Preparation and Characterization of Multi-Doped Porous Carbon Nanofibers from Carbonization in Different Atmospheres and Their Oxygen Electrocatalytic Properties Research
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of Bimetal NixCoy-ZIFs Nanocrystals
2.3. Preparation of Bimetal NixCoy-ZIFs/PAN Nanofibers
2.4. Preparation of Multi-Doped Porous Carbon Nanofibers
2.5. Material Characterization
2.6. Electrochemical Measurements
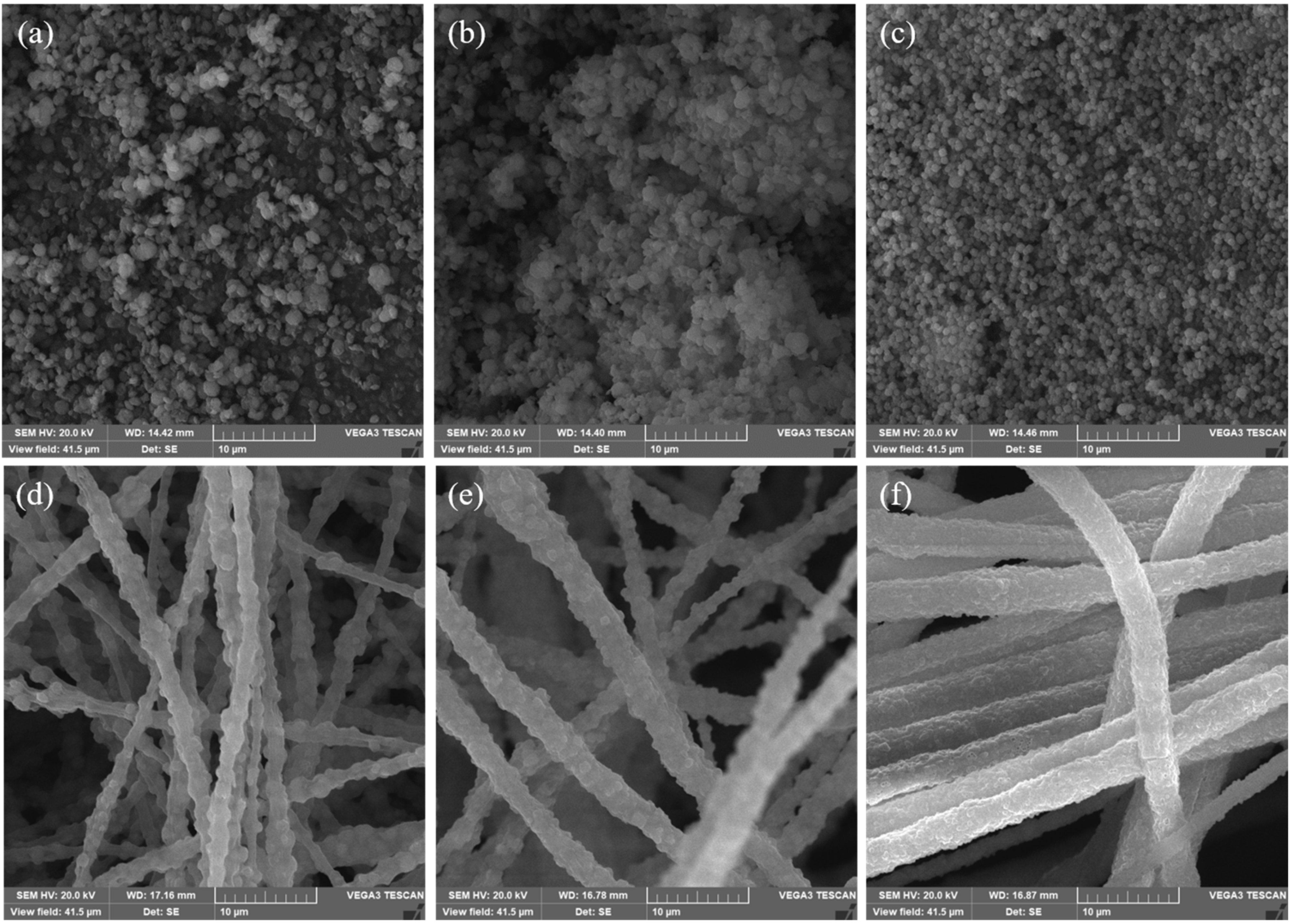
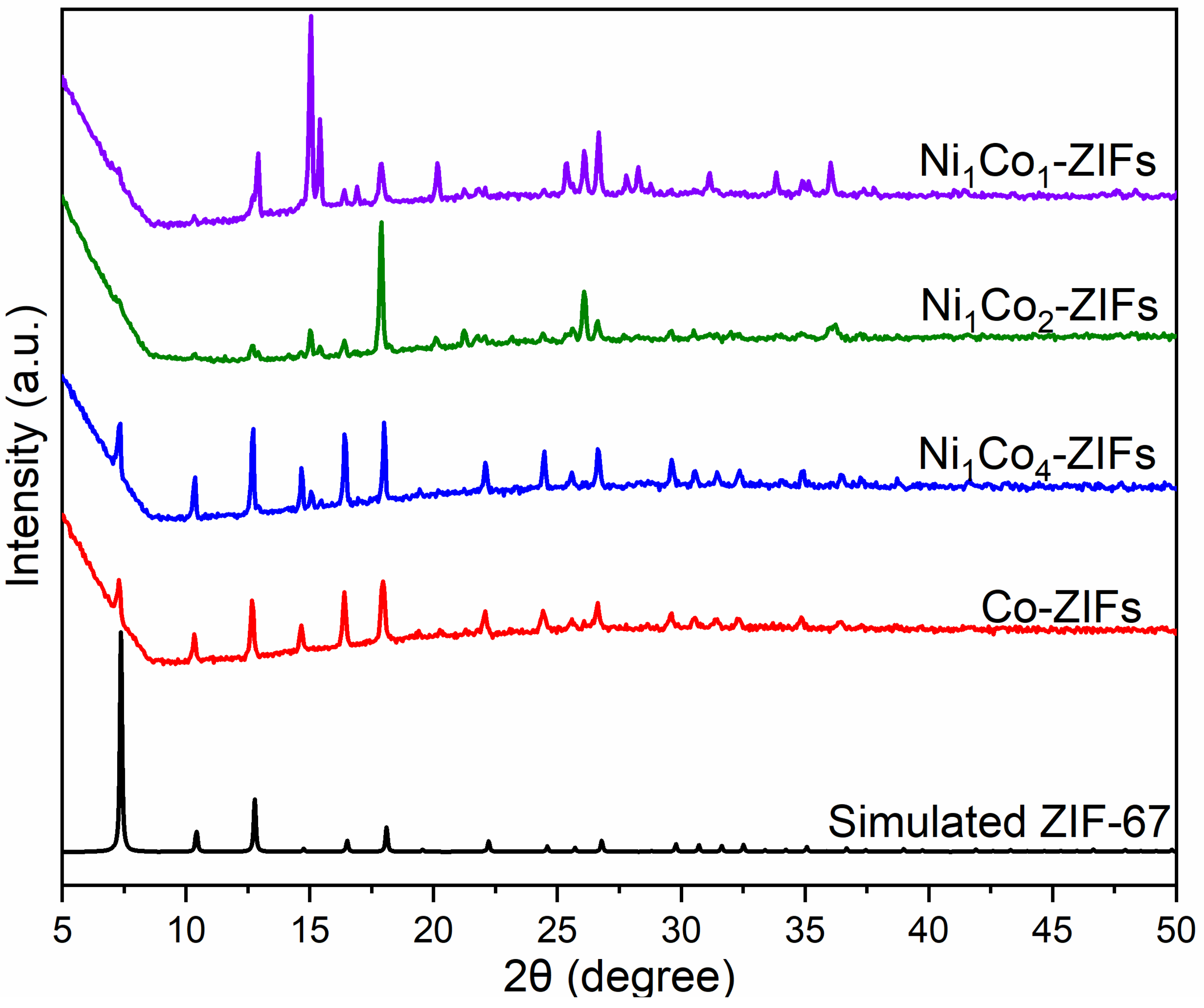
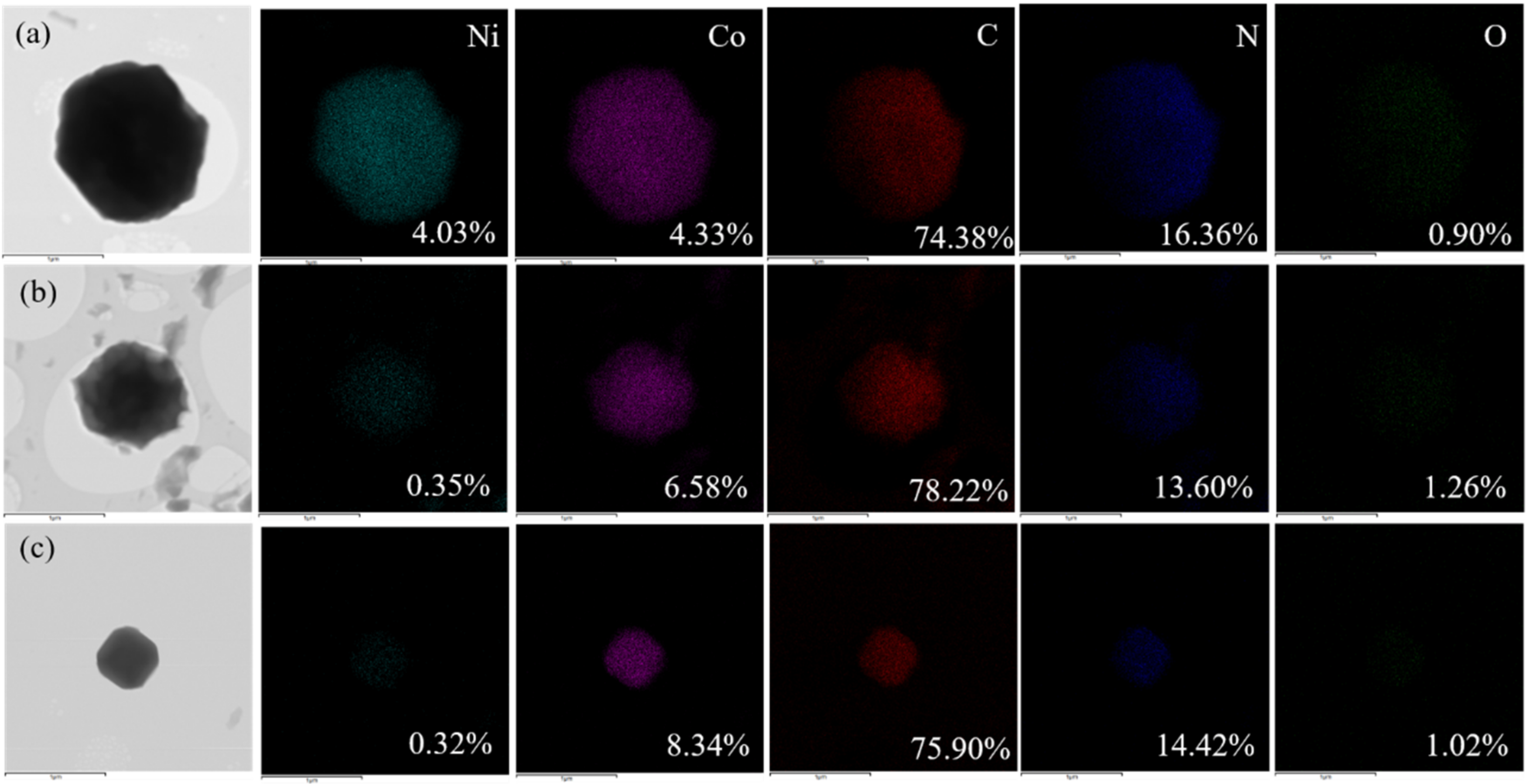
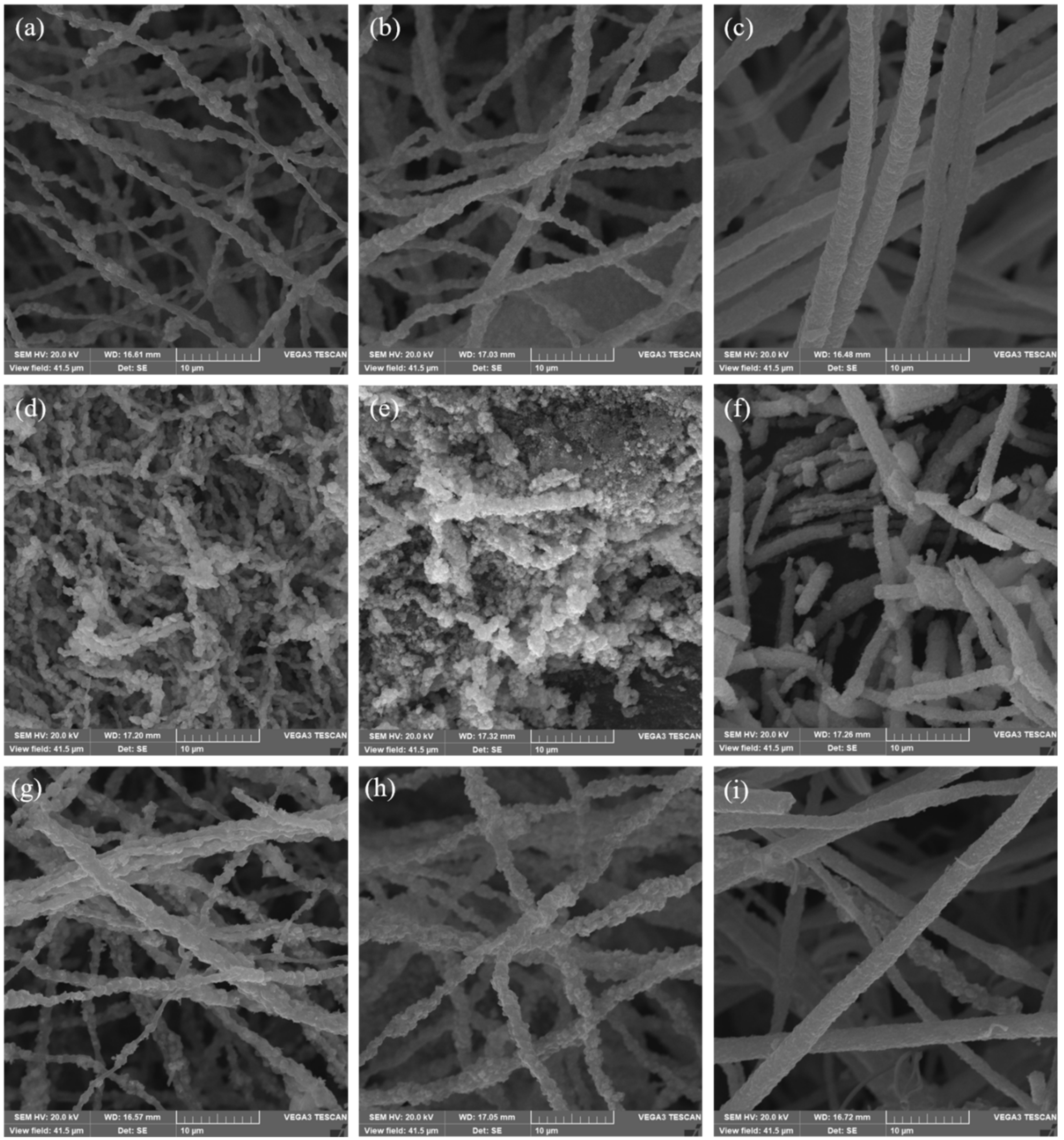
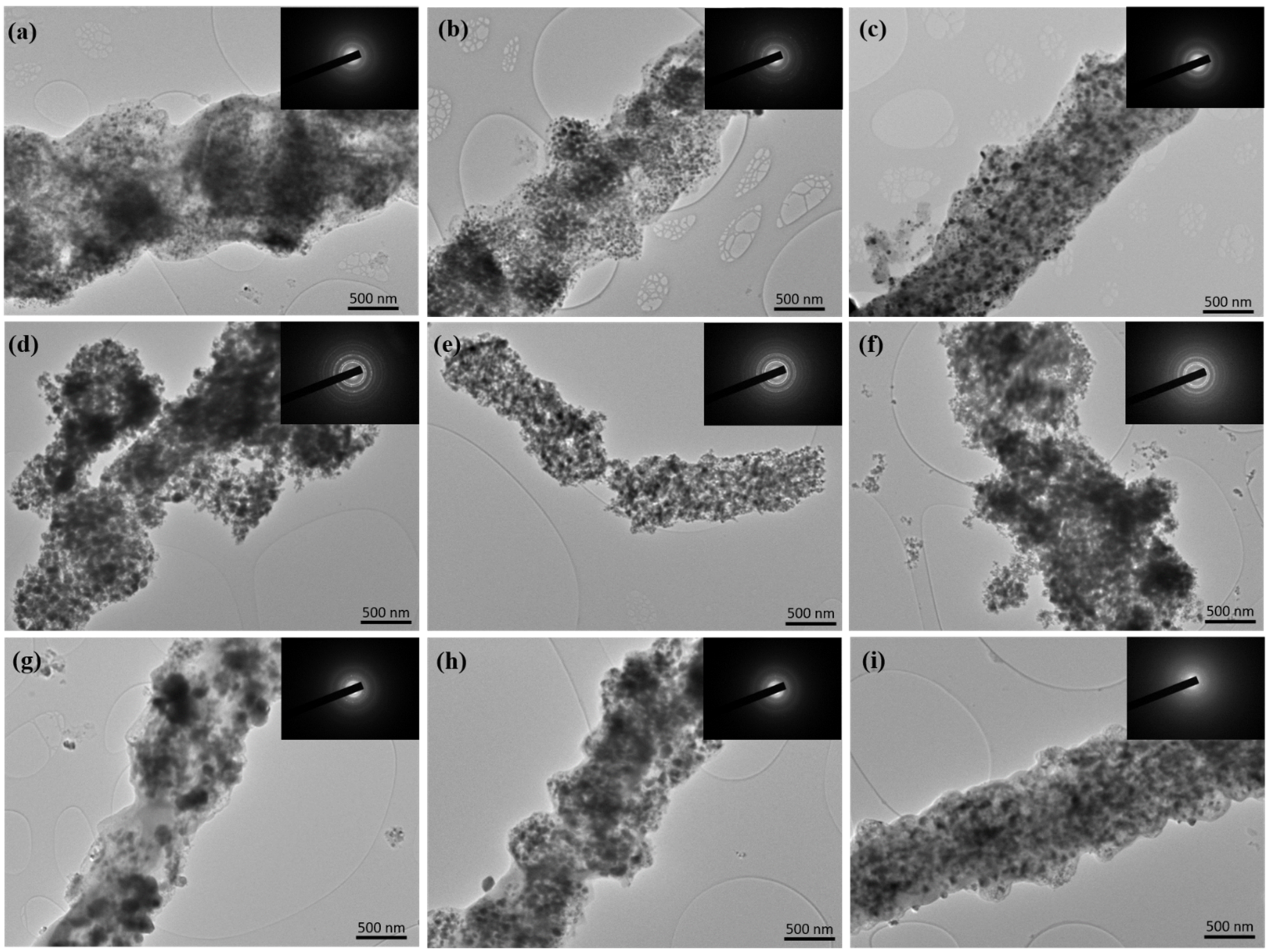
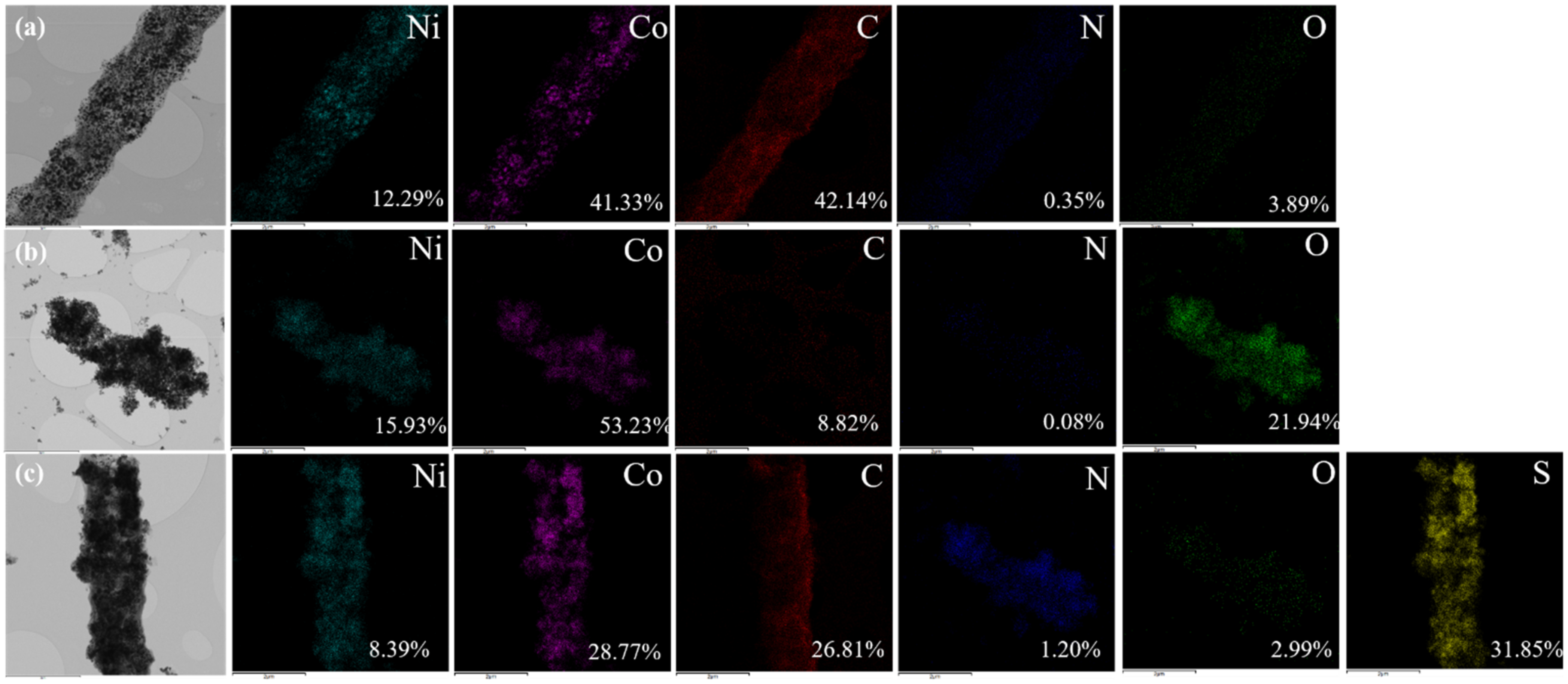
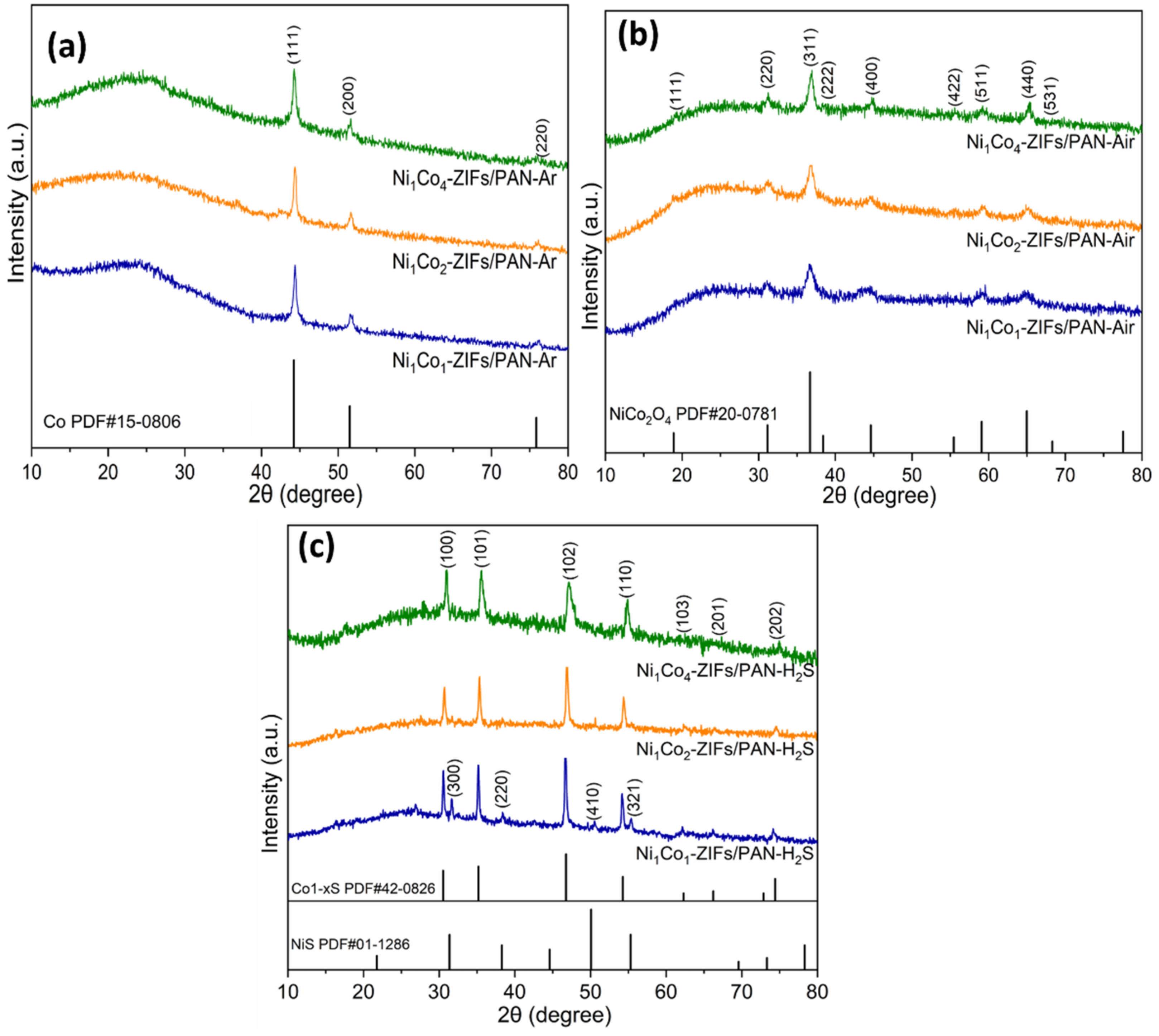
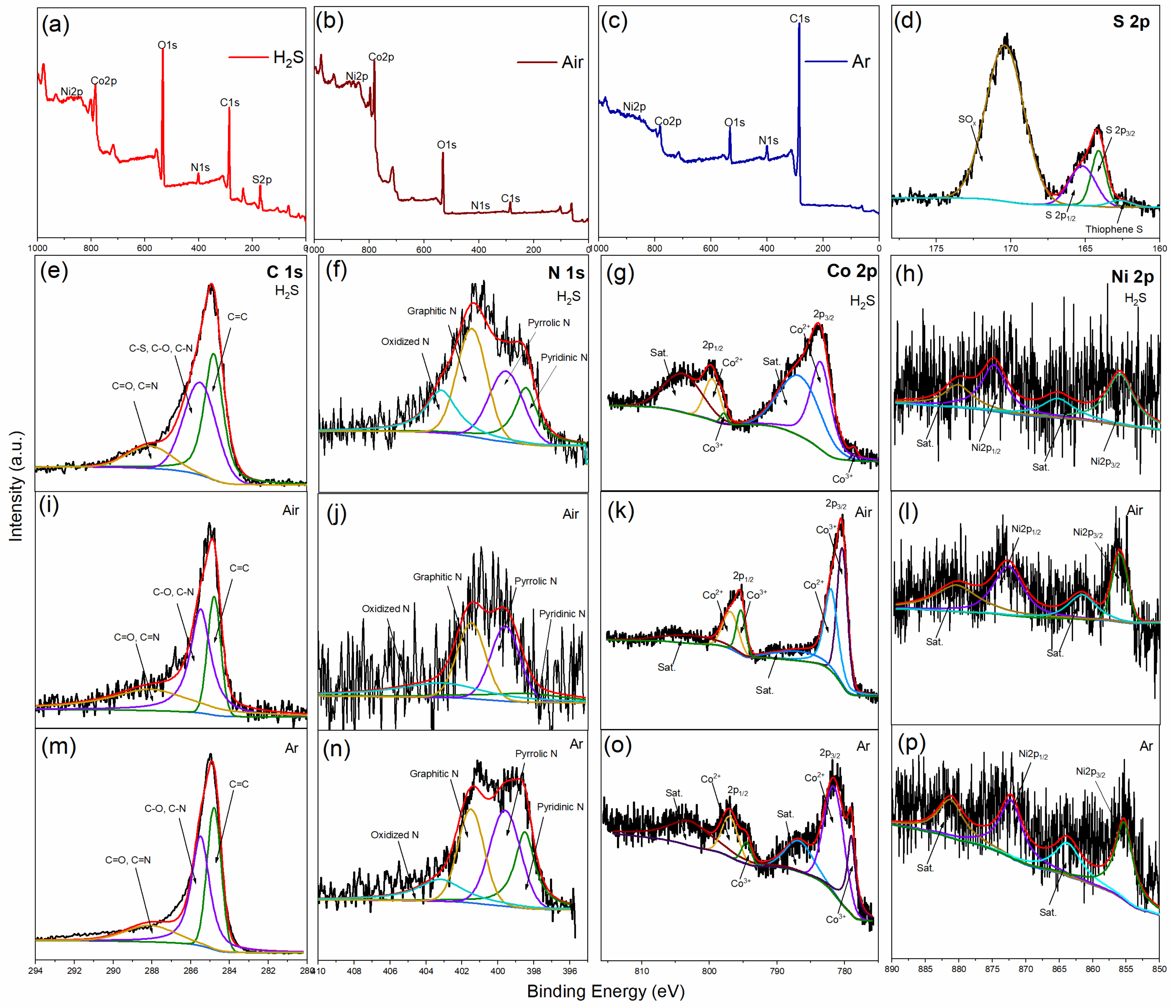
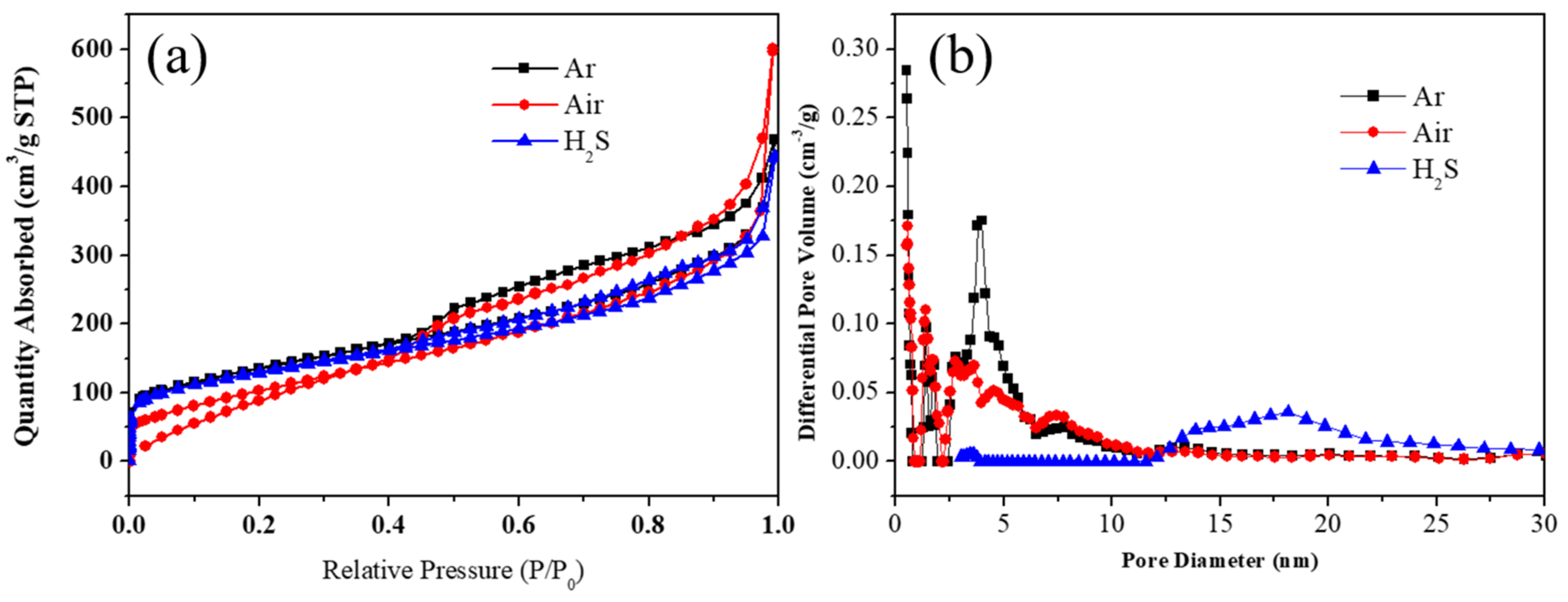
3. Results and Discussions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, G.; Santandreu, A.; Kellogg, W.; Gupta, S.; Ogoke, O.; Zhang, H.; Wang, H.-L.; Dai, L. Carbon nanocomposite catalysts for oxygen reduction and evolution reactions: From nitrogen doping to transition-metal addition. Nano Energy 2016, 29, 83–110. [Google Scholar] [CrossRef] [Green Version]
- Ruan, M.; Liu, J.; Song, P.; Xu, W. Meta-analysis of commercial Pt/C measurements for oxygen reduction reactions via data mining. Chin. J. Catal. 2022, 43, 116–121. [Google Scholar] [CrossRef]
- Gonzalez-Huerta, R.G.; Ramos-Sanchez, G.; Balbuena, P.B. Oxygen evolution in Co-doped RuO2 and IrO2: Experimental and theoretical insights to diminish electrolysis overpotential. J. Power Sources 2014, 268, 69–76. [Google Scholar] [CrossRef]
- Wu, X.; Niu, Y.; Feng, B.; Yu, Y.; Huang, X.; Zhong, C.; Hu, W.; Li, C.M. Mesoporous Hollow Nitrogen-Doped Carbon Nanospheres with Embedded MnFe2O4/Fe Hybrid Nanoparticles as Efficient Bifunctional Oxygen Electrocatalysts in Alkaline Media. ACS Appl. Mater. Interfaces 2018, 10, 20440–20447. [Google Scholar] [CrossRef]
- Ma, L.-L.; Hu, X.; Liu, W.-J.; Li, H.-C.; Lam, P.K.S.; Zeng, R.J.; Yu, H.-Q. Constructing N, P-dually doped biochar materials from biomass wastes for high-performance bifunctional oxygen electrocatalysts. Chemosphere 2021, 278, 130508. [Google Scholar] [CrossRef]
- Sun, J.; Lowe, S.E.; Zhang, L.; Wang, Y.; Pang, K.; Wang, Y.; Zhong, Y.; Liu, P.; Zhao, K.; Tang, Z. Ultrathin nitrogen-doped holey carbon@ graphene bifunctional electrocatalyst for oxygen reduction and evolution reactions in alkaline and acidic media. Angew. Chem. Int. Ed. 2018, 57, 16511–16515. [Google Scholar] [CrossRef]
- Morales, D.M.; Kazakova, M.A.; Dieckhöfer, S.; Selyutin, A.G.; Golubtsov, G.V.; Schuhmann, W.; Masa, J. Trimetallic Mn-Fe-Ni oxide nanoparticles supported on multi-walled carbon nanotubes as high-performance bifunctional ORR/OER electrocatalyst in alkaline media. Adv. Funct. Mater. 2020, 30, 1905992. [Google Scholar] [CrossRef]
- Zhang, W.; Yao, X.; Zhou, S.; Li, X.; Li, L.; Yu, Z.; Gu, L. ZIF-8/ZIF-67-derived Co-Nx-embedded 1D porous carbon nanofibers with graphitic carbon-encased Co nanoparticles as an efficient bifunctional electrocatalyst. Small 2018, 14, 1800423. [Google Scholar] [CrossRef]
- Meng, Y.; Huang, X.; Lin, H.; Zhang, P.; Gao, Q.; Li, W. Carbon-based nanomaterials as sustainable noble-metal-free electrocatalysts. Front. Chem. 2019, 7, 759. [Google Scholar] [CrossRef] [Green Version]
- Osgood, H.; Devaguptapu, S.V.; Xu, H.; Cho, J.; Wu, G. Transition metal (Fe, Co, Ni, and Mn) oxides for oxygen reduction and evolution bifunctional catalysts in alkaline media. Nano Today 2016, 11, 601–625. [Google Scholar] [CrossRef]
- Hu, C.; Paul, R.; Dai, Q.; Dai, L. Carbon-based metal-free electrocatalysts: From oxygen reduction to multifunctional electrocatalysis. Chem. Soc. Rev. 2021, 50, 11785–11843. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhao, B.; He, Y.-C.; Wen, H.-R.; Fu, X.-Z.; Sun, R.; Wong, C.-P. NiCo2O4 nanoframes with a nanosheet surface as efficient electrocatalysts for the oxygen evolution reaction. Mater. Chem. Front. 2018, 2, 1155–1164. [Google Scholar] [CrossRef]
- Faraji, M.; Arianpouya, N. NiCoFe-layered double hydroxides/MXene/N-doped carbon nanotube composite as a high performance bifunctional catalyst for oxygen electrocatalytic reactions in metal-air batteries. J. Electroanal. Chem. 2021, 901, 115797. [Google Scholar] [CrossRef]
- Lian, Y.; Shi, K.; Yang, H.; Sun, H.; Qi, P.; Ye, J.; Wu, W.; Deng, Z.; Peng, Y. Elucidation of Active Sites on S, N Codoped Carbon Cubes Embedding Co–Fe Carbides toward Reversible Oxygen Conversion in High-Performance Zinc–Air Batteries. Small 2020, 16, 1907368. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Luo, C.; Zhang, H.; Yang, L.; Huang, N.; Zhang, M.; Yu, H.; Sun, P.; Wang, L.; Lv, X.; et al. In-Situ derived Co1−xS@nitrogen-doped carbon nanoneedle array as a bifunctional electrocatalyst for flexible Zinc-air battery. J. Electroanal. Chem. 2021, 900, 115711. [Google Scholar] [CrossRef]
- Vijayakumar, E.; Ramakrishnan, S.; Sathiskumar, C.; Yoo, D.J.; Balamurugan, J.; Noh, H.S.; Kwon, D.; Kim, Y.H.; Lee, H. MOF-derived CoP-nitrogen-doped carbon@NiFeP nanoflakes as an efficient and durable electrocatalyst with multiple catalytically active sites for OER, HER, ORR and rechargeable zinc-air batteries. Chem. Eng. J. 2022, 428, 131115. [Google Scholar] [CrossRef]
- Kim, H.; Prasad Tiwari, A.; Mukhiya, T.; Kim, H.Y. Temperature-controlled in situ synthesized carbon nanotube-protected vanadium phosphate particle-anchored electrospun carbon nanofibers for high energy density symmetric supercapacitors. J. Colloid Interface Sci. 2021, 600, 740–751. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, J.; Cheng, H.; Li, G.; Cho, H.; Jiang, M.; Gao, Q.; Zhang, X. Developments of Advanced Electrospinning Techniques: A Critical Review. Adv. Mater. Technol. 2021, 6, 2100410. [Google Scholar] [CrossRef]
- Lei, Y.; Wang, Q.; Peng, S.; Ramakrishna, S.; Zhang, D.; Zhou, K. Electrospun Inorganic Nanofibers for Oxygen Electrocatalysis: Design, Fabrication, and Progress. Adv. Energy Mater. 2020, 10, 1902115. [Google Scholar] [CrossRef]
- Dapaah, M.F.; Niu, Q.; Yu, Y.-Y.; You, T.; Liu, B.; Cheng, L. Efficient persistent organic pollutant removal in water using MIL-metal–organic framework driven Fenton-like reactions: A critical review. Chem. Eng. J. 2022, 431, 134182. [Google Scholar] [CrossRef]
- Liu, B.; Wong-Foy, A.G.; Matzger, A.J. Rapid and enhanced activation of microporous coordination polymers by flowing supercritical CO2. Chem. Commun. 2013, 49, 1419–1421. [Google Scholar] [CrossRef]
- Dapaah, M.F.; Liu, B. Recent advances of supercritical CO2 in green synthesis and activation of metal–organic frameworks. J. Inorg. Organomet. Polym. Mater. 2020, 30, 581–595. [Google Scholar] [CrossRef]
- Dapaah, M.F.; Liu, B.; Cheng, L. Adsorption of organic compounds from aqueous solution by pyridine-2-carboxaldehyde grafted MIL-101(Cr)-NH2 metal-organic frameworks. J. Environ. Chem. Eng. 2021, 9, 105275. [Google Scholar] [CrossRef]
- Wang, H.-F.; Chen, L.; Pang, H.; Kaskel, S.; Xu, Q. MOF-derived electrocatalysts for oxygen reduction, oxygen evolution and hydrogen evolution reactions. Chem. Soc. Rev. 2020, 49, 1414–1448. [Google Scholar] [CrossRef]
- Muthurasu, A.; Tiwari, A.P.; Chhetri, K.; Dahal, B.; Kim, H.Y. Construction of iron doped cobalt- vanadate- cobalt oxide with metal-organic framework oriented nanoflakes for portable rechargeable zinc-air batteries powered total water splitting. Nano Energy 2021, 88, 106238. [Google Scholar] [CrossRef]
- Li, Y.-W.; Zhang, W.-J.; Li, J.; Ma, H.-Y.; Du, H.-M.; Li, D.-C.; Wang, S.-N.; Zhao, J.-S.; Dou, J.-M.; Xu, L. Fe-MOF-Derived Efficient ORR/OER Bifunctional Electrocatalyst for Rechargeable Zinc–Air Batteries. ACS Appl. Mater. Interfaces 2020, 12, 44710–44719. [Google Scholar] [CrossRef]
- Pan, Y.; Abazari, R.; Wu, Y.; Gao, J.; Zhang, Q. Advances in metal–organic frameworks and their derivatives for diverse electrocatalytic applications. Electrochem. Commun. 2021, 126, 107024. [Google Scholar] [CrossRef]
- Li, H.; Wu, G.; Cheng, G.; Shuai, Y.; Liu, S.; Liu, Y. CoNi nanoalloys embedded in N-doped carbon nanofibers derived from layered bimetal-organic framework and as efficient oxygen electrocatalyst. J. Alloys Compd. 2021, 888, 161588. [Google Scholar] [CrossRef]
- Chen, B.; Ma, G.; Zhu, Y.; Wang, J.; Xiong, W.; Xia, Y. Metal-organic-framework-derived bi-metallic sulfide on N, S-codoped porous carbon nanocomposites as multifunctional electrocatalysts. J. Power Sources 2016, 334, 112–119. [Google Scholar] [CrossRef]
- Niu, Q.; Chen, B.; Guo, J.; Nie, J.; Guo, X.; Ma, G. Flexible, Porous, and Metal–Heteroatom-Doped Carbon Nanofibers as Efficient ORR Electrocatalysts for Zn–Air Battery. Nano-Micro Lett. 2019, 11, 8. [Google Scholar] [CrossRef] [Green Version]
- Niu, Q.; Ola, O.; Chen, B.; Zhu, Y.; Xia, Y.; Ma, G. Metal Sulfide Nanoparticles Anchored N, S Co-doped Porous Carbon Nanofibers as Highly Efficient Bifunctional Electrocatalysts for Oxygen Reduction/Evolution Reactions. Int. J. Electrochem. Sci. 2020, 15, 4869–4883. [Google Scholar] [CrossRef]
- Niu, Q.; Ola, O.; Wang, N.; Chen, B.; Zhu, Y.; Xia, Y. Carbon encapsulated WS2 nanocomposites derived from ZIF-67@WS2 Core-Shell Nanoparticles and their electrocatalytic applications. Int. J. Electrochem. Sci. 2020, 15, 12370–12379. [Google Scholar] [CrossRef]
- Peng, W.; Jin, J.; Yang, S.; Shen, Z.; Wang, H.; Zhang, J.; Li, G. NiCo Alloy Nanoparticles Anchored on Carbon Nanotube-Decorated Carbon Nanorods as a Durable and Efficient Oxygen Electrocatalyst for Zinc-Air Flow Batteries. ACS Appl. Energy Mater. 2021, 4, 11041–11050. [Google Scholar] [CrossRef]
- Liu, C.; Zuo, P.; Jin, Y.; Zong, X.; Li, D.; Xiong, Y. Defect-enriched carbon nanofibers encapsulating NiCo oxide for efficient oxygen electrocatalysis and rechargeable Zn-air batteries. J. Power Sources 2020, 473, 228604. [Google Scholar] [CrossRef]
- Belkessam, C.; Bencherif, S.; Mechouet, M.; Idiri, N.; Ghilane, J. The Effect of Heteroatom Doping on Nickel Cobalt Oxide Electrocatalysts for Oxygen Evolution and Reduction Reactions. ChemPlusChem 2020, 85, 1710–1718. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Li, R.; Ma, G.; Gou, X.; Zhu, Y.; Xia, Y. Cobalt sulfide/N,S codoped porous carbon core–shell nanocomposites as superior bifunctional electrocatalysts for oxygen reduction and evolution reactions. Nanoscale 2015, 7, 20674–20684. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Yang, A.; Wang, A.; Yu, H.; Dai, J.; Zheng, Y. ZIF-67 derived Co, Fe, Ni co-doped porous carbon as an efficient electrocatalyst for hydrogen evolution reaction. J. Porous Mater. 2020, 27, 1685–1690. [Google Scholar] [CrossRef]
- Qiu, T.; Yang, J.-G.; Bai, X.-J.; Wang, Y.-L. The preparation of synthetic graphite materials with hierarchical pores from lignite by one-step impregnation and their characterization as dye absorbents. RSC Adv. 2019, 9, 12737–12746. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Gao, F.; Zhao, Y.; Liu, N.; Tan, T.; Wang, X. Two-dimensional CeO2/RGO composite-modified separator for lithium/sulfur batteries. Nanoscale Res. Lett. 2018, 13, 377. [Google Scholar] [CrossRef]
- Tang, Y.-J.; Liu, C.-H.; Huang, W.; Wang, X.-L.; Dong, L.-Z.; Li, S.-L.; Lan, Y.-Q. Bimetallic Carbides-Based Nanocomposite as Superior Electrocatalyst for Oxygen Evolution Reaction. ACS Appl. Mater. Interfaces 2017, 9, 16977–16985. [Google Scholar] [CrossRef]
- Hu, Z.; Guo, Z.; Zhang, Z.; Dou, M.; Wang, F. Bimetal zeolitic imidazolite framework-derived iron-, cobalt-and nitrogen-codoped carbon nanopolyhedra electrocatalyst for efficient oxygen reduction. ACS Appl. Mater. Interfaces 2018, 10, 12651–12658. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Sun, J.; Qin, Y.; Yang, H.; Zhang, S.; Liu, X.; Luo, J. Hollow CoFe-layered double hydroxide polyhedrons for highly efficient CO2 electrolysis. Sci. China Mater. 2021, 65, 536–542. [Google Scholar] [CrossRef]
- Gao, Y.; Mi, L.; Wei, W.; Cui, S.; Zheng, Z.; Hou, H.; Chen, W. Double metal ions synergistic effect in hierarchical multiple sulfide microflowers for enhanced supercapacitor performance. ACS Appl. Mater. Interfaces 2015, 7, 4311–4319. [Google Scholar] [CrossRef]
- Zhang, G.-Q.; Li, B.; Liu, M.-C.; Yuan, S.-K.; Niu, L.-Y. Liquid phase synthesis of CoP nanoparticles with high electrical conductivity for advanced energy storage. J. Nanomater. 2017, 2017, 9728591. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, D.; Wang, C.; Li, X.; Li, K.; Peng, Y.; Li, J. Facile surface improvement method for LaCoO3 for toluene oxidation. Catal. Sci. Technol. 2018, 8, 3166–3173. [Google Scholar] [CrossRef]
- Ren, J.-T.; Yuan, Z.-Y. Bifunctional electrocatalysts of cobalt sulfide nanocrystals in situ decorated on N, S-codoped porous carbon sheets for highly efficient oxygen electrochemistry. ACS Sustain. Chem. Eng. 2019, 7, 10121–10131. [Google Scholar] [CrossRef]
- Song, H.; Zhang, F.; Song, H.; Xu, X.; Li, F. The effect of neodymium content on dibenzothiophene HDS performance over a bulk Ni2P catalyst. Catal. Commun. 2015, 69, 59–62. [Google Scholar] [CrossRef]
- Dutta, S.; Indra, A.; Feng, Y.; Song, T.; Paik, U. Self-supported nickel iron layered double hydroxide-nickel selenide electrocatalyst for superior water splitting activity. ACS Appl. Mater. Interfaces 2017, 9, 33766–33774. [Google Scholar] [CrossRef]
- Peck, M.A.; Langell, M.A. Comparison of nanoscaled and bulk NiO structural and environmental characteristics by XRD, XAFS, and XPS. Chem. Mater. 2012, 24, 4483–4490. [Google Scholar] [CrossRef]
- Yin, J.; Li, Y.; Lv, F.; Lu, M.; Sun, K.; Wang, W.; Wang, L.; Cheng, F.; Li, Y.; Xi, P.; et al. Oxygen Vacancies Dominated NiS2/CoS2 Interface Porous Nanowires for Portable Zn–Air Batteries Driven Water Splitting Devices. Adv. Mater. 2017, 29, 1704681. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Ma, L.; Yadav, R.M.; Yang, Y.; Zhang, X.; Vajtai, R.; Lou, J.; Ajayan, P.M. Nitrogen-Doped Graphene with Pyridinic Dominance as a Highly Active and Stable Electrocatalyst for Oxygen Reduction. ACS Appl. Mater. Interfaces 2015, 7, 14763–14769. [Google Scholar] [CrossRef]
- Sheng, Z.-H.; Shao, L.; Chen, J.-J.; Bao, W.-J.; Wang, F.-B.; Xia, X.-H. Catalyst-Free Synthesis of Nitrogen-Doped Graphene via Thermal Annealing Graphite Oxide with Melamine and Its Excellent Electrocatalysis. ACS Nano 2011, 5, 4350–4358. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, H.; Yang, C.; Lu, J.; Zheng, L.; Liang, H.-P. Mesoporous carbon promoting the efficiency and stability of single atomic electrocatalysts for oxygen reduction reaction. Carbon 2022, 191, 393–402. [Google Scholar] [CrossRef]
- Liang, H.-W.; Zhuang, X.; Brüller, S.; Feng, X.; Müllen, K. Hierarchically porous carbons with optimized nitrogen doping as highly active electrocatalysts for oxygen reduction. Nat. Commun. 2014, 5, 4973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; Wang, C.; Hu, S.; Wu, H.; Feng, C.; Zhang, Y. Nitrogen, phosphorus co-doped mesoporous carbon materials as efficient catalysts for oxygen reduction reaction. Ionics 2019, 25, 4295–4303. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.; Ola, O.; Dapaah, M.F.; Lu, Y.; Niu, Q.; Cheng, L.; Wang, N.; Zhu, Y. Preparation and Characterization of Multi-Doped Porous Carbon Nanofibers from Carbonization in Different Atmospheres and Their Oxygen Electrocatalytic Properties Research. Nanomaterials 2022, 12, 832. https://doi.org/10.3390/nano12050832
Wang T, Ola O, Dapaah MF, Lu Y, Niu Q, Cheng L, Wang N, Zhu Y. Preparation and Characterization of Multi-Doped Porous Carbon Nanofibers from Carbonization in Different Atmospheres and Their Oxygen Electrocatalytic Properties Research. Nanomaterials. 2022; 12(5):832. https://doi.org/10.3390/nano12050832
Chicago/Turabian StyleWang, Tao, Oluwafunmilola Ola, Malcom Frimpong Dapaah, Yuhao Lu, Qijian Niu, Liang Cheng, Nannan Wang, and Yanqiu Zhu. 2022. "Preparation and Characterization of Multi-Doped Porous Carbon Nanofibers from Carbonization in Different Atmospheres and Their Oxygen Electrocatalytic Properties Research" Nanomaterials 12, no. 5: 832. https://doi.org/10.3390/nano12050832
APA StyleWang, T., Ola, O., Dapaah, M. F., Lu, Y., Niu, Q., Cheng, L., Wang, N., & Zhu, Y. (2022). Preparation and Characterization of Multi-Doped Porous Carbon Nanofibers from Carbonization in Different Atmospheres and Their Oxygen Electrocatalytic Properties Research. Nanomaterials, 12(5), 832. https://doi.org/10.3390/nano12050832






