Abstract
Solid oxide fuel cells (SOFC) are promising, environmentally friendly energy sources. Many works are devoted to the study of materials, individual aspects of SOFC operation, and the development of devices based on them. However, there is no work covering the entire spectrum of SOFC concepts and designs. In the present review, an attempt is made to collect and structure all types of SOFC that exist today. Structural features of each type of SOFC have been described, and their advantages and disadvantages have been identified. A comparison of the designs showed that among the well-studied dual-chamber SOFC with oxygen-ion conducting electrolyte, the anode-supported design is the most suitable for operation at temperatures below 800 °C. Other SOFC types that are promising for low-temperature operation are SOFC with proton-conducting electrolyte and electrolyte-free fuel cells. However, these recently developed technologies are still far from commercialization and require further research and development.
1. Introduction
The growing global energy demand coupled with the need to reduce emissions of environmentally harmful greenhouse gases have resulted in the search for new clean alternative energy sources. In this regard, fuel cells are attracting great attention. These are efficient and silent electrochemical devices that directly convert the chemical energy of a fuel into electrical energy without the limitations of the Carnot cycle. There are several types of fuel cells: alkaline fuel cells (AFC), phosphoric acid fuel cells (PAFC), molten carbonate fuel cells (MCFC), proton exchange membrane fuel cells (PEMFC), and solid oxide fuel cells (SOFC) [1]. The high operating temperature of SOFC (400–1000 °C) gives them certain advantages over other types of fuel cells. SOFC can use a wide range of hydrocarbons as fuel. Catalysts based on noble metals (for example, Pt) are not required for SOFC operation. Waste heat can be reused by cogeneration, which increases the overall efficiency of the system based on SOFC up to 90% [2]. In addition, all SOFC components are made of hard materials; therefore, they are not limited to plane geometry and can be shaped to any form.
Intensive research of SOFC has been going on for three decades. During this time, many electrolyte and electrode materials have been studied, and a large number of SOFC configurations have been proposed and implemented. The first works [3,4] in which the SOFC classification was considered divided the fuel cells according to their geometry. Subsequently, the initial classification was complicated [5,6], and the division of the cells into groups began to be carried out according to several criteria: temperature, form, supporting component, etc. However, a number of concepts such as single-chamber SOFC [7] and electrolyte-free fuel cell [8] are not considered in [5,6]. Although the standard criteria are applicable for these concepts, new division parameters must be introduced to correctly and unambiguously describe all SOFC designs. The expansion of the existing classification will make it possible to order the data on the features of each SOFC type and facilitate orientation in their diversity. In addition, the systematization of SOFC designs will help to identify unused versions and possibly indicate ways to solve technological problems by combining or adopting approaches used in different SOFC configurations.
In the presented work, we tried to collect all currently existing types of SOFC and highlight their advantages and disadvantages.
2. Classification of SOFC
The SOFC classification will be carried out according to several criteria: presence/absence of electrolyte, gas spaces separation, operating temperature, support types, and cell design. Individual fuel cells but not stacks will be used as the subject of classification, although it should be recognized that in many cases the advantages and disadvantages of a particular design are manifested precisely when cells are assembled into a stack.
2.1. Classification according to the Presence/Absence of Electrolyte
It is considered that the conventional SOFC structure is a three-layer one consisting of porous electrode layers (anode and cathode) separated by a dense electrolyte layer. However, in the last decade, electrolyte-free fuel cells (EFFC) have been developed. Sometimes, they are also called electrolyte-layer-free fuel cells, single-component fuel cells (SCFC), or non-electrolyte separator fuel cells (NEFC).
Three-layer solid oxide fuel cells (TL-SOFC) are divided into two large classes according to the type of charge carrier in the electrolyte: oxygen ions or protons. SOFC with oxygen-ion-conducting electrolyte are the most developed and have reached the commercialization stage. Usually, the abbreviation SOFC means exactly this type of fuel cell, and it is used without any reservations, but sometimes, when compared with other variants, the oxygen-ion-conducting electrolyte SOFCs are designated O-SOFC [9]. SOFC with proton-conducting electrolyte, which are marked in the literature as H-SOFC or PCFC [9,10], were mentioned already in [4], but their intensive development has been observed only in the last decade.
In O-SOFC (Figure 1a), oxygen ions move through the electrolyte from the cathode to the anode under the influence of the oxygen chemical potential gradient. To ensure continuous migration of O2− across the electrolyte, the oxygen on the cathode side must enter the electrolyte lattice from the gas phase, leave the electrolyte lattice on the anode side, and react with fuel. The cathodic reaction of converting O2 to O2−, known as oxygen reduction, involves the absorption of electrons, whereas electrons, H2O, and CO2 form at the anode when hydrogen or hydrocarbon fuel interacts with O2− supplied by the electrolyte. (Figure 1 shows only hydrogen for simplicity). The electrons released in the fuel oxidation reaction through an external load move to the cathode to participate in the oxygen reduction reaction, thereby generating an electric current.
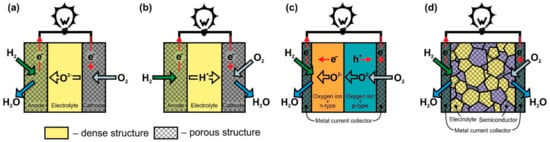
Figure 1.
Scheme of operation of (a) oxygen-ion conducting electrolyte SOFC (O-SOFC), (b) proton-conducting electrolyte SOFC (H-SOFC), (c) double-layer fuel cells (DLFC), and (d) single-layer fuel cells (SLFC).
The open-circuit voltage (OCV) of the fuel cell (when there is no current through the external load) depends on the gradient of the oxygen chemical potential from the cathode and anode sides, and the temperature and pressure in the system [11]. Fuel cell OCV is around 1.1 V at 900 °C with air as oxidant and hydrogen as fuel. When a current is passed, the voltage at the terminals of the fuel cell drops due to its internal resistance, which is the sum of ohmic, polarization, and concentration losses. The voltage on a load can be expressed as:
where I is the current, Ri is the ohmic resistance of the SOFC components, η is the voltage associated with polarization losses (overpotential), and ηconc is the voltage due to concentration losses. The electrolyte layer makes the main contribution to ohmic losses, since its conductivity is 2–3 orders of magnitude lower than that of electrode materials [4]. Polarization losses (or polarization resistance) are determined by the processes of current formation at the three phase boundary (electrolyte–electrode–gas), which depend on many parameters, such as composition, structure, physicochemical properties of the electrolyte and electrode materials, temperature, and oxygen partial pressure; in addition, they are largely determined by the morphology of the three phase boundary, which, in turn, is set by the prehistory and methods of making electrodes [12]. Concentration losses arise as a result of a change in the reagent concentration in the reaction zone due to the difficulty in delivering reagents (O2 and fuel) to the reaction site and the removal of reaction products (H2O, CO2) through a porous electrode. Concentration losses are small when high porosity and small thickness of the electrodes. The internal resistance of the SOFC should be minimized as much as possible to achieve high specific power.
Most often, oxide materials with a fluorite structure such as Y2O3 or Sc2O3 stabilized ZrO2 (YSZ or ScSZ, respectively), and Gd2O3- or Sm2O3-doped CeO2 (GDC or SmDC, respectively) are used as electrolytes for O-SOFC [13,14,15]. In the overwhelming majority of cases, a composite based on Ni is used as an anode material [16,17,18], and the most commonly cathode materials of O-SOFC are La1-xSrxMnO3 (LSM) [19] and La1-xSrxCo1-yFeyO3 (LSCF) [20]. At the same time, extensive research isbeing conducted to find new electrode materials [6,21,22,23,24,25]. Another area of research that has the prospect of improving SOFC performances is the creation of nanostructures [26,27]. In particular, the introduction of nanosized dense layers with mixed ion-electron conductivity at the cathode–electrolyte interface can significantly reduce the polarization resistance [28,29]. Nanostructuring of electrodes also results in an increase in their catalytic activity and allows the direct use of hydrocarbon fuels [30,31,32,33].
A single SOFC is not suitable for practical use due to its low OCV; therefore, individual cells are connected in a stack to generate a sufficiently high voltage and power. The connection is made using a component called interconnect, which must possess purely electronic conductivity (without oxygen-ion conductivity). The interconnect makes contribution to the internal resistance of the SOFC stack and is an important component together with the anode, cathode, and electrolyte. Consequently, the development of interconnections is also given much attention [6,34,35].
The operation principle of proton-conducting electrolyte SOFC (Figure 1b) is similar to the one of O-SOFC. The difference is that when the fuel is oxidized at the anode, H+ enters the electrolyte lattice, and after transferring through the electrolyte, it takes part in the oxygen reduction reaction with the formation of water. It is believed that the formation of water on the cathode side is the advantage of H-SOFC, since, in this case, there is no fuel dilution at the anode. In addition, proton-conducting materials such as SrCeO3, BaZrO3, and BaCeO3 exhibit higher conductivity than that of YSZ or GDC at temperatures of 350–600 °C due to the relatively low activation energy of proton migration in solid oxides [36,37]. Thus, H-SOFC must have a higher power than O-SOFC at low temperatures. However, the properties of the electrolytes and electrodes still have to be improved to completely implement this concept. The main issues associated with the development of proton-conducting electrolytes are to increase chemical stability (prevent interaction with CO2 and H2O), improve sinterability, and suppress electronic conductivity [38,39]. The greatest hopes for a decrease in polarization losses in H-SOFC are pinned on the development of a cathode material with mixed oxygen-ion-proton-electron triple conductivity [40]. Presently, H-SOFC research is being conducted at the laboratory level with hydrogen as a fuel [38,39].
Electrolyte-free fuel cells can be divided into two classes according to the number of layers of dissimilar materials used to them fabrication: double-layer fuel cells (DLFC) and single-layer fuel cells (SLFC).
The DLFC concept (Figure 1c) was proposed by B. Zhu’s group in [41] and developed in [42]. These are the only publications that we were able to find on this construction. DLFCs are formed from two materials with mixed oxygen-ion and electronic conductivity of n- or p-type. In Ref. [41], the anode and cathode layers were formed from composites to achieve the desired properties of materials. The p-n junction formed at the interface between the two materials prevents the transfer of electrons through the structure of the fuel cell and, in fact, acts as an electrolyte layer with oxygen-ionic conduction in the TL-SOFC.
The SLFC idea was proposed in [43] in 2000. It was based on the assumption that one material can perform the functions of all SOFC components (anode, electrolyte, cathode) due to different types of conductivity at different oxygen partial pressures. The conception was tested on La0.9Sr0.1InO3-δ, which has oxygen ionic conductivity, but at the same time, has p-type conductivity in an oxidizing atmosphere and n-type in a reducing atmosphere. The specific power of Pt/La0.9Sr0.1InO3-δ/Pt cell at 800 °C was 3 mW∙cm−2. B. Zhu et al. changed the approach to the formation of SLFC functional layer by making it a porous nanocomposite from materials with oxygen-ionic and semiconducting conduction (Figure 1d) [41,44]. To date, various two- and more-phase composites from materials with different conductivity types have been used for SLFC fabrication. A review of the materials can be found in [8,45]. In particular, ceria–carbonate electrolytes possess H+ and O2− conduction [41,46]; therefore, in Figure 1d the formation of H2O on the cathode side is shown.
The development of SLFC has been going on for ten years, but the operation principle is still not entirely clear. Two main mechanisms have been proposed to explain the effect of blocking the electron flow through the SLFC functional layer [47,48,49]. The first mechanism consists of the formation of a p-n bulk heterojunction in the center of the composite layer due to the fact that, when exposed to hydrogen and air, electrons and holes concentration zones appear near the fuel and oxidizing current collectors, respectively [47]. The second mechanism is associated with the formation of a Schottky junction between the semiconductor component of the functional layer and the metal current collector on the SLFC anode side [48]. In addition, the role of metal current collectors, which are most often made of Ni and Ag, remains unclear. Do they only serve to transport electrons or also function as electrodes in the SLFC? Nevertheless, it should be recognized that the elimination of the electrolyte layer from the fuel cell design gives the EFFC the following advantages over the conventional three-layer SOFC: (1) ease of manufacture, since only one layer needs to be formed, and (2) the problems of thermomechanical matching of the components are excluded. The developers also declare that polarization losses are reduced because there is no fixed electrode–electrolyte interface. Comparison of the characteristics of SLFC and TL-SOFC made from the same materials indicates that a single-layer structure has similar or even little higher specific power values than a three-layer structure [41,46,48].
A comparison of features of SOFC designs with different electrolytes and without ones is presented in Table 1.

Table 1.
Features of O-SOFC, H-SOFC, and FEFC.
2.2. Classification according to the Gas Spaces Separation
SOFC can be divided into three groups according to the criterion of supply of gas reagents: dual-chamber SOFC (DC-SOFC); single-chamber SOFC (SC-SOFC), which are also called “one-chamber”, “mixed-fuels”, or “mixed-reactant”; and no-chamber solid oxide fuel cells, which are most often called direct-flame SOFC (DF-SOFC) or flame fuel cells (FFC) (Figure 2).
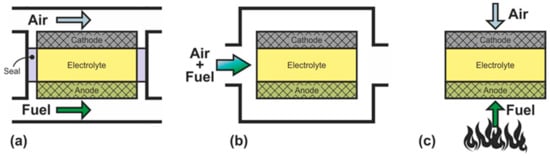
Figure 2.
Schematics of (a) dual-chamber SOFC, (b) single-chamber SOFC, and (c) no-chamber SOFC.
In DC-SOFC, the reactants are separated: the oxidant is fed to the cathode, and the fuel is fed to the anode without any mixing (Figure 2a). The operation principle of this configuration was discussed in detail above. It is only recalled that the electromotive force arises by the gradient of the oxygen partial pressure between the separate electrode chambers. Dual-chamber SOFC are considered to the conventional design, and the abbreviation SOFC usually denotes separate-reactant solid oxide fuel cells.
In SC-SOFC, a mixture of fuel and oxidizer is fed into the working chamber (Figure 2b) [50]. In this case, the operation principle is based on the selectivity of the electrodes for the respective reactions. The anode must be electrochemically active for fuel oxidation and inert to oxidant reduction, and the cathode must exhibit selective oxygen reduction and inertness to fuel. The open circuit voltage in SC-SOFC depends on both the electrocatalytic activity and the selectivity of the electrodes. Specific designs of fuel cells can be implemented because there is no need to hermetically isolate the electrodes from each other: SC-SOFC with coplanar electrodes or single-face SC-SOFC and fully porous SOFC (FP-SOFC) or all porous SOFC (Figure 3). In the design with coplanar electrodes (Figure 3a), the electrodes are formed on the same side of the electrolyte, which simplifies the fabrication of SC-SOFC, increases its thermomechanical stability, and allows the formation of several elements at once [51]. In FP-SOFC (Figure 3b,c), the electrolyte layer between the electrodes is porous, which makes the construction cheaper due to low electrolyte sintering temperatures [7]. However, a significant drawback of the specific SC-SOFC designs is the low specific power amounting to 1–40 mW·cm−2 at 750 °C [7,52,53]. Only in [54] was a specific power higher than 200 mW·cm−2 at 750 °C obtained for FP-SOFC. Moreover, in SOFC with coplanar electrodes, a strong degradation of characteristics is observed [7].
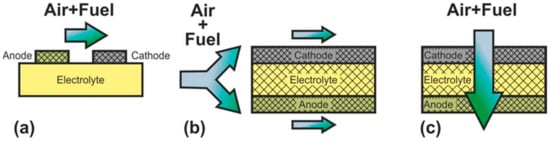
Figure 3.
Schematics of (a) SC-SOFC with coplanar electrodes and fully porous SOFC in (b) flow-by and (c) flow-through configuration.
In a mixed-reactant design proposed by M. Horiuchi et al. [55], the fuel cell is placed directly above the burning flame (Figure 2c). The anode is close to the fuel-rich flame, and the cathode has access to ambient air. In this case, the flame provides the fuel cell with heat, carries out the reforming of carbon-hydrogen fuel, and sets the difference in the oxygen partial pressure between the two electrodes by consuming oxygen at the anode. The operation principle of the DF-SOFC is close to the operation principle of the SC-SOFC since gas separation is not required. However, requirements for the selectivity of the catalysts are reduced because the DF-SOFC electrodes are placed in different atmospheres.
The mixed-reactant fuel cells (SC-SOFC and DF-SOFC) have several advantages over DC-SOFC [7,56], especially for small devices. The absence of the need to separate gas spaces results in increased thermomechanical stability and a simpler and compact design both of the fuel cell and the gas manifold. This, in turn, makes the fabrication of a single cell, and its collection in the stack is easier, whereas the formation of necessary effective sealing at high temperatures for separate-reactant SOFC is a challenge [57,58]. Moreover, the rigid connection of the cell to other stack components can result in mechanical stress and even breakage. Another advantage of mixed-reactant SOFC over DC-SOFC is the ability to maintain the operating temperature without the need to supply additional heat from outside. Herewith, DF-SOFC have a number of other advantages: the ability to use almost any hydrocarbon fuel, including gases, liquids, and solids, quick start-up, and the problem of the porous electrodes coking is less serious than that in SC-SOFC.
On the other hand, mixed-reactant SOFC have serious drawbacks that impede their practical use [7,56]. Electrode selectivity plays a key role in the functioning of SC-SOFC. However, fully selective materials have not been found yet. In particular, all SOFC cathode materials catalyze methane oxidation [59]. Due to parasitic reactions occurring at the electrodes, SC-SOFCs have a low electrical efficiency (~1%), as well as a low level of fuel utilization (about 10%) [7]. However, it has recently been shown that the use of a nanocomposite consisting of materials with different functions as a cathode can significantly increase its selectivity and thereby increase the efficiency of the entire SC-SOFC [60]. The electrical efficiency of DF-SOFC is even lower (0.45% [61]), which is associated not so much with the electrode selectivity but with the fact that the fuel is consumed in the combustion reaction. In addition, the significant material stresses arising from thermal load associated with placing the cell near an open flame are a particular problem for DF-SOFC. The use of an air/fuel mixture in SC-SOFC is a risk of ignition and/or explosion. Therefore, in SC-SOFC, hydrogen is not used, and most often, methane is used as a fuel. The separate-reactant SOFC are much safer and have significantly higher electrical efficiency (up to 60% [62]) and a level of fuel utilization (about 80% [62]). Apparently, precisely this huge difference in the efficiency, as well as the immaturity of the technology, are the reason why the mixed-reactant SOFC are not even mentioned in the classifications proposed in [3,4,5,6].
Table 2 summarizes the advantages and disadvantages of DC-SOFC, SC-SOFC, and DF-SOFC.

Table 2.
Features of DC-SOFC, SC-SOFC, and DF-SOFC.
Currently, DF-SOFC are fabricated based on oxygen-ion-conducting electrolytes [56]. To make SC-SOFC, oxygen-ion conducting electrolytes are also used in most cases [7], but there are single works on the use of proton conducting electrolytes (for example, [63,64]). It is obvious that EFFC operation in the condition of mixed reactants is impossible unless the current collectors possess selectivity to various reactions.
At the end of this section, it is worth mentioning the so-called flame-assisted fuel cells or flame fuel cells (FFC) [65,66,67]. This concept implies two devices integrated with each other: the combustion system and the SOFC itself. The premixed combustion system avoids complete oxidation of the fuel with excess air, which is present in conventional DF-SOFC. As a result, more fuel enters the SOFC anode for electrochemical power generation. Herewith, the air is separately supplied to the fuel cell cathode. Thus, from the point of view of classification, the SOFC operates in a dual-chamber mode. The FFC concept allows the use of a hydrocarbon fuel without any catalysts. However, the efficiency and level of fuel utilization of the FFC are low, although higher than those of the DF-SOFC. The highest electrical efficiency of 6% and fuel utilization coefficient of 23% of FFC have been achieved in [68].
2.3. Classification according to Operating Temperature
The first SOFC operated at temperatures of 900–1000 °C [3]. High operating temperatures ensured a low internal resistance of the fuel cell due to the high conductivity of the electrolyte and a high rate of electrode reactions and, accordingly, high specific power as well as the possibility of internal reforming of hydrocarbon fuel. However, high operating temperatures also cause a number of problems related to sealing, the morphological stability of electrodes, the chemical stability of cell components, and the heat resistance of accessories. These problems result in a high cost of cells and a reduction in their lifetime. Therefore, a strategy to reduce the operating temperatures of SOFC was adopted. Lower operating temperatures allow the use of new materials (in particular, steel interconnects [34]), reduce the SOFC cost, reduce degradation, and implement faster start-up.
At the present time, SOFC are usually divided into high-, medium-, and low-temperature categories. However, there is still no consensus on temperature ranges. In works [69,70,71] SOFC are divided only by medium temperature (500–750 °C) and high temperature (above 750 °C). The authors of [72] consider that the definitions of “low-temperature” and “medium-temperature” are a synonyms, and 800 °C is the upper limit of this temperature range. The majority of researchers dividing SOFCs into three temperature classes also define 800 °C as the boundary of medium–high temperatures [6,73,74,75]. Herewith, the boundary between low and medium temperatures varies: in [6,74] and [73,75], 650 and 600 °C are marked, respectively. It should be noted that, usually, the physical reasons for choosing a particular temperature as the range boundary are not explicitly indicated, which, most likely, is the reason for the differences.
The temperature of 800 °C between high- and medium-temperature ranges, accepted by most authors, implies the upper limit of the expediency of using steel interconnects for the stack manufacture [34]. In Ref. [70], it was proposed to make the possibility of implementing internal reforming of methane as a criterion for determining the lower operating temperature of SOFC. The authors of [70] decided that 500 °C is the lowest temperature at which methane internal reforming can occur on a suitable catalyst (catalyst was not specified), although it was recognized that this temperature is controversial. In a recent review [76], it was shown that methane internal reforming at the most common Ni-based anode can occur only at temperatures above 600 °C. Therefore, we propose to set the temperature of 600 °C as the boundary of the medium- and low-temperature region, thereby dividing SOFC into cells that can directly use methane as fuel and cells that require external reforming. Thus, in this work, SOFC operating in temperature ranges above 800 °C, from 600 to 800 °C, and below 600 °C are considered high-, medium-, and low-temperature SOFC, respectively.
The above arguments for lowering the operating temperature generally refer to well-studied separate-reactant O-SOFC. Alternative designs of SLFC, DLFC and H-SOFC have also been developed to reduce operating temperatures. H-SOFC have higher specific powers in the low-temperature region than those of O-SOFC due to more conductive electrolytes. The temperature range of H-SOFC research is 450–750 °C, and the maximum specific power of H-SOFC at 700 °C reaches ~1000 mW∙cm−2 [77,78]. The maximum temperature of SLFC testing also did not exceed 750 °C, and the obtained maximum specific powers at 550 °C varied in a wide range, from 200 to 1000 mW∙cm−2 [45]. DLFC studies were carried out at temperatures of about 550 °C, and the maximum specific powers were 560 and 280 mW∙cm−2 [41,42].
The development of mixed-reactant SOFC was aimed at simplifying the design and did not imply a decrease in operating temperature. The operating temperatures of SC-SOFC vary from 300 to 950 °C [7], and ones of DF-SOFC vary from 400 to 850 °C [79,80,81]. The data on the maximum specific power of SC-SOFC and DF-SOFC presented in the literature have a significant scatter from tens to several hundred mW∙cm−2. Their comparison is difficult since the power of mixed-reactant SOFC depends on not only the operating temperature, structure, and materials of the fuel cell, but also on the type of hydrocarbon fuel and the fuel–oxidizer ratio.
2.4. Classification according to Support Types
SOFC according to supporting component are usually divided into two large groups: self-supporting and external-supporting [5]. In a self-supporting SOFC, one of the components of the fuel cell (electrolyte, anode, or cathode) is the supporting element. In external-supporting SOFC, the supporting element is a porous inert substrate or a metal interconnect. Note that, due to the inherent feature of the design, the supporting substrate of the SC-SOFC can be gas-tight. SOFC schemes with different supporting components are shown in Figure 4.
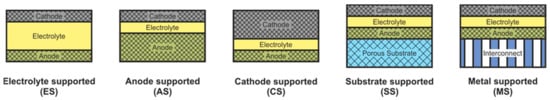
Figure 4.
Different types of cell support architectures for SOFC.
The advantages and disadvantages of SOFC with different supporting components are presented in Table 3 [5].

Table 3.
Features of SOFC with the different supporting components.
The supporting component in the overwhelming majority of the first SOFC was an electrolyte, and its thickness was about 0.2 mm [3,4]. The electrolyte-supported (ES) structure has relatively high strength and therefore has a low probability of mechanical failure, including due to re-oxidation of cermet anode based on Ni. However, the supporting electrolyte layer makes a significant contribution to the internal resistance of the SOFC. Since the conductivity of electrolytes has an exponential dependence on temperature [13], operating temperatures above 800 °C are required to achieve high specific power. Nevertheless, even now, the electrolyte supported design is popular among SOFC manufacturers [82,83,84].
The above-described tendency towards a decrease of the SOFC operating temperature resulted in the need to reduce the electrolyte thickness and transfer the function of the mechanical support to another component. The most widespread at present is the anode-supported (AS) structure of SOFC [85,86,87,88,89], which allows achieving high specific power at temperatures below 800 °C due to the thin electrolyte layer and high conductivity of the Ni-based anode [18]. In addition, this design is cheaper, because the NiO cost is lower than that of electrolyte and cathode materials. However, a significant drawback of the AS design is the possibility of anode re-oxidation, which is accompanied by a volume change of nickel by 41% [16] that can cause mechanical stresses and cell failure. Another problem of electrode supported designs is the limitation of the transfer of gas reagents through the thick, porous layer to the three-phase boundary, which can degrade the characteristics of the cell. In the anode supported structure, this problem is less acute than in the cathode supported (CS) one due to the already mentioned volumetric change of nickel during reduction. Moreover, the conductivity of cathode materials is lower than that of cermet anodes [90], which results in larger internal losses and, accordingly, to a lower specific power of CS SOFC in comparison with AS SOFC. The advantages of the cathode-supported structure include the phase stability of the supporting element (no oxidation–reduction cycles) and low ohmic losses on a thin electrolyte layer. CS SOFCs are not widely used, although this design was used to create the first kW-class generators developed by Simence/Westinghose [91]. It should be noted that the operating temperatures of industrial generators based on CS SOFC were above 900 °C.
The inert substrate-supported (SS) design allows the formation of thin electrolyte and electrode layers. This has to assistant in reducing the operating temperature and reaching of the high specific power of SOFC. Herewith, the supporting substrate can be made of a cheap material that is not usually used in the SOFC. In addition, the supporting substrate can be used as a carrier for a catalyst, allowing the conversion of hydrocarbon fuel into syngas [92]. On the other hand, the introduction of additional material into the SOFC composition increases the complexity of its design and manufacturing technology. Discontinuity of thin functional layers, which is very likely when they are formed on a porous substrate, can result in fuel cell failure. Despite these issues, industrial plants based on SS SOFC have been implemented [93,94] with operating temperatures above 900 °C.
The metal-supported (MS) design is attracting interest because of not only the low operating temperatures and potentially high specific power obtained by thin functional layers but also the high strength and electronic conductivity of the supporting component. However, the development of a technology of MS SOFC fabrication is not an easy task. A high sintering temperature is required for the formation of ceramic materials, while the metal substrate must not be overheated. Other serious problems of this design are the corrosion of the metal substrate under the SOFC operating conditions and the complexity in sealing operations. Advances in the development of interconnect supported SOFC are presented in [95,96]. The target operating temperatures of the MS SOFC are below 800 °C, but there are data on their testing at 850 °C [95].
The overwhelming majority of studies of H-SOFC are carried out on cells having an anode supported construction, which allows obtaining the highest power [39,78,97,98]. Only a few works were performed on cells with a supporting electrolyte [99] and supporting metal [100,101]. All studies of FEFC are currently performed on button cells, which can conditionally be classified as an electrolyte-supported design since the functional layer has a thickness of about 0.5–1 mm. The electrolyte- and anode-supported designs are manly used for DF-SOFC fabrication. A comparison of these two designs carried out in [79] showed that the maximum specific power of AS DF-SOFC (~475 mW∙cm−2) is almost four times higher than that of ES DF-SOFC (~121 mW∙cm−2). However, there are a few works are devoted to the development of metal supported DF-SOFC [81,102]. In Ref. [81], the peak specific power of 633 mW cm−2 was achieved on laboratory samples of MS DF-SOFC, whereas the specific power of the stack prototype tested on a commercial camping stove reached only 156 mW cm−2 [102], which is close to the one of ES DF-SOFC. Mostly, electrolytes and anodes are used as supporting components in SC-SOFC too [7,60,103,104,105]. In the single work [106] devoted to cathode-supported SC-SOFC, the peak specific power of only 9 mW∙cm−2 was obtained, whereas in AS SC-SOFC, power values of the order of 200–400 mW∙cm−2 are achieved [104,105]. A recent numerical simulation [107] has shown that the characteristics of a cathode-supported SC-SOFC should be less than those of an anode-supported SC-SOFC due to the difficulty of oxygen passing through the cathode layer to the reaction zone. The attempt of SC-SOFC forming on supporting dense substrates of MgO and stainless steel, which can be considered a supporting interconnect, was made in [108]. However, the internal resistance of the cells was very high. A numerical estimate of the residence time of the gas mixture in the cell has demonstrated that a structure with a supporting dense substrate for SC-SOFC is impractical [59]. No works describing SC-SOFC with a supporting porous substrate were found.
2.5. Classification according to Cell Design
Different geometric shapes of SOFC will be considered at the example of dual-chamber O-SOFC, since this group of fuel cells has the largest number of design options. Discussion of forms of other SOFC types will be carried out based on O-SOFC designs.
In accordance with the design, SOFC can be divided into planar, tubular, flat-tubular, and monolithic (Figure 5).
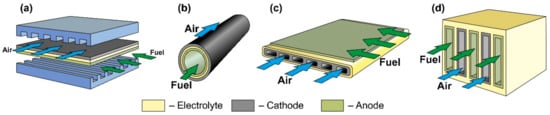
Figure 5.
Schematics of (a) planar, (b) tubular, (c) flat-tube, and (d) monolithic SOFC.
The planar design (Figure 5a) is the most common due to the ease of manufacturing cells and assembling them in a stack, relatively low cost, and high specific volumetric power achieved by close packing of cells and low ohmic losses on interconnects. The disadvantages of the planar design are the difficulty in forming a hermetic seal between the anode and cathode chambers at stack assembly, as well as low resistance to thermal stress. An anode is most often the supporting elements of planar SOFC, but an electrolyte is also a common support. Examples of commercial applications of AS and ES planar SOFC are presented in [70,83]. Planar SOFC with supporting metal are also used in kW class generators [109], whereas the research and development of CS and SS planar cells are carried out at the laboratory level (see, for example, [110,111]).
The tubular SOFC design (Figure 5b) ranks second in popularity [112], although the first stacks were assembled on tubular cells [3,4]. Its advantages include ease of sealing, as well as higher mechanical strength and higher resistance to thermal stress due to the symmetric circular geometry. On the other hand, tubular SOFC have a lower specific volumetric power than planar cells due to less dense packing and larger internal losses associated with long paths of connecting cells in a stack. Moreover, the manufacturing process of the tubular cells is more expensive. Today, as well as for the planar cells, the supporting anode design is the most widespread architecture of tubular SOFC [88], having displaced the cathode-supported design from the leading position. Although, as already mentioned, the first large stacks were assembled on tubular CS SOFC [91]. The tubular design with supporting porous substrate has also been commercially implemented [94], whereas there are few works on ES and IS tubular cells [113,114].
The flat-tube SOFC design (Figure 5c) is essentially a hybrid of planar and tubular ones and it is elaborated to combine the advantages of both SOFC types. In flat-tube SOFC, the sealing is carried out more easily than in a planar design, and the simplicity of assembling cells to a stack is preserved. At the same time, the specific volumetric power of the flat-tube design is higher than that of a tubular one, whereas high resistance to thermal stress is continued. However, the manufacture of flat-tube SOFC should be more complicated and expensive than of planar cells. As the supporting element for a flat-tube design, a cathode (we consider the DELTA design as flat-tube one) [91], an anode [115,116], and a porous substrate [117] are used. A detailed description of materials, fabrication methods, and characteristics of flat-tube SOFC can be found in a recent review [118].
The scheme of a monolithic SOFC design is shown in Figure 5d. It is necessary to clarify that, in works [4,5], where the classification by design was performed jointly for cells and stacks, the term “monolithic design” has a different meaning than in [3]. In Ref. [3], it was believed that the basis of a monolithic fuel cell is a supporting electrolyte with a system of gas channels on the walls of which electrodes are applied (Figure 5d). The electrodes of adjacent channels have opposite signs; therefore, such a monolithic SOFC can be considered a stack of parallel-connected cells. In Refs. [4,5], a monolithic design was meant as a stack assembled from several series-connected corrugated cells that formed gas channels (Figure 6a). In this work, we will adhere to the terminology of [3], since cells and not stacks are classified. In addition, the so-called honeycomb SOFC (Figure 6b) [119,120], which are often distinguished by a special design [75], correspond to the taken definition of a monolithic design. The monolithic design advantages are high thermomechanical strength and a quite high specific volumetric power. However, this design is troublesome to manufacture and has difficulties in sealing and organizing current collectors. Perhaps this is the reason for the small number of works devoted to monolithic SOFC. All investigated monolithic SOFCs had a self-supporting structure, with a supporting electrolyte [119,121], a cathode [120], and an anode [122]. A metal interconnect can be used as a supporting element for a monolithic SOFC, but this will further complicate the fabrication. Applying a supporting porous substrate is apparently impossible without changing the concept of a monolithic SOFC, since the internal contact between adjacent channels will be broken.
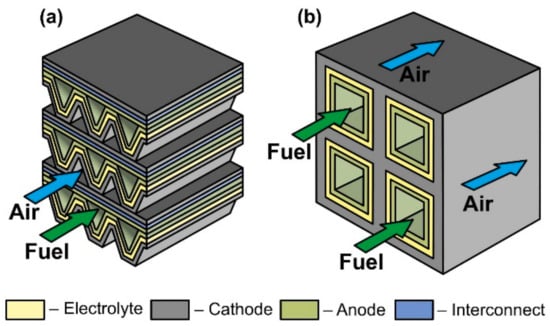
Figure 6.
Schematics of (a) monolithic stack and (b) cathode-supported honeycomb SOFC.
Table 4 summarizes the advantages and disadvantages of the four main SOFC designs.

Table 4.
Features of the different SOFC designs.
Among planar and tubular SOFC, microplanar and microtubular SOFC are distinguished, the development of which was carried out with an eye to mobile applications. The accentuation of these designs into separate groups is associated not only with the size of the fuel cells but with the features that arise when the size is reduced.
At the initial development stage of the concept of microplanar SOFC, these cells were usually called simply micro-SOFC [123], whereas now, they are commonly marked to as thin-film SOFC (TF-SOFC) [124,125]. In FT-SOFC, in contrast to large planar cells, the electrolyte layer thickness does not exceed 1 μm, which makes it possible to greatly reduce the operating temperature. The small electrolyte thickness is achieved due to the fact that the electrochemical part of the TF-SOFC is formed on the supporting substrate (Figure 7). There are two configurations of TF-SOFC: free-standing (Figure 7a) and porous substrate supported (Figure 7b).
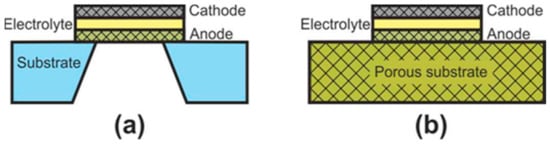
Figure 7.
Schematics of (a) free-standing FT-SOFC and (b) porous substrate supported FT-SOFC.
In free-standing TF-SOFC, an anode–electrolyte–cathode structure is formed over a hole in an inert material substrate (such as a silicon wafer). The main advantage of this structure is the use of very thin electrolytes with a thickness of tens of nanometres [125,126,127] that allows a reduction in the operating temperatures of TF-SOFCs to 300–500 °C. The highest peak power of free-standing TF-SOFCs of 1.3 W∙cm−2 at 450 °C [126] was achieved due to the combined effect of using a 60 nm-thick electrolyte and an increased effective area formed due to the three-dimensional architecture of the cell. However, in most works, the peak power is very modest, averaging 200–400 mW∙cm–2 [123,125]. In addition, free-standing TF-SOFC have a number of disadvantages: the warping of films during fabrication can result in to cracking; the low mechanical strength of the cathode–electrolyte–anode structure; the small active area of a single cell; manufacturing complexity; and the problem of scaling. Apparently, these drawbacks mean that this type of SOFC is practically not being developed now, although the work [128] proposes a manufacturing method of free-standing metal-supported TF-SOFC. Unfortunately, the cell characteristics are not given.
The fabrication of porous substrate supported TF-SOFCs is much simpler than that of free-standing TF-SOFC and consists of the serial formation of electrode and electrolyte layers on a substrate. The main technical issue at porous substrate supported TF-SOFC fabrication is to avoid the formation of defects in thin functional layers when they are deposited on a rough surface of the substrate. Therefore, material of the substrate is usually either a NiO-based composite, the porosity of which is formed/increased upon nickel reduction [123,125,129,130], or nanostructured anodized aluminum oxide (AAO) [123,125,131]. There are a number of works devoted to the development of metal supported TF-SOFC [132,133]. The values of specific power of porous substrate supported TF-SOFCs vary greatly in the literature, since the cells differ not only in the thickness of the electrolyte but also in the electrode materials. Most often, Pt is used as an electrode due to its low operating temperatures [123,127]. However, the development of nanostructured electrodes allows to abandon noble metals and achieve high SOFC characteristics [134,135,136]. The peak power of ~2.5 W∙cm−2 at 650 °C was achieved with a AAO-supported Ni-YSZ | YSZ | GDC | LSCF-YSZ cell with a thickness of about 4 µm [135].
From a classification point of view, a free-standing TF-SOFC is SS SOFC design and a porous substrate supported TF-SOFC can be AS, SS, and MS SOFC depending on the substrate material.
Microtubular SOFC are tubular cells, the outer diameter of which is less than 3 mm. This results in a higher specific volumetric power of the stack and a significant increase in thermal shock resistance [137,138]. Increased thermomechanical characteristics of microtubular SOFC ensure quick start-up and high resistance to thermal cycling. The disadvantages of microtubular SOFC are mainly related to their being assembled in a stack: (1) construction issues at the organization of the current collection and connecting individual cells with each other [139] and (2) sealing of the stack [140]. Nevertheless, in the last decade, microtubular SOFC, due to their advantages, have attracted more attention than standard tubular cells [112,138]. The most common supporting element for microtubular SOFC as well as for tubular cells is an anode [141,142]. Microtubular SOFC with other supporting elements are also researched but there are much fewer works [143,144,145,146]. In addition, nanotube SOFC with an outer diameter of less than 500 nm have been fabricated; however, the obtained specific power was very low (1.3 μW∙cm−2 at 550 °C) [147].
Currently, almost all studies of mixed-reactant fuel cells (DF-SOFC and SC-SOFC) have been carried out on button cells. There are only a few works on the use of microtubes to investigate SOFC in a single-chamber regime [148]. H-SOFCs are also studied mainly in the form of button cells [38,39]; however, there are several works to obtain sufficiently large anode supported planar cells [149,150]. In addition, AS H-SOFC were fabricated in tubular [151,152], thin film [153], and microtubular [78,154,155] designs.
3. Systematization of SOFC
Figure 8 shows a summary scheme of all types of SOFC realized to date. It should be emphasized that the previously considered SOFC divisions are equal, and the presented scheme does not have a strict hierarchical structure. The established dominance of the attributes by which the division was made is rather arbitrary, but we hope that this scheme clearly reflects the current state of developments in the SOFC field. The difference between the “button cell” and “planar SOFC” icons is the size of the cells. “Button cell” denotes small samples used in laboratory research, and “planar SOFC” denotes a large planar cell (linear dimension of several cm) suitable for making stacks. It can be seen that EFFC, mixed-reactant O-SOFC, and H-SOFC are still at the development stage, whereas dual-chamber O-SOFC are already used to manufacture industrial generators. Unfortunately, it is impossible to reflect in the scheme the number of works devoted to a particular type of SOFC design and, consequently, its demand. Single works concern DLFC [41,42], single-chamber H-SOFC [63,64], ES and MS H-SOFC [99,100] and CS O-SOFC [91,110,120,144], whereas the fabrication and testing of ES and AS O-SOFC is described in thousands of works. The temperature range for each type of SOFC in the scheme covers all data presented in the literature. On the one hand, this shows the temperature borders within which a certain SOFC type can function. On the other hand, this does not give a representation of the optimal operating temperature of this SOFC type. For example, the upper border of the SLFC operating range was set at 750 °C in accordance with [46], although studies of the characteristics of SLFC have mainly been carried out at 550 °C. Nevertheless, Figure 8 shows that the most well-developed O-SOFC used industrial generators operate at temperatures of 800 °C and above. Only AS O-SOFC stacks operate at lower temperatures [88,115]. Alternative designs such as EFFC and H-SOFC are promising for reducing operating temperatures; however, research and development are needed to reach a more mature state of these technologies.
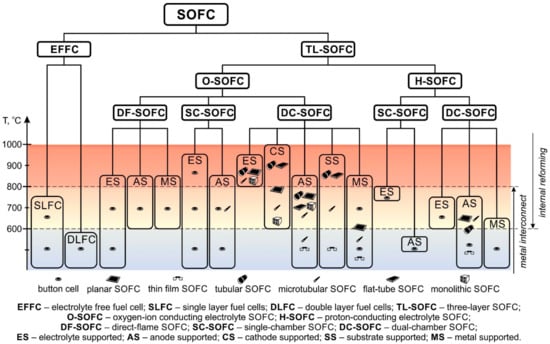
Figure 8.
Systematization of SOFC types.
4. Separate Designs and Concepts of SOFC
Several separated SOFC designs and concepts which do not mention earlier are presented in the literature. At the end of the review, these types of SOFC will be briefly considered, and their place in the proposed classification will be defined.
Sometimes, together with flat-tube and honeycomb SOFC, such designs as segmented-in-series or integrated planar SOFC and cone-shaped SOFC are discussed (Figure 9) [75]. Short current collectors inherent in these designs allow reducing the weight and cost of a fuel cell as well as improving its performance by low ohmic losses associated with the connection of the cells with each other. However, it must be emphasized that this is not some new type of separate cell design but a technique for connecting cells into a stack. The cone-shaped cells are a kind of tubular SOFC, whereas the segmented-in-series design was fabricated in both flat [93] and tubular [94] geometries.
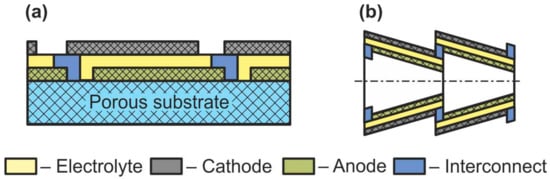
Figure 9.
Schematics of (a) segmented-in-series SOFC and (b) cone-shaped SOFC.
The concept of symmetric SOFC (SSOFC) is to replace different electrode materials (anodic and cathodic) of conventional SOFC on one material [156]. This simplifies and reduces the cost of fabricating fuel cells, since both electrodes can be fired in one thermal cycle. In addition, the use of the same material for the anode and cathode diminishes the problems of thermomechanical compatibility of SOFC components by the formation of the same electrode–electrolyte boundaries. Another advantage of the symmetric SOFC concept is the ability to solve the issues associated with sulfur poisoning and carbon deposition by changing the direction of gas flows to oxidize these substances. Any design of a dual-chamber SOFC is suitable for the implementation of SSOFC concept, since the term “symmetric” means the same electrode materials and not the configuration of the cell itself. However, mixed-reactant SOFC with identical electrodes will not function, since it is impossible for one material to have selectivity to different reactions. The development of an electrode material that must simultaneously satisfy all the requirements for cathode and anode of SOFC is an obstacle to the realization of symmetric SOFC [156,157,158].
Another noteworthy concept is reversible SOFC (RSOFC or RSOC), which implies that a solid-state electrochemical device can operate both in the fuel cell mode and in the electrolysis mode [159,160]. In the first mode, RSOFC operates on the SOFC principle converting fuel into electricity. In the second mode, the RSOFC operates as a solid oxide electrolysis cell (SOEC), consuming energy and generating hydrogen (fuel) from water. Thus, RSOFCs can “preserve” excess electricity in the form of chemical energy of the produced substances (mainly hydrogen) and, if necessary (during peak electricity demand), convert the fuel back into electricity. As in the case of SSOFC, RSOFC can be implemented in any separate-reactant SOFC design. Today, pilot plants of RSOFC are already being tested [160]. However, a number of problems still need to be solved for the commercialization of RSOFC: an exact understanding of cell behavior and its degradation when switching modes, the selection of materials and operating parameters suitable for reversible operation, a connection of RSOFC to existing networks, and reducing the cost.
5. Conclusions
A brief description of all SOFC configurations developed today is presented in the review. To cover all SOFC concepts, the standard SOFC classification is supplemented by division according to such criteria as presence/absence of electrolyte and gas spaces separation. Herewith, the types of SOFC that are usually not mentioned in the classifications (electrolyte-free fuel cell and mixed-reactant SOFC) have been considered along with other types of SOFC from standpoint of standard criteria: operating temperature, support types, and geometry. This has made it possible to compare the various designs. It is shown that the most developed group of SOFC are separate-reactant fuel cells with oxygen-ion-conducting electrolytes. Among them, the most popular design is an anode-supported one, which permits one to achieve high specific powers at temperatures below 800 °C. However, electrolyte-free SOFC and proton-conducting electrolyte SOFC, the intensive development of which began recently, have a greater potential for reducing operating temperatures than standard dual-chamber O-SOFC. All SOFC types have some drawbacks; therefore, further research and new ideas are necessary for the practical mass implementation of this technology.
Author Contributions
K.A.K.: funding acquisition, project administration; A.V.N.: conceptualization, investigation, writing—review and editing, writing—original draft preparation; K.Z.B.: investigation; N.B.P.: investigation, writing—review and editing; A.M.K.: investigation, writing—review and editing; M.M.K.: investigation; G.D.K.: investigation; N.A.: investigation. All authors have read and agreed to the published version of the manuscript.
Funding
The work was fulfilled in the frame of project No. AP09261208 supported by the Ministry of Education and Science of Kazakhstan. This work was performed as part of State Task No. 122011200363-9.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kirubakaran, A.; Jain, S.; Nema, R.N. A review on fuel cell technologies and power electronic interface. Renew. Sust. Energ. Rev. 2009, 13, 2430–2440. [Google Scholar] [CrossRef]
- Ramadhani, F.; Hussain, M.A.; Mokhlis, H. A comprehensive review and technical guideline for optimal design and operations of fuel cell-based cogeneration systems. Processes 2019, 7, 950. [Google Scholar] [CrossRef] [Green Version]
- Perfil’ev, M.V.; Demin, A.K.; Kuzin, B.L.; Lipilin, A.S. Vysokotemperaturnyj Jelektroliz Gazov; Nauka: Moscow, Russia, 1988; 232p, ISBN 5-02-001399-4. [Google Scholar]
- Minh, N.Q. Ceramic fuel cells. J. Am. Ceram. Soc. 1993, 76, 563–588. [Google Scholar] [CrossRef]
- Minh, N.Q. Solid oxide fuel cell technology—Features and applications. Solid State Ion. 2004, 174, 271–277. [Google Scholar] [CrossRef]
- Solid Oxide Fuel Cells: From Materials to System Modeling; Ni, M.; Zhao, T.S. (Eds.) UK RSC Publishing: Cambridge, UK, 2013; 523p. [Google Scholar] [CrossRef]
- Kuhn, M.; Napporn, T.W. Single-chamber solid oxide fuel cell technology—From its origins to today’s state of the art. Energies 2010, 3, 57–134. [Google Scholar] [CrossRef]
- Zhu, B.; Raza, R.; Fan, L.; Sun, C. (Eds.) Solid Oxide Fuel Cells: From Electrolyte-Based to Electrolyte-Free Devices; Wiley-VCH: Weinheim, Germany, 2020; 488p, ISBN 978-3-527-81278-3. [Google Scholar]
- Bello, I.T.; Zhai, S.; Zhao, S.; Li, Z.; Yu, N.; Ni, M. Scientometric review of proton-conducting solid oxide fuel cells. Int. J. Hydrogen Energy 2021, 46, 37406–37428. [Google Scholar] [CrossRef]
- Hossain, S.; Abdalla, A.M.; Jamain, S.N.B.; Zaini, J.H.; Azad, A.K. A review on proton conducting electrolytes for clean energy and intermediate temperature-solid oxide fuel cells. Renew. Sust. Energ. Rev. 2017, 79, 750–764. [Google Scholar] [CrossRef]
- Singhal, S.C.; Kendall, K. (Eds.) High Temperature Solid Oxide Fuel Cells: Fundamentals, Design and Applications; Elsevier Ltd.: Oxford, UK, 2003; 405p, ISBN 1856173879. [Google Scholar]
- Kan, W.H.; Samson, A.J.; Thangadurai, V. Trends in electrode development for next generation solid oxide fuel cells. J. Mater. Chem. A 2016, 4, 17913–17932. [Google Scholar] [CrossRef] [Green Version]
- Fergus, J.W. Electrolytes for solid oxide fuel cells. J. Power Sources 2006, 162, 30–40. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, X.; Wang, X.; Yu, J.; Li, L. A review of zirconia-based solid electrolytes. Ionics 2016, 22, 2249–2262. [Google Scholar] [CrossRef]
- Jaiswal, N.; Tanwar, K.; Suman, R.; Kumar, D.; Upadhyay, S.; Parkash, O. A brief review on ceria based solid electrolytes for solid oxide fuel cells. J. Alloys Compd. 2019, 781, 984–1005. [Google Scholar] [CrossRef]
- Prakash, B.S.; Kumar, S.S.; Aruna, S.T. Properties and development of Ni/YSZ as an anode material in solid oxide fuel cell: A review. Renew. Sust. Energ. Rev. 2014, 36, 149–179. [Google Scholar] [CrossRef]
- Ng, K.H.; Rahman, H.A.; Somalu, M.R. Review: Enhancement of composite anode materials for low-temperature solid oxide fuels. Int. J. Hydrogen Energy 2019, 44, 30692–30704. [Google Scholar] [CrossRef]
- Liu, Y.; Shao, Z.; Mori, T.; Jiang, S.P. Development of nickel based cermet anode materials in solid oxide fuel cells—Now and future. Mater. Rep. Energy 2021, 1, 100003. [Google Scholar] [CrossRef]
- Jiang, S.P. Development of lanthanum strontium manganite perovskite cathode materials of solid oxide fuel cells: A review. J. Mater. Sci. 2008, 43, 6799–6833. [Google Scholar] [CrossRef]
- Jiang, S.P. Development of lanthanum strontium cobalt ferrite perovskite electrodes of solid oxide fuel cells—A review. Int. J. Hydrogen Energy 2019, 44, 7448–7493. [Google Scholar] [CrossRef]
- Curia, M.; Silva, E.R.; Furtado, J.G.M.; Ferraz, H.C.; Secchi, A.R. Anodes for SOFC: Review of material selection, interface and electrochemical phenomena. Quim. Nova 2021, 44, 86–97. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, W.; Ding, D.; Liu, M.; Ciucci, F.; Tade, M.; Shao, Z. Advances in cathode materials for solid oxide fuel cells: Complex oxides without alkaline earth metal elements. Adv. Energy Mater. 2015, 5, 1500537. [Google Scholar] [CrossRef]
- Jacobs, R.; Mayeshiba, T.; Booske, J.; Morgan, D. Material discovery and design principles for stable, high activity perovskite cathodes for solid oxide fuel cells. Adv. Energy Mater. 2018, 8, 1702708. [Google Scholar] [CrossRef] [Green Version]
- Ding, P.; Li, W.; Zhao, H.; Wu, C.; Zhao, L.; Dong, B.; Wang, S. Review on Ruddlesden–Popper perovskites as cathode for solid oxide fuel cells. J. Phys. Mater. 2021, 4, 022002. [Google Scholar] [CrossRef]
- Ahmad, M.Z.; Ahmad, S.H.; Chen, R.S.; Ismail, A.F.; Hazan, R.; Baharuddin, N.A. Review on recent advancement in cathode material for lower and intermediate temperature solid oxide fuel cells application. Int. J. Hydrogen Energy 2021, 47, 1103–1120. [Google Scholar]
- Fan, L.; Zhu, B.; Su, P.-C.; He, C. Nanomaterials and technologies for low temperature solid oxide fuel cells: Recent advances, challenges and opportunities. Nano Energy 2018, 45, 148–176. [Google Scholar]
- Jo, S.; Sharma, B.; Park, D.-H.; Myung, J. Materials and nano-structural processes for use in solid oxide fuel cells: A review. J. Korean Ceram. Soc. 2020, 57, 135–151. [Google Scholar]
- Janga, I.; Kima, S.; Kima, C.; Yoon, H.; Song, T. Enhancement of oxygen reduction reaction through coating a nano-web-structured La0.6Sr0.4Co0.2Fe0.8O3-δ thin-film as a cathode/electrolyte interfacial layer for lowering the operating temperature of solid oxide fuel cells. J. Power Sources 2018, 392, 123–128. [Google Scholar]
- Pavzderin, N.B.; Solovyev, A.A.; Nikonov, A.V.; Shipilova, A.V.; Rabotkin, S.V.; Semenov, V.A.; Grenaderov, A.S.; Oskomov, K.V. Formation of a dense La(Sr)Fe(Ga)O3 interlayer at the electrolyte/porous cathode interface by magnetron sputtering and its effect on the cathode characteristics. Russ. J. Electrochem. 2021, 57, 519–525. [Google Scholar]
- Develos-Bagarinao, K.; de Vero, J.; Kishimoto, H.; Ishiyama, T.; Yamaji, K.; Horita, T.; Yokokawa, H. Multilayered LSC and GDC: An approach for designing cathode materials with superior oxygen exchange properties for solid oxide fuel cells. Nano Energy 2018, 52, 369–380. [Google Scholar]
- Zhang, Y.; Xu, N.; Fan, H.; Han, M. La0.6Sr0.4Co0.2Fe0.8O3-δ nanoparticles modified Ni-based anode for direct methane-fueled SOFCs. Energy Procedia 2019, 158, 2250–2255. [Google Scholar]
- Pei, K.; Zhou, Y.; Xu, K.; He, Z.; Chen, Y.; Zhang, W.; Yoo, S.; Zhao, B.; Yuan, W.; Liu, M.; et al. Enhanced Cr-tolerance of an SOFC cathode by an efficient electro-catalyst coating. Nano Energy 2020, 72, 104704. [Google Scholar]
- Venancio, S.A.; Sarruf, B.J.M.; Gomes, G.G.; de Miranda, P.E.V. Multifunctional macroporous solid oxide fuel cell anode with active nanosized ceramic electrocatalyst. Int. J. Hydrogen Energy 2020, 45, 5501–5511. [Google Scholar]
- Wu, J.; Liu, X.J. Recent development of SOFC metallic interconnect. Mater. Sci. Technol. 2010, 26, 293–305. [Google Scholar] [CrossRef]
- Mah, J.C.W.; Muchtar, A.; Somalu, M.R.; Ghazali, M.J. Metallic interconnects for solid oxide fuel cell: A review on protective coating and deposition techniques. Int. J. Hydrogen Energy 2017, 42, 9219–9229. [Google Scholar] [CrossRef]
- Fabbri, E.; Pergolesi, D.; Traversa, E. Materials challenges toward proton-conducting oxide fuel cells: A critical review. Chem. Soc. Rev. 2010, 39, 4355–4369. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Gao, J.; Zhao, Z.; Amoroso, J.; Tong, J.; Brinkman, K.S. Review: Recent progress in low-temperature proton-conducting ceramics. J. Mater. Sci. 2019, 54, 9291–9312. [Google Scholar]
- Singh, B.; Ghosh, S.; Aich, S.; Roy, B. Low temperature solid oxide electrolytes (LT-SOE): A review. J. Power Sources 2017, 339, 103–135. [Google Scholar] [CrossRef]
- Zhang, W.; Hu, Y.H. Progress in proton-conducting oxides as electrolytes for low-temperature solid oxide fuel cells: From materials to devices. Energy Sci. Eng. 2021, 9, 984–1011. [Google Scholar] [CrossRef]
- Yang, G.; Su, C.; Shi, H.; Zhu, Y.; Song, Y.; Zhou, W.; Shao, Z. Toward reducing the operation temperature of solid oxide fuel cells: Our past 15 years of efforts in cathode development. Energy Fuels 2020, 34, 15169–15194. [Google Scholar] [CrossRef]
- Zhu, B.; Raza, R.; Qin, H.; Liu, Q.; Fan, L. Fuel cells based on electrolyte and non-electrolyte separators. Energy Environ. Sci. 2011, 4, 2986–2992. [Google Scholar] [CrossRef]
- Wang, G.; Wu, X.; Cai, Y.; Ji, Y.; Yaqub, A.; Zhu, B. Design, fabrication and characterization of a double layer solid oxide fuel cell (DLFC). J. Power Sources 2016, 332, 8–15. [Google Scholar] [CrossRef]
- He, H.P.; Huang, X.J.; Chen, L.Q. A practice of single layer solid oxide fuel cell. Ionics 2000, 6, 64–69. [Google Scholar] [CrossRef]
- Zhu, B.; Ma, Y.; Wang, X.; Raza, R.; Qin, H.; Fan, L. A fuel cell with a single component functioning simultaneously as the electrodes and electrolyte. Electrochem. Commun. 2011, 13, 225–227. [Google Scholar] [CrossRef]
- Hu, E.; Jiang, Z.; Fan, L.; Singh, M.; Wang, F.; Raza, R.; Sajid, M.; Wang, J.; Kim, J.S.; Zhu, B. Junction and energy band on novel semiconductor-based fuel cells. iScience 2021, 24, 102191. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Tian, L.; Li, J.; Zhao, Y.; Tian, Y.; Li, Y. Single-layer fuel cell based on a composite of Ce0.8Sm0.2O2−δ–Na2CO3 and a mixed ionic and electronic conductor Sr2Fe1.5Mo0.5O6−δ. J. Power Sources 2014, 249, 270–276. [Google Scholar] [CrossRef]
- Zhu, B.; Lund, P.; Raza, R.; Patakangas, J.; Huang, Q.-A.; Fan, L.; Singh, M. A new energy conversion technology based on nano-redox and nano-device processes. Nano Energy 2013, 2, 1179–1185. [Google Scholar] [CrossRef]
- Zhu, B.; Lund, P.D.; Raza, R.; Ma, Y.; Fan, L.; Afzal, M.; Patakangas, J.; He, Y.; Zhao, Y.; Tan, W.; et al. Schottky junction effect on high performance fuel cells based on nanocomposite materials. Adv. Energy Mater. 2015, 5, 1401895. [Google Scholar] [CrossRef]
- Zhu, B.; Mi, Y.; Xia, C.; Wang, B.; Kim, J.-S.; Lund, P.; Li, T. A nanoscale perspective on solid oxide and semiconductor membrane fuel cells: Materials and technology. Energy Mater. 2021, 1, 100002. [Google Scholar]
- Yano, M.; Tomita, A.; Sano, M.; Hibino, T. Recent advances in single-chamber solid oxide fuel cells: A review. Solid State Ion. 2007, 177, 3351–3359. [Google Scholar] [CrossRef]
- Jacques-Bedard, X.; Napporn, T.W.; Roberge, R.; Meunier, M. Coplanar electrodes design for a single-chamber SOFC. J. Electrochem. Soc. 2007, 154, B305–B309. [Google Scholar] [CrossRef]
- Kamvar, M.; Ghassemi, M.; Rezaei, M. Effect of catalyst layer configuration on single chamber solid oxide fuel cell performance. Appl. Therm. Eng. 2016, 100, 98–104. [Google Scholar] [CrossRef]
- Guo, Y.; Bessaa, M.; Aguado, S.; Steil, M.C.; Rembelski, D.; Rieu, M.; Viricelle, J.-P.; Benameur, N.; Guizard, C.; Tardivat, C.; et al. An all porous solid oxide fuel cell (SOFC): A bridging technology between dual and single chamber SOFCs. Energy Environ. Sci. 2013, 6, 2119–2123. [Google Scholar] [CrossRef]
- Guo, Y.M.; Largiller, G.; Guizard, C.; Tardivat, C.; Farrusseng, D. Coke-free operation of an all porous solid oxide fuel cell (AP-SOFC) used as an O2 supply device. J. Mater. Chem. A 2015, 3, 2684–2689. [Google Scholar] [CrossRef]
- Horiuchi, M.; Suganuma, S.; Watanabe, M. Electrochemical power generation directly from combustion flame of gases, liquids, and solids. J. Electrochem. Soc. 2004, 151, A1402–A1405. [Google Scholar] [CrossRef]
- Shi, Y.; Cai, N.; Cao, T.; Zhang, J. (Eds.) High-Temperature Electrochemical Energy Conversion and Storage: Fundamentals and Applications; CRC Press: London, UK, 2018; 223p, ISBN 9780367889838. [Google Scholar]
- Mahapatra, M.K.; Lu, K. Glass-based seals for solid oxide fuel and electrolyzer cells—A review. Mater. Sci. Eng. R. Rep. 2010, 67, 65–85. [Google Scholar] [CrossRef]
- Singh, K.; Walia, T. Review on silicate and borosilicate-based glass sealants and their interaction with components of solid oxide fuel cell. Int. J. Energy Res. 2021, 45, 20559–20582. [Google Scholar]
- Riess, I.J. On the single chamber solid oxide fuel cells. Power Sources 2008, 175, 325–337. [Google Scholar] [CrossRef]
- Bedon, A.; Viricelle, J.P.; Rieu, M.; Mascotto, S.; Glisenti, A. Single chamber Solid Oxide Fuel Cells selective electrodes: A real chance with brownmillerite-based nanocomposites. Int. J. Hydrogen Energy. 2021, 46, 14735–14747. [Google Scholar]
- Vogler, M.; Barzan, D.; Kronemayer, H.; Schulz, C.; Horiuchi, M.; Suganuma, S.; Tokutake, Y.; Warnatz, J.; Bessler, W.G. Direct-flame solid-oxide fuel cell (DFFC): A thermally self-sustained, air self-breathing, hydrocarbon-operated SOFC System in a simple, no-chamber setup. ECS Trans. 2007, 7, 555–564. [Google Scholar] [CrossRef]
- Behling, N.H. Fuel cells. In Current Technology Challenges and Future Research Needs; Elsevier: Amsterdam, The Netherlnds, 2013; 685p. [Google Scholar] [CrossRef]
- van Rij, L.N.; Le, J.; van Landschoot, R.C.; Schoonman, J.A. A novel Ni-CERMET electrode based on a proton conducting electrolyte. J. Mater. Sci. 2001, 36, 1069–1076. [Google Scholar] [CrossRef]
- Fabbri, E.; D’Epifanio, A.; Sanna, S.; Bartolomeo, E.D.; Balestrino, G.; Licoccia, S.; Traversa, E. A novel single chamber solid oxide fuel cell based on chemically stable thin films of Y-doped BaZrO3 proton conducting electrolyte. Energy Environ. Sci. 2010, 3, 618–621. [Google Scholar] [CrossRef]
- Wang, K.; Milcarek, R.J.; Zeng, P.; Ahn, J. Flame-assisted fuel cells running methane. Int. J. Hydrogen Energy 2015, 40, 4659–4665. [Google Scholar]
- Milcarek, R.J.; Wang, K.; Falkenstein-Smith, R.L.; Ahn, J. Micro-tubular flame-assisted fuel cells for micro-combined heat and power systems. J. Power Sources 2016, 306, 148–151. [Google Scholar]
- Milcarek, R.J.; Ahn, J. Micro-tubular flame-assisted fuel cells running methane, propane and butane: On soot, efficiency and power density. Energy 2019, 169, 776–782. [Google Scholar]
- Wang, Y.; Shi, Y.; Cao, T.; Zeng, H.; Cai, N.; Ye, X.; Wang, S. A flame fuel cell stack powered by a porous media combustor. Int. J. Hydrogen Energy 2018, 43, 22595–22603. [Google Scholar]
- Steele, B.C.H. Material science and engineering: The enabling technology for the commercialisation of fuel cell systems. J. Mater. Sci. 2001, 36, 1053–1068. [Google Scholar] [CrossRef]
- Brett, D.J.L.; Atkinson, A.; Brandon, N.P.; Skinner, S.J. Intermediate temperature solid oxide fuel cells. Chem. Soc. Rev. 2008, 37, 1568–1578. [Google Scholar] [CrossRef]
- Kaur, G. (Ed.) Intermediate Temperature Solid Oxide Fuel Cells; Elsevier: Amsterdam, The Netherlnds, 2020; 516p. [Google Scholar] [CrossRef]
- Ferrari, M.L.; Damo, U.M.; Turan, A.; Sanchez, D. Hybrid Systems Based on Solid Oxide Fuel Cells; Wiley: Hoboken, NJ, USA, 2017; 325p. [Google Scholar] [CrossRef]
- Weber, A.; Ivers-Tiffee, E.J. Materials and concepts for solid oxide fuel cells (SOFCs) in stationary and mobile applications. Power Sour. 2004, 127, 273–283. [Google Scholar] [CrossRef]
- Wachsman, E.D.; Lee, K.T. Lowering the temperature of solid oxide fuel cells. Science 2011, 334, 935–939. [Google Scholar] [CrossRef]
- Timurkutluk, B.; Timurkutluk, C.; Mat, M.D.; Kaplan, Y. A review on cell/stack designs for high performance solid oxide fuel cells. Renew. Sustain. Energy Rev. 2016, 56, 1101–1121. [Google Scholar] [CrossRef]
- Qiu, P.; Sun, S.; Yang, X.; Chen, F.; Xiong, C.; Jia, L.; Li, J. A review on anode on-cell catalyst reforming layer for direct methane solid oxide fuel cells. Int. J. Hydrogen Energy 2021, 46, 25208–25224. [Google Scholar] [CrossRef]
- Duan, C.; Tong, J.; Shang, M.; Nikodemski, S.; Sanders, M.; Ricote, S.; Almansoori, A.; O’Hayre, R. Readily processed protonic ceramic fuel cells with high performance at low temperatures. Science 2015, 349, 1321–1326. [Google Scholar] [CrossRef]
- Chen, C.; Dong, Y.; Li, L.; Wang, Z.; Liu, M.; Rainwater, B.H.; Bai, Y. Electrochemical properties of micro-tubular intermediate temperature solid oxide fuel cell with novel asymmetric structure based on BaZr0.1Ce0.7Y0.1Yb0.1O3-δ proton conducting electrolyte. Int. J. Hydrogen Energy 2019, 44, 16887–16897. [Google Scholar] [CrossRef]
- Wang, K.; Zeng, P.; Ahn, J. High performance direct flame fuel cell using a propane flame. Proc. Combust. Inst. 2011, 33, 3431–3437. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, L.; Luo, L.; Wu, Y.; Liu, L.; Shi, J. The study of portable direct-flame solid oxide fuel cell (DF-SOFC) stack with butane fuel. J. Fuel Chem. Technol. 2014, 42, 1135–1139. [Google Scholar] [CrossRef]
- Tucker, M.C.; Ying, A.S. Metal-supported solid oxide fuel cells operated in direct-flame configuration. Int. J. Hydrogen Energy 2017, 42, 24426–24434. [Google Scholar]
- Mai, A.; Iwanschitz, B.; Weissen, U.; Denzler, R.; Haberstock, D.; Nerlich, V.; Sfeir, J.; Schuler, A. Status of Hexis SOFC stack development and the Galileo 1000 N micro-CHP system. ECS Trans. 2009, 25, 149–158. [Google Scholar] [CrossRef]
- Singh, A.; Ghuman, J.S.; Kumar, R. Bloom Energy for producing electricity. Int. J. Power Syst. Oper. Energy Manag. 2014, 4, 3. [Google Scholar] [CrossRef]
- Kwon, Y.; Han, Y.J. Fabrication of electrolyte-supported solid oxide fuel cells using a tape casting process. Ceram. Soc. Jpn. 2020, 128, 310–316. [Google Scholar] [CrossRef]
- Williams, M.C.; Strakey, J.P.; Surdoval, W.A.; Wilson, L.C. Solid oxide fuel cell technology development in the U.S. Solid State Ion. 2006, 177, 2039–2044. [Google Scholar] [CrossRef]
- McConnell, V.P. Versa Power’s SOFC could scale to MW for SECA, and work in transport hybrids. Fuel Cells Bull. 2007, 2007, 12–16. [Google Scholar] [CrossRef]
- Yoo, Y.-S.; Lee, T.; Choi, J.H.; Park, T.-S.; Oh, J.-M.; Kim, C.-Y. Fabrication and demonstration of 1kW class SOFC stack and system for residential power generation application. J. Fuel Cell Sci. Technol. 2009, 6, 021008. [Google Scholar] [CrossRef]
- Santori, G.; Brunetti, E.; Polonara, F. Experimental characterization of an anode-supported tubular SOFC generator fueled with hydrogen, including a principal component analysis and a multi-linear regression. Int. J. Hydrogen Energy 2011, 36, 8435–8449. [Google Scholar] [CrossRef] [Green Version]
- Harboe, S.; Schreiber, A.; Margaritis, N.; Blum, L.; Guillon, O.; Menzler, N.H. Manufacturing cost model for planar 5 kWel SOFC stacks at Forschungszentrum Julich. Int. J. Hydrogen Energy 2020, 45, 8015–8030. [Google Scholar] [CrossRef]
- Tsipis, E.V.; Kharton, V.V. Electrode materials and reaction mechanisms in solid oxide fuel cells: A brief review. II. Electrochemical behavior vs. materials science aspects. J. Solid State Electrochem. 2008, 12, 1367–1391. [Google Scholar] [CrossRef]
- Huang, K.; Singhal, S.C. Cathode-supported tubular solid oxide fuel cell technology: A critical review. J. Power Sources 2013, 237, 84–97. [Google Scholar] [CrossRef]
- Zhao, K.; Kim, B.-H.; Du, Y.; Xu, Q.; Ahn, B.-G. Ceria catalyst for inert-substrate supported tubular solid oxide fuel cells running on methane fuel. J. Power Sources 2016, 314, 10–17. [Google Scholar] [CrossRef]
- Gardner, F.J.; Day, M.J.; Brandon, N.P.; Pashley, M.N.; Cassidy, M. SOFC technology development at Rolls-Royce. J. Power Sources 2000, 86, 122–129. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Ando, Y.; Kabata, T.; Nishiura, M.; Tomida, K.; Matake, N. Extremely high-efficiency thermal power system-solid oxide fuel cell (SOFC) triple combined-cycle system. Mitsubishi Heavy Ind. Tech. Rev. 2011, 48, 9–15. [Google Scholar]
- Krishnan, V.V. Recent developments in metal-supported solid oxide fuel cells. Wiley Interdiscip. Rev. Energy Environ. 2017, 6, e246. [Google Scholar] [CrossRef]
- Tucker, M.C. Progress in metal-supported solid oxide electrolysis cells: A review. Int. J. Hydrogen Energy 2020, 45, 24203–24218. [Google Scholar] [CrossRef]
- Sun, W.; Liu, M.; Liu, W. Chemically stable yttrium and tin co-doped barium zirconate electrolyte for next generation high performance proton-conducting solid oxide fuel cells. Adv. Energy Mater. 2013, 3, 1041–1050. [Google Scholar] [CrossRef]
- Azad, A.K.; Abdalla, A.M.; Afif, A.; Azad, A.; Afroze, S.; Idris, A.C.; Park, J.-Y.; Saqib, M.; Radenahmad, N.; Hossain, S.; et al. Improved mechanical strength, proton conductivity and power density in an ‘all-protonic’ ceramic fuel cell at intermediate temperature. Sci. Rep. 2021, 11, 19382. [Google Scholar]
- Hwang, S.H.; Kim, S.K.; Nam, J.T.; Park, J.S. Fabrication of an electrolyte-supported protonic ceramic fuel cell with nano-sized powders of Ni-composite anode. Int. J. Hydrogen Energy 2021, 46, 1076–1084. [Google Scholar] [CrossRef]
- Stange, M.; Stefan, E.; Denonville, C.; Larring, Y.; Rørvik, P.M.; Haugsrud, R. Development of novel metal-supported proton ceramic electrolyser cell with thin film BZY15-Ni electrode and BZY15 electrolyte. Int. J. Hydrogen Energy 2017, 42, 13454–13462. [Google Scholar] [CrossRef]
- Wang, R.; Byrne, C.; Tucker, M.C. Assessment of co-sintering as a fabrication approach for metal-supported proton-conducting solid oxide cells. Solid State Ion. 2019, 332, 25–33. [Google Scholar]
- Tucker, M.C. Personal power using metal-supported solid oxide fuel cells operated in a camping stove flame. Int. J. Hydrogen Energy. 2018, 43, 8991–8998. [Google Scholar]
- Sayan, Y.; Venkatesan, V.; Guk, E.; Wu, H.; Kim, J.-S. Single-step fabrication of an anode supported planar single-chamber solid oxide fuel cell. Int. J. Appl. Ceram. Technol. 2018, 15, 1375–1387. [Google Scholar]
- Tian, Y.; Lü, Z.; Wang, Z.; Wei, B.; Guo, X.; Wu, P. Effect of the angle between gas flow direction and electrode on single-chamber SOFC stacks. J. Solid State Electr. 2019, 23, 1651–1657. [Google Scholar]
- Tian, Y.; Wu, P.; Zhang, X.; Guo, X.; Ding, L. Performance of a linear array solid oxide fuel cell micro-stack operated in single-chamber conditions. Ionics 2020, 26, 6217–6224. [Google Scholar]
- Choi, I.; Kim, J.-S.; Venkatesan, V.; Ranaweera, M. Fabrication and evaluation of a novel wavy single chamber solid oxide fuel cell via in-situ monitoring of curvature evolution. Appl. Energy 2017, 195, 1038–1046. [Google Scholar]
- Kamvar, M.; Ghassemi, M.; Steinberger-Wilckens, R. The numerical investigation of a planar single chamber solid oxide fuel cell performance with a focus on the support types. Int. J. Hydrogen Energy 2020, 45, 7077–7087. [Google Scholar]
- Raz, S.; Jak, M.J.G.; Schoonman, J.; Riess, I. Supported mixed-gas fuel cells. Solid State Ion. 2002, 149, 335–341. [Google Scholar] [CrossRef]
- Udomsilp, D.; Rechberger, J.; Neubauer, R.; Bischof, C. Metal-supported solid oxide fuel cells with exceptionally high power density for range extender systems. Cell Rep. Phys. Sci. 2020, 1, 100072. [Google Scholar] [CrossRef]
- Liu, T.; Lin, J.; Liu, T.; Wu, H.; Xia, C.; Chen, C.; Zhan, Z. Tailoring the pore structure of cathode supports for improving. J. Electroceram. 2018, 40, 138–143. [Google Scholar] [CrossRef]
- Ji, S.; Cho, G.Y.; Yu, W.; Su, P.C.; Lee, M.H.; Cha, S.W. Plasma-enhanced atomic layer deposition of nanoscale yttria-stabilized zirconia electrolyte for solid oxide fuel cells with porous substrate. ACS Appl. Mater. Interfaces 2015, 7, 2998–3002. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Gou, Y.; Qiao, J.; Sun, W.; Wang, Z.; Sun, K. Recent progress of tubular solid oxide fuel cell: From materials to applications. J. Power Sources 2020, 477, 228693. [Google Scholar] [CrossRef]
- Ivanov, V.V.; Lipilin, A.S.; Kotov, Y.A.; Khrustov, V.R.; Shkerin, S.N.; Paranin, S.N.; Spirin, A.V.; Kaygorodov, A.S. Formation of a thin-layer electrolyte for SOFC by magnetic pulse compaction of tapes cast of nanopowders. J. Power Sources 2006, 159, 605–612. [Google Scholar] [CrossRef]
- Han, Z.; Yang, Z.; Han, M. Fabrication of metal-supported tubular solid oxide fuel cell by phase-inversion method and in situ reduction. Int. J. Hydrogen Energy 2016, 41, 10935–10941. [Google Scholar] [CrossRef]
- Lim, T.H.; Park, J.L.; Lee, S.B.; Park, S.J.; Song, R.H.; Shin, D.R. Fabrication and operation of a 1 kW class anode-supported flat tubular SOFC stack. Int. J. Hydrogen Energy 2010, 35, 9687–9692. [Google Scholar] [CrossRef]
- Park, S.; Sammes, N.M.; Song, K.H.; Kim, T.; Chung, J.S. Monolithic flat tubular types of solid oxide fuel cells with integrated electrode and gas channels. Int. J. Hydrogen Energy 2017, 42, 1154–1160. [Google Scholar] [CrossRef]
- Mushtaq, U.; Kim, D.W.; Yun, U.J.; Lee, J.W.; Lee, S.B.; Park, S.J.; Song, R.H.; Kim, G.; Lim, T.H. Effect of cathode geometry on the electrochemical performance of flat tubular segmented-in-series (SIS) solid oxide fuel cell. Int. J. Hydrogen Energy 2015, 40, 6207–6215. [Google Scholar] [CrossRef]
- Khan, M.Z.; Iltaf, A.; Ishfaq, H.A.; Khan, F.N.; Tanveer, W.H.; Song, R.H.; Mehran, M.T.; Saleem, M.; Hussain, A.; Masaud, Z. Flat-tubular solid oxide fuel cells and stacks: A review. J. Asian Ceram. Soc. 2021, 9, 745–770. [Google Scholar] [CrossRef]
- Zha, S.; Zhang, Y.; Liu, M. Functionally graded cathodes fabricated by sol-gel/slurry coating for honeycomb SOFCs. Solid State Ionics 2005, 176, 25–31. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Shimizu, S.; Suzuki, T.; Fujishiro, Y.; Awano, M. Fabrication and evaluation of a novel cathode-supported honeycomb SOFC stack. Mater. Lett. 2009, 63, 2577–2580. [Google Scholar] [CrossRef]
- Ruiz-Morales, J.C.; Marrero-Lopez, D.; Pena-Martinez, J.; Canales-Vazquez, J.; Road, J.J.; Segarrad, M.; Savvina, S.N.; Núnez, P. Performance of a novel type of electrolyte-supported solid oxide fuel cell with honeycomb structure. J. Power Sources 2010, 195, 516–521. [Google Scholar] [CrossRef]
- Ikeda, S.; Nakajima, H.; Kitahara, T. Enhancement of fuel transfer in anode-supported honeycomb solid oxide fuel cells. J. Phys. Conf. Ser. 2016, 745, 032082. [Google Scholar] [CrossRef] [Green Version]
- Evans, A.; Bieberle-Hutter, A.; Rupp, J.L.M.; Gauckler, L.J. Review on microfabricated micro-solid oxide fuel cell membranes. J. Power Sources 2009, 194, 119–129. [Google Scholar] [CrossRef]
- Lee, Y.H.; Chang, I.; Cho, G.Y.; Park, J.; Yu, W.; Tanveer, W.H.; Cha, S.W. Thin film solid oxide fuel cells operating below 600 °C: A Review. Int. J. Precis. Eng. Manuf-Green Technol. 2018, 5, 441–453. [Google Scholar]
- Baek, J.D.; Chang, I.; Su., P.C. Thin-film solid oxide fuel cells. In Materials for Energy, 1st ed.; Zhang, S., Ed.; CRC Press: Boca Raton, FL, USA, 2021; pp. 239–283. [Google Scholar]
- An, J.; Kim, Y.-B.; Gür, T.M.; Park, J.; Prinz, F.B. 3-D nanostructured bilayer solid oxide fuel cell with 1.3 W/cm2 at 450 °C. Nano Lett. 2013, 13, 4551–4555. [Google Scholar]
- Baek, J.D.; Liu, K.Y.; Su, P.C. A functional micro-solid oxide fuel cell with 10 nm-thick freestanding electrolyte. J. Mater. Chem. A 2017, 5, 18414–18419. [Google Scholar] [CrossRef]
- Wells, M.P.; Lovett, A.J.; Chalklen, T.; Baiutti, F.; Tarancón, A.; Wang, X.; Ding, J.; Wang, H.; Kar-Narayan, S.; Acosta, M.; et al. Route to high-performance micro-solid oxide fuel cells on metallic substrates. ACS Appl. Mater. Interfaces 2021, 13, 4117–4125. [Google Scholar]
- Kang, S.; Su, P.C.; Park, Y.I.; Saito, Y.; Prinz, F.B. Thin-film solid oxide fuel cells on porous nickel substrates with multistage nanohole array. J. Electrochem. Soc. 2006, 153, A554–A559. [Google Scholar] [CrossRef]
- Kang, S.; Lee, J.; Cho, G.Y.; Kim, Y.; Lee, S.; Cha, S.W.; Bae, J. Scalable fabrication process of thin-film solid oxide fuel cells with an anode functional layer design and a sputtered electrolyte. Int. J. Hydrogen Energy 2020, 45, 33980–33992. [Google Scholar]
- Cho, G.Y.; Yu, W.; Lee, Y.H.; Lee, Y.; Tanveer, W.H.; Kim, Y.; Lee, S.; Cha, S.W. Effects of nanoscale PEALD YSZ interlayer for AAO based thin film solid oxide fuel cells. Int. J. Precis. Eng. Manuf-Green Technol. 2020, 7, 423–430. [Google Scholar]
- Kim, K.J.; Park, B.H.; Kim, S.J.; Lee, Y.; Bae, H.; Choi, G.M. Micro solid oxide fuel cell fabricated on porous stainless steel: A new strategy for enhanced thermal cycling ability. Sci. Rep. 2016, 6, 22443. [Google Scholar] [PubMed]
- Reolon, R.P.; Sanna, S.; Xu, Y.; Lee, I.; Bergmann, C.P.; Prydsa, N.; Esposito, V. Effects of accelerated degradation on metal supported thin film-based solid oxide fuel cells. J. Mater. Chem. A 2018, 6, 7887–7896. [Google Scholar]
- Lee, M.S.; Lee, S.; Jeong, W.; Ryu, S.; Yu, W.; Lee, Y.H.; Cho, G.Y.; Cha, S.W. Nanoporous nickel thin film anode optimization for low-temperature solid oxide fuel cells. Int. J. Hydrogen Energy 2021, 46, 36445–36453. [Google Scholar]
- Yu, W.; Lim, Y.; Lee, S.; Pandiyan, A.; Cho, G.; Cha, S.W. Low-temperature, high-performance thin-film solid oxide fuel cells with tailored nano-column structures of a sputtered Ni anode. J. Mater. Chem. A 2020, 41, 21668–21679. [Google Scholar]
- Lee, Y.H.; Ren, H.; Wu, E.A.; Fullerton, E.E.; Meng, Y.S.; Minh, N.Q. All-sputtered, superior power density thin-film solid oxide fuel cells with a novel nanofibrous ceramic cathode. Nano Lett. 2020, 20, 2943–2949. [Google Scholar]
- Kendall, K. Progress in microtubular solid oxide fuel cells. Int. J. Appl. Ceram. Technol. 2010, 7, 1–9. [Google Scholar] [CrossRef]
- Zhang, X.; Jin, Y.; Li, D.; Xiong, Y. A review on recent advances in micro-tubular solid oxide fuel cells. J. Power Sources 2021, 506, 230135. [Google Scholar] [CrossRef]
- Howe, K.S.; Thompson, G.J.; Kendall, K. Micro-tubular solid oxide fuel cells and stacks. J. Power Sources 2011, 196, 1677–1686. [Google Scholar] [CrossRef]
- Lawlor, V.; Griesser, S.; Buchinger, G.; Olabi, A.G.; Cordiner, S.; Meissner, D. Review of the micro-tubular solid oxide fuel cell Part I. Stack design issues and research activities. J. Power Sources 2009, 193, 387–399. [Google Scholar] [CrossRef]
- Jamil, S.M.; Othman, M.H.D.; Rahman, M.A.; Jaafar, J.; Ismail, A.F. Anode supported micro-tubular SOFC fabricated with mixed particle size electrolyte via phase-inversion technique. Int. J. Hydrogen Energy 2017, 42, 9188–9201. [Google Scholar] [CrossRef]
- Nikonov, A.V.; Spirin, A.V.; Lipilin, A.S.; Khrustov, V.R.; Paranin, S.N. Fabrication of microtubular solid oxide fuel cells by film compaction and co-sintering. Russ. J. Electrochem. 2018, 54, 547–553. [Google Scholar] [CrossRef]
- Hsieh, W.S.; Lin, P.; Wang, S.F. Characteristics of electrolyte supported micro-tubular solid oxide fuel cells with GDC-ScSZ bilayer electrolyte. Int. J. Hydrogen Energy 2014, 39, 17267–17274. [Google Scholar] [CrossRef]
- Meng, X.; Yang, N.; Gong, X.; Yin, Y.; Ma, Z.-F.; Tan, X.; Shao, Z.; Liu, S. Novel cathode-supported hollow fibers for light weight micro-tubular solid oxide fuel cells with an active cathode functional layer. J. Mater. Chem. A 2015, 3, 1017–1022. [Google Scholar] [CrossRef]
- Sumi, H.; Shimada, H.; Yamaguchi, Y.; Yamaguchi, T. Effect of anode thickness on polarization resistance for metal-supported microtubular solid oxide fuel cells. J. Electrochem. Soc. 2017, 164, F243–F247. [Google Scholar] [CrossRef]
- Hedayat, N.; Panthi, D.; Du, Y. Inert substrate-supported microtubular solid oxide fuel cells based on highly porous ceramic by low-temperature co-sintering. Ceram. Int. 2019, 45, 579–587. [Google Scholar] [CrossRef]
- Motoyama, M.; Chao, C.C.; An, J.; Jung, H.J.; Gur, T.M.; Prinz, F.B. Nanotubular array solid oxide fuel cell. ACS Nano 2014, 8, 340–351. [Google Scholar] [CrossRef]
- Akhtar, N.; Decent, S.P.; Loghin, D.; Kendall, K. Mixed-reactant, micro-tubular solid oxide fuel cells: An experimental study. J. Power Sources 2009, 193, 39–48. [Google Scholar] [CrossRef]
- An, H.; Lee, H.-W.; Kim, B.-K.; Son, J.-W.; Yoon, K.J.; Kim, H.; Shin, D.; Ji, H.-I.; Lee, J.-H. A 5×5 cm2 protonic ceramic fuel cell with a power density of 1.3 W cm–2 at 600 °C. Nat. Energy 2018, 3, 870–875. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, H.; Li, Y.; Xing, J.; Zhang, Z.; Ding, X.; Zhang, B.; Zhou, J.; Wang, S. Fabrication and performance of anode-supported proton conducting solid oxide fuel cells based on BaZr0.1Ce0.7Y0.1Yb0.1O3-δ electrolyte by multi-layer aqueous-based co-tape casting. J. Power Sources 2021, 506, 229922. [Google Scholar] [CrossRef]
- Zhu, L.; O’Hayre, R.; Sullivan, N.P. High performance tubular protonic ceramic fuel cells via highly-scalable extrusion process. Int. J. Hydrogen Energy 2021, 46, 27784–27792. [Google Scholar] [CrossRef]
- Hanifi, A.R.; Sandhu, N.K.; Etsell, T.H.; Luo, J.L.; Sarkar, P. Fabrication and characterization of a tubular ceramic fuel cell based on BaZr0.1Ce0.7Y0.1Yb0.1O3-d proton conducting electrolyte. J. Power Sources 2017, 341, 264–269. [Google Scholar] [CrossRef]
- Li, Y.; Wang, S.; Su, P.-C. Proton-conducting micro-solid oxide fuel cells with improved cathode reactions by a nanoscale thin film gadolinium-doped ceria interlayer. Sci. Rep. 2016, 6, 22369. [Google Scholar]
- Ren, C.; Wang, S.; Liu, T.; Lin, Y.; Chen, F. Fabrication of microtubular solid oxide fuel cells using sulfur-free polymer binder via a phase inversion method. J. Power Sources 2015, 290, 1–7. [Google Scholar] [CrossRef]
- Chen, C.; Dong, Y.; Li, L.; Wang, Z.; Liu, M.; Rainwater, B.H.; Bai, Y. High performance of anode supported BaZr0.1Ce0.7Y0.1Yb0.1O3-δ proton-conducting electrolyte micro-tubular cells with asymmetric structure for IT-SOFCs. J. Electroanal. Chem. 2019, 844, 49–57. [Google Scholar]
- Ruiz-Morales, J.C.; Marrero-Lopez, D.; Canales-Vazquez, J.; Irvine, J.T.S. Symmetric and reversible solid oxide fuel cells. RSC Adv. 2011, 1, 1403–1414. [Google Scholar] [CrossRef]
- Su, C.; Wang, W.; Liu, M.; Tade, M.O.; Shao, Z. Progress and prospects in symmetrical solid oxide fuel cells with two identical electrodes. Adv. Energy Mater. 2015, 5, 1500188. [Google Scholar] [CrossRef]
- Zhao, Z.; Qi, H.; Tang, S.; Zhang, C.; Wang, X.; Cheng, M.; Shao, Z. A highly active and stable hybrid oxygen electrode for reversible solid oxide cells. Int. J. Hydrogen Energy. 2021, 46, 36012–36022. [Google Scholar]
- Mogensen, M.B.; Chen, M.; Frandsen, H.L.; Graves, C.; Hansen, J.B.; Hansen, K.V.; Hauch, A.; Jacobsen, T.; Jensen, S.H.; Skafte, T.L.; et al. Reversible solid-oxide cells for clean and sustainable energy. Clean Energy 2019, 3, 175–201. [Google Scholar] [CrossRef]
- Bianchi, F.R.; Bosio, B. Operating principles, performance and technology readiness level of reversible solid oxide cells. Sustainability 2021, 13, 4777. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).