Compound Heat Transfer Augmentation of a Shell-and-Coil Ice Storage Unit with Metal-Oxide Nano Additives and Connecting Plates
Abstract
:1. Introduction
2. Computational Simulation
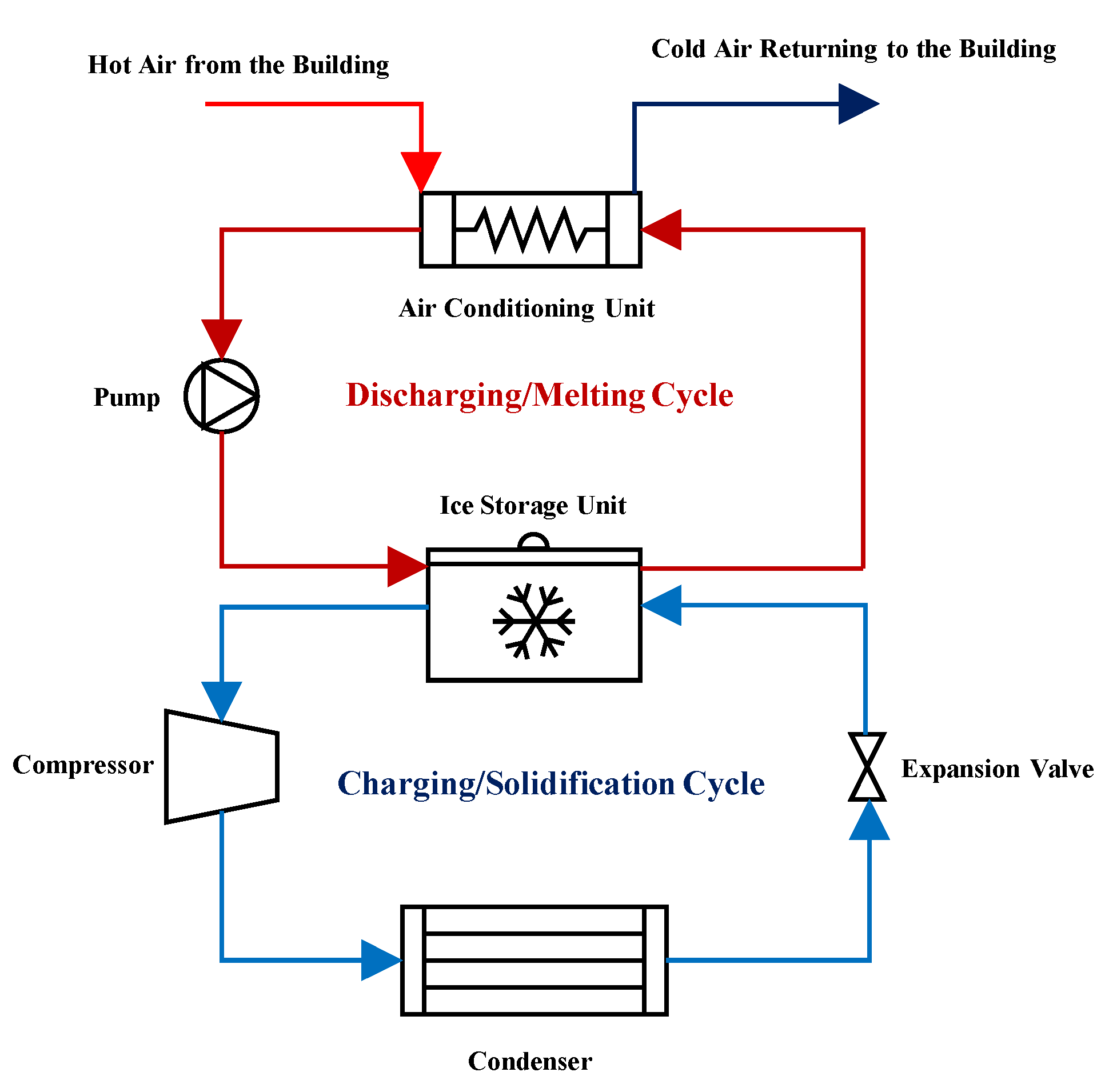
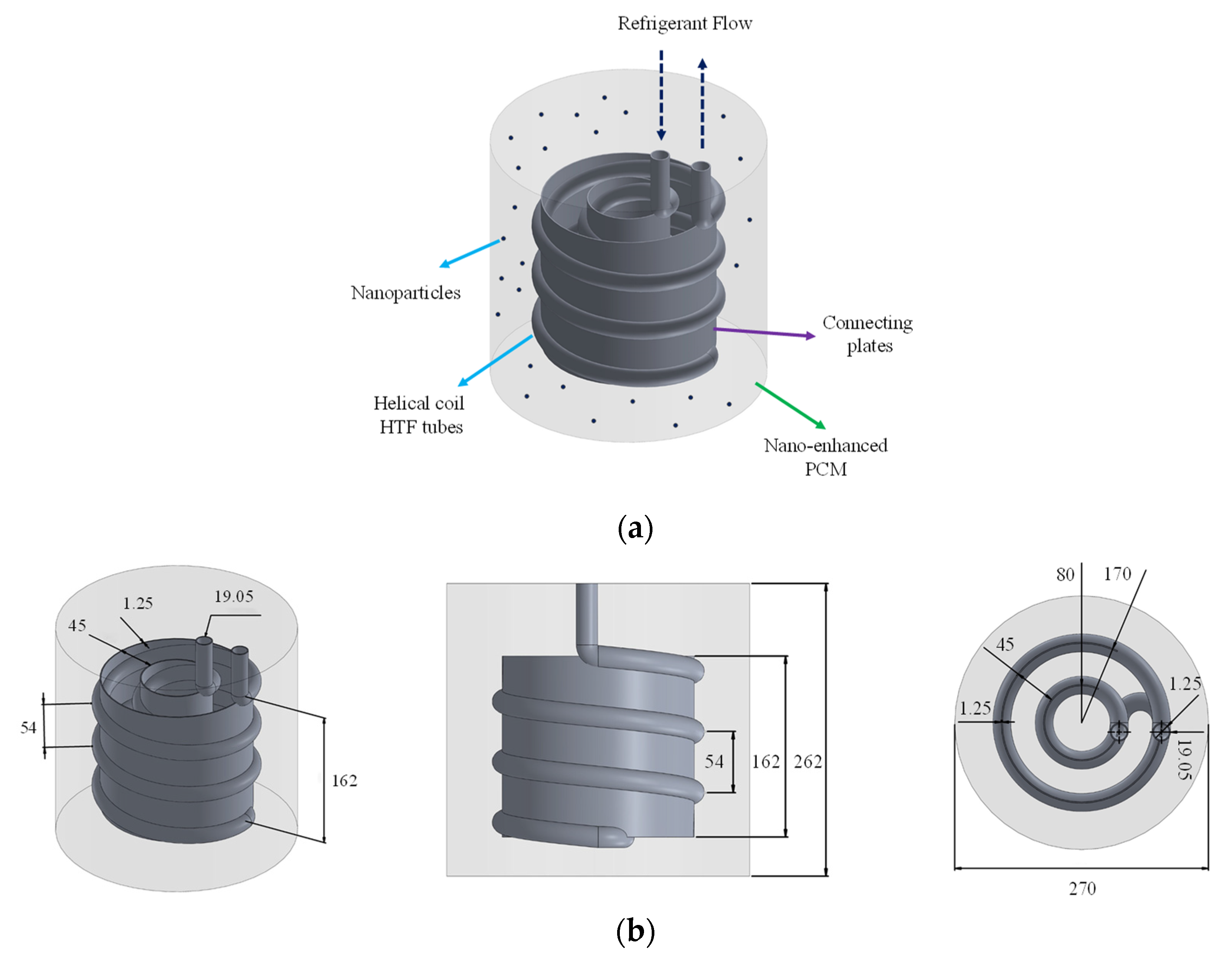
2.1. Problem Description
- The coolant helical coils carry the coolant flow to cool down the storage;
- The water in the container acts as the PCM and freezes as the heat in the container is absorbed by the coolant tubes;
- The plates are used as fins to distribute the coldness of the tubes more uniformly in the container;
- Nanomaterial additives are added to the PCM to thermally enhance it and improve its thermal conductivity.
- The process is transient;
- The flow within the entire system is incompressible and laminar;
- Various thermophysical values are applied for ice and water;
- The volume change throughout the process is ignored;
- The Boussinesq approximation is utilized to contemplate the results of natural convection;
- The NPs are homogeneously dispersed within the PCM using an ultrasonic homogenizer;
- The insulation layers over the storage are thick enough to impose a heat flux of zero.
2.2. Governing Equations, Solution Condition, and Method
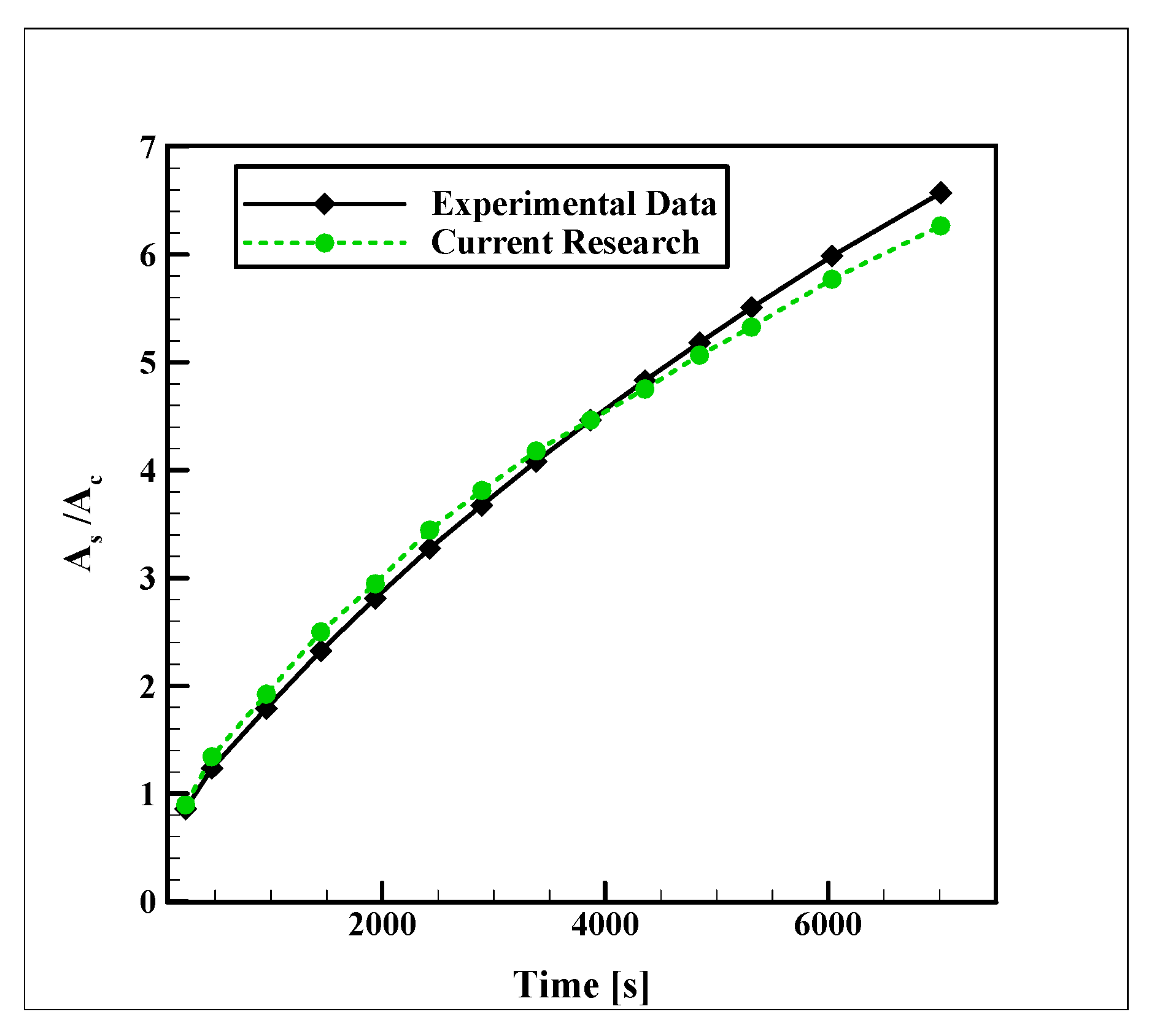
2.3. Validation and Sensitivity Analyses
3. Results
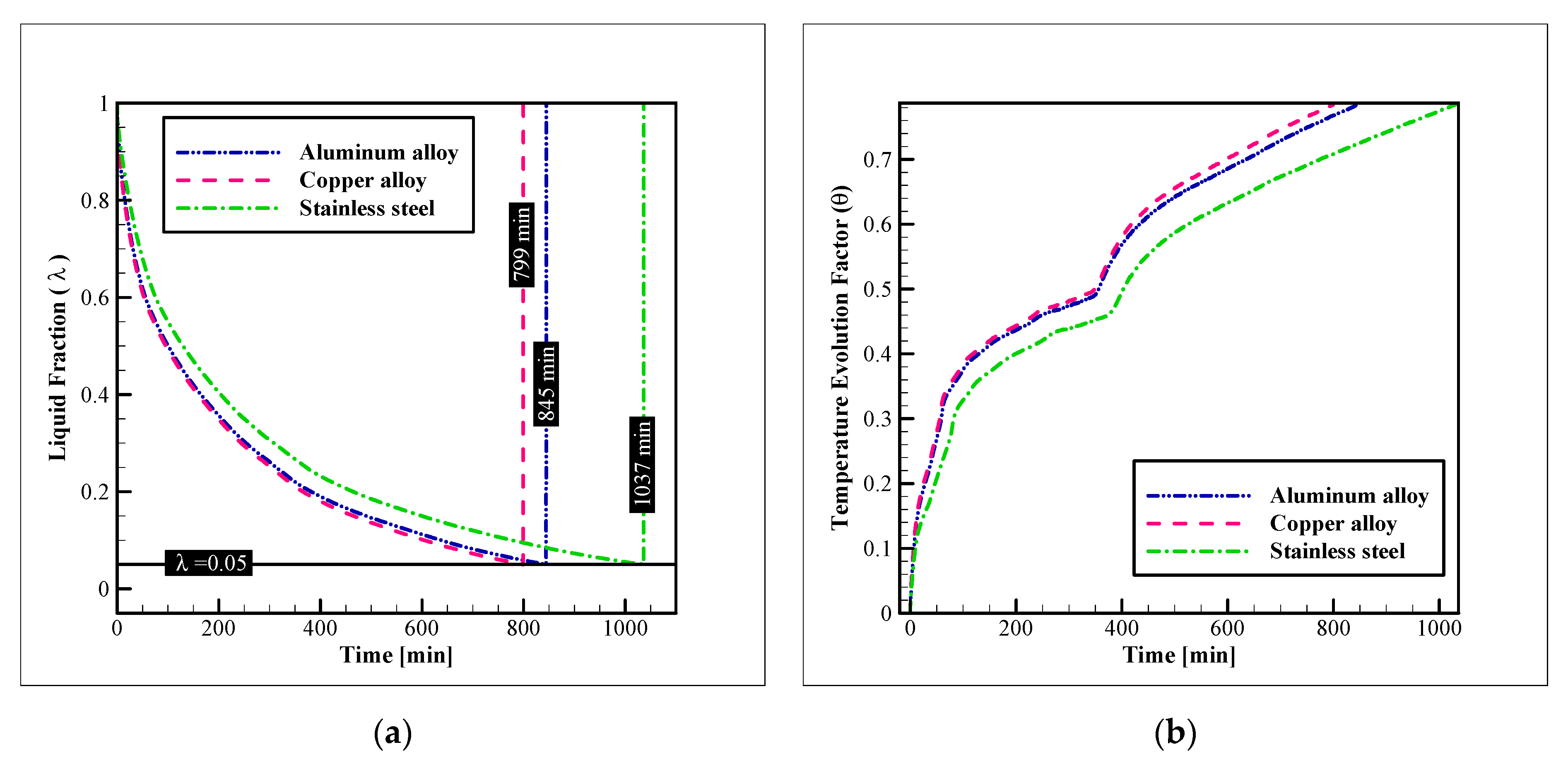
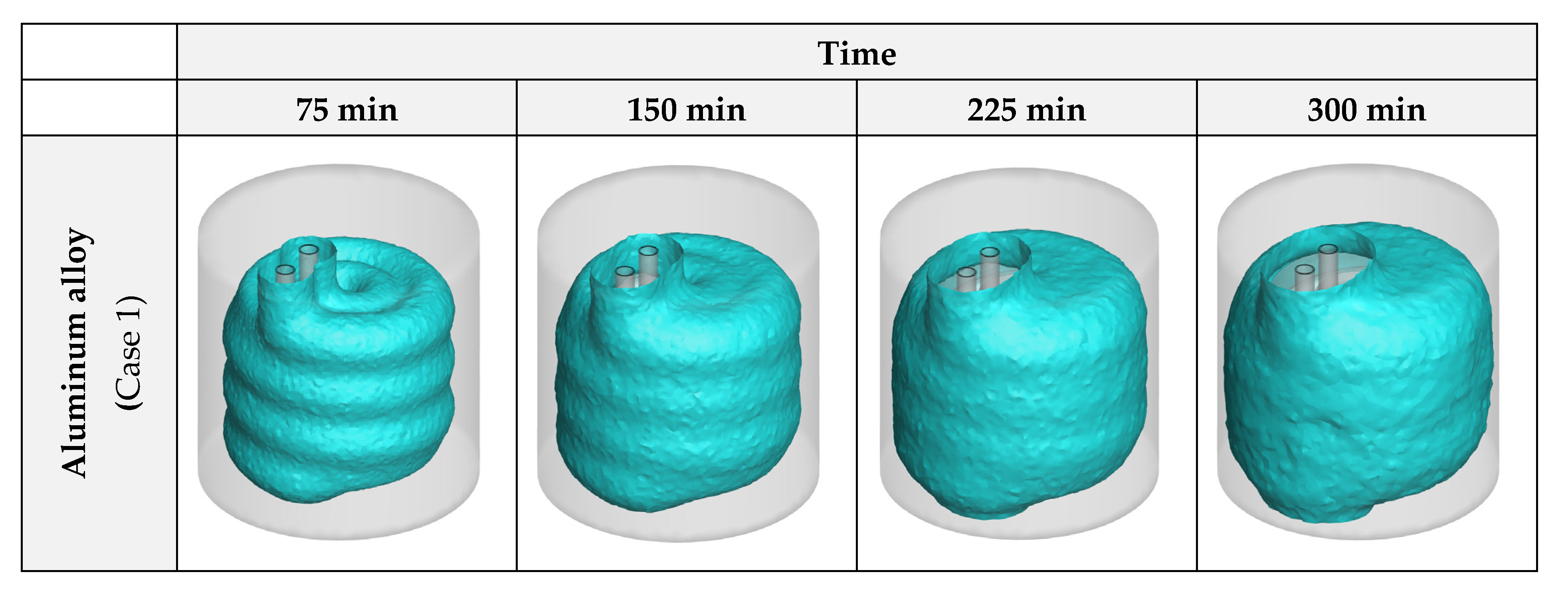
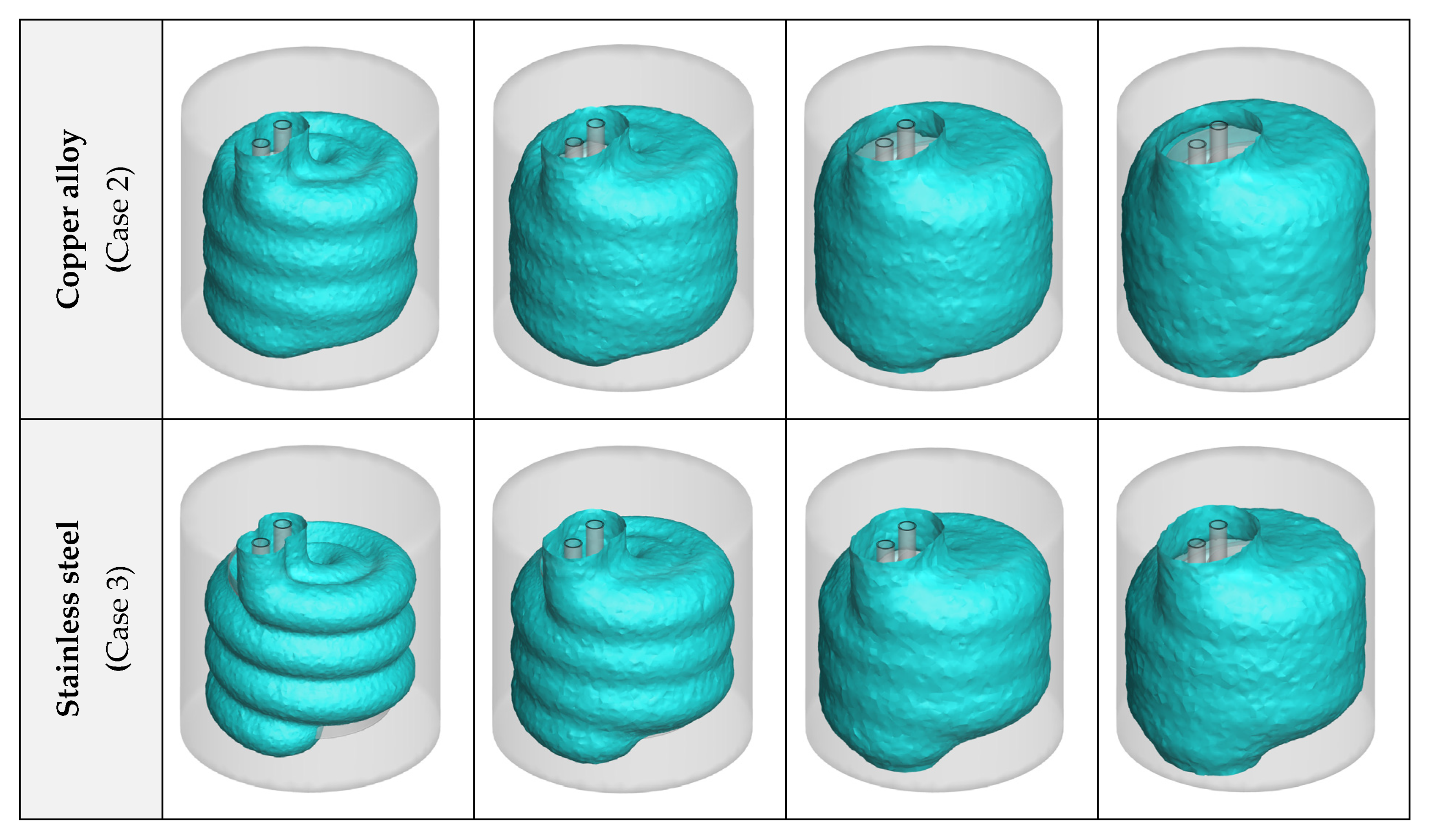
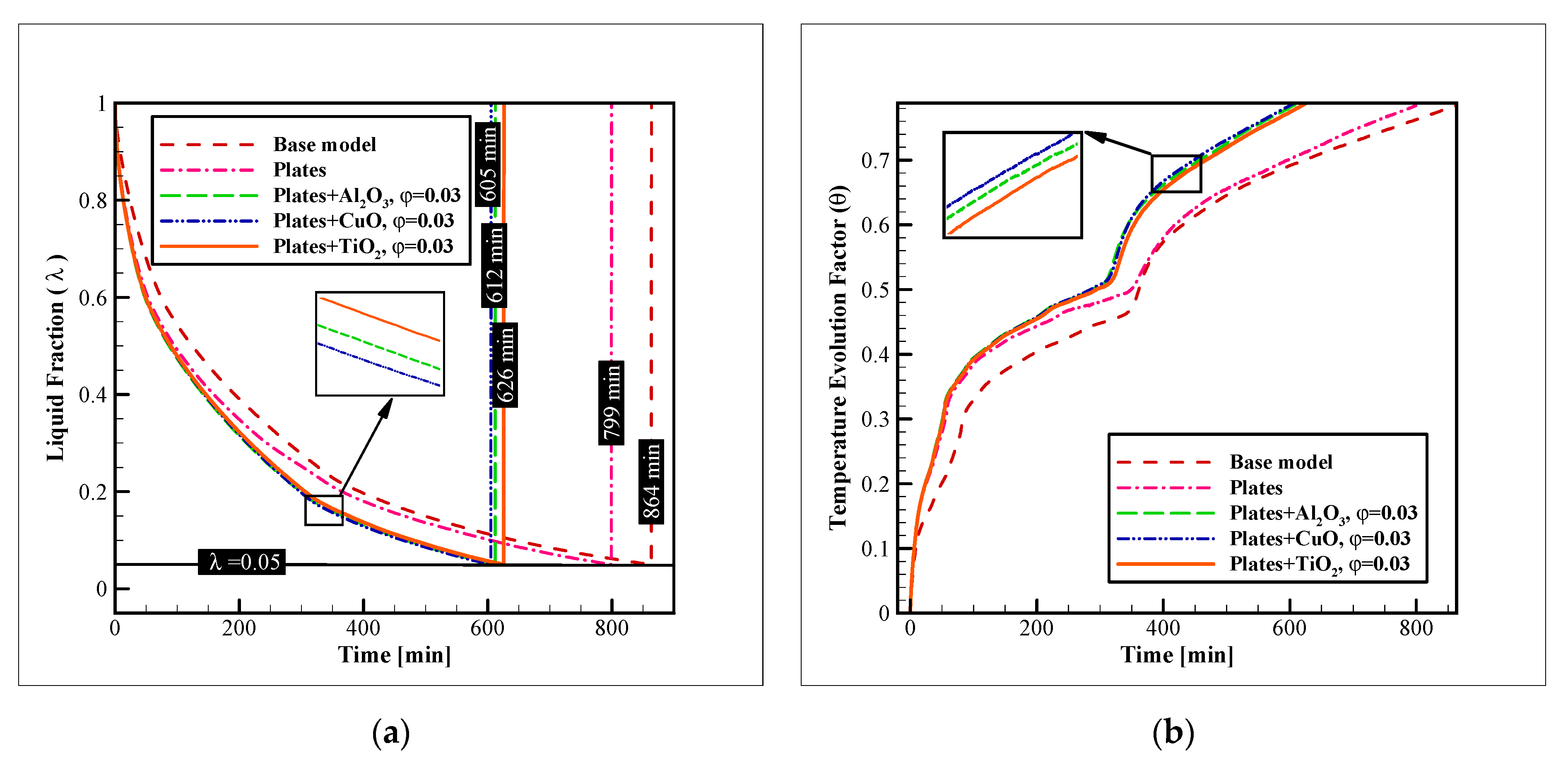
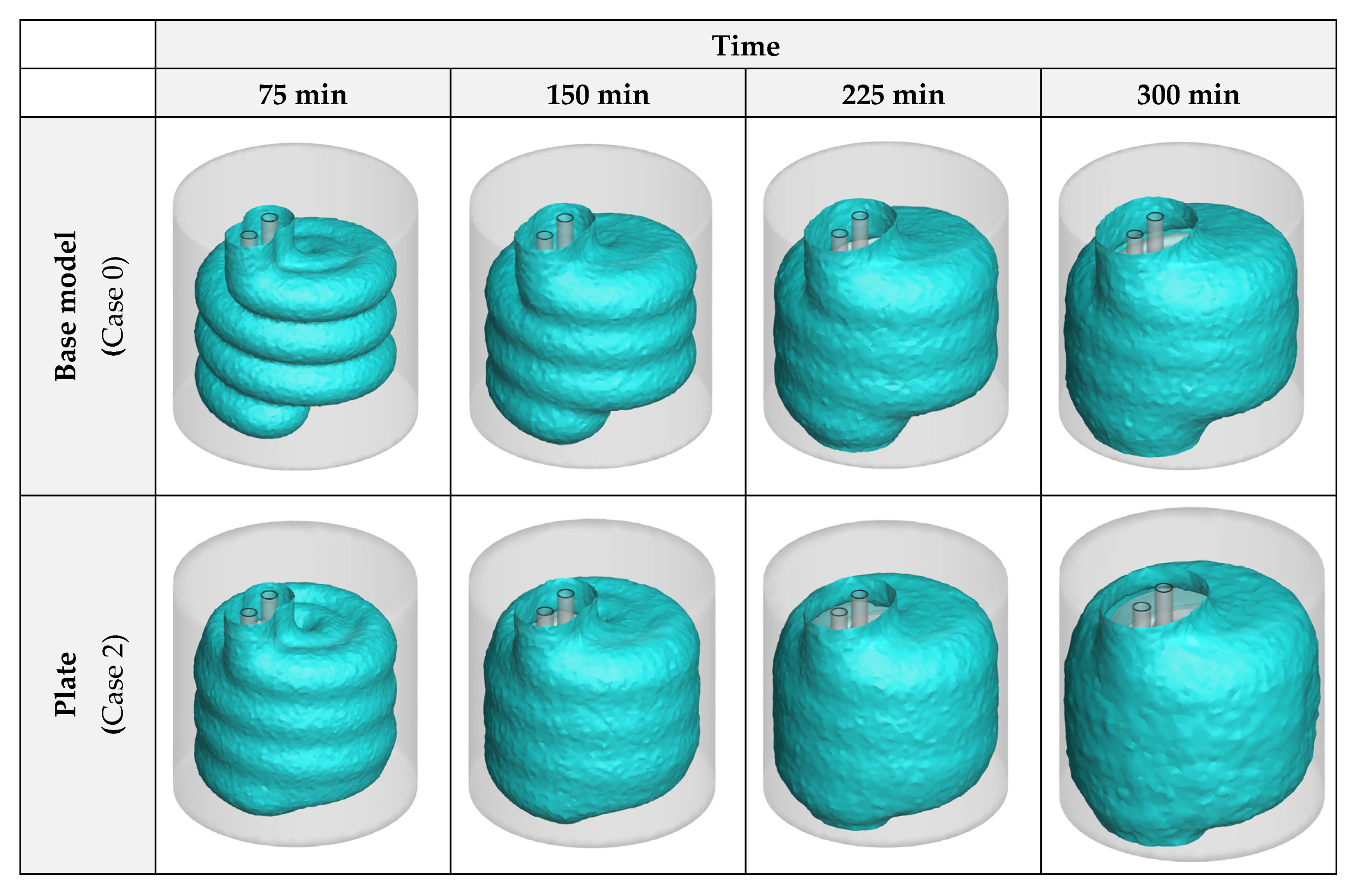
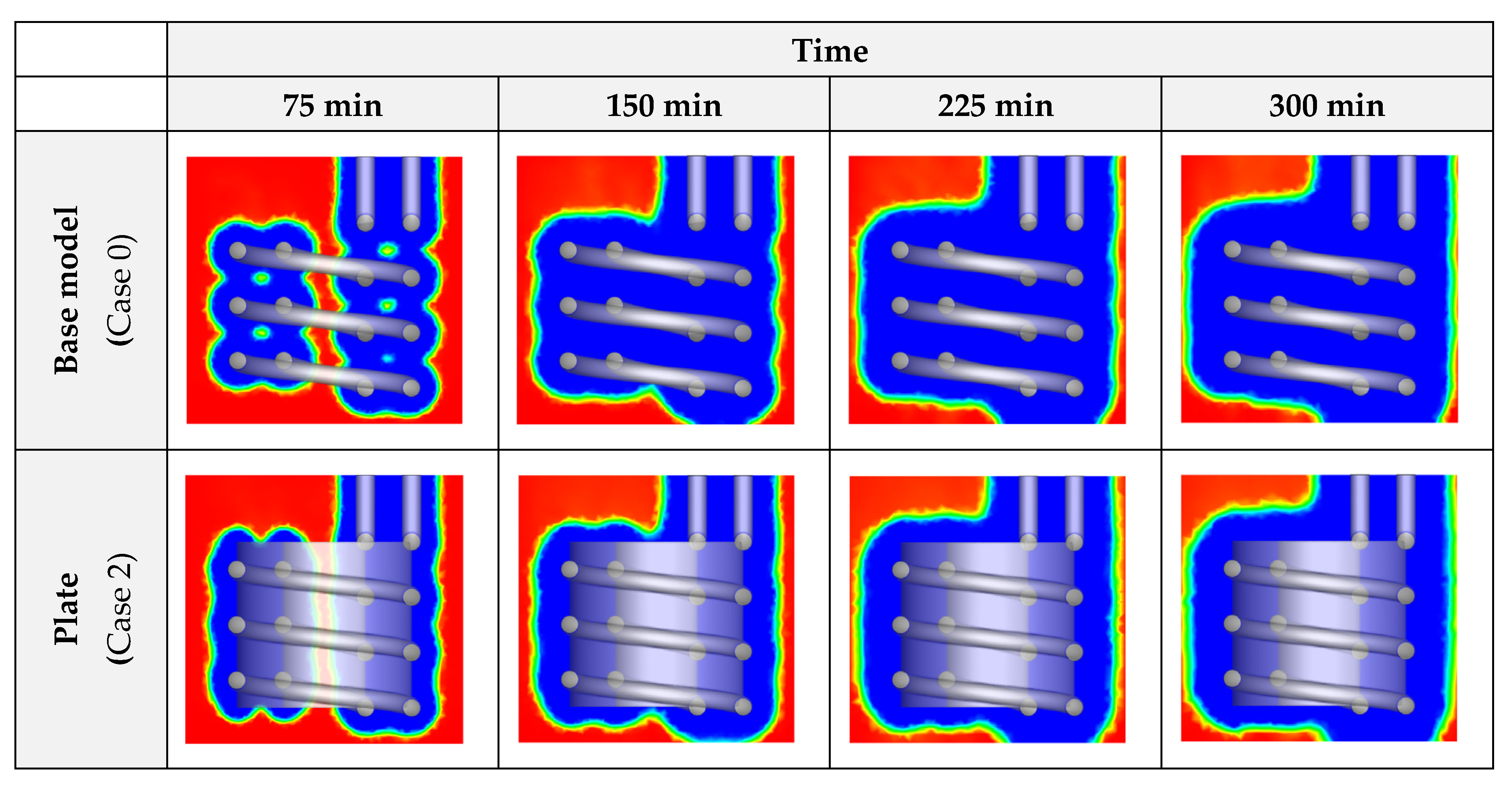
3.1. Heat Exchanger Material
3.2. Nanoparticle Material
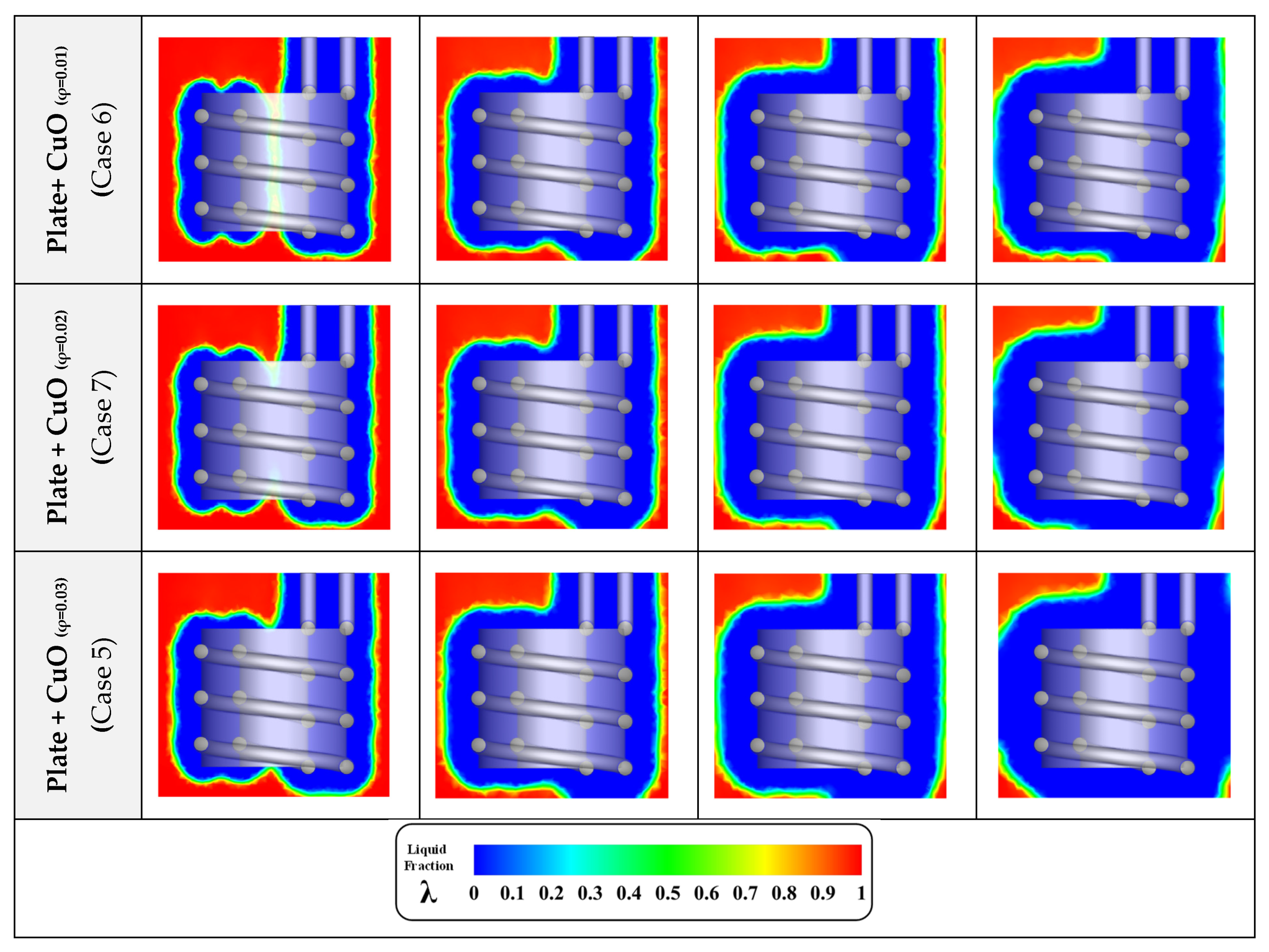
3.3. Nanoparticle Concentration
4. Conclusions and Recommendations
- Considering the thermal performance with different HX materials, copper alloy C11000 presents the best results, however, the aluminum alloy 6061 has a similar thermal performance (just 5.44% lower). Selecting aluminum alloy for the HX is cheaper and makes the HX almost 70% lighter;
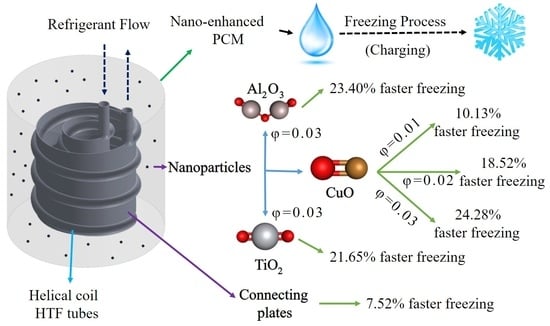
- The compound heat transfer augmentation of the ice storage systems with nano additives and connecting plates is significantly influential and can lead to enhancements up to 29.97% in the complete freezing time;
- The effects of NPs are higher than the plates, as the plates lose their influence after a certain amount of time since they are covered by ice, however, the NPs constantly improve the process until it ends;
- Among the tested NPs, CuO leads to the best results. However, the results with Al2O3 or even TiO2 are not much different;
- The concentration of NPs is considerably more effective on the freezing time than the material of the NPs.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| Mushy zone constant [kg·m−3·s−1] | |
| Specific heat [J·kg−1 K−1] | |
| The diameter [mm] | |
| Gravity [m·s−2] | |
| Specific enthalpy [J·kg−1] | |
| Latent heat of fusion [J·kg−1] | |
| Thermal conductivity [W·m−1·K−1] | |
| m | Mass [kg] |
| Reynolds Number | |
| Temperature [K] | |
| Time [s] or [hr] | |
| Volume [m3] | |
| Velocity vector [m·s−1] | |
| Velocity magnitude [m·s−1] | |
| Greek Symbols | |
| Thermal expansion coefficient [K−1] | |
| Temperature evolution factor | |
| Liquid fraction | |
| Dynamic viscosity [Pa·s] | |
| Density [kg·m−3] | |
| Nanoparticle volume fraction | |
| Subscripts | |
| 0 | Reference |
| b | Base fluid |
| cool | Coolant |
| f | Fluid |
| i | In |
| init | Initial |
| lat | Latent |
| liq | Liquid |
| liquidus | Liquidus |
| NEPCM | Nano-enhanced PCM |
| np | Nanoparticles |
References
- Huggins, R.A. Energy Storage; Springer: Berlin, Germany, 2010; Volume 406. [Google Scholar]
- Baou, M.; Afsharpanah, F.; Delavar, M.A. Numerical study of enhancing vehicle radiator performance using different porous fin configurations and materials. Heat Transf. Asian Res. 2020, 49, 502–518. [Google Scholar] [CrossRef]
- Sircar, A.; Rayavarapu, K.; Bist, N.; Yadav, K.; Singh, S. Applications of nanoparticles in enhanced oil recovery. Pet. Res. 2021. [Google Scholar] [CrossRef]
- Noghrehabadi, A.; Hajidavaloo, E.; Moravej, M. Experimental investigation of efficiency of square flat-plate solar collector using SiO2/water nanofluid. Case Stud. Therm. Eng. 2016, 8, 378–386. [Google Scholar] [CrossRef] [Green Version]
- Jamshidi, N.; Farhadi, M.; Sedighi, K.; Ganji, D.D. Optimization of design parameters for nanofluids flowing inside helical coils. Int. Commun. Heat Mass Transf. 2012, 39, 311–317. [Google Scholar] [CrossRef]
- Mutuku, W.N. Ethylene glycol (EG)-based nanofluids as a coolant for automotive radiator. Asia Pac. J. Comput. Eng. 2016, 3, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Al-Jethelah, M.; Tasnim, S.H.; Mahmud, S.; Dutta, A. Nano-PCM filled energy storage system for solar-thermal applications. Renew. Energy 2018, 126, 137–155. [Google Scholar] [CrossRef]
- Leong, K.Y.; Rahman, M.R.A.; Gurunathan, B.A. Nano-enhanced phase change materials: A review of thermo-physical properties, applications and challenges. J. Energy Storage 2019, 21, 18–31. [Google Scholar] [CrossRef]
- ul Hasnain, F.; Irfan, M.; Khan, M.M.; Khan, L.A.; Ahmed, H.F. Melting performance enhancement of a phase change material using branched fins and nanoparticles for energy storage applications. J. Energy Storage 2021, 38, 102513. [Google Scholar] [CrossRef]
- Al-Madhhachi, H.; Al-Najideen, M. Thermal, environmental, and cost analysis of effective solar portable vaccine refrigerator by COMSOL Multiphysics. Heat Transf. 2021, 50, 179–195. [Google Scholar] [CrossRef]
- Najim, F.T.; Mohammed, H.I.; Al-Najjar, H.M.T.; Thangavelu, L.; Mahmoud, M.Z.; Mahdi, J.M.; Tiji, M.E.; Yaïci, W.; Talebizadehsardari, P. Improved Melting of Latent Heat Storage Using Fin Arrays with Non-Uniform Dimensions and Distinct Patterns. Nanomaterials 2022, 12, 403. [Google Scholar] [CrossRef]
- Alnakeeb, M.A.; Salam, M.A.A.; Hassab, M.A. Eccentricity optimization of an inner flat-tube double-pipe latent-heat thermal energy storage unit. Case Stud. Therm. Eng. 2021, 25, 100969. [Google Scholar] [CrossRef]
- Leng, Z.; Yuan, Y.; Cao, X.; Wang, J.; Haghighat, F. Melting and solidification performance in two horizontal shell-and-tube heat exchangers with different structures. Int. J. Energy Res. 2020, 44, 11288–11301. [Google Scholar] [CrossRef]
- Li, M.; Mahdi, J.M.; Mohammed, H.I.; Bokov, D.O.; Mahmoud, M.Z.; Naghizadeh, A.; Talebizadehsardari, P.; Yaïci, W. Solidification Enhancement in a Multi-Tube Latent Heat Storage System for Efficient and Economical Production: Effect of Number, Position and Temperature of the Tubes. Nanomaterials 2021, 11, 3211. [Google Scholar] [CrossRef] [PubMed]
- Shahsavar, A.; Goodarzi, A.; Talebizadehsardari, P.; Arıcı, M. Numerical investigation of a double-pipe latent heat thermal energy storage with sinusoidal wavy fins during melting and solidification. Int. J. Energy Res. 2021, 45, 20934–20948. [Google Scholar] [CrossRef]
- Mahdavi, A.; Moghaddam, M.A.E.; Mahmoudi, A. Simultaneous charging and discharging of multi-tube heat storage systems using copper fins and Cu nanoparticles. Case Stud. Therm. Eng. 2021, 27, 101343. [Google Scholar] [CrossRef]
- Li, P.; Wang, F.; Hamali, W.; Althobaiti, S.; Musa, A.; Abu-Hamdeh, N. Thermal behavior of heat storage system with incorporating nanomaterial. Case Stud. Therm. Eng. 2022, 31, 101827. [Google Scholar] [CrossRef]
- Mundra, S.S.; Pardeshi, S.S. Analysis of phase change material inside horizontally oriented heat storage unit: A numerical and experimental approach. Case Stud. Therm. Eng. 2022, 31, 101831. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, Z.; Li, D.; Wu, Y.; Arıcı, M. Seasonal thermal performance analysis of glazed window filled with paraffin including various nanoparticles. Int. J. Energy Res. 2020, 44, 3008–3019. [Google Scholar] [CrossRef]
- Afsharpanah, F.; Ajarostaghi, S.S.M.; Sedighi, K. The influence of geometrical parameters on the ice formation enhancement in a shell and double coil ice storage system. SN Appl. Sci. 2019, 1, 1264. [Google Scholar] [CrossRef] [Green Version]
- Afsharpanah, F.; Pakzad, K.; Ajarostaghi, S.S.M.; Poncet, S.; Sedighi, K. Accelerating the charging process in a shell and dual coil ice storage unit equipped with connecting plates. Int. J. Energy Res. 2022, 1–19. [Google Scholar] [CrossRef]
- Bergman, T.L.; Incropera, F.P.; DeWitt, D.P.; Lavine, A.S. Fundamentals of Heat and Mass Transfer; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Voller, V.R.; Prakash, C. A fixed grid numerical modelling methodology for convection-diffusion mushy region phase-change problems. Int. J. Heat Mass Transf. 1987, 30, 1709–1719. [Google Scholar] [CrossRef]
- Fallah Najafabadi, M.; Talebi Rostami, H.; Farhadi, M. Analysis of a twisted double-pipe heat exchanger with lobed cross-section as a novel heat storage unit for solar collectors using phase-change material. Int. Commun. Heat Mass Transf. 2021, 128, 105598. [Google Scholar] [CrossRef]
- Sasaguchi, K.; Kusano, K.; Viskanta, R. A numerical analysis of solid-liquid phase change heat transfer around a single and two horizontal, vertically spaced cylinders in a rectangular cavity. Int. J. Heat Mass Transf. 1997, 40, 1343–1354. [Google Scholar] [CrossRef]
- Sun, X.; Liu, H.; Duan, X.; Guo, H.; Li, Y.; Qiao, J.; Liu, Q.; Liu, J. Effect of hydrogen enrichment on the flame propagation, emissions formation and energy balance of the natural gas spark ignition engine. Fuel 2022, 307, 121843. [Google Scholar] [CrossRef]

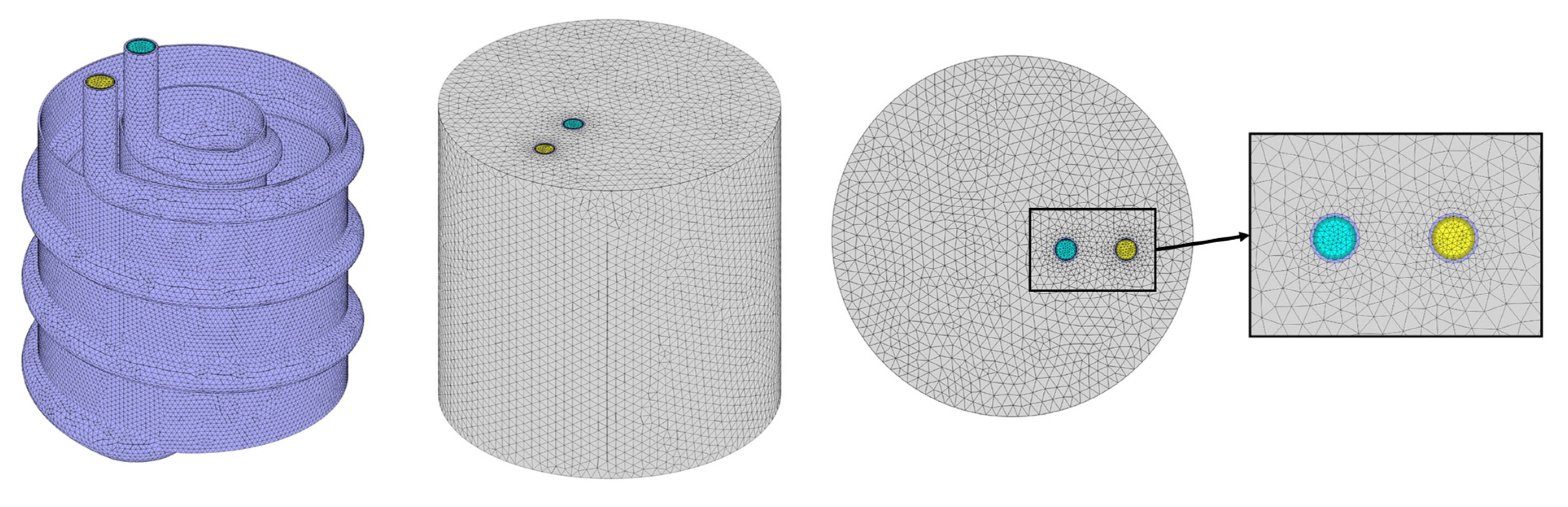


| Property | PCM | HTF | HX | NPs | |||||
|---|---|---|---|---|---|---|---|---|---|
| Water | Ethylene Glycol Solution | Aluminum Alloy 6061 | Copper Alloy C 11000 | Stainless Steel AISI 304 | Al2O3 | Cuo | TiO2 | ||
| Phase | |||||||||
| Solid | Liquid | ||||||||
| ρ [kg·m−3] | 917 | 999.8 | 8933 | 2700 | 8890 | 7900 | 3970 | 6510 | 4250 |
| µ [Pa·s] | - | 0.00162 | - | - | - | - | - | - | - |
| cp [J·kg−1·K−1] | 2217 | 4180 | 385 | 963 | 385 | 477 | 765 | 540 | 686.2 |
| k [W·m−1·K−1] | 1.918 | 0.578 | 401 | 202.3 | 388 | 14.9 | 40 | 18 | 8.9538 |
| hsf [J·kg−1] | 334,000 | - | - | - | - | - | - | - | |
| Tsolidus [K] | 273.15 | - | - | - | - | - | - | - | |
| Tliquidus [K] | 273.15 | - | - | - | - | - | - | - | |
| β [K −1] | - | −6.733353 × 10−5 | - | - | - | - | 0.85 | 0.85 | 0.9 |
| Studied Parameter | Case Number | HX Material | NP Type | NP Concentration |
|---|---|---|---|---|
| φ [%] | ||||
| HX material | 1 | Aluminum alloy 6061 | - | 0% |
| 2 | Copper alloy C11000 | |||
| 3 | Stainless steel AISI 304 | |||
| NP type | 2 | Copper alloy C11000 | - | 3% |
| 4 | Al2O3 | |||
| 5 | CuO | |||
| 6 | TiO2 | |||
| NP concentration [%] | 2 | Copper alloy C11000 | CuO | 0% |
| 7 | 1% | |||
| 8 | 2% | |||
| 5 | 3% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Afsharpanah, F.; Mousavi Ajarostaghi, S.S.; Akbarzadeh Hamedani, F.; Saffari Pour, M. Compound Heat Transfer Augmentation of a Shell-and-Coil Ice Storage Unit with Metal-Oxide Nano Additives and Connecting Plates. Nanomaterials 2022, 12, 1010. https://doi.org/10.3390/nano12061010
Afsharpanah F, Mousavi Ajarostaghi SS, Akbarzadeh Hamedani F, Saffari Pour M. Compound Heat Transfer Augmentation of a Shell-and-Coil Ice Storage Unit with Metal-Oxide Nano Additives and Connecting Plates. Nanomaterials. 2022; 12(6):1010. https://doi.org/10.3390/nano12061010
Chicago/Turabian StyleAfsharpanah, Farhad, Seyed Soheil Mousavi Ajarostaghi, Farzam Akbarzadeh Hamedani, and Mohsen Saffari Pour. 2022. "Compound Heat Transfer Augmentation of a Shell-and-Coil Ice Storage Unit with Metal-Oxide Nano Additives and Connecting Plates" Nanomaterials 12, no. 6: 1010. https://doi.org/10.3390/nano12061010
APA StyleAfsharpanah, F., Mousavi Ajarostaghi, S. S., Akbarzadeh Hamedani, F., & Saffari Pour, M. (2022). Compound Heat Transfer Augmentation of a Shell-and-Coil Ice Storage Unit with Metal-Oxide Nano Additives and Connecting Plates. Nanomaterials, 12(6), 1010. https://doi.org/10.3390/nano12061010