Application of Green Gold Nanoparticles in Cancer Therapy and Diagnosis
Abstract
:1. Introduction
2. AuNPs; In Vitro Characterization (In Vitro)
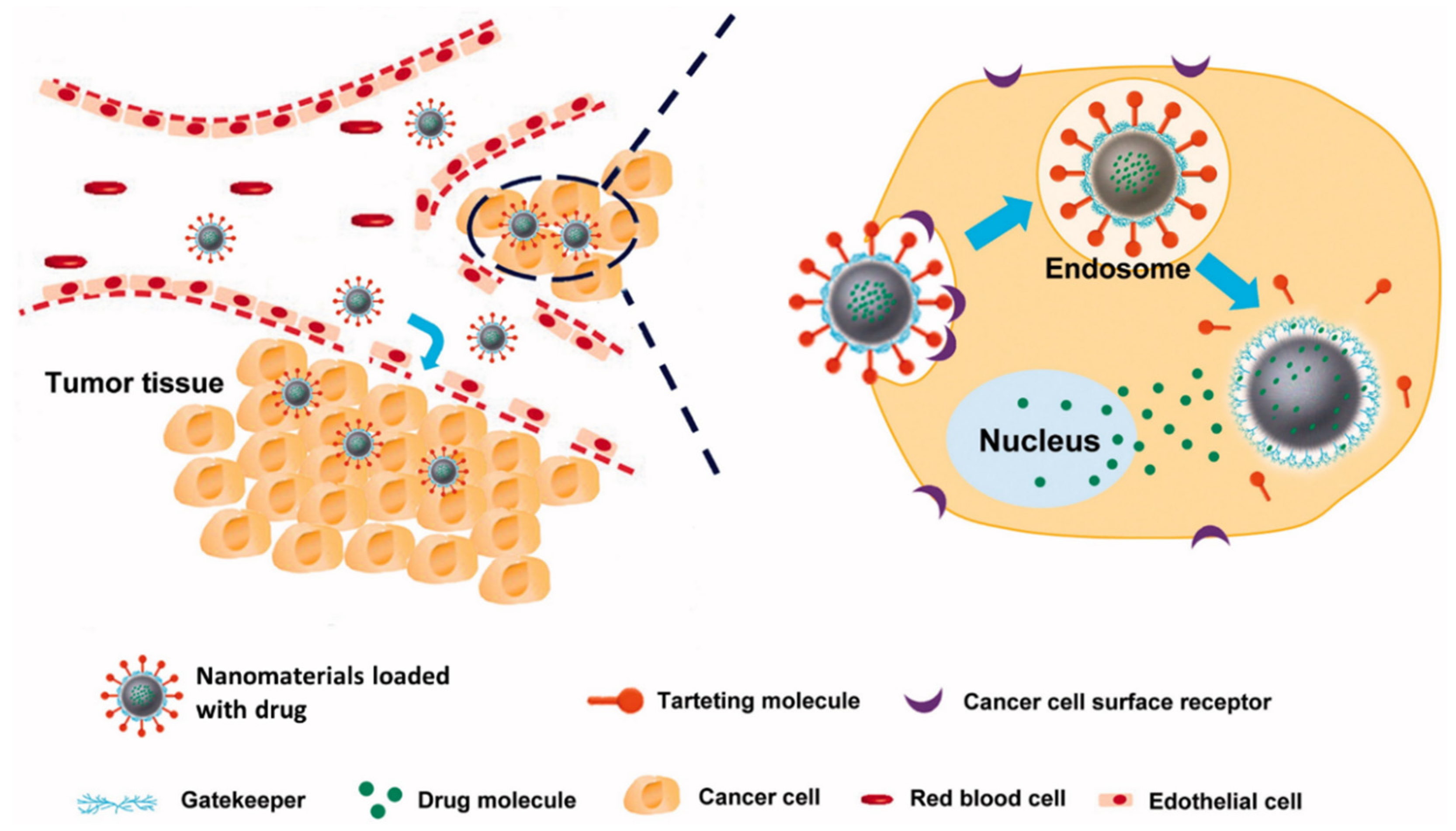
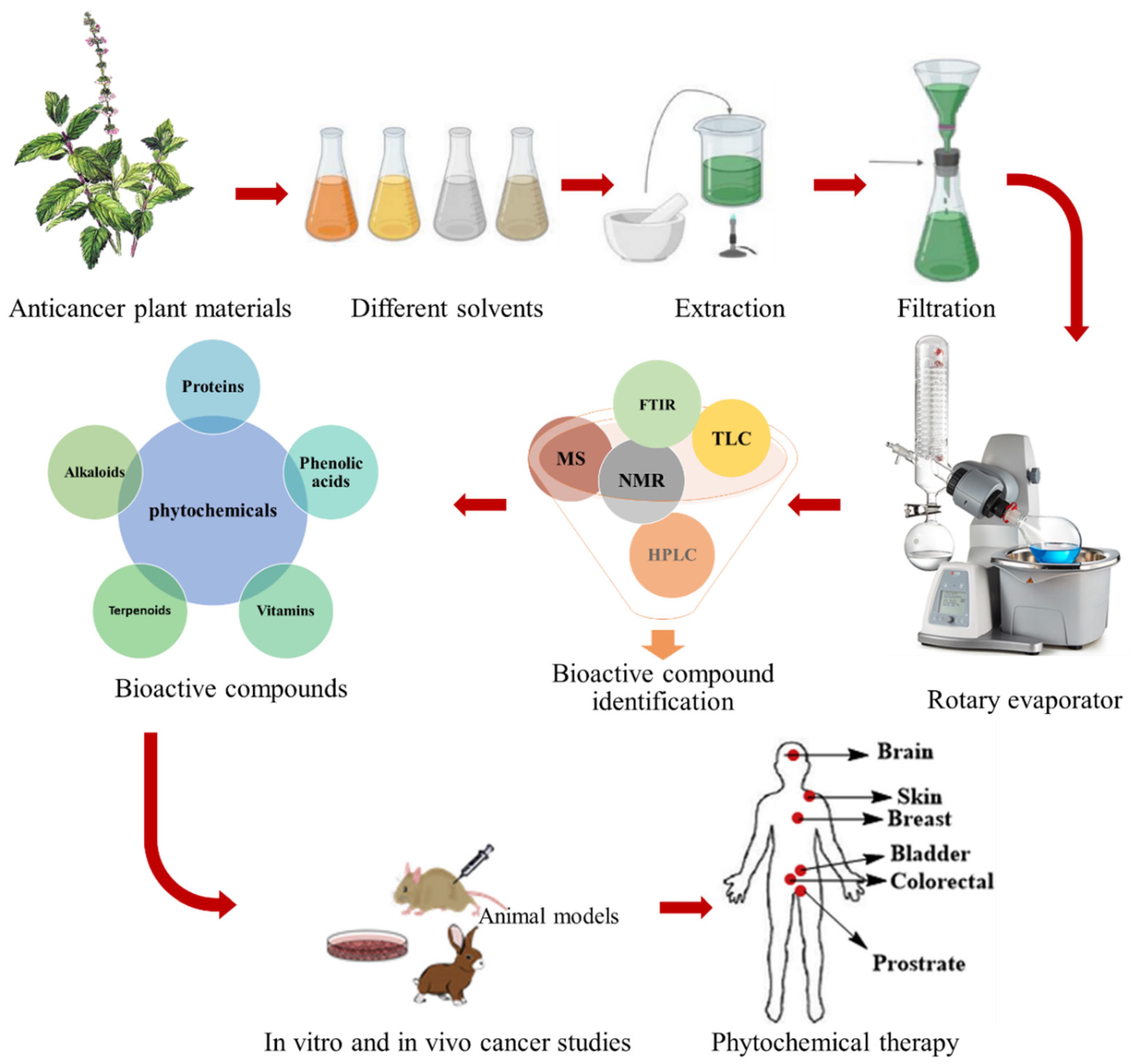
3. Prospective Application of Plant-Based Materials in Cancer Therapy
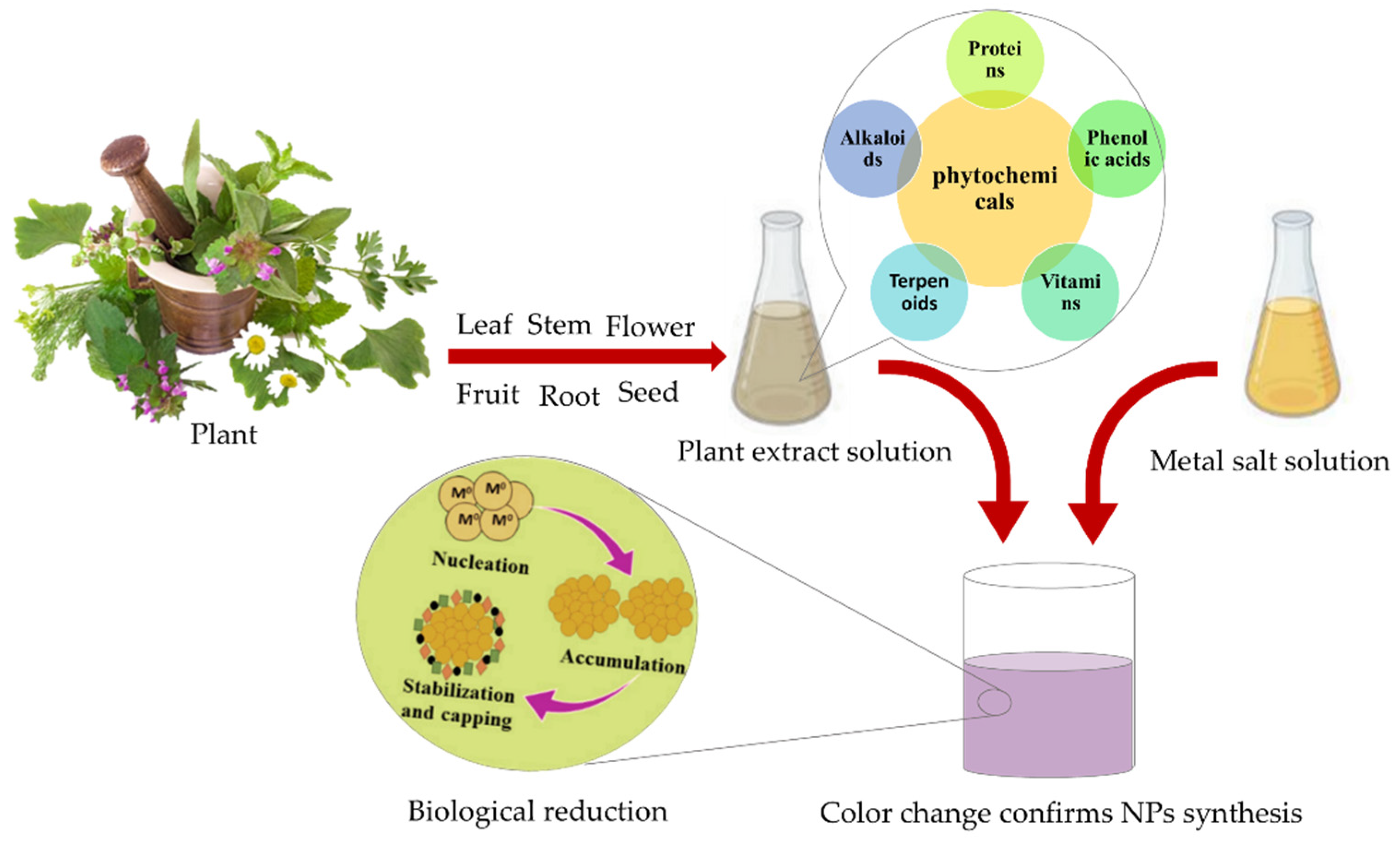
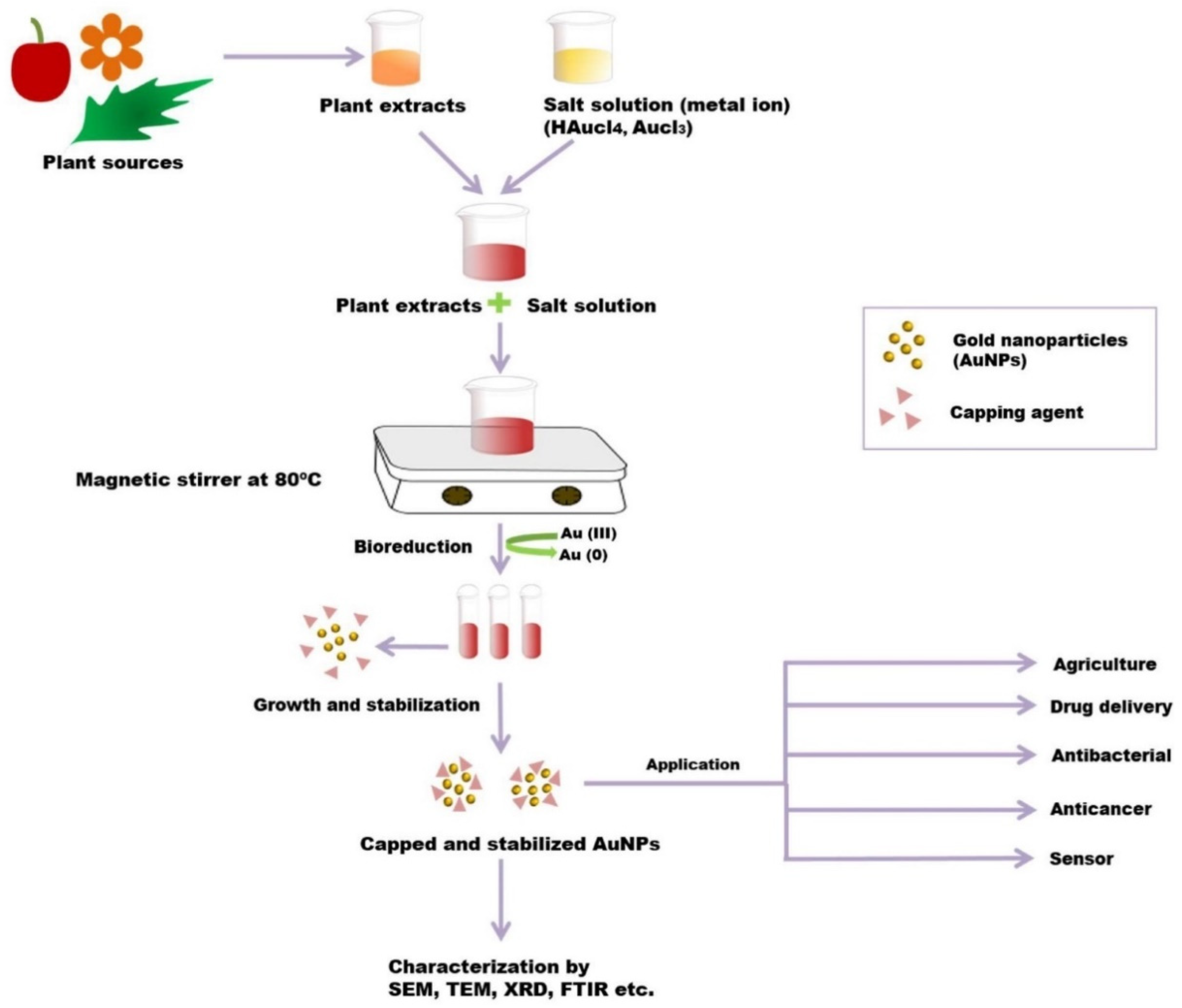
4. Green Synthesis of AuNPs
5. Green Synthesis of AuNPs for Cancer Theranostics
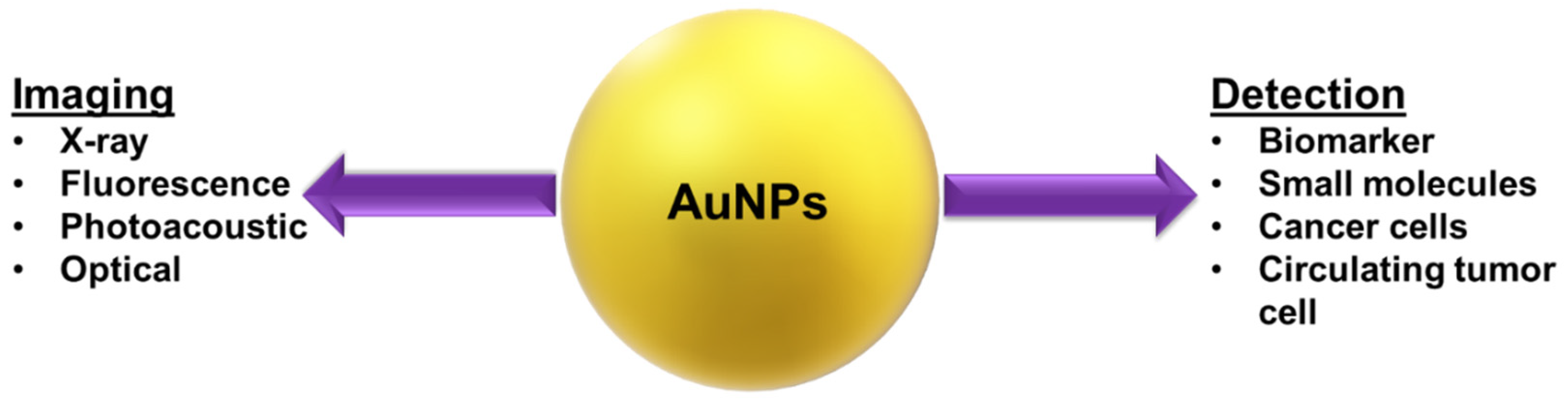
5.1. Plant-Based AuNPs for Cancer Detection
5.1.1. Fluorescent-Plant-Based Markers for Detecting Cancers
5.1.2. Other Plant-Based Markers for Detecting Cancers
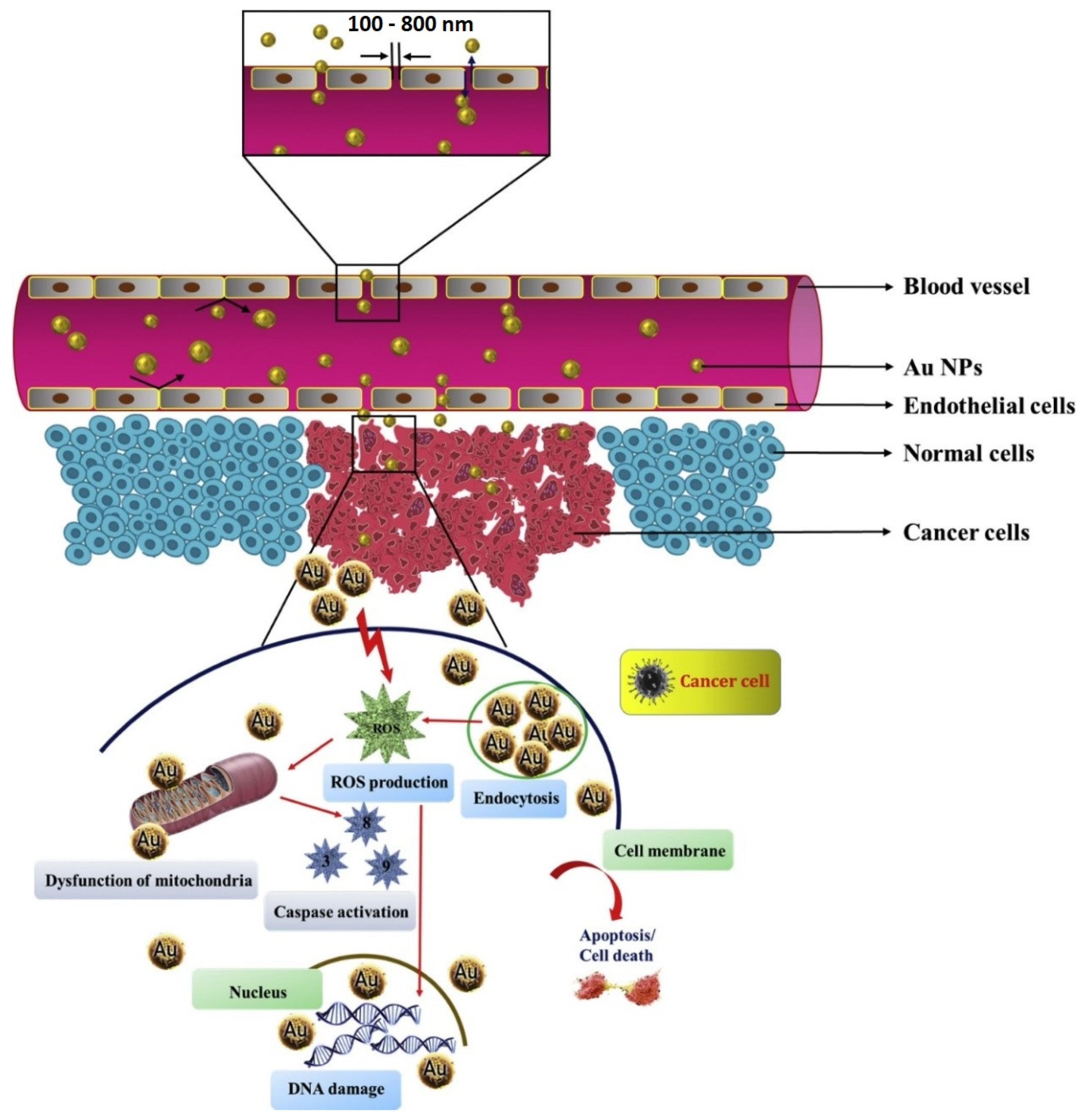
5.2. Plant-Based AuNPs for Cancer Treatment
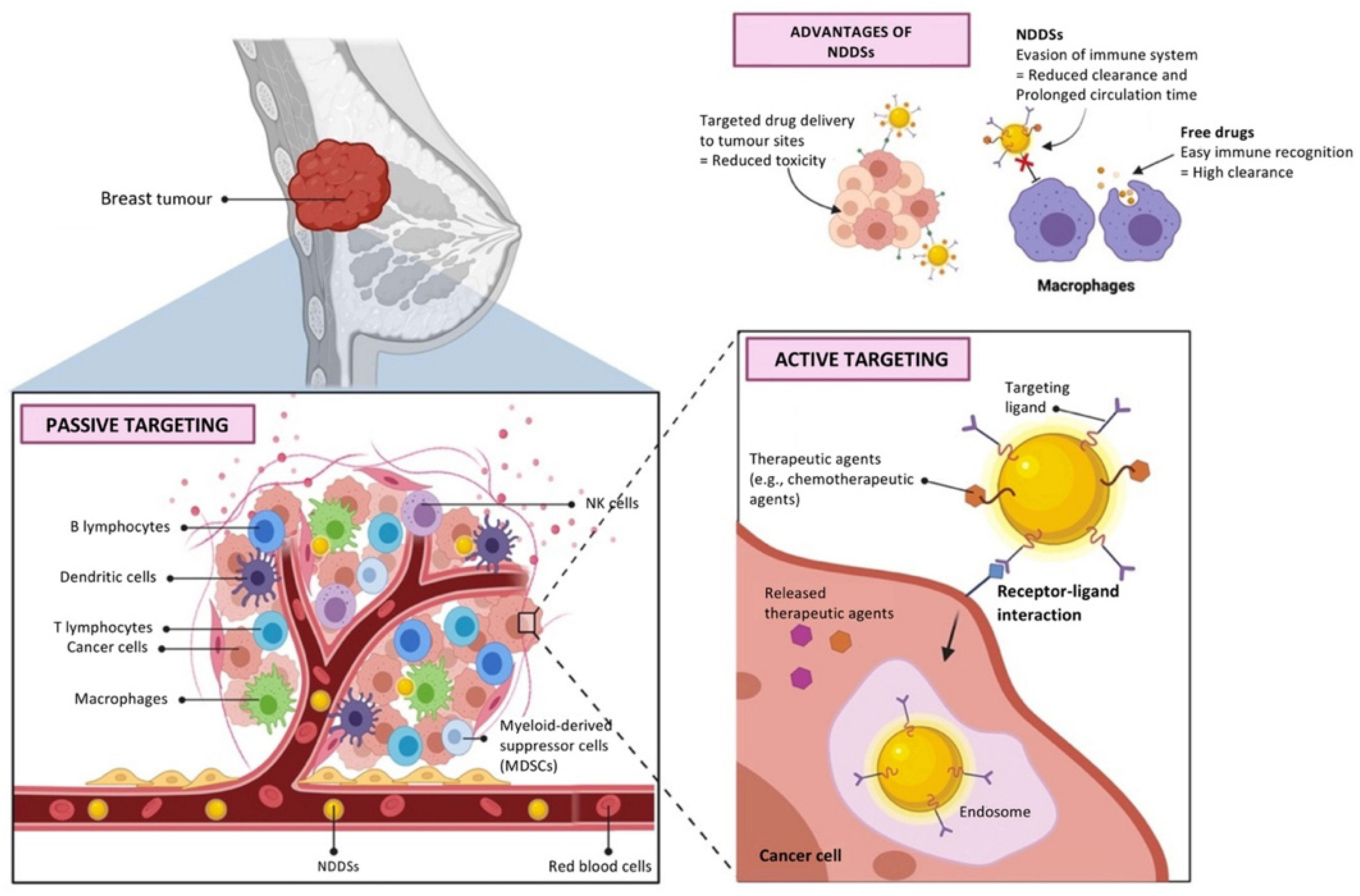
5.2.1. Breast Cancer
5.2.2. Cervical Cancer
5.2.3. Liver Cancer
5.2.4. Colon Cancer
5.2.5. Lung Cancer
5.2.6. Hematological Malignancies
5.2.7. Other Cancers
6. Challenges and Opportunities
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviation
| BRP | Brazilian red propolis |
| NPs | nanoparticles |
| PBMCs | peripheral blood mononuclear cells |
| CNTs | carbon nanotubes |
| NMs | nanomaterials |
| MNPs | metal nanoparticles |
| NIR | near-infrared |
| SEM | scanning electron microscopy |
| TEM | transmission electron microscopy |
| DLS | dynamic light scattering |
| CT | computed tomography |
| CDI | color Doppler ultrasound imaging |
| SPR | surface plasmon resonance |
| siRNA | short interfering ribonucleic acid |
| HPV | human papillomavirus |
| RME | receptor-mediated endocytosis |
| PSA | prostate-specific antigen |
| CEA | carcinoembryonic antigen |
| ctDNA | circulating tumor DNA |
| ctRNA | circulating tumor DNA |
| PET | positron emission computed tomography |
| MRI | magnetic resonance imaging |
| AuNPs | gold nanoparticles |
| rGO | reduced graphene oxide |
| LOD | limit of detection |
| LDH | lactate dehydrogenase |
| HCC | hepatocellular carcinoma |
| TUNEL | terminal deoxynucleotidyl transferase biotin-dUTP nick end labeling |
| DPPH | 2,2-diphenyl-1-picryl-hydrazyl-hydrate |
| ROS | reactive oxygen species |
| DAPI | 4′,6-diamidino-2-phenylindole |
| Ao/EtBr | Acridine orange/Ethidium bromide |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide |
| IC50 | half-maximal inhibitory concentration |
| PI | propidium iodide |
| CCK8 | Cell Counting Kit 8 |
| TUNEL | terminal deoxynucleotidyl transferase dUTP nick end labeling |
| GSH | glutathione |
| SPIONs | superparamagnetic iron oxide nanoparticles |
| RT-PCR | reverse transcription-polymerase chain reaction |
| FTIR | Fourier transform infrared spectroscopy |
| UV-Vis | ultraviolet-visible |
| GC-MS | gas chromatography-mass spectrometry |
| PARP | poly (ADP-ribose) polymerase |
| HRTEM | high-resolution transmission electron microscopy |
| XRD | X-ray diffraction |
| EDX | energy-dispersive X-Ray analysis |
| XPS | X-ray photoelectron spectroscopy |
| DPPH | 2,2-diphenylpicrylhydrazyl |
| CC50 | 50% cytotoxic concentration |
| qPCR | quantitative polymerase chain reaction |
| FESEM | field-emission scanning electron microscopy |
| EDS | energy-dispersive spectroscopy |
| IFN-γ | interferon gamma |
| IFN-α | interferon alfa |
| TNF-α | tumor necrosis factor-alpha |
| WBC | white blood cells |
| HUVECs | human umbilical vein endothelial cells |
References
- Iqbal, J.; Abbasi, B.A.; Mahmood, T.; Kanwal, S.; Ali, B.; Shah, S.A.; Khalil, A.T. Plant-derived anticancer agents: A green anticancer approach. Asian Pac. J. Trop. Biomed. 2017, 7, 1129–1150. [Google Scholar] [CrossRef]
- Leng, F.; Liu, F.; Yang, Y.; Wu, Y.; Tian, W. Strategies on Nanodiagnostics and Nanotherapies of the Three Common Cancers. Nanomaterials 2018, 8, 202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. The Top 10 Causes of Death. 2019. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 22 February 2022).
- Hussain, W.; Ullah, M.; Dastagir, G.; Badshah, L. Quantitative ethnobotanical appraisal of medicinal plants used by inhabitants of lower Kurram, Kurram agency, Pakistan. Avicenna J. Phytomed. 2018, 8, 313–329. [Google Scholar] [PubMed]
- Lebech, A.-M.; Gaardsting, A.; Loft, A.; Graff, J.; Markova, E.; Bertelsen, A.K.; Madsen, J.L.; Andersen, K.F.; von Benzon, E.; Helms, M.; et al. Whole-body 18F-FDG PET/CT is superior to CT as first-line diagnostic imaging in patients referred with serious nonspecific symptoms or signs of cancer: A randomized prospective study of 200 patients. J. Nucl. Med. 2017, 58, 1058–1064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lambregts, D.M.J.; Cappendijk, V.C.; Maas, M.; Beets, G.L.; Beets-Tan, R.G.H. Value of MRI and diffusion-weighted MRI for the diagnosis of locally recurrent rectal cancer. Eur. Radiol. 2011, 21, 1250–1258. [Google Scholar] [CrossRef] [Green Version]
- Kalff, V.; Hicks, R.J.; E Ware, R.; Hogg, A.; Binns, D.; McKenzie, A.F. The clinical impact of 18F-FDG PET in patients with suspected or confirmed recurrence of colorectal cancer: A prospective study. J. Nucl. Med. 2002, 43, 492–499. [Google Scholar]
- Talerman, A.; Haije, W.; Baggerman, L. Serum alphafetoprotein (AFP) in patients with germ cell tumors of the gonads and extragonadal sites: Correlation between endodermal sinus (yolk sac) tumor and raised serum AFP. Cancer 1980, 46, 380–385. [Google Scholar] [CrossRef]
- Zeh, G. Oligo-Aminoferrocenes for Cancer Treatment; Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU): Erlangen, Germany, 2020. [Google Scholar]
- Xie, Y.-H.; Chen, Y.-X.; Fang, J.-Y. Comprehensive review of targeted therapy for colorectal cancer. Signal Transduct. Target. Ther. 2020, 5, 22. [Google Scholar] [CrossRef]
- Martin, T.A.; Ye, L.; Sanders, A.J.; Lane, J.; Jiang, W.G. Cancer invasion and metastasis: Molecular and cellular perspective. In Madame Curie Bioscience Database [Internet]; Landes Bioscience: Austin, TX, USA, 2013. [Google Scholar]
- Mauro, N.; Scialabba, C.; Agnello, S.; Cavallaro, G.; Giammona, G. Folic acid-functionalized graphene oxide nanosheets via plasma etching as a platform to combine NIR anticancer phototherapy and targeted drug delivery. Mater. Sci. Eng. C 2020, 107, 110201. [Google Scholar] [CrossRef]
- Ma, X.; Tao, H.; Yang, K.; Feng, L.; Cheng, L.; Shi, X.; Li, Y.; Guo, L.; Liu, Z. A functionalized graphene oxide-iron oxide nanocomposite for magnetically targeted drug delivery, photothermal therapy, and magnetic resonance imaging. Nano Res. 2012, 5, 199–212. [Google Scholar] [CrossRef]
- Shi, W. Application of Multifunctional Nanomaterials Combined with Sports Rehabilitation Training in the Diagnosis and Treatment of Cardiovascular Diseases. Integr. Ferroelectr. 2021, 216, 81–93. [Google Scholar] [CrossRef]
- Pippa, N.; Chronopoulos, D.D.; Stellas, D.; Fernández-Pacheco, R.; Arenal, R.; Demetzos, C.; Tagmatarchis, N. Design and development of multi-walled carbon nanotube-liposome drug delivery platforms. Int. J. Pharm. 2017, 528, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Madani, S.Z.M.; Safaee, M.M.; Gravely, M.; Silva, C.; Kennedy, S.; Bothun, G.D.; Roxbury, D. Carbon nanotube–liposome complexes in hydrogels for controlled drug delivery via near-infrared laser stimulation. ACS Appl. Nano Mater. 2020, 4, 331–342. [Google Scholar] [CrossRef]
- Senapati, S.; Mahanta, A.K.; Kumar, S.; Maiti, P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct. Target. Ther. 2018, 3, 7. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Q.; Lin, Y.; Han, N.; Li, X.; Geng, H.; Wang, X.; Cui, Y.; Wang, S. Mesoporous carbon nanomaterials in drug delivery and biomedical application. Drug Deliv. 2017, 24, 94–107. [Google Scholar] [CrossRef]
- Thambiraj, S.; Hema, S.; Shankaran, D.R. Functionalized gold nanoparticles for drug delivery applications. Mater. Today Proc. 2018, 5, 16763–16773. [Google Scholar] [CrossRef]
- Patel, D.K.; Senapati, S.; Mourya, P.; Singh, M.M.; Aswal, V.K.; Ray, B.; Maiti, P. Functionalized graphene tagged polyurethanes for corrosion inhibitor and sustained drug delivery. ACS Biomater. Sci. Eng. 2017, 3, 3351–3363. [Google Scholar] [CrossRef]
- Qi, Z.; Shi, J.; Zhang, Z.; Cao, Y.; Li, J.; Cao, S. PEGylated graphene oxide-capped gold nanorods/silica nanoparticles as multifunctional drug delivery platform with enhanced near-infrared responsiveness. Mater. Sci. Eng. C 2019, 104, 109889. [Google Scholar] [CrossRef]
- Kalimuthu, K.; Lubin, B.C.; Bazylevich, A.; Gellerman, G.; Shpilberg, O.; Luboshits, G.; Firer, M.A. Gold nanoparticles stabilize peptide-drug-conjugates for sustained targeted drug delivery to cancer cells. J. Nanobiotechnology 2018, 16, 1–3. [Google Scholar] [CrossRef]
- Kashyap, D.; Tuli, H.S.; Yerer, M.B.; Sharma, A.; Sak, K.; Srivastava, S.; Pandey, A.; Garg, V.K.; Sethi, G.; Bishayee, A. Natural product-based nanoformulations for cancer therapy: Opportunities and challenges. Semin. Cancer Biol. 2021, 69, 5–23. [Google Scholar] [CrossRef]
- Tuasha, N.; Petros, B.; Asfaw, Z. Medicinal plants used by traditional healers to treat malignancies and other human ailments in Dalle District, Sidama Zone, Ethiopia. J. Ethnobiol. Ethnomed. 2018, 14, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, A.; Ali, S.; Murad, W.; Hayat, K.; Siraj, S.; Jawad, M.; Khan, R.A.; Uddin, J.; Al-Harrasi, A.; Khan, A. Phytochemical and pharmacological uses of medicinal plants to treat cancer: A case study from Khyber Pakhtunkhwa, North Pakistan. J. Ethnopharmacol. 2021, 281, 114437. [Google Scholar] [CrossRef] [PubMed]
- Saiful Yazan, L.; Muhamad Zali, M.F.S.; Mohd Ali, R.; Zainal, N.A.; Esa, N.; Sapuan, S.; Ong, Y.S.; Tor, Y.S.; Gopalsamy, B.; Voon, F.L. Chemopreventive properties and toxicity of Kelulut honey in Sprague Dawley rats induced with Azoxymethane. BioMed Res. Int. 2016, 2016, 4036926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sargazi, S.; Moudi, M.; Kooshkaki, O.; Mirinejad, S.; Saravani, R. Hydro-alcoholic extract of Achillea Wilhelmsii C. Koch reduces the expression of cell death-associated genes while inducing DNA damage in HeLa cervical cancer cells. Iran. J. Med. Sci. 2020, 45, 359–367. [Google Scholar] [PubMed]
- Sargazi, M.L.; Saravani, R.; Shahraki, A. Hydroalcoholic extract of Levisticum officinale increases cGMP signaling pathway by down-regulating PDE5 expression and induction of apoptosis in MCF-7 and MDA-MB-468 breast cancer cell lines. Iran. Biomed. J. 2019, 23, 280. [Google Scholar]
- Hayat, K.; Khan, J.; Khan, A.; Ullah, S.; Ali, S.; Salahuddin; Fu, Y. Ameliorative Effects of Exogenous Proline on Photosynthetic Attributes, Nutrients Uptake, and Oxidative Stresses under Cadmium in Pigeon Pea (Cajanus cajan L.). Plants 2021, 10, 796. [Google Scholar] [CrossRef]
- Seca, A.M.; Pinto, D.C. Plant secondary metabolites as anticancer agents: Successes in clinical trials and therapeutic application. Int. J. Mol. Sci. 2018, 19, 263. [Google Scholar] [CrossRef] [Green Version]
- Gupta, R.; Xie, H. Nanoparticles in daily life: Applications, toxicity and regulations. J. Environ. Pathol. Toxicol. Oncol. 2018, 37, 209–230. [Google Scholar] [CrossRef]
- Hua, S.; De Matos, M.B.; Metselaar, J.M.; Storm, G. Current trends and challenges in the clinical translation of nanoparticulate nanomedicines: Pathways for translational development and commercialization. Front. Pharmacol. 2018, 9, 790. [Google Scholar] [CrossRef]
- Sadi, M.H.; Shahidi Bonjar, G.H. Plants used in folkloric medicine of Iran are exquisite bio-resources in production of silver nanoparticles. IET Nanobiotechnol. 2017, 11, 300–309. [Google Scholar] [CrossRef]
- Hamidian, K.; Sarani, M.; Barani, M.; Khakbaz, F. Cytotoxic performance of green synthesized Ag and Mg dual doped ZnO NPs using Salvadora persica extract against MDA-MB-231 and MCF-10 cells. Arab. J. Chem. 2022, 15, 103792. [Google Scholar] [CrossRef]
- Shu, M.; He, F.; Li, Z.; Zhu, X.; Ma, Y.; Zhou, Z.; Yang, Z.; Gao, F.; Zeng, M. Biosynthesis and antibacterial activity of silver nanoparticles using yeast extract as reducing and capping agents. Nanoscale Res. Lett. 2020, 15, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akl, B.; Nader, M.M.; El-Saadony, M. Biosynthesis of silver nanoparticles by Serratia marcescens ssp sakuensis and its antibacterial application against some pathogenic bacteria. J. Agric. Chem. Biotechnol. 2020, 11, 1–8. [Google Scholar] [CrossRef]
- Kumari, S.; Tehri, N.; Gahlaut, A.; Hooda, V. Actinomycetes mediated synthesis, characterization, and applications of metallic nanoparticles. Inorg. Nano-Met. Chem. 2020, 51, 1386–1395. [Google Scholar] [CrossRef]
- Naimi-Shamel, N.; Pourali, P.; Dolatabadi, S. Green synthesis of gold nanoparticles using Fusarium oxysporum and antibacterial activity of its tetracycline conjugant. J. De Mycol. Med. 2019, 29, 7–13. [Google Scholar] [CrossRef]
- Khanna, P.; Kaur, A.; Goyal, D. Algae-based metallic nanoparticles: Synthesis, characterization and applications. J. Microbiol. Methods 2019, 163, 105656. [Google Scholar] [CrossRef]
- Kanwar, R.; Rathee, J.; Salunke, D.B.; Mehta, S.K. Green nanotechnology-driven drug delivery assemblies. ACS Omega 2019, 4, 8804–8815. [Google Scholar] [CrossRef] [Green Version]
- Chugh, H.; Sood, D.; Chandra, I.; Tomar, V.; Dhawan, G.; Chandra, R. Role of gold and silver nanoparticles in cancer nano-medicine. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1210–1220. [Google Scholar] [CrossRef]
- Rónavári, A.; Igaz, N.; Adamecz, D.I.; Szerencsés, B.; Molnar, C.; Kónya, Z.; Pfeiffer, I.; Kiricsi, M. Green silver and gold nanoparticles: Biological synthesis approaches and potentials for biomedical applications. Molecules 2021, 26, 844. [Google Scholar] [CrossRef]
- Ismail, E.H.; Saqer, A.M.A.; Assirey, E.; Naqvi, A.; Okasha, R.M. Successful Green Synthesis of Gold Nanoparticles using a Corchorus olitorius Extract and Their Antiproliferative Effect in Cancer Cells. Int. J. Mol. Sci. 2018, 19, 2612. [Google Scholar] [CrossRef] [Green Version]
- Fazal, S.; Jayasree, A.; Sasidharan, S.; Koyakutty, M.; Nair, S.V.; Menon, D. Green synthesis of anisotropic gold nanoparticles for photothermal therapy of cancer. ACS Appl. Mater. Interfaces 2014, 6, 8080–8089. [Google Scholar] [CrossRef] [PubMed]
- Folorunso, A.; Akintelu, S.; Oyebamiji, A.K.; Ajayi, S.; Abiola, B.; Abdusalam, I.; Morakinyo, A. Biosynthesis, characterization and antimicrobial activity of gold nanoparticles from leaf extracts of Annona muricata. J. Nanostructure Chem. 2019, 9, 111–117. [Google Scholar] [CrossRef] [Green Version]
- Noruzi, M. Biosynthesis of gold nanoparticles using plant extracts. Bioprocess Biosyst. Eng. 2015, 38, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Joshi, M.; Bhattacharyya, A.; Ali, S.W. Characterization techniques for nanotechnology applications in textiles. Indian J. Fibre Text. Res. 2008, 33, 304–317. [Google Scholar]
- Lin, P.-C.; Lin, S.; Wang, P.C.; Sridhar, R. Techniques for physicochemical characterization of nanomaterials. Biotechnol. Adv. 2014, 32, 711–726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Gao, X.; Chen, Z.; Chen, Y.; Chen, H. Preparation, characterization and application of polysaccharide-based metallic nanoparticles: A review. Polymers 2017, 9, 689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alshammari, A.; Angela, K. Influence of single use and combination of reductants on the size, morphology and growth steps of gold nanoparticles in colloidal mixture. Open J. Phys. Chem. 2012, 2, 24975. [Google Scholar] [CrossRef] [Green Version]
- Sulaiman, G.M.; Waheeb, H.M.; Jabir, M.S.; Khazaal, S.H.; Dewir, Y.H.; Naidoo, Y. Hesperidin loaded on gold nanoparticles as a drug delivery system for a successful biocompatible, anti-cancer, anti-inflammatory and phagocytosis inducer model. Sci. Rep. 2020, 10, 9362. [Google Scholar] [CrossRef] [PubMed]
- Karthick, V.; Ganesh Kumar, V.; Maiyalagan, T.; Deepa, R.; Govindaraju, K.; Rajeswari, A.; Stalin Dhas, T. Green synthesis of well dispersed nanoparticles using leaf extract of medicinally useful Adhatoda vasica nees. Micro Nanosyst. 2012, 4, 192–198. [Google Scholar] [CrossRef]
- Zhaleh, M.; Zangeneh, A.; Goorani, S.; Seydi, N.; Zangeneh, M.M.; Tahvilian, R.; Pirabbasi, E. In vitro and in vivo evaluation of cytotoxicity, antioxidant, antibacterial, antifungal, and cutaneous wound healing properties of gold nanoparticles produced via a green chemistry synthesis using Gundelia tournefortii L. as a capping and reducing agent. Appl. Organomet. Chem. 2019, 33, e5015. [Google Scholar] [CrossRef]
- Erjaee, H.; Rajaian, H.; Nazifi, S. Synthesis and characterization of novel silver nanoparticles using Chamaemelum nobile extract for antibacterial application. Adv. Nat. Sci. Nanosci. Nanotechnol. 2017, 8, 025004. [Google Scholar] [CrossRef]
- Boyd, R.D.; Cuenat, A.; Meli, F.; Klein, T.; Frase, C.G.; Gleber, G.; Krumrey, M.; Duta, A.; Duta, S.; Hogstrom, R. Good practice guide for the determination of the size distributions of spherical nanoparticle samples. In Good Practice Guide No. 119; National Physical Laboratory: London, UK, 2011. [Google Scholar]
- Karimi-Maleh, H.; Cellat, K.; Arıkan, K.; Savk, A.; Karimi, F.; Şen, F. Palladium–Nickel nanoparticles decorated on Functionalized-MWCNT for high precision non-enzymatic glucose sensing. Mater. Chem. Phys. 2020, 250, 123042. [Google Scholar] [CrossRef]
- Scimeca, M.; Bischetti, S.; Lamsira, H.K.; Bonfiglio, R.; Bonanno, E. Energy Dispersive X-ray (EDX) microanalysis: A powerful tool in biomedical research and diagnosis. Eur. J. Histochem. EJH 2018, 62, 2841. [Google Scholar] [CrossRef] [PubMed]
- Ndeh, N.T.; Maensiri, S.; Maensiri, D. The effect of green synthesized gold nanoparticles on rice germination and roots. Adv. Nat. Sci. Nanosci. Nanotechnol. 2017, 8, 035008. [Google Scholar] [CrossRef] [Green Version]
- Kuruppu, A.I.; Paranagama, P.; Goonasekara, C.L. Medicinal plants commonly used against cancer in traditional medicine formulae in Sri Lanka. Saudi Pharm. J. 2019, 27, 565–573. [Google Scholar] [CrossRef]
- Amjad, M.S.; Zahoor, U.; Bussmann, R.W.; Altaf, M.; Gardazi, S.M.H.; Abbasi, A.M. Ethnobotanical survey of the medicinal flora of Harighal, Azad Jammu & Kashmir, Pakistan. J. Ethnobiol. Ethnomed. 2020, 16, 65. [Google Scholar] [CrossRef]
- Schaal, B. Plants and people: Our shared history and future. Plants People Planet. 2019, 1, 14–19. [Google Scholar] [CrossRef]
- Delgoda, R.; Murray, J. Evolutionary perspectives on the role of plant secondary metabolites. In Pharmacognosy; Elsevier: Amsterdam, The Netherlands, 2017; pp. 93–100. [Google Scholar]
- Kabera, J.N.; Semana, E.; Mussa, A.R.; He, X. Plant secondary metabolites: Biosynthesis, classification, function and pharmacological properties. J. Pharm. Pharmacol. 2014, 2, 377–392. [Google Scholar]
- Fulda, S.; Efferth, T. Selected secondary plant metabolites for cancer therapy. World J. Tradit. Chin. Med. 2015, 1, 24–28. [Google Scholar] [CrossRef]
- Qu, Y.; Safonova, O.; De Luca, V. Completion of the canonical pathway for assembly of anticancer drugs vincristine/vinblastine in Catharanthus roseus. Plant J. 2019, 97, 257–266. [Google Scholar] [CrossRef] [Green Version]
- Zhou, R.-s.; Wang, X.-W.; Sun, Q.-f.; Ye, Z.J.; Liu, J.-w.; Zhou, D.-H.; Tang, Y. Anticancer effects of emodin on HepG2 cell: Evidence from bioinformatic analysis. BioMed Res. Int. 2019, 2019, 3065818. [Google Scholar] [CrossRef] [PubMed]
- Zein, N.; Aziz, S.W.; El-Sayed, A.S.; Sitohy, B. Comparative cytotoxic and anticancer effect of Taxol derived from Aspergillus terreus and Taxus brevifolia. Biosci. Res. 2019, 16, 1500–1509. [Google Scholar]
- Imran, M.; Salehi, B.; Sharifi-Rad, J.; Aslam Gondal, T.; Saeed, F.; Imran, A.; Shahbaz, M.; Tsouh Fokou, P.V.; Umair Arshad, M.; Khan, H. Kaempferol: A key emphasis to its anticancer potential. Molecules 2019, 24, 2277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodenak-Kladniew, B.; Castro, M.A.; Crespo, R.; Galle, M.; de Bravo, M.G. Anti-cancer mechanisms of linalool and 1, 8-cineole in non-small cell lung cancer A549 cells. Heliyon 2020, 6, e05639. [Google Scholar] [CrossRef]
- Balkrishna, A.; Das, S.K.; Pokhrel, S.; Joshi, A.; Verma, S.; Sharma, V.K.; Sharma, V.; Sharma, N.; Joshi, C. Colchicine: Isolation, LC–MS QTof screening, and anticancer activity study of Gloriosa superba seeds. Molecules 2019, 24, 2772. [Google Scholar] [CrossRef] [Green Version]
- Satari, A.; Ghasemi, S.; Habtemariam, S.; Asgharian, S.; Lorigooini, Z. Rutin: A Flavonoid as an Effective Sensitizer for Anticancer Therapy; Insights into Multifaceted Mechanisms and Applicability for Combination Therapy. Evid.-Based Complement. Altern. Med. 2021, 2021, 9913179. [Google Scholar] [CrossRef]
- Almatroodi, S.A.; Alsahli, M.A.; Almatroudi, A.; Verma, A.K.; Aloliqi, A.; Allemailem, K.S.; Khan, A.A.; Rahmani, A.H. Potential therapeutic targets of quercetin, a plant flavonol, and its role in the therapy of various types of cancer through the modulation of various cell signaling pathways. Molecules 2021, 26, 1315. [Google Scholar] [CrossRef]
- Ijaz, S.; Akhtar, N.; Khan, M.S.; Hameed, A.; Irfan, M.; Arshad, M.A.; Ali, S.; Asrar, M. Plant derived anticancer agents: A green approach towards skin cancers. Biomed. Pharmacother. 2018, 103, 1643–1651. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Sureda, A.; Tenore, G.C.; Daglia, M.; Sharifi-Rad, M.; Valussi, M.; Tundis, R.; Sharifi-Rad, M.; Loizzo, M.R.; Ademiluyi, A.O. Biological activities of essential oils: From plant chemoecology to traditional healing systems. Molecules 2017, 22, 70. [Google Scholar] [CrossRef]
- Duan, H.; Wang, D.; Li, Y. Green chemistry for nanoparticle synthesis. Chem. Soc. Rev. 2015, 44, 5778–5792. [Google Scholar] [CrossRef]
- Khan, M.; Shaik, M.R.; Adil, S.F.; Khan, S.T.; Al-Warthan, A.; Siddiqui, M.R.H.; Tahir, M.N.; Tremel, W. Plant extracts as green reductants for the synthesis of silver nanoparticles: Lessons from chemical synthesis. Dalton Trans. 2018, 47, 11988–12010. [Google Scholar] [CrossRef] [PubMed]
- Ogunyemi, S.O.; Abdallah, Y.; Zhang, M.; Fouad, H.; Hong, X.; Ibrahim, E.; Masum, M.M.I.; Hossain, A.; Mo, J.; Li, B. Green synthesis of zinc oxide nanoparticles using different plant extracts and their antibacterial activity against Xanthomonas oryzae pv. oryzae. Artif. Cells Nanomed. Biotechnol. 2019, 47, 341–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alalaiwe, A. The clinical pharmacokinetics impact of medical nanometals on drug delivery system. Nanomed. Nanotechnol. Biol. Med. 2019, 17, 47–61. [Google Scholar] [CrossRef] [PubMed]
- Birla, S.; Tiwari, V.; Gade, A.; Ingle, A.; Yadav, A.; Rai, M. Fabrication of silver nanoparticles by Phoma glomerata and its combined effect against Escherichia coli, Pseudomonas aeruginosa and Staphylococcus aureus. Lett. Appl. Microbiol. 2009, 48, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.; Yadav, A.; Gade, A. CRC 675—current trends in phytosynthesis of metal nanoparticles. Crit. Rev. Biotechnol. 2008, 28, 277–284. [Google Scholar] [CrossRef]
- Lee, K.X.; Shameli, K.; Yew, Y.P.; Teow, S.-Y.; Jahangirian, H.; Rafiee-Moghaddam, R.; Webster, T.J. Recent developments in the facile bio-synthesis of gold nanoparticles (AuNPs) and their biomedical applications. Int. J. Nanomed. 2020, 15, 275–300. [Google Scholar] [CrossRef]
- Bharadwaj, K.K.; Rabha, B.; Pati, S.; Sarkar, T.; Choudhury, B.K.; Barman, A.; Bhattacharjya, D.; Srivastava, A.; Baishya, D.; Edinur, H.A. Green Synthesis of Gold Nanoparticles Using Plant Extracts as Beneficial Prospect for Cancer Theranostics. Molecules 2021, 26, 6389. [Google Scholar] [CrossRef]
- Amendola, V.; Pilot, R.; Frasconi, M.; Marago, O.M.; Iatì, M.A. Surface plasmon resonance in gold nanoparticles: A review. J. Phys. Condens. Matter 2017, 29, 203002. [Google Scholar] [CrossRef]
- Ajnai, G.; Chiu, A.; Kan, T.; Cheng, C.-C.; Tsai, T.-H.; Chang, J. Trends of gold nanoparticle-based drug delivery system in cancer therapy. J. Exp. Clin. Med. 2014, 6, 172–178. [Google Scholar] [CrossRef]
- Kong, F.-Y.; Zhang, J.-W.; Li, R.-F.; Wang, Z.-X.; Wang, W.-J.; Wang, W. Unique roles of gold nanoparticles in drug delivery, targeting and imaging applications. Molecules 2017, 22, 1445. [Google Scholar] [CrossRef] [Green Version]
- Bindhu, M.; Umadevi, M. Antibacterial activities of green synthesized gold nanoparticles. Mater. Lett. 2014, 120, 122–125. [Google Scholar] [CrossRef]
- Barai, A.C.; Paul, K.; Dey, A.; Manna, S.; Roy, S.; Bag, B.G.; Mukhopadhyay, C. Green synthesis of Nerium oleander-conjugated gold nanoparticles and study of its in vitro anticancer activity on MCF-7 cell lines and catalytic activity. Nano Converg. 2018, 5, 10. [Google Scholar] [CrossRef] [PubMed]
- Hassanen, E.I.; Korany, R.M.; Bakeer, A.M. Cisplatin-conjugated gold nanoparticles-based drug delivery system for targeting hepatic tumors. J. Biochem. Mol. Toxicol. 2021, 35, e22722. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Han, B.; Wang, Y.; Shi, H.; Zhao, J.; Li, G. Gold Nanoparticles-based Bio-Sensing Methods for Tumor-related Biomedical Applications in Bodily Fluids. Curr. Nanosci. 2020, 16, 425–440. [Google Scholar] [CrossRef]
- Kim, H.S.; Lee, D.Y. Photothermal therapy with gold nanoparticles as an anticancer medication. J. Pharm. Investig. 2017, 47, 19–26. [Google Scholar] [CrossRef]
- Patra, N.; Dehury, N.; Pal, A.; Behera, A.; Patra, S. Preparation and mechanistic aspect of natural xanthone functionalized gold nanoparticle. Mater. Sci. Eng. C 2018, 90, 439–445. [Google Scholar] [CrossRef]
- Siddique, S.; Chow, J.C. Gold nanoparticles for drug delivery and cancer therapy. Appl. Sci. 2020, 10, 3824. [Google Scholar] [CrossRef]
- Yap, K.M.; Sekar, M.; Fuloria, S.; Wu, Y.S.; Gan, S.H.; Rani, N.N.I.M.; Subramaniyan, V.; Kokare, C.; Lum, P.T.; Begum, M.Y. Drug Delivery of Natural Products Through Nanocarriers for Effective Breast Cancer Therapy: A Comprehensive Review of Literature. Int. J. Nanomed. 2021, 16, 7891–7941. [Google Scholar] [CrossRef]
- Gardea-Torresdey, J.L.; Gomez, E.; Peralta-Videa, J.R.; Parsons, J.G.; Troiani, H.; Jose-Yacaman, M. Alfalfa sprouts: A natural source for the synthesis of silver nanoparticles. Langmuir 2003, 19, 1357–1361. [Google Scholar] [CrossRef]
- Monopoli, M.P.; Åberg, C.; Salvati, A.; Dawson, K.A. Biomolecular coronas provide the biological identity of nanosized materials. Nat. Nanotechnol. 2012, 7, 779–786. [Google Scholar] [CrossRef]
- Singh, P.; Pandit, S.; Mokkapati, V.; Garg, A.; Ravikumar, V.; Mijakovic, I. Gold nanoparticles in diagnostics and therapeutics for human cancer. Int. J. Mol. Sci. 2018, 19, 1979. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Crawford, B.M.; Vo-Dinh, T. Gold nanoparticles-mediated photothermal therapy and immunotherapy. Immunotherapy 2018, 10, 1175–1188. [Google Scholar] [CrossRef] [PubMed]
- Galper, M.W.; Saung, M.T.; Fuster, V.; Roessl, E.; Thran, A.; Proksa, R.; Fayad, Z.A.; Cormode, D.P. Effect of computed tomography scanning parameters on gold nanoparticle and iodine contrast. Investig. Radiol. 2012, 47, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Santos-Martinez, M.J.; Rahme, K.; Corbalan, J.J.; Faulkner, C.; Holmes, J.D.; Tajber, L.; Medina, C.; Radomski, M.W. Pegylation increases platelet biocompatibility of gold nanoparticles. J. Biomed. Nanotechnol. 2014, 10, 1004–1015. [Google Scholar] [CrossRef]
- Cormode, D.P.; Skajaa, T.; van Schooneveld, M.M.; Koole, R.; Jarzyna, P.; Lobatto, M.E.; Calcagno, C.; Barazza, A.; Gordon, R.E.; Zanzonico, P. Nanocrystal core high-density lipoproteins: A multimodality contrast agent platform. Nano Lett. 2008, 8, 3715–3723. [Google Scholar] [CrossRef] [Green Version]
- Arifin, D.R.; Long, C.M.; Gilad, A.A.; Alric, C.; Roux, S.; Tillement, O.; Link, T.W.; Arepally, A.; Bulte, J.W. Trimodal gadolinium-gold microcapsules containing pancreatic islet cells restore normoglycemia in diabetic mice and can be tracked by using US, CT, and positive-contrast MR imaging. Radiology 2011, 260, 790–798. [Google Scholar] [CrossRef] [Green Version]
- Cai, Q.-Y.; Kim, S.H.; Choi, K.S.; Kim, S.Y.; Byun, S.J.; Kim, K.W.; Park, S.H.; Juhng, S.K.; Yoon, K.-H. Colloidal gold nanoparticles as a blood-pool contrast agent for X-ray computed tomography in mice. Investig. Radiol. 2007, 42, 797–806. [Google Scholar] [CrossRef]
- Alford, R.; Simpson, H.M.; Duberman, J.; Hill, G.C.; Ogawa, M.; Regino, C.; Kobayashi, H.; Choyke, P.L. Toxicity of organic fluorophores used in molecular imaging: Literature review. Mol. Imaging 2009, 8, 341–354. [Google Scholar] [CrossRef] [Green Version]
- Roy, N.K.; Monisha, J.; Padmavathi, G.; Das, A.; Gupta, S.; Ramakrishnan, E.; Kotoky, J.; Kunnumakkara, A.B. Rapid biosynthesis of gold nanoparticles using aqueous-ethanoic leaf extract of heartleaf moonseed: Characterization and effect of ph on its synthesis. Curr. Nanomater. 2017, 2, 3–10. [Google Scholar] [CrossRef]
- Gong, F.; Yang, N.; Wang, X.; Zhao, Q.; Chen, Q.; Liu, Z.; Cheng, L. Tumor microenvironment-responsive intelligent nanoplatforms for cancer theranostics. Nano Today 2020, 32, 100851. [Google Scholar] [CrossRef]
- Usman, A.I.; Aziz, A.A.; Noqta, O.A. Application of green synthesis of gold nanoparticles: A review. J. Teknol. 2019, 81. [Google Scholar] [CrossRef] [Green Version]
- Song, J.; Kim, J.; Hwang, S.; Jeon, M.; Jeong, S.; Kim, C.; Kim, S. “Smart” gold nanoparticles for photoacoustic imaging: An imaging contrast agent responsive to the cancer microenvironment and signal amplification via pH-induced aggregation. Chem. Commun. 2016, 52, 8287–8290. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Wang, X.; Burda, C.; Basilion, J.P. Recent Development of Gold Nanoparticles as Contrast Agents for Cancer Diagnosis. Cancers 2021, 13, 1825. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yang, M.; Zhu, Y.; Mao, C. Metallic nanoclusters for cancer imaging and therapy. Curr. Med. Chem. 2018, 25, 1379–1396. [Google Scholar] [CrossRef]
- Patra, C.R.; Mukherjee, S.; Kotcherlakota, R. Biosynthesized silver nanoparticles: A step forward for cancer theranostics? Nanomedicine 2014, 9, 1445–1448. [Google Scholar] [CrossRef]
- Gurunathan, S.; Han, J.; Park, J.H.; Kim, J.-H. A green chemistry approach for synthesizing biocompatible gold nanoparticles. Nanoscale Res. Lett. 2014, 9, 248. [Google Scholar] [CrossRef] [Green Version]
- Peng, H.-S.; Chiu, D.T. Soft fluorescent nanomaterials for biological and biomedical imaging. Chem. Soc. Rev. 2015, 44, 4699–4722. [Google Scholar] [CrossRef] [Green Version]
- Braeken, Y.; Cheruku, S.; Ethirajan, A.; Maes, W. Conjugated polymer nanoparticles for bioimaging. Materials 2017, 10, 1420. [Google Scholar] [CrossRef] [Green Version]
- Lang, M.; Stober, F.; Lichtenthaler, H. Fluorescence emission spectra of plant leaves and plant constituents. Radiat. Environ. Biophys. 1991, 30, 333–347. [Google Scholar] [CrossRef]
- Lagorio, M.G.; Cordon, G.B.; Iriel, A. Reviewing the relevance of fluorescence in biological systems. Photochem. Photobiol. Sci. 2015, 14, 1538–1559. [Google Scholar] [CrossRef] [Green Version]
- Kotcherlakota, R.; Nimushakavi, S.; Roy, A.; Yadavalli, H.C.; Mukherjee, S.; Haque, S.; Patra, C.R. Biosynthesized gold nanoparticles: In vivo study of near-infrared fluorescence (NIR)-based bio-imaging and cell labeling applications. ACS Biomater. Sci. Eng. 2019, 5, 5439–5452. [Google Scholar] [CrossRef] [PubMed]
- Diaz, B.; Sánchez-Espinel, C.; Arruebo, M.; Faro, J.; de Miguel, E.; Magadan, S.; Yaguee, C.; Fernández-Pacheco, R.; Ibarra, M.R.; Santamaria, J. Assessing methods for blood cell cytotoxic responses to inorganic nanoparticles and nanoparticle aggregates. Small 2008, 4, 2025–2034. [Google Scholar] [CrossRef] [PubMed]
- Raynal, I.; Prigent, P.; Peyramaure, S.; Najid, A.; Rebuzzi, C.; Corot, C. Macrophage endocytosis of superparamagnetic iron oxide nanoparticles: Mechanisms and comparison of ferumoxides and ferumoxtran-10. Investig. Radiol. 2004, 39, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, M.; Hofmann, H.; Rothen-Rutishauser, B.; Petri-Fink, A. Assessing the in vitro and in vivo toxicity of superparamagnetic iron oxide nanoparticles. Chem. Rev. 2012, 112, 2323–2338. [Google Scholar] [CrossRef] [Green Version]
- JesÚs, M.; AlcÁntara, D.; PenadÉs, S. Cell response to magnetic glyconanoparticles: Does the carbohydrate matter? IEEE Trans. Nanobiosci. 2007, 6, 275–281. [Google Scholar]
- Narayanan, S.; Sathy, B.N.; Mony, U.; Koyakutty, M.; Nair, S.V.; Menon, D. Biocompatible magnetite/gold nanohybrid contrast agents via green chemistry for MRI and CT bioimaging. ACS Appl. Mater. Interfaces 2012, 4, 251–260. [Google Scholar] [CrossRef]
- Chanda, N.; Shukla, R.; Zambre, A.; Mekapothula, S.; Kulkarni, R.R.; Katti, K.; Bhattacharyya, K.; Fent, G.M.; Casteel, S.W.; Boote, E.J. An effective strategy for the synthesis of biocompatible gold nanoparticles using cinnamon phytochemicals for phantom CT imaging and photoacoustic detection of cancerous cells. Pharm. Res. 2011, 28, 279–291. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, S.; Vinothkumar, B.; Prashanthi, S.; Bangal, P.R.; Sreedhar, B.; Patra, C.R. Potential therapeutic and diagnostic applications of one-step in situ biosynthesized gold nanoconjugates (2-in-1 system) in cancer treatment. RSC Adv. 2013, 3, 2318–2329. [Google Scholar] [CrossRef]
- Morel, A.-L.; Giraud, S.; Bialecki, A.; Moustaoui, H.; de La Chapelle, M.L.; Spadavecchia, J. Green extraction of endemic plants to synthesize gold nanoparticles for theranostic applications. Front. Lab. Med. 2017, 1, 158–171. [Google Scholar] [CrossRef]
- Robles-Escajeda, E.; Lerma, D.; Nyakeriga, A.M.; Ross, J.A.; Kirken, R.A.; Aguilera, R.J.; Varela-Ramirez, A. Searching in mother nature for anti-cancer activity: Anti-proliferative and pro-apoptotic effect elicited by green barley on leukemia/lymphoma cells. PLoS ONE 2013, 8, e73508. [Google Scholar] [CrossRef] [Green Version]
- Xue, N.; Zhou, C.; Chu, Z.; Chen, L.; Jia, N. Barley leaves mediated biosynthesis of Au nanomaterials as a potential contrast agent for computed tomography imaging. Sci. China Technol. Sci. 2021, 64, 433–440. [Google Scholar] [CrossRef]
- Koo, K.M.; Soda, N.; Shiddiky, M.J. Magnetic nanomaterial–based electrochemical biosensors for the detection of diverse circulating cancer biomarkers. Curr. Opin. Electrochem. 2021, 25, 100645. [Google Scholar] [CrossRef]
- Zhang, Y.; Lyu, H. Application of biosensors based on nanomaterials in cancer cell detection. In Proceedings of the The 2021 2nd International Conference on Internet of Things, Artificial Intelligence and Mechanical Automation (IoTAIMA 2021), Hangzhou, China, 14–16 May 2021; p. 012149. [Google Scholar]
- Qian, L.; Li, Q.; Baryeh, K.; Qiu, W.; Li, K.; Zhang, J.; Yu, Q.; Xu, D.; Liu, W.; Brand, R.E. Biosensors for early diagnosis of pancreatic cancer: A review. Transl. Res. 2019, 213, 67–89. [Google Scholar] [CrossRef] [PubMed]
- Falkowski, P.; Lukaszewski, Z.; Gorodkiewicz, E. Potential of surface plasmon resonance biosensors in cancer detection. J. Pharm. Biomed. Anal. 2021, 194, 113802. [Google Scholar] [CrossRef]
- Sun, T.; Pan, H.; Mei, Y.; Zhang, P.; Zeng, D.; Liu, X.; Rong, S.; Chang, D. Electrochemical sensor sensitive detection of chloramphenicol based on ionic-liquid-assisted synthesis of de-layered molybdenum disulfide/graphene oxide nanocomposites. J. Appl. Electrochem. 2019, 49, 261–270. [Google Scholar] [CrossRef]
- Hajian, R.; Mehrayin, Z.; Mohagheghian, M.; Zafari, M.; Hosseini, P.; Shams, N. Fabrication of an electrochemical sensor based on carbon nanotubes modified with gold nanoparticles for determination of valrubicin as a chemotherapy drug: Valrubicin-DNA interaction. Mater. Sci. Eng. C 2015, 49, 769–775. [Google Scholar] [CrossRef]
- Khan, M.Z.H.; Liu, X.; Tang, Y.; Zhu, J.; Hu, W.; Liu, X. A glassy carbon electrode modified with a composite consisting of gold nanoparticle, reduced graphene oxide and poly (L-arginine) for simultaneous voltammetric determination of dopamine, serotonin and L-tryptophan. Microchim. Acta 2018, 185, 439. [Google Scholar] [CrossRef]
- da Silva, W.; Ghica, M.E.; Brett, C.M. Gold nanoparticle decorated multiwalled carbon nanotube modified electrodes for the electrochemical determination of theophylline. Anal. Methods 2018, 10, 5634–5642. [Google Scholar] [CrossRef]
- Nazarpour, S.; Hajian, R.; Sabzvari, M.H. A novel nanocomposite electrochemical sensor based on green synthesis of reduced graphene oxide/gold nanoparticles modified screen printed electrode for determination of tryptophan using response surface methodology approach. Microchem. J. 2020, 154, 104634. [Google Scholar] [CrossRef]
- Barabadi, H.; Ovais, M.; Shinwari, Z.K.; Saravanan, M. Anti-cancer green bionanomaterials: Present status and future prospects. Green Chem. Lett. Rev. 2017, 10, 285–314. [Google Scholar] [CrossRef] [Green Version]
- Soto, K.M.; Mendoza, S.; López-Romero, J.M.; Gasca-Tirado, J.R.; Manzano-Ramírez, A. Gold nanoparticles: Synthesis, application in colon cancer therapy and new approaches-review. Green Chem. Lett. Rev. 2021, 14, 665–678. [Google Scholar] [CrossRef]
- Rao, P.V.; Nallappan, D.; Madhavi, K.; Rahman, S.; Jun Wei, L.; Gan, S.H. Phytochemicals and biogenic metallic nanoparticles as anticancer agents. Oxidative Med. Cell. Longev. 2016, 2016, 3685671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anwar, A.; Ovais, M.; Khan, A.; Raza, A. Docetaxel-loaded solid lipid nanoparticles: A novel drug delivery system. IET Nanobiotechnol. 2017, 11, 621–629. [Google Scholar]
- Jain, N.; Jain, P.; Rajput, D.; Patil, U.K. Green synthesized plant-based silver nanoparticles: Therapeutic prospective for anticancer and antiviral activity. Micro Nano Syst. Lett. 2021, 9, 5. [Google Scholar] [CrossRef]
- Vinay, S.; Sumedha, H.; Shashank, M.; Nagaraju, G.; Chandrasekhar, N. In vitro antibacterial, antioxidant and cytotoxic potential of gold nanoparticles synthesized using novel Elaeocarpus ganitrus seeds extract. J. Sci. Adv. Mater. Devices 2021, 6, 127–133. [Google Scholar] [CrossRef]
- Vemuri, S.K.; Banala, R.R.; Mukherjee, S.; Uppula, P.; Subbaiah, G.; AV, G.R.; Malarvilli, T. Novel biosynthesized gold nanoparticles as anti-cancer agents against breast cancer: Synthesis, biological evaluation, molecular modelling studies. Mater. Sci. Eng. C 2019, 99, 417–429. [Google Scholar] [CrossRef]
- Karmous, I.; Pandey, A.; Haj, K.B.; Chaoui, A. Efficiency of the green synthesized nanoparticles as new tools in cancer therapy: Insights on plant-based bioengineered nanoparticles, biophysical properties, and anticancer roles. Biol. Trace Elem. Res. 2019, 196, 330–342. [Google Scholar] [CrossRef]
- Singh, N.; Das, M.K.; Ansari, A.; Mohanta, D.; Rajamani, P. Biogenic nanosized gold particles: Physico-chemical characterization and its anticancer response against breast cancer. Biotechnol. Rep. 2021, 30, e00612. [Google Scholar] [CrossRef]
- Hosny, M.; Fawzy, M. Instantaneous phytosynthesis of gold nanoparticles via Persicaria salicifolia leaf extract, and their medical applications. Adv. Powder Technol. 2021, 32, 2891–2904. [Google Scholar] [CrossRef]
- Devendiran, R.M.; Chinnaiyan, S.k.; Yadav, N.K.; Moorthy, G.K.; Ramanathan, G.; Singaravelu, S.; Sivagnanam, U.T.; Perumal, P.T. Green synthesis of folic acid-conjugated gold nanoparticles with pectin as reducing/stabilizing agent for cancer theranostics. RSC Adv. 2016, 6, 29757–29768. [Google Scholar] [CrossRef]
- Hoshyar, R.; Khayati, G.R.; Poorgholami, M.; Kaykhaii, M. A novel green one-step synthesis of gold nanoparticles using crocin and their anti-cancer activities. J. Photochem. Photobiol. B Biol. 2016, 159, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Vinayagam, R.; Santhoshkumar, M.; Lee, K.E.; David, E.; Kang, S.G. Bioengineered gold nanoparticles using Cynodon dactylon extract and its cytotoxicity and antibacterial activities. Bioprocess Biosyst. Eng. 2021, 44, 1253–1262. [Google Scholar] [CrossRef] [PubMed]
- Uzma, M.; Sunayana, N.; Raghavendra, V.B.; Madhu, C.S.; Shanmuganathan, R.; Brindhadevi, K. Biogenic synthesis of gold nanoparticles using Commiphora wightii and their cytotoxic effects on breast cancer cell line (MCF-7). Process Biochem. 2020, 92, 269–276. [Google Scholar] [CrossRef]
- Li, S.; Al-Misned, F.A.; El-Serehy, H.A.; Yang, L. Green synthesis of gold nanoparticles using aqueous extract of Mentha Longifolia leaf and investigation of its anti-human breast carcinoma properties in the in vitro condition. Arab. J. Chem. 2021, 14, 102931. [Google Scholar] [CrossRef]
- Kiran, M.; Kumar, C.R.; Shwetha, U.; Onkarappa, H.; Betageri, V.; Latha, M. Green synthesis and characterization of gold nanoparticles from Moringa oleifera leaves and assessment of antioxidant, antidiabetic and anticancer properties. Chem. Data Collect. 2021, 33, 100714. [Google Scholar] [CrossRef]
- Hosny, M.; Fawzy, M.; Abdelfatah, A.M.; Fawzy, E.E.; Eltaweil, A.S. Comparative study on the potentialities of two halophytic species in the green synthesis of gold nanoparticles and their anticancer, antioxidant and catalytic efficiencies. Adv. Powder Technol. 2021, 32, 3220–3233. [Google Scholar] [CrossRef]
- Divakaran, D.; Lakkakula, J.R.; Thakur, M.; Kumawat, M.K.; Srivastava, R. Dragon fruit extract capped gold nanoparticles: Synthesis and their differential cytotoxicity effect on breast cancer cells. Mater. Lett. 2019, 236, 498–502. [Google Scholar] [CrossRef]
- KS, U.S.; Govindaraju, K.; Prabhu, D.; Arulvasu, C.; Karthick, V.; Changmai, N. Anti-proliferative effect of biogenic gold nanoparticles against breast cancer cell lines (MDA-MB-231 & MCF-7). Appl. Surf. Sci. 2016, 371, 415–424. [Google Scholar]
- Qian, L.; Su, W.; Wang, Y.; Dang, M.; Zhang, W.; Wang, C. Synthesis and characterization of gold nanoparticles from aqueous leaf extract of Alternanthera sessilis and its anticancer activity on cervical cancer cells (HeLa). Artif. Cells Nanomed. Biotechnol. 2019, 47, 1173–1180. [Google Scholar] [CrossRef] [Green Version]
- Golemis, E.A.; Scheet, P.; Beck, T.N.; Scolnick, E.M.; Hunter, D.J.; Hawk, E.; Hopkins, N. Molecular mechanisms of the preventable causes of cancer in the United States. Genes Dev. 2018, 32, 868–902. [Google Scholar] [CrossRef] [Green Version]
- Ke, Y.; Al Aboody, M.S.; Alturaiki, W.; Alsagaby, S.A.; Alfaiz, F.A.; Veeraraghavan, V.P.; Mickymaray, S. Photosynthesized gold nanoparticles from Catharanthus roseus induces caspase-mediated apoptosis in cervical cancer cells (HeLa). Artif. Cells Nanomed. Biotechnol. 2019, 47, 1938–1946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baharara, J.; Ramezani, T.; Divsalar, A.; Mousavi, M.; Seyedarabi, A. Induction of apoptosis by green synthesized gold nanoparticles through activation of caspase-3 and 9 in human cervical cancer cells. Avicenna J. Med. Biotechnol. 2016, 8, 75–83. [Google Scholar] [PubMed]
- Kajani, A.A.; Bordbar, A.-K.; Esfahani, S.H.Z.; Razmjou, A. Gold nanoparticles as potent anticancer agent: Green synthesis, characterization, and in vitro study. RSC Adv. 2016, 6, 63973–63983. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2021. CA: A Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Li, J.; Zhong, C.; Zhang, X.; Bao, Y. Green synthesis of gold nanoparticles from Dendrobium officinale and its anticancer effect on liver cancer. Drug Deliv. 2021, 28, 985–994. [Google Scholar] [CrossRef]
- Rajeshkumar, S.; Malarkodi, C.; Al Farraj, D.A.; Elshikh, M.S.; Roopan, S.M. Employing sulphated polysaccharide (fucoidan) as medium for gold nanoparticles preparation and its anticancer study against HepG2 cell lines. Mater. Today Commun. 2021, 26, 101975. [Google Scholar] [CrossRef]
- Ji, Y.; Cao, Y.; Song, Y. Green synthesis of gold nanoparticles using a Cordyceps militaris extract and their antiproliferative effect in liver cancer cells (HepG2). Artif. Cells Nanomed. Biotechnol. 2019, 47, 2737–2745. [Google Scholar] [CrossRef] [Green Version]
- Perveen, K.; Husain, F.M.; Qais, F.A.; Khan, A.; Razak, S.; Afsar, T.; Alam, P.; Almajwal, A.M.; Abulmeaty, M. Microwave-Assisted Rapid Green Synthesis of Gold Nanoparticles Using Seed Extract of Trachyspermum ammi: ROS Mediated Biofilm Inhibition and Anticancer Activity. Biomolecules 2021, 11, 197. [Google Scholar] [CrossRef]
- MR, K.P.; Iyer, P.R. Antiproliferative effects on tumor cells of the synthesized gold nanoparticles against Hep2 liver cancer cell line. Egypt. Liver J. 2020, 10, 15. [Google Scholar]
- Kumar, S.U.; Gopinath, P.; Negi, Y.S. Synthesis and bio-evaluation of xylan-5-fluorouracil-1-acetic acid conjugates as prodrugs for colon cancer treatment. Carbohydr. Polym. 2017, 157, 1442–1450. [Google Scholar]
- Xia, Y.; You, P.; Xu, F.; Liu, J.; Xing, F. Novel functionalized selenium nanoparticles for enhanced anti-hepatocarcinoma activity in vitro. Nanoscale Res. Lett. 2015, 10, 349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González-Ballesteros, N.; Prado-López, S.; Rodríguez-González, J.; Lastra, M.; Rodríguez-Argüelles, M. Green synthesis of gold nanoparticles using brown algae Cystoseira baccata: Its activity in colon cancer cells. Colloids Surf. B Biointerfaces 2017, 153, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, N.; Shomali, T.; Hosseinzadeh, S.; Raouf Fard, F.; Pourmontaseri, M.; Fazeli, M. Green synthesis of gold nanoparticles by using Ferula persica Willd. gum essential oil: Production, characterization and in vitro anti-cancer effects. J. Pharm. Pharmacol. 2020, 72, 1013–1025. [Google Scholar] [CrossRef] [PubMed]
- Abel, E.E.; Poonga, P.R.J.; Panicker, S.G. Characterization and in vitro studies on anticancer, antioxidant activity against colon cancer cell line of gold nanoparticles capped with Cassia tora SM leaf extract. Appl. Nanosci. 2016, 6, 121–129. [Google Scholar] [CrossRef] [Green Version]
- Malaikolundhan, H.; Mookkan, G.; Krishnamoorthi, G.; Matheswaran, N.; Alsawalha, M.; Veeraraghavan, V.P.; Krishna Mohan, S.; Di, A. Anticarcinogenic effect of gold nanoparticles synthesized from Albizia lebbeck on HCT-116 colon cancer cell lines. Artif. Cells Nanomed. Biotechnol. 2020, 48, 1206–1213. [Google Scholar] [CrossRef]
- Liu, Q.; Wu, F.; Chen, Y.; Alrashood, S.T.; Alharbi, S.A. Anti-human colon cancer properties of a novel chemotherapeutic supplement formulated by gold nanoparticles containing Allium sativum L. leaf aqueous extract and investigation of its cytotoxicity and antioxidant activities. Arab. J. Chem. 2021, 14, 103039. [Google Scholar] [CrossRef]
- Datkhile, K.D.; Patil, S.R.; Durgawale, P.P.; Patil, M.N.; Hinge, D.D.; Jagdale, N.J.; Deshmukh, V.N.; More, A.L. Biogenic synthesis of gold nanoparticles using Argemone mexicana L. and their cytotoxic and genotoxic effects on human colon cancer cell line (HCT-15). J. Genet. Eng. Biotechnol. 2021, 19, 9. [Google Scholar] [CrossRef]
- Miri, A.; Darroudi, M.; Entezari, R.; Sarani, M. Biosynthesis of gold nanoparticles using Prosopis farcta extract and its in vitro toxicity on colon cancer cells. Res. Chem. Intermed. 2018, 44, 3169–3177. [Google Scholar] [CrossRef]
- Mata, R.; Nakkala, J.R.; Sadras, S.R. Polyphenol stabilized colloidal gold nanoparticles from Abutilon indicum leaf extract induce apoptosis in HT-29 colon cancer cells. Colloids Surf. B Biointerfaces 2016, 143, 499–510. [Google Scholar] [CrossRef]
- Elumalai, D.; Suman, T.; Hemavathi, M.; Swetha, C.; Kavitha, R.; Arulvasu, C.; Kaleena, P. Biofabrication of gold nanoparticles using Ganoderma lucidum and their cytotoxicity against human colon cancer cell line (HT-29). Bull. Mater. Sci. 2021, 44, 132. [Google Scholar] [CrossRef]
- Haga, A.; Takahashi, W.; Aoki, S.; Nawa, K.; Yamashita, H.; Abe, O.; Nakagawa, K. Classification of early stage non-small cell lung cancers on computed tomographic images into histological types using radiomic features: Interobserver delineation variability analysis. Radiol. Phys. Technol. 2018, 11, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Hu, N.; Han, L.; Pi, Y.; Gao, Y.; Chen, K. Anticancer activity of green synthesised gold nanoparticles from Marsdenia tenacissima inhibits A549 cell proliferation through the apoptotic pathway. Artif. Cells Nanomed. Biotechnol. 2019, 47, 4012–4019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vijayakumar, S.; Vaseeharan, B.; Malaikozhundan, B.; Gopi, N.; Ekambaram, P.; Pachaiappan, R.; Velusamy, P.; Murugan, K.; Benelli, G.; Kumar, R.S. Therapeutic effects of gold nanoparticles synthesized using Musa paradisiaca peel extract against multiple antibiotic resistant Enterococcus faecalis biofilms and human lung cancer cells (A549). Microb. Pathog. 2017, 102, 173–183. [Google Scholar] [CrossRef]
- Anand, K.; Gengan, R.; Phulukdaree, A.; Chuturgoon, A. Agroforestry waste Moringa oleifera petals mediated green synthesis of gold nanoparticles and their anti-cancer and catalytic activity. J. Ind. Eng. Chem. 2015, 21, 1105–1111. [Google Scholar] [CrossRef]
- Tiloke, C.; Phulukdaree, A.; Anand, K.; Gengan, R.M.; Chuturgoon, A.A. Moringa oleifera gold nanoparticles modulate oncogenes, tumor suppressor genes, and caspase-9 splice variants in a549 cells. J. Cell. Biochem. 2016, 117, 2302–2314. [Google Scholar] [CrossRef] [PubMed]
- El-Borady, O.M.; Fawzy, M.; Hosny, M. Antioxidant, anticancer and enhanced photocatalytic potentials of gold nanoparticles biosynthesized by common reed leaf extract. Appl. Nanosci. 2021, 1–12. [Google Scholar] [CrossRef]
- Abdoli, M.; Arkan, E.; Shekarbeygi, Z.; Khaledian, S. Green synthesis of gold nanoparticles using Centaurea behen leaf aqueous extract and investigating their antioxidant and cytotoxic effects on acute leukemia cancer cell line (THP-1). Inorg. Chem. Commun. 2021, 129, 108649. [Google Scholar] [CrossRef]
- Zhao, P.; El-kott, A.; Ahmed, A.E.; Khames, A.; Zein, M.A. Green synthesis of gold nanoparticles (Au NPs) using Tribulus terrestris extract: Investigation of its catalytic activity in the oxidation of sulfides to sulfoxides and study of its anti-acute leukemia activity. Inorg. Chem. Commun. 2021, 131, 108781. [Google Scholar] [CrossRef]
- Chang, Y.; Zheng, C.; Chinnathambi, A.; Alahmadi, T.A.; Alharbi, S.A. Cytotoxicity, anti-acute leukemia, and antioxidant properties of gold nanoparticles green-synthesized using Cannabis sativa L leaf aqueous extract. Arab. J. Chem. 2021, 14, 103060. [Google Scholar] [CrossRef]
- Ahmeda, A.; Zangeneh, A.; Zangeneh, M.M. Green synthesis and chemical characterization of gold nanoparticle synthesized using Camellia sinensis leaf aqueous extract for the treatment of acute myeloid leukemia in comparison to daunorubicin in a leukemic mouse model. Appl. Organomet. Chem. 2020, 34, e5290. [Google Scholar] [CrossRef]
- Zangeneh, M.M.; Zangeneh, A. Novel green synthesis of Hibiscus sabdariffa flower extract conjugated gold nanoparticles with excellent anti-acute myeloid leukemia effect in comparison to daunorubicin in a leukemic rodent model. Appl. Organomet. Chem. 2020, 34, e5271. [Google Scholar] [CrossRef]
- Chen, J.; Li, Y.; Fang, G.; Cao, Z.; Shang, Y.; Alfarraj, S.; Alharbi, S.A.; Li, J.; Yang, S.; Duan, X. Green synthesis, characterization, cytotoxicity, antioxidant, and anti-human ovarian cancer activities of Curcumae kwangsiensis leaf aqueous extract green-synthesized gold nanoparticles. Arab. J. Chem. 2021, 14, 103000. [Google Scholar] [CrossRef]
- Wang, L.; Xu, J.; Yan, Y.; Liu, H.; Karunakaran, T.; Li, F. Green synthesis of gold nanoparticles from Scutellaria barbata and its anticancer activity in pancreatic cancer cell (PANC-1). Artif. Cells Nanomed. Biotechnol. 2019, 47, 1617–1627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, R.; Pei, Q.; Shou, T.; Zhang, W.; Hu, J.; Li, W. Apoptotic effect of green synthesized gold nanoparticles from Curcuma wenyujin extract against human renal cell carcinoma A498 cells. Int. J. Nanomed. 2019, 14, 4091–4103. [Google Scholar] [CrossRef] [Green Version]
- Wu, F.; Zhu, J.; Li, G.; Wang, J.; Veeraraghavan, V.P.; Krishna Mohan, S.; Zhang, Q. Biologically synthesized green gold nanoparticles from Siberian ginseng induce growth-inhibitory effect on melanoma cells (B16). Artif. Cells Nanomed. Biotechnol. 2019, 47, 3297–3305. [Google Scholar] [CrossRef] [Green Version]
- Patil, M.P.; Jin, X.; Simeon, N.C.; Palma, J.; Kim, D.; Ngabire, D.; Kim, N.-H.; Tarte, N.H.; Kim, G.-D. Anticancer activity of Sasa borealis leaf extract-mediated gold nanoparticles. Artif. Cells Nanomed. Biotechnol. 2018, 46, 82–88. [Google Scholar] [CrossRef] [Green Version]
- Yun, Z.; Chinnathambi, A.; Alharbi, S.A.; Jin, Z. Biosynthesis of gold nanoparticles using Vetex negundo and evaluation of pro-apoptotic effect on human gastric cancer cell lines. J. Photochem. Photobiol. B Biol. 2020, 203, 111749. [Google Scholar] [CrossRef]
- Botteon, C.; Silva, L.; Ccana-Ccapatinta, G.; Silva, T.; Ambrosio, S.; Veneziani, R.; Bastos, J.; Marcato, P. Biosynthesis and characterization of gold nanoparticles using Brazilian red propolis and evaluation of its antimicrobial and anticancer activities. Sci. Rep. 2021, 11, 1974. [Google Scholar] [CrossRef]
- Nambiar, S.; Osei, E.; Fleck, A.; Darko, J.; Mutsaers, A.J.; Wettig, S. Synthesis of curcumin-functionalized gold nanoparticles and cytotoxicity studies in human prostate cancer cell line. Appl. Nanosci. 2018, 8, 347–357. [Google Scholar] [CrossRef]
- Libutti, S.; Paciotti, G.; Myer, L.; Haynes, R.; Gannon, W.; Walker, M.; Seidel, G.; Byrnes, A.; Yuldasheva, N.; Tamarkin, L. Results of a completed phase I clinical trial of CYT-6091: A pegylated colloidal gold-TNF nanomedicine. J. Clin. Oncol. 2009, 27, 3586. [Google Scholar] [CrossRef]
- Mukherjee, S.; Sau, S.; Madhuri, D.; Bollu, V.S.; Madhusudana, K.; Sreedhar, B.; Banerjee, R.; Patra, C.R. Green synthesis and characterization of monodispersed gold nanoparticles: Toxicity study, delivery of doxorubicin and its bio-distribution in mouse model. J. Biomed. Nanotechnol. 2016, 12, 165–181. [Google Scholar] [CrossRef] [PubMed]
- Shunmugam, R.; Balusamy, S.R.; Kumar, V.; Menon, S.; Lakshmi, T.; Perumalsamy, H. Biosynthesis of gold nanoparticles using marine microbe (Vibrio alginolyticus) and its anticancer and antioxidant analysis. J. King Saud Univ.-Sci. 2021, 33, 101260. [Google Scholar] [CrossRef]
- Mukherjee, S.; Nethi, S.K.; Patra, C.R. Green synthesized gold nanoparticles for future biomedical applications. In Particulate Technology for Delivery of Therapeutics; Springer: Berlin/Heidelberg, Germany, 2017; pp. 359–393. [Google Scholar]
- Desai, N. Challenges in development of nanoparticle-based therapeutics. AAPS J. 2012, 14, 282–295. [Google Scholar] [CrossRef] [Green Version]
- Moghimi, S.M.; Hunter, A.C.; Murray, J.C. Long-circulating and target-specific nanoparticles: Theory to practice. Pharmacol. Rev. 2001, 53, 283–318. [Google Scholar] [PubMed]
- Spyratou, E.; Makropoulou, M.; Efstathopoulos, E.P.; Georgakilas, A.G.; Sihver, L. Recent advances in cancer therapy based on dual mode gold nanoparticles. Cancers 2017, 9, 173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Yuan, F. Delivery of viral vectors to tumor cells: Extracellular transport, systemic distribution, and strategies for improvement. Ann. Biomed. Eng. 2006, 34, 114–127. [Google Scholar] [CrossRef]
- Yuan, F.; Leunig, M.; Huang, S.K.; Berk, D.A.; Papahadjopoulos, D.; Jain, R.K. Mirovascular permeability and interstitial penetration of sterically stabilized (stealth) liposomes in a human tumor xenograft. Cancer Res. 1994, 54, 3352–3356. [Google Scholar]
- Bhattarai, B.; Zaker, Y.; Bigioni, T.P. Green synthesis of gold and silver nanoparticles: Challenges and opportunities. Curr. Opin. Green Sustain. Chem. 2018, 12, 91–100. [Google Scholar] [CrossRef]
- Hassanisaadi, M.; Bonjar, G.H.S.; Rahdar, A.; Pandey, S.; Hosseinipour, A.; Abdolshahi, R. Environmentally safe biosynthesis of gold nanoparticles using plant water extracts. Nanomaterials 2021, 11, 2033. [Google Scholar] [CrossRef]
- Del Pino, P.; Pelaz, B.; Zhang, Q.; Maffre, P.; Nienhaus, G.U.; Parak, W.J. Protein corona formation around nanoparticles–from the past to the future. Mater. Horiz. 2014, 1, 301–313. [Google Scholar] [CrossRef]
- Bonjar, L.S. “Nanogold detoxifying machine” to remove idle nanogold particles from blood stream of cancer patients treated with antibody-nanogold therapeutics. Med. Hypotheses 2013, 80, 601–605. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Ahmad, F.; Koivisto, J.T.; Kellomäki, M. Green synthesis of controlled size gold and silver nanoparticles using antioxidant as capping and reducing agent. Colloid Interface Sci. Commun. 2020, 39, 100322. [Google Scholar] [CrossRef]
| Type of Extract | Plant Material | Particle Size of AuNPs | Cell line/Exposure Time (h) | Outcome (IC50) | Ref. |
|---|---|---|---|---|---|
| Aqueous extract | Cynodon dactylon | 22–34 nm | MCF-7/24 | 31.34 μg/mL | [148] |
| Dendrobium officinale | 30 nm | HepG2/24 | Maximum inhibition at 200 μg/mL | [161] | |
| Cystoseira baccata | 8.4 nm | HT-29/48 | 49.61 µM | [168] | |
| Caco-2/48 | 79.03 µM | ||||
| Siberian ginseng | 200 nm | B16/24 | CC50 = 10 μg/mL | [191] | |
| Aqueous leaf extract | Commiphora wightii | 20.2 nm | MCF-7/24 | 66.11 μg/mL | [149] |
| Albizia lebbeck | 20–30 nm | HCT-116/24 | 48 μg/mL | [171] | |
| Argemone mexicana | 20–40 nm | HCT-15/24 and 48 | 20.53 μg/mL | [173] | |
| 12.03 μg/mL | |||||
| Prosopis farcta | 25 nm | HT-29/72 | 419.7 µg/mL | [174] | |
| Moringa oleifera | 10–20 nm | A549/24 | 98.46 μg/mL | [181] | |
| SNO/24 | 92.01 μg/mL | ||||
| Phragmites australis | 18 nm | A549/72 | 129 μg/mL | [182] | |
| Centaurea behen | <50 nm | THP-1/24 | 25 μg/mL | [183] | |
| Cannabis sativa | 18.6 nm | MOLT-3 TALL-104/24 (Jurkat, Clone E6-1)/48 J.RT3-T3.5/72 | 329 μg/mL | [185] | |
| 381 μg/mL | |||||
| 502 μg/mL | |||||
| 567 μg/mL | |||||
| Curcumae | 8–25 nm | PA-1/48 | 153 μg/mL | [188] | |
| Kwangsiensis | SW-626/48 | 166 μg/mL | |||
| SK-OV-3/48 | 204 μg/mL | ||||
| Leaf extract | Mentha Longifolia | 36.4 nm | MCF-7/48 | 264 μg/mL | [150] |
| Hs 578Bst/48 | 269 μg/mL | ||||
| Hs 319.T/48 | 224 μg/mL | ||||
| UACC-3133/48 | 201 μg/mL | ||||
| Moringa oliefera | 35–51 nm | MCF-7/24 | 67.92 μg/mL | [151] | |
| Mimosa pudica | 12.5 nm | MCF-7/48 | 6 μg/mL | [154] | |
| MDA-MB-231/48 | 4 μg/mL | ||||
| Catharanthus roseus | 25–35 nm | HeLa/24 | 5 μg/mL | [157] | |
| Alternanthera Sessilis | 20–40 nm | HeLa/24 | Concentration-dependent cell death (10–15 μg/mL) | [155] | |
| Zataria multiflora | 10–42 nm | HeLa/48 | 100 μg/mL | [158] | |
| Abutilon indicum | 1–20 nm | HT-29/24 and 48 | 210 μg/mL | [175] | |
| 180 μg/mL | |||||
| Sasa borealis | 10–30 nm | AGS/24 | 120 μg/mL | [192] | |
| Stems | Atriplex halimus | 2–10 nm | MCF-7/48 | 47.03 μg/mL | [152] |
| Chenopodium amperosidies | ~40 nm | 22 μg/mL | |||
| Pulp extract | Dragon fruit | 10–20 nm | MCF-7/24 | 80% inhibition at highest dose (500 μg/mL after 48 h exposure) | [153] |
| MDA-MB-231/48 | No significant effect | ||||
| Annona muricata | 20–30 nm | Hep2, 24 | 10.94 μg/mL | [165] | |
| Ethanolic, aqueous | Taxus baccata | <20 nm | MCF-7/48 and 72 | Maximum cell mortality in Hela cells, followed by MCF-7 and Caov-4. | [159] |
| Caov-4/48 and 72 | |||||
| HeLa/48 and 72 | |||||
| Isolated from seaweed | Fuciodan | 31 nm | HepG2/24 | Maximum inhibition at 100 μg/mL | [162] |
| Biomass | Cordyceps | 15–20 nm | HepG2/24 | 10 and 12.5 μg/mL | [163] |
| Militaris | |||||
| Essential oil | Ferula persica | 37.05 nm | CT26/24 | 0.0024 mg/mL | [169] |
| Leaf powder | Cassia | 57 nm | Col320/24 | Maximum inhibition at 75 μg/mL | [170] |
| tora | |||||
| Fruit body | Ganoderma lucidum | 1–100 nm | HT-29/24 | 84.58 μg/mL | [176] |
| Aqueous peel extract | Musa paradisiaca | 50 nm | A549/24 | 58 μg/mL | [179] |
| Aqueous flower extract | Tribulus terrestris | 10–15 nm | THP-1/72 | 468 μg/mL | [184] |
| Plant extract | Scutellaria barbata | 0.4–1 μm | PANC-1/24, 48, and 72 | Maximum inhibition at 100 μg/mL | [189] |
| Marsdenia tenacissima | 50 nm | A549/72 | 15 μg/mL | [178] | |
| Seed extract | Elaeocarpus ganitrus | 30.34 nm | PC-3 | 64.23 μg/mL | [141] |
| Trachyspermum ammi | 16.63 nm | HepG2, 48 | 92.453 µg/mL | [164] | |
| Aqueous rhizome extract | Curcuma wenyujin | 200 nm | A498/24 | CC50 = 25 μg/mL | [190] |
| SW-156/24 | CC50 = 40 μg/mL | ||||
| Ethanolic extract | Vitex negundo | 30 nm | AGS/24, 48 and 72 | 15 and 20 μg/mL | [193] |
| Extract and fractions | Brazilian red propolis | 8–15 nm | T24/24 | AuNPs prepared from extract showed the highest cytotoxicity | [194] |
| PC-3/24 | |||||
| Aqueous root extract | Crocus sativus | 15 nm | PC-3/24 and 48 | AuNPs prepared from curcumin showed effective cytotoxicity at | [195] |
| - | Vibrio alginolyticus | 100–150 nm | HCA-7/24 | 15 μg/mL | [198] |
| Curcumin | 5–25 nm | MCF-7/36 MDA-MB-231/36 | Combinations of AuNPs showed higher anticancer activity as compared to individual AuNPs | [142] | |
| Turmeric | 3–20 nm | ||||
| Quercetin | 15–60 nm | ||||
| Crocin | 4–10 nm | MCF-7/24 | 1.8 mg/mL | [147] | |
| MCF-7/43 | 1.2 mg/mL |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sargazi, S.; Laraib, U.; Er, S.; Rahdar, A.; Hassanisaadi, M.; Zafar, M.N.; Díez-Pascual, A.M.; Bilal, M. Application of Green Gold Nanoparticles in Cancer Therapy and Diagnosis. Nanomaterials 2022, 12, 1102. https://doi.org/10.3390/nano12071102
Sargazi S, Laraib U, Er S, Rahdar A, Hassanisaadi M, Zafar MN, Díez-Pascual AM, Bilal M. Application of Green Gold Nanoparticles in Cancer Therapy and Diagnosis. Nanomaterials. 2022; 12(7):1102. https://doi.org/10.3390/nano12071102
Chicago/Turabian StyleSargazi, Saman, Ushna Laraib, Simge Er, Abbas Rahdar, Mohadeseh Hassanisaadi, Muhammad Nadeem Zafar, Ana M. Díez-Pascual, and Muhammad Bilal. 2022. "Application of Green Gold Nanoparticles in Cancer Therapy and Diagnosis" Nanomaterials 12, no. 7: 1102. https://doi.org/10.3390/nano12071102
APA StyleSargazi, S., Laraib, U., Er, S., Rahdar, A., Hassanisaadi, M., Zafar, M. N., Díez-Pascual, A. M., & Bilal, M. (2022). Application of Green Gold Nanoparticles in Cancer Therapy and Diagnosis. Nanomaterials, 12(7), 1102. https://doi.org/10.3390/nano12071102










