New Insights into the Magnetic Properties of CoFe2O4@SiO2@Au Magnetoplasmonic Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples Preparation
2.2. Characterization
3. Results
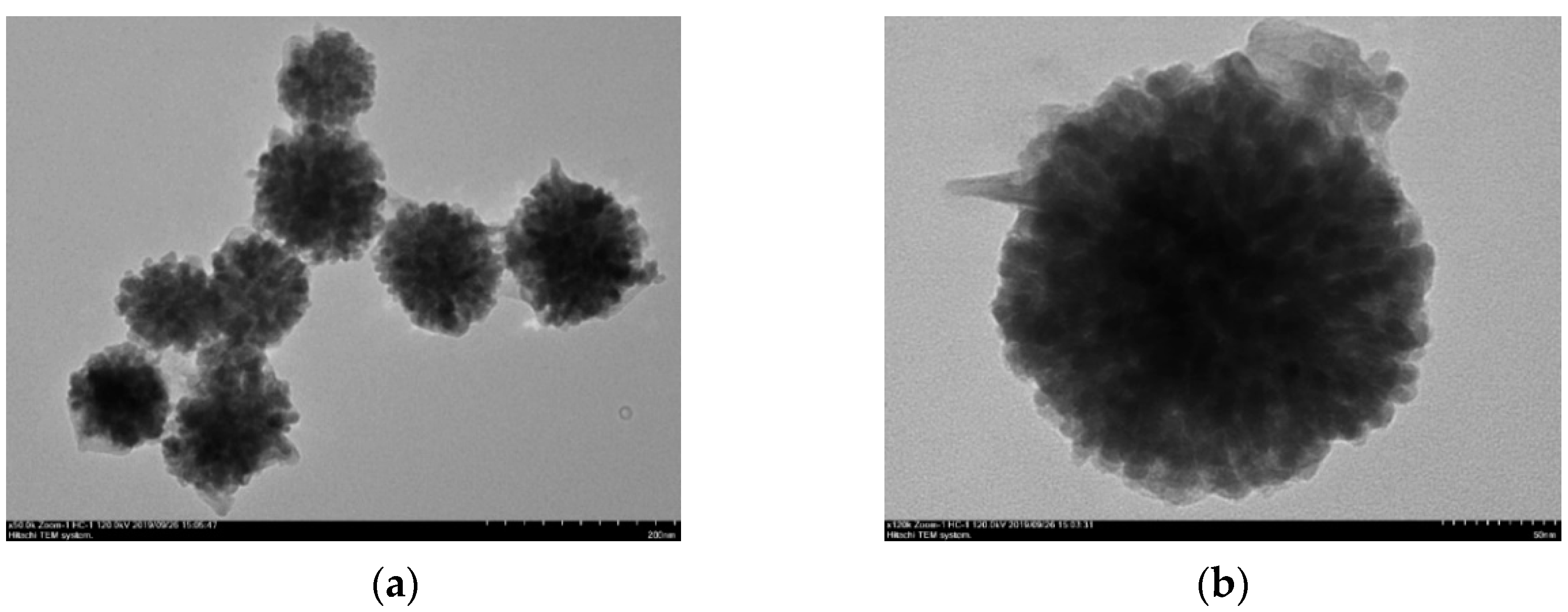
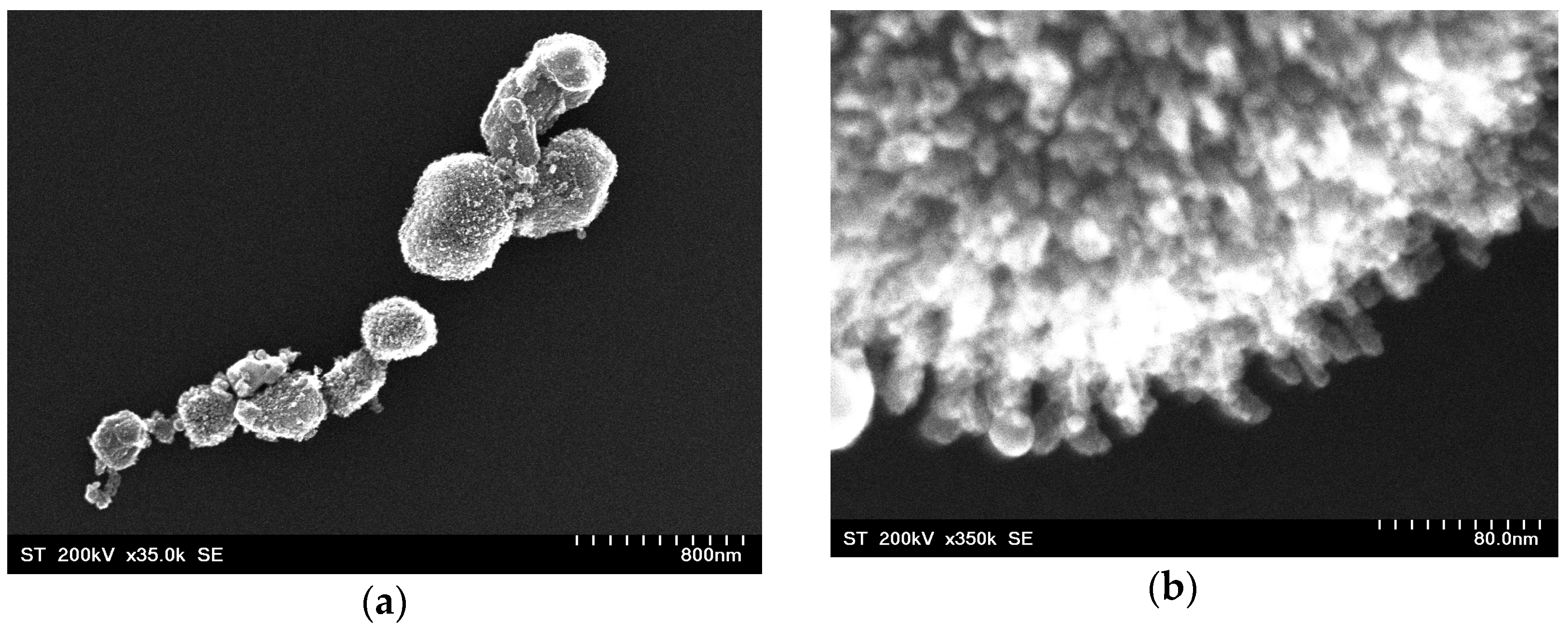
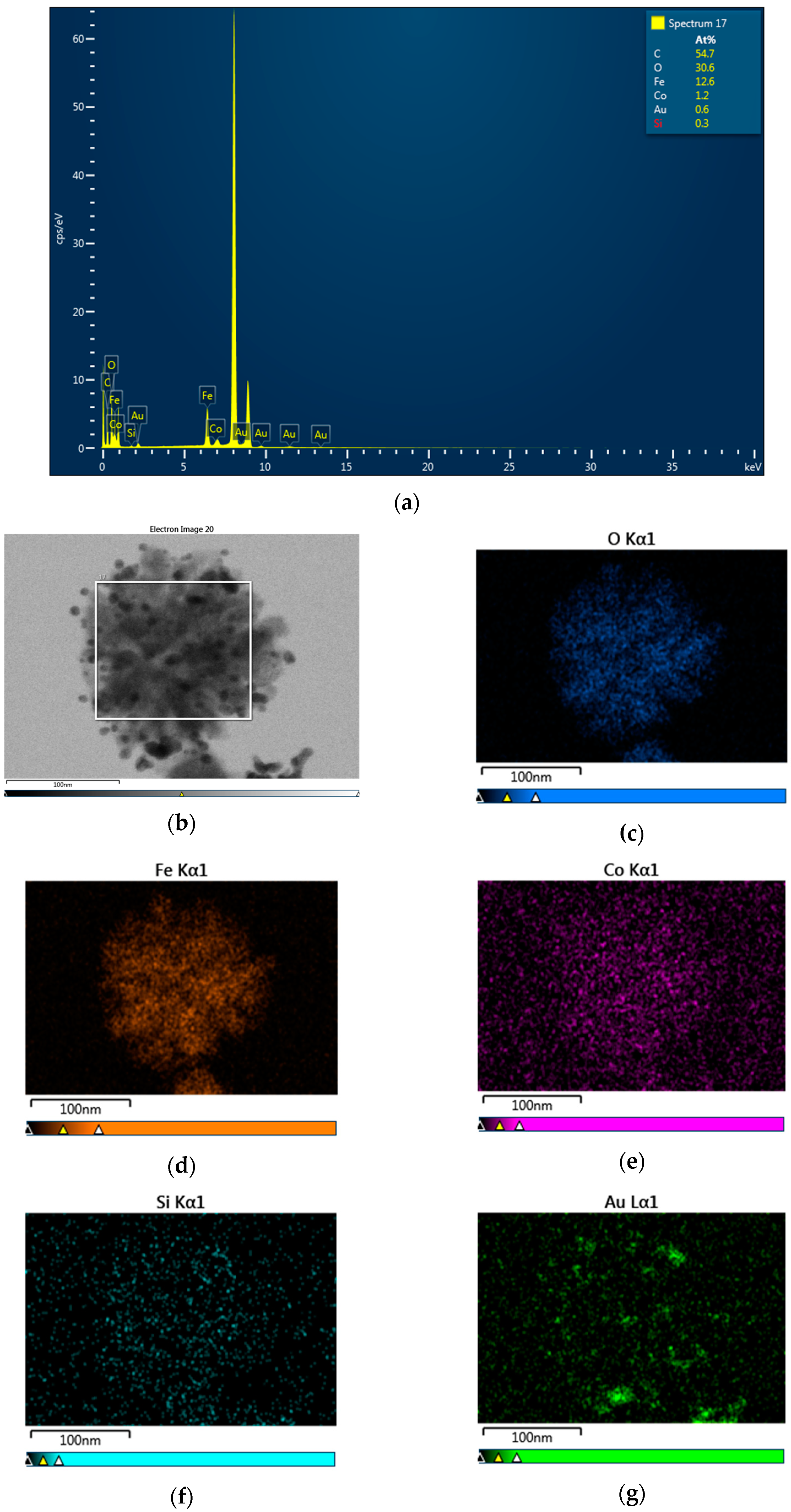
3.1. Morphology and Crystal Structure
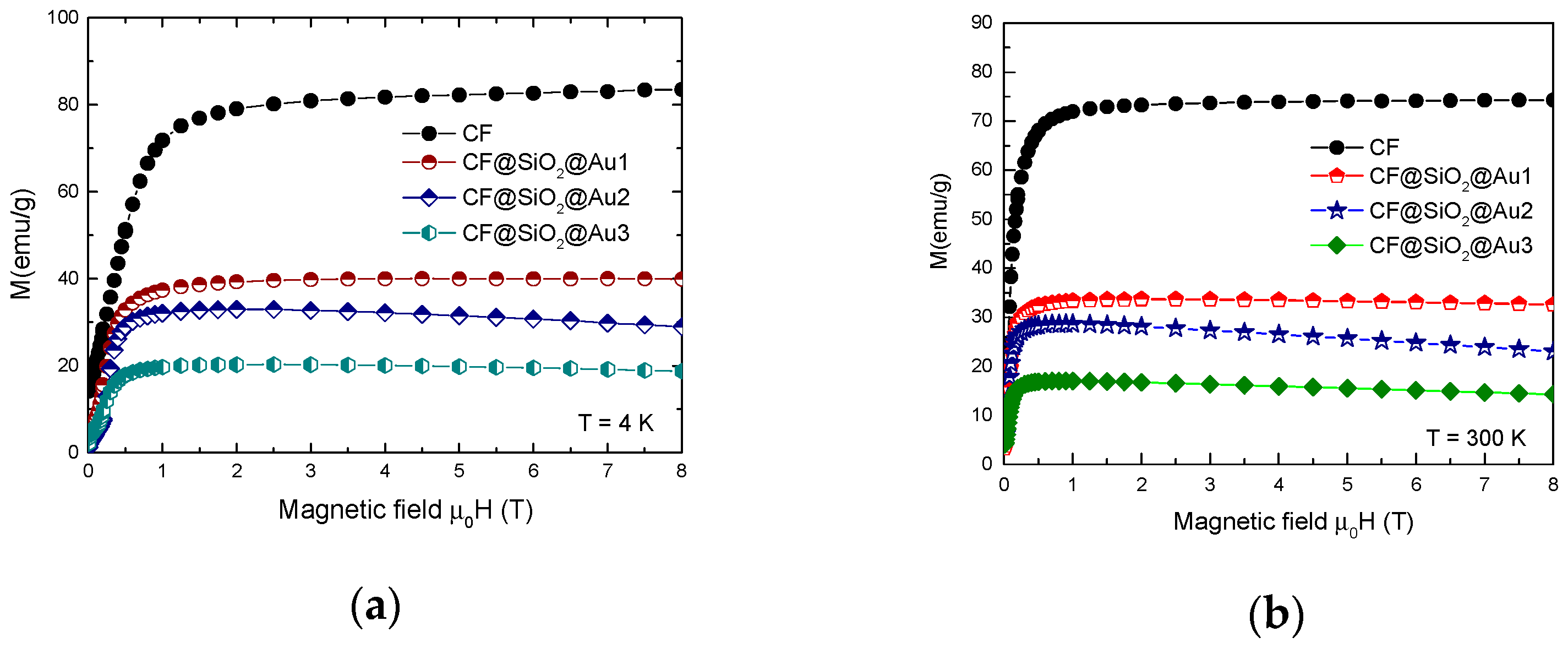
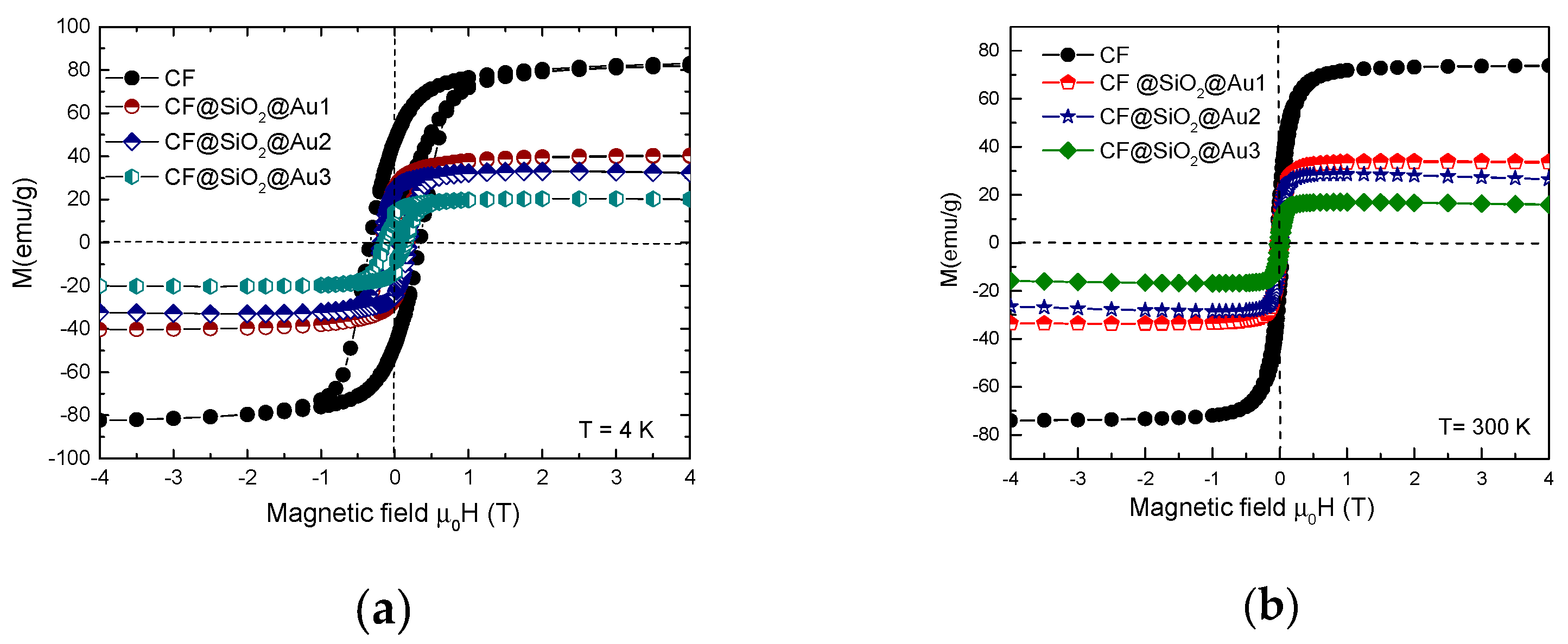
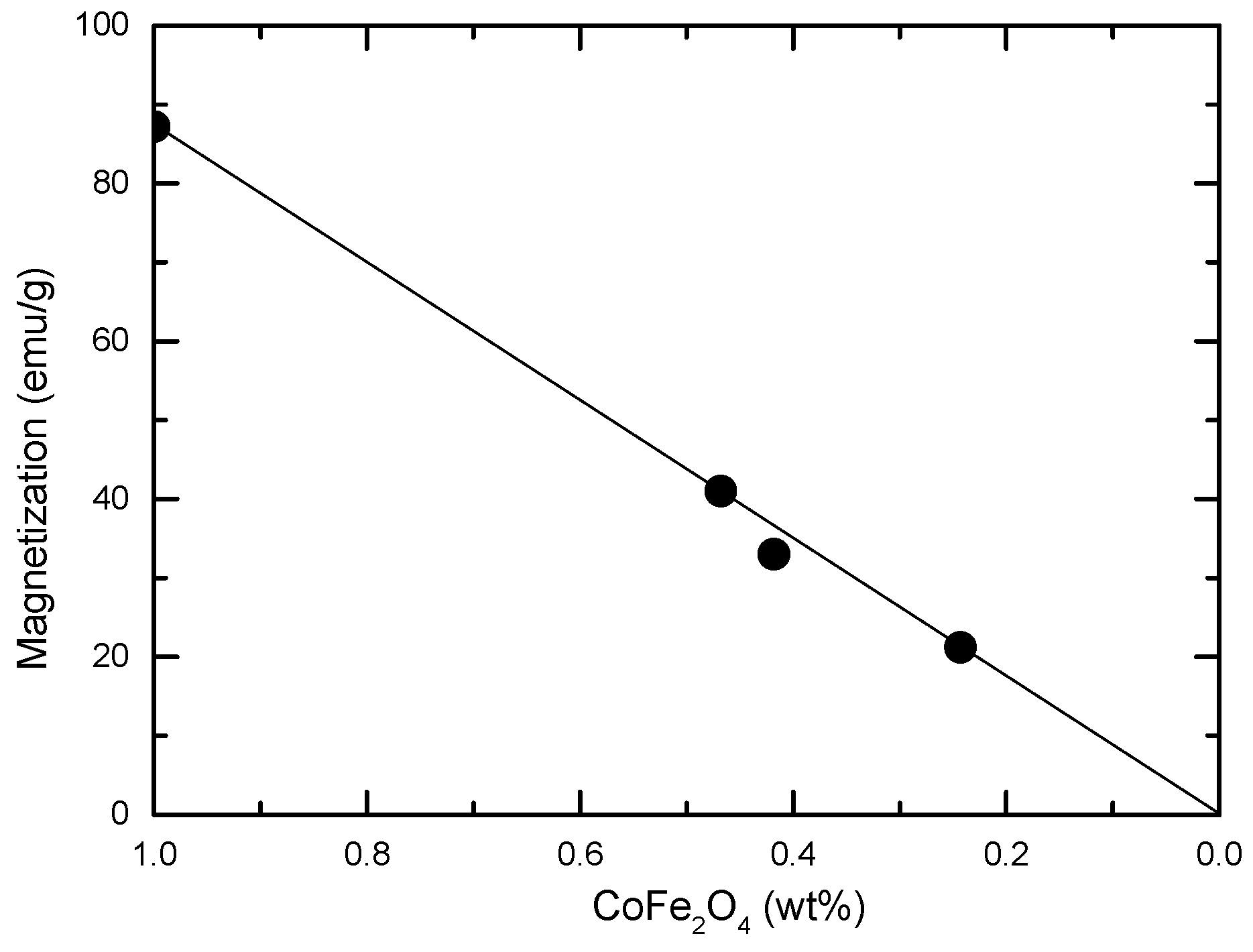
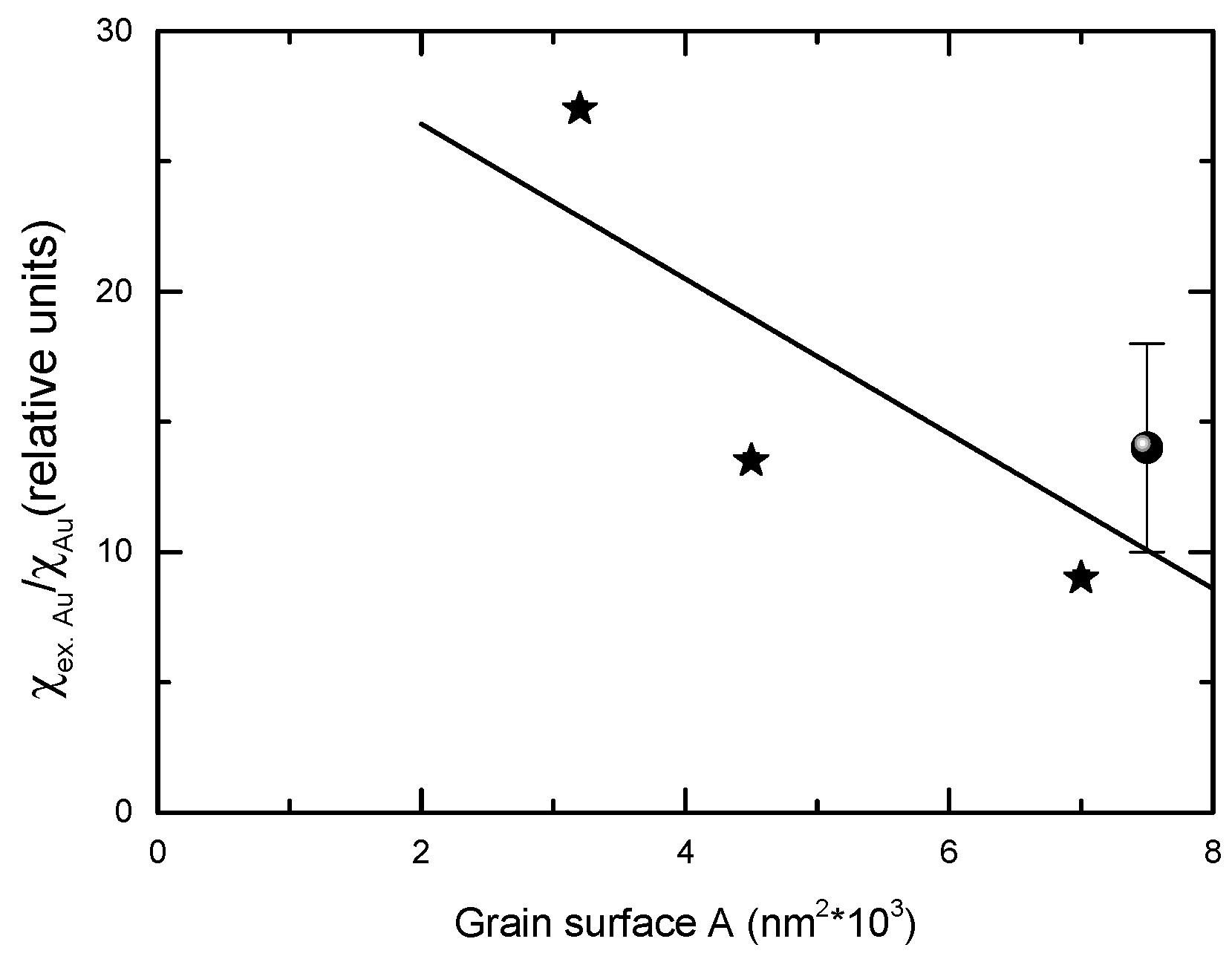
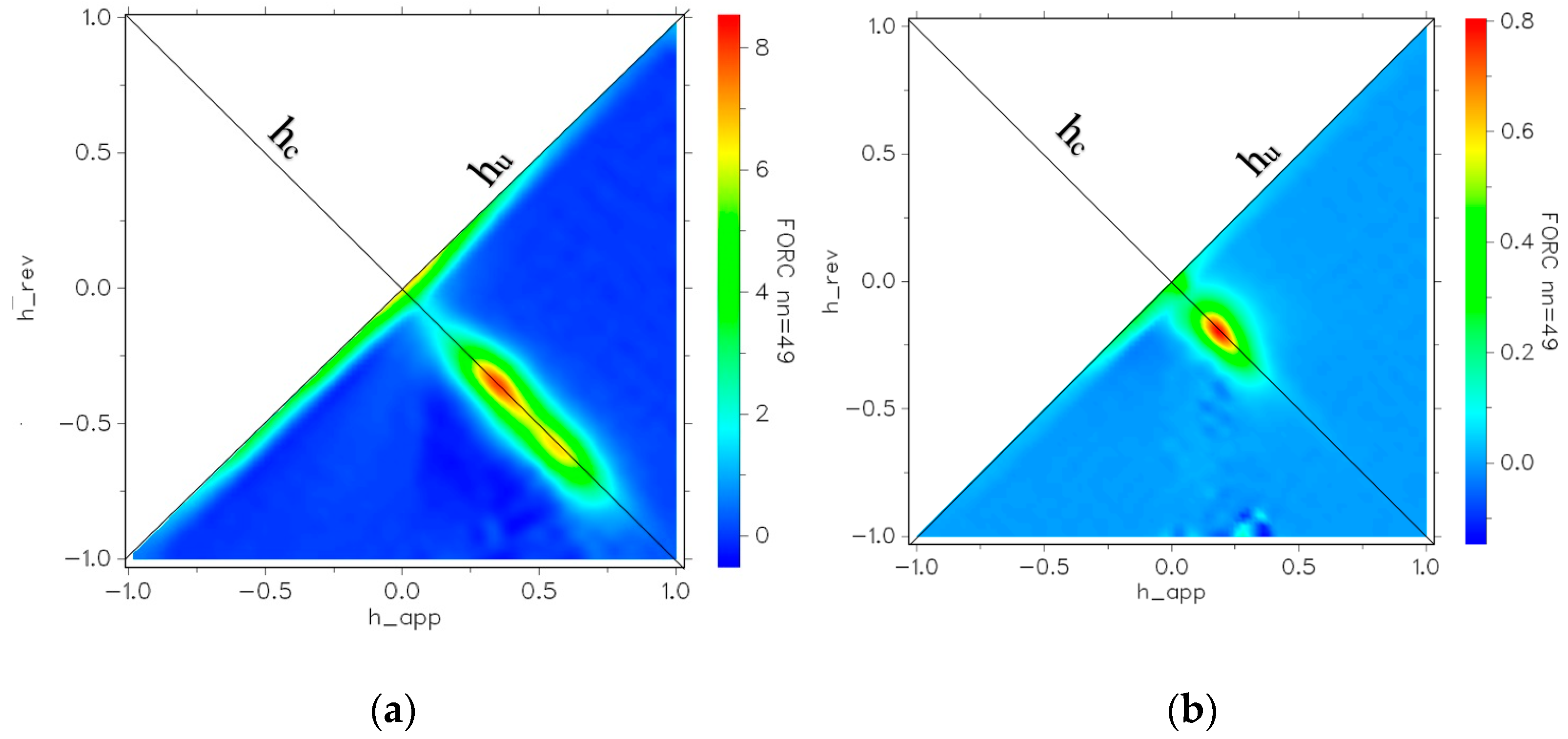
3.2. Magnetic Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sawatzky, G.A.; Van Der Woude, F.; Morrish, A.H. Mössbauer Study of Several Ferrimagnetic Spinels. Phys. Rev. 1969, 187, 747–757. [Google Scholar] [CrossRef]
- Srivastava, C.M.; Srinivasan, G.; Nanadikar, N.G. Exchange constants in spinel ferrites. Phys. Rev. B 1979, 19, 499–508. [Google Scholar] [CrossRef] [Green Version]
- Chinnasamy, C.N.; Jeyadevan, B.; Shinoda, K.; Tohji, K.; Djayaprawira, D.J.; Takahashi, M.; Joseyphus, J.; Narayanasamy, A. Unusually high coercivity and critical single-domain size of nearly monodispersed CoFe2O4 nanoparticles. Appl. Phys. Lett. 2003, 83, 2862–2864. [Google Scholar] [CrossRef]
- Sun, Q.-C.; Birkel, C.S.; Cao, J.; Tremel, W.; Musfeldt, J.L. Spectroscopic signature of the superparamagnetic transition and surface spin disorder in CoFe2O4 nanoparticles. ACS Nano 2012, 6, 4876–4883. [Google Scholar] [CrossRef] [PubMed]
- Peddis, D.; Yaacoub, N.; Ferretti, M.; Martinelli, A.; Piccaluga, G.; Musinu, A.; Cannas, C.; Navarra, G.; Greneche, J.-M.; Fiorani, D. Cationic distribution and spin canting in CoFe2O4 nanoparticles. J. Phys. Condens. Matter 2011, 23, 426004. [Google Scholar] [CrossRef]
- Peddis, D.; Cannas, C.; Musinu, A.; Piccaluga, G. Coexistence of superparmagnetism and spin-glass like magnetic ordering phenomena in a CoFe2O4−SiO2 nanocomposite. J. Phys. Chem. C 2008, 112, 5141–5147. [Google Scholar] [CrossRef]
- Chiu, C.-W.; Lin, P.-H. Core/shell Ag@silicate nanoplatelets and poly(Vinyl Alcohol) spherical nanohybrids fabricated by coaxial electrospraying as highly sensitive SERS substrates. RSC Adv. 2016, 6, 67204–67211. [Google Scholar] [CrossRef]
- Prabhakaran, T.; Mangalaraja, R.; Denardin, J.; Jiménez, J.A. The effect of reaction temperature on the structural and magnetic properties of nano CoFe2O4. Ceram. Int. 2017, 43, 5599–5606. [Google Scholar] [CrossRef]
- Kiran, B.L.; Rami, R.Y.V. Synthesis and characterization of magnetically core-shell structured CoFe2O4/SiO2 nanoparticles; Their enhanced antibacterial and electrocatalytic properties&rdquo. Coll. Surf. A Physicochem. Eng. Asp. 2020, 598, 124806. [Google Scholar] [CrossRef]
- Lin, Y.-S.; Haynes, C.L. Synthesis and characterization of biocompatible and size-tunable multifunctional porous silica nanoparticles. Chem. Mater. 2009, 21, 3979–3986. [Google Scholar] [CrossRef]
- Cannas, C.; Musinu, A.; Ardu, A.; Orrù, F.; Peddis, D.; Casu, M.; Sanna, R.; Angius, F.; Diaz, G.; Piccaluga, G. CoFe2O4 and CoFe2O4/SiO2 core/shell nanoparticles: Magnetic and spectroscopic study. Chem. Mater. 2010, 22, 3353–3361. [Google Scholar] [CrossRef]
- Parma, A.; Freris, I.; Riello, P.; Cristofori, D.; Fernández, C.D.J.; Amendola, V.; Meneghetti, M.; Benedetti, A. Structural and magnetic properties of mesoporous SiO2 nanoparticles impregnated with iron oxide or cobalt-iron oxide nanocrystals. J. Mater. Chem. 2012, 22, 19276–19288. [Google Scholar] [CrossRef]
- Carlà, F.; Campo, G.; Sangregorio, C.; Caneschi, A.; Fernández, C.D.J.; Cabrera, L.I. Electrochemical characterization of core@shell CoFe2O4/Au composite. J. Nanoparticle Res. 2013, 15, 1–16. [Google Scholar] [CrossRef]
- Zhang, X.; Niu, Y.; Zhao, J.; Li, Y. Fabrication, structures and molecule detection of gold films coated on γ-Fe2O3@SiO2 ellipsoid ordered arrays. Coll. Surf. A Physicochem. Eng. Asp. 2017, 520, 343–347. [Google Scholar] [CrossRef]
- Kassim, S.; Tahrin, R.A.A.; Harun, N.A. Metallic Core-Shell Photonic Crystals for Surface-Enhanced Raman Scattering (SERS). Plasmonics 2020, 15, 1499–1505. [Google Scholar] [CrossRef]
- Li, Y.; Duan, W.; Wei, J. CoFe2O4/Ag nanostructures as highly sensitive surface-enhanced raman scattering substrates. ACS Appl. Nano Mater. 2021, 4, 4181–4188. [Google Scholar] [CrossRef]
- Huynh, K.-H.; Hahm, E.; Noh, M.; Lee, J.-H.; Pham, X.-H.; Lee, S.; Kim, J.; Rho, W.-Y.; Chang, H.; Kim, D.; et al. Recent advances in surface-enhanced raman scattering magnetic plasmonic particles for bioapplications. Nanomaterials 2021, 11, 1215. [Google Scholar] [CrossRef] [PubMed]
- Dudric, R.; Souca, G.; Szatmári, Á.; Szilárd, T.; Nitica, S.; Iacovita, C.; Moldovan, A.I.; Stiufiuc, R.; Tetean, R.; Burzo, E. Magnetite nanoparticles for medical applications. AIP Conf. Proc. 2020, 2218, 030014. [Google Scholar] [CrossRef]
- Souca, G.; Dudric, R.; Iacovita, C.; Moldovan, A.; Frentiu, T.; Stiufiuc, R.; Lucaciu, C.M.; Tetean, R.; Burzo, E. Physical properties of zn doped Fe3O4 nanoparticles. J. Optoelectron. Adv. Mater. 2020, 22, 298–302. [Google Scholar]
- Tejada, J.; Martinez, B.; Labarta, A.; Chudnovsky, E.M. Correlated spin glass generated by structural disorder in the amorphous Dy6Fe74B20 alloy. Phys. Rev. B 1991, 44, 7698. [Google Scholar] [CrossRef] [Green Version]
- Serrate, D.; De Teresa, J.M.; Algarabel, P.A.; Ibarra, M.R.; Galibert, J. Intergrain magnetoresistance up to 50 T in the half-metallic(Ba0.8Sr0.2)2FeMoO6double perovskite: Spin-glass behavior of the grain boundary. Phys. Rev. B 2005, 71, 104409. [Google Scholar] [CrossRef]
- Burzo, E.; Balasz, I.; Valeanu, M.; Pop, I. The effects of thermal treatment on the physical properties of Sr2FeMo1−xMxO6 perovskite with M=W, Ta and 0.3. J. Alloy. Compd. 2011, 509, 105–113. [Google Scholar] [CrossRef]
- Burzo, E. Magnetic and transport properties of double perovskites. Stud. Univ. Babes-Bolyai. Chem. 2021, 66, 63–72. [Google Scholar] [CrossRef]
- Concas, G.; Spano, G.; Cannas, C.; Musinu, A.; Peddis, D.; Piccaluga, G. Inversion degree and saturation magnetization of different nanocrystalline cobalt ferrites. J. Magn. Magn. Mater. 2009, 321, 1893–1897. [Google Scholar] [CrossRef]
- Venturini, J.; Tonelli, A.M.; Wermuth, T.B.; Zampiva, R.Y.S.; Arcaro, S.; Viegas, A.D.C.; Bergmann, C.P. Excess of cations in the sol-gel synthesis of cobalt ferrite (CoFe2O4): A pathway to switching the inversion degree of spinels. J. Magn. Magn. Mater. 2019, 482, 1–8. [Google Scholar] [CrossRef]
- Caruntu, D.; Caruntu, G.; O’Connor, C.J. Magnetic properties of variable-sized Fe3O4 nanoparticles synthesized from non-aqueous homogeneous solutions of polyols. J. Phys. D Appl. Phys. 2007, 40, 5801–5809. [Google Scholar] [CrossRef] [Green Version]
- Stoner, E.C.; Wohlfarth, E.P. A mechanism of magnetic hysteresis in heterogeneous alloys. Philos. Trans. R. Soc. Lond. Ser. A Math. Phys. Sci. 1948, 240, 599–642. [Google Scholar] [CrossRef]
- Gittleman, J.I.; Abeles, B.; Bozowski, S. Superparamagnetism and relaxation effects in granular Ni-SiO2 and Ni-Al2O3 films. Phys. Rev. B 1974, 9, 3891. [Google Scholar] [CrossRef]
- Omelyanchik, A.; Salvador, M.; D’Orazio, F.; Mameli, V.; Cannas, C.; Fiorani, D.; Musinu, A.; Rivas, M.; Rodionova, V.; Varvaro, G.; et al. Magnetocrystalline and surface anisotropy in CoFe2O4 nanoparticles. Nanomaterials 2020, 10, 1288. [Google Scholar] [CrossRef] [PubMed]
- Kondrashova, N.; Valtsifer, V.; Strelnikov, V.; Mitrofanov, V.; Uporov, S. Magnetic Properties of Siilica with Mesoporesd as MCM-48. 2015. Available online: https://www.ariel.ac.il/sites/conf/mmt/ws2015/service%20files/papers/124-130.pdf (accessed on 19 February 2022).
- Henry, W.G.; Rogers, J.L. XXI. The magnetic susceptibilities of copper, silver and gold and errors in the Gouy method. Philos. Mag. 1956, 1, 223–236. [Google Scholar] [CrossRef]
- Van Rhee, P.G.; Zijlstra, P.; Verhagen, T.G.A.; Aarts, J.; Katsnelson, M.I.; Maan, J.C.; Orrit, M.; Christianen, P.C.M. Giant magnetic susceptibility of gold nanorods detected by magnetic alignment. Phys. Rev. Lett. 2013, 111, 127202. [Google Scholar] [CrossRef] [Green Version]
- Hernando, A.; Ayuela, A.; Crespo, P.; Echenique, P.M. Giant diamagnetism of gold nanorods. New J. Phys. 2014, 16, 073043. [Google Scholar] [CrossRef] [Green Version]
- Roda-Llordes, M.; Gonzalez-Ballestero, C.; López, A.E.R.; Martínez-Pérez, M.J.; Luis, F.; Romero-Isart, O. Quantum size effects in the magnetic susceptibility of a metallic nanoparticle. Phys. Rev. B 2021, 104, L100407. [Google Scholar] [CrossRef]
- Kouhpanji, M.R.Z.; Stadler, B.J.H. Beyond the qualitative description of complex magnetic nanoparticle arrays using FORC measurement. Nano Express 2020, 1, 010017. [Google Scholar] [CrossRef]
- Muxworthy, A.; Heslop, D.; Williams, W. Influence of magnetostatic interactions on first-order-reversal-curve (FORC) diagrams: A micromagnetic approach. Geophys. J. Int. 2004, 158, 888–897. [Google Scholar] [CrossRef] [Green Version]
- Tanasa, R.; Stancu, A. Statistical Characterization of the FORC Diagram. IEEE Trans. Magn. 2006, 42, 3246–3248. [Google Scholar] [CrossRef]
- Dodrill, B.; Ohodnicki, P.; McHenry, M.; Leary, A. High-Temperature First-Order-Reversal-Curve (FORC) Study of Magnetic Nanoparticle Based Nanocomposite Materials. MRS Adv. 2017, 2, 2669–2674. [Google Scholar] [CrossRef]
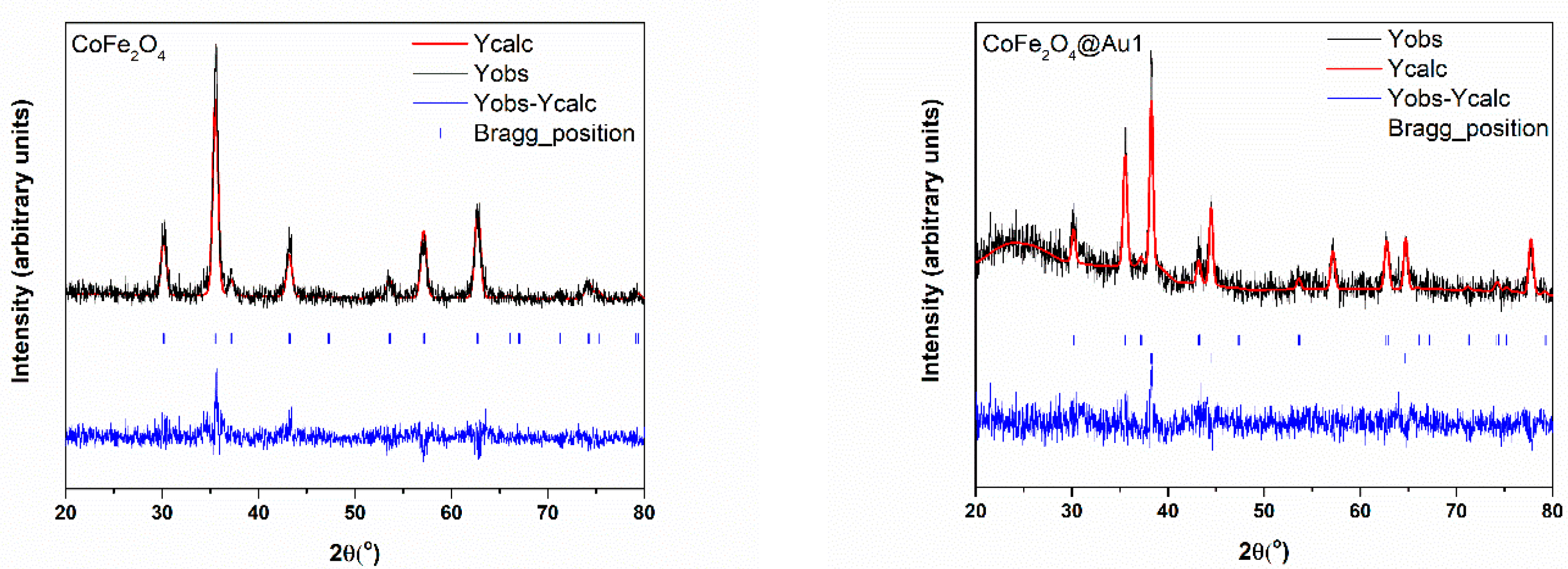
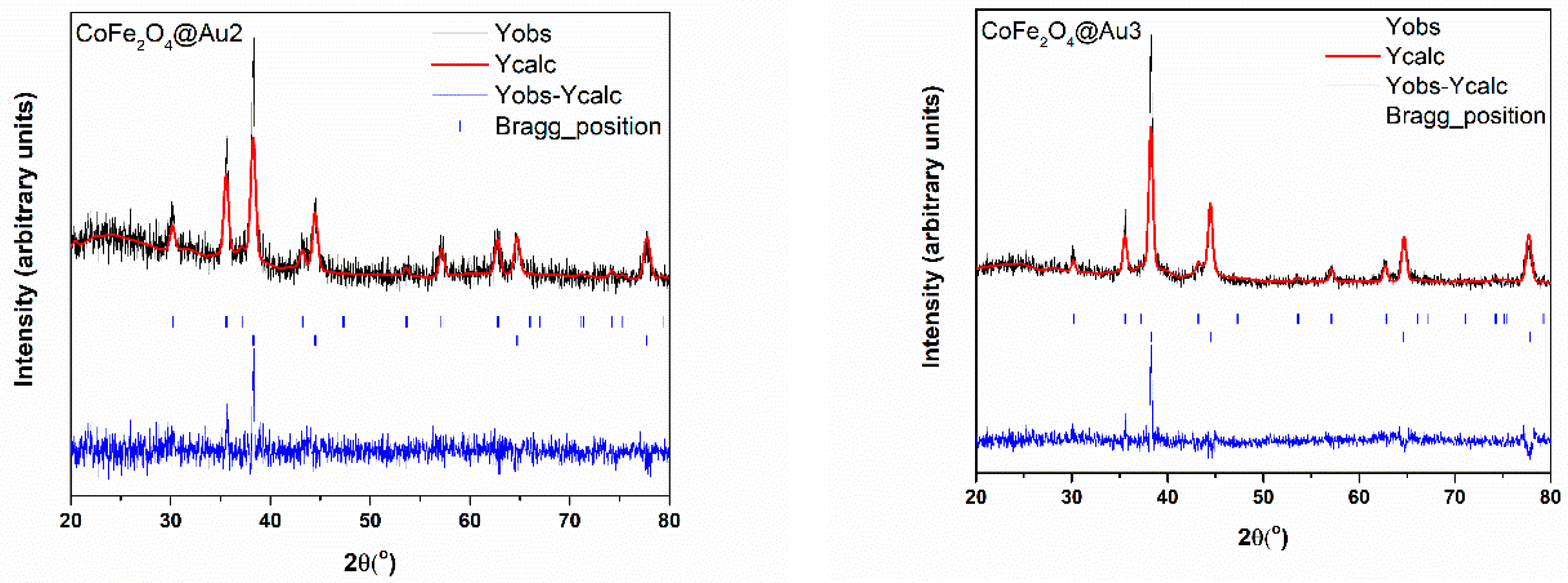
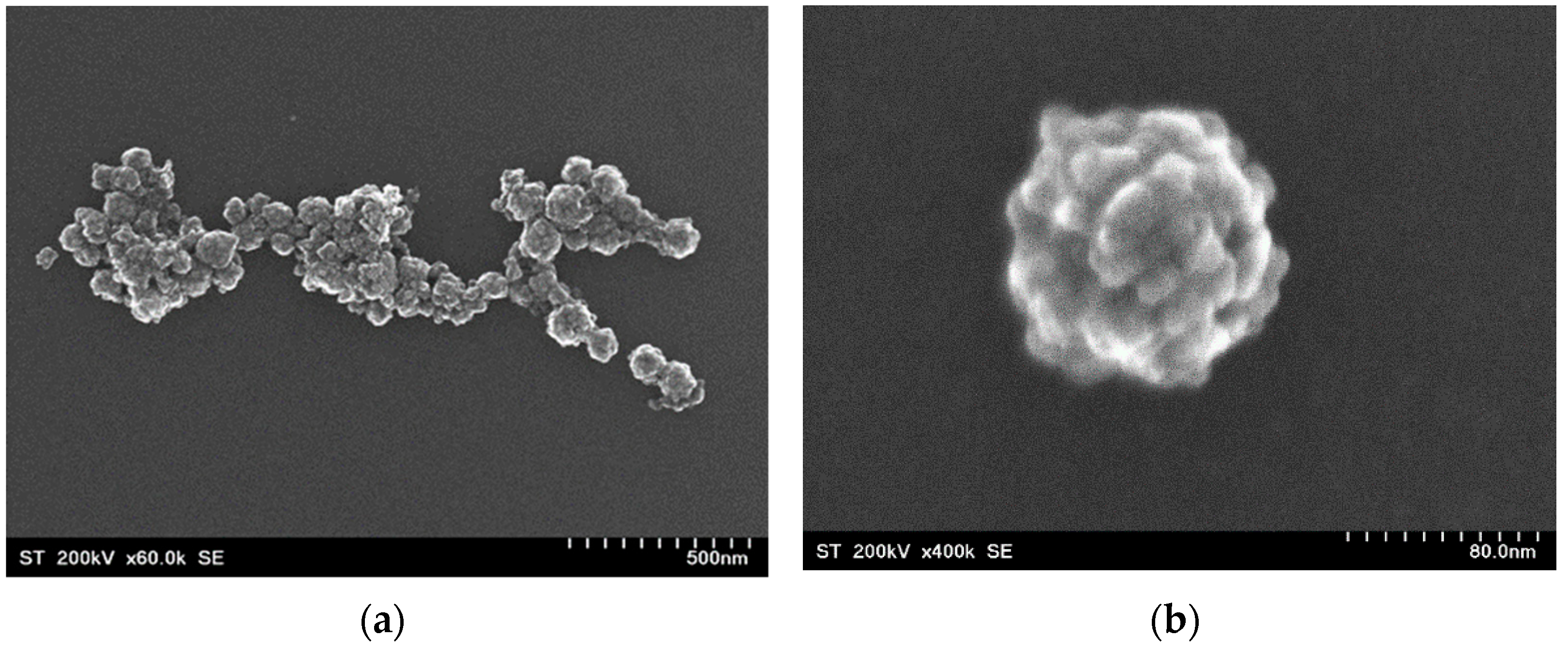
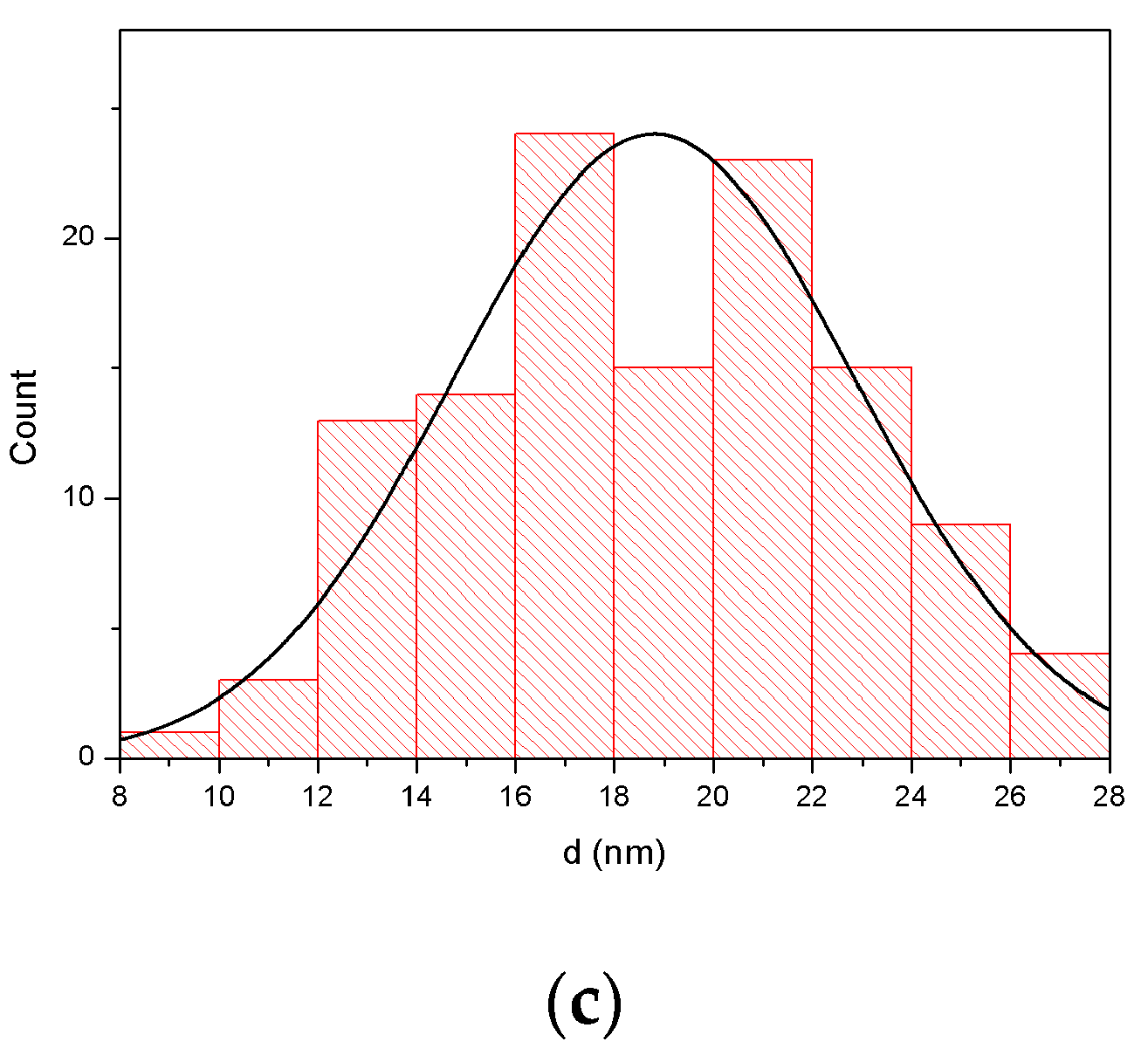

| Nanostructure | CoFe2O4 | Au1 | Au2 | Au3 | |
|---|---|---|---|---|---|
| Composition (weight %) | CoFe2O4 | 100 | 46.8 | 41.8 | 24.3 |
| SiO2 | - | 11.0 | 11.6 | 6.3 | |
| Au | - | 42.2 | 46.6 | 69.4 | |
| Lattice parameter (nm) | CoFe2O4 core | 0.8379 (9) | 0.8375 (4) | 0.8372 (9) | 0.8376 (9) |
| Crystallite size (nm | 14.2 (2) | 20.2 (3) | 17.3 (2) | 20.2 (2) | |
| Lattice parameter (nm) | Au shell | - | 0.4073 (2) | 0.4073 (9) | 0.4074 (7) |
| Crystallite size (nm | - | 23.57 (2) | 15.86 (4) | 18.88 (6) | |
| Nanocomposite magnetization/CoFe2O4 weight percent (emu/g) | 86.9 | 86.1 | 84.5 | 85.5 | |
| Coercive field HC(T) | T = 4.2 k | 0.35 | 0.20 | 0.28 | 0.19 |
| T = 300 k | 0.04 | 0.03 | 0.03 | 0.030 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bortnic, R.; Szatmari, A.; Souca, G.; Hirian, R.; Dudric, R.; Barbu-Tudoran, L.; Toma, V.; Știufiuc, R.; Tetean, R.; Burzo, E. New Insights into the Magnetic Properties of CoFe2O4@SiO2@Au Magnetoplasmonic Nanoparticles. Nanomaterials 2022, 12, 942. https://doi.org/10.3390/nano12060942
Bortnic R, Szatmari A, Souca G, Hirian R, Dudric R, Barbu-Tudoran L, Toma V, Știufiuc R, Tetean R, Burzo E. New Insights into the Magnetic Properties of CoFe2O4@SiO2@Au Magnetoplasmonic Nanoparticles. Nanomaterials. 2022; 12(6):942. https://doi.org/10.3390/nano12060942
Chicago/Turabian StyleBortnic, Rareș, Adam Szatmari, Gabriela Souca, Răzvan Hirian, Roxana Dudric, Lucian Barbu-Tudoran, Valentin Toma, Rareș Știufiuc, Romulus Tetean, and Emil Burzo. 2022. "New Insights into the Magnetic Properties of CoFe2O4@SiO2@Au Magnetoplasmonic Nanoparticles" Nanomaterials 12, no. 6: 942. https://doi.org/10.3390/nano12060942
APA StyleBortnic, R., Szatmari, A., Souca, G., Hirian, R., Dudric, R., Barbu-Tudoran, L., Toma, V., Știufiuc, R., Tetean, R., & Burzo, E. (2022). New Insights into the Magnetic Properties of CoFe2O4@SiO2@Au Magnetoplasmonic Nanoparticles. Nanomaterials, 12(6), 942. https://doi.org/10.3390/nano12060942









