Multifunctionalized Mesostructured Silica Nanoparticles Containing Mn2 Complex for Improved Catalase-Mimicking Activity in Water
Abstract
:1. Introduction
2. Materials and Methods
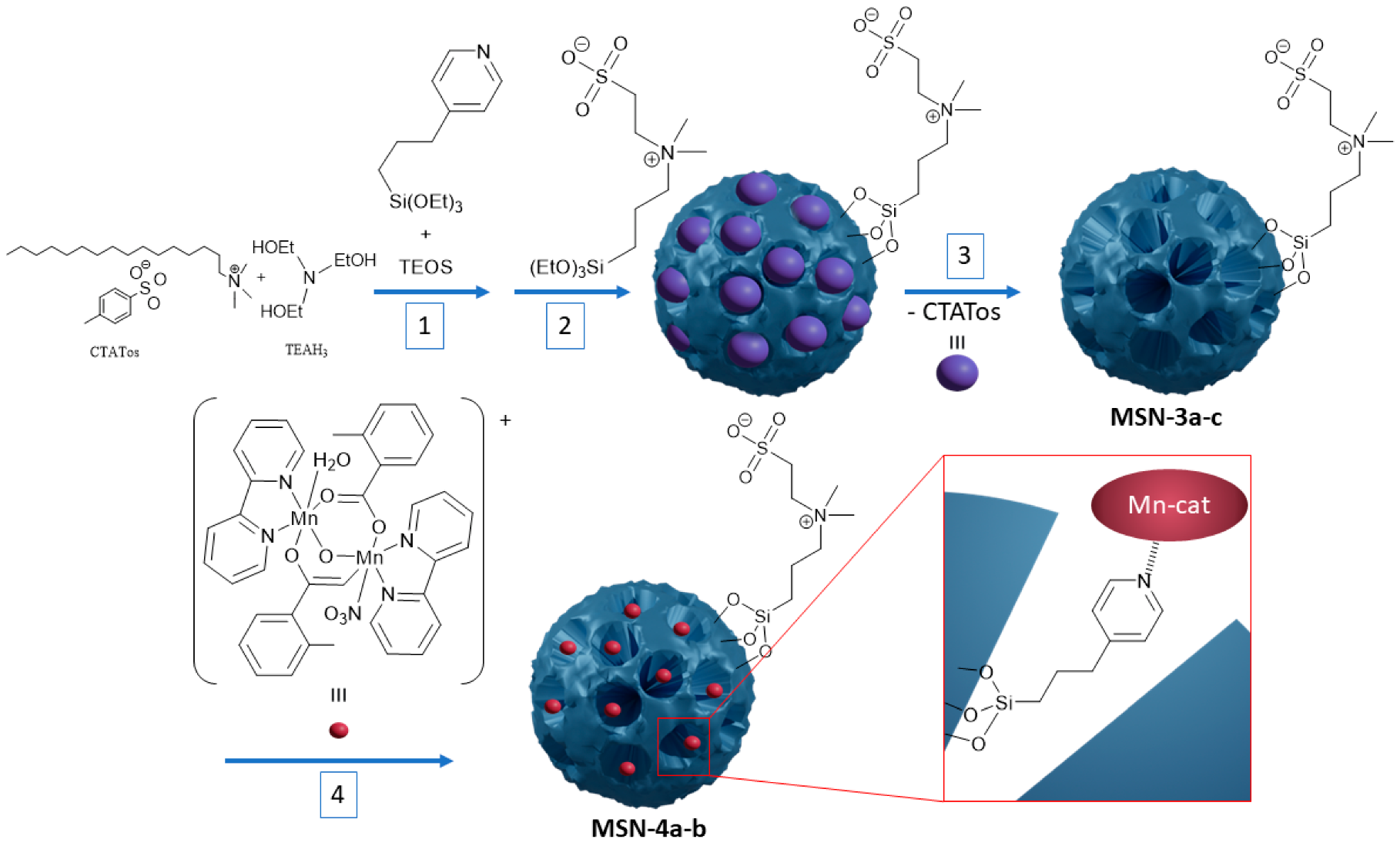
2.1. Synthsis of the Mesoporous Silica Nanoparticles
2.2. Encapsulation of the Dinuclear Manganese Complex
2.3. Nanoparticle’s Characterization
2.4. Resistance to Protein-Induced Aggregation and Colloidal Stability
2.5. Catalytic Tests
3. Results and Discussion
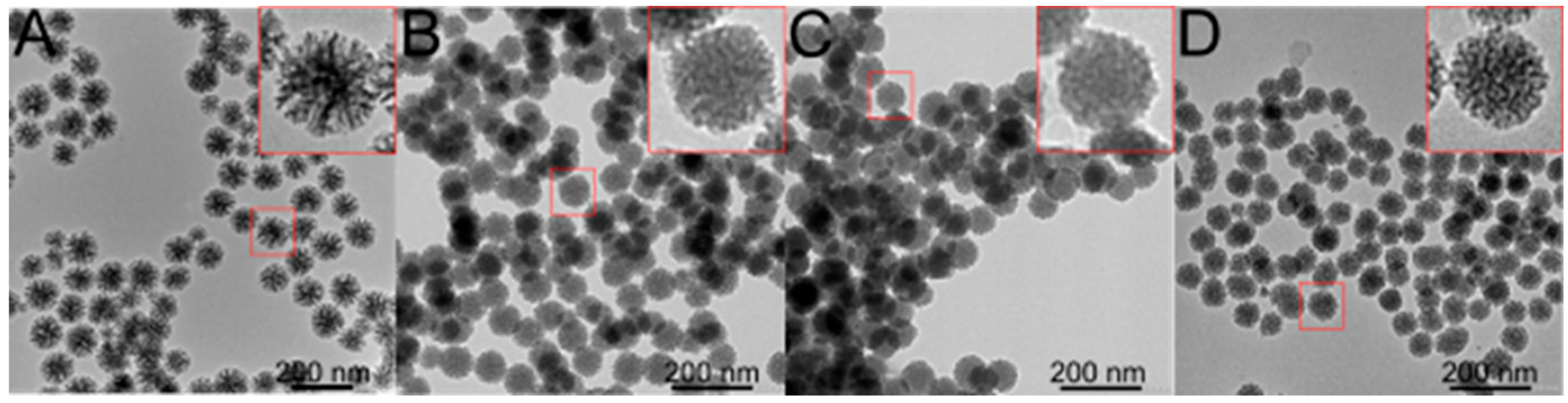
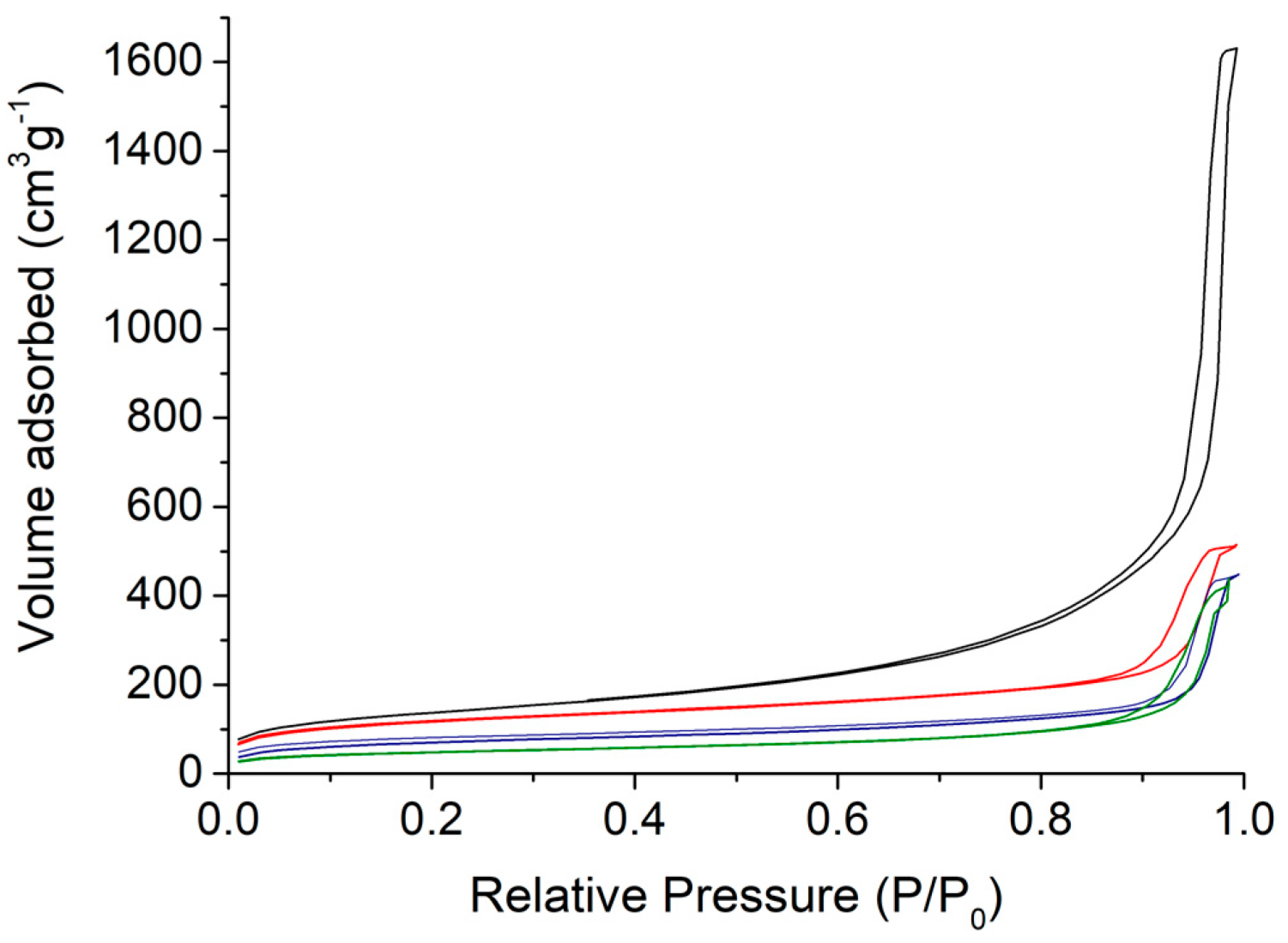
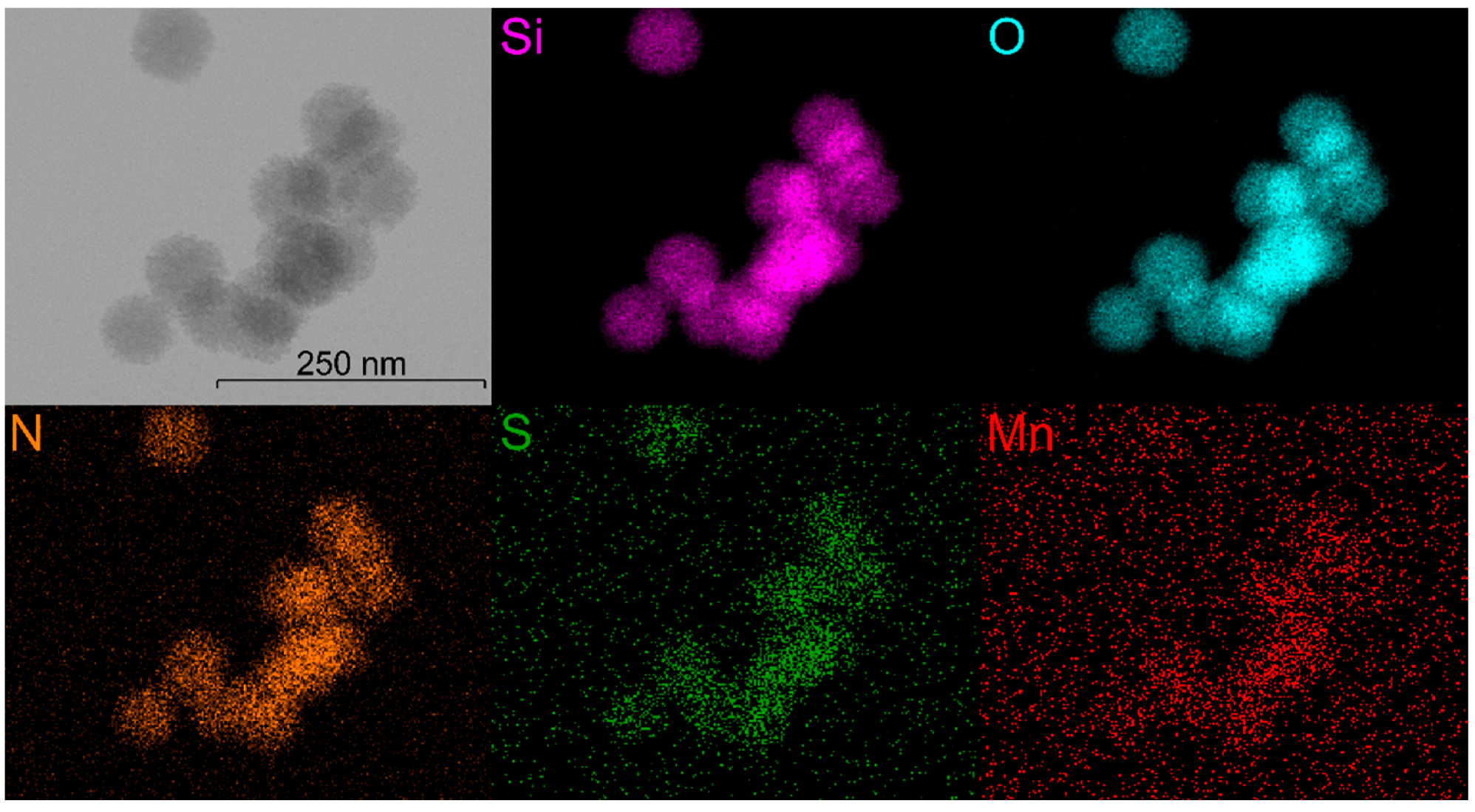
3.1. Synthesis and Characterisations
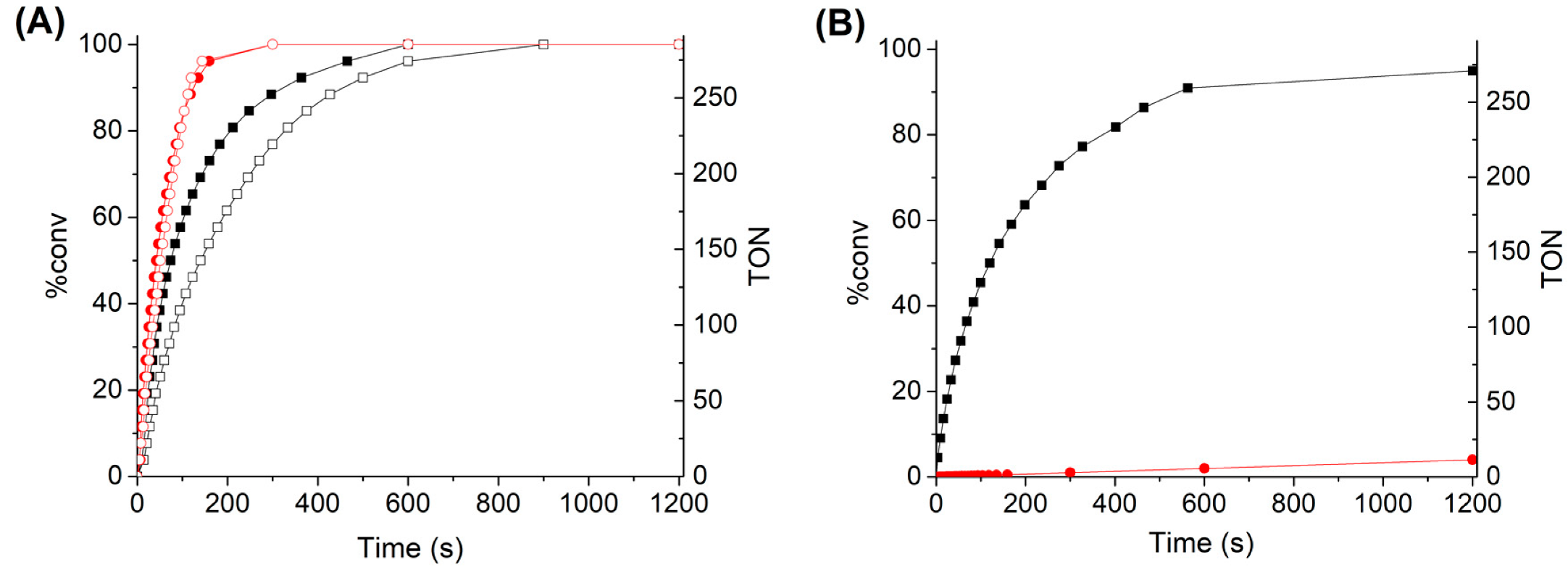
3.2. H2O2 Catalytic Dismutation Reaction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Vivero-Escoto, J.L.; Slowing, I.I.; Trewyn, B.G.; Lin, V.S. Mesoporous silica nanoparticles for intracellular controlled drug delivery. Small 2010, 6, 1952–1967. [Google Scholar] [CrossRef] [PubMed]
- Albela, B.; Bonneviot, L. Surface molecular engineering in the confined space of templated porous silica. New J. Chem. 2016, 40, 4115–4131. [Google Scholar] [CrossRef]
- Li, X.; Yuan, J.; Du, J.; Sui, H.; He, L. Functionalized Ordered Mesoporous Silica by Vinyltriethoxysilane for the Removal of Volatile Organic Compounds through Adsorption/Desorption Process. Ind. Eng. Chem. Res. 2020, 59, 3511–3520. [Google Scholar] [CrossRef]
- Molnar, A.; Rac, B. Organic Transformations over Silica Materials Modified by Covalently Bonded Surface Functional Groups. Curr. Org. Chem. 2006, 10, 1697–1726. [Google Scholar] [CrossRef]
- Narayan, R.; Nayak, U.A.-O.; Raichur, A.M.; Garg, S. Mesoporous Silica Nanoparticles: A Comprehensive Review on Synthesis and Recent Advances. Pharmaceutics 2018, 10, 118. [Google Scholar] [CrossRef] [Green Version]
- Rahikkala, A.; Pereira, S.A.P.; Figueiredo, P.; Passos, M.L.C.; Araújo, A.R.T.S.; Saraiva, M.L.M.F.S.; Santos, H.A. Mesoporous Silica Nanoparticles for Targeted and Stimuli-Responsive Delivery of Chemotherapeutics: A Review. Adv. Biosyst. 2018, 2, 1800020. [Google Scholar] [CrossRef]
- Shylesh, S.; Samuel, P.P.; Sisodiya, S.; Singh, A.P. Periodic Mesoporous Silicas and Organosilicas: An Overview towards Catalysis. Catal. Surv. Asia 2008, 12, 266. [Google Scholar] [CrossRef]
- Singh, B.; Na, J.; Konarova, M.; Wakihara, T.; Yamauchi, Y.; Salomon, C.; Gawande, M.B. Functional Mesoporous Silica Nanomaterials for Catalysis and Environmental Applications. Bull. Chem. Soc. Jpn. 2020, 93, 1459–1496. [Google Scholar] [CrossRef]
- Tang, F.; Li, L.; Chen, D. Mesoporous Silica Nanoparticles: Synthesis, Biocompatibility and Drug Delivery. Adv. Mater. 2012, 24, 1504–1534. [Google Scholar] [CrossRef]
- Kecht, J.; Schlossbauer, A.; Bein, T. Selective Functionalization of the Outer and Inner Surfaces in Mesoporous Silica Nanoparticles. Chem. Mater. 2008, 20, 7207–7214. [Google Scholar] [CrossRef]
- Von Baeckmann, C.; Guillet-Nicolas, R.; Renfer, D.; Kählig, H.; Kleitz, F. A Toolbox for the Synthesis of Multifunctionalized Mesoporous Silica Nanoparticles for Biomedical Applications. ACS Omega 2018, 3, 17496–17510. [Google Scholar] [CrossRef]
- Huh, S.; Wiench, J.W.; Trewyn, B.G.; Song, S.; Pruski, M.; Lin, V.S.Y. Tuning of particle morphology and pore properties in mesoporous silicas with multiple organic functional groups. Chem. Commun. 2003, 18, 2364–2365. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Regí, M.; Balas, F.; Arcos, D. Mesoporous Materials for Drug Delivery. Angew. Chem. Int. Ed. 2007, 46, 7548–7558. [Google Scholar] [CrossRef] [PubMed]
- Nel, A.E.; Mädler, L.; Velegol, D.; Xia, T.; Hoek, E.M.V.; Somasundaran, P.; Klaessig, F.; Castranova, V.; Thompson, M. Understanding biophysicochemical interactions at the nano–bio interface. Nat. Mater. 2009, 8, 543–557. [Google Scholar] [CrossRef]
- Wu, S.-H.; Hung, Y.; Mou, C.-Y. Mesoporous silica nanoparticles as nanocarriers. Chem. Commun. 2011, 47, 9972–9985. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.C.; Cunha, Â.; Trindade, T.; Tomé, J.P.C. The role of surface functionalization of silica nanoparticles for bioimaging. J. Innov. Opt. Health Sci. 2016, 9, 1630005. [Google Scholar] [CrossRef] [Green Version]
- Huang, R.; Shen, Y.-W.; Guan, Y.-Y.; Jiang, Y.-X.; Wu, Y.; Rahman, K.; Zhang, L.-J.; Liu, H.-J.; Luan, X. Mesoporous silica nanoparticles: Facile surface functionalization and versatile biomedical applications in oncology. Acta Biomater. 2020, 116, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, S.K.; Selvaraj, S. Mesoporous silica nanoparticles: Importance of surface modifications and its role in drug delivery. RSC Adv. 2014, 4, 14328–14334. [Google Scholar] [CrossRef]
- Wieszczycka, K.; Staszak, K.; Woźniak-Budych, M.J.; Litowczenko, J.; Maciejewska, B.M.; Jurga, S. Surface functionalization–The way for advanced applications of smart materials. Coord. Chem. Rev. 2021, 436, 213846. [Google Scholar] [CrossRef]
- Gößl, D.; Singer, H.; Chiu, H.-Y.; Schmidt, A.; Lichtnecker, M.; Engelke, H.; Bein, T. Highly active enzymes immobilized in large pore colloidal mesoporous silica nanoparticles. New J. Chem. 2019, 43, 1671–1680. [Google Scholar] [CrossRef]
- Du, X.; Qiao, S.Z. Dendritic Silica Particles with Center-Radial Pore Channels: Promising Platforms for Catalysis and Biomedical Applications. Small 2015, 11, 392–413. [Google Scholar] [CrossRef] [PubMed]
- Polshettiwar, V.; Cha, D.; Zhang, X.; Basset, J.M. High-Surface-Area Silica Nanospheres (KCC-1) with a Fibrous Morphology. Angew. Chem. Int. Ed. 2010, 49, 9652–9656. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Jiao, X.; Yang, H. Hydrophobic core/hydrophilic shell structured mesoporous silica nanospheres: Enhanced adsorption of organic compounds from water. Langmuir 2013, 29, 1228–1237. [Google Scholar] [CrossRef] [PubMed]
- Dai, Q.; Yang, L.; Wang, Y.; Cao, X.; Yao, C.; Xu, X. Surface charge-controlled electron transfer and catalytic behavior of immobilized cytochrome P450 BM3 inside dendritic mesoporous silica nanoparticles. Anal. Bioanal. Chem. 2020, 412, 4703–4712. [Google Scholar] [CrossRef]
- Dardouri, R.; Navarro Yerga, R.M.; Mota, N.; Albela, B.; Bonneviot, L.; Zina, M.S. Lower methane combustion temperature on palladium nanoparticles anchored on TiOx subnano-islets in stellate mesoporous silica nanospheres. New J. Chem. 2020, 44, 906–919. [Google Scholar] [CrossRef]
- Fihri, A.; Cha, D.; Bouhrara, M.; Almana, N.; Polshettiwar, V. Fibrous Nano-Silica (KCC-1)-Supported Palladium Catalyst: Suzuki Coupling Reactions Under Sustainable Conditions. ChemSusChem 2012, 5, 85–89. [Google Scholar] [CrossRef]
- Fihri, A.; Bouhrara, M.; Patil, U.; Cha, D.; Saih, Y.; Polshettiwar, V. Fibrous Nano-Silica Supported Ruthenium (KCC-1/Ru): A Sustainable Catalyst for the Hydrogenolysis of Alkanes with Good Catalytic Activity and Lifetime. ACS Catal. 2012, 2, 1425–1431. [Google Scholar] [CrossRef]
- Polshettiwar, V.; Thivolle-Cazat, J.; Taoufik, M.; Stoffelbach, F.; Norsic, S.; Basset, J.-M. “Hydro-metathesis” of Olefins: A Catalytic Reaction Using a Bifunctional Single-Site Tantalum Hydride Catalyst Supported on Fibrous Silica (KCC-1) Nanospheres. Angew. Chem. Int. Ed. 2011, 50, 2747–2751. [Google Scholar] [CrossRef]
- Park, D.S.; Yun, D.; Choi, Y.; Kim, T.Y.; Oh, S.; Cho, J.-H.; Yi, J. Effect of 3D open-pores on the dehydration of n-butanol to di-n-butyl ether (DNBE) over a supported heteropolyacid catalyst. Chem. Eng. J. 2013, 228, 889–895. [Google Scholar] [CrossRef]
- Bouhrara, M.; Ranga, C.; Fihri, A.; Shaikh, R.R.; Sarawade, P.; Emwas, A.-H.; Hedhili, M.N.; Polshettiwar, V. Nitridated Fibrous Silica (KCC-1) as a Sustainable Solid Base Nanocatalyst. ACS Sustain. Chem. Eng. 2013, 1, 1192–1199. [Google Scholar] [CrossRef]
- Fernández, G.; Corbella, M.; Aullón, G.; Maestro, M.A.; Mahía, J. New Dinuclear MnIII Compounds with 2-MeC6H4COO and 2-FC6H4COO Bridges–Effect of Terminal Monodentate Ligands (H2O, ClO4– and NO3–) on the Magnetic Properties. Eur. J. Inorg. Chem. 2007, 2007, 1285–1296. [Google Scholar] [CrossRef]
- Garcia-Cirera, B.; Corbella, M.; Bonneviot, L.; Albela, B. Bio-inspired manganese mesoporous silica hybrid material as a water compatible antioxidant. Microporous Mesoporous Mater. 2018, 261, 150–157. [Google Scholar] [CrossRef]
- Zhang, K.; Xu, L.L.; Jiang, J.G.; Calin, N.; Lam, K.F.; Zhang, S.J.; Wu, H.H.; Wu, G.D.; Albela, B.; Bonneviot, L.; et al. Facile large-scale synthesis of monodisperse mesoporous silica nanospheres with tunable pore structure. J. Am. Chem. Soc. 2013, 135, 2427–2430. [Google Scholar] [CrossRef] [PubMed]
- Estephan, Z.G.; Jaber, J.A.; Schlenoff, J.B. Zwitterion-stabilized silica nanoparticles: Toward nonstick nano. Langmuir 2010, 26, 16884–16889. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Song, H.; Yang, Y.; Liu, Y.; Tang, J.; Yu, C. Kinetically Controlled Dendritic Mesoporous Silica Nanoparticles: From Dahlia- to Pomegranate-like Structures by Micelle Filling. Chem. Mater. 2018, 30, 5770–5776. [Google Scholar] [CrossRef]
- Xiong, L.; Du, X.; Shi, B.; Bi, J.; Kleitz, F.; Qiao, S.Z. Tunable stellate mesoporous silica nanoparticles for intracellular drug delivery. J. Mater. Chem. B 2015, 3, 1712–1721. [Google Scholar] [CrossRef]
- Chen, S.; Chen, S.; Jiang, S.; Mo, Y.; Tang, J.; Ge, Z. Synthesis and characterization of siloxane sulfobetaine antimicrobial agents. Surf. Sci. 2011, 605, L25–L28. [Google Scholar] [CrossRef]
- Pombo Garcia, K.; Zarschler, K.; Barbaro, L.; Barreto, J.A.; O’Malley, W.; Spiccia, L.; Stephan, H.; Graham, B. Zwitterionic-coated "stealth" nanoparticles for biomedical applications: Recent advances in countering biomolecular corona formation and uptake by the mononuclear phagocyte system. Small 2014, 10, 2516–2529. [Google Scholar] [CrossRef]



| Sample | TEOS | 1,4-pyr | SBS |
|---|---|---|---|
| MSN | 1 | 0 | 0 |
| MSN-1a | 0.99 | 0.01 | 0 |
| MSN-1b | 0.95 | 0.05 | 0 |
| MSN-1c | 0.9 | 0.1 | 0 |
| MSN-2 | 1 | 0 | 0.04 |
| MSN-3a | 0.99 | 0.01 | 0.04 |
| MSN-3b | 0.95 | 0.05 | 0.04 |
| MSN-3c | 0.9 | 0.1 | 0.04 |
| Sample | Composition b | M (g mol−1) | SBET (m2 g−1) | Pore Size (nm) | dTEM (nm) |
|---|---|---|---|---|---|
| MSNa | (SiO2)1(CTA)0.005 | 62.7 | 547 | 19.4 | 100.4 ± 10.8 |
| MSN-1a | (SiO2)1(CTA)0.005(1,4-pyr)0.008 | 63.2 | 480 | 12.6 | 79.3 ± 8.4 |
| MSN-1b | (SiO2)1(CTA)0.006(1,4-pyr)0.047 | 72.2 | 442 | 7.7 | 63.8 ± 5.7 |
| MSN-1c | (SiO2)1(CTA)0.007(1,4-pyr)0.090 | 82.1 | 407 | - | 63.7 ± 6.3 |
| MSN-2a | (SiO2)1(CTA)0.005 (SBS)0.030 | 70.8 | 480 | 17.1 | 102.9 ± 13.2 |
| MSN-3a | (SiO2)1(CTA)0.005(1,4-pyr)0.017(SBS)0.030 | 74.5 | 360 | 14.5 | 81.3 ± 1.8 |
| MSN-3b | (SiO2)1(CTA)0.001(1,4-pyr)0.048(SBS)0.028 | 79.7 | 423 | 7.6 | 66.8 ± 5.7 |
| MSN-3c | (SiO2)1(CTA)0.002(1,4-pyr)0.096(SBS)0.027 | 90.1 | 400 | - | 72.7 ± 6.3 |
| MSN-4a | (SiO2)1(CTA)0.001(1,4-pyr)0.096(SBS)0.027(Mn-cat)0.0096 | 98.4 | 235 | - | 72.5 ± 5.8 |
| MSN-4b | (SiO2)1(CTA)0.001(1,4-pyr)0.096(SBS)0.027(Mn-cat)0.048 | 136.1 | 166 | - | 71.1 ± 5.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pelluau, T.; Sene, S.; Garcia-Cirera, B.; Albela, B.; Bonneviot, L.; Larionova, J.; Guari, Y. Multifunctionalized Mesostructured Silica Nanoparticles Containing Mn2 Complex for Improved Catalase-Mimicking Activity in Water. Nanomaterials 2022, 12, 1136. https://doi.org/10.3390/nano12071136
Pelluau T, Sene S, Garcia-Cirera B, Albela B, Bonneviot L, Larionova J, Guari Y. Multifunctionalized Mesostructured Silica Nanoparticles Containing Mn2 Complex for Improved Catalase-Mimicking Activity in Water. Nanomaterials. 2022; 12(7):1136. https://doi.org/10.3390/nano12071136
Chicago/Turabian StylePelluau, Tristan, Saad Sene, Beltzane Garcia-Cirera, Belen Albela, Laurent Bonneviot, Joulia Larionova, and Yannick Guari. 2022. "Multifunctionalized Mesostructured Silica Nanoparticles Containing Mn2 Complex for Improved Catalase-Mimicking Activity in Water" Nanomaterials 12, no. 7: 1136. https://doi.org/10.3390/nano12071136
APA StylePelluau, T., Sene, S., Garcia-Cirera, B., Albela, B., Bonneviot, L., Larionova, J., & Guari, Y. (2022). Multifunctionalized Mesostructured Silica Nanoparticles Containing Mn2 Complex for Improved Catalase-Mimicking Activity in Water. Nanomaterials, 12(7), 1136. https://doi.org/10.3390/nano12071136








