Biogenic Synthesis of Silver Nanoparticles Using Phagnalon niveum and Its In Vivo Anti-Diabetic Effect against Alloxan-Induced Diabetic Wistar Rats
Abstract
1. Introduction
2. Material and Methods
2.1. Chemicals and Instruments
2.2. Plant Collection
2.3. Extract Preparations of Phagnalon niveum Edgew
2.4. Phytochemical Screening
2.5. Test
2.5.1. Test for Alkaloids
2.5.2. Test for Glycosides
2.5.3. Test for Saponins
2.5.4. Testing for Proteins and Amino Acids
Protein Biuret Test
Test for Amino Acids
2.5.5. Test for Phytosterols
2.5.6. Test for Phenols
2.5.7. Test for Flavonoids
2.5.8. Test for Terpenoids
2.5.9. Test for Tannins
2.5.10. Test for Quinones
2.5.11. Test for Coumarins
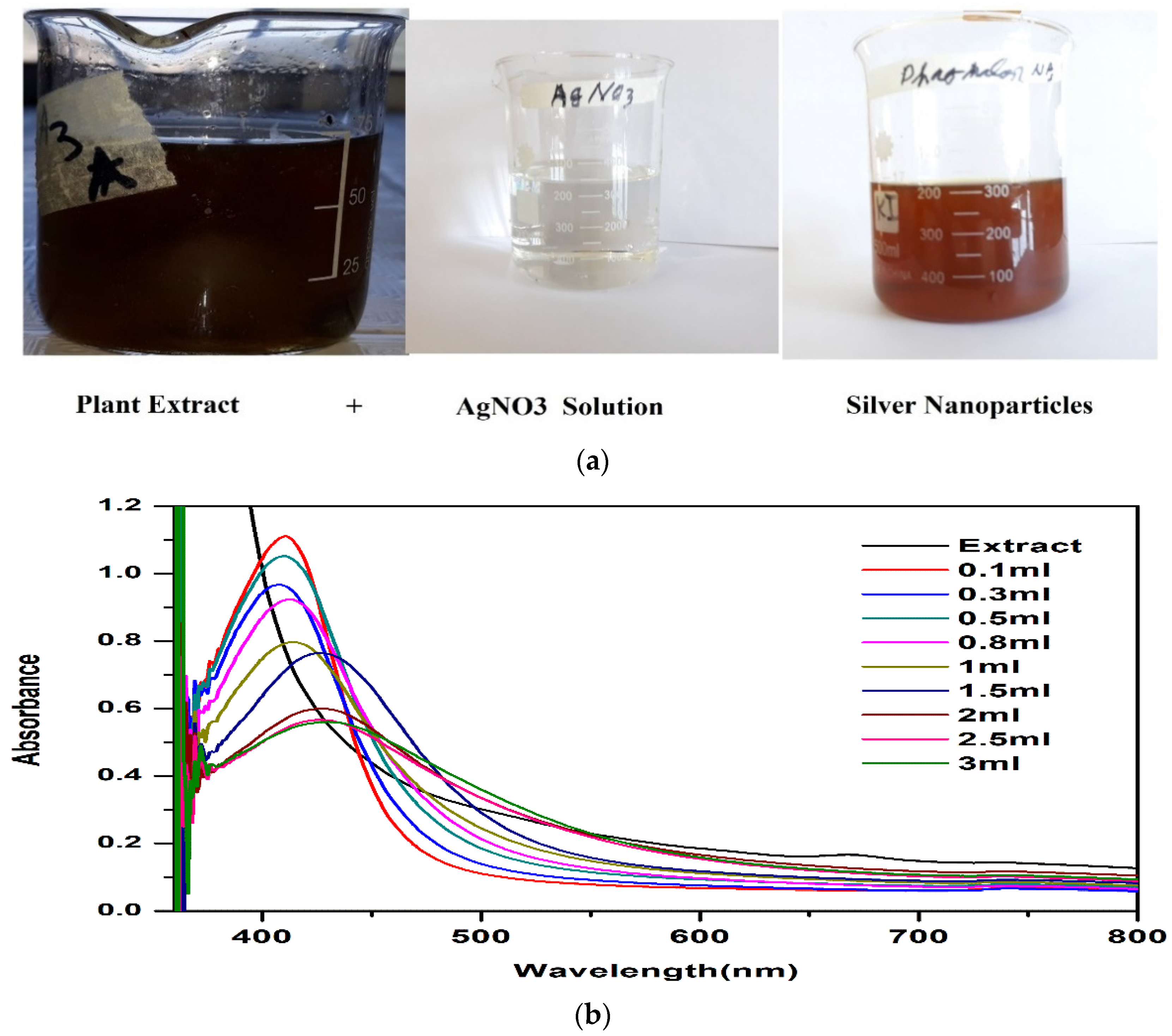
2.6. Synthesis of Silver Nanoparticles
2.7. Characterisation of Silver Nanoparticles
2.8. Induction of Diabetes
2.9. Acute Toxicity
2.10. Experimental Treatments in Animals and Ethics
2.11. Oral Glucose Tolerance Test
2.12. Serological Analysis
2.13. Urine Analysis
2.14. Statistical Analysis
3. Results
3.1. Phytochemical Analysis
3.2. Synthesis and Characterisation of Silver Nanoparticles
3.2.1. Green Synthesis of AgNPs
3.2.2. UV-Vis Spectroscopy
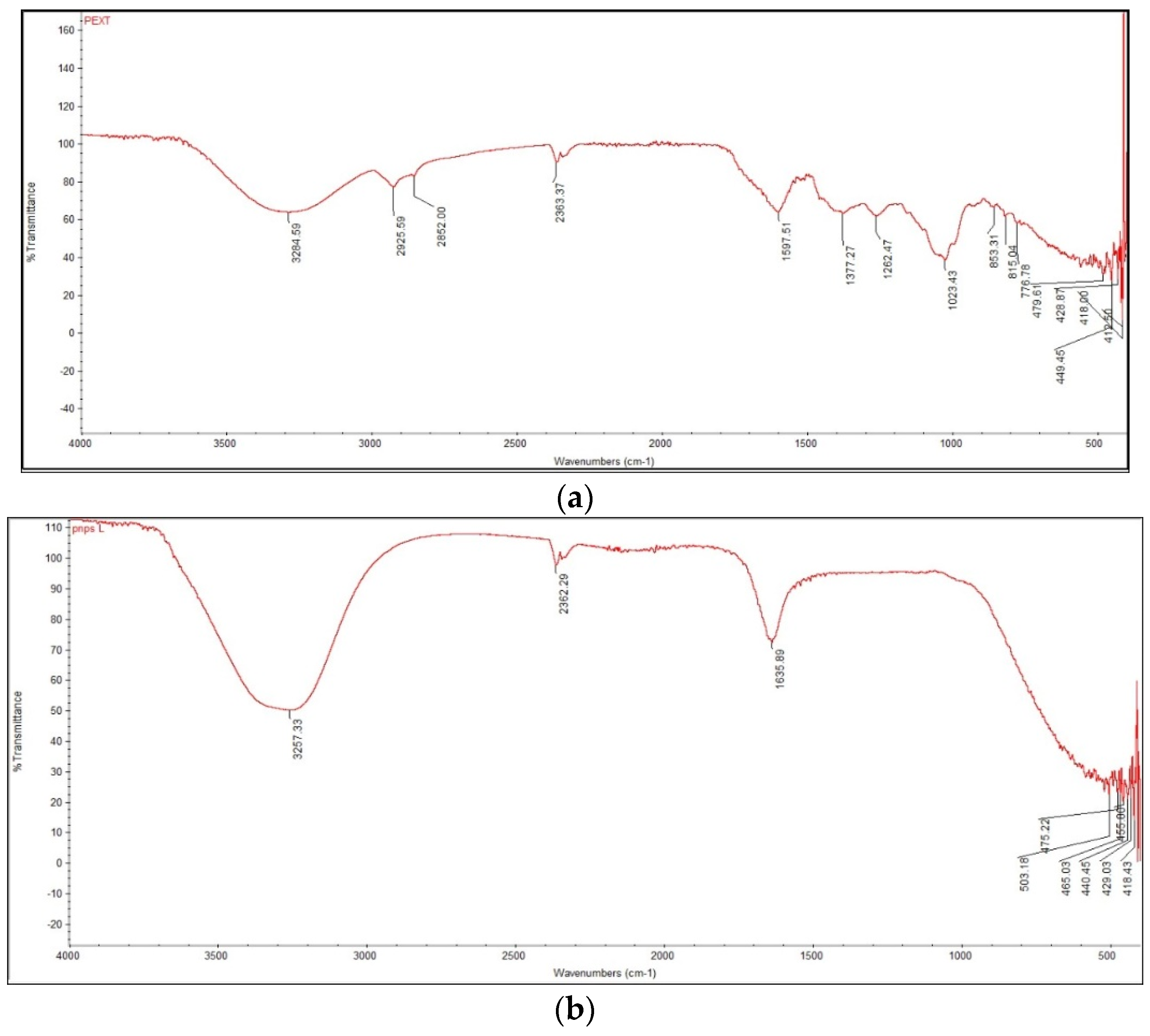
3.2.3. Fourier-Transform Infrared Spectroscopy
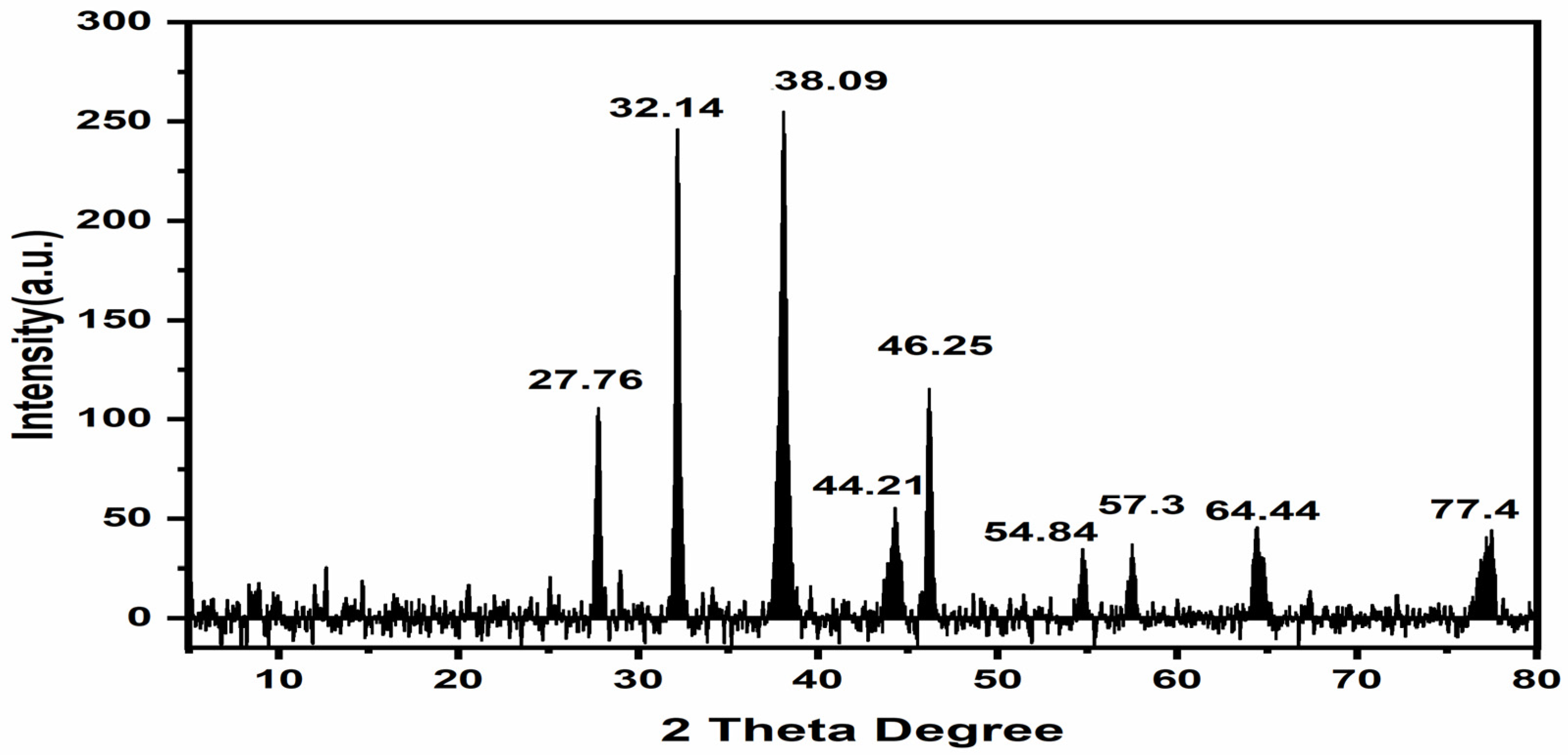
3.2.4. XRD Analysis
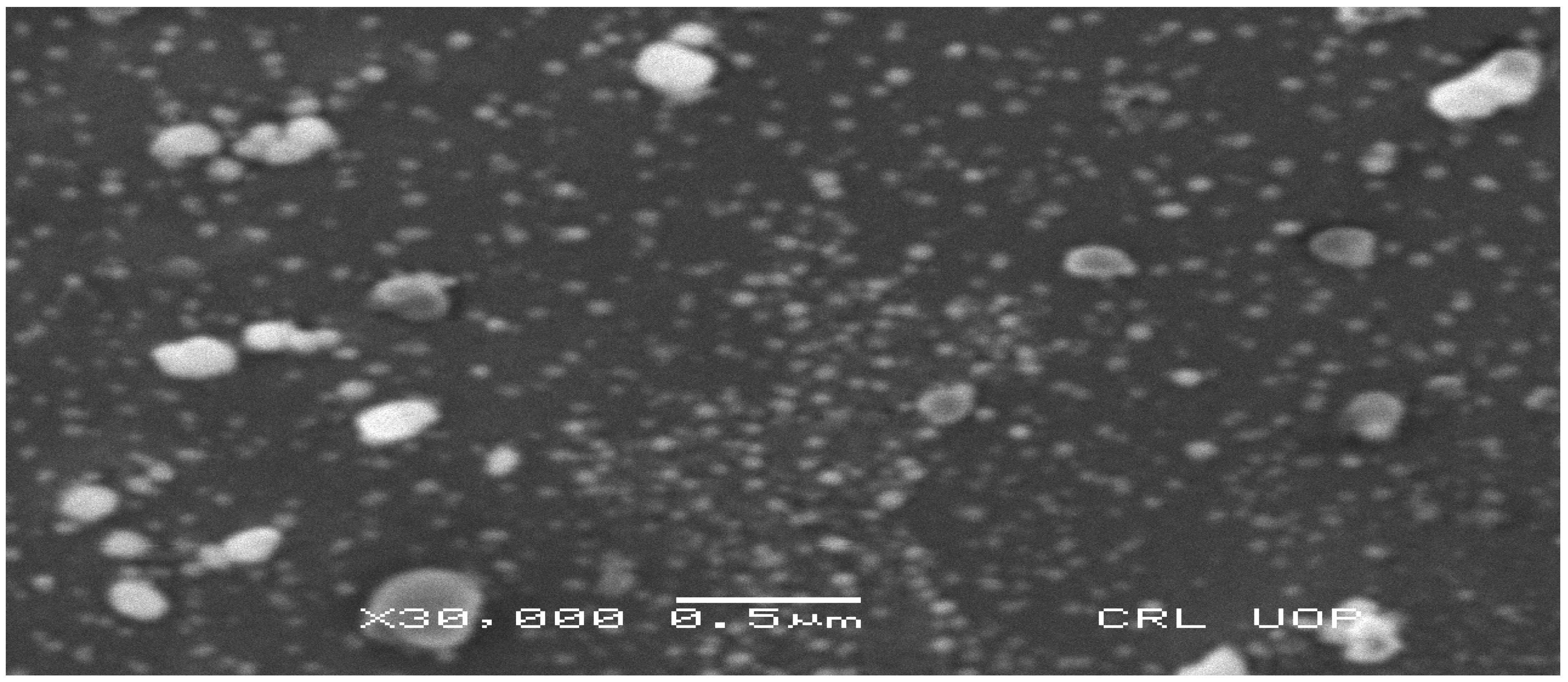
3.2.5. SEM Analysis
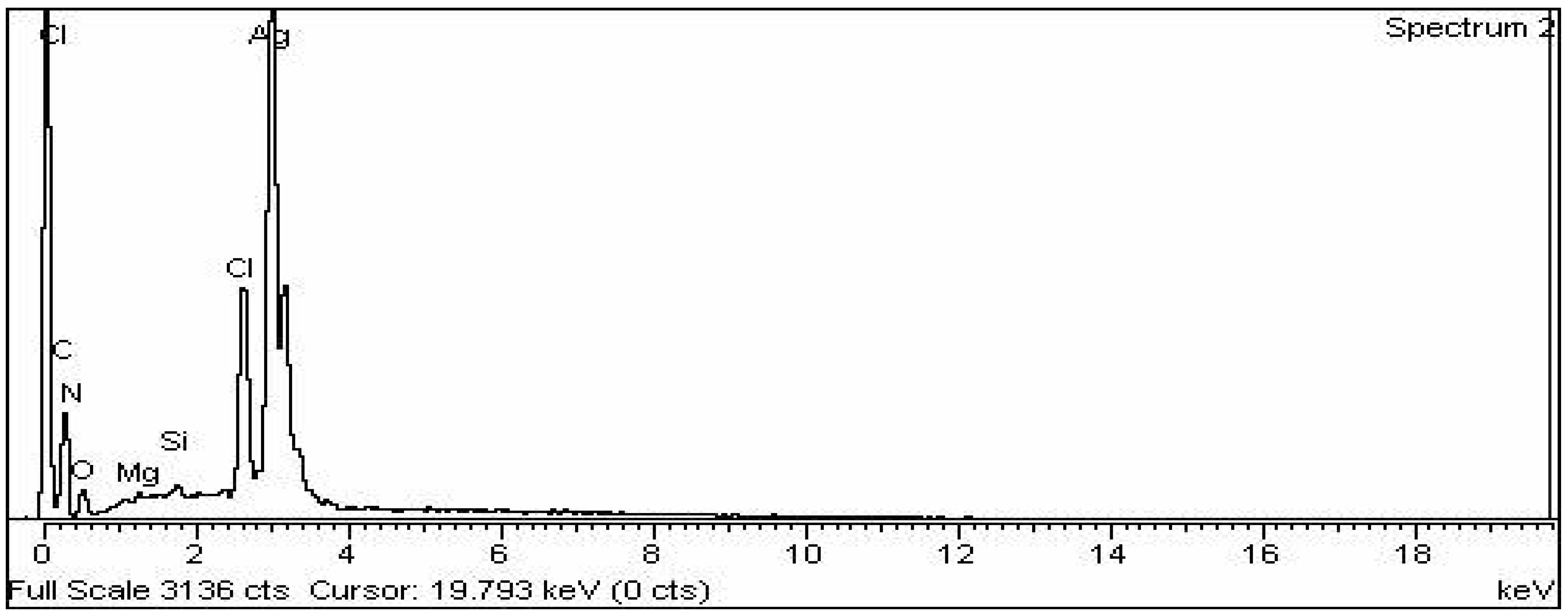
3.2.6. EDX Analysis
3.3. Acute Toxicity Test
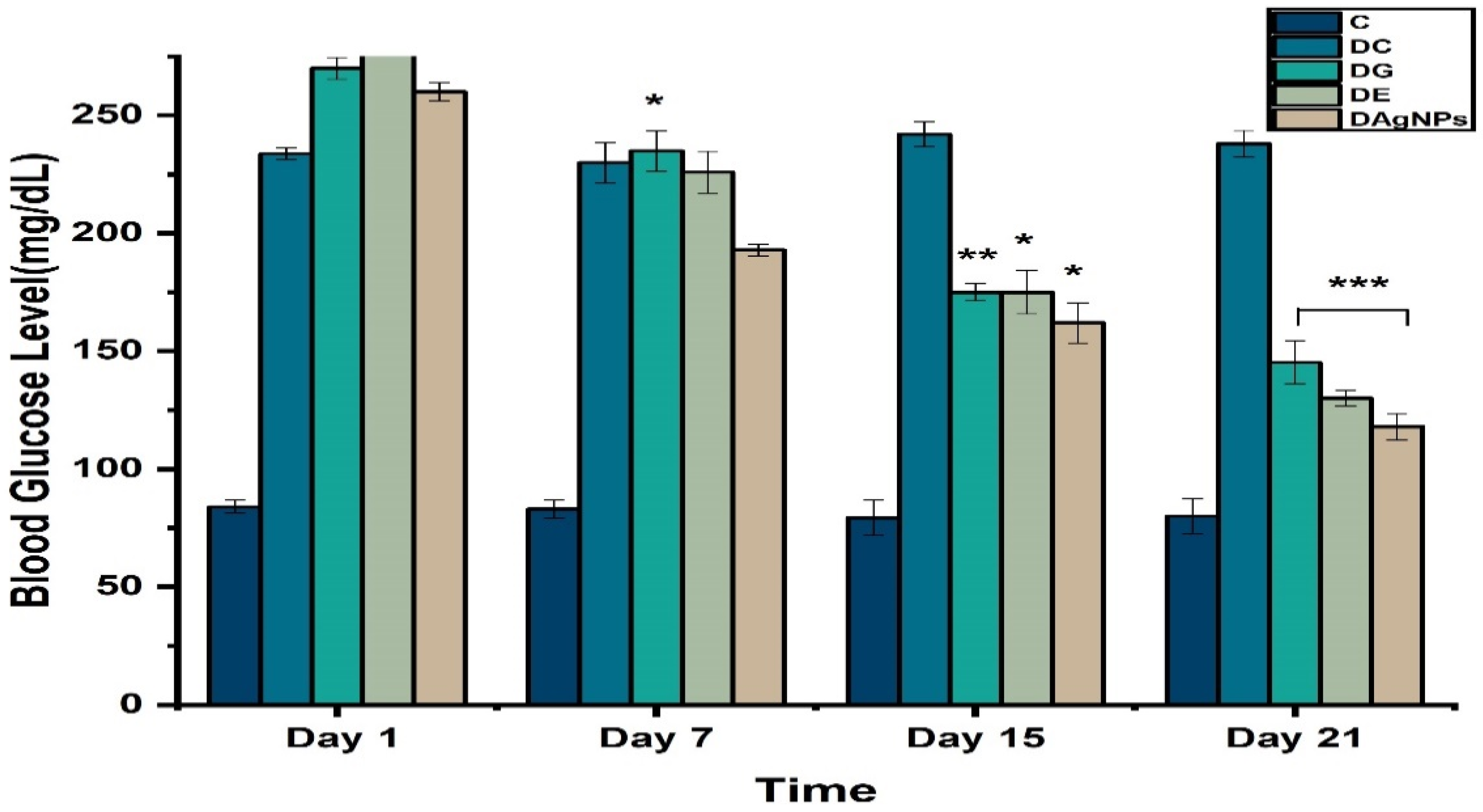
3.4. Blood Glucose Level
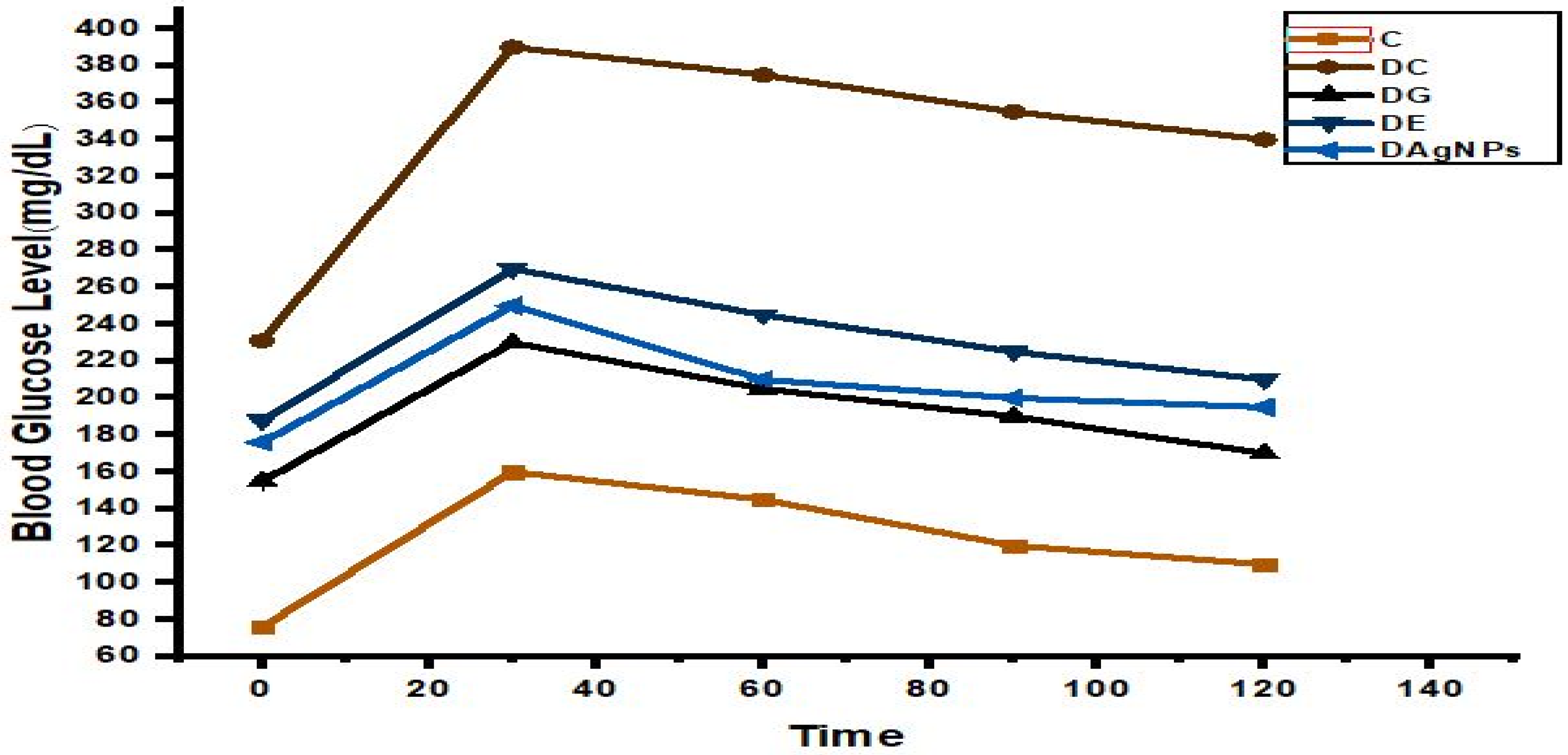
3.5. Oral Glucose Tolerance Test
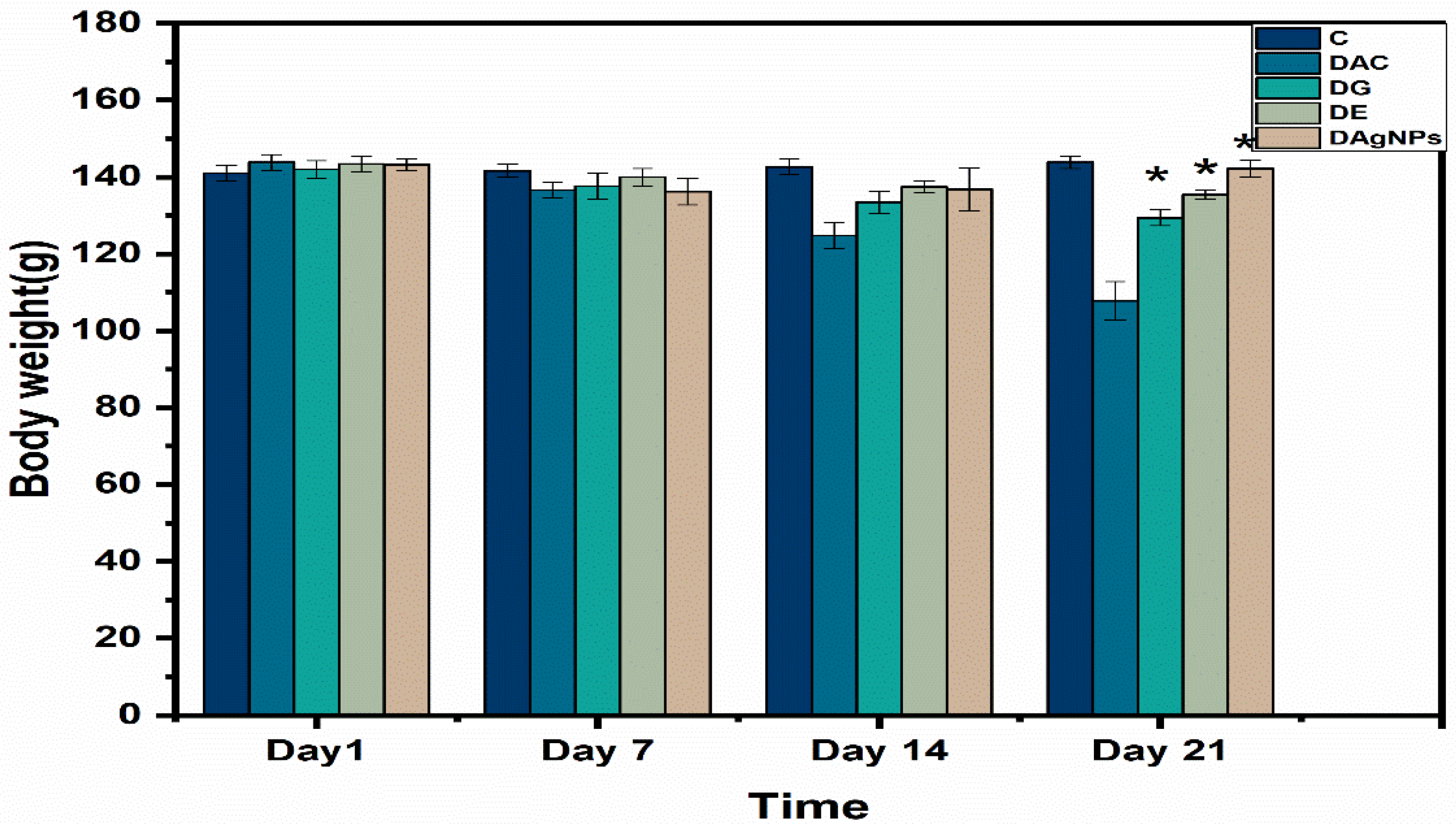
3.6. Body Weight
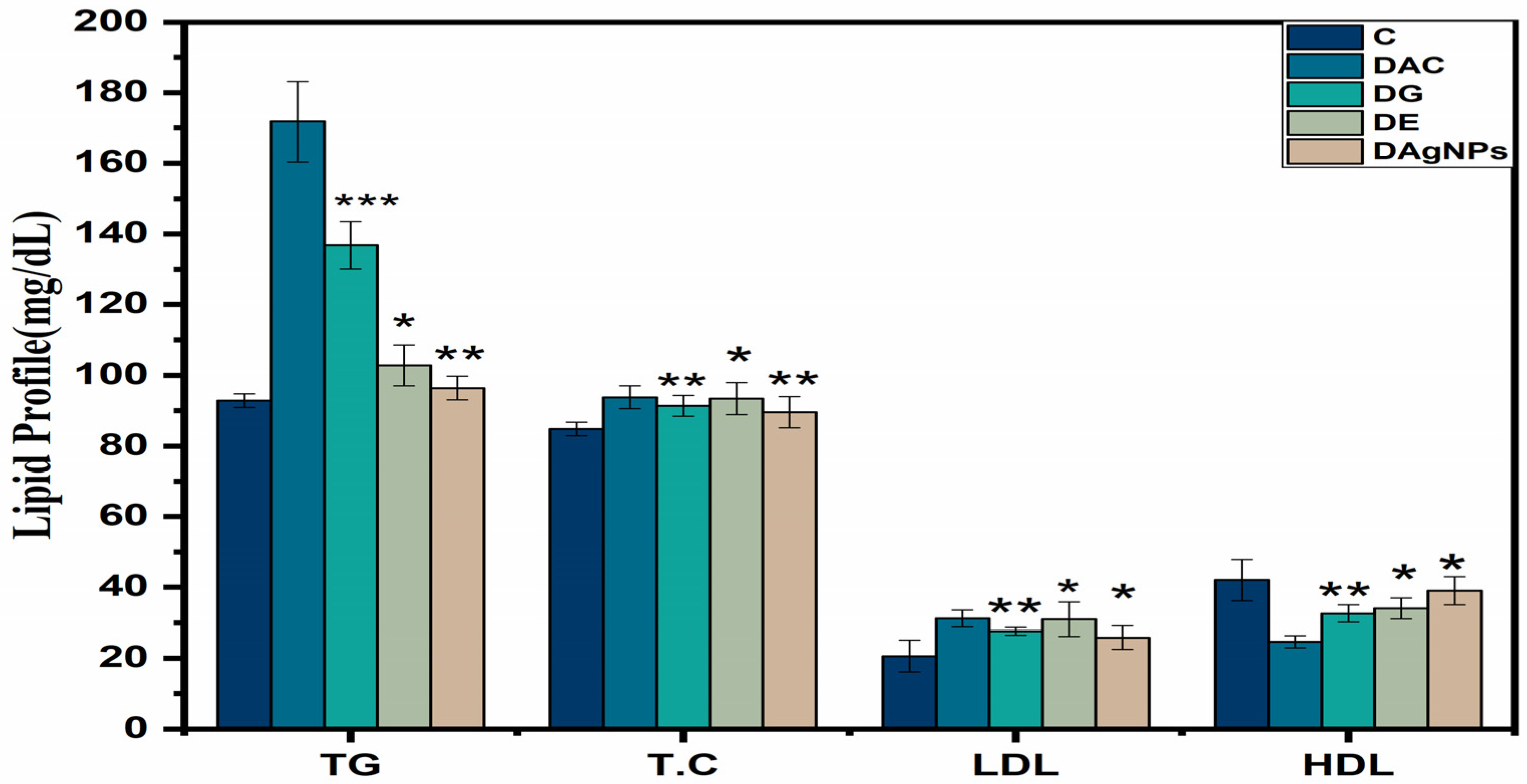
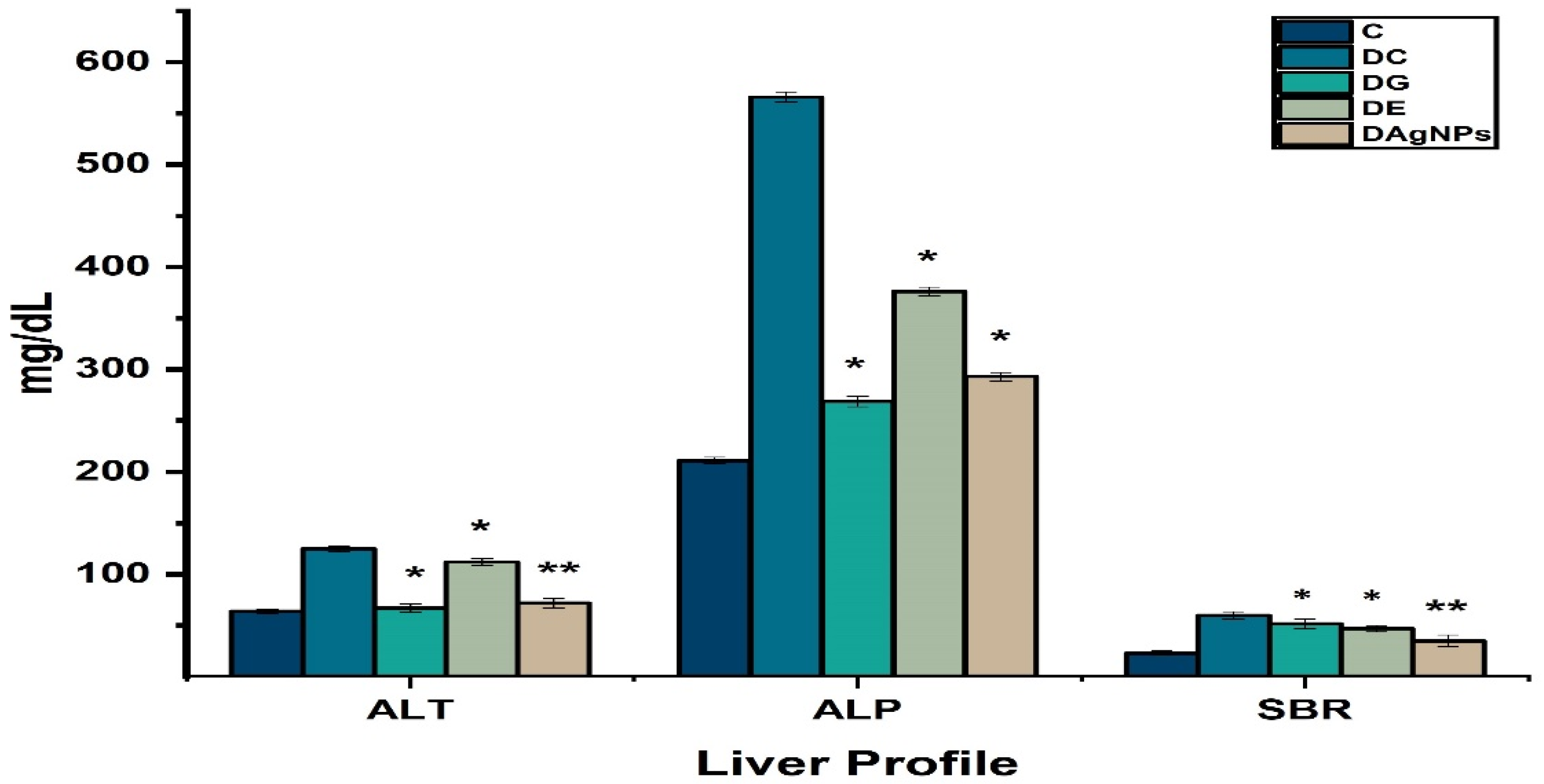
3.7. Serological and Urine Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ALB | Albumin protein |
| ALP | Alkaline phosphatase |
| ALT | Alanine aminotransferase |
| BIL | Bilirubin |
| BLO | Blood |
| BU | Blood urea |
| C | Control |
| C | Carbon |
| Ca | Calcium |
| Cl | Chlorine |
| Creat | Creatinine |
| ddH2O | Double-distilled water |
| DC | Diabetic control |
| DE | Diabetic extract |
| DG | Diabetic glibenclamide |
| DAgNPs | Diabetic silver nanoparticles |
| DPP4 | Di peptidyl peptidase enzyme 4 |
| EDX | Energy-dispersive X-ray spectroscopy |
| FTIR | Fourier Transform Infrared spectroscopy |
| GLU | Glucose |
| HDLc | High-density lipoprotein |
| KET | Ketone |
| LDLc | Low-density lipoprotein |
| LEU | Leukocytes |
| Mg | Magnesium |
| N | Nitrogen |
| NaOH | Sodium hydroxide |
| NIT | Nitrite |
| O | Oxygen |
| pH | Power of hydrogen ion |
| PRO | Protein |
| SBR | Serum bilirubin |
| SEM | Scanning Electron Microscopy |
| SGLT-2 | Sodium–glucose transport protein 2 |
| SG | Specific gravity |
| Si | Silicon |
| T1D | Type-1 diabetes mellitus |
| T2D | Type-2 diabetes mellitus |
| TGs | Tri glycerides |
| URO | Urobilinogen |
| UV-Vis | Ultraviolet-Visible |
| XRD | X-ray diffraction |
References
- Santhoshkumar, J.; Rajeshkumar, S.; Kumar, S.V. Phyto-Assisted synthesis, characterization and applications of gold Nanoparticles—A review. Biochem. Biophys. Rep. 2017, 11, 46–57. [Google Scholar] [CrossRef]
- Preethi, R.; Padma, P.R. Anticancer activity of silver nanobioconjugates synthesized from Piper betle leaves extract and its active compound eugenol. Int. J. Pharm. Pharm. Sci. 2016, 8, 201–205. [Google Scholar] [CrossRef]
- Agarwal, H.; Kumar, S.V.; Rajeshkumar, S. A review on green synthesis of zinc oxide nanoparticles—An Eco-Friendly approach. Resour. Technol. 2017, 3, 406–413. [Google Scholar] [CrossRef]
- Menon, S.; Rajeshkumar, S.; Kumar, V. A review on biogenic synthesis of gold nanoparticles, characterization, and its applications. Resour. Technol. 2017, 3, 516–527. [Google Scholar] [CrossRef]
- Iravani, S. Green synthesis of metal nanoparticles using plants. Green Chem. 2011, 13, 2638–2650. [Google Scholar] [CrossRef]
- Zhang, Z.; Cai, H.; Liu, Z.; Yao, P. Effective enhancement of hypoglycemic effect of insulin by liver-targeted nanoparticles containing cholic acid-Modified chitosan derivative. Mol. Pharm. 2016, 13, 2433–2442. [Google Scholar] [CrossRef]
- Arvanag, F.M.; Bayrami, A.; Habibi-Yangjeh, A.; Pouran, S.R. A comprehensive study on antidiabetic and antibacterial activities of ZnO nanoparticles biosynthesized using Silybum marianum L seed extract. Mater. Sci. Eng. C 2019, 97, 397–405. [Google Scholar] [CrossRef]
- Hussein, J.; Attia, M.F.; El Bana, M.; El-Daly, S.M.; Mohamed, N.; El-Khayat, Z.; El-Naggar, M.E. Solid state synthesis of docosahexaenoic acid-loaded zinc oxide nanoparticles as a potential antidiabetic agent in rats. Int. J. Biol. Macromol. 2019, 140, 1305–1314. [Google Scholar] [CrossRef]
- Dhas, T.S.; Kumar, V.G.; Karthick, V.; Vasanth, K.; Singaravelu, G.; Govindaraju, K. Effect of biosynthesized gold nanoparticles by Sargassum swartzii in alloxan induced diabetic rats. Enzyme Microb. Technol. 2016, 95, 100–106. [Google Scholar] [CrossRef]
- Veiseh, O.; Tang, B.C.; Whitehead, K.A.; Anderson, D.G.; Langer, R. Managing diabetes with nanomedicine: Challenges and opportunities. Nat. Rev. Drug Discov. 2015, 14, 45–57. [Google Scholar] [CrossRef]
- Malapermal, V.; Botha, I.; Krishna, S.B.N.; Mbatha, J.N. Enhancing antidiabetic and antimicrobial performance of Ocimum basilicum, and Ocimum sanctum (L.) using silver nanoparticles. Saudi J. Biol. Sci. 2017, 24, 1294–1305. [Google Scholar] [CrossRef] [PubMed]
- Samadder, A.; Khuda-Bukhsh, A.R. Nanotechnological approaches in diabetes treatment: A new horizon. World J. Transl. Med. 2014, 3, 84–95. [Google Scholar] [CrossRef]
- Thorve, V.S.; Kshirsagar, A.D.; Vyawahare, N.S.; Joshi, V.S.; Ingale, K.G.; Mohite, R.J. Diabetes-induced erectile dysfunction: Epidemiology, pathophysiology and management. J. Diabetes Complicat. 2011, 25, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Wright, E., Jr.; Scism-Bacon, J.L.; Glass, L.C. Oxidative stress in type 2 diabetes: The role of fasting and postprandial glycaemia. Int. J. Clin. Pract. 2006, 60, 308–314. [Google Scholar] [CrossRef]
- Anderson, M.; Powell, J.; Campbell, K.M.; Taylor, J.R. Optimal management of type 2 diabetes in patients with increased risk of hypoglycemia. Diabetes Metab. Syndr. Obes. Targets Ther. 2014, 7, 85. [Google Scholar]
- Volpe, C.M.O.; Villar-Delfino, P.H.; dos Anjos, P.M.F.; Nogueira-Machado, J.A. Cellular death, reactive oxygen species (ROS) and diabetic complications. Cell Death Dis. 2018, 9, 119. [Google Scholar] [CrossRef]
- Ali, S.I.; Qaiser, M.; Abid, R. Flora Pakistan; No. 210: Asteraceae (II) inuleae, Plucheeae, Gnaphalieae; MBG Press: St. Louis, MO, USA, 2003; Volume 210. [Google Scholar]
- Nisar-Ul-Haq, M.; Wazir, S.M.; Ullah, F.; Khan, R.A.; Shah, M.S.; Khatak, A. Phytochemical and biological evaluation of defatted seeds of Jatropha curcas. Sains Malays. 2016, 45, 1435–1442. [Google Scholar]
- Jayapriya, G.; Shoba, F.G. Screening for phytochemical activity of Urechites lutea plant. Asian J. Plant Sci. Res. 2014, 4, 20–24. [Google Scholar]
- Savithramma, N.; Rao, M.L.; Suhrulatha, D. Screening of medicinal plants for secondary metabolites. Middle-East J. Sci. Res. 2011, 8, 579–584. [Google Scholar]
- Khan, N.A.; Niaz, A.; Zaman, M.I.; Khan, F.A.; Nisar-Ul-Haq, M.; Tariq, M. Sensitive and selective colorimetric detection of Pb2+ by silver nanoparticles synthesized from Aconitum violaceum plant leaf extract. Mater. Res. Bull. 2018, 102, 330–336. [Google Scholar] [CrossRef]
- Prakash, P.; Gnanaprakasam, P.; Emmanuel, R.; Arokiyaraj, S.; Saravanan, M. Green synthesis of silver nanoparticles from leaf extract of Mimusops elengi, Linn. for enhanced antibacterial activity against multi drug resistant clinical isolates. Colloids Surf. B Biointerfaces 2013, 108, 255–259. [Google Scholar] [CrossRef]
- Das, G.; Patra, J.K.; Debnath, T.; Ansari, A.; Shin, H.-S. Investigation of antioxidant, antibacterial, antidiabetic, and cytotoxicity potential of silver nanoparticles synthesized using the outer peel extract of Ananas comosus (L.). PLoS ONE 2019, 14, e0220950. [Google Scholar] [CrossRef]
- Sengottaiyan, A.; Aravinthan, A.; Sudhakar, C.; Selvam, K.; Srinivasan, P.; Govarthanan, M.; Manoharan, K.; Selvankumar, T. Synthesis and characterization of Solanum nigrum-Mediated silver nanoparticles and its protective effect on alloxan-Induced diabetic rats. J. Nanostruct. Chem. 2016, 6, 41–48. [Google Scholar] [CrossRef]
- Xu, Z.P.; Zeng, Q.H.; Lu, G.Q.; Yu, A.B. Inorganic nanoparticles as carriers for efficient cellular delivery. Chem. Eng. Sci. 2006, 61, 1027–1040. [Google Scholar] [CrossRef]
- Raut, N.A.; Gaikwad, N.J. Antidiabetic activity of hydro-Ethanolic extract of Cyperus rotundus in alloxan induced diabetes in rats. Fitoterapia 2006, 77, 585–588. [Google Scholar] [CrossRef]
- Vessal, M.; Hemmati, M.; Vasei, M. Antidiabetic effects of quercetin in streptozocin-induced diabetic rats. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2003, 135, 357–364. [Google Scholar] [CrossRef]
- Mansour, H.A.; Newairy, A.-S.A.; Yousef, M.I.; Sheweita, S.A. Biochemical study on the effects of some Egyptian herbs in alloxan-Induced diabetic rats. Toxicology 2002, 170, 221–228. [Google Scholar] [CrossRef]
- Hossain, M.A.; Nagooru, M.R. Biochemical profiling and total flavonoids contents of leaves crude extract of endemic medicinal plant Corydyline terminalis L. Kunth. Pharmacogn. J. 2011, 3, 25–30. [Google Scholar] [CrossRef]
- Akharaiyi, F.C. Antibacterial, phytochemical and antioxidant activities of Datura metel. Int. J. PharmTech Res. 2011, 3, 478–483. [Google Scholar]
- Suresh, S.N.; Nagarajan, N. Antimicrobial activity and preliminary phytochemical analysis of Begonia malabarica Lam. J. Pure Appl. Microbiol. 2009, 3, 801–803. [Google Scholar]
- Gonzalez-Guevara, J.L.; Gonzalez-Lavaut, J.A.; Pino-Rodriguez, S.; Garcia-Torres, M.; Carballo-Gonzalez, M.T.; Echemendia-Arana, O.A.; Molina-Torres, J.; Prieto-Gonzalez, S. Phytochemical screening and in vitro antiherpetic activity of four Erythtroxylum species. Acta Farm. Bonaer 2004, 23, 506–509. [Google Scholar]
- Anyasor, G.N.; Ogunwenmo, O.; Oyelana, O.A.; Akpofunure, B.E. Phytochemical constituents and antioxidant activities of aqueous and methanol stem extracts of Costus afer Ker Gawl. (Costaceae). Afr. J. Biotechnol. 2010, 9, 4880–4884. [Google Scholar]
- Lee, C.P.-D.; Hsiu, S.-L.; Hou, Y.-C. Flavonoids in herbs: Biological fates and potential interactions with xenobiotics. J. Food Drug Anal. 2002, 10, 3. [Google Scholar] [CrossRef]
- Igbinosa, O.O.; Igbinosa, E.O.; Aiyegoro, O.A. Antimicrobial activity and phytochemical screening of stem bark extracts from Jatropha curcas (Linn). Afr. J. Pharm. Pharmacol. 2009, 3, 58–62. [Google Scholar]
- Thitilertdecha, N.; Teerawutgulrag, A.; Rakariyatham, N. Antioxidant and antibacterial activities of Nephelium lappaceum L. extracts. LWT-Food Sci. Technol. 2008, 41, 2029–2035. [Google Scholar] [CrossRef]
- Barile, E.; Bonanomi, G.; Antignani, V.; Zolfaghari, B.; Sajjadi, S.E.; Scala, F.; Lanzotti, V. Saponins from Allium minutiflorum with antifungal activity. Phytochemistry 2007, 68, 596–603. [Google Scholar] [CrossRef]
- Ayoola, G.A.; Coker, H.A.; Adesegun, S.A.; Adepoju-Bello, A.A.; Obaweya, K.; Ezennia, E.C.; Atangbayila, T.O. Phytochemical screening and antioxidant activities of some selected medicinal plants used for malaria therapy in Southwestern Nigeria. Trop. J. Pharm. Res. 2008, 7, 1019–1024. [Google Scholar]
- Vadlapudi, V.; Kaladhar, D. Antimicrobial study of plant extracts of Datura metel L. against some important disease causing pathogens. Asian Pac. J. Trop. Dis. 2012, 2, S94–S97. [Google Scholar] [CrossRef]
- Sekar, D.; Kolanjinathan, K.; Saranraj, P.; Gajendiran, K. Screening of Phyllanthus amarus, Acalypha indica and Datura metel for its antimicrobial activity against selected pathogens. Int. J. Pharm. Biol. Arch. 2012, 3, 1231–1236. [Google Scholar]
- Zayed, M.F.; Eisa, W.H.; Shabaka, A.A. Malva parviflora extract assisted green synthesis of silver nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 98, 423–428. [Google Scholar] [CrossRef]
- Chanda, S. Silver nanoparticles (medicinal plants mediated): A new generation of antimicrobials to combat microbial pathogens—A review. In Microbial Pathogens and Strategies for Combating Them: Science, Technology and Education; Formatex Research Center: Badajoz, Spain, 2013; pp. 1314–1323. [Google Scholar]
- Pant, G.; Nayak, N.; Prasuna, R.G. Enhancement of antidandruff activity of shampoo by biosynthesized silver nanoparticles from Solanum trilobatum plant leaf. Appl. Nanosci. 2013, 3, 431–439. [Google Scholar] [CrossRef]
- Roopan, S.M.; Madhumitha, G.; Rahuman, A.A.; Kamaraj, C.; Bharathi, A.; Surendra, T.V. Low-Cost and Eco-Friendly Phyto-Synthesis of silver nanoparticles using Cocos nucifera coir extract and its larvicidal activity. Ind. Crops Prod. 2013, 43, 631–635. [Google Scholar] [CrossRef]
- Vivek, R.; Thangam, R.; Muthuchelian, K.; Gunasekaran, P.; Kaveri, K.; Kannan, S. Green biosynthesis of silver nanoparticles from Annona squamosa leaf extract and its in vitro cytotoxic effect on MCF-7 cells. Process Biochem. 2012, 47, 2405–2410. [Google Scholar] [CrossRef]
- El-Bindary, A.; Anwar, Z.; El-Shafaie, T. Effect of silicon dioxide nanoparticles on the assessment of quercetin flavonoid using Rhodamine B Isothiocyanate dye. J. Mol. Liq. 2021, 323, 114607. [Google Scholar] [CrossRef]
- El-Gammal, O.A.; Mohamed, F.S.; Rezk, G.N.; El-Bindary, A.A. Synthesis, characterization, catalytic, DNA binding and antibacterial activities of Co (II), Ni (II) and Cu (II) complexes with new Schiff base ligand. J. Mol. Liq. 2021, 326, 115223. [Google Scholar] [CrossRef]
- El-Bindary, M.A.; El-Bindary, A.A. Synthesis, characterization, DNA binding, and biological action of dimedone arylhydrazone chelates. Appl. Organomet. Chem. 2022, e6576. [Google Scholar] [CrossRef]
- Thirunavoukkarasu, M.; Balaji, U.; Behera, S.; Panda, P.K.; Mishra, B.K. Biosynthesis of silver nanoparticle from leaf extract of Desmodium gangeticum (L.) DC. and its biomedical potential. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 116, 424–427. [Google Scholar] [CrossRef]
- Zaheer, Z.; Rafiuddin, H.S. Bio-Conjugated silver nanoparticles: From Ocimum sanctum and role of cetyltrimethyl ammonium bromide. Colloids Surf. B Biointerfaces 2013, 108, 90–94. [Google Scholar] [CrossRef]
- Charbgoo, F.; Ahmad, M.B.; Darroudi, M. Cerium oxide nanoparticles: Green synthesis and biological applications. Int. J. Nanomed. 2017, 12, 1401. [Google Scholar] [CrossRef]
- Gylling, H.; Miettinen, T.A. Cholesterol absorption, synthesis, and LDL metabolism in NIDDM. Diabetes Care 1997, 20, 90–95. [Google Scholar] [CrossRef]
- Ragini, V.; KVSRG, P.; Bharathi, K. Antidiabetic and antioxidant activity of Shorea tumbuggaia Rox. Int. J. Innov. Pharm. Res. 2011, 2, 113–121. [Google Scholar]
- Attanayake, A.P.; Jayatilaka, K.A.P.W.; Pathirana, C.; Mudduwa, L.K.B. Study of antihyperglycaemic activity of medicinal plant extracts in alloxan induced diabetic rats. Anc. Sci. Life 2013, 32, 193. [Google Scholar] [CrossRef] [PubMed]
- Fröde, T.S.; Medeiros, Y.S. Animal models to test drugs with potential antidiabetic activity. J. Ethnopharmacol. 2008, 115, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Swanston-Flatt, S.K.; Day, C.; Bailey, C.J.; Flatt, P.R. Traditional plant treatments for diabetes. Studies in normal and streptozotocin diabetic mice. Diabetologia 1990, 33, 462–464. [Google Scholar] [CrossRef] [PubMed]
- Eleazu, C.O.; Eleazu, K.C.; Ironkwe, A.; Iroaganachi, M.A. Effect of Livingstone potato (Plectranthus esculenthus NE Br) on diabetes and its complications in streptozotocin induced diabetes in rats. Diabetes Metab. J. 2014, 38, 366–374. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shaik, A.H.; Rasool, S.N.; Reddy, A.V.K.; Kareem, M.A.; Krushna, G.S.; Devi, K.L. Cardioprotective effect of HPLC standardized ethanolic extract of Terminalia pallida fruits against isoproterenol-induced myocardial infarction in albino rats. J. Ethnopharmacol. 2012, 141, 33–40. [Google Scholar] [CrossRef]
- Shanmugasundaram, E.R.B.; Rajeswari, G.; Baskaran, K.; Kumar, B.R.R.; Shanmugasundaram, K.R.; Ahmath, B.K. Use of Gymnema sylvestre leaf extract in the control of blood glucose in insulin-dependent diabetes mellitus. J. Ethnopharmacol. 1990, 30, 281–294. [Google Scholar] [CrossRef]


| Tests | Present (+) | Absent (−) |
|---|---|---|
| Phenols | + | |
| Proteins | + | |
| Glycosides | - | |
| Tannins | + | |
| Flavonoids | + | |
| Alkaloids | + | |
| Saponins | - | |
| Quinones | + | |
| Sterols | + | |
| Moline Test for Carbohydrates | + | |
| Amino acids | + | |
| Terpenoides | + | |
| Coumarins | + |
| LEU | NIT | URO | PRO | PH | BLO | SG | KET | BIL | GLU | |
|---|---|---|---|---|---|---|---|---|---|---|
| C | - | - | - | - | 5 | - | 1 | - | - | - |
| DC | 15 | + | 1 | 30 ± 0.3 | 5 | ++ | 1.01 | 15 ± 1.5 | 1 | 250 ± 15 |
| DG | 15 | + | 1 | 30 ± 0.4 | 6 | ++ | 1.05 | 15 ± 1.6 | 1 | - |
| DE | - | - | 1 | 15 | 5.5 | + | 1.01 | 15 ± 1.7 | 1 | - |
| DAgNPs | - | - | 1 | 15 | 5.3 | + | 1.05 | 15 ± 1.9 | 1 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ul Haq, M.N.; Shah, G.M.; Gul, A.; Foudah, A.I.; Alqarni, M.H.; Yusufoglu, H.S.; Hussain, M.; Alkreathy, H.M.; Ullah, I.; Khan, A.M.; et al. Biogenic Synthesis of Silver Nanoparticles Using Phagnalon niveum and Its In Vivo Anti-Diabetic Effect against Alloxan-Induced Diabetic Wistar Rats. Nanomaterials 2022, 12, 830. https://doi.org/10.3390/nano12050830
Ul Haq MN, Shah GM, Gul A, Foudah AI, Alqarni MH, Yusufoglu HS, Hussain M, Alkreathy HM, Ullah I, Khan AM, et al. Biogenic Synthesis of Silver Nanoparticles Using Phagnalon niveum and Its In Vivo Anti-Diabetic Effect against Alloxan-Induced Diabetic Wistar Rats. Nanomaterials. 2022; 12(5):830. https://doi.org/10.3390/nano12050830
Chicago/Turabian StyleUl Haq, Muhammad Nisar, Ghulam Mujtaba Shah, Alia Gul, Ahmed Ibrahim Foudah, Mohammad Hamed Alqarni, Hasan Soliman Yusufoglu, Masroor Hussain, Huda Mohammed Alkreathy, Ihsan Ullah, Amir Muhammad Khan, and et al. 2022. "Biogenic Synthesis of Silver Nanoparticles Using Phagnalon niveum and Its In Vivo Anti-Diabetic Effect against Alloxan-Induced Diabetic Wistar Rats" Nanomaterials 12, no. 5: 830. https://doi.org/10.3390/nano12050830
APA StyleUl Haq, M. N., Shah, G. M., Gul, A., Foudah, A. I., Alqarni, M. H., Yusufoglu, H. S., Hussain, M., Alkreathy, H. M., Ullah, I., Khan, A. M., Jamil, S., Ahmed, M., & Khan, R. A. (2022). Biogenic Synthesis of Silver Nanoparticles Using Phagnalon niveum and Its In Vivo Anti-Diabetic Effect against Alloxan-Induced Diabetic Wistar Rats. Nanomaterials, 12(5), 830. https://doi.org/10.3390/nano12050830









