Carbon Quantum Dots’ Synthesis with a Strong Chemical Claw for Five Transition Metal Sensing in the Irving–Williams Series
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Synthesis Part
2.3. Characterization Part
2.4. Detection of Metal Ions by CQDs
2.5. The Calculation of the Calibration Curve and the Model of Linear Regression
3. Results
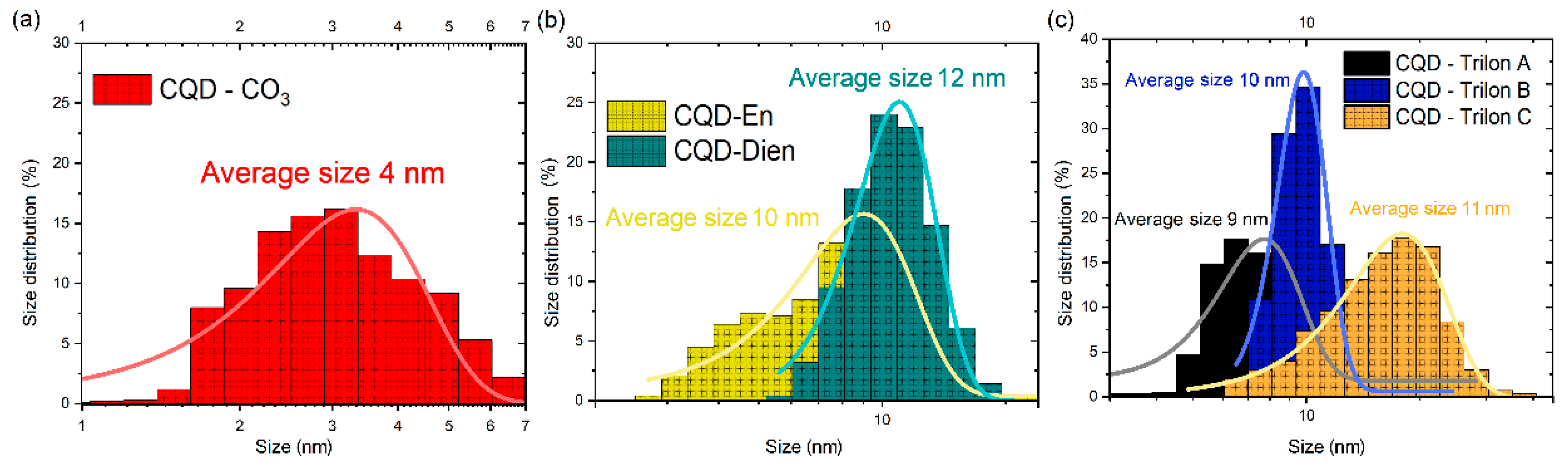
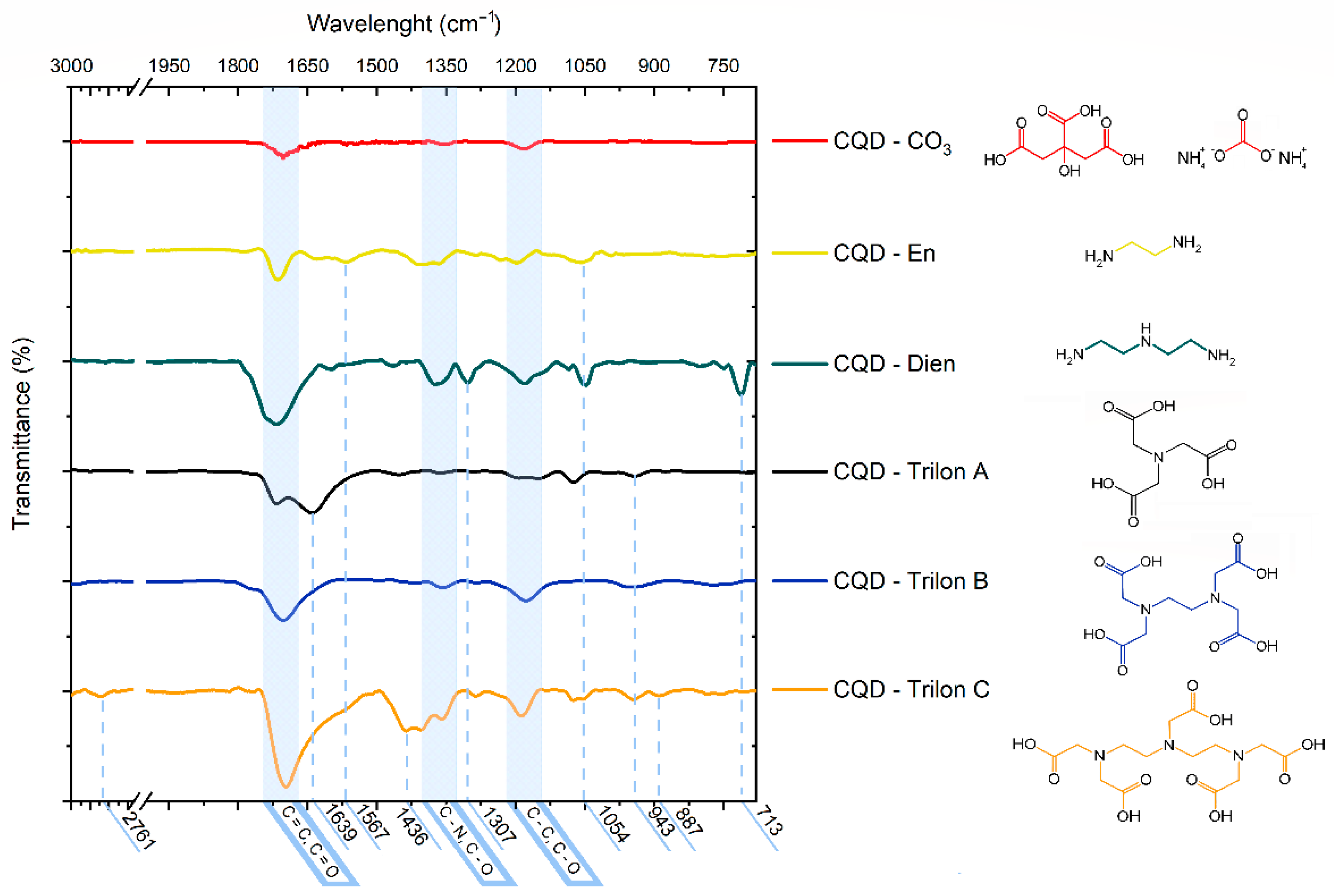
3.1. Characterization of CQD-CO3 and CQD-En, Dien, Trilon A, B, C Functionalized Particles
3.2. The Assumption in Claw Formation on CQDs’ Surface
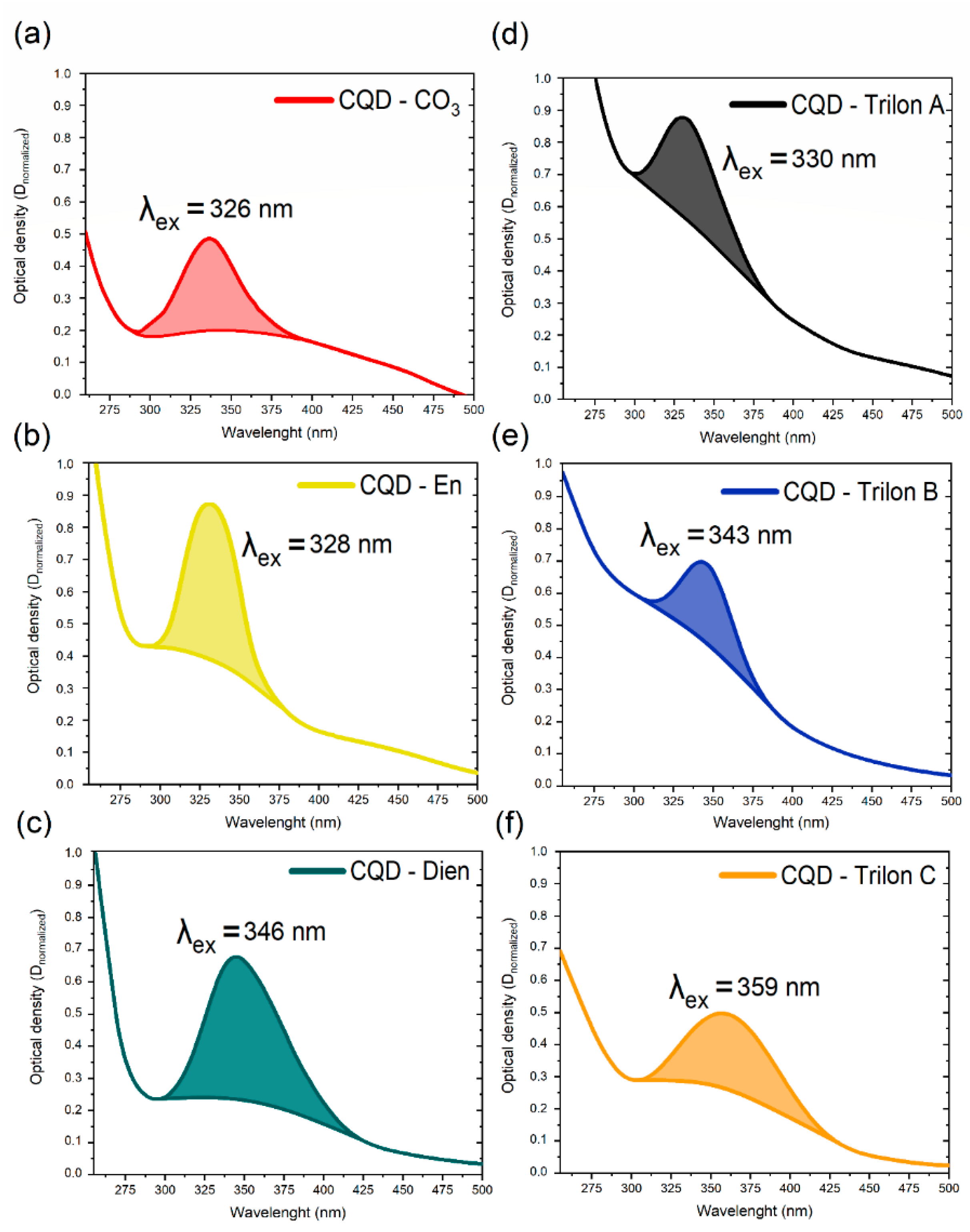
3.3. ABS Spectroscopy for CQDs—Family
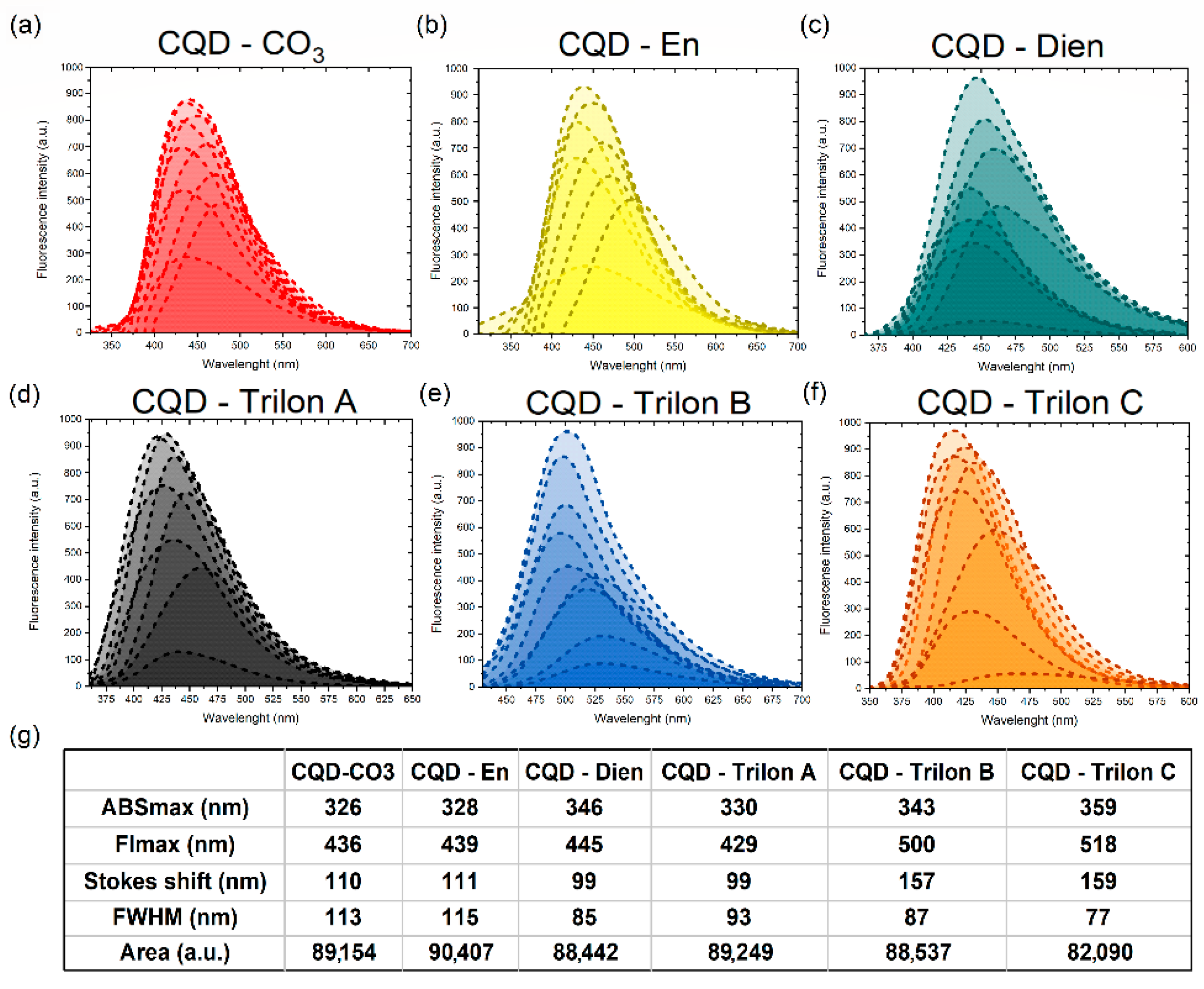
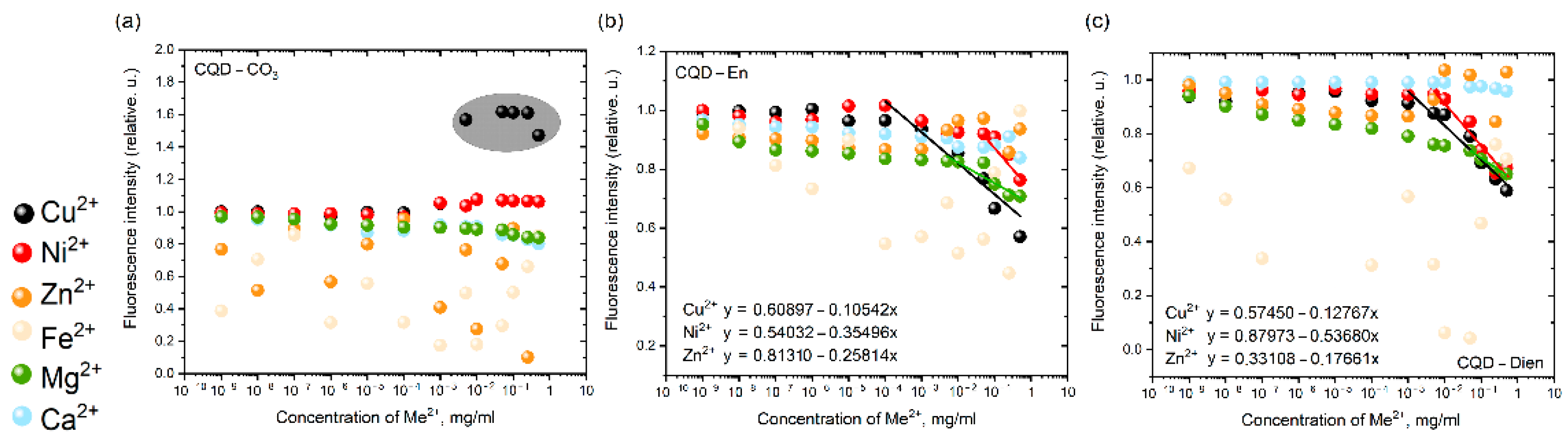
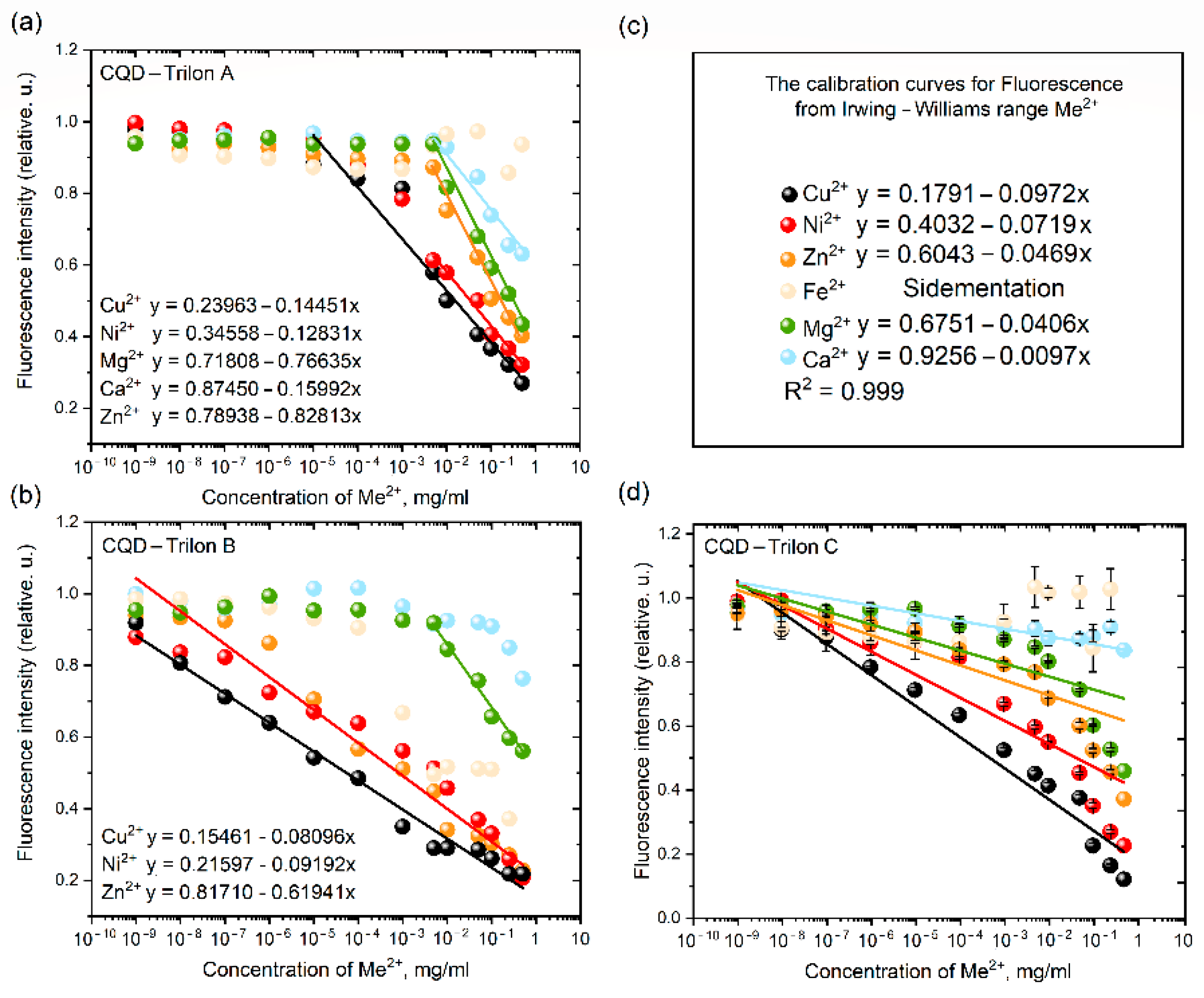
3.4. Fl Spectroscopy for CQDs—Family
4. Discussion
The Relationships of CQDs’ Surface in Ionic Solutions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Corsi, I.; Winther-Nielsen, M.; Sethi, R.; Punta, C.; Della Torre, C.; Libralato, G.; Lofrano, G.; Sabatini, L.; Aiello, M.; Fiordi, L.; et al. Ecofriendly nanotechnologies and nanomaterials for environmental applications: Key issue and consensus recommendations for sustainable and ecosafe nanoremediation. Ecotoxicol. Environ. Saf. 2018, 154, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Titirici, M.-M.; White, R.J.; Brun, N.; Budarin, V.L.; Su, D.S.; del Monte, F.; Clark, J.H.; MacLachlan, M.J. Sustainable carbon materials. Chem. Soc. Rev. 2014, 44, 250–290. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Jiang, Y.; Sun, X.; Bai, Z.; Zhang, Y.; Zhou, X. Surface modification and chemical functionalization of carbon dots: A review. Mikrochim. Acta 2018, 185, 424. [Google Scholar] [CrossRef] [PubMed]
- Viter, R.; Iatsunskyi, I. Metal Oxide Nanostructures in Sensing. In Nanomaterials Design for Sensing Applications; Zenkina, O.V., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; Chapter 2; pp. 41–91. [Google Scholar]
- Lou, Y.; Zhao, Y.; Chen, J.; Zhu, J.-J. Metal ions optical sensing by semiconductor quantum dots. J. Mater. Chem. C 2014, 2, 595–613. [Google Scholar] [CrossRef]
- Das, R.; Bandyopadhyay, R.; Pramanik, P. Carbon quantum dots from natural resource: A review. Mater. Today Chem. 2018, 8, 96–109. [Google Scholar] [CrossRef]
- Molaei, M.J. Principles, mechanisms, and application of carbon quantum dots in sensors: A review. Anal. Methods 2020, 12, 1266–1287. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, A. Carbon quantum dots: Synthesis, properties and applications. J. Mater. Chem. C 2014, 2, 6921–6939. [Google Scholar] [CrossRef] [Green Version]
- Sabet, M.; Mahdavi, K. Green synthesis of high photoluminescence nitrogen-doped carbon quantum dots from grass via a simple hydrothermal method for removing organic and inorganic water pollutions. Appl. Surf. Sci. 2019, 463, 283–291. [Google Scholar] [CrossRef]
- Cailotto, S.; Amadio, E.; Facchin, M.; Selva, M.; Pontoglio, E.; Rizzolio, F.; Riello, P.; Toffoli, G.; Benedetti, A.; Perosa, A. Carbon Dots from Sugars and Ascorbic Acid: Role of the Precursors on Morphology, Properties, Toxicity, and Drug Uptake. ACS Med. Chem. Lett. 2018, 9, 832–837. [Google Scholar] [CrossRef]
- Feng, H.; Qian, Z. Functional Carbon Quantum Dots: A Versatile Platform for Chemosensing and Biosensing. Chem. Rec. 2017, 18, 491–505. [Google Scholar] [CrossRef] [PubMed]
- Tian, R.; Hu, S.; Wu, L.; Chang, Q.; Yang, J.; Liu, J. Tailoring surface groups of carbon quantum dots to improve photoluminescence behaviors. Appl. Surf. Sci. 2014, 301, 156–160. [Google Scholar] [CrossRef]
- Yang, F.; LeCroy, G.E.; Wang, P.; Liang, W.; Chen, J.; Fernando, K.A.S.; Bunker, C.E.; Qian, H.; Sun, Y.-P. Functionalization of Carbon Nanoparticles and Defunctionalization—Toward Structural and Mechanistic Elucidation of Carbon “Quantum” Dots. J. Phys. Chem. C 2016, 120, 25604–25611. [Google Scholar] [CrossRef]
- Gao, W.; Song, H.; Wang, X.; Liu, X.; Pang, X.; Zhou, Y.; Gao, B.; Peng, X. Carbon Dots with Red Emission for Sensing of Pt2+, Au3+, and Pd2+ and Their Bioapplications in Vitro and in Vivo. ACS Appl. Mater. Interfaces 2018, 10, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Devi, P.; Rajput, P.; Thakur, A.; Kim, K.-H.; Kumar, P. Recent advances in carbon quantum dot-based sensing of heavy metals in water. TrAC Trends Anal. Chem. 2019, 114, 171–195. [Google Scholar] [CrossRef]
- Wiśniewski, M.; Czarnecka, J.; Bolibok, P.; Świdziński, M.; Roszek, K. New Insight into the Fluorescence Quenching of Nitrogen-Containing Carbonaceous Quantum Dots—From Surface Chemistry to Biomedical Applications. Materials 2021, 14, 2454. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.; Yang, G.; Wan, X. Ultra-high quantum yield nitrogen-doped carbon quantum dots and their versatile application in fluorescence sensing, bioimaging and anti-counterfeiting. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 253, 119583. [Google Scholar] [CrossRef] [PubMed]
- Nazri, N.A.A.; Azeman, N.H.; Luo, Y.; Bakar, A.A.A. Carbon quantum dots for optical sensor applications: A review. Opt. Laser Technol. 2021, 139, 106928. [Google Scholar] [CrossRef]
- Ng, H.K.M.; Lim, G.K.; Leo, C.P. Comparison between hydrothermal and microwave-assisted synthesis of carbon dots from biowaste and chemical for heavy metal detection: A review. Microchem. J. 2021, 165, 106116. [Google Scholar] [CrossRef]
- Kurian, M.; Paul, A. Recent trends in the use of green sources for carbon dot synthesis–A short review. Carbon Trends 2021, 3, 100032. [Google Scholar] [CrossRef]
- Das, P.; Maruthapandi, M.; Saravanan, A.; Natan, M.; Jacobi, G.; Banin, E.; Gedanken, A. Carbon Dots for Heavy-Metal Sensing, pH-Sensitive Cargo Delivery, and Antibacterial Applications. ACS Appl. Nano Mater. 2020, 3, 11777–11790. [Google Scholar] [CrossRef]
- Das, P.; Ganguly, S.; Bose, M.; Ray, D.; Ghosh, S.; Mondal, S.; Aswal, V.K.; Das, A.K.; Banerjee, S.; Das, N.C. Surface quaternized nanosensor as a one-arrow-two-hawks approach for fluorescence turn “on–off–on” bifunctional sensing and antibacterial activity. New J. Chem. 2019, 43, 6205–6219. [Google Scholar] [CrossRef]
- Mintz, K.J.; Zhou, Y.; Leblanc, R.M. Recent development of carbon quantum dots regarding their optical properties, photoluminescence mechanism, and core structure. Nanoscale 2019, 11, 4634–4652. [Google Scholar] [CrossRef]
- Hines, D.A.; Kamat, P.V. Recent Advances in Quantum Dot Surface Chemistry. ACS Appl. Mater. Interfaces 2014, 6, 3041–3057. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Wang, R.; Li, G.; Chen, C.; Chi, Y.; Chen, G. Polyamine-Functionalized Carbon Quantum Dots as Fluorescent Probes for Selective and Sensitive Detection of Copper Ions. Anal. Chem. 2012, 84, 6220–6224. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Liang, H. Nickel ion detection by imidazole modified carbon dots. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 211, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Buffle, J. Complexation Reactions in Aquatic Systems: An Analytical Approach; Halsted Press Distributor: New York, NY, USA, 1988. [Google Scholar]
- Varadwaj, P.R.; Varadwaj, A.; Jin, B.-Y. Ligand(s)-to-metal charge transfer as a factor controlling the equilibrium constants of late first-row transition metal complexes: Revealing the Irving–Williams thermodynamical series. Phys. Chem. Chem. Phys. 2015, 17, 805–811. [Google Scholar] [CrossRef] [PubMed]
- Leussing, D.L. The estimation of the stabilities of bivalent transition metal complexes and deviations from the irving-williams order. Talanta 1960, 4, 264–267. [Google Scholar] [CrossRef]
- Zu, F.; Yan, F.; Bai, Z.; Xu, J.; Wang, Y.; Huang, Y.; Zhou, X. The quenching of the fluorescence of carbon dots: A review on mechanisms and applications. Mikrochim. Acta 2017, 184, 1899–1914. [Google Scholar] [CrossRef]
- Rojas-Valencia, O.; Regules-Carrasco, M.; Hernández-Fuentes, J.; Germán, C.R.-S.; Estrada-Flores, M.; Villagarcía-Chávez, E. Synthesis of blue emissive carbon quantum dots from Hibiscus Sabdariffa flower: Surface functionalization analysis by FT-IR spectroscopy. Materialia 2021, 19, 101182. [Google Scholar] [CrossRef]
- Tepliakov, N.V.; Kundelev, E.V.; Khavlyuk, P.D.; Xiong, Y.; Leonov, M.Y.; Zhu, W.; Baranov, A.V.; Fedorov, A.V.; Rogach, A.L.; Rukhlenko, I.D. sp2–sp3-Hybridized Atomic Domains Determine Optical Features of Carbon Dots. ACS Nano 2019, 13, 10737–10744. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Li, W.; Wu, Q.; Liu, Y.; Liu, S. Hydrothermal synthesis of nitrogen-doped carbon quantum dots from microcrystalline cellulose for the detection of Fe3+ ions in an acidic environment. RSC Adv. 2017, 7, 44144–44153. [Google Scholar] [CrossRef] [Green Version]
- Limosani, F.; Bauer, E.M.; Cecchetti, D.; Biagioni, S.; Orlando, V.; Pizzoferrato, R.; Prosposito, P.; Carbone, M. Top-Down N-Doped Carbon Quantum Dots for Multiple Purposes: Heavy Metal Detection and Intracellular Fluorescence. Nanomaterials 2021, 11, 2249. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Zhang, J.; Zhang, Y.; Xiao, Y.; Shi, Y.; Chen, Y.; Ding, L.; Xu, W. Influence of Group Modification at the Edges of Carbon Quantum Dots on Fluorescent Emission. Nanoscale Res. Lett. 2019, 14, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Liang, Y.; Shen, Y.; Liu, C.; Ren, X. Effects of chemical bonds between nitrogen and its neighbor carbon atoms on fluorescence properties of carbon quantum dots. J. Lumin 2018, 197, 285–290. [Google Scholar] [CrossRef]
- Ding, H.; Li, X.-H.; Chen, X.-B.; Wei, J.-S.; Li, X.-B.; Xiong, H.-M. Surface states of carbon dots and their influences on luminescence. J. Appl. Phys. 2020, 127, 231101. [Google Scholar] [CrossRef]
- Stachowska, J.D.; Murphy, A.; Mellor, C.; Fernandes, D.; Gibbons, E.N.; Krysmann, M.J.; Kelarakis, A.; Burgaz, E.; Moore, J.; Yeates, S.G. A rich gallery of carbon dots based photoluminescent suspensions and powders derived by citric acid/urea. Sci. Rep. 2021, 11, 10554. [Google Scholar] [CrossRef] [PubMed]
- Burstein, E.A.; Emelyanenko, V.I. Log-Normal Description of Fluorescence Spectra of Organic Fluorophores. Photochem. Photobiol. 1996, 64, 316–320. [Google Scholar] [CrossRef]
- Anatomy of Fluorescence Spectra. Available online: https://www.thermofisher.com/ru/ru/home/life-science/cell-analysis/cell-analysis-learning-center/molecular-probes-school-of-fluorescence/fluorescence-basics/anatomy-fluorescence-spectra.html (accessed on 25 January 2022).
- Niu, W.-J.; Li, Y.; Zhu, R.-H.; Shan, D.; Fan, Y.-R.; Zhang, X.-J. Ethylenediamine-assisted hydrothermal synthesis of nitrogen-doped carbon quantum dots as fluorescent probes for sensitive biosensing and bioimaging. Sens. Actuators B Chem. 2015, 218, 229–236. [Google Scholar] [CrossRef]
- Devi, S.; Gupta, R.K.; Paul, A.K.; Kumar, V.; Sachdev, A.; Gopinath, P.; Tyagi, S. Ethylenediamine mediated luminescence enhancement of pollutant derivatized carbon quantum dots for intracellular trinitrotoluene detection: Soot to shine. RSC Adv. 2018, 8, 32684–32694. [Google Scholar] [CrossRef] [Green Version]
- Łoczechin, A.; Séron, K.; Barras, A.; Giovanelli, E.; Belouzard, S.; Chen, Y.-T.; Metzler-Nolte, N.; Boukherroub, R.; Dubuisson, J.; Szunerits, S. Functional Carbon Quantum Dots as Medical Countermeasures to Human Coronavirus. ACS Appl. Mater. Interfaces 2019, 11, 42964–42974. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Xie, Y.; Wei, Y.; Yang, Y.; Liu, X.; Chen, Y.; Xu, B. An Efficient Synthesis and Photoelectric Properties of Green Carbon Quantum Dots with High Fluorescent Quantum Yield. Nanomaterials 2020, 10, 82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Yu, H.; Bian, T.; Zhao, Y.; Zhou, C.; Shang, L.; Liu, Y.; Wu, L.-Z.; Tung, C.-H.; Zhang, T. Highly luminescent nitrogen-doped carbon quantum dots as effective fluorescent probes for mercuric and iodide ions. J. Mater. Chem. C 2015, 3, 1922–1928. [Google Scholar] [CrossRef]
- Wang, Y.; Chang, X.; Jing, N.; Zhang, Y. Hydrothermal synthesis of carbon quantum dots as fluorescent probes for the sensitive and rapid detection of picric acid. Anal. Methods 2018, 10, 2775–2784. [Google Scholar] [CrossRef]
- Feng, X.T.; Zhang, F.; Wang, Y.L.; Zhang, Y.; Yang, Y.Z.; Liu, X.G. Luminescent carbon quantum dots with high quantum yield as a single white converter for white light emitting diodes. Appl. Phys. Lett. 2015, 107, 213102. [Google Scholar] [CrossRef]
- Devi, S.; Kaur, A.; Sarkar, S.; Vohra, S.; Tyagi, S. Synthesis and characterization of highly luminescent N-doped carbon quantum dots for metal ion sensing. Integr. Ferroelectr. 2018, 186, 32–39. [Google Scholar] [CrossRef]
- Freire, R.; Le, N.D.; Jiang, Z.; Kim, C.S.; Rotello, V.M.; Fechine, P. NH2-rich Carbon Quantum Dots: A protein-responsive probe for detection and identification. Sens. Actuators B Chem. 2018, 255, 2725–2732. [Google Scholar] [CrossRef]
- Santiago, S.R.M.; Lin, T.N.; Chang, C.H.; Wong, Y.A.; Lin, C.A.J.; Yuan, C.T.; Shen, J.L. Synthesis of N-doped graphene quantum dots by pulsed laser ablation with diethylenetriamine (DETA) and their photoluminescence. Phys. Chem. Chem. Phys. 2017, 19, 22395–22400. [Google Scholar] [CrossRef] [PubMed]
- Aghamali, A.; Khosravi, M.; Hamishehkar, H.; Modirshahla, N.; Behnajady, M.A. Synthesis and characterization of high efficient photoluminescent sunlight driven photocatalyst of N-Carbon Quantum Dots. J. Lumin. 2018, 201, 265–274. [Google Scholar] [CrossRef]
- Guo, Y.; Zhao, W. Hydrothermal synthesis of highly fluorescent nitrogen-doped carbon quantum dots with good biocompatibility and the application for sensing ellagic acid. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 240, 118580. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Singh, V.; Yadav, P.K.; Chandra, S.; Bano, D.; Kumar, V.; Koch, B.; Talat, M.; Hasan, S.H. Bright-blue-emission nitrogen and phosphorus-doped carbon quantum dots as a promising nanoprobe for detection of Cr(vi) and ascorbic acid in pure aqueous solution and in living cells. New J. Chem. 2018, 42, 12990–12997. [Google Scholar] [CrossRef]
- Kalaiyarasan, G. Determination of vitamin B12 via pH-dependent quenching of the fluorescence of nitrogen doped carbon quantum dots. Mikrochim. Acta 2017, 184, 3883–3891. [Google Scholar] [CrossRef]
- Wang, M.; Xia, Y.; Qiu, J.; Ren, X. Carbon quantum dots embedded mesoporous silica for rapid fluorescent detection of acidic gas. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 206, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Ren, X.; Zhu, L.; Xia, Y.; Qiu, J. Preparation of mesoporous silica/carbon quantum dots composite and its application in selective and sensitive Hg2+ detection. Microporous Mesoporous Mater. 2019, 284, 378–384. [Google Scholar] [CrossRef]
- Lv, J.; Fu, L.; Zeng, B.; Tang, M.; Li, J. Synthesis and Acidizing Corrosion Inhibition Performance of N-Doped Carbon Quantum Dots. Russ. J. Appl. Chem. 2019, 92, 848–856. [Google Scholar] [CrossRef]
- Bao, W.; Ma, H.; Wang, N.; He, Z. pH-sensitive carbon quantum dots−doxorubicin nanoparticles for tumor cellular targeted drug delivery. Polym. Adv. Technol. 2019, 30, 2664–2673. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, X.; Wu, L.; Wu, W.; Zheng, Y.; Lin, L.; Weng, S.; Lin, X. Nitrogen-doped carbon quantum dots as an antimicrobial agent against Staphylococcus for the treatment of infected wounds. Colloids Surf. B Biointerfaces 2019, 179, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Jiao, Y.; Cheng, C.; Hua, J.; Yang, Y. Nitrogen-doped carbon quantum dots as a fluorescence probe combined with magnetic solid-phase extraction purification for analysis of folic acid in human serum. Anal. Bioanal. Chem. 2017, 409, 7063–7075. [Google Scholar] [CrossRef] [PubMed]
- Tian, R.; Zhong, S.; Wu, J.; Geng, Y.; Zhou, B.; Wang, Q.; Jiang, W. Facile preparation and the stepwise formation mechanistic investigation of gram-scale nitrogen-doped graphene quantum dots. J. Mater. Chem. C 2017, 5, 9174–9180. [Google Scholar] [CrossRef]
- Lee, H.J.; Jana, J.; Ngo, Y.-L.T.; Wang, L.L.; Chung, J.S.; Hur, S.H. The effect of solvent polarity on emission properties of carbon dots and their uses in colorimetric sensors for water and humidity. Mater. Res. Bull. 2019, 119, 110564. [Google Scholar] [CrossRef]
- Zhuo, S.; Gao, L.; Zhang, P.; Du, J.; Zhu, C. Living cell imaging and sensing of hydrogen sulfide using high-efficiency fluorescent Cu-doped carbon quantum dots. New J. Chem. 2018, 42, 19659–19664. [Google Scholar] [CrossRef]
- Bharathi, D.; Siddlingeshwar, B.; Krishna, R.H.; Singh, V.; Kottam, N.; Divakar, D.D.; Alkheraif, A.A. Green and Cost Effective Synthesis of Fluorescent Carbon Quantum Dots for Dopamine Detection. J. Fluoresc. 2018, 28, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Jeong, G.; Lee, J.M.; Lee, J.A.; Praneerad, J.; Choi, C.A.; Supchocksoonthorn, P.; Roy, A.K.; Chae, W.-S.; Paoprasert, P.; Yeo, M.K.; et al. Microwave-assisted synthesis of multifunctional fluorescent carbon quantum dots from A4/B2 polyamidation monomer sets. Appl. Surf. Sci. 2021, 542, 148471. [Google Scholar] [CrossRef]
- Bharathi, D.; Krishna, R.H.; Siddlingeshwar, B.; Divakar, D.D.; Alkheraif, A.A. Understanding the interaction of carbon quantum dots with CuO and Cu2O by fluorescence quenching. J. Hazard. Mater. 2019, 369, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, S.; Guan, Y.; Li, H.; Fang, J.; Zhang, P.; Du, J.; Zhu, C. Facile fabrication of fluorescent Fe-doped carbon quantum dots for dopamine sensing and bioimaging application. Analyst 2019, 144, 656–662. [Google Scholar] [CrossRef]
- Shi, Y.; Pan, Y.; Zhong, J.; Yang, J.; Zheng, J.; Cheng, J.; Song, R.; Yi, C. Facile synthesis of gadolinium (III) chelates functionalized carbon quantum dots for fluorescence and magnetic resonance dual-modal bioimaging. Carbon 2015, 93, 742–750. [Google Scholar] [CrossRef]
- Han, T.; Yan, T.; Li, Y.; Cao, W.; Pang, X.; Huang, Q.; Wei, Q. Eco-friendly synthesis of electrochemiluminescent nitrogen-doped carbon quantum dots from diethylene triamine pentacetate and their application for protein detection. Carbon 2015, 91, 144–152. [Google Scholar] [CrossRef]
- Zhou, J.; Han, T.; Ma, H.; Yan, T.; Pang, X.; Li, Y.; Wei, Q. A novel electrochemiluminescent immunosensor based on the quenching effect of aminated graphene on nitrogen-doped carbon quantum dots. Anal. Chim. Acta 2015, 889, 82–89. [Google Scholar] [CrossRef]
- Gao, A.; Kang, Y.-F.; Yin, X.-B. Red fluorescence-magnetic resonance dual modality imaging applications of gadolinium containing carbon quantum dots with excitation independent emission. New J. Chem. 2017, 41, 3422–3431. [Google Scholar] [CrossRef]
- Tarasenka, N.; Stupak, A.; Tarasenko, N.; Chakrabarti, S.; Mariotti, D. Structure and Optical Properties of Carbon Nanoparticles Generated by Laser Treatment of Graphite in Liquids. ChemPhysChem 2017, 18, 1074–1083. [Google Scholar] [CrossRef]
- Soni, H.; Pamidimukkala, P. Liquid crystalline multilayer graphene quantum dots with hackelite structures: Characterisation and application for sensing nitrophenols. Sens. Actuators B Chem. 2018, 268, 100–107. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Y.; Yang, Z.; Wang, Y.; Rao, H.; Yue, G.; Wu, C.; Lu, C.; Wang, X. A ratiometric fluorescence and colorimetric dual-mode assay for H2O2 and xanthine based on Fe, N co-doped carbon dots. Dyes Pigments 2020, 180, 108486. [Google Scholar] [CrossRef]
- Shi, Y.; Li, C.; Liu, S.; Liu, Z.; Zhu, J.; Yang, J.; Hu, X. Facile synthesis of fluorescent carbon dots for determination of curcumin based on fluorescence resonance energy transfer. RSC Adv. 2015, 5, 64790–64796. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yakusheva, A.; Sayapina, A.; Luchnikov, L.; Arkhipov, D.; Karunakaran, G.; Kuznetsov, D. Carbon Quantum Dots’ Synthesis with a Strong Chemical Claw for Five Transition Metal Sensing in the Irving–Williams Series. Nanomaterials 2022, 12, 806. https://doi.org/10.3390/nano12050806
Yakusheva A, Sayapina A, Luchnikov L, Arkhipov D, Karunakaran G, Kuznetsov D. Carbon Quantum Dots’ Synthesis with a Strong Chemical Claw for Five Transition Metal Sensing in the Irving–Williams Series. Nanomaterials. 2022; 12(5):806. https://doi.org/10.3390/nano12050806
Chicago/Turabian StyleYakusheva, Anastasia, Anastasia Sayapina, Lev Luchnikov, Dmitry Arkhipov, Gopalu Karunakaran, and Denis Kuznetsov. 2022. "Carbon Quantum Dots’ Synthesis with a Strong Chemical Claw for Five Transition Metal Sensing in the Irving–Williams Series" Nanomaterials 12, no. 5: 806. https://doi.org/10.3390/nano12050806
APA StyleYakusheva, A., Sayapina, A., Luchnikov, L., Arkhipov, D., Karunakaran, G., & Kuznetsov, D. (2022). Carbon Quantum Dots’ Synthesis with a Strong Chemical Claw for Five Transition Metal Sensing in the Irving–Williams Series. Nanomaterials, 12(5), 806. https://doi.org/10.3390/nano12050806







