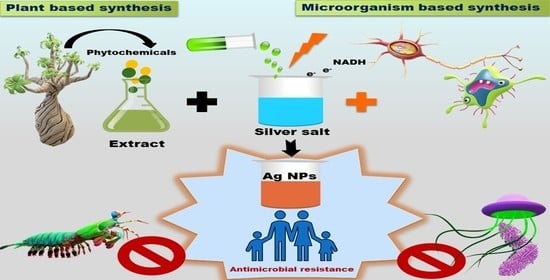
Recent Advances in Green Synthesis of Ag NPs for Extenuating Antimicrobial Resistance
Abstract
:1. Introduction
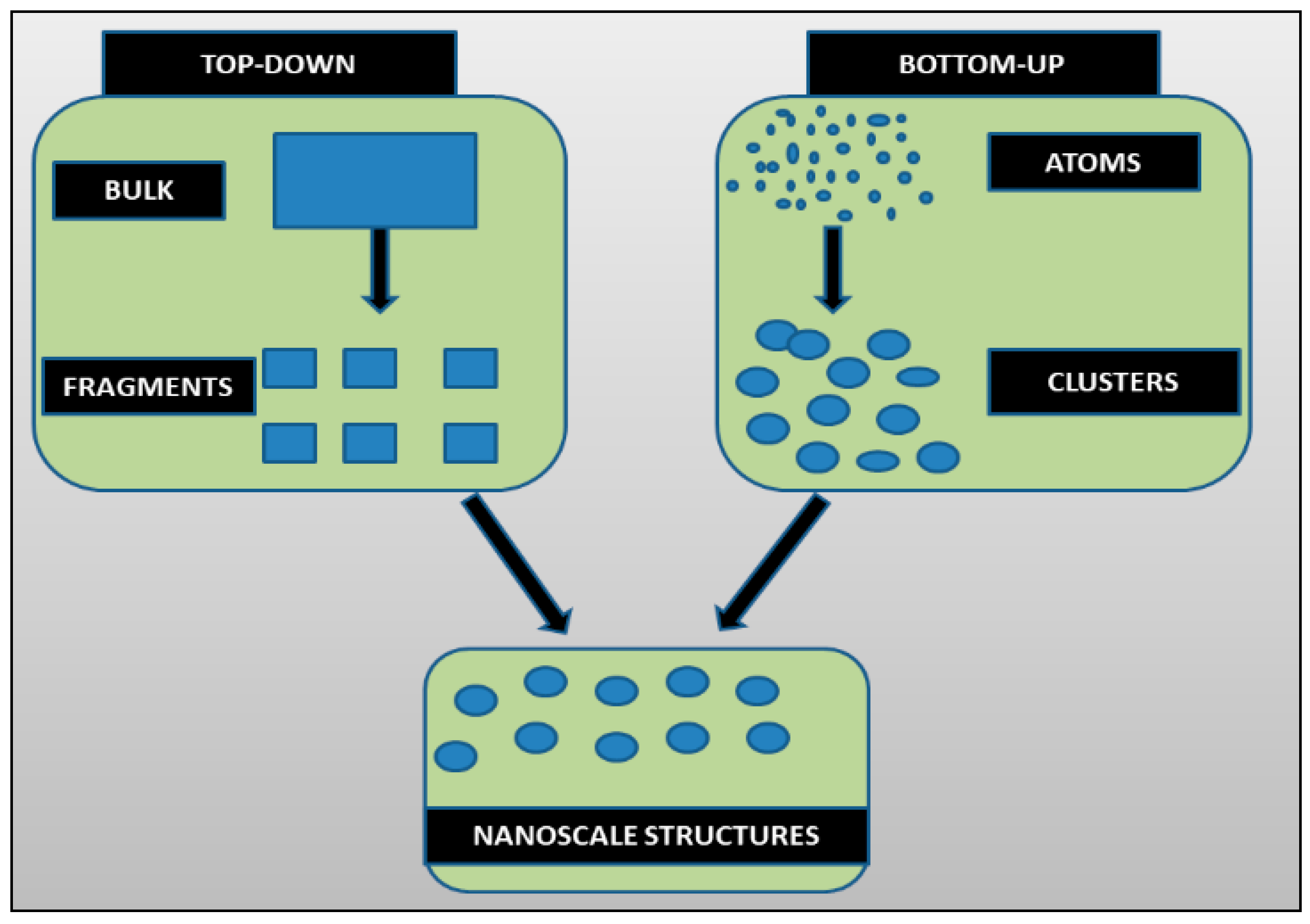
Methods of AgNPs Synthesis
2. Synthesis of Ag-NPs via Biological Methods
- (a)
- The use of microorganisms such as yeasts, bacteria, fungus, and actinomycetes in the production of chemicals; and
- (b)
- Extract of different parts of plants.
2.1. AgNPs Synthesis via Bacteria
- Geobacter spp., Arthrobacter gangotriensis;
- Bacillus cereus, Antarctica Bacillus amyloliquefaciens;
- Corynebacterium sp. SH09, and Shewanellaoneidensis;
- Pseudomonas proteolytica, Aeromonas sp. SH10 Phaeocystis;
- Escherichia coli, Lactobacillus case;
- Bacillus cecembensis, Enterobacter cloacae, and Bacillus indicus.
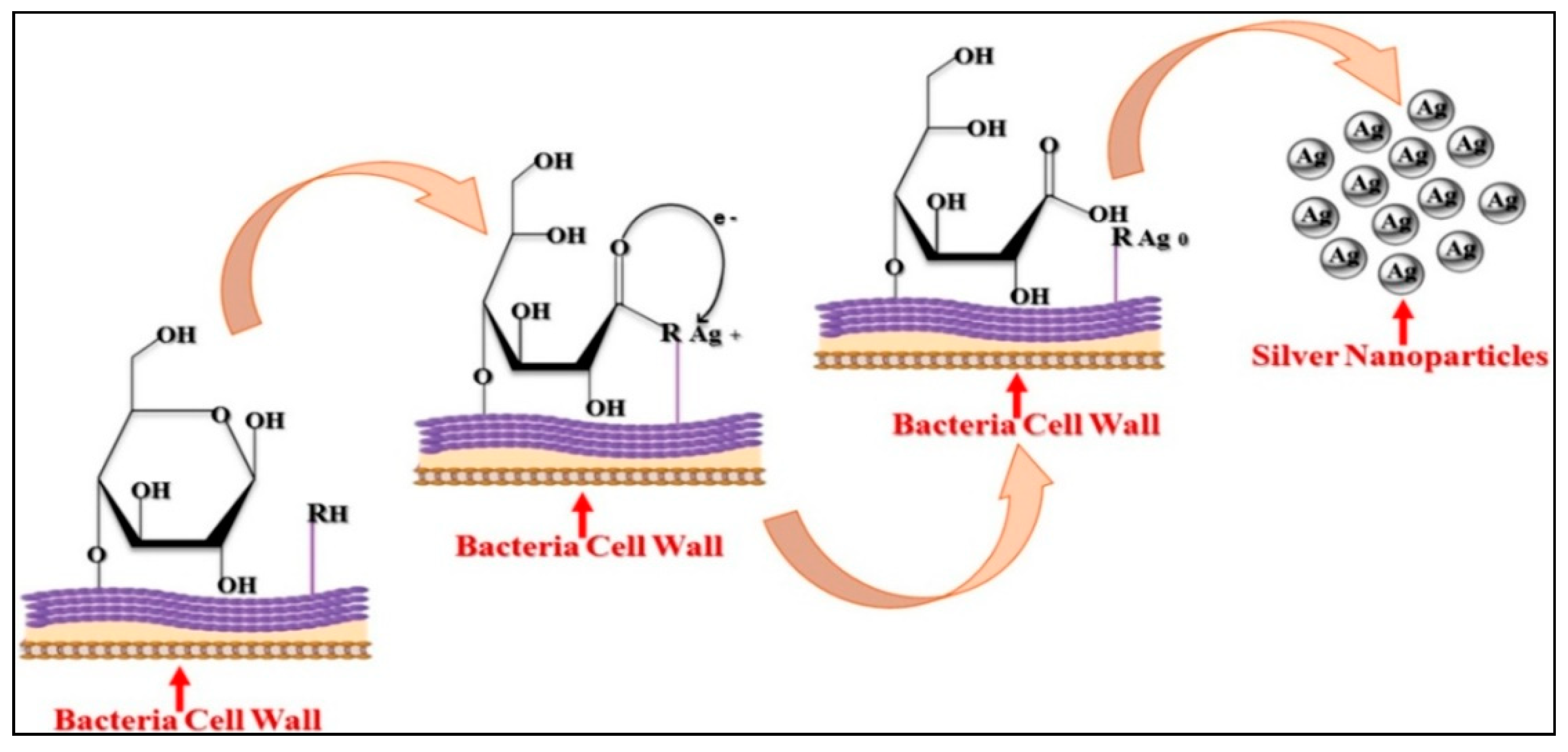
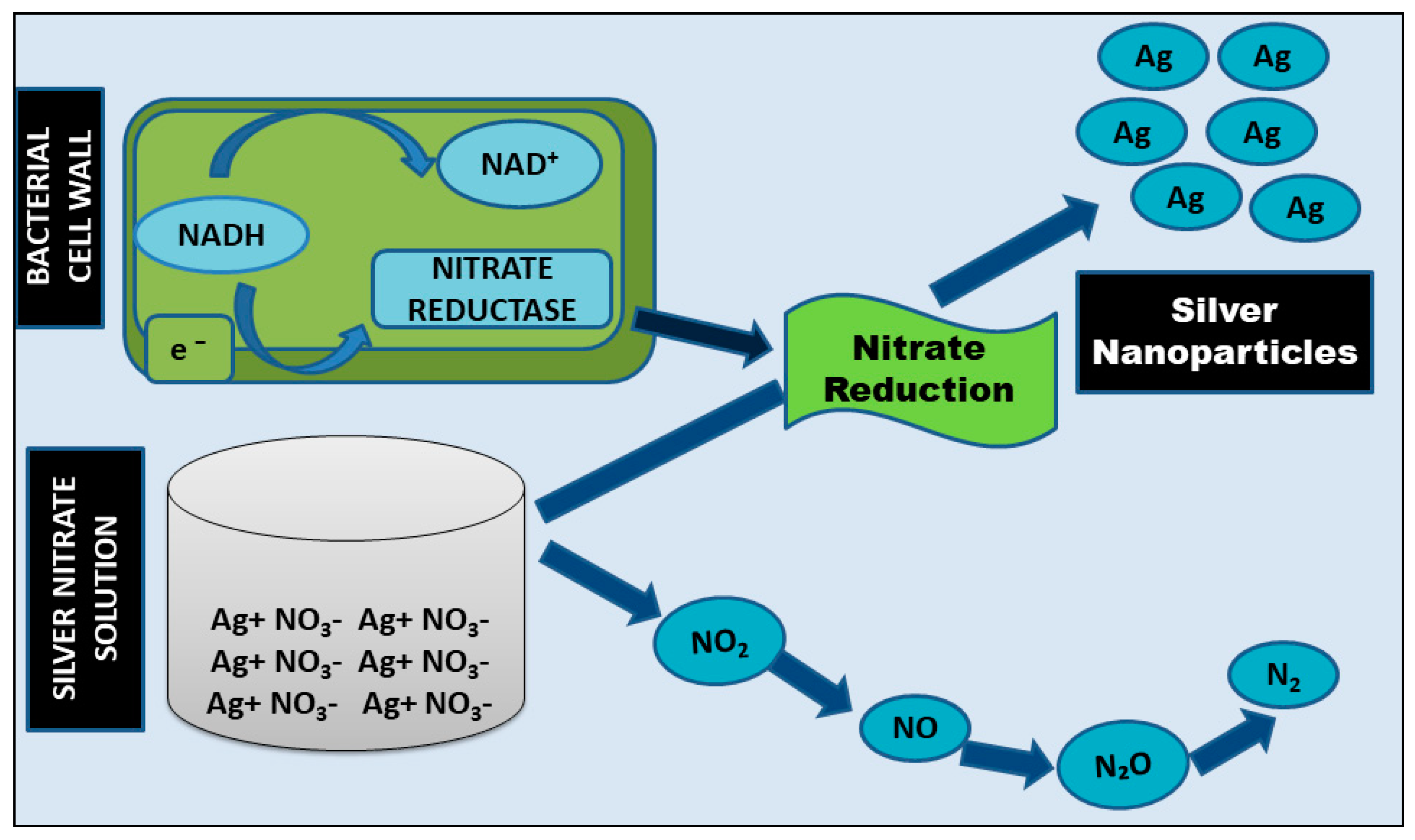
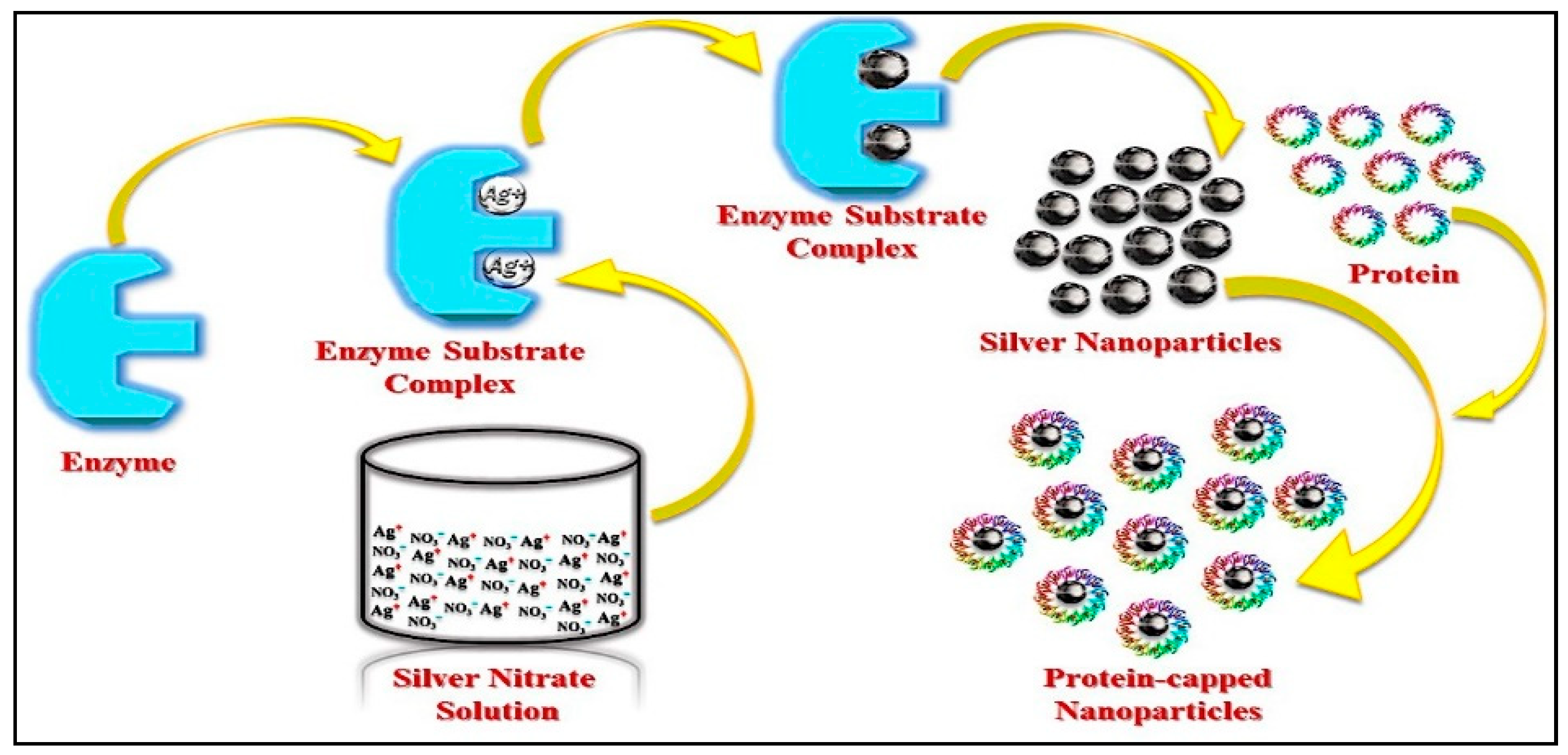
2.1.1. Intracellular Method and Mechanism
2.1.2. Extracellular Method and Mechanism
2.2. Synthesis by Using Fungi
Mechanism of Synthesis
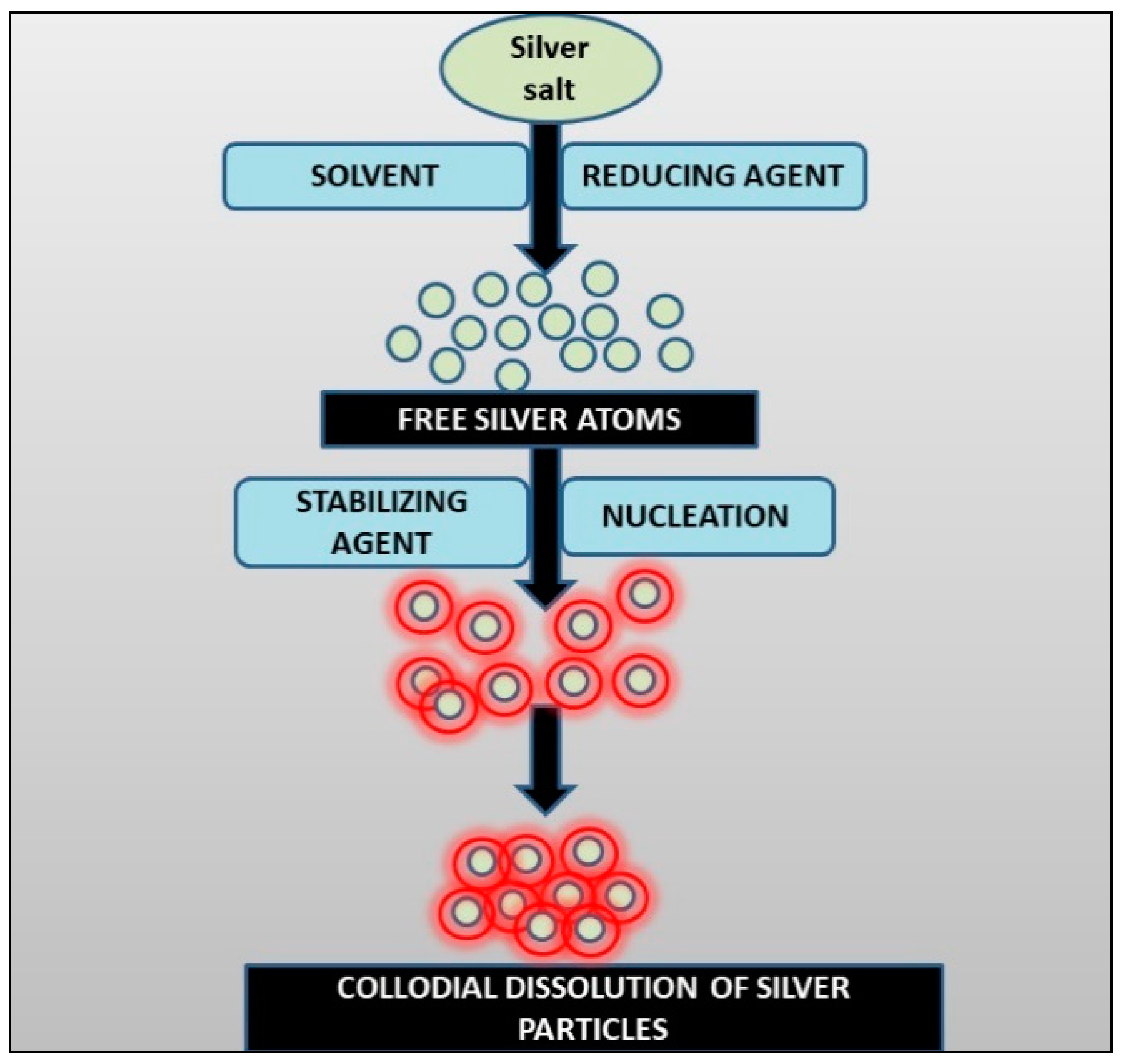
2.3. Synthesis of AgNPs by Using Plants
Mechanism of Synthesis
2.4. Key Factors for Efficient, Economical, and Reliable Preparation of AgNPs
3. Antimicrobial Activity of Ag NPs
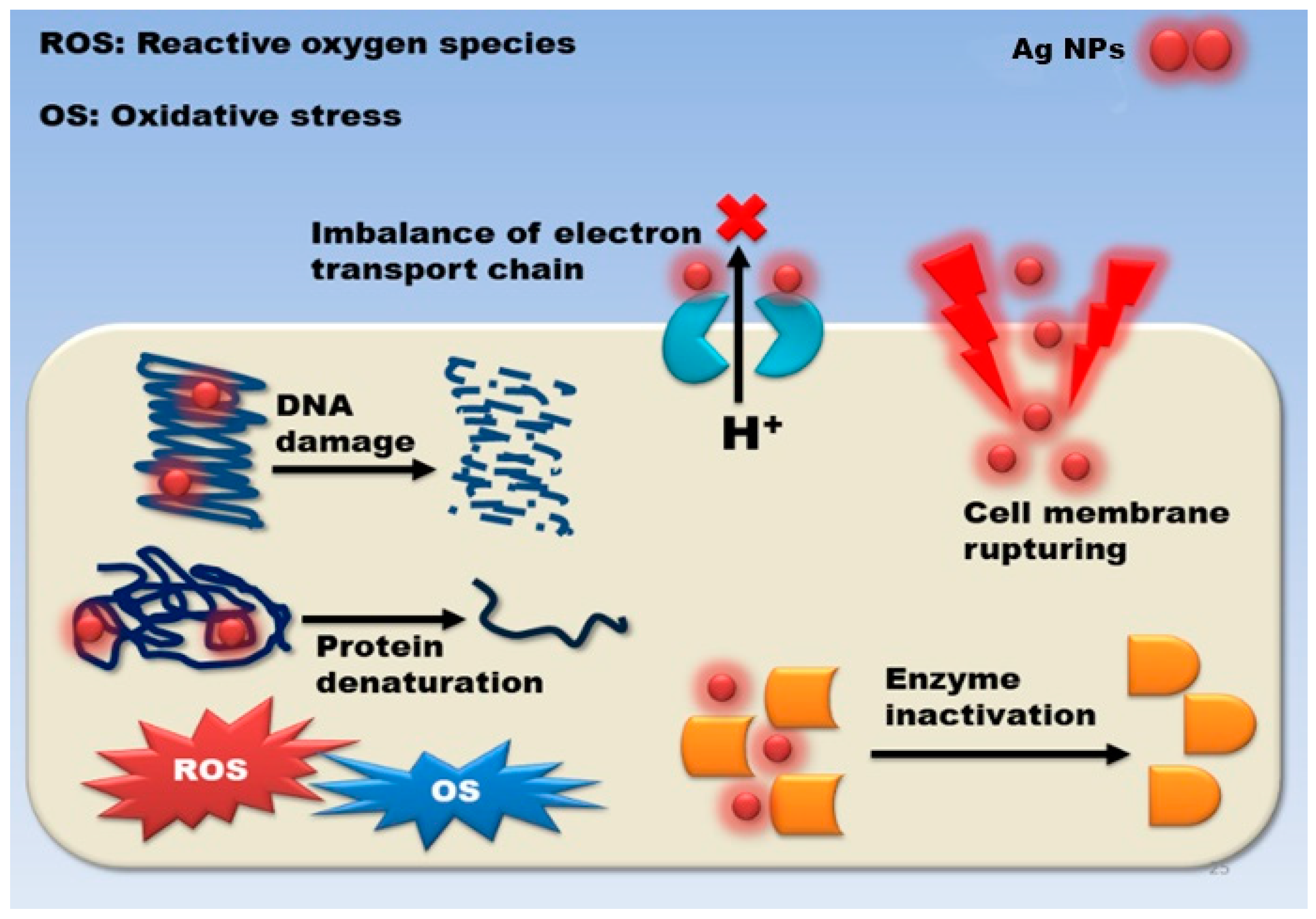
3.1. Mechanism of Action
3.2. Damage to the Cell Wall and Membrane
3.3. Intracellular Penetration and Damage
3.4. Oxidative Stress
4. Toxicology of Silver Nanoparticles to Human Health
5. Future Challenges
6. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mba, I.E.; Nweze, E.I. Nanoparticles as therapeutic options for treating multidrug-resistant bacteria: Research progress, challenges, and prospects. World J. Microbiol. Biotechnol. 2021, 37. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, C.; Le, C.; Tuttobene, M.R.; Subils, T.; Martinez, J.; Sieira, R.; Papp-Wallace, K.M.; Keppetipola, N.; Bonomo, R.A.; Actis, L.A.; et al. Human pleural fluid and human serum albumin modulate the behavior of a hypervirulent and multidrug-resistant (MDR) acinetobacter Baumannii representative strain. Pathogens 2021, 10, 471. [Google Scholar] [CrossRef] [PubMed]
- Amaro, F.; Morón, Á.; Díaz, S.; Martín-González, A.; Gutiérrez, J.C. Metallic nanoparticles—friends or foes in the battle against antibiotic-resistant bacteria? Microorganisms 2021, 9, 364. [Google Scholar] [CrossRef]
- Morgan, D.J.; Okeke, I.N.; Laxminarayan, R.; Perencevich, E.N.; Weisenberg, S. Non-prescription antimicrobial use worldwide: A systematic review. Lancet Infect. Dis. 2011, 11, 692–701. [Google Scholar] [CrossRef] [Green Version]
- Abbasi, E.; Milani, M.; Aval, S.F.; Kouhi, M.; Akbarzadeh, A.; Nasrabadi, H.T.; Nikasa, P.; Joo, S.W.; Hanifehpour, Y.; Nejati-Koshki, K.; et al. Silver nanoparticles: Synthesis methods, bio-applications and properties. Crit. Rev. Microbiol. 2016, 42, 173–180. [Google Scholar] [CrossRef]
- Mostafavi, E.; Medina-Cruz, D.; Vernet-Crua, A.; Chen, J.; Cholula-Díaz, J.L.; Guisbiers, G.; Webster, T.J. Green nanomedicine: The path to the next generation of nanomaterials for diagnosing brain tumors and therapeutics? Expert Opin. Drug Deliv. 2021, 18, 715–736. [Google Scholar] [CrossRef]
- Iqbal, T.; Raza, A.; Zafar, M.; Afsheen, S.; Kebaili, I.; Alrobei, H. Plant-mediated green synthesis of zinc oxide nanoparticles for novel application to enhance the shelf life of tomatoes. Appl. Nanosci. 2022, 12, 179–191. [Google Scholar] [CrossRef]
- Dhumale, V.A.; Gangwar, R.K.; Pande, N. Importance of gold nanoparticles for detection of toxic heavy metal ions and vital role in biomedical applications. Mater. Res. Innov. 2021, 25, 354–362. [Google Scholar] [CrossRef]
- Sridhar, K.; Inbaraj, B.S.; Chen, B.H. Recent advances on nanoparticle based strategies for improving carotenoid stability and biological activity. Antioxidants 2021, 10, 713. [Google Scholar] [CrossRef] [PubMed]
- Huq, M.A.; Ashrafudoulla, M.; Rahman, M.M.; Balusamy, S.R.; Akter, S. Green Synthesis and Potential Antibacterial Applications of Bioactive Silver Nanoparticles: A Review. Polymers 2022, 14, 742. [Google Scholar] [CrossRef]
- Rafique, M.; Sadaf, I.; Rafique, M.S.; Tahir, M.B. A review on green synthesis of silver nanoparticles and their applications. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1272–1291. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.; Hu, X.; Yan, H.; Sun, Y. Surface functionalization of nanostructured Cu/Ag-deposited polypropylene fiber by magnetron sputtering. E-Polymers 2021, 21, 140–150. [Google Scholar] [CrossRef]
- Valerini, D.; Tammaro, L.; Vitali, R.; Guillot, G.; Rinaldi, A. Sputter-deposited ag nanoparticles on electrospun pcl scaffolds: Morphology, wettability and antibacterial activity. Coatings 2021, 11, 345. [Google Scholar] [CrossRef]
- Natsuki, J. A Review of Silver Nanoparticles: Synthesis Methods, Properties and Applications. Int. J. Mater. Sci. Appl. 2015, 4, 325. [Google Scholar] [CrossRef]
- Qais, F.A.; Shafiq, A.; Khan, H.M.; Husain, F.M.; Khan, R.A.; Alenazi, B.; Alsalme, A.; Ahmad, I. Antibacterial effect of silver nanoparticles synthesized using Murraya koenigii (L.) against multidrug-resistant pathogens. Bioinorg. Chem. Appl. 2019, 2019. [Google Scholar] [CrossRef] [Green Version]
- MODAN, E.M.; PLĂIAȘU, A.G. Advantages and Disadvantages of Chemical Methods in the Elaboration of Nanomaterials. Ann. “Dunarea Jos” Univ. Galati. Fascicle IX Metall. Mater. Sci. 2020, 43, 53–60. [Google Scholar] [CrossRef]
- Singh, J.; Dutta, T.; Kim, K.H.; Rawat, M.; Samddar, P.; Kumar, P. “Green” synthesis of metals and their oxide nanoparticles: Applications for environmental remediation. J. Nanobiotechnol. 2018, 16. [Google Scholar] [CrossRef] [PubMed]
- Dawadi, S.; Katuwal, S.; Gupta, A.; Lamichhane, U.; Thapa, R.; Jaisi, S.; Lamichhane, G.; Bhattarai, D.P.; Parajuli, N. Current Research on Silver Nanoparticles: Synthesis, Characterization, and Applications. J. Nanomater. 2021, 2021. [Google Scholar] [CrossRef]
- Koul, B.; Poonia, A.K.; Yadav, D.; Jin, J.O. Microbe-mediated biosynthesis of nanoparticles: Applications and future prospects. Biomolecules 2021, 11, 886. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, T. A modern review of silver nanoparticles mediated plant extracts and its potential bioapplications. Int. J. Bot. Stud. 2021, 6, 170–175. [Google Scholar]
- De Silva, C.; Nawawi, N.M.; Karim, M.M.A.; Gani, S.A.; Masarudin, M.J.; Gunasekaran, B.; Ahmad, S.A. The mechanistic action of biosynthesised silver nanoparticles and its application in aquaculture and livestock industries. Animals 2021, 11, 2097. [Google Scholar] [CrossRef] [PubMed]
- El-Bendary, M.A.; Afifi, S.S.; Moharam, M.E.; Abo El-Ola, S.M.; Salama, A.; Omara, E.A.; Shaheen, M.N.F.; Hamed, A.A.; Gawdat, N.A. Biosynthesis of silver nanoparticles using isolated Bacillus subtilis: Characterization, antimicrobial activity, cytotoxicity, and their performance as antimicrobial agent for textile materials. Prep. Biochem. Biotechnol. 2020, 0, 54–68. [Google Scholar] [CrossRef] [PubMed]
- Monowar, T.; Rahman, M.S.; Bhore, S.J.; Sathasivam, K.V. Endophytic bacteria enterobacter hormaechei fabricated silver nanoparticles and their antimicrobial activity. Pharmaceutics 2021, 13, 511. [Google Scholar] [CrossRef]
- Gordienko, M.G.; Palchikova, V.V.; Kalenov, S.V.; Lebedev, E.A.; Belov, A.A.; Menshutina, N.V. The alginate–chitosan composite sponges with biogenic Ag nanoparticles produced by combining of cryostructuration, ionotropic gelation and ion replacement methods. Int. J. Polym. Mater. Polym. Biomater. 2020, 0, 1–11. [Google Scholar] [CrossRef]
- Vala, A.K.; Trivedi, H.; Gosai, H.; Panseriya, H.; Dave, B. Biosynthesized Silver Nanoparticles and Their Therapeutic Applications, 1st ed.; Elsevier B.V.: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Ghosh, V. Marine Bioresources as Potential Source for Synthesis of Nanoparticles. Encycl. Mar. Biotechnol. 2020, 1521–1534. [Google Scholar] [CrossRef]
- Orizola, J.; Ríos-Silva, M.; Muñoz-Villagrán, C.; Vargas, E.; Vásquez, C.; Arenas, F. In vitro biosynthesis of Ag, Au and Te-containing nanostructures by Exiguobacterium cell-free extracts. BMC Biotechnol. 2020, 20, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Al-Hamoud, K.; Liaqat, Z.; Shaik, M.R.; Adil, S.F.; Kuniyil, M.; Alkhathlan, H.Z.; Al-Warthan, A.; Siddiqui, M.R.H.; Mondeshki, M.; et al. Synthesis of au, ag, and au–ag bimetallic nanoparticles using pulicaria undulata extract and their catalytic activity for the reduction of 4-nitrophenol. Nanomaterials 2020, 10, 1885. [Google Scholar] [CrossRef] [PubMed]
- Syame, S.M.; Mansour, A.S.; Khalaf, D.D.; Ibrahim, E.S.; Gaber, E.S. Green Synthesis of Silver Nanoparticles Using Lactic Acid Bacteria: Assessment of Antimicrobial Activity. World’s Vet. J. 2020, 10, 625–633. [Google Scholar] [CrossRef]
- Patil, S.P.; Kumbhar, S.T. Vitex negundo assisted green synthesis of metallic nanoparticles with different applications: A mini review. Futur. J. Pharm. Sci. 2020, 6. [Google Scholar] [CrossRef]
- Gloria Martin, K.D.; Vergara Padilla, K.G. Sunlight Mediated Synthesis of Silver Nanoparticles by Bacillus sp and Its Antibacterial Property. Orient. J. Chem. 2020, 36, 419–424. [Google Scholar] [CrossRef]
- Yorseng, K.; Siengchin, S.; Ashok, B.; Rajulu, A.V. Nanocomposite egg shell powder with in situ generated silver nanoparticles using inherent collagen as reducing agent. J. Bioresour. Bioprod. 2020, 5, 101–107. [Google Scholar] [CrossRef]
- Awwad, A.M.; Salem, N.M.; Aqarbeh, M.M.; Abdulaziz, F.M. Green synthesis, characterization of silver sulfide nanoparticles and antibacterial activity evaluation. Chem. Int. 2019, 6, 42–48. [Google Scholar] [CrossRef]
- Ramakrishnan, A.; Gokul, R.; Pandimadevi, M. Preparation and characterisation of nanofibres from bio cellulose and neem-AgNP bio composites for wound healing. Int. J. Biomed. Nanosci. Nanotechnol. 2020, 4, 80. [Google Scholar] [CrossRef]
- Arifin, D.C.V.; Saragih, D.I.; Santosa, S.J. Antibacterial activity of silver nanoparticles synthesized using tyrosine as capping and reducing agent. Int. J. Emerg. Trends Eng. Res. 2020, 8, 2414–2421. [Google Scholar] [CrossRef]
- Mamangkey, J.; Suryanto, D.; Munir, E.; Mustopa, A.Z. Antibacterial and Antioxidant Activity of Newly Keratinolytic Bacteria, Azotobacter chroococcum B4. Int. J. PharmTech Res. 2020, 13, 123–127. [Google Scholar] [CrossRef]
- Geoprincy, G.; Vidhya Srri, B.N.; Poonguzhali, U.; Nagendra Gandhi, N.; Renganathan, S. A review on green synthesis of silver nanoparticles. Asian J. Pharm. Clin. Res. 2013, 6, 8–12. [Google Scholar]
- Salouti, M.; Derakhshan, F.K. Phytosynthesis of Nanoscale Materials; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 9780128153222. [Google Scholar]
- Kobayashi, R.K.T.; Nishio, E.K.; Scandorieiro, S.; Saikawa, G.I.A.; Da Rocha, S.P.D.; Nakazato, G. Metallic Nanoparticles as a Potential Antimicrobial for Catheters and Prostheses; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 9780128184356. [Google Scholar]
- Baruah, D.; Yadav, R.N.S.; Yadav, A.; Das, A.M. Alpinia nigra fruits mediated synthesis of silver nanoparticles and their antimicrobial and photocatalytic activities. J. Photochem. Photobiol. B Biol. 2019, 201, 111649. [Google Scholar] [CrossRef] [PubMed]
- Akl, B.; Nader, M.; El-Saadony, M. Biosynthesis of Silver Nanoparticles by Serratia marcescens ssp sakuensis and its Antibacterial Application against some Pathogenic Bacteria. J. Agric. Chem. Biotechnol. 2020, 11, 1–8. [Google Scholar] [CrossRef]
- Elangovan, M.; Muju, G.; Anantharaman, P. Biosynthesis of Silver Nanoparticles from Platymonas sp. and Its Antibacterial Activity Against Biofouling Causing Bacterial Strains. J. Biol. Act. Prod. Nat. 2019, 9, 269–277. [Google Scholar] [CrossRef]
- Arya, G.; Mankamna Kumari, R.; Pundir, R.; Chatterjee, S.; Gupta, N.; Kumar, A.; Chandra, R.; Nimesh, S. Versatile biomedical potential of biosynthesized silver nanoparticles from Acacia nilotica bark. J. Appl. Biomed. 2019, 17, 115–124. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Saravanakumar, K.; Jin, T.; Wang, M.H. Mycosynthesis, characterization, anticancer and antibacterial activity of silver nanoparticles from endophytic fungus Talaromyces purpureogenus. Int. J. Nanomed. 2019, 14, 3427–3438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minh Dat, N.; Linh, V.N.P.; Huy, L.A.; Huong, N.T.; Tu, T.H.; Phuong, N.T.L.; Nam, H.M.; Thanh Phong, M.; Hieu, N.H. Fabrication and antibacterial activity against Pseudomonas aeruginosa and Staphylococcus aureus of silver nanoparticle decorated reduced graphene oxide nanocomposites. Mater. Technol. 2019, 34, 369–375. [Google Scholar] [CrossRef]
- Roy, A.; Bulut, O.; Some, S.; Mandal, A.K.; Yilmaz, M.D. Green synthesis of silver nanoparticles: Biomolecule-nanoparticle organizations targeting antimicrobial activity. RSC Adv. 2019, 9, 2673–2702. [Google Scholar] [CrossRef] [Green Version]
- Ratan, Z.A.; Haidere, M.F.; Nurunnabi, M.; Shahriar, S.M.; Ahammad, A.J.S.; Shim, Y.Y.; Reaney, M.J.T.; Cho, J.Y. Green chemistry synthesis of silver nanoparticles and their potential anticancer effects. Cancers 2020, 12, 855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Rubaye, H.I.; Al-Rubaye, B.K.; Al-Abodi, E.E.; Yousif, E.I. Green Chemistry Synthesis of Modified Silver Nanoparticles. J. Phys. Conf. Ser. 2020, 1664, 1–26. [Google Scholar] [CrossRef]
- Seetharaman, P.K.; Chandrasekaran, R.; Periakaruppan, R.; Gnanasekar, S.; Sivaperumal, S.; Abd-Elsalam, K.A.; Valis, M.; Kuca, K. Functional attributes of myco-synthesized silver nanoparticles from endophytic fungi: A new implication in biomedical applications. Biology 2021, 10, 473. [Google Scholar] [CrossRef]
- Khan, I.H.; Javaid, A.; Ahmed, D. Trichoderma viride Controls Macrophomina phaseolina through its DNA disintegration and Production of Antifungal Compounds. Int. J. Agric. Biol. 2021, 25, 888–894. [Google Scholar] [CrossRef]
- Štular, D.; Savio, E.; Simončič, B.; Šobak, M.; Jerman, I.; Poljanšek, I.; Ferri, A.; Tomšič, B. Multifunctional antibacterial and ultraviolet protective cotton cellulose developed by in situ biosynthesis of silver nanoparticles into a polysiloxane matrix mediated by sumac leaf extract. Appl. Surf. Sci. 2021, 563. [Google Scholar] [CrossRef]
- Al-hosaini, K.A.; Azhar, A. Silver Nanoparticle and their Antimicrobial Properties. EC Pharmacol. Toxicol. 2021, 9, 24–31. [Google Scholar]
- Dawoud, T.M.; Yassin, M.A.; El-Samawaty, A.R.M.; Elgorban, A.M. Silver nanoparticles synthesized by Nigrospora oryzae showed antifungal activity. Saudi J. Biol. Sci. 2021, 28, 1847–1852. [Google Scholar] [CrossRef]
- Al-khattaf, F.S. Gold and silver nanoparticles: Green synthesis, microbes, mechanism, factors, plant disease management and environmental risks. Saudi J. Biol. Sci. 2021, 28, 3624–3631. [Google Scholar] [CrossRef]
- Kahraman, T.; Elif Korcan, S.; Liman, R.; Hakkı Ciğerci, İ.; Acikbas, Y.; Konuk, M.; Uysal Akkuş, G. Synthesis, Characterization, and Optimization of Green Silver Nanoparticles Using Neopestalotiopsis clavispora and Evaluation of Its Antibacterial, Antibiofilm, and Genotoxic Effects. EuroBiotech J. 2021, 5, 109–122. [Google Scholar] [CrossRef]
- Rai, M.; Bonde, S.; Golinska, P.; Trzcińska-Wencel, J.; Gade, A.; Abd-Elsalam, K.; Shende, S.; Gaikwad, S.; Ingle, A.P. Fusarium as a novel fungus for the synthesis of nanoparticles: Mechanism and applications. J. Fungi 2021, 7, 139. [Google Scholar] [CrossRef] [PubMed]
- Santos, T.S.; Silva, T.M.; Cardoso, J.C.; de Albuquerque-Júnior, R.L.C.; Zielinska, A.; Souto, E.B.; Severino, P.; da Costa Mendonça, M. Biosynthesis of Silver Nanoparticles Mediated by Entomopathogenic Fungi: Antimicrobial Resistance, Nanopesticides, and Toxicity. Antibiotics 2021, 10, 852. [Google Scholar] [CrossRef] [PubMed]
- El-Mekkawy, R.M.; Almanaa, T.N.; Yassin, M.A.; Rabie, G.; Saleh, N. Silver nanoparticles (AgNPs) biosynthesized by Aspergillus flavus KF946095; their characterization and antibacterial activity. J. Pure Appl. Microbiol. 2021, 15, 105–113. [Google Scholar] [CrossRef]
- Singh, A.; Gaurav, S.S.; Shukla, G.; Rani, P. Evaluation of mycosilver nanofungicides as potential control agent against phytophthora infestans. Plant Cell Biotechnol. Mol. Biol. 2021, 22, 157–168. [Google Scholar]
- Guilger-Casagrande, M.; Germano-Costa, T.; Bilesky-José, N.; Pasquoto-Stigliani, T.; Carvalho, L.; Fraceto, L.F.; de Lima, R. Influence of the capping of biogenic silver nanoparticles on their toxicity and mechanism of action towards Sclerotinia sclerotiorum. J. Nanobiotechnol. 2021, 19, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Gnanasangeetha, D.; Suresh, M. A review on green synthesis of metal and metal oxide nanoparticles. Nat. Environ. Pollut. Technol. 2020, 19, 1789–1800. [Google Scholar] [CrossRef]
- Potbhare, A.K.; Chouke, P.B.; Mondal, A.; Thakare, R.U.; Mondal, S.; Chaudhary, R.G.; Rai, A.R. Rhizoctonia solani assisted biosynthesis of silver nanoparticles for antibacterial assay. Mater. Today Proc. 2019, 29, 939–945. [Google Scholar] [CrossRef]
- Sopan Namdev, N.; Pravin Onkar, P. Green Synthesis of Silver Nanoparticles: An Eco- Friendly Approach. Nano Biomed. Eng. 2020, 12, 281–296. [Google Scholar] [CrossRef]
- Çağrı Mehmetoğlu, A.; Sezer, E.; Erol, S. Development of antimicrobial whey protein-based film containing silver nanoparticles biosynthesised by Aspergillus Niger. Int. J. Food Sci. Technol. 2021, 56, 965–973. [Google Scholar] [CrossRef]
- Rizwan, M.; Amin, S.; Kudaibergenova, B.M.; Rauf, A.; Siddique, M.; Ullah, K.; Bawazeer, S.; Farooq, U.; Mabkhot, Y.N.; Ramadan, M.F. Green synthesis and antimicrobial potential of silver Nanoparticles with Boerhavia procumbens extract. J. Pure Appl. Microbiol. 2020, 14, 1437–1451. [Google Scholar] [CrossRef]
- Huang, W.; Yan, M.; Duan, H.; Bi, Y.; Cheng, X.; Yu, H. Synergistic Antifungal Activity of Green Synthesized Silver Nanoparticles and Epoxiconazole against Setosphaeria turcica. J. Nanomater. 2020, 2020. [Google Scholar] [CrossRef] [Green Version]
- Almaary, K.S.; Sayed, S.R.M.; Abd-Elkader, O.H.; Dawoud, T.M.; El Orabi, N.F.; Elgorban, A.M. Complete green synthesis of silver-nanoparticles applying seed-borne Penicillium duclauxii. Saudi J. Biol. Sci. 2020, 27, 1333–1339. [Google Scholar] [CrossRef] [PubMed]
- Soliman, A.M.; Abdel-Latif, W.; Shehata, I.H.; Fouda, A.; Abdo, A.M.; Ahmed, Y.M. Green Approach to Overcome the Resistance Pattern of Candida spp. Using Biosynthesized Silver Nanoparticles Fabricated by Penicillium chrysogenum F9. Biol. Trace Elem. Res. 2021, 199, 800–811. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.; De Klerk, C.; Kim, J.; Kang, M.; Fosso-Kankeu, E. Eco friendly approach for synthesis, characterization and biological activities of milk protein stabilized silver nanoparticles. Polymers 2020, 12, 1418. [Google Scholar] [CrossRef] [PubMed]
- Guilger-Casagrande, M.; de Lima, R. Synthesis of Silver Nanoparticles Mediated by Fungi: A Review. Front. Bioeng. Biotechnol. 2019, 7, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-bahrani, R.M. Green synthesized of silver nanoparticles and study their properties and applications. J. Biotechnol. Res. Cent. 2019, 13, 5–9. [Google Scholar]
- Barbosa, A.C.M.S.; Costa Silva, L.P.; Ferraz, C.M.; Tobias, F.L.; De Araújo, J.V.; Loureiro, B.; Braga, G.M.A.M.; Veloso, F.B.R.; Soares, F.E.D.F.; Fronza, M.; et al. Nematicidal activity of silver nanoparticles from the fungus Duddingtonia flagrans. Int. J. Nanomed. 2019, 14, 2341–2348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madhankumar, R.; Sivasankar, P.; Kalaimurugan, D.; Murugesan, S. Antibacterial and Larvicidal Activity of Silver Nanoparticles Synthesized by the Leaf Extract of Andrographis serpyllifolia Wight. J. Clust. Sci. 2020, 31, 719–726. [Google Scholar] [CrossRef]
- Noshad, A.; Iqbal, M.; Folkers, L.; Hetherington, C.; Khan, A.; Numan, M.; Ullah, S. Antibacterial Effect of Silver Nanoparticles (AgNPs) Synthesized from Trichoderma Harzianum against Clavibacter Michiganensis. J. Nano Res. 2019, 58, 10–19. [Google Scholar] [CrossRef]
- Bagur, H.; Poojari, C.C.; Melappa, G.; Rangappa, R.; Chandrasekhar, N.; Somu, P. Biogenically Synthesized Silver Nanoparticles Using Endophyte Fungal Extract of Ocimum tenuiflorum and Evaluation of Biomedical Properties. J. Clust. Sci. 2020, 31, 1241–1255. [Google Scholar] [CrossRef]
- Gemishev, O.T.; Panayotova, M.I.; Panayotov, V.T. Biosynthesis of silver nanoparticles by cell-free extract from Trichoderma reesei—Study on the influence of growth media. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1117, 012007. [Google Scholar] [CrossRef]
- Avilala, J.; Golla, N. Antibacterial and Antiviral Properties of Silver Nanoparticles Synthesized By Marine Actinomycetes. Int. J. Pharm. Sci. Res. 2019, 10, 1223–1228. [Google Scholar] [CrossRef]
- Lee, S.H.; Jun, B.H. Silver nanoparticles: Synthesis and application for nanomedicine. Int. J. Mol. Sci. 2019, 20, 865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rani, P.; Kumar, V.; Pal, P.; Singh, A.; Zhang, W. Highly stable AgNPs prepared via a novel green approach for catalytic and photocatalytic removal of biological and non-biological pollutants. Environ. Int. 2020, 143, 105924. [Google Scholar] [CrossRef] [PubMed]
- Wani, I.A. Review—Recent Advances in Biogenic Silver Nanoparticles & NanoComposite Based Plasmonic-Colorimetric and Electrochemical Sensors. ECS J. Solid State Sci. Technol. 2021, 10, 047003. [Google Scholar] [CrossRef]
- Jain, R.; Mendiratta, S.; Kumar, L.; Srivastava, A. Green synthesis of iron nanoparticles using Artocarpus heterophyllus peel extract and their application as a heterogeneous Fenton-like catalyst for the degradation of Fuchsin Basic dye. Curr. Res. Green Sustain. Chem. 2021, 4, 100086. [Google Scholar] [CrossRef]
- Safipour Afshar, A.; Saeid Nematpour, F. Evaluation of the Cytotoxic Activity of Biosynthesized Silver Nanoparticles Using Berberis vulgaris Leaf Extract. Jentashapir J. Cell. Mol. Biol. 2021, 12, 1–7. [Google Scholar] [CrossRef]
- Swara, B.J.; Al-barzinji, I.M. Effects of Nano Silver Particles and Gibberellic Acid on Growth and Some Physiological Characteristics of Dalbergia Sissoo Roxb. Sci. J. Univ. Zakho 2021, 9, 102–108. [Google Scholar] [CrossRef]
- Arif, R.; Uddin, R. A review on recent developments in the biosynthesis of silver nanoparticles and its biomedical applications. Med. Devices Sens. 2021, 4, 1–20. [Google Scholar] [CrossRef]
- Bahattab, O.; Khan, I.; Bawazeer, S.; Rauf, A.; Qureshi, M.N.; Al-Awthan, Y.S.; Muhammad, N.; Khan, A.; Akram, M.; Islam, M.N.; et al. Synthesis and biological activities of alcohol extract of black cumin seeds ( Bunium persicum )-based gold nanoparticles and their catalytic applications. Green Process. Synth. 2021, 10, 440–455. [Google Scholar] [CrossRef]
- Vivehananthan, K.; Weligodage, H. Effect of Different Process Parameters on the formation of Silver Nanoparticles using Crude and Modified Neem ( Azadirachta indica ) Leaf Extracts. USJP-Acad. J. 2021, 1, 266–276. [Google Scholar] [CrossRef]
- Chen, Z.; Niu, J.; Guo, Z.; Sui, X.; Xu, N.; Kareem, H.A.; Hassan, M.U.; Zhang, Q.; Cui, J.; Wang, Q. Integrating transcriptome and physiological analyses to elucidate the essential biological mechanisms of graphene phytotoxicity of alfalfa (Medicago sativa L.). Ecotoxicol. Environ. Saf. 2021, 220, 112348. [Google Scholar] [CrossRef]
- Lashin, I.; Fouda, A.; Gobouri, A.A.; Azab, E.; Mohammedsaleh, Z.M.; Makharita, R.R. Antimicrobial and in vitro cytotoxic efficacy of biogenic silver nanoparticles (Ag-nps) fabricated by callus extract of solanum incanum L. Biomolecules 2021, 11, 341. [Google Scholar] [CrossRef]
- Sharma, Y.; Kawatra, A.; Sharma, V.; Dhull, D.; Kaushik, S.; Yadav, J.P.; Kaushik, S. In-vitro and in-silico evaluation of the anti-chikungunya potential of Psidium guajava leaf extract and their synthesized silver nanoparticles. VirusDisease 2021, 32, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Vyavahare, S.; Padole, N.N. A REVIEW: SILVER NANOPARTICLES IN WOUND HEALING. Eur. J. Pharm. Med. Res. 2021, 8, 212–218. [Google Scholar]
- Kyzioł, A.; Łukasiewicz, S.; Sebastian, V.; Kuśtrowski, P.; Kozieł, M.; Majda, D.; Cierniak, A. Towards plant-mediated chemistry – Au nanoparticles obtained using aqueous extract of Rosa damascena and their biological activity in vitro. J. Inorg. Biochem. 2021, 214. [Google Scholar] [CrossRef]
- Göl, F.; Aygün, A.; Seyrankaya, A.; Gür, T.; Yenikaya, C.; Şen, F. Green synthesis and characterization of Camellia sinensis mediated silver nanoparticles for antibacterial ceramic applications. Mater. Chem. Phys. 2020, 250. [Google Scholar] [CrossRef]
- Al-Shaheen, M.A.S.; Owaid, M.N.; Muslim, R.F. Synthesis and Characterization of Zinc Nanoparticles by Natural Organic Compounds Extracted from Licorice Root and their Influence on Germination of Sorghum bicolor Seeds. Jordan J. Biol. Sci. 2020, 13, 559–565. [Google Scholar]
- Tehri, N.; Vashishth, A.; Gahlaut, A.; Hooda, V. Biosynthesis, antimicrobial spectra and applications of silver nanoparticles: Current progress and future prospects. Inorg. Nano-Metal Chem. 2020, 52, 1–19. [Google Scholar] [CrossRef]
- Fahmy, S.A.; Preis, E.; Bakowsky, U.; Azzazy, H.M.E.S. Platinum Nanoparticles: Green Synthesis and Biomedical Applications. Molecules 2020, 25, 4981. [Google Scholar] [CrossRef] [PubMed]
- Meena, R.K.; Meena, R.; Arya, D.K.; Jadoun, S.; Hada, R.; Kumari, R. Synthesis of Silver Nanoparticles by Phyllanthus emblica Plant Extract and Their Antibacterial Activity. Mater. Sci. Res. India 2020, 17, 136–145. [Google Scholar] [CrossRef]
- Anees Ahmad, S.; Sachi Das, S.; Khatoon, A.; Tahir Ansari, M.; Afzal, M.; Saquib Hasnain, M.; Kumar Nayak, A. Bactericidal activity of silver nanoparticles: A mechanistic review. Mater. Sci. Energy Technol. 2020, 3, 756–769. [Google Scholar] [CrossRef]
- Balogun, S.W.; James, O.O.; Sanusi, Y.K.; Olayinka, O.H. Green synthesis and characterization of zinc oxide nanoparticles using bashful (Mimosa pudica), leaf extract: A precursor for organic electronics applications. SN Appl. Sci. 2020, 2, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, D.H.; Lee, J.S.; Park, K.D.; Ching, Y.C.; Nguyen, X.T.; Phan, V.H.G.; Thi, T.T.H. Green silver nanoparticles formed by Phyllanthus urinaria, Pouzolzia zeylanica, and scoparia dulcis leaf extracts and the antifungal activity. Nanomaterials 2020, 10, 542. [Google Scholar] [CrossRef] [Green Version]
- Manik, U.P.; Nande, A.; Raut, S.; Dhoble, S.J. Green synthesis of silver nanoparticles using plant leaf extraction of Artocarpus heterophylus and Azadirachta indica. Results Mater. 2020, 6, 100086. [Google Scholar] [CrossRef]
- Jeyaprakash, K.; AlSalhi, M.S.; Devanesan, S. Anticancer and antioxidant efficacy of silver nanoparticles synthesized from fruit of Morinda citrifolia Linn on Ehrlich ascites carcinoma mice. J. King Saud Univ.-Sci. 2020, 32, 3181–3186. [Google Scholar] [CrossRef]
- Tag, H.M. Green Synthesis and Characterization of Silver Nanoparticles Using Bauhinia Variegate Leaves Aqueous Extract. Biomed. J. Sci. Tech. Res. 2020, 29. [Google Scholar] [CrossRef]
- Bhardwaj, K.; Dhanjal, D.S.; Sharma, A.; Nepovimova, E.; Kalia, A.; Thakur, S.; Bhardwaj, S.; Chopra, C.; Singh, R.; Verma, R.; et al. Conifer-derived metallic nanoparticles: Green synthesis and biological applications. Int. J. Mol. Sci. 2020, 21, 9028. [Google Scholar] [CrossRef]
- Maktumsab, M.T.; Guled, M.B. Plant archives. Plant Arch. 2017, 17, 261–266. [Google Scholar]
- Ankegowda, V.M.; Kollur, S.P.; Prasad, S.K.; Pradeep, S.; Dhramashekara, C.; Jain, A.S.; Prasad, A.; Srinivasa, C.; Sridhara Setty, P.B.; Gopinath, S.M.; et al. Phyto-Mediated Synthesis of Silver Nanoparticles Using Terminalia chebula Fruit Extract and Evaluation of Its Cytotoxic and Antimicrobial Potential. Molecules 2020, 25, 5042. [Google Scholar] [CrossRef] [PubMed]
- Mikhailova, E.O. Silver Nanoparticles: Mechanism of Action and Probable Bio-Application. J. Funct. Biomater. 2020, 11, 84. [Google Scholar] [CrossRef] [PubMed]
- Akintelu, S.A.; Bo, Y.; Folorunso, A.S. A review on synthesis, optimization, mechanism, characterization, and antibacterial application of silver nanoparticles synthesized from plants. J. Chem. 2020, 2020. [Google Scholar] [CrossRef]
- Salmen, S.H.; Alharbi, S.A. Silver nanoparticles synthesized biogenically from Aloe fleurentiniorum extract: Characterization and antibacterial activity. Green Chem. Lett. Rev. 2020, 13, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Hassan, O.M.; Ibraheem, I.J.; Adil, B.H.; Obaid, A.S.; Salih, T.A.; Obaid, A.S. Synthesis of Silver Nanoparticles by ecofriendly nvironmental method using Piper nigrum, Ziziphus spina-christi, and Eucalyptusglobulus extract. J. Phys. Conf. Ser. 2020, 1530. [Google Scholar] [CrossRef]
- Valsalam, S.; Agastian, P.; Esmail, G.A.; Ghilan, A.K.M.; Al-Dhabi, N.A.; Arasu, M.V. Biosynthesis of silver and gold nanoparticles using Musa acuminata colla flower and its pharmaceutical activity against bacteria and anticancer efficacy. J. Photochem. Photobiol. B Biol. 2019, 201, 111670. [Google Scholar] [CrossRef] [PubMed]
- Bahrami-Teimoori, B.; Nikparast, Y.; Hojatianfar, M.; Akhlaghi, M.; Ghorbani, R.; Pourianfar, H.R. Characterisation and antifungal activity of silver nanoparticles biologically synthesised by Amaranthus retroflexus leaf extract. J. Exp. Nanosci. 2017, 12, 129–139. [Google Scholar] [CrossRef] [Green Version]
- Hamidi, A.; Taghavizadeh Yazdi, M.E.; Amiri, M.S.; Hosseini, H.A.; Darroudi, M. Biological synthesis of silver nanoparticles in Tribulus terrestris L. extract and evaluation of their photocatalyst, antibacterial, and cytotoxicity effects. Res. Chem. Intermed. 2019, 45, 2915–2925. [Google Scholar] [CrossRef]
- Bhuvaneswari, T.S.; Thirugnanam, T.; Thirumurugan, V. Phytomediated synthesis of silver nanoparticles using Cassia auriculata L: Evaluation of antibacterial and antifungal activity. Asian J. Pharm. Pharmacol. 2019, 5, 326–331. [Google Scholar] [CrossRef]
- Boomi, P.; Ganesan, R.M.; Poorani, G.; Gurumallesh Prabu, H.; Ravikumar, S.; Jeyakanthan, J. Biological synergy of greener gold nanoparticles by using Coleus aromaticus leaf extract. Mater. Sci. Eng. C 2019, 99, 202–210. [Google Scholar] [CrossRef]
- Chandhru, M.; Logesh, R.; Rani, S.K.; Ahmed, N.; Vasimalai, N. One-pot green route synthesis of silver nanoparticles from jack fruit seeds and their antibacterial activities with escherichia coli and salmonella bacteria. Biocatal. Agric. Biotechnol. 2019, 20, 101241. [Google Scholar] [CrossRef]
- Chaudhary, S.; Rohilla, D.; Umar, A.; Kaur, N.; Shanavas, A. Synthesis and characterizations of luminescent copper oxide nanoparticles: Toxicological profiling and sensing applications. Ceram. Int. 2019, 45, 15025–15035. [Google Scholar] [CrossRef]
- Eisa, W.H.; Zayed, M.F.; Anis, B.; Abbas, L.M.; Ali, S.S.M.; Mostafa, A.M. Clean production of powdery silver nanoparticles using Zingiber officinale: The structural and catalytic properties. J. Clean. Prod. 2019, 241, 118398. [Google Scholar] [CrossRef]
- Aritonang, H.F.; Koleangan, H.; Wuntu, A.D. Synthesis of silver nanoparticles using aqueous extract of medicinal plants’ (impatiens balsamina and lantana camara) fresh leaves and analysis of antimicrobial activity. Int. J. Microbiol. 2019, 2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Izwanie, N.; Basri, H.; Harun, Z. Biosynthesized nanoparticles from aloe vera: A brief review towards membrane technology. Malays. J. Fundam. Appl. Sci. 2019, 15, 895–902. [Google Scholar]
- Kalaiselvi, D.; Mohankumar, A.; Shanmugam, G.; Nivitha, S.; Sundararaj, P. Green synthesis of silver nanoparticles using latex extract of Euphorbia tirucalli: A novel approach for the management of root knot nematode, Meloidogyne incognita. Crop Prot. 2019, 117, 108–114. [Google Scholar] [CrossRef]
- Anandan, M.; Poorani, G.; Boomi, P.; Varunkumar, K.; Anand, K.; Chuturgoon, A.A.; Saravanan, M.; Gurumallesh Prabu, H. Green synthesis of anisotropic silver nanoparticles from the aqueous leaf extract of Dodonaea viscosa with their antibacterial and anticancer activities. Process Biochem. 2019, 80, 80–88. [Google Scholar] [CrossRef]
- Nayak, S.; Sajankila, S.P.; Rao, C.V.; Hegde, A.R.; Mutalik, S. Biogenic synthesis of silver nanoparticles using Jatropha curcas seed cake extract and characterization: Evaluation of its antibacterial activity. Energy Sources Part A Recover. Util. Environ. Eff. 2019, 43, 3415–3423. [Google Scholar] [CrossRef]
- Thakur, B.K.; Kumar, A.; Kumar, D. Green synthesis of titanium dioxide nanoparticles using Azadirachta indica leaf extract and evaluation of their antibacterial activity. S. Afr. J. Bot. 2019, 124, 223–227. [Google Scholar] [CrossRef]
- Ajitha, B.; Reddy, Y.A.K.; Jeon, H.J.; Ahn, C.W. Synthesis of silver nanoparticles in an eco-friendly way using Phyllanthus amarus leaf extract: Antimicrobial and catalytic activity. Adv. Powder Technol. 2018, 29, 86–93. [Google Scholar] [CrossRef]
- Labanni, A.; Zulhadjri, Z.; Handayani, D.; Ohya, Y.; Arief, S. The effect of monoethanolamine as stabilizing agent in Uncaria gambir Roxb. mediated synthesis of silver nanoparticles and its antibacterial activity. J. Dispers. Sci. Technol. 2020, 41, 1480–1487. [Google Scholar] [CrossRef]
- Ezealisiji, K.M.; Siwe-Noundou, X.; Maduelosi, B.; Nwachukwu, N.; Krause, R.W.M. Green synthesis of zinc oxide nanoparticles using Solanum torvum (L) leaf extract and evaluation of the toxicological profile of the ZnO nanoparticles–hydrogel composite in Wistar albino rats. Int. Nano Lett. 2019, 9, 99–107. [Google Scholar] [CrossRef] [Green Version]
- Huq, M.A. Green synthesis of silver nanoparticles using pseudoduganella eburnea MAHUQ-39 and their antimicrobial mechanisms investigation against drug resistant human pathogens. Int. J. Mol. Sci. 2020, 21, 1510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nayak, D.; Ashe, S.; Rauta, P.R.; Kumari, M.; Nayak, B. Bark extract mediated green synthesis of silver nanoparticles: Evaluation of antimicrobial activity and antiproliferative response against osteosarcoma. Mater. Sci. Eng. C 2016, 58, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Mittal, A.K.; Tripathy, D.; Choudhary, A.; Aili, P.K.; Chatterjee, A.; Singh, I.P.; Banerjee, U.C. Bio-synthesis of silver nanoparticles using Potentilla fulgens Wall. ex Hook. and its therapeutic evaluation as anticancer and antimicrobial agent. Mater. Sci. Eng. C 2015, 53, 120–127. [Google Scholar] [CrossRef]
- Hamouda, R.A.; Hussein, M.H.; Abo-elmagd, R.A.; Bawazir, S.S. Synthesis and biological characterization of silver nanoparticles derived from the cyanobacterium Oscillatoria limnetica. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- Sukweenadhi, J.; Setiawan, K.I.; Avanti, C.; Kartini, K.; Rupa, E.J.; Yang, D.C. Scale-up of green synthesis and characterization of silver nanoparticles using ethanol extract of Plantago major L. leaf and its antibacterial potential. S. Afr. J. Chem. Eng. 2021, 38, 1–8. [Google Scholar] [CrossRef]
- Nahar, K.; Yang, D.C.; Rupa, E.J.; Khatun, K.; Al-Reza, S.M. Eco-friendly synthesis of silver nanoparticles from clerodendrum viscosum leaf extract and its antibacterial potential. Nanomed. Res. J. 2020, 5, 276–287. [Google Scholar] [CrossRef]
- Khatami, M.; Zafarnia, N.; Heydarpoor Bami, M.; Sharifi, I.; Singh, H. Antifungal and antibacterial activity of densely dispersed silver nanospheres with homogeneity size which synthesized using chicory: An in vitro study. J. Mycol. Med. 2018, 28, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Adibkia, K.; Dizaj, S.M.; Zarrintan, M.H.; Lotfipour, F.; Barzegar-Jalali, M. Antimicrobial activity of the metals and metal oxide nanoparticles. Mater. Sci. Eng. C 2014, 44, 278–284. [Google Scholar] [CrossRef]
- Borase, H.P.; Salunke, B.K.; Salunkhe, R.B.; Patil, C.D.; Hallsworth, J.E.; Kim, B.S.; Patil, S.V. Plant extract: A promising biomatrix for ecofriendly, controlled synthesis of silver nanoparticles. Appl. Biochem. Biotechnol. 2014, 173, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Hetta, H.F.; Al-Kadmy, I.M.S.; Khazaal, S.S.; Abbas, S.; Suhail, A.; El-Mokhtar, M.A.; Ellah, N.H.A.; Ahmed, E.A.; Abd-ellatief, R.B.; El-Masry, E.A.; et al. Antibiofilm and antivirulence potential of silver nanoparticles against multidrug-resistant Acinetobacter baumannii. Sci. Rep. 2021, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Jahan, I.; IȘILDAK, I. Lemon Peel Extract for Synthesizing Non-Toxic Silver Nanoparticles through One-Step Microwave-Accelerated Scheme. Dergipark. Org. Tr 2021, 4, 1–10. [Google Scholar]
- Siddiqui, S.; Rai, P.K.; Singh, R.; Kumar, D. Evaluation of antimicrobial activity and biofilm inhibition potential of Nelumbo nucifera Seed Extract Encapsulated Silver Nanoparticle. Int. J. Pharm. Res. 2021, 13. [Google Scholar] [CrossRef]
- Abdel-Azeem, A.; Nada, A.A.; O’Donovan, A.; Thakur, V.K.; Elkelish, A. Mycogenic silver nanoparticles from endophytic Trichoderma atroviride with antimicrobial activity. J. Renew. Mater. 2020, 8, 171–185. [Google Scholar] [CrossRef]
- Martínez-Flores, H.E.; Contreras-Chávez, R.; Garnica-Romo, M.G. Effect of Extraction Processes on Bioactive Compounds from Pleurotus ostreatus and Pleurotus djamor: Their Applications in the Synthesis of Silver Nanoparticles. J. Inorg. Organomet. Polym. Mater. 2021, 31, 1406–1418. [Google Scholar] [CrossRef]
- Torabfam, M. Microwave - assisted green synthesis of silver nanoparticles using dried extracts of Chlorella vulgaris and antibacterial activity studies. Green Process. Synth. 2020, 9, 283–293. [Google Scholar] [CrossRef]
- Behdad, R.; Pargol, M.; Mirzaie, A.; Karizi, S.Z.; Noorbazargan, H.; Akbarzadeh, I. Efflux pump inhibitory activity of biologically synthesized silver nanoparticles against multidrug-resistant Acinetobacter baumannii clinical isolates. J. Basic Microbiol. 2020, 60, 494–507. [Google Scholar] [CrossRef] [PubMed]
- Estevez, M.B.; Raffaelli, S.; Mitchell, S.G.; Faccio, R.; Alborés, S. Biofilm eradication using biogenic silver nanoparticles. Molecules 2020, 25, 2023. [Google Scholar] [CrossRef]
- Salisu, I.B.; Abubakar, A.S.; Abdullahi, M. A Novel Biosynthesis, Characterization and Antimicrobial Activity of Silver Nanoparticles Using Leaves Extract of Aloe vera Plant. Int. J. Sci. Res. 2014, 3, 311–314. [Google Scholar]
- Magdy, A.; Sadaka, E.; Hanafy, N.; El-Magd, M.A.; Allahloubi, N.; El Kemary, M. Green tea ameliorates the side effects of the silver nanoparticles treatment of Ehrlich ascites tumor in mice. Mol. Cell. Toxicol. 2020, 16, 271–282. [Google Scholar] [CrossRef]
- Biswal, S.K.; Behera, M.; Rout, A.S.; Tripathy, A. Green synthesis of silver nanoparticles using raw fruit extract of mimusops elengi and their antimicrobial study. Biointerface Res. Appl. Chem. 2021, 11, 10040–10051. [Google Scholar] [CrossRef]
- Babele, P.K.; Singh, A.K.; Srivastava, A. Bio-Inspired Silver Nanoparticles Impose Metabolic and Epigenetic Toxicity to Saccharomyces cerevisiae. Front. Pharmacol. 2019, 10, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Lagashetty, A.; Patil, M.K.; Ganiger, S.K. Green Synthesis, Characterization, and Thermal Study of Silver Nanoparticles by Achras sapota, Psidium guajava, and Azadirachta indica Plant Extracts. Plasmonics 2019, 14, 1219–1226. [Google Scholar] [CrossRef]
- Al-Daami, Q.; Al-Mashhedy, L. Hypoglycemic effect by assay some glucoregulatory enzymes and hematological parameters using silver nanoparticles of peel Raphanus sativus L aqueas extract in male Rats. J. Phys. Conf. Ser. 2019, 1294. [Google Scholar] [CrossRef]
- Jogaiah, S.; Kurjogi, M.; Abdelrahman, M.; Hanumanthappa, N.; Tran, L.S.P. Ganoderma applanatum-mediated green synthesis of silver nanoparticles: Structural characterization, and in vitro and in vivo biomedical and agrochemical properties. Arab. J. Chem. 2019, 12, 1108–1120. [Google Scholar] [CrossRef]
- Priyadarshini, S.; Sulava, S.; Bhol, R.; Jena, S. Green synthesis of silver nanoparticles using Azadirachta indica and Ocimum sanctum leaf extract. Curr. Sci. 2019, 117, 1300–1307. [Google Scholar] [CrossRef]
- Vanti, G.L.; Kurjogi, M.; Basavesha, K.N.; Teradal, N.L.; Masaphy, S.; Nargund, V.B. Synthesis and antibacterial activity of solanum torvum mediated silver nanoparticle against Xxanthomonas axonopodis pv.punicae and Ralstonia solanacearum. J. Biotechnol. 2020, 309, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Lim, D.H.; Lim, H.J.; Kwon, T.; Choi, J.S.; Jeong, S.; Choi, I.H.; Cheon, J. Size dependent macrophage responses and toxicological effects of Ag nanoparticles. Chem. Commun. 2011, 47, 4382–4384. [Google Scholar] [CrossRef] [PubMed]
- Jacob, J.M.; John, M.S.; Jacob, A.; Abitha, P.; Kumar, S.S.; Rajan, R.; Natarajan, S.; Pugazhendhi, A. Bactericidal coating of paper towels via sustainable biosynthesis of silver nanoparticles using Ocimum sanctum leaf extract. Mater. Res. Express 2019, 6. [Google Scholar] [CrossRef]
- Saravanan, M.; Arokiyaraj, S.; Lakshmi, T.; Pugazhendhi, A. Synthesis of silver nanoparticles from Phenerochaete chrysosporium (MTCC-787) and their antibacterial activity against human pathogenic bacteria. Microb. Pathog. 2018, 117, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Tran, Q.H.; Nguyen, V.Q.; Le, A. Corrigendum: Silver nanoparticles: Synthesis, properties, toxicology, applications and perspectives (Adv. Nat. Sci: Nanosci. Nanotechnol. 4 033001). Adv. Nat. Sci. Nanosci. Nanotechnol. 2018, 9, 049501. [Google Scholar] [CrossRef]
- Panyala, N.R.; Peña-Méndez, E.M.; Havel, J. Silver or silver nanoparticles: A hazardous threat to the environment and human health? J. Appl. Biomed. 2008, 6, 117–129. [Google Scholar] [CrossRef] [Green Version]
- Castellini, C.; Ruggeri, S.; Mattioli, S.; Bernardini, G.; Macchioni, L.; Moretti, E.; Collodel, G. Long-term effects of silver nanoparticles on reproductive activity of rabbit buck. Syst. Biol. Reprod. Med. 2014, 60, 143–150. [Google Scholar] [CrossRef] [Green Version]
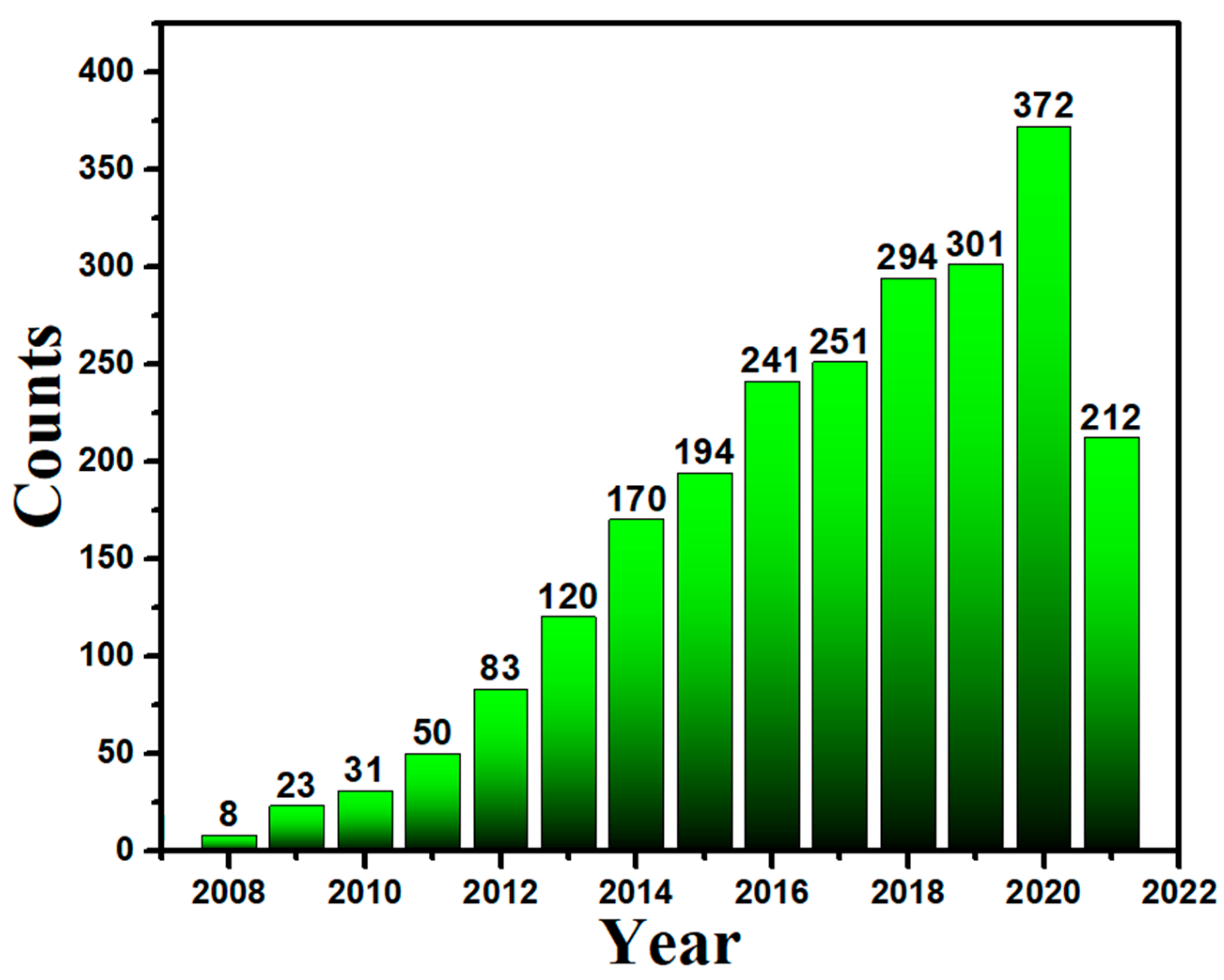

| Year | S/N | Bacterial Species | Method | Size (nm) | Morphology | References |
|---|---|---|---|---|---|---|
| 2021 | 1. | Serratia nematodiphila | Intracellular | 10–31 | Spherical | [18] |
| 2. | Cyanobacteria Spirulina platensis and actinobacteria Streptomyces spp. 211A | Intracellular | 7–15 | - | [19] | |
| 3. | Klebsiella pneumonia | Extracellular | 1–6 | Spherical | [20] | |
| 4. | Bacillus indicus | Extracellular | - | Spherical | [21] | |
| 5. | Bacillus subtilis (MTCC441) | Intracellular | 10–100 | Spherical | [22] | |
| 6. | Endosymbiotic Bacterium | Intracellular | 10–60 | Cubic, spherical, hexagonal, crystalline, and oval | [23] | |
| 7. | Penicillium glabrum (MTCC 1985) | Extracellular | 26–32 | Spherical | [24] | |
| 8. | Bacillus strain CS 11 | Extracellular | 45 ± 0.15 | FCC, Spherical | [25] | |
| 2020 | 9. | Marine Ochrobactrum sp. | Intracellular | 35–85 | Spherical | [26] |
| 10. | Exiguobacterium mexicanum | Extracellular | 5–40 | - | [27] | |
| 11. | Actinobacteria | Intracellular | 5–50 | Spherical | [28] | |
| 12. | Lactobacillus strains | Intracellular | 15–500 | Cluster triangular, hexagonal, and crystalline | [29] | |
| 13. | Pseudomonas proteolytica, Bacillus cecembensis | Extracellular | 6–13 | Spherical | [30] | |
| 14. | Rhodococcus sp. | Intracellular | 5–50 | Spherical | [26] | |
| 15. | Bacillus sp. | Extracellular | 5–15 | Crystalline | [31] | |
| 16. | Bacillus licheniformis | Extracellular | 8–63 | Spherical | [32] | |
| 17. | Shewanellao neidensis | Intracellular | 4 ± 1.5 | Spherical | [33] | |
| 18. | Gluconacetobacter xylinus | Intracellular | 40–100 | Spherical | [34] | |
| 19. | Bacillus subtilis | Extracellular | 5–60 | Spherical | [35] | |
| 20. | Nocardiopsissp.MBRC-1 | Intracellular | 45 ± 0.15 | Spherical | [36] | |
| 21. | Pseudomonas stutzeri AG259 | Extracellular | 35–200 | Cluster equilateral triangular, and hexagonal | [37] | |
| 2019 | 22. | Klebsiella pneumonia, Escherichia coli, and Enterobacter cloacae | Extracellular | 28–122 | Spherical | [38] |
| 23. | Aeromonas sp. THG-FG1.2 | Extracellular | 8–16 | fcc spherical | [39] | |
| 24. | Escherichia coli DH5a | Extracellular | 10–100 | Spherical | [40] | |
| 25. | Pseudomonas putida NCIM 2650 | Extracellular | 70 | Spherical | [41] | |
| 26. | Vibrio alginolyticus | Intracellular | 50–100 | Crystalline, spherical | [42] | |
| 27. | Lactobacillus casei | Intracellular | 20–50 | Spherical | [43] | |
| 28. | Deinococcus radiodurans | Extracellular | 4–50 | Spherical | [44] | |
| 29. | Bacillus pumilus, B. persicus, and B. licheniformis | Extracellular | 77–92 | Spherical | [41] | |
| 30. | Staphylococcus aureus | Extracellular | 160–180 | Spherical | [45] |
| Year | S/N | Fungal Species | Method | Size | Morphology | References |
|---|---|---|---|---|---|---|
| 2021 | 1. | Endophytic fungus | Intracellular | 10–25 | Polydispersedspherical, hexagonal, and spherical | [49] |
| 2. | Trichoderma viride | Extracellular | 5–40 | Spherical | [50] | |
| 3. | Schizophyllum commune | Intracellular and extracellular | 51–93 | Spherical | [51] | |
| 4. | Humicola sp. | Extracellular | 5–25 | Spherical | [52] | |
| 5. | Penicillium citrinum | Extracellular | - | Uniform spherical | [53] | |
| 6. | Rhizopus stolonifer | Extracellular | 9.47 | Spherical | [54] | |
| 7. | Cladosporium cladosporioides | Intracellular | 10–100 | Spherical | [55] | |
| 8. | Fusarium semitectum | Extracellular | 1–50 | Ellipsoid, polydispersed spherical | [56] | |
| 9. | Filamentous fungus | Extracellular | 58.35 ± 17.88 | - | [57] | |
| 10. | Aspergillus flavus | Extracellular | 8.92 | - | [58] | |
| 11. | Cladosporium sphaerospermum | Extracellular | 15.1 ± 1 | Spherical | [55] | |
| 12. | Arthroderma fulvum | Intracellular | 20.56 | Spherical | [59] | |
| 13. | Sclerotinia sclerotiorum MTCC 8785 | Extracellular | 10–15 | Spherical | [60] | |
| 2020 | 14. | Penicillium brecompactum | Intracellular | 23–105 | Crystalline spherical | [61] |
| 15. | Rhizoctonia solani | Intracellular | 2–22 | Spherical | [62] | |
| 16. | Rhizopus nigricans | Extracellular | 35–40 | Round | [61] | |
| 17. | Alternaria alternate | Extracellular | 32.5 | Polydispersed, spherical | [63] | |
| 18. | Aspergillus niger | Extracellular | 1–20 | Polydispersed, spherical | [64] | |
| 19. | Penicillium hrysogenumad Aspergillus oryzae | Extracellular | 6–100, 14–76 | Spherical | [65] | |
| 20. | Cryphonectria sp. | Extracellular | 30–70 | - | [66] | |
| 21. | Penicillium sp. | Extracellular | 25–30 | Spherical | [67] | |
| 22. | Penicillium sp. | Extracellular | 58.35 ± 17.88 | - | [68] | |
| 23. | Aspergillus fumigates | Extracellular | 5–25 | Spherical | [69] | |
| 2019 | 24. | Guignardia mngifera | Extracellular | 5–30 | Spherical | [70] |
| 25. | Cariolus versicolor | Intracellular | 25–75 | Spherical | [71] | |
| 26. | Duddingtonia flagrans | Extracellular | 30–409 | Spherical | [72] | |
| 27. | Isaria fumosorosea | Extracellular | 51.31–111.02 | Spherical | [73] | |
| 28. | Penicillium purpurogenum | Intracellular | 8–10 | Spherical | [70] | |
| 29. | Fusarium solani | Extracellular | 5–35 | Spherical | [15] | |
| 30. | Trichoderma harzianum | Extracellular | 34.77 | Ellipsoidal, spherical | [74] | |
| 31. | Aspergillus fumigates | Extracellular | 5–25 | Spherical | [72] | |
| 32. | Endophytic fungus | Extracellular | 25–30 | Spherical | [75] | |
| 33. | Phoma glomerata | Extracellular | 60–80 | Spherical | [15] | |
| 34. | Trichoderma reesei | Extracellular | 5–50 | Random | [76] | |
| 35. | Fusarium acuminatum | Extracellular | 13 | Spherical | [77] |
| Year | S/N | Plant Name | Size (nm) | Morphology | References |
|---|---|---|---|---|---|
| 2021 | 1. | Phaseolus vulgaris | 10–20 | Spherical | [79] |
| 2. | Ficus Benjamina | 20–30 | – | [80] | |
| 3. | Magnolia Kobus | 50–500 | FCC | [20] | |
| 4. | Pinus thunbergii | 5–50 | Triangular, hexagonal | [81] | |
| 5. | Ficus panda | 12–36 | Spherical | [82] | |
| 6. | Dalbergia sissoo | 5–55 | Spherical | [83] | |
| 7. | Musa balbisiana, Azadirachta indica, and Ocimum tenuiflorum | 100 | Spherical, triangular, and cuboidal | [84] | |
| 8. | Buniumpersicum | 20–50 | Spherical | [85] | |
| 9. | Acalypha Indica | 20–30 | - | [86] | |
| 10. | Medicago Sativa | 2–20 | Spherical | [87] | |
| 11. | Sesuvium portulacastrum L. | 5–20 | Spherical | [88] | |
| 12. | Cyamopsis tetragonaloba | 8 | Spherical | [89] | |
| 13. | Pine, persimmon, ginkgo, magnolia, and Platanus | 15–500 | _ | [90] | |
| 14. | Rosa Damascena | - | Spherical | [91] | |
| 2020 | 15. | Camellia Sinensis | 4 | Spheroidal | [92] |
| 16. | Sorghum bicolor | 10 | Spheroidal | [93] | |
| 17. | Jatropha gossypifolia | 62 | Spherical | [94] | |
| 18. | Coffee Arabica | 20–30 | Spherical, Ellipsoidal | [41] | |
| 19. | Prunus yedoensis | 20–70 | Spherical and oval | [95] | |
| 20. | Emblica Officinalis | 10–20 | - | [96] | |
| 21. | Vitex negundo | 10–30 | Spheroidal | [30] | |
| 22. | Cinnamomum camhora | 55–80 | Triangular or spherical | [97] | |
| 23. | Mimosa pudica | 25–60 | Spherical | [98] | |
| 24. | Camellia Sinensis | 20 | Spheroidal | [92] | |
| 25. | Euphorbia lacteal | 186 | Spherical | [99] | |
| 26. | Azadirachta Indica | 50–100 | Spherical | [100] | |
| 27. | Morinda citrifolia | 30–55 | Spherical | [101] | |
| 28. | Jatropha curcas | 10–20 | Spherical | [97] | |
| 29. | Bauhinia variegate | 38–65 | Spherical, triangle, truncated triangles, and decahedrons | [102] | |
| 30. | Pinus thunbergii | 20–100 | Spheroidal | [103] | |
| 31. | Pulicaria glutinosa | 40–60 | Spherical | [28] | |
| 32. | Nyctanthes arbor-tristis | 50–80 | Spherical | [104] | |
| 33. | Terminalia chebula | 25 | Spherical, ovoid | [105] | |
| 34. | Dioscorea bulbifera | 8–20 | FCC | [106] | |
| 35. | Elaeagnus latifolia | 30–50 | Spherical | [107] | |
| 36. | Vigna sp. L. | 24.35 | Spherical | [108] | |
| 37. | Piper nigrum | 5–50 | FCC | [109] | |
| 2019 | 38. | Musa acuminata | - | Agglomerated form | [110] |
| 39. | Amaranthus retroflexus | 10–32 | Spherical | [111] | |
| 40. | Tribulus Terrestris L. | 16–28 | Spherical | [112] | |
| 41. | Cassia auriculata | 20–40 | Spherical | [113] | |
| 42. | Adenium obesum | 10–30 | Spherical | [40] | |
| 43. | Coleus aromaticus | 40–50 | Spherical | [114] | |
| 44. | Artocarpus heterophyllus Lam | 10.78 | Irregular | [115] | |
| 45. | Vigna radiate | 5–30 | Spherical, oval | [116] | |
| 46. | Zingiber officinale | 10–20 | - | [117] | |
| 47. | Lantana Camara | 14–27 | Spherical | [118] | |
| 48. | Aloe vera | 20 | Spherical | [119] | |
| 49. | Hevea brasiliensis | 2–100 | Spherical | [120] | |
| 50. | Dodonaea viscosa | 16 | Spheroidal | [121] | |
| 51. | Murraya koenigii | 20–35 | Spheroidal | [15] | |
| 52. | Jatropha curcas | 73 | Spherical | [122] | |
| 53. | Pedilanthus tithymaloides | 123 | Spherical | [46] | |
| 54. | Euphorbia prostrate | 25–80 | Rod | [123] | |
| 55. | Syzygium aromaticum | - | - | [124] | |
| 56. | Tinospora cordifolia | 55–80 | Aggregated | [125] | |
| 57. | Solanum torvum | 14 | Spheroidal | [126] | |
| 58. | Murraya koenigii | 10–25 | Spheroidal | [15] | |
| 59. | Ocimum tenuiflorum | 7–15 | Spherical and ovoid | [75] |
| Year | S/N | Organism | Biological Entity | Size (nm) | Morphology | Method | References |
|---|---|---|---|---|---|---|---|
| 2021 | 1. | Fungi | Penicillium sp. | 25 | Spherical | Agar well diffusion method | [53] |
| 2. | Fungi | Arthroderma fulvum | 15.5 | Spherical | Brothmicro-dilution method | [18] | |
| 3. | Fungi | Penicillium aculeatum | 4–55 | Spherical | Disk diffusion method | [37] | |
| 4. | Bacteria | Acinetobacter baumannii | 37–168 | Spherical | Broth micro-dilution method | [136] | |
| 5. | Plant | Artocarpus altilis | 20–45 | Spherical | Agar well diffusion method | [81] | |
| 6. | Plant | Convolvulus arvensis | 28 | Spherical | Disc diffusion and broth macro-dilution method | [137] | |
| 7. | Plant | Erythrina suberosa | 15–34 | Spherical | Agar cup and broth micro-dilution methods | [37] | |
| 8. | Plant | Psidium guajava | 20–25 | Spherical | Agar well diffusion 1method | [89] | |
| 9. | Plant | Nelumbo Nucifera | 12.9 | Quasi–Spherical | Broth dilution method | [138] | |
| 10. | Plant | Boerhaavia diffusa | 25 | Spherical | Agar well diffusion | [20] | |
| 11. | Plant | Alpinia katsumadai | 12.6 | Quasi-Spherical | Broth dilution method | [104] | |
| 12. | Fungi | Curvularia lunata | 64.3 | Spherical | Disk diffusion assay | [139] | |
| 13. | Fungi | Pleurotus ostreatus | 10–40 | Spherical | Disk diffusion and broth micro-dilution methods | [140] | |
| 2020 | 14. | Fungi | Rhodotorula glutinis | 15–220 | Spherical | Agar well diffusion and broth microdilution methods | [109] |
| 15. | Bacteria | Pseudomonas deceptionensis | 127 | Spherical | Agar well diffusion method | [141] | |
| 16 | Bacteria | Acinetobacter baumannii | 37–168 | Spherical | Broth micro-dilution method | [142] | |
| 17. | Bacteria | Phenerochaete Chrysosporium | - | Spherical and oval | Agar well diffusion method | [143] | |
| 18. | Bacteria | Bacillus endophyticus | 4.8–6.6 | Spherical | Agar well diffusion method | [144] | |
| 19. | Plant | tea | 10–20 | Spherical | Disk and broth dilution methods | [145] | |
| 20. | Plant | Eucalyptus globules | 1.9–4.3 | Spherical | Agar well diffusion and broth dilution methods | [109] | |
| 21. | Plant | Mimusops elengi | 55–83 | Spherical | Disk diffusion method | [146] | |
| 2019 | 22. | Fungi | Saccharomyces cerevisiae | 5–50 | Spherical | Brothmicro-dilution method | [147] |
| 23. | Fungi | Guignardia mangiferae | 5–30 | Spherical | Agar well | [70] | |
| 24. | Fungi | Penicillium polonicum | 10–15 | Spherical | Agar well diffusion | [148] | |
| 25. | Fungi | Raphanus sativus | 4–30 | Spherical | Disk diffusion method | [149] | |
| 26. | Fungi | Ganoderma applanatum | 133 | Spherical | Agar welldiffusionmethod | [150] | |
| 27. | Bacteria | Weissella oryzae | 150 | Spherical | Disk diffusion method | [43] | |
| 28. | Bacteria | Ochrobactrum anthropi | 38–85 | Spherical | Agar well diffusion method | [46] | |
| 29. | Plant | Elephantopus scaber | 37 | Spherical | Agar well diffusion method | [46] | |
| 30. | Plant | Ocimum sanctum | ~15 | Spherical | Disc and dilution approach | [151] | |
| 31. | Plant | Musa paradisiacal | 23.7 | Spherical | Agar well diffusion and broth dilution approaches | [46] | |
| 32. | Plant | Dalbergia spinosa | 18 | Spherical | Disk diffusion and broth microdilution methods | [15] | |
| 33. | Plant | Emblica officinalis | 15 | Spherical | Disk diffusion method | [152] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parmar, S.; Kaur, H.; Singh, J.; Matharu, A.S.; Ramakrishna, S.; Bechelany, M. Recent Advances in Green Synthesis of Ag NPs for Extenuating Antimicrobial Resistance. Nanomaterials 2022, 12, 1115. https://doi.org/10.3390/nano12071115
Parmar S, Kaur H, Singh J, Matharu AS, Ramakrishna S, Bechelany M. Recent Advances in Green Synthesis of Ag NPs for Extenuating Antimicrobial Resistance. Nanomaterials. 2022; 12(7):1115. https://doi.org/10.3390/nano12071115
Chicago/Turabian StyleParmar, Simerjeet, Harwinder Kaur, Jagpreet Singh, Avtar Singh Matharu, Seeram Ramakrishna, and Mikhael Bechelany. 2022. "Recent Advances in Green Synthesis of Ag NPs for Extenuating Antimicrobial Resistance" Nanomaterials 12, no. 7: 1115. https://doi.org/10.3390/nano12071115
APA StyleParmar, S., Kaur, H., Singh, J., Matharu, A. S., Ramakrishna, S., & Bechelany, M. (2022). Recent Advances in Green Synthesis of Ag NPs for Extenuating Antimicrobial Resistance. Nanomaterials, 12(7), 1115. https://doi.org/10.3390/nano12071115