Effectiveness of Direct Laser Interference Patterning and Peptide Immobilization on Endothelial Cell Migration for Cardio-Vascular Applications: An In Vitro Study
Abstract
1. Introduction
2. Materials and Methods
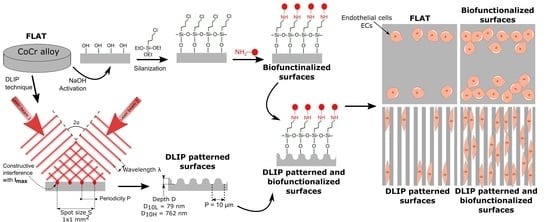
2.1. CoCr Alloy Surfaces
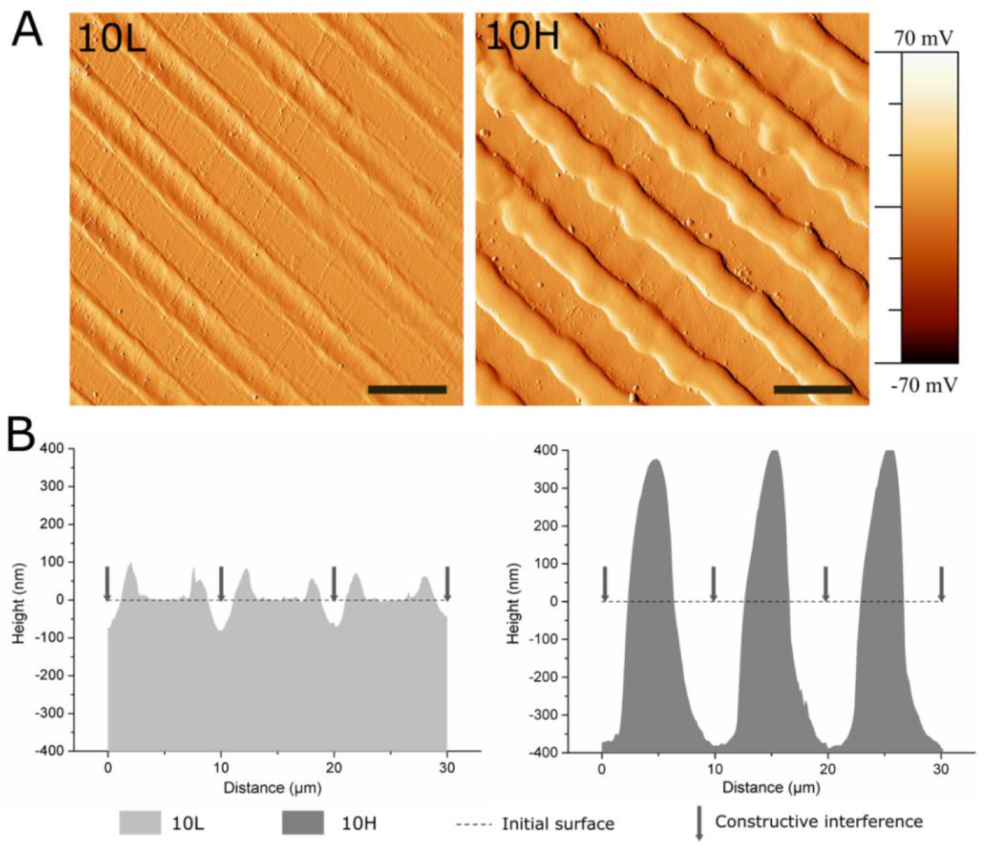
2.2. DLIP Patterning
2.3. Functionalization of CoCr Surfaces
2.3.1. Solid-Phase Peptide Synthesis
2.3.2. Protocol of Immobilization
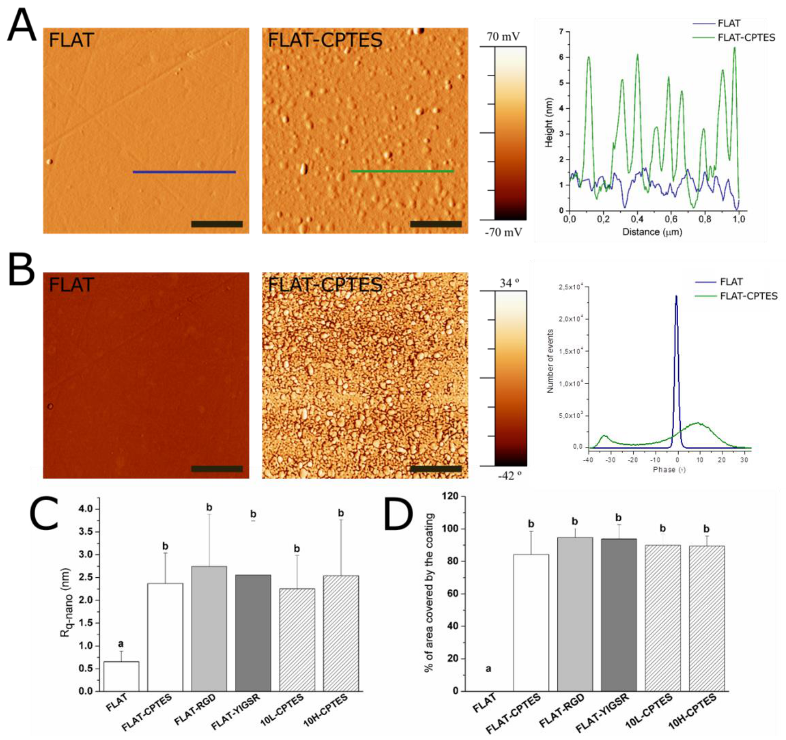
2.4. Physicochemical Characterization of the Patterned Surfaces
2.5. Physicochemical Characterization of the Biofunctionalized Surfaces
2.6. Biological Characterization
2.6.1. Endothelial Cells
2.6.2. Cell Adhesion
2.6.3. Wound Healing Migration Study
2.6.4. Cells Proliferation
2.7. Statistical Analysis
3. Results and Discussion
3.1. Fabrication of Linear Patterned CoCr by DLIP and Surface Characterization
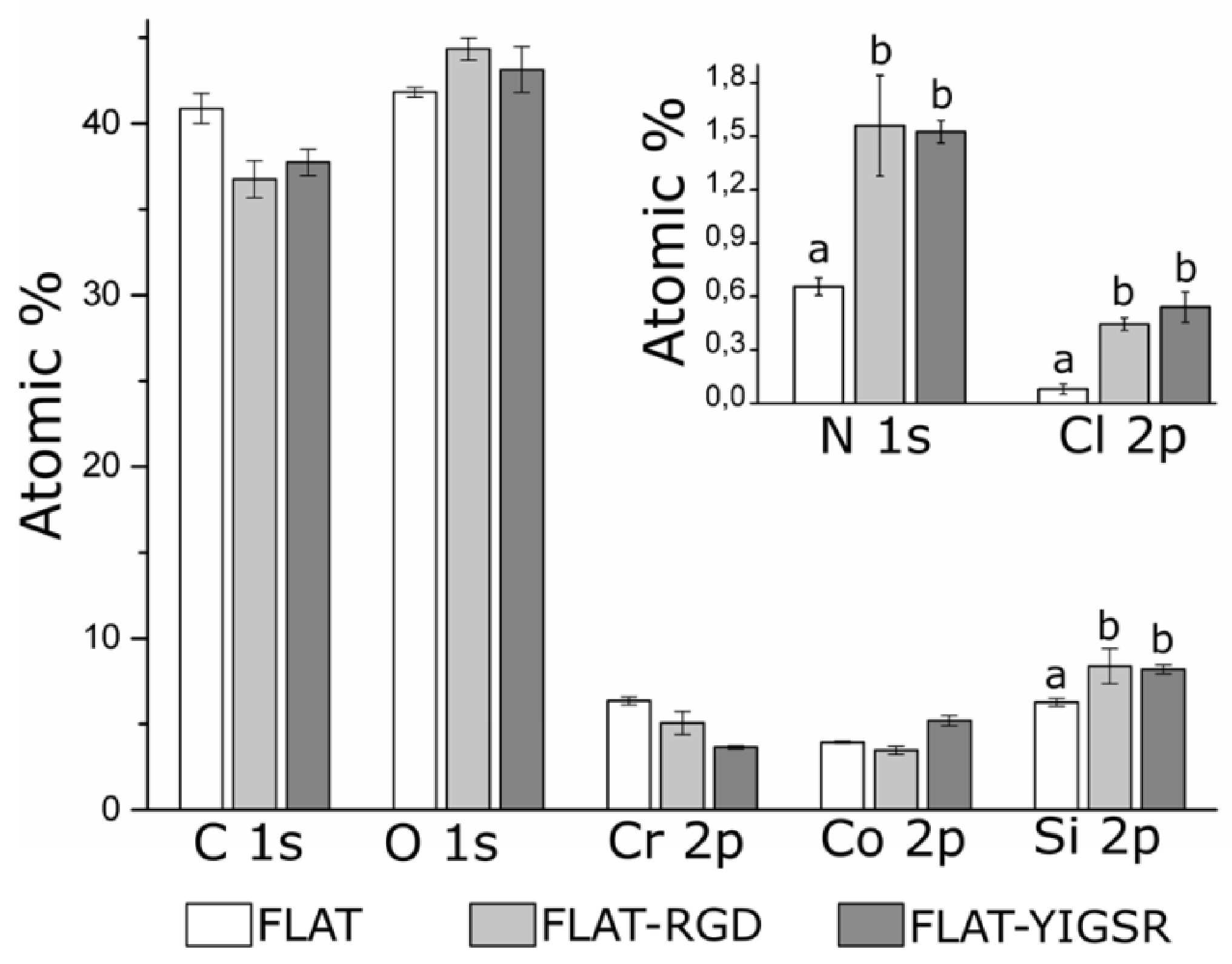
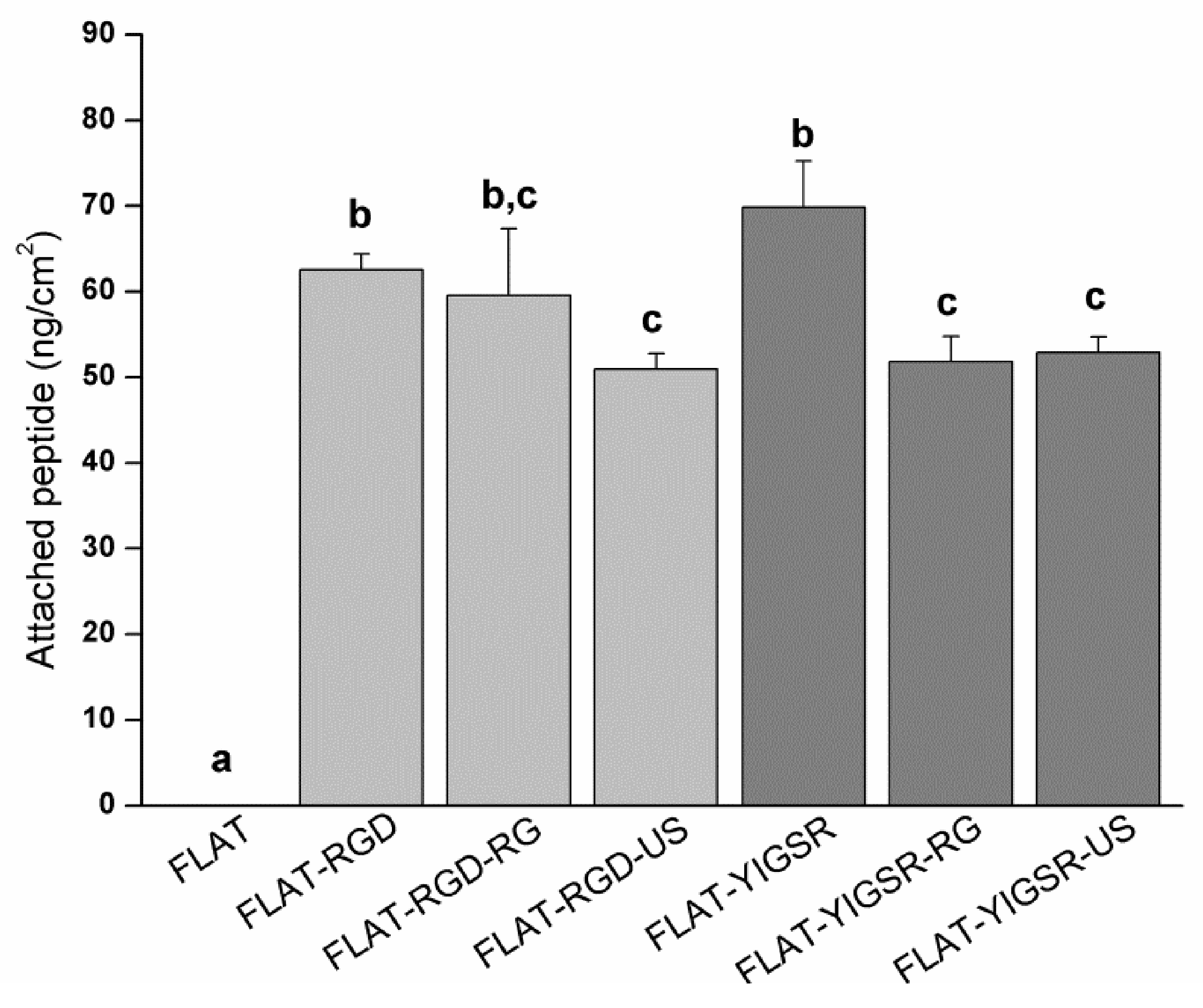
3.2. Biofunctionalization of CoCr and Surface Characterization
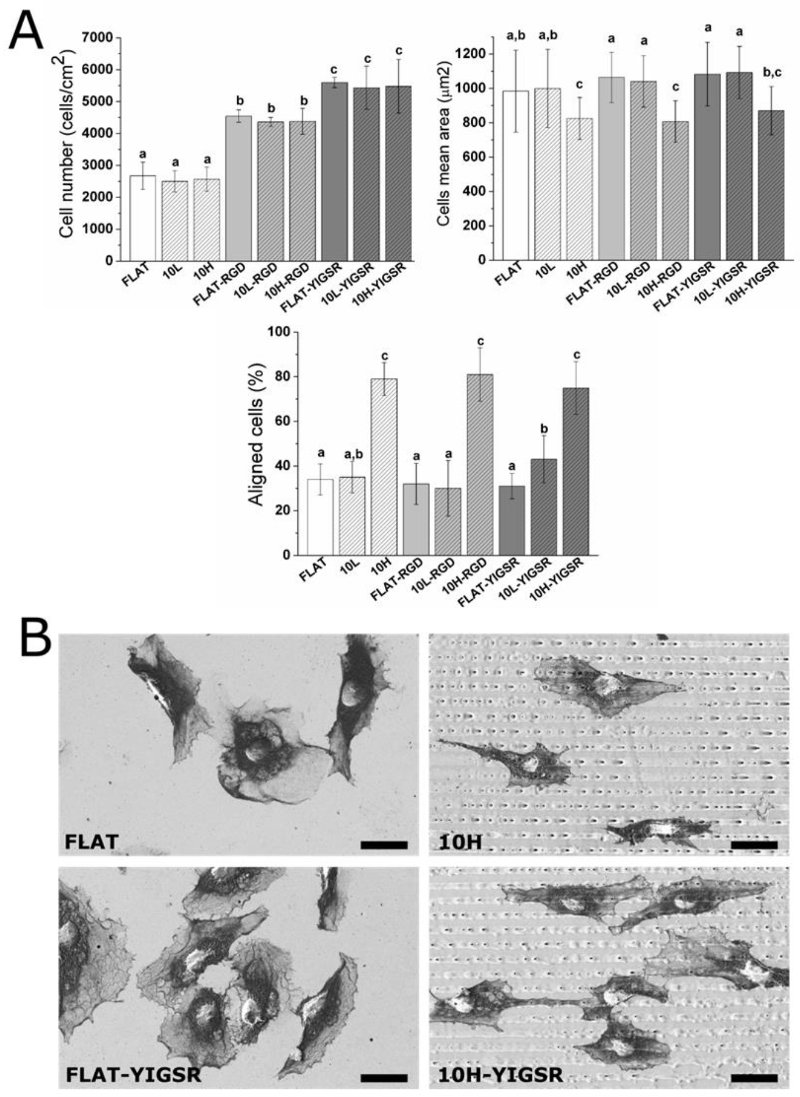
3.3. HUVECs Adhesion and Alignment
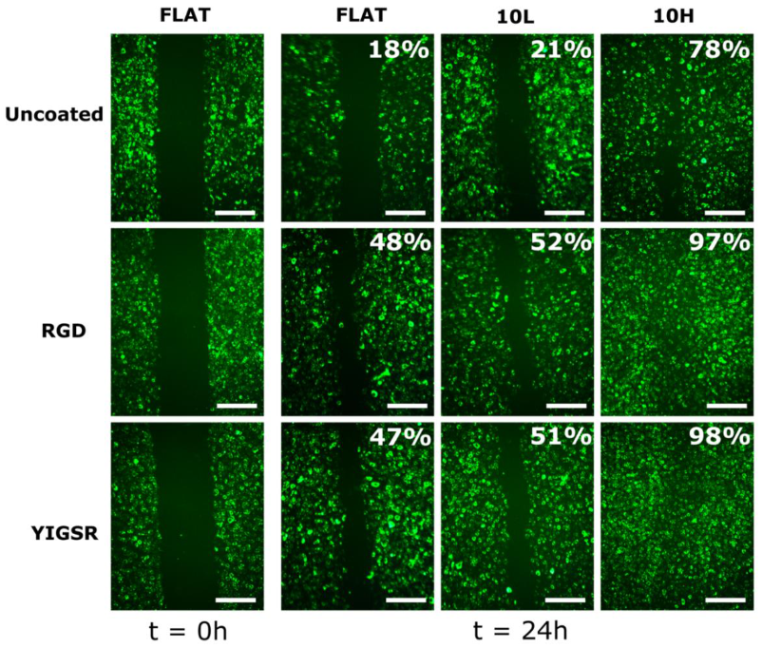
3.4. HUVECs Migration
3.5. HUVECs Proliferation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Piccolo, R.; Bonaa, K.H.; Efthimiou, O.; Varenne, O.; Baldo, A.; Urban, P.; Kaiser, C.; Remkes, W.; Räber, L.; de Belder, A.; et al. Drug-Eluting or Bare-Metal Stents for Percutaneous Coronary Intervention: A Systematic Review and Individual Patient Data Meta-Analysis of Randomised Clinical Trials. Lancet 2019, 393, 2503–2510. [Google Scholar] [CrossRef]
- Torii, S.; Jinnouchi, H.; Sakamoto, A.; Kutyna, M.; Cornelissen, A.; Kuntz, S.; Guo, L.; Mori, H.; Harari, E.; Paek, K.H.; et al. Drug-eluting coronary stents: Insights from preclinical and pathology studies. Nat. Rev. Cardiol. 2020, 17, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Rykowska, I.; Nowak, I.; Nowak, R. Drug-Eluting Stents and Balloons—Materials, Structure Designs, and Coating Techniques: A Review. Molecules 2020, 25, 4624. [Google Scholar] [CrossRef] [PubMed]
- Lemos, P.A.; Bienert, I. The Supralimus® sirolimus-eluting stent. Expert Rev. Med. Devices 2013, 10, 295–300. [Google Scholar] [CrossRef]
- Habib, A.; Finn, A.V. Endothelialization of drug eluting stents and its impact on dual anti-platelet therapy duration. Pharmacol. Res. 2015, 93, 22–27. [Google Scholar] [CrossRef]
- Kang, S.H.; Chae, I.H.; Park, J.J.; Lee, H.S.; Kang, D.Y.; Hwang, S.S.; Youn, T.J.; Kim, H.S. Stent Thrombosis With Drug-Eluting Stents and Bioresorbable Scaffolds: Evidence From a Network Meta-Analysis of 147 Trials. JACC Cardiovasc. Interv. 2016, 9, 1203–1212. [Google Scholar] [CrossRef]
- Polimeni, A.; Sorrentino, S.; Spaccarotella, C.; Mongiardo, A.; Sabatino, J.; de Rosa, S.; Gori, T.; Indolfi, C. Stent Thrombosis After Percutaneous Coronary Intervention: From Bare-Metal to the Last Generation of Drug-Eluting Stents. Cardiol. Clin. 2020, 38, 639–647. [Google Scholar] [CrossRef]
- Mas-Moruno, C.; Su, B.; Dalby, M.J. Multifunctional Coatings and Nanotopographies: Toward Cell Instructive and Antibacterial Implants. Adv. Health Mater. 2019, 8, e1801103. [Google Scholar] [CrossRef]
- Oliver-Cervelló, L.; Martin-Gómez, H.; Mas-Moruno, C. New trends in the development of multifunctional peptides to functionalize biomaterials. J. Pept. Sci. 2021, 28, e3335. [Google Scholar] [CrossRef]
- de Mel, A.; Jell, G.; Stevens, M.M.; Seifalian, A.M. Biofunctionalization of Biomaterials for Accelerated in Situ Endothelialization: A Review. Biomacromolecules 2008, 9, 2969–2979. [Google Scholar] [CrossRef]
- von der Mark, K.; Park, J. Engineering Biocompatible Implant Surfaces: Part II: Cellular Recognition of Biomaterial Surfaces: Lessons from Cell–Matrix Interactions. Prog. Mater. Sci. 2013, 58, 327–381. [Google Scholar] [CrossRef]
- Heath, D.E. Promoting Endothelialization of Polymeric Cardiovascular Biomaterials. Macromol. Chem. Phys. 2017, 218, 1600574. [Google Scholar] [CrossRef]
- Davis, G.E.; Senger, D.R. Endothelial Extracellular Matrix: Biosynthesis, Remodeling, and Functions during Vascular Morphogenesis and Neovessel Stabilization. Circ. Res. 2005, 97, 1093–1107. [Google Scholar] [CrossRef]
- Dong, H.G.; Yoon, K.J.; Sun, Y.P.; Yong, D.P.; Park, K.D. Heparin-Conjugated Star-Shaped PLA for Improved Bio-compatibility. J. Biomed. Mater. Res. Part A 2008, 86, 842–848. [Google Scholar]
- Ahn, Y.K.; Jeong, M.H.; Kim, J.W.; Kim, S.H.; Cho, J.H.; Cho, J.G.; Park, C.S.; Juhng, S.W.; Park, J.C.; Kang, J.C. Preventive effects of the heparin-coated stent on restenosis in the porcine model. Catheter. Cardiovasc. Interv. 1999, 48, 324–330. [Google Scholar] [CrossRef]
- De Scheerder, I.; Wang, K.; Wilczek, K.; Meuleman, D.; Van Amsterdam, R.; Vogel, G.; Piessens, J.; Van de Werf, F. Experimental Study of Thrombogenicity and Foreign Body Reaction Induced by Heparin-Coated Coronary Stents. Circulation 1997, 95, 1549–1553. [Google Scholar] [CrossRef]
- Ali, S.; Saik, J.E.; Gould, D.J.; Dickinson, M.E.; West, J.L. Immobilization of Cell-Adhesive Laminin Peptides in Degradable PEGDA Hydrogels Influences Endothelial Cell Tubulogenesis. BioResearch Open Access 2013, 2, 241–249. [Google Scholar] [CrossRef]
- Kumar, T.R.S.; Krishnan, L.K. Fibrin-mediated endothelial cell adhesion to vascular biomaterials resists shear stress due to flow. J. Mater. Sci. Mater. Electron. 2002, 13, 751–755. [Google Scholar] [CrossRef]
- Hersel, U.; Dahmen, C.; Kessler, H. RGD modified polymers: Biomaterials for stimulated cell adhesion and beyond. Biomaterials 2003, 24, 4385–4415. [Google Scholar] [CrossRef]
- Pagel, M.; Beck-Sickinger, A.G. Multifunctional biomaterial coatings: Synthetic challenges and biological activity. Biol. Chem. 2017, 398, 3–22. [Google Scholar] [CrossRef]
- Collier, J.H.; Segura, T. Evolving the use of peptides as components of biomaterials. Biomaterials 2011, 32, 4198–4204. [Google Scholar] [CrossRef]
- Pierschbacher, M.D.; Ruoslahti, E. Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature 1984, 309, 30–33. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, Z.; Song, L.; Zhao, Q.; Zhang, J.; Li, D.; Wang, S.; Han, J.; Zheng, X.L.; Yang, Z.; et al. Endothelialization and Patency of RGD-Functionalized Vascular Grafts in a Rabbit Carotid Artery Model. Biomaterials 2012, 33, 2880–2891. [Google Scholar] [CrossRef]
- Hoesli, C.A.; Garnier, A.; Juneau, P.-M.; Chevallier, P.; Duchesne, C.; Laroche, G. A fluorophore-tagged RGD peptide to control endothelial cell adhesion to micropatterned surfaces. Biomaterials 2014, 35, 879–890. [Google Scholar] [CrossRef]
- Hubbell, J.; Massia, S.P.; Desai, N.P.; Drumheller, P.D. Endothelial Cell-Selective Materials for Tissue Engineering in the Vascular Graft Via a New Receptor. Nat. Biotechnol. 1991, 9, 568–572. [Google Scholar] [CrossRef]
- Veiseh, M.; Veiseh, O.; Martin, M.C.; Asphahani, F.; Zhang, M. Short Peptides Enhance Single Cell Adhesion and Viability on Microarrays. Langmuir 2007, 23, 4472–4479. [Google Scholar] [CrossRef]
- Ren, X.; Feng, Y.; Guo, J.; Wang, H.; Li, Q.; Yang, J.; Hao, X.; Lv, J.; Ma, N.; Li, W. Surface Modification and Endotheli-alization of Biomaterials as Potential Scaffolds for Vascular Tissue Engineering Applications. Chem. Soc. Rev. 2015, 44, 5680–5742. [Google Scholar] [CrossRef]
- Wang, H.; Feng, Y.; Yang, J.; Guo, J.; Zhang, W. Targeting REDV peptide functionalized polycationic gene carrier for enhancing the transfection and migration capability of human endothelial cells. J. Mater. Chem. B 2015, 3, 3379–3391. [Google Scholar] [CrossRef]
- Jun, H.-W.; West, J.L. Modification of polyurethaneurea with PEG and YIGSR peptide to enhance endothelialization without platelet adhesion. J. Biomed. Mater. Res. 2005, 72, 131–139. [Google Scholar] [CrossRef]
- Massia, S.P.; Hubbell’, J.A. Covalent Surface Immobilization of Arg-Gly-Asp-and Tyr-Lle-Gly-Ser-Arg-Containing Peptides to Obtain Well-Defined Cell-Adhesive Substrates. Anal. Biochem. 1990, 187, 292–301. [Google Scholar] [CrossRef]
- Jun, H.-W.; West, J.L. Endothelialization of Microporous YIGSR/PEG-Modified Polyurethaneurea. Tissue Eng. 2005, 11, 1133–1140. [Google Scholar] [CrossRef] [PubMed]
- Castellanos, M.I.; Mas-Moruno, C.; Grau, A.; Serra-Picamal, X.; Trepat, X.; Albericio, F.; Joner, M.; Gil, F.J.; Ginebra, M.-P.; Manero, J.M.; et al. Functionalization of CoCr surfaces with cell adhesive peptides to promote HUVECs adhesion and proliferation. Appl. Surf. Sci. 2017, 393, 82–92. [Google Scholar] [CrossRef]
- Schieber, R.; Lasserre, F.; Hans, M.; Yagüe, M.F.; Díaz-Ricart, M.; Escolar, G.; Ginebra, M.-P.; Mücklich, F.; Pegueroles, M. Direct Laser Interference Patterning of CoCr Alloy Surfaces to Control Endothelial Cell and Platelet Response for Cardiovascular Applications. Adv. Health Mater. 2017, 6, 1700327. [Google Scholar] [CrossRef] [PubMed]
- Zorlutuna, P.; Rong, Z.; Vadgama, P.; Hasirci, V. Influence of nanopatterns on endothelial cell adhesion: Enhanced cell retention under shear stress. Acta Biomater. 2009, 5, 2451–2459. [Google Scholar] [CrossRef] [PubMed]
- Biela, S.A.; Su, Y.; Spatz, J.P.; Kemkemer, R. Different sensitivity of human endothelial cells, smooth muscle cells and fibroblasts to topography in the nano–micro range. Acta Biomater. 2009, 5, 2460–2466. [Google Scholar] [CrossRef] [PubMed]
- Liliensiek, S.J.; Wood, J.A.; Yong, J.; Auerbach, R.; Nealey, P.F.; Murphy, C.J. Modulation of Human Vascular Endo-thelial Cell Behaviors by Nanotopographic Cues. Biomaterials 2010, 31, 5418–5426. [Google Scholar] [CrossRef] [PubMed]
- Morgan, J.T.; Wood, J.A.; Shah, N.M.; Hughbanks, M.L.; Russell, P.; Barakat, A.I.; Murphy, C.J. Integration of basal topographic cues and apical shear stress in vascular endothelial cells. Biomaterials 2012, 33, 4126–4135. [Google Scholar] [CrossRef]
- Falconnet, D.; Csucs, G.; Grandin, H.M.; Textor, M. Surface engineering approaches to micropattern surfaces for cell-based assays. Biomaterials 2006, 27, 3044–3063. [Google Scholar] [CrossRef]
- Tan, C.H.; Muhamad, N.; Abdullah, M.M.A.B. Surface Topographical Modification of Coronary Stent: A Review. IOP Conf. Ser. Mater. Sci. Eng. 2017, 209, 012031. [Google Scholar] [CrossRef]
- Lu, J.; Rao, M.; MacDonald, N.C.; Khang, D.; Webster, T.J. Improved endothelial cell adhesion and proliferation on patterned titanium surfaces with rationally designed, micrometer to nanometer features. Acta Biomater. 2008, 4, 192–201. [Google Scholar] [CrossRef]
- Ermis, M.; Antmen, E.; Hasirci, V. Micro and Nanofabrication methods to control cell-substrate interactions and cell behavior: A review from the tissue engineering perspective. Bioact. Mater. 2018, 3, 355–369. [Google Scholar] [CrossRef]
- Mücklich, F.; Lasagni, A.; Daniel, C. Laser Interference Metallurgy—Using interference as a tool for micro/nano structuring. Int. J. Mater. Res. 2006, 97, 1337–1344. [Google Scholar] [CrossRef]
- Minguela, J.; Müller, D.W.; Mücklich, F.; Llanes, L.; Ginebra, M.P.; Roa, J.J.; Mas-Moruno, C. Peptidic Biofunctionali-zation of Laser Patterned Dental Zirconia: A Biochemical-Topographical Approach. Mater. Sci. Eng. C 2021, 125, 112096. [Google Scholar] [CrossRef]
- Mas-Moruno, C.; Fraioli, R.; Albericio, F.; Manero, J.M.; Gil, F.J. Novel Peptide-Based Platform for the Dual Presenta-tion of Biologically Active Peptide Motifs on Biomaterials. ACS Appl. Mater. Interfaces 2014, 6, 6525–6536. [Google Scholar] [CrossRef]
- Frank, A.O.; Otto, D.-C.E.; Mas-Moruno, C.; Schiller, H.B.; Marinelli, L.; Cosconati, S.; Bochen, D.-C.A.; Vossmeyer, D.; Zahn, G.; Stragies, R.; et al. Conformational Control of Integrin-Subtype Selectivity in isoDGR Peptide Motifs: A Biological Switch. Angew. Chem. Int. Ed. 2010, 49, 9278–9281. [Google Scholar] [CrossRef]
- Mas-Moruno, C.; Beck, J.G.; Doedens, L.; Frank, A.O.; Marinelli, L.; Cosconati, S.; Novellino, E.; Kessler, H. Increasing αvβ3 Selectivity of the Anti-Angiogenic Drug Cilengitide by N-Methylation. Angew. Chem. Int. Ed. 2011, 50, 9496–9500. [Google Scholar] [CrossRef]
- Smith, R.M.; Hansen, D.E. The PH-Rate Profile for the Hydrolysis of a Peptide Bond. J. Am. Chem. Soc. 1998, 120, 8910–8913. [Google Scholar] [CrossRef]
- Horcas, I.; Fernández, R.; Gómez-Rodriguez, J.M.; Colchero, J.; Gomez-Herrero, J.; Baro, A.M. WSXM: A software for scanning probe microscopy and a tool for nanotechnology. Rev. Sci. Instrum. 2007, 78, 13705. [Google Scholar] [CrossRef]
- International Organization for Standardization. Biological Evaluation of Medical Devices. Part 15: Identification and Quantification of Degradation Products from Metals and Alloys; International Organization for Standardization: Geneva, Switzerland, 2019. [Google Scholar]
- Tzoneva, R.; Heuchel, M.; Groth, T.; Altankov, G.; Albrecht, W.; Paul, D. Fibrinogen Adsorption and Platelet Interac-tions on Polymer Membranes. J. Biomater. Sci. Polym. Ed. 2002, 13, 1033–1050. [Google Scholar] [CrossRef]
- Sevilla, P.; Godoy, M.; Salvagni, E.; Rodríguez, D.; Gil, F.J. Biofunctionalization of Titanium Surfaces for Osseinte-gration Process Improvement. J. Phys. Conf. Ser. 2010, 252, 012009. [Google Scholar]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Kiefer, K.; Akpınar, G.; Haidar, A.; Ikier, T.; Akkan, C.K.; Akman, E.; Lee, J.; Miró, M.M.; Kaçar, E.; Demir, A.; et al. Al2O3micro- and nanostructures affect vascular cell response. RSC Adv. 2016, 6, 17460–17469. [Google Scholar] [CrossRef]
- Lee, P.P.; Cerchiari, A.; Desai, T.A. Nitinol-Based Nanotubular Coatings for the Modulation of Human Vascular Cell Function. Nano Lett. 2014, 14, 5021–5028. [Google Scholar] [CrossRef]
- Csaderova, L.; Martines, E.; Seunarine, K.; Gadegaard, N.; Wilkinson, C.D.W.; Riehle, M.O. A Biodegradable and Biocompatible Regular Nanopattern for Large-Scale Selective Cell Growth. Small 2010, 6, 2755–2761. [Google Scholar] [CrossRef]
- Liang, C.; Hu, Y.; Wang, H.; Xia, D.; Li, Q.; Zhang, J.; Yang, J.; Li, B.; Li, H.; Han, D.; et al. Biomimetic cardiovascular stents for in vivo re-endothelialization. Biomaterials 2016, 103, 170–182. [Google Scholar] [CrossRef]
- Schieber, R.; Raymond, Y.; Caparrós, C.; Bou, J.; Acero, E.H.; Guebitz, G.M.; Canal, C.; Pegueroles, M. Functionalization Strategies and Fabrication of Solvent-Cast PLLA for Bioresorbable Stents. Appl. Sci. 2021, 11, 1478. [Google Scholar] [CrossRef]
- Navarro, L.; Luna, J.; Rintoul, I. Surface conditioning of cardiovascular 316l stainless steel stents: A review. Surf. Rev. Lett. 2017, 24, 1730002. [Google Scholar] [CrossRef]
- Allmen, M.V.; Blatter, A. Laser Beam Interactions with Materials Physical Principles and Applications; Springer Series in Materials Science; Springer: Berlin/Heidelberg, Germany, 1988; Volume 2, p. 232. [Google Scholar]
- Dayan, A.D.; Paine, A.J. Mechanisms of chromium toxicity, carcinogenicity and allergenicity: Review of the literature from 1985 to 2000. Hum. Exp. Toxicol. 2001, 20, 439–451. [Google Scholar] [CrossRef]
- Chen, Q.; Thouas, G.A. Metallic implant biomaterials. Mater. Sci. Eng. R Rep. 2015, 87, 1–57. [Google Scholar] [CrossRef]
- Madl, A.K.; Kovochich, M.; Liong, M.; Finley, B.L.; Paustenbach, D.J.; Oberdörster, G. Toxicology of wear particles of cobalt-chromium alloy metal-on-metal hip implants Part II: Importance of physicochemical properties and dose in animal and in vitro studies as a basis for risk assessment. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1285–1298. [Google Scholar] [CrossRef]
- Schnitzer, C.; Ripperger, S. Influence of Surface Roughness on Streaming Potential Method. Chem. Eng. Technol. 2008, 31, 1696–1700. [Google Scholar] [CrossRef]
- Mas-Moruno, C.; Dorfner, P.M.; Manzenrieder, F.; Neubauer, S.; Reuning, U.; Burgkart, R.; Kessler, H. Behavior of Primary Human Osteoblasts on Trimmed and Sandblasted Ti6Al4V Surfaces Functionalized with Integrin Avb3-Selective Cyclic RGD Peptides. J. Biomed. Mater. Res. Part A 2013, 101, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Tan, G.; Zhang, L.; Ning, C.; Liu, X.; Liao, J. Preparation and characterization of APTES films on modification titanium by SAMs. Thin Solid Film. 2011, 519, 4997–5001. [Google Scholar] [CrossRef]
- Kim, J.; Seidler, P.; Wan, L.S.; Fill, C. Formation, structure, and reactivity of amino-terminated organic films on silicon substrates. J. Colloid Interface Sci. 2009, 329, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Sargeant, T.D.; Rao, M.S.; Koh, C.-Y.; Stupp, S.I. Covalent functionalization of NiTi surfaces with bioactive peptide amphiphile nanofibers. Biomaterials 2008, 29, 1085–1098. [Google Scholar] [CrossRef]
- Healy, K.; Ducheyne, P. Oxidation kinetics of titanium thin films in model physiologic environments. J. Colloid Interface Sci. 1992, 150, 404–417. [Google Scholar] [CrossRef]
- Rezania, A.; Johnson, R.; Lefkow, A.R.; Healy, K.E. Bioactivation of Metal Oxide Surfaces. 1. Surface Characterization and Cell Response. Langmuir 1999, 15, 6931–6939. [Google Scholar] [CrossRef]
- Yang, Y.; Porté, M.-C.; Marmey, P.; El Haj, A.J.; Amédée, J.; Baquey, C. Covalent bonding of collagen on poly(L-lactic acid) by gamma irradiation. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2003, 207, 165–174. [Google Scholar] [CrossRef]
- Song, K.H.; Kwon, K.W.; Song, S.; Suh, K.-Y.; Doh, J. Dynamics of T cells on endothelial layers aligned by nanostructured surfaces. Biomaterials 2012, 33, 2007–2015. [Google Scholar] [CrossRef]
- Palmaz, J.C.; Benson, A.; Sprague, E.A. Influence of Surface Topography on Endothelialization of Intravascular Metallic Material. J. Vasc. Interv. Radiol. 1999, 10, 439–444. [Google Scholar] [CrossRef]
- Ranjan, A.; Webster, T.J. Increased endothelial cell adhesion and elongation on micron-patterned nano-rough poly(dimethylsiloxane) films. Nanotechnology 2009, 20, 305102. [Google Scholar] [CrossRef]
- Yu, F.; Mücklich, F.; Li, P.; Shen, H.; Mathur, S.; Lehr, C.-M.; Bakowsky, U. In Vitro Cell Response to a Polymer Surface Micropatterned by Laser Interference Lithography. Biomacromolecules 2005, 6, 1160–1167. [Google Scholar] [CrossRef]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. Cell Junctions, Cell Adhesion, and the Extracellular Matrix. In Molecular Biology of the Cell; W. W. Norton & Co: New York, NY, USA, 2019; Chapter 19. [Google Scholar]
- Teichmann, J.; Morgenstern, A.; Seebach, J.; Schnittler, H.-J.; Werner, C.; Pompe, T. The control of endothelial cell adhesion and migration by shear stress and matrix-substrate anchorage. Biomaterials 2012, 33, 1959–1969. [Google Scholar] [CrossRef]
- Bouta, E.M.; McCarthy, C.W.; Keim, A.; Wang, H.B.; Gilbert, R.J.; Goldman, J. Biomaterial guides for lymphatic endothelial cell alignment and migration. Acta Biomater. 2011, 7, 1104–1113. [Google Scholar] [CrossRef][Green Version]
- McClatchey, A.I.; Yap, A.S. Contact inhibition (of proliferation) redux. Curr. Opin. Cell Biol. 2012, 24, 685–694. [Google Scholar] [CrossRef]
- Cho, E.H.; Dai, Y. SIRT1 controls cell proliferation by regulating contact inhibition. Biochem. Biophys. Res. Commun. 2016, 478, 868–872. [Google Scholar] [CrossRef]
- Bønaa, K.H.; Mannsverk, J.; Wiseth, R.; Aaberge, L.; Myreng, Y.; Nygård, O.; Nilsen, D.W.; Kløw, N.-E.; Uchto, M.; Trovik, T.; et al. Drug-Eluting or Bare-Metal Stents for Coronary Artery Disease. N. Engl. J. Med. 2016, 375, 1242–1252. [Google Scholar] [CrossRef]
- Scafa Udriste, A.; Niculescu, A.-G.; Grumezescu, A.M.; Bädilä, E. Cardiovascular Stents: A Review of Past, Current, and Emerging Devices. Materials 2021, 14, 2498. [Google Scholar] [CrossRef]
- Wei, Y.; Ji, Y.; Xiao, L.-L.; Lin, Q.-K.; Xu, J.-P.; Ren, K.-F.; Ji, J. Surface engineering of cardiovascular stent with endothelial cell selectivity for in vivo re-endothelialisation. Biomaterials 2013, 34, 2588–2599. [Google Scholar] [CrossRef]
- Kouvroukoglou, S.; Dee, K.C.; Bizios, R.; McIntire, L.V.; Zygourakis, K. Endothelial Cell Migration on Surfaces Modified with Immobilized Adhesive Peptides. Biomaterials 2000, 21, 1725–1733. [Google Scholar] [CrossRef]
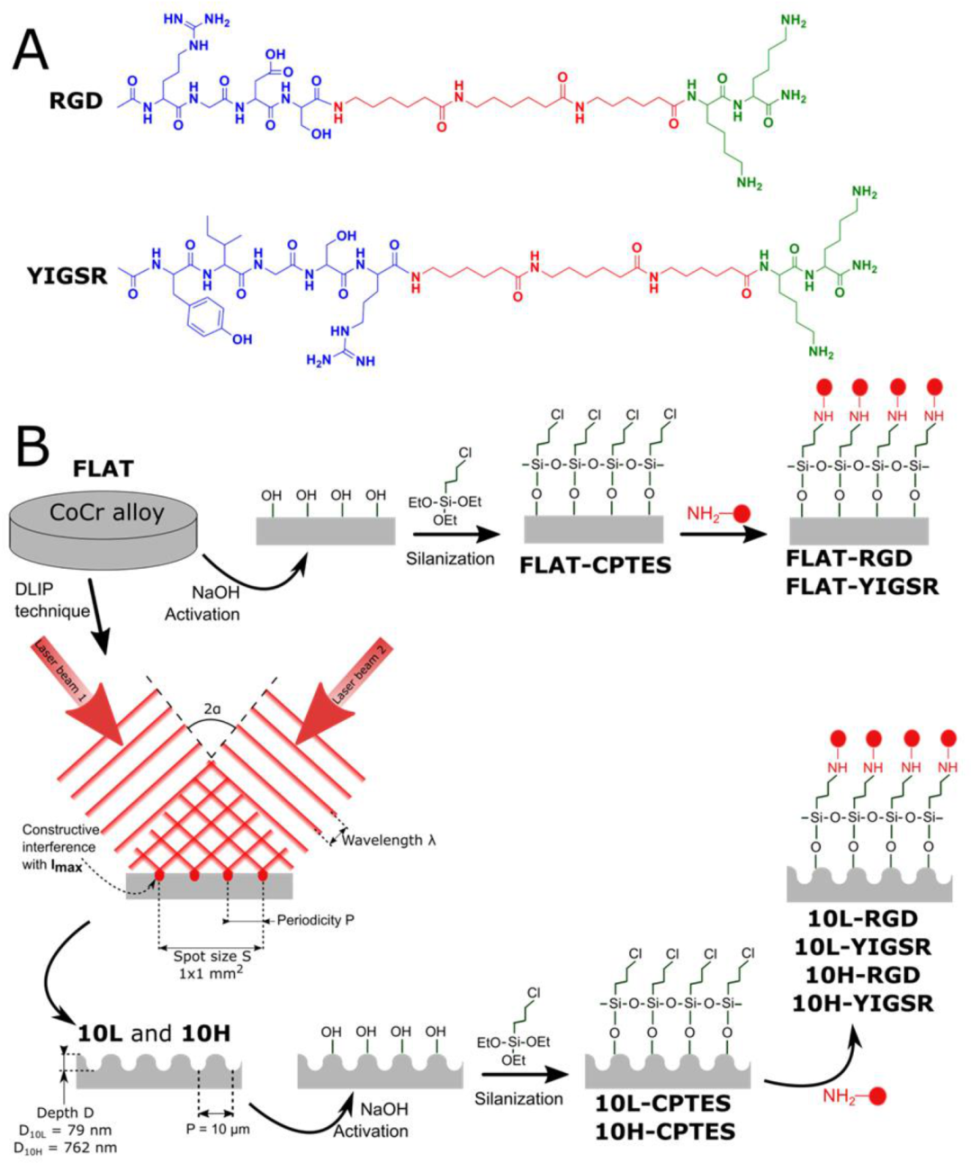

| Code | Sequence | tR (min) a | Purity (%) a | Calculated m/z | Experimental m/z d [M + H]+ |
|---|---|---|---|---|---|
| RGD | Ac-Arg-Gly-Asp-Ser-(Ahx)3-Lys-Lys-NH2 | 2.955 b | 93 | 1069.66 | 1069.68 |
| RGD–CF | CF-Arg-Gly-Asp-Ser-(Ahx)3-Lys-Lys-NH2 | 4.345 b | 98 | 1385.70 | 1385.66 |
| YIGSR | Ac-Tyr-Ile-Gly-Ser-Arg-(Ahx)3-Lys-Lys-NH2 | 5.228 c | 98 | 1230.78 | 1230.75 |
| YIGSR–CF | CF-Tyr-Ile-Gly-Ser-Arg-(Ahx)3-Lys-Lys-NH2 | 5.503 c | 99 | 1546.82 | 1546.80 |
| Periodicity (P) | Depth (D) | Rq-micro | Rq-nano | ||
|---|---|---|---|---|---|
| µm | nm | nm | nm | ||
| FLAT | - | - | 7 ± 1 | - | 0.66 ± 0.23 |
| Valley | 0.54 ± 0.17 | ||||
| 10L | 10.0 ± 0.1 | 79 ± 12 | 33 ± 4 | Peak | 0.78 ± 0.31 |
| Valley | 0.79 ± 0.22 | ||||
| 10H | 10.0 ± 0.1 | 762 ± 83 | 304 ± 25 | Peak | 0.79 ± 0.17 |
| Corrosion Potential | Ion Release | Zeta Potential | ||||
|---|---|---|---|---|---|---|
| Co Ions | Cr Ions | Ni Ions | IEP | ζ at pH 7.4 | ||
| mV | ng | Ng | ng | pH | mV | |
| FLAT | 1014.3 ± 10.1 | 47.5 ± 16.0 | 1.6 ± 0.3 | 12.4 ± 3.8 | 4.9 ± 0.0 | −34.5 ± 2.9 |
| 10L | 1017.6 ± 9.5 | 23.3 ± 6.3 | 1.5 ± 0.2 | 9.4 ± 2.8 | 4.8 ± 0.0 | −39.7 ± 0.2 |
| 10H | 1031.7 ± 6.1 | 77.8 ± 8.6 | 4.3 ± 0.3 | 17.5 ± 1.0 | 4.5 ± 0.0 | −11.5 ± 1.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schieber, R.; Mas-Moruno, C.; Lasserre, F.; Roa, J.J.; Ginebra, M.-P.; Mücklich, F.; Pegueroles, M. Effectiveness of Direct Laser Interference Patterning and Peptide Immobilization on Endothelial Cell Migration for Cardio-Vascular Applications: An In Vitro Study. Nanomaterials 2022, 12, 1217. https://doi.org/10.3390/nano12071217
Schieber R, Mas-Moruno C, Lasserre F, Roa JJ, Ginebra M-P, Mücklich F, Pegueroles M. Effectiveness of Direct Laser Interference Patterning and Peptide Immobilization on Endothelial Cell Migration for Cardio-Vascular Applications: An In Vitro Study. Nanomaterials. 2022; 12(7):1217. https://doi.org/10.3390/nano12071217
Chicago/Turabian StyleSchieber, Romain, Carlos Mas-Moruno, Federico Lasserre, Joan Josep Roa, Maria-Pau Ginebra, Frank Mücklich, and Marta Pegueroles. 2022. "Effectiveness of Direct Laser Interference Patterning and Peptide Immobilization on Endothelial Cell Migration for Cardio-Vascular Applications: An In Vitro Study" Nanomaterials 12, no. 7: 1217. https://doi.org/10.3390/nano12071217
APA StyleSchieber, R., Mas-Moruno, C., Lasserre, F., Roa, J. J., Ginebra, M.-P., Mücklich, F., & Pegueroles, M. (2022). Effectiveness of Direct Laser Interference Patterning and Peptide Immobilization on Endothelial Cell Migration for Cardio-Vascular Applications: An In Vitro Study. Nanomaterials, 12(7), 1217. https://doi.org/10.3390/nano12071217