Application of Copper Nanoparticles in Dentistry
Abstract
1. Introduction
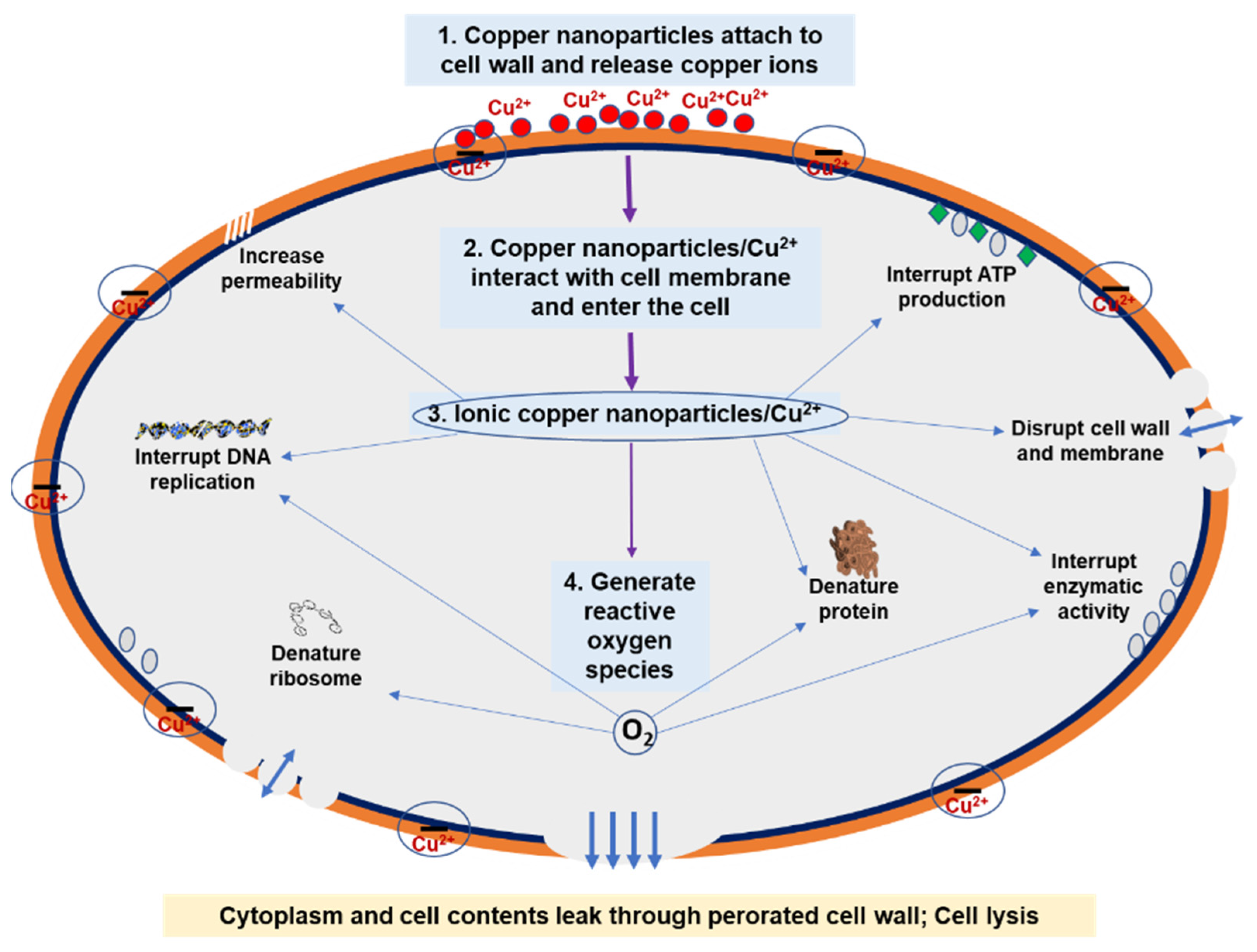
2. Antibacterial Mechanism of Copper Nanoparticles
- Attach to the cell wall and release copper ions.
- Adhere to the cell membrane and enter the cell.
- Disrupt the cell wall and membrane, denature protein, interrupt enzymatic activity, interrupt deoxyribonucleic acid (DNA) replication, and interrupt adenosine triphosphate (ATP) production.
- Generate reactive oxygen species. Reactive oxygen species further interrupt DNA replication and initiate the breakdown of DNA, denature ribosomes, and denature protein.
3. Copper Nanoparticles in Dental Materials
3.1. Copper Nanoparticles in Dental Metals and Alloys
3.2. Copper Nanoparticles in Dental Polymers and Resins
3.3. Copper Nanoparticles in Dental Cements
3.4. Copper Nanoparticles in Miscellaneous Dental Materials
4. Biocompatibility Studies of Copper Nanoparticles
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chandraleka, S.; Ramya, K.; Chandramohan, G.; Dhanasekaran, D.; Priyadharshini, A.; Panneerselvam, A. Antimicrobial mechanism of copper (II) 1,10-phenanthroline and 2,2′-bipyridyl complex on bacterial and fungal pathogens. J. Saudi Chem. Soc. 2014, 18, 953–962. [Google Scholar] [CrossRef]
- O’Gorman, J.; Humphreys, H. Application of copper to prevent and control infection. Where are we now? J. Hosp. Infect. 2012, 81, 217–223. [Google Scholar] [CrossRef]
- Ingle, A.P.; Duran, N.; Rai, M. Bioactivity, mechanism of action, and cytotoxicity of copper-based nanoparticles: A review. Appl. Microbiol. Biotechnol. 2014, 98, 1001–1009. [Google Scholar] [CrossRef]
- Khan, F.A. Biotechnology Fundamentals, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2017; ISBN 9780815370048. [Google Scholar]
- Ermini, M.L.; Voliani, V. Antimicrobial nano-agents: The copper age. ACS Nano 2021, 15, 6008–6029. [Google Scholar] [CrossRef]
- Ramyadevi, J.; Jeyasubramanian, K.; Marikani, A.; Rajakumar, G.; Rahuman, A.A. Synthesis and antimicrobial activity of copper nanoparticles. Mater. Lett. 2012, 71, 114–116. [Google Scholar] [CrossRef]
- Wei, Y.; Chen, S.; Kowalczyk, B.; Huda, S.; Gray, T.P.; Grzybowski, B.A. Synthesis of stable, low-dispersity copper nanoparticles and nanorods and their antifungal and catalytic properties. J. Phys. Chem. C Nanomater. Interfaces 2010, 114, 15612–15616. [Google Scholar] [CrossRef]
- Maleki Dizaj, S.; Barzegar-Jalali, M.; Zarrintan, M.H.; Adibkia, K.; Lotfipour, F. Calcium carbonate nanoparticles as cancer drug delivery system. Expert Opin. Drug Deliv. 2015, 12, 1649–1660. [Google Scholar] [CrossRef]
- Anu, K.; Maleeka Begum, S.; Rajesh, G.; Renuka Devi, K. Wet Biochemical Synthesis of Copper Oxide Nanoparticles Coated on Titanium Dental Implants. Int. J. Adv. Res. Sci. Eng. Technol. 2016, 3, 1191–1194. [Google Scholar]
- Nezhad, S.S.; Khorasgani, M.R.; Emtiazi, G.; Yaghoobi, M.M.; Shakeri, S. Isolation of copper oxide (CuO) nanoparticles resistant Pseudomonas strains from soil and investigation on possible mechanism for resistance. World J. Microbiol. Biotechnol. 2014, 30, 809–817. [Google Scholar] [CrossRef]
- Chatterjee, A.K.; Sarkar, R.K.; Chattopadhyay, A.P.; Aich, P.; Chakraborty, R.; Basu, T. A simple robust method for synthesis of metallic copper nanoparticles of high antibacterial potency against E. coli. Nanotechnology 2012, 23, 085103. [Google Scholar] [CrossRef] [PubMed]
- Essa, A.M.M.; Khallaf, M.K. Antimicrobial potential of consolidation polymers loaded with biological copper nanoparticles. BMC Microbiol. 2016, 16, 144. [Google Scholar] [CrossRef]
- Zakharova, O.V.; Godymchuk, A.Y.; Gusev, A.A.; Gulchenko, S.I.; Vasyukova, I.A.; Kuznetsov, D.V. Considerable variation of antibacterial activity of Cu nanoparticles suspensions depending on the storage time, dispersive medium, and particle sizes. BioMed Res. Int. 2015, 2015, 412530. [Google Scholar] [CrossRef]
- Ruparelia, J.P.; Chatterjee, A.K.; Duttagupta, S.P.; Mukherji, S. Strain specificity in antimicrobial activity of silver and copper nanoparticles. Acta Biomater. 2008, 4, 707–716. [Google Scholar] [CrossRef]
- Yoon, K.Y.; Byeon, J.H.; Park, J.H.; Hwang, J. Susceptibility constants of Escherichia coli and Bacillus subtilis to silver and copper nanoparticles. Sci. Total Environ. 2007, 373, 572–575. [Google Scholar] [CrossRef] [PubMed]
- Renne, W.G.; Lindner, A.; Mennito, A.S.; Agee, K.A.; Pashley, D.H.; Willett, D.; Sentelle, D.; Defee, M.; Schmidt, M.; Sabatini, C. Antibacterial properties of copper iodide-doped glass ionomer-based materials and effect of copper iodide nanoparticles on collagen degradation. Clin. Oral Investig. 2017, 21, 369–379. [Google Scholar] [CrossRef] [PubMed]
- ALGhanem, A.; Fernandes, G.; Visser, M.; Dziak, R.; Renné, W.G.; Sabatini, C. Biocompatibility and Bond Degradation of Poly-Acrylic Acid Coated Copper Iodide-Adhesives. Dent. Mater. 2017, 33, e336–e347. [Google Scholar] [CrossRef]
- Argueta-Figueroa, L.; Scougall-Vilchis, R.J.; Morales-Luckie, R.A.; Olea-Mejia, O.F. An evaluation of the antibacterial properties and shear bond strength of copper nanoparticles as a nanofiller in orthodontic adhesive. Aust. Orthod. J. 2015, 31, 42–48. [Google Scholar] [CrossRef]
- Gosau, M.; Haupt, M.; Thude, S.; Strowitzki, M.; Schminke, B.; Buergers, R. Antimicrobial effect and biocompatibility of novel metallic nanocrystalline implant coatings: Novel antibacterial implant surfaces. J. Biomed. Mater. Res. B Appl. Biomater. 2016, 104, 1571–1579. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Fu, S.; Yang, L.; Qin, G.; Zhang, E. A Nano-Structured TiO2/CuO/Cu2O Coating on Ti-Cu Alloy with Dual Function of Antibacterial Ability and Osteogenic Activity. J. Mater. Sci. Technol. 2022, 97, 201–212. [Google Scholar] [CrossRef]
- Vincent, M.; Duval, R.E.; Hartemann, P.; Engels-Deutsch, M. Contact killing and antimicrobial properties of copper. J. Appl. Microbiol. 2018, 124, 1032–1046. [Google Scholar] [CrossRef] [PubMed]
- Milanino, R. Copper in Medicine and Personal Care: A Historical Overview. In Copper and the Skin; Clinical & Basic Science Series; Informa Healthcare: New York, NY, USA, 2006. [Google Scholar]
- Jin, S.; Ren, L.; Yang, K. Bio-functional Cu containing biomaterials: A New Way to enhance bio-adaption of biomaterials. J. Mater. Sci. Technol. 2016, 32, 835–839. [Google Scholar] [CrossRef]
- Jiang, W.; Mashayekhi, H.; Xing, B. Bacterial toxicity comparison between nano- and micro-scaled oxide particles. Environ. Pollut. 2009, 157, 1619–1625. [Google Scholar] [CrossRef]
- Azam, A.; Ahmed, A.S.; Oves, M.; Khan, M.; Memic, A. Size-dependent antimicrobial properties of CuO nanoparticles against Gram-positive and negative bacterial strains. Int. J. Nanomed. 2012, 7, 3527–3535. [Google Scholar] [CrossRef]
- Amro, N.A.; Kotra, L.P.; Wadu-Mesthrige, K.; Bulychev, A.; Mobashery, S.; Liu, G.-Y. High-resolution atomic force microscopy studies of the Escherichia coli outer membrane: Structural basis for permeability. Langmuir 2000, 16, 2789–2796. [Google Scholar] [CrossRef]
- Tran, N.; Mir, A.; Mallik, D.; Sinha, A.; Nayar, S.; Webster, T.J. Bactericidal effect of iron oxide nanoparticles on Staphylococcus aureus. Int. J. Nanomed. 2010, 5, 277–283. [Google Scholar]
- Applerot, G.; Lellouche, J.; Lipovsky, A.; Nitzan, Y.; Lubart, R.; Gedanken, A.; Banin, E. Understanding the antibacterial mechanism of CuO nanoparticles: Revealing the route of induced oxidative stress. Small 2012, 8, 3326–3337. [Google Scholar] [CrossRef]
- Fang, J.; Lyon, D.Y.; Wiesner, M.R.; Dong, J.; Alvarez, P.J.J. Effect of a fullerene water suspension on bacterial phospholipids and membrane phase behavior. Environ. Sci. Technol. 2007, 41, 2636–2642. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.M.; Feris, K.; Bell, J.; Wingett, D.G.; Hanley, C.; Punnoose, A. Selective toxicity of zinc oxide nanoparticles to prokaryotic and eukaryotic systems. Appl. Phys. Lett. 2007, 90, 2139021–2139023. [Google Scholar] [CrossRef]
- Kim, J.S.; Kuk, E.; Yu, K.N.; Kim, J.-H.; Park, S.J.; Lee, H.J.; Kim, S.H.; Park, Y.K.; Park, Y.H.; Hwang, C.-Y.; et al. Antimicrobial effects of silver nanoparticles. Nanomedicine 2007, 3, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Morones, J.R.; Elechiguerra, J.L.; Camacho, A.; Holt, K.; Kouri, J.B.; Ramírez, J.T.; Yacaman, M.J. The bactericidal effect of silver nanoparticles. Nanotechnology 2005, 16, 2346–2353. [Google Scholar] [CrossRef] [PubMed]
- Lok, C.-N.; Ho, C.-M.; Chen, R.; He, Q.-Y.; Yu, W.-Y.; Sun, H.; Tam, P.K.-H.; Chiu, J.-F.; Che, C.-M. Proteomic analysis of the mode of antibacterial action of silver nanoparticles. J. Proteome Res. 2006, 5, 916–924. [Google Scholar] [CrossRef]
- Sondi, I.; Salopek-Sondi, B. Silver nanoparticles as antimicrobial agent: A case study on E. coli as a model for Gram-negative bacteria. J. Colloid Interface Sci. 2004, 275, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Raffi, M.; Mehrwan, S.; Bhatti, T.M.; Akhter, J.I.; Hameed, A.; Yawar, W.; ul Hasan, M.M. Investigations into the antibacterial behavior of copper nanoparticles against Escherichia coli. Ann. Microbiol. 2010, 60, 75–80. [Google Scholar] [CrossRef]
- Ren, G.; Hu, D.; Cheng, E.W.C.; Vargas-Reus, M.A.; Reip, P.; Allaker, R.P. Characterisation of Copper Oxide Nanoparticles for Antimicrobial Applications. Int. J. Antimicrob. Agents 2009, 33, 587–590. [Google Scholar] [CrossRef]
- Tee, J.K.; Ong, C.N.; Bay, B.H.; Ho, H.K.; Leong, D.T. Oxidative stress by inorganic nanoparticles. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2016, 8, 414–438. [Google Scholar] [CrossRef]
- Cai, X.; Zhang, B.; Liang, Y.; Zhang, J.; Yan, Y.; Chen, X.; Wu, Z.; Liu, H.; Wen, S.; Tan, S.; et al. Study on the antibacterial mechanism of copper ion- and neodymium ion-modified α-zirconium phosphate with better antibacterial activity and lower cytotoxicity. Colloids Surf. B Biointerfaces 2015, 132, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Borkow, G. Using copper to fight microorganisms. Curr. Chem. Biol. 2012, 6, 93–103. [Google Scholar] [CrossRef]
- Wu, X.; Ye, L.; Liu, K.; Wang, W.; Wei, J.; Chen, F.; Liu, C. Antibacterial properties of mesoporous copper-doped silica xerogels. Biomed. Mater. 2009, 4, 045008. [Google Scholar] [CrossRef]
- Warnes, S.L.; Summersgill, E.N.; Keevil, C.W. Inactivation of murine norovirus on a range of copper alloy surfaces is accompanied by loss of capsid integrity. Appl. Environ. Microbiol. 2015, 81, 1085–1091. [Google Scholar] [CrossRef] [PubMed]
- Bleichert, P.; Espírito Santo, C.; Hanczaruk, M.; Meyer, H.; Grass, G. Inactivation of bacterial and viral biothreat agents on metallic copper surfaces. Biometals 2014, 27, 1179–1189. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Pi, Q.M.; You, H.H.; Li, J.Q.; Wang, P.C.; Yang, X.; Wu, Y. A Smart Multi-Functional Coating Based on Anti-Pathogen Micelles Tethered with Copper Nanoparticles via a Biosynthesis Method Using L-Vitamin C. RSC Adv. 2018, 8, 18272–18283. [Google Scholar] [CrossRef]
- Sagripanti, J.L.; Routson, L.B.; Lytle, C.D. Virus inactivation by copper or iron ions alone and in the presence of peroxide. Appl. Environ. Microbiol. 1993, 59, 4374–4376. [Google Scholar] [CrossRef] [PubMed]
- Grass, G.; Rensing, C.; Solioz, M. Metallic copper as an antimicrobial surface. Appl. Environ. Microbiol. 2011, 77, 1541–1547. [Google Scholar] [CrossRef] [PubMed]
- Bengtsson, U.G.; Hylander, L.D. Increased mercury emissions from modern dental amalgams. Biometals 2017, 30, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Al Sarraj, Z.S.A.; Atiyah, R.I. Preparation and characterization of high-copper restorative dental alloys corrected. Adv. Mater. Res. 2011, 324, 69–72. [Google Scholar]
- Berry, T.G.; Osborne, J.S.; Summitt, J.B. Clinical evaluation of high-copper amalgams. Am. J. Dent. 1995, 8, 122–124. [Google Scholar] [PubMed]
- Hasheminezhad, A.; Zebarjad, S.M.; Sajjadi, S.A.; Rahanjam, L. Effect of copper content on compressive strength and microstructure of dental amalgams. Engineering 2012, 4, 155–159. [Google Scholar] [CrossRef][Green Version]
- Sun, D.; Xu, D.; Yang, C.; Shahzad, M.B.; Sun, Z.; Xia, J.; Zhao, J.; Gu, T.; Yang, K.; Wang, G. An investigation of the antibacterial ability and cytotoxicity of a novel cu-bearing 317L stainless steel. Sci. Rep. 2016, 6, 29244. [Google Scholar] [CrossRef]
- Guan, J.; Guo, L.; Fu, Y.; Chai, H. In vitro evaluation of antibacterial activity and cytocompatibility of antibacterial stainless steel containing copper. J. Biomed. Eng. 2013, 30, 333–337. [Google Scholar]
- Chai, H.; Guo, L.; Wang, X.; Fu, Y.; Guan, J.; Tan, L.; Ren, L.; Yang, K. Antibacterial effect of 317L stainless steel contained copper in prevention of implant-related infection in vitro and in vivo. J. Mater. Sci. Mater. Med. 2011, 22, 2525–2535. [Google Scholar] [CrossRef]
- Liu, R.; Tang, Y.; Liu, H.; Zeng, L.; Ma, Z.; Li, J.; Zhao, Y.; Ren, L.; Yang, K. Effects of combined chemical design (Cu addition) and topographical modification (SLA) of Ti-Cu/SLA for promoting osteogenic, angiogenic and antibacterial activities. J. Mater. Sci. Technol. 2020, 47, 202–215. [Google Scholar] [CrossRef]
- Zhuang, Y.; Zhang, S.; Yang, K.; Ren, L.; Dai, K. Antibacterial activity of copper-bearing 316L stainless steel for the prevention of implant-related infection. J. Biomed. Mater. Res. B Appl. Biomater. 2020, 108, 484–495. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Ren, L.; Zhang, Y.; Xue, N.; Yang, K.; Zhong, M. Antibacterial activity against Porphyromonas gingivalis and biological characteristics of antibacterial stainless steel. Colloids Surf. B Biointerfaces 2013, 105, 51–57. [Google Scholar] [CrossRef]
- Li, M.; Nan, L.; Liang, C.; Sun, Z.; Yang, L.; Yang, K. Antibacterial behavior and related mechanisms of martensitic Cu-bearing stainless steel evaluated by a mixed infection model of Escherichia coli and Staphylococcus aureus in vitro. J. Mater. Sci. Technol. 2021, 62, 139–147. [Google Scholar] [CrossRef]
- Yan, X.; Wan, P.; Tan, L.; Zhao, M.; Qin, L.; Yang, K. Corrosion and biological performance of biodegradable magnesium alloys mediated by low copper addition and processing. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 93, 565–581. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Fu, X.; Pan, H.; Wan, P.; Wang, L.; Tan, L.; Wang, K.; Zhao, Y.; Yang, K.; Chu, P.K. Biodegradable Mg-Cu alloys with enhanced osteogenesis, angiogenesis, and long-lasting antibacterial effects. Sci. Rep. 2016, 6, 27374. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Li, M.; Liu, R.; Ren, L.; Zhang, Y.; Pan, H.; Zhao, Y.; Yang, K. In vitro study on an antibacterial Ti-5Cu alloy for medical application. J. Mater. Sci. Mater. Med. 2016, 27, 91. [Google Scholar] [CrossRef]
- Bai, B.; Zhang, E.; Liu, J.; Zhu, J. The anti-bacterial activity of titanium-copper sintered alloy against Porphyromonas gingivalis in vitro. Dent. Mater. J. 2016, 35, 659–667. [Google Scholar] [CrossRef]
- Guo, S.; Lu, Y.; Wu, S.; Liu, L.; He, M.; Zhao, C.; Gan, Y.; Lin, J.; Luo, J.; Xu, X.; et al. Preliminary study on the corrosion resistance, antibacterial activity and cytotoxicity of selective-laser-melted Ti6Al4V-xCu alloys. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 72, 631–640. [Google Scholar] [CrossRef]
- Li, M.; Ma, Z.; Zhu, Y.; Xia, H.; Yao, M.; Chu, X.; Wang, X.; Yang, K.; Yang, M.; Zhang, Y.; et al. Toward a molecular understanding of the antibacterial mechanism of copper-bearing titanium alloys against Staphylococcus aureus. Adv. Healthc. Mater. 2016, 5, 557–566. [Google Scholar] [CrossRef]
- Liu, R.; Memarzadeh, K.; Chang, B.; Zhang, Y.; Ma, Z.; Allaker, R.P.; Ren, L.; Yang, K. Antibacterial effect of copper-bearing titanium alloy (Ti-Cu) against Streptococcus mutans and Porphyromonas gingivalis. Sci. Rep. 2016, 6, 29985. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zhou, Y.; Xu, M.; Han, P.; Chen, L.; Chang, J.; Xiao, Y. Copper-containing mesoporous bioactive glass scaffolds with multifunctional properties of angiogenesis capacity, osteostimulation and antibacterial activity. Biomaterials 2013, 34, 422–433. [Google Scholar] [CrossRef] [PubMed]
- Bari, A.; Bloise, N.; Fiorilli, S.; Novajra, G.; Vallet-Regí, M.; Bruni, G.; Torres-Pardo, A.; González-Calbet, J.M.; Visai, L.; Vitale-Brovarone, C. Copper-containing mesoporous bioactive glass nanoparticles as multifunctional agent for bone regeneration. Acta Biomater. 2017, 55, 493–504. [Google Scholar] [CrossRef] [PubMed]
- Koohkan, R.; Hooshmand, T.; Tahriri, M.; Mohebbi-Kalhori, D. Synthesis, characterization and in vitro bioactivity of mesoporous copper silicate bioactive glasses. Ceram. Int. 2018, 44, 2390–2399. [Google Scholar] [CrossRef]
- Fowler, L.; Masia, N.; Cornish, L.A.; Chown, L.H.; Engqvist, H.; Norgren, S.; Öhman-Mägi, C. Development of antibacterial Ti-Cu(x) alloys for dental applications: Effects of ageing for alloys with up to 10 wt% Cu. Materials 2019, 12, 4017. [Google Scholar] [CrossRef]
- Rosenbaum, J.; Versace, D.L.; Abbad-Andallousi, S.; Pires, R.; Azevedo, C.; Cénédese, P.; Dubot, P. Antibacterial properties of nanostructured Cu-TiO2 surfaces for dental implants. Biomater. Sci. 2017, 5, 455–462. [Google Scholar] [CrossRef]
- Astasov-Frauenhoffer, M.; Koegel, S.; Waltimo, T.; Zimmermann, A.; Walker, C.; Hauser-Gerspach, I.; Jung, C. Antimicrobial efficacy of copper-doped titanium surfaces for dental implants. J. Mater. Sci. Mater. Med. 2019, 30, 84. [Google Scholar] [CrossRef]
- Morales, A.G.; Jofre, J.; Perez-Tijerina, E.; Sólis-Pomar, S.; Sánchez-Sanhueza, G.; Mf, M. Effect of titanium coated with 3 different types of copper nanoparticles in the oral biofilm formation. J. Dent. Health Oral Disord. Ther. 2021, 12, 1–6. [Google Scholar]
- Liu, H.; Tang, Y.; Zhang, S.; Liu, H.; Wang, Z.; Li, Y.; Wang, X.; Ren, L.; Yang, K.; Qin, L. Anti-infection mechanism of a novel dental implant made of titanium-copper (TiCu) alloy and its mechanism associated with oral microbiology. Bioact. Mater. 2022, 8, 381–395. [Google Scholar] [CrossRef]
- Hameed, H.A. Evaluation of Antibacterial Properties of Copper Nanoparticles Surface Coating on Titanium Dental Implant. J. Pharm. Sci. Res 2018, 10, 1157–1160. [Google Scholar]
- Gil, F.J.; Planell, J.A. Effect of copper addition on the superelastic behavior of Ni-Ti shape memory alloys for orthodontic applications. J. Biomed. Mater. Res. 1999, 48, 682–688. [Google Scholar] [CrossRef]
- Sachdeva, R.C.L.; Miyazaki, S.; Farzin-Nia, F. Orthodontic Archwire and Method of Moving Teeth. U.S. Patent Application No. 5,044,947, 3 September 1991. [Google Scholar]
- Darabara, M.S.; Bourithis, L.I.; Zinelis, S.; Papadimitriou, G.D. Metallurgical characterization, galvanic corrosion, and ionic release of orthodontic brackets coupled with Ni-Ti archwires. J. Biomed. Mater. Res. B Appl. Biomater. 2007, 81B, 126–134. [Google Scholar] [CrossRef]
- Pompei-Reynolds, R.C.; Kanavakis, G. Interlot variations of transition temperature range and force delivery in copper-nickel-titanium orthodontic wires. Am. J. Orthod. Dentofacial Orthop. 2014, 146, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Kusy, R.P.; Whitley, J.Q. Thermal and mechanical characteristics of stainless steel, titanium-molybdenum, and nickel-titanium archwires. Am. J. Orthod. Dentofacial Orthop. 2007, 131, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Phukaoluan, A.; Khantachawana, A.; Kaewtatip, P.; Dechkunakorn, S.; Anuwongnukroh, N.; Santiwong, P.; Kajornchaiyakul, J. Comparison of friction forces between stainless orthodontic steel brackets and TiNi wires in wet and dry conditions. Int. Orthod. 2017, 15, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Pandis, N.; Polychronopoulou, A.; Eliades, T. Alleviation of mandibular anterior crowding with copper-nickel-titanium vs. nickel-titanium wires: A double-blind randomized control trial. Am. J. Orthod. Dentofac. Orthop. 2009, 136, 152.e1–152.e7. [Google Scholar] [CrossRef]
- Shoeib, A.; Shaker, M.; Abdeen, R.; El-Segai, A. Evaluation of silver and copper oxide NANOPARTICLES release from different prosthetic materials incorporated with nanoparticles. Egypt. Dent. J. 2019, 65, 2849–2855. [Google Scholar] [CrossRef]
- Marovic, D.; Haugen, H.J.; Negovetic Mandic, V.; Par, M.; Zheng, K.; Tarle, Z.; Boccaccini, A.R. Incorporation of copper-doped mesoporous bioactive glass nanospheres in experimental dental composites: Chemical and mechanical characterization. Materials 2021, 14, 2611. [Google Scholar] [CrossRef]
- Gutiérrez, M.F.; Malaquias, P.; Hass, V.; Matos, T.P.; Lourenço, L.; Reis, A.; Loguercio, A.D.; Farago, P.V. The role of copper nanoparticles in an etch-and-rinse adhesive on antimicrobial activity, mechanical properties and the durability of resin-dentine interfaces. J. Dent. 2017, 61, 12–20. [Google Scholar] [CrossRef]
- Sabatini, C.; Mennito, A.S.; Wolf, B.J.; Pashley, D.H.; Renné, W.G. Incorporation of bactericidal poly-acrylic acid modified copper iodide particles into adhesive resins. J. Dent. 2015, 43, 546–555. [Google Scholar] [CrossRef]
- Foley, J.; Evans, D.J.; Lloyd, C.H.; Blackwell, A. Black copper phosphate cement: Does it have a future? Eur. J. Prosthodont. Restor. Dent. 2001, 9, 67–71. [Google Scholar] [PubMed]
- Foley, J.; Blackwell, A. Ion Release from Copper Phosphate Cement and Influence on Streptococcus mutans Growth in vitro: A Comparative Study. Caries Res. 2003, 37, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Foley, J.; Evans, D.J.; Blackwell, A. Restoration of primary teeth: A study of copper phosphate cement. Health Bull. 2001, 59, 45–48. [Google Scholar]
- Song, H.B.; Wang, X.; Patton, J.R.; Stansbury, J.W.; Bowman, C.N. Kinetics and mechanics of photo-polymerized triazole-containing thermosetting composites via the copper(I)-catalyzed azide-alkyne cycloaddition. Dent. Mater. 2017, 33, 621–629. [Google Scholar] [CrossRef]
- Aguilar-Perez, D.; Vargas-Coronado, R.; Cervantes-Uc, J.M.; Rodriguez-Fuentes, N.; Aparicio, C.; Covarrubias, C.; Alvarez-Perez, M.; Garcia-Perez, V.; Martinez-Hernandez, M.; Cauich-Rodriguez, J.V. Antibacterial activity of a glass ionomer cement doped with copper nanoparticles. Dent. Mater. J. 2020, 39, 389–396. [Google Scholar] [CrossRef]
- Rau, J.V.; Wu, V.M.; Graziani, V.; Fadeeva, I.V.; Fomin, A.S.; Fosca, M.; Uskoković, V. The Bone Building Blues: Self-hardening copper-doped calcium phosphate cement and its in vitro assessment against mammalian cells and bacteria. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 79, 270–279. [Google Scholar] [CrossRef]
- Lin, Z.; Cao, Y.; Zou, J.; Zhu, F.; Gao, Y.; Zheng, X.; Wang, H.; Zhang, T.; Wu, T. Improved osteogenesis and angiogenesis of a novel copper ions doped calcium phosphate cement. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 114, 111032. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, H.; He, F.; Wu, T.; Zhou, L.; Ye, J. Concentration-dependent osteogenic and angiogenic biological performances of calcium phosphate cement modified with copper ions. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 99, 1199–1212. [Google Scholar] [CrossRef]
- Wassmann, T.; Schubert, A.; Malinski, F.; Rosentritt, M.; Krohn, S.; Techmer, K.; Bürgers, R. The antimicrobial and cytotoxic effects of a copper-loaded zinc oxide phosphate cement. Clin. Oral Investig. 2020, 24, 3899–3909. [Google Scholar] [CrossRef]
- Thomas, B.; Gautam, A.; Prasad, B.R.; Kumari, S. Evaluation of micronutrient (zinc, copper, and iron) levels in periodontitis patients with and without diabetes mellitus type 2: A biochemical study. Indian J. Dent. Res. 2013, 24, 468–473. [Google Scholar]
- Thomas, B.; Ramesh, A.; Suresh, S.; Prasad, B.R. A comparative evaluation of antioxidant enzymes and selenium in the serum of periodontitis patients with diabetes mellitus type 2. Contemp. Clin. Dent. 2013, 4, 176–180. [Google Scholar]
- Sundaram, G.; Ramakrishnan, T.; Parthasarathy, H.; Moses, J.; Lalitha, T. Evaluation of micronutrient (zinc, magnesium, and copper) levels in serum and glycemic status after nonsurgical periodontal therapy in type 2 diabetic patients with chronic periodontitis. Contemp. Clin. Dent. 2017, 8, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Dannan, A.; Hanno, Y. The relationship between periodontal diseases and plasma level of copper and magnesium. Dentistry 2018, 8, 1000471. [Google Scholar] [CrossRef]
- Herman, M.; Golasik, M.; Piekoszewski, W.; Walas, S.; Napierala, M.; Wyganowska-Swiatkowska, M.; Kurhanska-Flisykowska, A.; Wozniak, A.; Florek, E. Essential and toxic metals in oral fluid-a potential role in the diagnosis of periodontal diseases. Biol. Trace Elem. Res. 2016, 173, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.R.; Naftel, S.J.; Nelson, A.J.; Edwards, M.; Mithoowani, H.; Stakiw, J. Synchrotron radiation analysis of possible correlations between metal status in human cementum and periodontal disease. J. Synchrotron Radiat. 2010, 17, 263–267. [Google Scholar] [CrossRef]
- Manea, A.; Nechifor, M. Research on plasma and saliva levels of some bivalent cations in patients with chronic periodontitis (salivary cations in chronic periodontitis). Rev. Med. Chir. Soc. Med. Nat. Iasi 2014, 118, 439–449. [Google Scholar] [PubMed]
- González, J.P.; Covarrubias, C.; Cádiz, M.; Corral, C.; Cuadra, F.; Fuentevilla, I. Design of Antimicrobial Release Systems Based on Chitosan and Copper Nanoparticles for Localized Periodontal Therapy. J. Dent. Oral Disord. 2016, 2, 1035. [Google Scholar]
- Majbauddin, A.; Kodani, I.; Ryoke, K. The effect of bamboo leaf extract solution and sodium copper chlorophyllin solution on growth and volatile sulfur compounds production of oral malodor associated some anaerobic periodontal bacteria. Yonago Acta Med. 2015, 58, 129–136. [Google Scholar]
- Meto, A.; Droboniku, E.; Blasi, E.; Colombari, B.; Tragaj, E.; Cervino, G.; Fiorillo, L.; Meto, A. Copper–calcium hydroxide and permanent electrophoretic current for treatment of apical periodontitis. Materials 2021, 14, 678. [Google Scholar] [CrossRef]
- Gould, S.W.J.; Fielder, M.D.; Kelly, A.F.; Morgan, M.; Kenny, J.; Naughton, D.P. The antimicrobial properties of copper surfaces against a range of important nosocomial pathogens. Ann. Microbiol. 2009, 59, 151–156. [Google Scholar] [CrossRef]
- Sierra, M.; Sanhueza, A.; Alcántara, R.; Sánchez, G. Antimicrobial Evaluation of Copper Sulfate (II) on Strains of Enterococcus Faecalis. In Vitro Study. J. Oral Res. 2013, 2, 114–118. [Google Scholar] [CrossRef]
- Sánchez-Sanhueza, G.; Rebolledo, S.; López, J.; Encalada, M.; Bello-Toledo, H.; Rojas, D.; Medinam, C.; Melendrez, M.F. Synthesis of copper nanowires and their antimicrobial activity on strains isolated persistent endodontic infections. J. Nanosci. Nanotechnol. 2018, 18, 4507–4514. [Google Scholar] [CrossRef]
- Sánchez-Sanhueza, G.; Alcántara-Dufeu, R.; Carrillo, L.; Mansilla, H.; Novoa, C.; Bello-Toledo, H. Ex vivo Effect of Copper Sulfate on Enterococcus faecalis in Root Canals. Int. J. Odontostomatol. 2015, 9, 505–510. [Google Scholar] [CrossRef]
- Meto, A.; Colombari, B.; Sala, A.; Pericolini, E.; Meto, A.; Peppoloni, S.; Blasi, E. Antimicrobial and antibiofilm efficacy of a copper/calcium hydroxide-based endodontic paste against Staphylococcus aureus, Pseudomonas aeruginosa and Candida albicans. Dent. Mater. J. 2019, 38, 591–603. [Google Scholar] [CrossRef]
- Zhang, F.; Zhou, M.; Gu, W.; Shen, Z.; Ma, X.; Lu, F.; Yang, X.; Zheng, Y.; Gou, Z. Zinc-/copper-substituted dicalcium silicate cement: Advanced biomaterials with enhanced osteogenesis and long-term antibacterial properties. J. Mater. Chem. B Mater. Biol. Med. 2020, 8, 1060–1070. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, A.; Renaudin, G.; Charbonnel, N.; Nedelec, J.-M.; Forestier, C.; Descamps, S. Copper-doped Biphasic Calcium Phosphate powders: Dopant release, cytotoxicity and antibacterial properties. Materials 2021, 14, 2393. [Google Scholar] [CrossRef] [PubMed]
- Gomes, S.; Vichery, C.; Descamps, S.; Martinez, H.; Kaur, A.; Jacobs, A.; Nedelec, J.-M.; Renaudin, G. Cu-doping of calcium phosphate bioceramics: From mechanism to the control of cytotoxicity. Acta Biomater. 2018, 65, 462–474. [Google Scholar] [CrossRef]
- Zhang, W.; Chang, Q.; Xu, L.; Li, G.; Yang, G.; Ding, X.; Wang, X.; Cui, D.; Jiang, X. Graphene oxide-copper nanocomposite-coated porous CaP scaffold for vascularized bone regeneration via activation of hif-1α. Adv. Healthc. Mater. 2016, 5, 1299–1309. [Google Scholar] [CrossRef]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial biofilms: A common cause of persistent infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Xu, D.; Yang, C.; Chen, J.; Shahzad, M.B.; Sun, Z.; Zhao, J.; Gu, T.; Yang, K.; Wang, G. Inhibition of Staphylococcus aureus biofilm by a copper-bearing 317L-Cu stainless steel and its corrosion resistance. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 69, 744–750. [Google Scholar] [CrossRef]
- Chen, A.F.; Parvizi, J. Antibiotic-loaded bone cement, and periprosthetic joint infection. J. Long Term Eff. Med. Implant. 2014, 24, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Yang, Y.; Lu, J.; Wang, C.; Xie, Y.; Zheng, X.; Yao, Z.; Zhang, C. Sustained release vancomycin-coated titanium alloy using a novel electrostatic dry powder coating technique may be a potential strategy to reduce implant-related infection. Biosci. Trends 2017, 11, 346–354. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, D.; Pan, X.; Wang, L. Characterization of the complexation between Al3+ and extracellular polymeric substances prepared from alga-bacteria biofilm. Chin. J. Appl. Environ. Biol 2009, 15, 347–350. [Google Scholar]
- Zhao, W.; Walker, S.L.; Huang, Q.; Cai, P. Contrasting effects of extracellular polymeric substances on the surface characteristics of bacterial pathogens and cell attachment to soil particles. Chem. Geol. 2015, 410, 79–88. [Google Scholar] [CrossRef]
- Xia, C.; Ma, X.; Zhang, X.; Li, K.; Tan, J.; Qiao, Y.; Liu, X. Enhanced physicochemical and biological properties of C/Cu dual ions implanted medical titanium. Bioact. Mater. 2020, 5, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Hou, C.; Mao, Y.; Yang, L.; Tamaddon, M.; Zhang, J.; Qu, X.; Liu, C.; Su, B.; Lu, X. Characteristics of novel Ti-10Mo-xCu alloy by powder metallurgy for potential biomedical implant applications. Bioact. Mater. 2020, 5, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Hadeel, S.; Ahmed, M. Evaluation of anti-fungal effect of nano-particles within different types of acrylic resin denture bases. Egypt. Dent. J. 2015, 61, 3443–3449. [Google Scholar]
- Torres-Rosas, R.; Torres-Gómez, N.; García-Contreras, R.; Scougall-Vilchis, R.; Domínguez-Díaz, L.; Argueta-Figueroa, L. Copper nanoparticles as nanofillers in an adhesive resin system: An in vitro study. Dent. Med. Probl. 2020, 57, 239–246. [Google Scholar] [CrossRef]
- Nourollahi, M.; Meryon, S.D. The antibacterial properties of four elements released from dental restorative materials. Int. Endod. J. 1989, 22, 9–16. [Google Scholar] [CrossRef]
- Grossman, E.S.; Dip, H.; Witcomb, M.J.; Matejka, J.M. Influence of amalgams, bases, and varnish on seal composition at restoration tooth interfaces. J. Prosthet. Dent. 1995, 73, 290–298. [Google Scholar] [CrossRef]
- Dionysopoulos, P.; Kotsanos, N.; Koliniotou-Koubia, E.; Papagodiannis, Y. Secondary caries formation in vitro around fluoride-releasing restorations. Oper. Dent. 1994, 19, 183–188. [Google Scholar]
- Habibovic, P.; Barralet, J.E. Bioinorganics and biomaterials: Bone repair. Acta Biomater. 2011, 7, 3013–3026. [Google Scholar] [CrossRef] [PubMed]
- Willis, M.S.; Monaghan, S.A.; Miller, M.L.; McKenna, R.W.; Perkins, W.D.; Levinson, B.S.; Bhushan, V.; Kroft, S.H. Zinc-induced copper deficiency: A report of three cases initially recognized on bone marrow examination: A report of three cases initially recognized on bone marrow examination. Am. J. Clin. Pathol. 2005, 123, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Ran, S.; Wang, J.; Jiang, W.; Zhu, C.; Liang, J. Assessment of dentinal tubule invasion capacity of Enterococcus faecalis under stress conditions ex vivo. Int. Endod. J. 2015, 48, 362–372. [Google Scholar] [CrossRef]
- Hohscheidt, G.L.; Böttcher, D.E.; Fatturi Parolo, C.C.; Montagner, F.; Grecca, F.S. Response of E. faecalis biofilms to different associations of auxiliary substances during root canal preparation: A confocal laser microscopy analysis: Irrigation protocols during endodontics. Microsc. Res. Tech. 2013, 76, 658–662. [Google Scholar] [CrossRef] [PubMed]
- Colvin, V.L. The potential environmental impact of engineered nanomaterials. Nat. Biotechnol. 2003, 21, 1166–1170. [Google Scholar] [CrossRef] [PubMed]
- Georgopoulos, P.G.; Roy, A.; Yonone-Lioy, M.J.; Opiekun, R.E.; Lioy, P.J. Environmental copper: Its dynamics and human exposure issues. J. Toxicol. Environ. Health B Crit. Rev. 2001, 4, 341–394. [Google Scholar] [CrossRef]
- Fahmy, B.; Cormier, S.A. Copper oxide nanoparticles induce oxidative stress and cytotoxicity in airway epithelial cells. Toxicol. Vitr. 2009, 23, 1365–1371. [Google Scholar] [CrossRef]
- Cho, W.-S.; Duffin, R.; Poland, C.A.; Duschl, A.; Oostingh, G.J.; Macnee, W.; Bradley, M.; Megson, I.L.; Donaldson, K. Differential pro-inflammatory effects of metal oxide nanoparticles and their soluble ions in vitro and in vivo; zinc and copper nanoparticles, but not their ions, recruit eosinophils to the lungs. Nanotoxicology 2012, 6, 22–35. [Google Scholar] [CrossRef]
- Rodhe, Y.; Skoglund, S.; Odnevall Wallinder, I.; Potácová, Z.; Möller, L. Copper-based nanoparticles induce high toxicity in leukemic HL60 cells. Toxicol. Vitr. 2015, 29, 1711–1719. [Google Scholar] [CrossRef]
- Siddiqui, M.A.; Alhadlaq, H.A.; Ahmad, J.; Al-Khedhairy, A.A.; Musarrat, J.; Ahamed, M. Copper oxide nanoparticles induced mitochondria mediated apoptosis in human hepatocarcinoma cells. PLoS ONE 2013, 8, e69534. [Google Scholar] [CrossRef] [PubMed]
- Kahru, A.; Savolainen, K. Potential hazard of nanoparticles: From properties to biological and environmental effects. Toxicology 2010, 269, 89–91. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, B.M.; Ali, S.F.; Murdock, R.C.; Hussain, S.M.; Srivatsan, M. Copper nanoparticles exert size and concentration dependent toxicity on somatosensory neurons of rat. Nanotoxicology 2010, 4, 150–160. [Google Scholar] [CrossRef]
- Xu, J.; Li, Z.; Xu, P.; Xiao, L.; Yang, Z. Nanosized copper oxide induces apoptosis through oxidative stress in podocytes. Arch. Toxicol. 2013, 87, 1067–1073. [Google Scholar] [CrossRef]
- Chen, Z.; Meng, H.; Xing, G.; Chen, C.; Zhao, Y.; Jia, G.; Wang, T.; Yuan, H.; Ye, C.; Zhao, F.; et al. Acute toxicological effects of copper nanoparticles in vivo. Toxicol. Lett. 2006, 163, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Manna, P.; Ghosh, M.; Ghosh, J.; Das, J.; Sil, P.C. Contribution of nano-copper particles to in vivo liver dysfunction and cellular damage: Role of IκBα/NF-κB, MAPKs and mitochondrial signal. Nanotoxicology 2012, 6, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Lei, R.; Wu, C.; Yang, B.; Ma, H.; Shi, C.; Wang, Q.; Wang, Q.; Yuan, Y.; Liao, M. Integrated metabolomic analysis of the nano-sized copper particle-induced hepatotoxicity and nephrotoxicity in rats: A rapid in vivo screening method for nanotoxicity. Toxicol. Appl. Pharmacol. 2008, 232, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Chen, Z.; Xing, G.; Yuan, H.; Chen, C.; Zhao, F.; Zhang, C.; Zhao, Y. Ultrahigh reactivity provokes nanotoxicity: Explanation of oral toxicity of nano-copper particles. Toxicol. Lett. 2007, 175, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Zietz, B.P.; Dieter, H.H.; Lakomek, M.; Schneider, H.; Kessler-Gaedtke, B.; Dunkelberg, H. Epidemiological investigation on chronic copper toxicity to children exposed via the public drinking water supply. Sci. Total Environ. 2003, 302, 127–144. [Google Scholar] [CrossRef]
- Bertinato, J.; L’Abbé, M.R. Maintaining copper homeostasis: Regulation of copper-trafficking proteins in response to copper deficiency or overload. J. Nutr. Biochem. 2004, 15, 316–322. [Google Scholar] [CrossRef]
- Sarkar, A.; Das, J.; Manna, P.; Sil, P.C. Nano-copper induces oxidative stress and apoptosis in kidney via both extrinsic and intrinsic pathways. Toxicology 2011, 290, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Chaves, S.B.; Lacava, L.M.; Lacava, Z.G.M.; Silva, O.; Pelegrini, F.; Buske, N.; Gansau, C.; Morais, P.C.; Azevedo, R.B. Light microscopy and magnetic resonance characterization of a DMSA-coated magnetic fluid in mice. IEEE Trans. Magn. 2002, 38, 3231–3233. [Google Scholar] [CrossRef]
- Nizami, M.Z.I.; Xu, V.W.; Yin, I.X.; Yu, O.Y.; Chu, C.-H. Metal and Metal Oxide Nanoparticles in Caries Prevention: A Review. Nanomaterials 2021, 11, 3446. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Sanhueza, G.; Fuentes-Rodríguez, D.; Bello-Toledo, H. Copper Nanoparticles as Potential Antimicrobial Agent in Disinfecting Root Canals: A Systematic Review. Int. J. Odontostomatol. 2016, 10, 547–554. [Google Scholar] [CrossRef]
- Allaker, R.P. The Use of Antimicrobial Nanoparticles to Control Oral Infections. In Nano-Antimicrobials; Springer: Berlin/Heidelberg, Germany, 2012; pp. 395–425. ISBN 9783642244278. [Google Scholar]
| Materials [Reference(s)] | Properties | Applications | Functions |
|---|---|---|---|
| Dental metals and alloys | |||
| Copper-coated metal [44,45] | Offer antimicrobial properties | Denture framework | Prevent stomatitis |
| Copper amalgam alloy [46,47,48,49] | Improve microstructural Improve mechanical properties | Amalgam restoration | Prevent corrosion |
| Copper-linked alloy [50,51,52,53,54,55,56] | Offer antimicrobial properties | Dental implant | Prevent implantitis |
| Magnesium–copper alloy [57,58] | Offer antimicrobial properties | Dental implant | Prevent implantitis |
| Titanium–copper alloy [57,58,59,60,61,62,63,64,65,66,67,68,69,70,71] | Offer antimicrobial properties | Dental implant | Prevent implantitis |
| Titanium–hydroxyapatite–copper alloy [72] | Offer antimicrobial properties | Dental Implant | Prevent implantitis |
| Nickel–titanium–copper alloy [73,74,75,76,77,78,79] | Enhance mechanical properties Enhance thermal properties Prevent galvanic corrosion Reduce alloy aging | Orthodontic bracket Orthodontic archwire | Facilitate orthodontic tooth movement |
| Dental polymers and resins | |||
| Copper-nanoparticle-incorporated acrylic resin [80] | Offer antimicrobial properties | Denture soft liner | Prevent stomatitis |
| Copper-doped mesoporous bioactive glass nanosphere acrylic resin [81] | Facilitate copper-ion release Offer antimicrobial properties | Denture acrylic base | Prevent stomatitis |
| Copper nanoparticles with adhesive resin [82] | Offer antimicrobial properties | Dental adhesive | Prevent secondary caries |
| Polyacrylic acid–copper iodide nanoparticles with adhesive resin [83] | Offer antimicrobial properties | Dental adhesive | Prevent secondary caries |
| Dental Cements | |||
| Copper [84,85,86] | Offer antimicrobial properties | Lining materials | Prevent secondary caries |
| Copper(I)-catalysed azide-alkyne cycloaddition composites [87] | Reduce shrinkage stress | Composite resin restoration | Prevent secondary caries |
| Copper nanoparticles incorporated in glass ionomer cement [88] | Offer antimicrobial properties | Glass ionomer restoration | Prevent secondary caries |
| Copper ions releasing blue calcium phosphate cement [89] | Offer antimicrobial properties Improve cytocompatibility | Regenerative dental material | Promote bone formation |
| Functionalized copper phosphate nanoparticles [90,91] | Increase vascularization Enhance bone regeneration | Regenerative dental material | Promote bone formation |
| Copper-modified zinc oxide phosphate [92] | Reduce marginal gap | Luting cement | Prevent secondary caries |
| Miscellaneousdental materials | |||
| Copper nanoparticles [93,94,95,96,97,98,99,100,101,102] | Enhance anti-inflammatory effects | Periodontal therapy | Prevent inflammation |
| Copper-based substance [103,104,105,106,107] | Offer antimicrobial properties | Endodontic irrigation solution Endodontic paste | Prevent apical reinfection |
| Nano copper-nonstoichiometric dicalcium silicate [108] | Offer antimicrobial properties Facilitate tissue regeneration | Regenerative dental material | Promote bone formation |
| Copper-doped biphasic calcium phosphate [109,110] | Improve bone regeneration Act as a bone substitution | Synthetic bone graft material | Promote bone formation |
| Graphene oxide copper nanocomposite [111] | Enhance bone regeneration | Regenerative dental material | Promote bone formation |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, V.W.; Nizami, M.Z.I.; Yin, I.X.; Yu, O.Y.; Lung, C.Y.K.; Chu, C.H. Application of Copper Nanoparticles in Dentistry. Nanomaterials 2022, 12, 805. https://doi.org/10.3390/nano12050805
Xu VW, Nizami MZI, Yin IX, Yu OY, Lung CYK, Chu CH. Application of Copper Nanoparticles in Dentistry. Nanomaterials. 2022; 12(5):805. https://doi.org/10.3390/nano12050805
Chicago/Turabian StyleXu, Veena Wenqing, Mohammed Zahedul Islam Nizami, Iris Xiaoxue Yin, Ollie Yiru Yu, Christie Ying Kei Lung, and Chun Hung Chu. 2022. "Application of Copper Nanoparticles in Dentistry" Nanomaterials 12, no. 5: 805. https://doi.org/10.3390/nano12050805
APA StyleXu, V. W., Nizami, M. Z. I., Yin, I. X., Yu, O. Y., Lung, C. Y. K., & Chu, C. H. (2022). Application of Copper Nanoparticles in Dentistry. Nanomaterials, 12(5), 805. https://doi.org/10.3390/nano12050805









