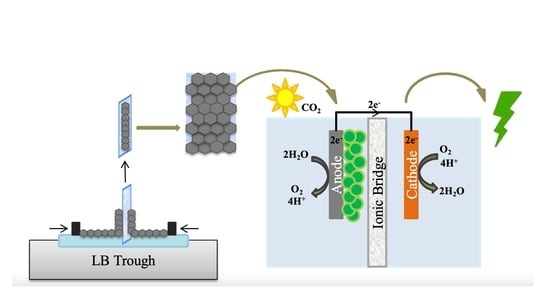
Langmuir–Blodgett Graphene-Based Films for Algal Biophotovoltaic Fuel Cells
Abstract
:1. Introduction
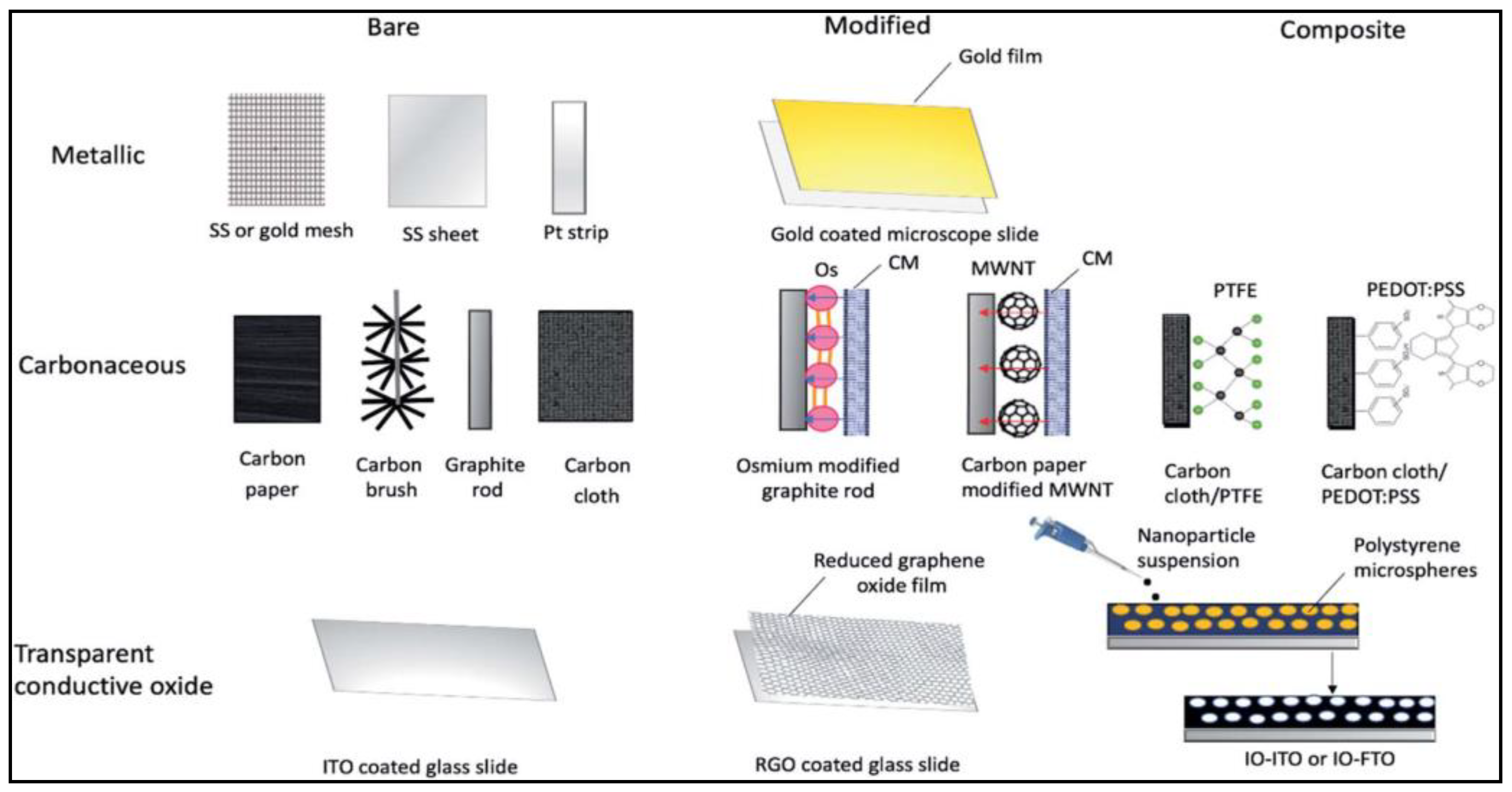
2. Requirements of Anode Materials for BPV
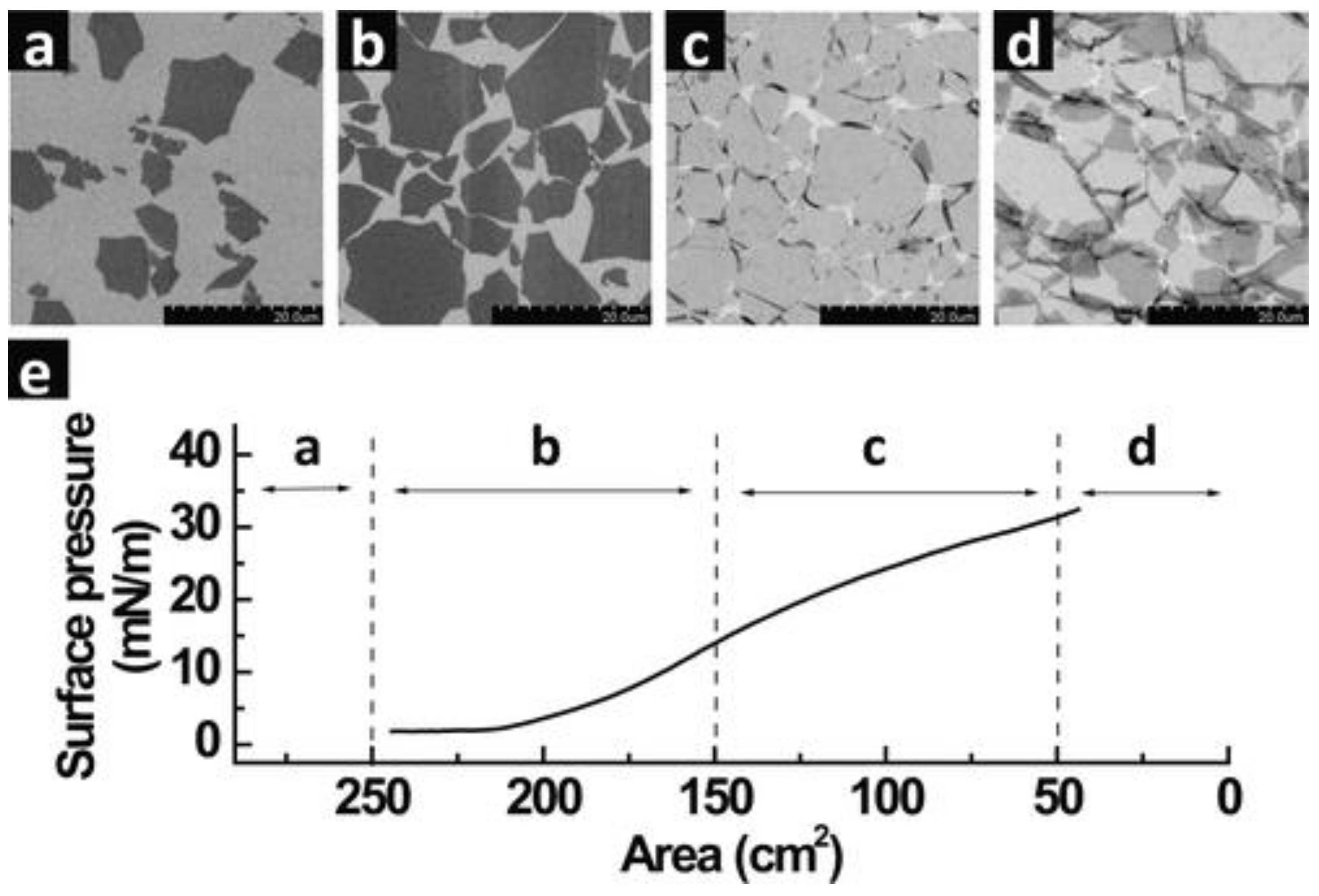
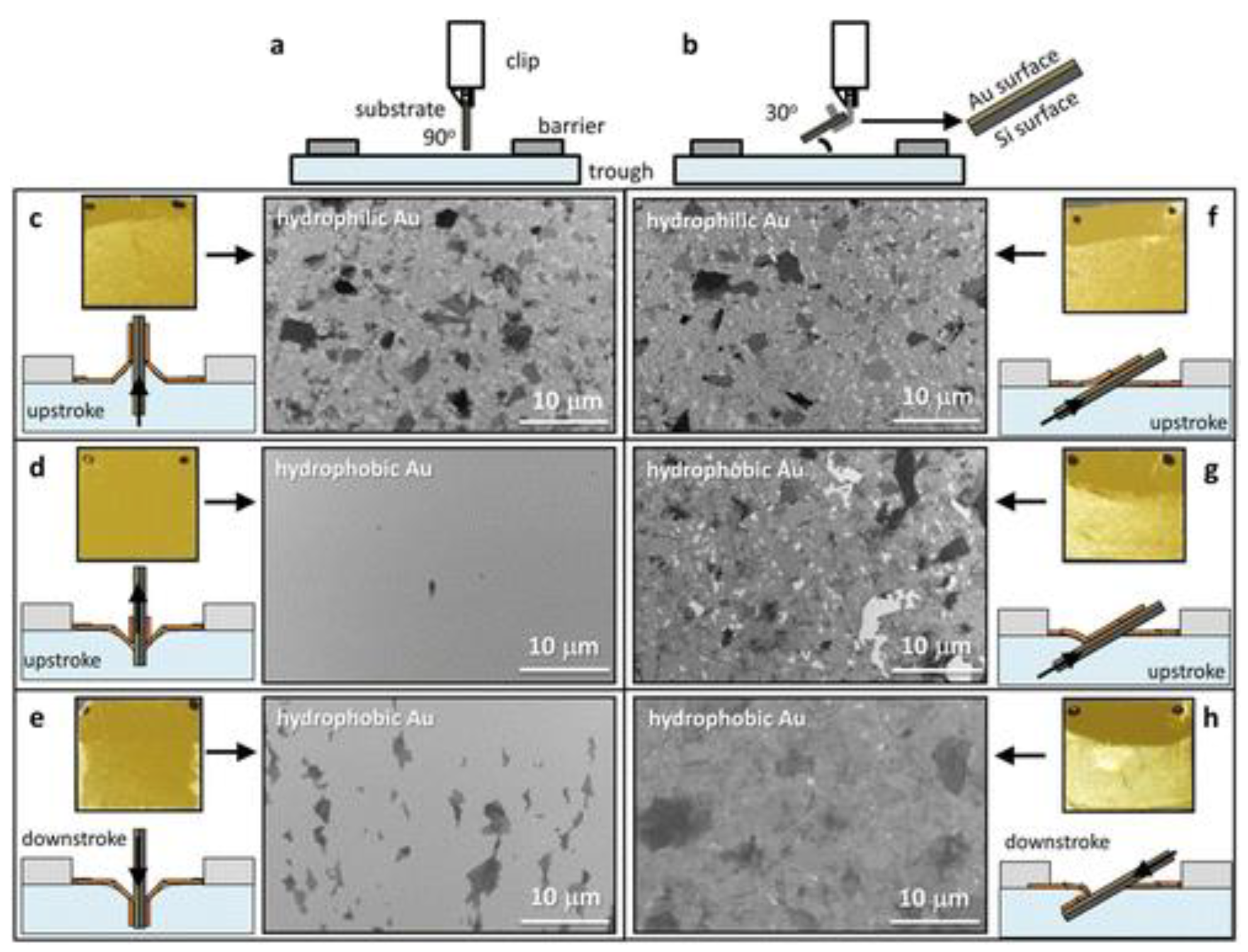
3. Fundamentals of the LB Technique and Its Applications for Graphene-Based Films
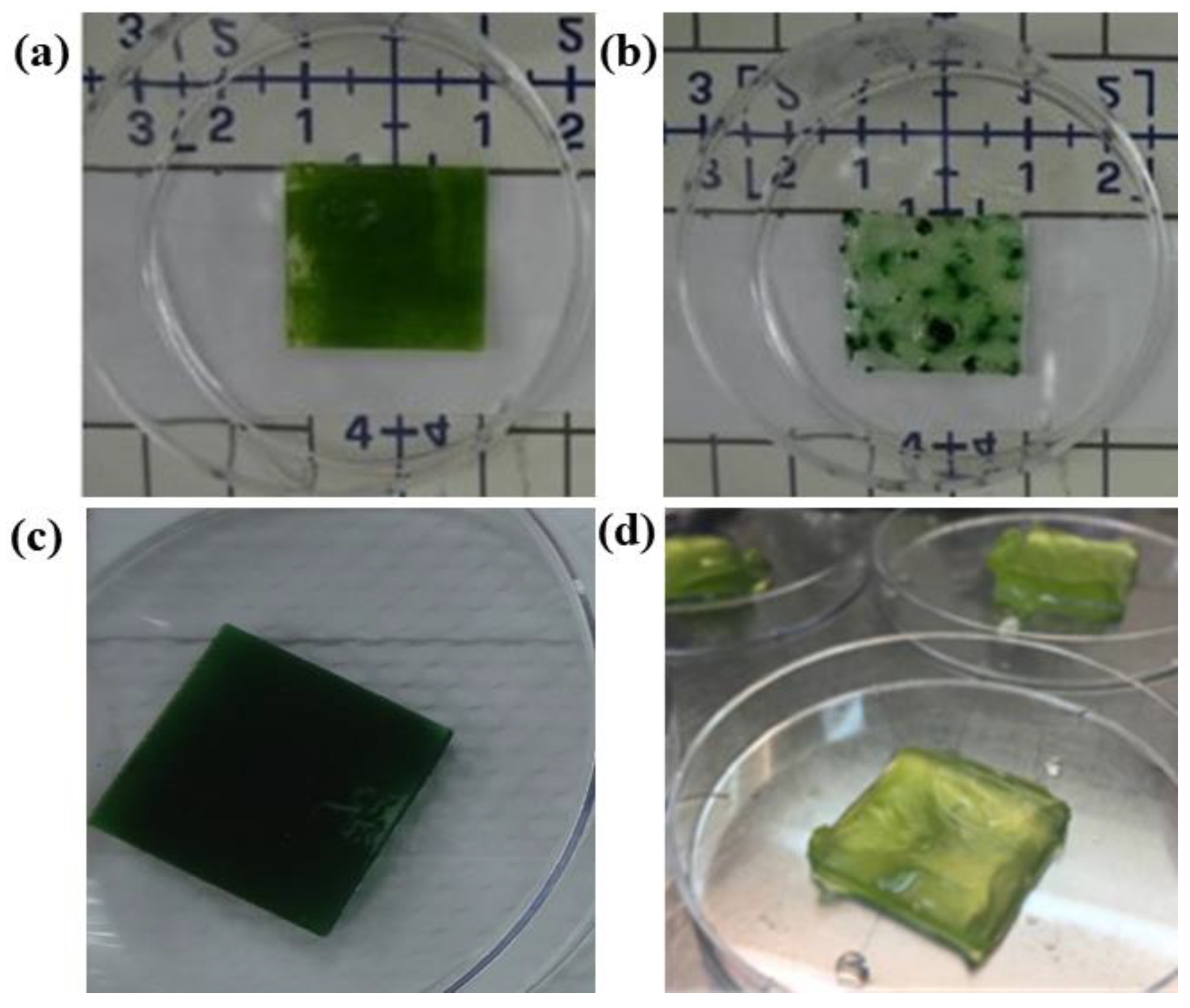
4. Recent Research Efforts
4.1. Graphene-Based LB Films for Fuel Cell Applications
4.2. Progress in Algae-Based Biophotovolatics
5. Electrochemical Studies
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anam, M.; Gomes, H.I.; Rivers, G.; Gom, R.L.; Wildman, R. Evaluation of photoanode materials used in biophotovoltaic systems for renewable energy generation. Sustain. Energy Fuels 2021, 5, 4209. [Google Scholar] [CrossRef]
- Tschörtner, J.; Lai, B.; Krömer, J.O. Biophotovoltaics: Green Power Generation from Sunlight and Water. Front. Microbiol. 2019, 10, 866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sayed, E.T.; Abdelkareem, M.A.; Obaideen, K.; Elsaid, K.; Wilberforce, T.; Maghrabie, H.M.; Olabi, A.G. Progress in plant-based bioelectrochemical systems and their connection with sustainable development goals. Carbon Resour. Convers. 2021, 4, 169–183. [Google Scholar] [CrossRef]
- Paul, S.; Bera, S.; Dasgupta, R.; Mondal, S.; Roy, S. Review on the recent structural advances in open and closed systems for carbon capture through algae. Energy Nexus 2021, 4, 100032. [Google Scholar] [CrossRef]
- He, M.; Rosenbaum, Z.; Angenent, L.T. Light energy to bioelectricity: Photosynthetic microbial fuel cells. Curr. Opin. Biotechnol. 2010, 21, 259. [Google Scholar]
- McCormick, A.J.; Bombelli, P.; Scott, A.M.; Philips, A.J.; Smith, A.G.; Fisher, A.C.; Howe, C.J. Photosynthetic biofilms in pure culture harness solar energy in a mediatorless bio-photovoltaic (BPV) cell system. Energy Environ. Sci. 2011, 4, 4699. [Google Scholar] [CrossRef]
- Bombelli, P.; Bradley, R.W.; Scott, A.M.; Philips, A.J.; McCormick, A.J.; Cruz, S.M.; Anderson, A.; Yunus, K.; Bendall, D.S.; Cameron, P.J.; et al. Quantitative analysis of the factors limiting solar power transduction by Synechocystis sp. PCC 6803 in biological photovoltaic devices. Energy Environ. Sci. 2011, 4, 4690. [Google Scholar] [CrossRef]
- Miao, L.; Jia, R.; Wang, Y.; Kong, C.P.; Wang, J.; Eglitis, R.I.; Zhang, H.X. Certain doping concentrations caused half-metallic graphene. J. Saudi Chem. Soc. 2017, 21, 111. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and Graphene Oxide: Synthesis, Properties, and Applications. Adv. Mater. 2010, 22, 3906. [Google Scholar] [CrossRef]
- Kim, J.; Cote, L.J.; Kim, F.; Yuan, W.; Shull, K.R.; Huang, J.J. Graphene Oxide Sheets at Interfaces. Am. Chem. Soc. 2010, 132, 8180. [Google Scholar] [CrossRef]
- Zheng, Q.; Shi, L.; Yang, J. Langmuir-Blodgett assembly of ultra-large graphene oxide films for transparent electrodes. Trans. Nonferrous Met. Soc. China 2012, 22, 2504. [Google Scholar] [CrossRef]
- Khan, Z.U.; Kausar, A.; Ullah, H.; Badshah, A.; Khan, W.U. A review of graphene oxide, graphene buckypaper, and polymer/graphene composites: Properties and fabrication techniques. J. Plast. Film Sheeting 2016, 32, 336. [Google Scholar] [CrossRef]
- Coroş, M.; Pogăcean, F.; Măgeruşan, L.; Socaci, C.; Pruneanu, S. A brief overview on synthesis and applications of graphene and graphene-based nanomaterials. Front. Mater. Sci. 2019, 13, 23. [Google Scholar] [CrossRef]
- Becerril, H.A.; Mao, J.; Liu, Z.; Stoltenberg, R.M.; Bao, Z.; Chen, Y. Evaluation of solution-processed reduced graphene oxide films as transparent conductors. ACS Nano 2008, 2, 463. [Google Scholar] [CrossRef]
- Eda, G.; Fanchini, G.; Chhowalla, M. Large-area ultrathin films of reduced graphene oxide as a transparent and flexible electronic material. Nat. Nanotechnol. 2008, 3, 270. [Google Scholar] [CrossRef]
- Pei, S.; Zhao, J.; Du, J.; Ren, W.; Cheng, H.M. Direct reduction of graphene oxide films into highly conductive and flexible graphene films by hydrohalic acids. Carbon 2010, 48, 4466. [Google Scholar] [CrossRef]
- Li, X.; Zhang, G.; Bai, X.; Sun, X.; Wang, X.; Wang, E.; Dai, H. Highly conducting graphene sheets and Langmuir-Blodgett films. Nat. Nanotechnol. 2008, 3, 538. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Q.; Ip, W.H.; Lin, X.; Yousefi, N.; Yeung, K.K.; Li, Z.; Kim, J.-K. Transparent Conductive Films Consisting of Ultralarge Graphene Sheets Produced by Langmuir–Blodgett Assembly. ACS Nano 2011, 5, 6039. [Google Scholar] [CrossRef]
- Li, G.; Huang, B.; Pan, Z.; Su, X.; Shao, Z.; An, L. Advances in three-dimensional graphene-based materials: Configurations, preparation and application in secondary metal (Li, Na, K, Mg, Al)-ion batteries. Energy Environ. Sci. 2019, 12, 2030. [Google Scholar] [CrossRef]
- Xu, Y.; Lin, Z.; Huang, X.; Wang, Y.; Huang, Y.; Duan, X. Functionalized Graphene Hydrogel-Based High-Performance Supercapacitors. Adv. Mat. 2013, 25, 5779. [Google Scholar] [CrossRef]
- Wu, Z.S.; Sun, Y.; Tan, Y.Z.; Yang, S.; Feng, X.; Müllen, K.J. Three-dimensional graphene-based macro- and mesoporous frameworks for high-performance electrochemical capacitive energy storage. Am. Chem. Soc. 2012, 134, 19532. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Zhang, Y.; Li, P.; Gao, C. Strong, Conductive, Lightweight, Neat Graphene Aerogel Fibers with Aligned Pores. ACS Nano 2012, 6, 7103. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liu, J.; Hu, Y.; Cheng, H.; Hu, C.; Jiang, C.; Jiang, L.; Cao, A.; Qu, L. Highly Compression-Tolerant Supercapacitor Based on Polypyrrole-mediated Graphene Foam Electrodes. Adv. Mater. 2012, 25, 591–595. [Google Scholar] [CrossRef] [PubMed]
- Fang, Q.; Chen, B.J. Self-assembly of graphene oxide aerogels by layered double hydroxides cross-linking and their application in water purification. Mater. Chem. A 2014, 2, 8941. [Google Scholar] [CrossRef]
- Ye, S.; Feng, J. Self-Assembled Three-Dimensional Hierarchical Graphene/Polypyrrole Nanotube Hybrid Aerogel and Its Application for Supercapacitors. ACS Appl. Mater. Interfaces 2014, 6, 9671. [Google Scholar] [CrossRef]
- Wang, J.; Liang, S.; Ma, L.; Ding, S.; Yu, X.; Zhou, L.; Wang, Q. One-pot synthesis of CdS–reduced graphene oxide 3D composites with enhanced photocatalytic properties. Cryst. Eng. Commun. 2014, 16, 399. [Google Scholar] [CrossRef]
- Patil, U.M.; Sohn, J.S.; Kulkarni, S.B.; Lee, S.C.; Park, H.G.; Gurav, K.V.; Kim, J.H.; Jun, S.C. Enhanced Supercapacitive Performance of Chemically Grown Cobalt–Nickel Hydroxides on Three-Dimensional Graphene Foam Electrodes. ACS Appl. Mater. Interfaces 2014, 6, 2450. [Google Scholar] [CrossRef]
- Li, Z.; Liu, P.; Yun, G.; Shi, K.; Lv, X.; Li, K.; Xing, J.; Yang, B. 3D (Three-dimensional) sandwich-structured of ZnO (zinc oxide)/rGO (reduced graphene oxide)/ZnO for high performance supercapacitors. Energy 2014, 69, 266. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Fal′ko, V.I.; Colombo, L.; Gellert, P.R.; Schwab, M.G.; Kim, K. A Roadmap for Graphene. Nature 2012, 490, 192. [Google Scholar] [CrossRef]
- Fang, Q.; Shen, Y.; Chen, B. Synthesis, decoration and properties of three-dimensional graphene-based macrostructures: A review. Chem. Eng. J. 2015, 264, 753. [Google Scholar] [CrossRef]
- Petty, M.C. Langmuir-Blodgett Films: An Introduction; Cambridge University Press: Cambridge, UK, 1996. [Google Scholar]
- Mirley, C.L.; Koberstein, J.T. Preparation of Ultrathin Metal Oxide Films from Langmuir-Blodgett Layers. Langmuir 1995, 11, 2837–2839. [Google Scholar] [CrossRef]
- Hussain, S.A.; Dey, B.; Bhattacharjee, D.; Mehta, N. Unique supramolecular assembly through Langmuir – Blodgett (LB) technique. Heliyon 2018, 4, e01038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaafar, M.M.; Ciniciato, G.P.M.K.; Ibrahim, S.A.; Phang, S.M.; Yunus, K.; Fisher, A.C.; Iwamoto, M.; Periasamy, V. Preparation of a Three-Dimensional Reduced Graphene Oxide Film by Using the Langmuir–Blodgett Method. Langmuir 2015, 31, 10426. [Google Scholar] [CrossRef] [PubMed]
- Yaqoob, A.A.; Ibrahim, M.N.M.; Rafatullah, M.; Chua, Y.S.; Ahmad, A.; Umar, K. Recent Advances in Anodes for Microbial Fuel Cells: An Overview. Materials 2020, 13, 2078. [Google Scholar] [CrossRef] [PubMed]
- Ng, F.L.; Jaafar, M.M.; Phang, S.M.; Chan, Z.; Salleh, N.A.; Azmi, S.Z.; Yunus, K.; Fisher, A.C.; Periasamy, V. Reduced Graphene Oxide Anodes for Potential Application in Algae Biophotovoltaic Platforms. Sci. Rep. 2014, 4, 7562. [Google Scholar] [CrossRef] [Green Version]
- Cote, L.J.; Kim, F.; Huang, J. Langmuir−Blodgett Assembly of Graphite Oxide Single Layers. J. Am. Chem. Soc. 2009, 131, 1043–1049. [Google Scholar] [CrossRef]
- Velázquez, M.M.; Alejo, T.; López-Díaz, D.; Martín-García, B.; Merchán, M.D. Two-Dimensional Materials—Synthesis, Characterization and Potential Applications; In TechOpen Limited: London, UK, 2016. [Google Scholar]
- Kim, J.; Cote, L.J.; Huang, J. Two Dimensional Soft Material: New Faces of Graphene Oxide. Acc.Chem. Res. 2012, 45, 1356. [Google Scholar] [CrossRef]
- Lerf, A.; He, H.; Forster, M.; Klinowski, J. Structure of Graphite Oxide Revisited. J. Phys. Chem. B 1998, 102, 4477. [Google Scholar] [CrossRef]
- Wen, X.; Garland, C.W.; Hwa, T.; Kardar, M.; Kokufuta, E.; Li, Y.; Orkisz, M.; Tanaka, T. Crumpled and collapsed conformations in graphite oxide membranes. Nature 1992, 355, 426. [Google Scholar] [CrossRef]
- Zheng, Q.; Shi, L.; Ma, P.C.; Xue, Q.; Li, J.; Tang, Z.; Yang, J. Structure control of ultra-large graphene oxide sheets by the Langmuir–Blodgett method. RSC Adv. 2013, 3, 4680. [Google Scholar] [CrossRef]
- Xu, L.; Tetreault, A.R.; Khaligh, H.H.; Goldthorpe, I.A.; Wettig, S.D.; Pope, M.A. Continuous Langmuir–Blodgett Deposition and Transfer by Controlled Edge-to-Edge Assembly of Floating 2D Materials. Langmuir 2019, 35, 51. [Google Scholar] [CrossRef] [PubMed]
- Harrison, K.L.; Biedermann, L.B.; Zavadil, K.R. Mechanical Properties of Water-Assembled Graphene Oxide Langmuir Monolayers: Guiding Controlled Transfer. Langmuir 2015, 31, 9825–9832. [Google Scholar] [CrossRef] [PubMed]
- Kouloumpis, A.; Thomou, E.; Chalmpes, N.; Dimos, K.; Spyrou, K.; Bourlinos, A.B.; Koutselas, I.; Gournis, D.; Rudolf, P. Graphene/Carbon Dot Hybrid Thin Films Prepared by a Modified Langmuir–Schaefer Method. ACS Omega 2017, 2, 2090. [Google Scholar] [CrossRef] [Green Version]
- Bae, S.; Kim, H.; Lee, Y.; Xu, X.; Park, J.S.; Zheng, Y.; Balakrishnan, J.; Lei, T.; Kim, H.R.; Song, Y.I.; et al. Roll-to-roll production of 30-inch graphene films for transparent électrodes. Nat. Nanotechnol. 2010, 5, 574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, B.; Xing, M.; Zhang, J. Recent advances in three-dimensional graphene based materials for catalysis applications. Chem. Soc. Rev. 2018, 47, 2165. [Google Scholar] [CrossRef]
- Chen, Z.; Ren, W.; Gao, L.; Liu, B.; Pei, S.; Cheng, H.M. Three-dimensional flexible and conductive interconnected graphene networks grown by chemical vapour deposition. Nat. Mater. 2011, 10, 424. [Google Scholar] [CrossRef]
- Ma, Y.; Chen, Y. Three-dimensional graphene networks: Synthesis, properties and applications. Natl. Sci. Rev. 2015, 2, 40. [Google Scholar] [CrossRef] [Green Version]
- Singhvi, R.; Stephanopoulos, G.; Wang, D.I.C. Effects of substratum morphology on cell physiology. Biotechnol. Bioeng. 1994, 43, 764. [Google Scholar] [CrossRef]
- Brauker, J.H.; Carr-Brendel, V.E.; Martinson, L.A.; Crudele, J.; Johnston, W.D.; Johnson, R.C. Neovascularization of synthetic membranes directed by membrane microarchitecture. J. Biomed. Mater. Res. 1995, 29, 1517. [Google Scholar] [CrossRef]
- Bobyn, J.D.; Wilson, G.J.; MacGregor, D.C.; Pilliar, R.M.; Weatherly, G.C. Effect of pore size on the peel strength of attachment of fibrous tissue to porous-surfaced implants. J. Biomed. Mat. Res. 1982, 16, 571. [Google Scholar] [CrossRef]
- Clark, P.; Connolly, P.; Curtis, A.S.; Dow, J.A.; Wilkinson, C.D. Topographical control of cell behaviour. I. Simple step cues. Development 1990, 108, 635. [Google Scholar] [CrossRef] [PubMed]
- Rosengren, A.; Bjursten, L.M.; Danielsen, N.; Persson, H.; Kober, M.J. Tissue reactions to polyethylene implants with different surface topography. Mater. Sci. Mater. Med. 1999, 10, 75. [Google Scholar] [CrossRef] [PubMed]
- Flemming, R.G.; Murphy, C.J.; Abrams, G.A.; Goodman, S.L.; Nealey, P.F. Effects of synthetic micro- and nano-structured surfaces on cell behavior. Biomaterials 1999, 20, 573. [Google Scholar] [CrossRef]
- Sure, S.; Torriero, A.A.J.; Gaur, A.; Li, L.H.; Chen, Y.; Tripathi, C.; Adholeya, A.; Ackland, M.L.; Kochar, M. Inquisition of Microcystis aeruginosa and Synechocystis Nanowires: Characterization and Modelling. Antonie Leeuwenhoek 2015, 108, 1213. [Google Scholar] [CrossRef]
- Bombelli, P.; Müller, T.; Herling, T.W.; Howe, C.J.; Knowles, T.P.J. A High Power-Density, Mediator-Free, Microfluidic Biophotovoltaic Device for Cyanobacterial Cells. Adv. Energy Mater. 2015, 5, 1401299. [Google Scholar] [CrossRef] [Green Version]
- Bateson, P.; Fleet, J.; Riseley, A.; Janeva, E.; Marcella, A.; Farinea, C.; Kuptsova, M.; Pueyo, N.V.; Howe, C.J.; Bombelli, P.; et al. Electrochemical Characterisation of Bio-Bottle-Voltaic (BBV) Systems Operated with Algae and Built with Recycled Materials. Biology 2018, 7, 26. [Google Scholar] [CrossRef] [Green Version]
- Nishio, K.; Hashimoto, K.; Watanabe, K. Light/electricity conversion by a self-organized photosynthetic biofilm in a single-chamber reactor. Appl. Microbiol. Biotechnol. 2010, 86, 957. [Google Scholar] [CrossRef]
- Gonzalez-Aravena, A.C.; Yunus, K.; Zhang, L.; Norling, B.; Fisher, A.C. Tapping into cyanobacteria electron transfer for higher exoelectrogenic activity by imposing iron limited growth. RSC Adv. 2018, 8, 20263. [Google Scholar] [CrossRef] [Green Version]
- Bradley, R.W.; Bombelli, P.; Rowden, S.J.L.; Howe, C.J. Biological photovoltaics: Intra- and extra-cellular electron transport by cyanobacteria. Biochem. Soc. Trans. 2012, 40, 1302. [Google Scholar] [CrossRef] [Green Version]
- Ng, F.L.; Phang, S.M.; Periasamy, V.; Yunus, K.; Fisher, A.C. Evaluation of Algal Biofilms on Indium Tin Oxide (ITO) for Use in Biophotovoltaic Platforms Based on Photosynthetic Performance. PLoS ONE 2014, 9, e97643. [Google Scholar] [CrossRef] [Green Version]
- Ng, F.L.; Phang, S.M.; Periasamy, V.; Yunus, K.; Fisher, A.C. Enhancement of Power Output by using Alginate Immobilized Algae in Biophotovoltaic Devices. Sci. Rep. 2017, 7, 16237. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.R.; Wakayama, T.; Nakamura, C.; Miyake, J.; Zorin, N.A.; Qian, D.J. Electrochemical properties of carbon nanotubes–hydrogenase conjugates Langmuir–Blodgett films. Electrochim. Acta 2007, 52, 3222. [Google Scholar] [CrossRef]
- Huang, M.; Mao, X.; Chen, D.; He, S.; He, S.; Liu, W.; Xu, J.; Miao, S. Amphiphilic Molecules of Nb18: Synthesis, Langmuir-Blodgett Films Construction and Applications in Electro-Catalysts. J. Electrochem. Soc. 2015, 162, H229. [Google Scholar] [CrossRef]
- Kondalkar, V.V.; Mali, S.S.; Kharade, R.R.; Mane, R.M.; Patil, P.S.; Hong, C.K.; Kim, J.H.; Choudhury, S.; Bhosale, P.N. Langmuir–Blodgett self organized nanocrystalline tungsten oxide thin films for electrochromic performance. RSC Adv. 2015, 5, 26923. [Google Scholar] [CrossRef]
- Paul, S.; Pearson, C.; Molloy, A.; Cousins, M.A.; Green, M.; Kolliopoulou, S.; Dimitrakis, P.; Normand, P.; Tsoukalas, D.; Petty, M. Langmuir-Blodgett film deposition of metallic nanoparticles and their application to electronic memory structures. Nano Lett. 2003, 3, 533. [Google Scholar] [CrossRef]
- Nalwa, H.S.; Kakuta, A. Organometallic Langmuir-Blodgett films for electronics and photonics. Appl. Organomet. Chem. 1992, 6, 645. [Google Scholar] [CrossRef]
- Gür, B.; Işık, M.; Kıranşan, K.D.; Alanyalıoğlu, M.; Beydemir, Ş.; Meral, K. High enzymatic activity preservation of malate dehydrogenase immobilized in a Langmuir–Blodgett film and its electrochemical biosensor application for malic acid detection. RSC Adv. 2016, 6, 79792. [Google Scholar] [CrossRef]
- Ibrahim, S.A.; Jaafar, M.M.; Ng, F.L.; Phang, S.M.; Kumar, G.G.; Majid, W.H.A.; Periasamy, V. Plasma-treated Langmuir–Blodgett reduced graphene oxide thin film for applications in biophotovoltaics. Appl. Phys. A 2018, 124, 59. [Google Scholar] [CrossRef]
- Shin, H.; Kim, T.; Seo, I.; Kim, S.; Kim, Y.J.; Hong, H.; Park, Y.; Jeong, H.M.; Kim, K.; Ryu, W. Fabrication of scalable and flexible bio-photoanodes by electrospraying thylakoid/graphene oxide composites. Appl. Surf. Sci. 2019, 481, 1. [Google Scholar] [CrossRef]
- Pankratova, G.; Pankratov, D.; di Bari, C.; Goñi-Urtiaga, A.; Toscano, M.D.; Chi, Q.; de Lacey, A.L. Three-Dimensional Graphene Matrix-Supported and Thylakoid Membrane-Based High-Performance Bioelectrochemical Solar Cell. ACS Appl. Energy Mater. 2018, 1, 319. [Google Scholar] [CrossRef]
- Cai, P.; Li, G.; Yang, Y.; Su, X.; Zhang, Z. Co-assembly of thylakoid and graphene oxide as a photoelectrochemical composite film for enhanced mediated electron transfer. Colloids Surf. A 2018, 555, 37. [Google Scholar] [CrossRef]
- Senthilkumar, N.; Sheet, S.; Sathishkumar, Y.; Lee, Y.S.; Phang, S.M.; Periasamy, V.; Gnanakumar, G. Titania/reduced graphene oxide composite nanofibers for the direct extraction of photosynthetic electrons from microalgae for biophotovoltaic cell applications. Appl. Phys. A 2018, 124, 769. [Google Scholar] [CrossRef]

| Electrode | Catalyst | Fabrication Method | Types of FCs | Maximum Current/ Power Efficiency | Reference |
|---|---|---|---|---|---|
| ITO-PEN a ITO-PEN a | TM b/GO c TM b | Electro-spraying | Photosynthetic electrons from plant | 3.92 μA/cm2 0.41 μA/cm2 | [71] |
| GC d | TM b/rGO e | Electro-reduction and electro-deposition | Photobioelectrochemical FCs | 5.24 µA/cm2 | [72] |
| ITO f | GO c-TM b rGO e-TM b | Drop-casting | Photoelectrochemical | 21.37 µA/cm2 18.95 µA/cm2 | [73] |
| CC g | TiO2/rGO e | Electro-spinning | BPV | 34.66 mW/m2 | [74] |
| Glass | rGO e film | LB method | Biological | - | [34] |
| ITO f | rGO e film | LB method | BPV | 6.94 × 105 W/m2 | [70] |
| ITO f | rGO e film | LB method | BPV | 0.14 mW/m2 | [62] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Periasamy, V.; Jaafar, M.M.; Chandrasekaran, K.; Talebi, S.; Ng, F.L.; Phang, S.M.; kumar, G.G.; Iwamoto, M. Langmuir–Blodgett Graphene-Based Films for Algal Biophotovoltaic Fuel Cells. Nanomaterials 2022, 12, 840. https://doi.org/10.3390/nano12050840
Periasamy V, Jaafar MM, Chandrasekaran K, Talebi S, Ng FL, Phang SM, kumar GG, Iwamoto M. Langmuir–Blodgett Graphene-Based Films for Algal Biophotovoltaic Fuel Cells. Nanomaterials. 2022; 12(5):840. https://doi.org/10.3390/nano12050840
Chicago/Turabian StylePeriasamy, Vengadesh, Muhammad Musoddiq Jaafar, Karthikeyan Chandrasekaran, Sara Talebi, Fong Lee Ng, Siew Moi Phang, Georgepeter Gnana kumar, and Mitsumasa Iwamoto. 2022. "Langmuir–Blodgett Graphene-Based Films for Algal Biophotovoltaic Fuel Cells" Nanomaterials 12, no. 5: 840. https://doi.org/10.3390/nano12050840
APA StylePeriasamy, V., Jaafar, M. M., Chandrasekaran, K., Talebi, S., Ng, F. L., Phang, S. M., kumar, G. G., & Iwamoto, M. (2022). Langmuir–Blodgett Graphene-Based Films for Algal Biophotovoltaic Fuel Cells. Nanomaterials, 12(5), 840. https://doi.org/10.3390/nano12050840