar TI, Bondaruk AA, Zheleznova EK, Dong M, Aloraini DA, et al. A Study of Ta2O5 Nanopillars with Ni Tips Prepared by Porous Anodic Alumina Through-Mask Anodization. Nanomaterials. 2022; 12(8):1344. https://doi.org/10.3390/nano12081344
Chicago/Turabian StyleVorobjova, Alla I., Daria I. Tishkevich, Elena A. Outkina, Dmitry L. Shimanovich, Ihar U. Razanau, Tatiana I. Zubar, Anastasia A. Bondaruk, Ekaterina K. Zheleznova, Mengge Dong, Dalal A. Aloraini, and et al. 2022. "A Study of Ta2O5 Nanopillars with Ni Tips Prepared by Porous Anodic Alumina Through-Mask Anodization" Nanomaterials 12, no. 8: 1344. https://doi.org/10.3390/nano12081344
APA StyleVorobjova, A. I., Tishkevich, D. I., Outkina, E. A., Shimanovich, D. L., Razanau, I. U., Zubar, T. I., Bondaruk, A. A., Zheleznova, E. K., Dong, M., Aloraini, D. A., Sayyed, M. I., Almuqrin, A. H., Silibin, M. V., Trukhanov, S. V., & Trukhanov, A. V. (2022). A Study of Ta2O5 Nanopillars with Ni Tips Prepared by Porous Anodic Alumina Through-Mask Anodization. Nanomaterials, 12(8), 1344. https://doi.org/10.3390/nano12081344












