The Effects of the Temperature and Termination(-O) on the Friction and Adhesion Properties of MXenes Using Molecular Dynamics Simulation
Abstract
:1. Introduction
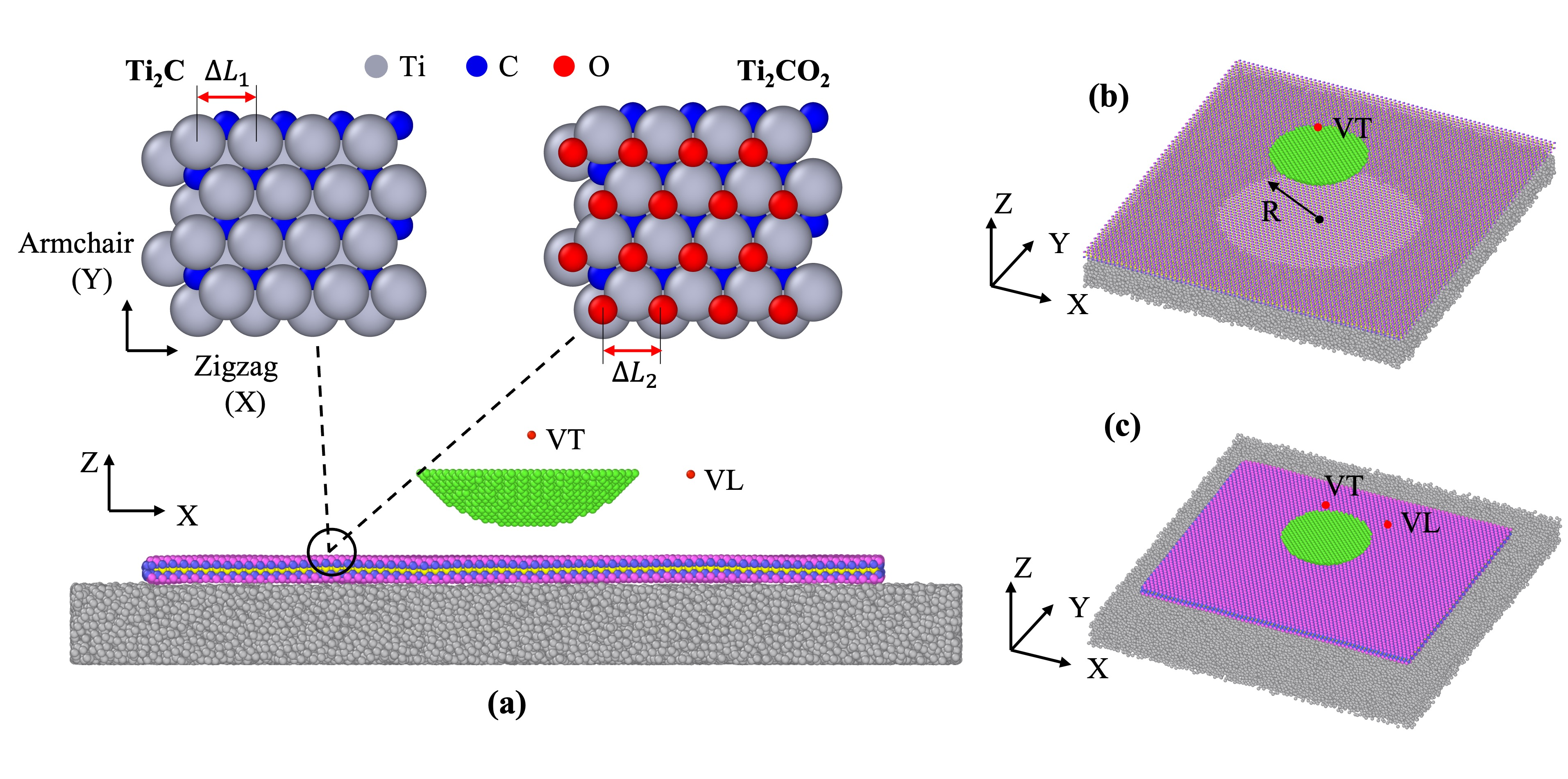
2. Materials and Methods
3. Results and Discussion
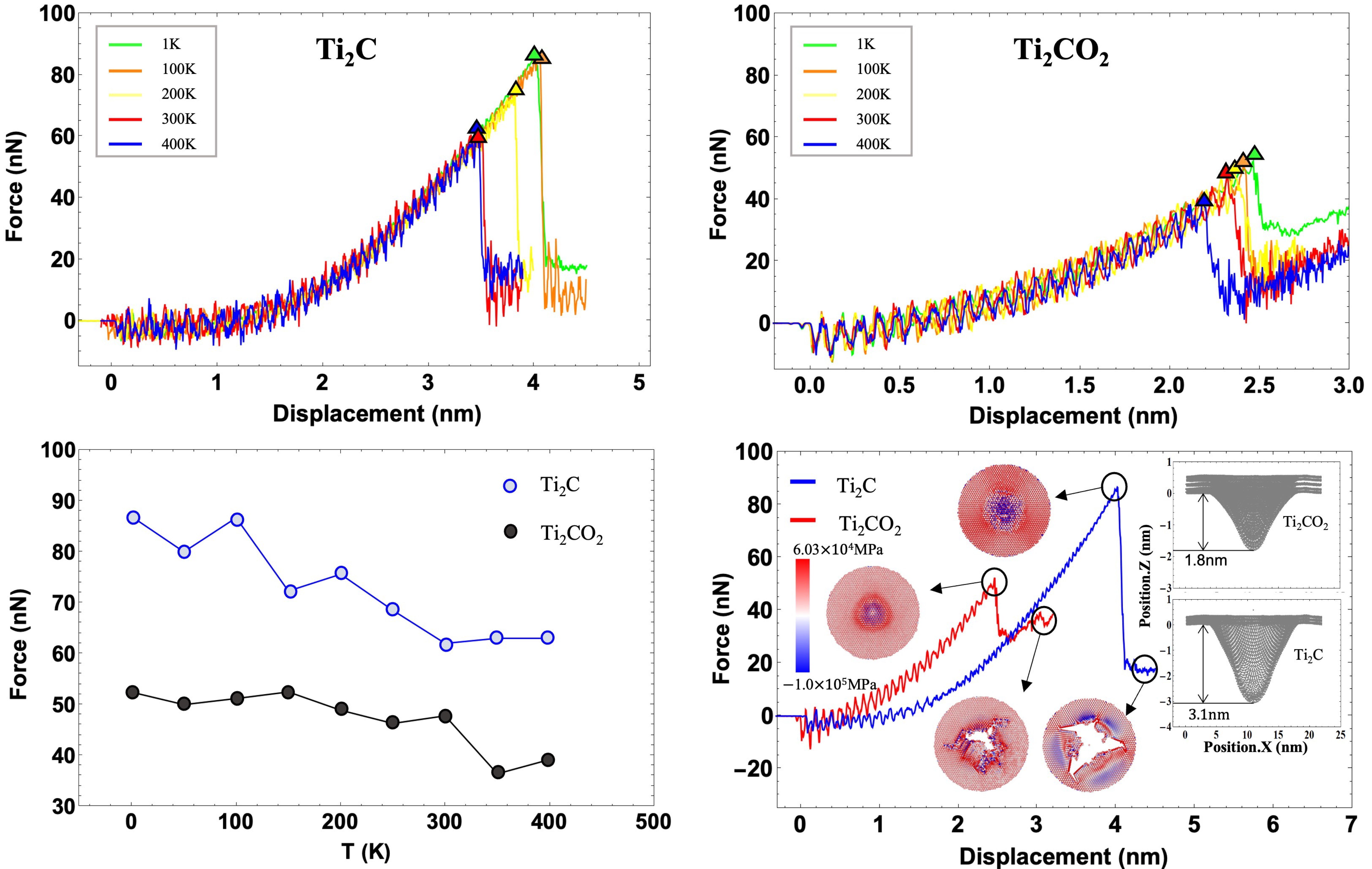
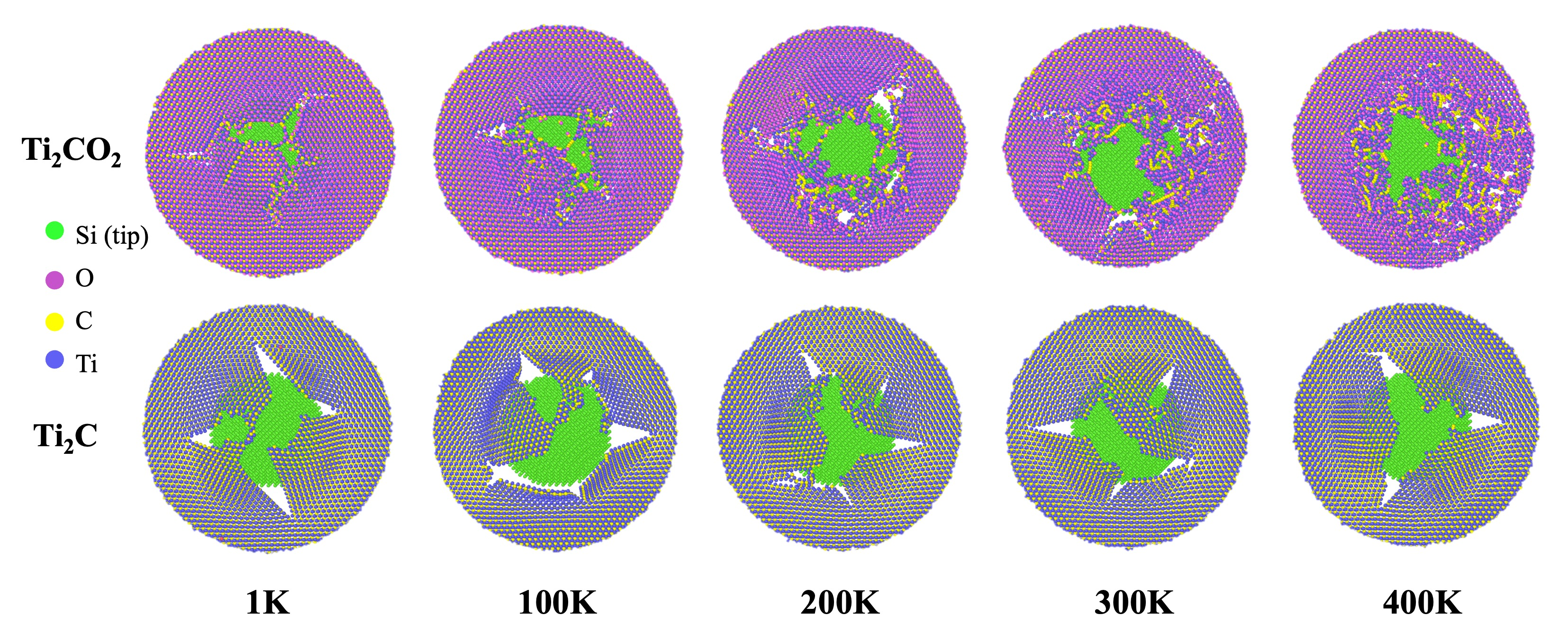
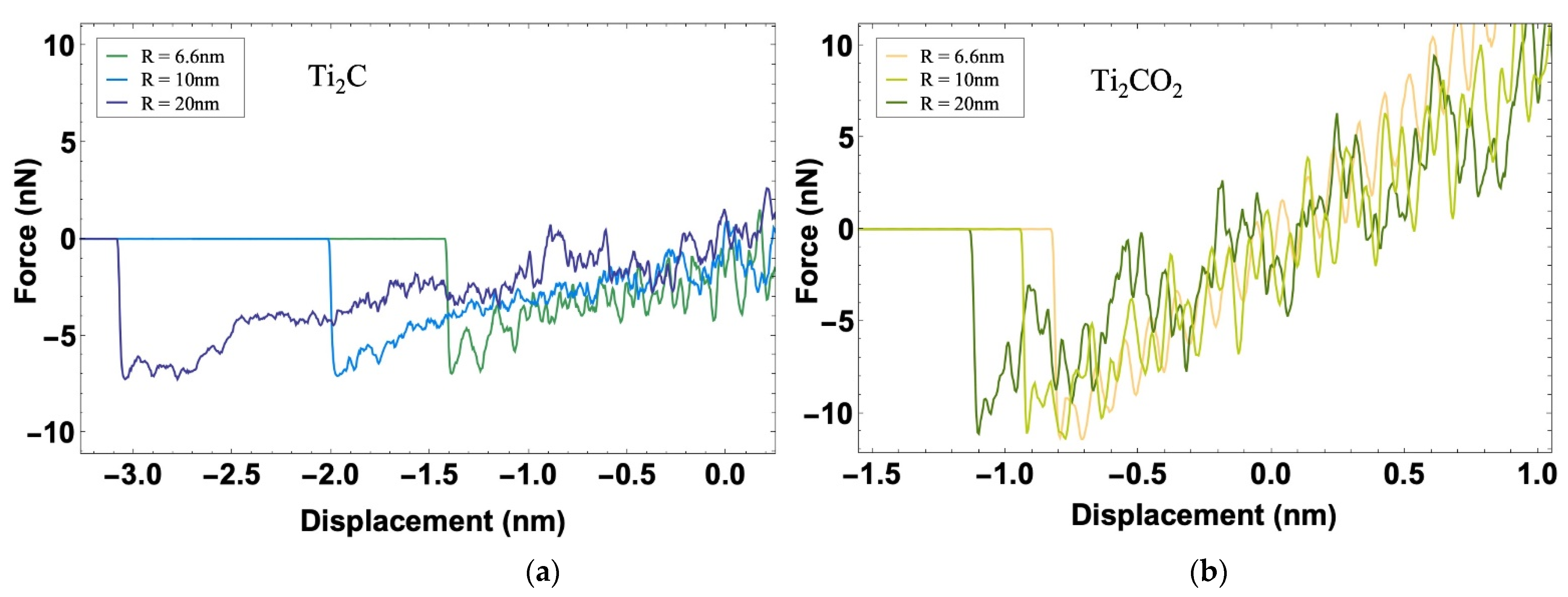
3.1. Indentation Simulation
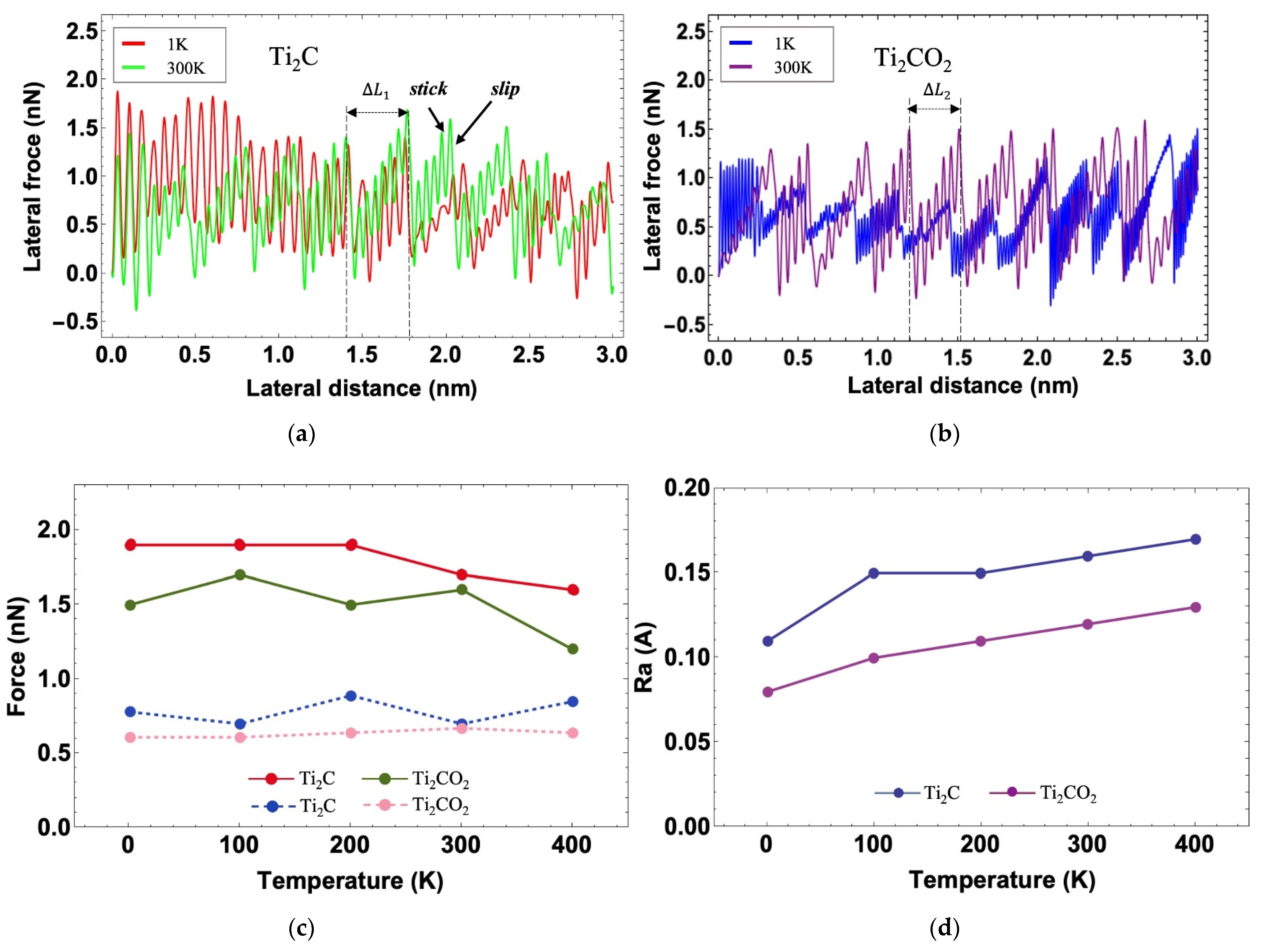
3.2. Friction Simulation
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fu, B.; Sun, J.; Wang, C.; Shang, C.; Xu, L.; Li, J.; Zhang, H. MXenes: Synthesis, Optical Properties, and Applications in Ultrafast Photonics. Small 2021, 17, 2006054. [Google Scholar] [CrossRef]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-Dimensional Nanocrystals Produced by Exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef] [Green Version]
- Anasori, B.; Lukatskaya, M.R.; Gogotsi, Y. 2D metal carbides and nitrides (MXenes) for energy storage. Nat. Rev. Mater. 2017, 2, 16098. [Google Scholar] [CrossRef]
- Lian, P.; Dong, Y.; Wu, Z.S.; Zheng, S.; Wang, S.; Sun, C.; Qin, J.; Shi, X.; Bao, X. Alkalized Ti3C2 MXene Nanoribbons with Expanded Interlayer Spacing for High-Capacity Sodium and Potassium Ion Batteries. Nano Energy 2017, 40, 1–8. [Google Scholar] [CrossRef]
- Lukatskaya, M.R.; Kota, S.; Lin, Z.; Zhao, M.Q.; Shpigel, N.; Levi, M.D.; Halim, J.; Taberna, P.L.; Barsoum, M.W.; Simon, P.; et al. Ultra-High-Rate Pseudocapacitive Energy Storage in Two-Dimensional Transition Metal Carbides. Nat. Energy 2017, 6, 17105. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, N.; Li, L.; Hu, X.; Zou, Z.; Wang, J.; Luo, S.; Gao, Y. A Highly Flexible and Sensitive Piezoresistive Sensor Based on MXene with Greatly Changed Interlayer Distances. Nat. Commun. 2017, 8, 1207. [Google Scholar] [CrossRef] [Green Version]
- Wu, F.; Zheng, H.; Wang, W.; Wu, Q.; Zhang, Q.; Guo, J.; Pu, B.; Shi, X.; Li, J.; Chen, X.; et al. Rapid Eradication of Antibiotic-Resistant Bacteria and Biofilms by MXene and near-Infrared Light through Photothermal Ablation. Sci. China Mater. 2021, 64, 748–7587. [Google Scholar] [CrossRef]
- Liu, J.; Peng, W.; Li, Y.; Zhang, F.; Fan, X. 2D MXene-Based Materials for Electrocatalysis. Trans. Tianjin Univ. 2020, 26, 149–171. [Google Scholar] [CrossRef] [Green Version]
- Cui, C.; Cheng, R.; Zhang, H.; Zhang, C.; Ma, Y.; Shi, C.; Fan, B.; Wang, H.; Wang, X. Ultrastable MXene@Pt/SWCNTs’ Nanocatalysts for Hydrogen Evolution Reaction. Adv. Funct. Mater. 2020, 30, 2000693. [Google Scholar] [CrossRef]
- Li, Z.; Qi, Z.; Wang, S.; Ma, T.; Zhou, L.; Wu, Z.; Luan, X.; Lin, F.Y.; Chen, M.; Miller, J.T.; et al. In Situ Formed Pt3Ti Nanoparticles on a Two-Dimensional Transition Metal Carbide (MXene) Used as Efficient Catalysts for Hydrogen Evolution Reactions. Nano Lett. 2019, 19, 5102–5108. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, H.B.; Sun, R.; Liu, Y.; Liu, Z.; Zhou, A.; Yu, Z.Z. Hydrophobic, Flexible, and Lightweight MXene Foams for High-Performance Electromagnetic-Interference Shielding. Adv. Mater. 2017, 29, 1702367. [Google Scholar] [CrossRef] [PubMed]
- Lukatskaya, M.R.; Mashtalir, O.; Ren, C.E.; Dall’Agnese, Y.; Rozier, P.; Taberna, P.L.; Gogotsi, Y. Cation Intercalation and High Volumetric Capacitance of Two-Dimensional Titanium Carbide. Science 2013, 341, 1502–1505. [Google Scholar] [CrossRef] [Green Version]
- Mashtalir, O.; Naguib, M.; Mochalin, V.N.; Dall’Agnese, Y.; Heon, M.; Barsoum, M.W.; Gogotsi, Y. Intercalation and Delamination of Layered Carbides and Carbonitrides. Nat. Commun. 2013, 4, 1716. [Google Scholar] [CrossRef]
- Zhang, N.; Hong, Y.; Yazdanparast, S.; Zaeem, M.A. Superior Structural, Elastic and Electronic Properties of 2D Titanium Nitride MXenes over Carbide MXenes: A Comprehensive First Principles Study. 2D Mater. 2018, 5, 045004. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.; Li, W. High-Thermal-Stability and High-Thermal-Conductivity Ti3C2Tx MXene/Poly (Vinyl Alcohol) (PVA) Composites. ACS Omega 2018, 3, 2609–2617. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Zhang, Q.; Yan, L.; Wu, G.; Hu, M.; Lin, X.; Yuan, K.; Yang, X. Ultrafast Flash Energy Conductance at MXene-Surfactant Interface and Its Molecular Origins. Adv. Mater. Interfaces 2019, 6, 1901461. [Google Scholar] [CrossRef]
- Khazaei, M.; Arai, M.; Sasaki, T.; Chung, C.Y.; Venkataramanan, N.S.; Estili, M.; Sakka, Y.; Kawazoe, Y. Novel Electronic and Magnetic Properties of Two-Dimensional Transition Metal Carbides and Nitrides. Adv. Funct. Mater. 2013, 23, 2185–2192. [Google Scholar] [CrossRef]
- Hong, N.V.M.; Huang, H.; Zhou, K.; Lee, P.S.; Que, W.; Xu, J.Z.; Kong, L.B. Recent Progress in Layered Transition Metal Carbides and/or Nitrides (MXenes) and Their Composites: Synthesis and Applications. J. Mater. Chem. A 2017, 5, 3039–3068. [Google Scholar] [CrossRef]
- Verger, L.; Xu, C.; Natu, V.; Cheng, H.M.; Ren, W.; Barsoum, M.W. Overview of the Synthesis of MXenes and Other Ultrathin 2D Transition Metal Carbides and Nitrides. Curr. Opin. Solid State Mater. Sci. 2019, 23, 149–163. [Google Scholar] [CrossRef]
- Fu, Z.H.; Zhang, Q.F.; Legut, D.; Si, C.; Germann, T.C.; Lookman, T.; Du, S.Y.; Francisco, J.S.; Zhang, R.F. Stabilization and Strengthening Effects of Functional Groups in Two-Dimensional Titanium Carbide. Phys. Rev. B 2016, 94, 104103. [Google Scholar] [CrossRef]
- Guo, Z.; Zhou, J.; Si, C.; Sun, Z. Flexible Two-Dimensional Tin+1Cn (n = 1, 2 and 3) and Their Functionalized MXenes Predicted by Density Functional Theories. Phys. Chem. Chem. Phys. 2015, 17, 15348–15354. [Google Scholar] [CrossRef]
- Ashton, M.; Mathew, K.; Hennig, R.G.; Sinnott, S.B. Predicted Surface Composition and Thermodynamic Stability of MXenes in Solution. J. Phys. Chem. C 2016, 120, 3550–3556. [Google Scholar] [CrossRef]
- Yu, H.; Xu, K.; Zhang, Z.; Weng, J.; Wu, J. Oxygen Functionalization-Induced Crossover in the Tensile Properties of Thinnest 2D Ti2C MXene. J. Phys. Chem. C 2021, 9, 2416–2425. [Google Scholar] [CrossRef]
- Borysiuk, V.N.; Mochalin, V.N.; Gogotsi, Y. Molecular Dynamic Study of the Mechanical Properties of Two-Dimensional Titanium Carbides Tin+1Cn (MXenes). Nanotechnology 2015, 26, 265705. [Google Scholar] [CrossRef] [Green Version]
- Naguib, M.; Come, J.; Dyatkin, B.; Presser, V.; Taberna, P.L.; Simon, P.; Barsoum, M.W.; Gogotsi, Y. MXene: A Promising Transition Metal Carbide Anode for Lithium-Ion Batteries. Electrochem. Commun. 2012, 16, 61–64. [Google Scholar] [CrossRef] [Green Version]
- Liang, X.; Garsuch, A.; Nazar, L.F. Sulfur Cathodes Based on Conductive MXene Nanosheets for High-Performance Lithium-Sulfur Batteries. Angew. Chem. 2015, 127, 3979–3983. [Google Scholar] [CrossRef]
- Malaki, M.; Varma, R.S. Mechanotribological Aspects of MXene-Reinforced Nanocomposites. Adv. Mater. 2020, 32, 2003154. [Google Scholar] [CrossRef] [PubMed]
- Kurtoglu, M.; Naguib, M.; Gogotsi, Y.; Barsoum, M.W. First Principles Study of Two-Dimensional Early Transition Metal Carbides. MRS Commun. 2012, 2, 133–137. [Google Scholar] [CrossRef]
- Guo, Y.; Zhou, X.; Wang, D.; Xu, X.; Xu, Q. Nanomechanical Properties of Ti3C2 Mxene. Langmuir 2019, 35, 14481–14485. [Google Scholar] [CrossRef]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the Elastic Properties and Intrinsic Strength of Monolayer Graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef]
- Li, S.; Li, Q.; Carpick, R.W.; Gumbsch, P.; Liu, X.Z.; Ding, X.; Sun, J.; Li, J. The Evolving Quality of Frictional Contact with Graphene. Nature 2016, 539, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Dong, Y.; Perez, D.; Martini, A.; Carpick, R.W. Speed Dependence of Atomic Stick-Slip Friction in Optimally Matched Experiments and Molecular Dynamics Simulations. Phys. Rev. Lett. 2011, 106, 126101. [Google Scholar] [CrossRef] [Green Version]
- Yan, X.; Sharma, P. Time-Scaling in Atomistics and the Rate-Dependent Mechanical Behavior of Nanostructures. Nano Lett. 2016, 16, 3487–3492. [Google Scholar] [CrossRef] [PubMed]
- Evstigneev, M.; Reimann, P. Rate Description of the Stick-Slip Motion in Friction Force Microscopy Experiments. Phys. Rev. E-Stat. Nonlinear Soft Matter Phys. 2005, 71, 056119. [Google Scholar] [CrossRef]
- Yan, X.; Cao, P.; Tao, W.; Sharma, P.; Park, H.S. Atomistic Modeling at Experimental Strain Rates and Timescales. J. Phys. D Appl. Phys. 2016, 49, 493002. [Google Scholar] [CrossRef] [Green Version]
- Yan, X.; Gouissem, A.; Guduru, P.R.; Sharma, P. Elucidating the Atomistic Mechanisms Underpinning Plasticity in Li-Si Nanostructures. Phys. Rev. Mater. 2017, 1, 055401. [Google Scholar] [CrossRef]
- Lipatov, A.; Lu, H.; Alhabeb, M.; Anasori, B.; Gruverman, A.; Gogotsi, Y.; Sinitskii, A. Elastic Properties of 2D Ti3C2Tx MXene Monolayers and Bilayers. Sci. Adv. 2018, 4, eaat0491. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Sun, N.; Liu, Y.; Jiang, C. Anisotropic Frictional Properties between Ti3C2Tx MXene/SiO2 Layer-Dependent Heterojunctions. J. Sci. Adv. Mater. Devices 2021, 6, 488–493. [Google Scholar] [CrossRef]
- Zhou, X.; Guo, Y.; Wang, D.; Xu, Q. Nano Friction and Adhesion Properties on Ti3C2 and Nb2C MXene Studied by AFM. Tribol. Int. 2021, 153, 106646. [Google Scholar] [CrossRef]
- Osti, N.C.; Naguib, M.; Ostadhossein, A.; Xie, Y.; Kent, P.R.C.; Dyatkin, B.; Rother, G.; Heller, W.T.; van Duin, A.C.T.; Gogotsi, Y.; et al. Effect of Metal Ion Intercalation on the Structure of MXene and Water Dynamics on Its Internal Surfaces. ACS Appl. Mater. Interfaces 2016, 8, 8859–8863. [Google Scholar] [CrossRef]
- Jhon, Y.I.; Byun, Y.T.; Lee, J.H.; Jhon, Y.M. Robust Mechanical Tunability of 2D Transition Metal Carbides via Surface Termination Engineering: Molecular Dynamics Simulation. Appl. Surf. Sci. 2020, 532, 147380. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, Y.; Zhang, Y.; Sun, J.; Hu, M.; Yan, X.; Yuan, K.; Yang, X.; Li, J. Surface Oxidation Modulates the Interfacial and Lateral Thermal Migration of MXene (Ti3C2Tx) Flakes. J. Phys. Chem. Lett. 2020, 11, 9521–9527. [Google Scholar] [CrossRef]
- White, D.; Jih-Heng, H.U.; Johnston, H.L. The Intermolecular Force Constants of Fluorine. J. Chem. Phys. 1953, 21, 1149–1152. [Google Scholar] [CrossRef]
- Arkundato, A.; Hasan, M.; Pramutadi, A.; Rivai, A.K.; Su’ud, Z. Thermodynamics and Structural Properties of Ti3SiC2 in Liquid Lead Coolant. J. Phys. Conf. Ser. 2020, 1493, 012026. [Google Scholar] [CrossRef]
- Stillinger, F.H.; Weber, T.A. Computer Simulation of Local Order in Condensed Phases of Silicon. Phys. Rev. B 1985, 8, 5262–5271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plimpton, S. Fast parallel algorithms for short-range molecular dynamics. J. Comput. Phys. 1995, 117, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Stukowski, A. Visualization and analysis of atomistic simulation data with OVITO—The Open Visualization Tool. Model. Simul. Mater. Sci. Eng. 2009, 18, 015012. [Google Scholar] [CrossRef]
- Wei, C.; Wu, C. Nonlinear Fracture of Two-Dimensional Transition Metal Carbides (MXenes). Eng. Fract. Mech. 2020, 230, 106978. [Google Scholar] [CrossRef]
- Jacobs, T.D.B.; Ryan, K.E.; Keating, P.L.; Grierson, D.S.; Lefever, J.A.; Turner, K.T.; Harrison, J.A.; Carpick, R.W. The Effect of Atomic-Scale Roughness on the Adhesion of Nanoscale Asperities: A Combined Simulation and Experimental Investigation. Tribol. Lett. 2013, 50, 81–93. [Google Scholar] [CrossRef]
- Maugis, D. Adhesion of Spheres: The JKR-DMT Transition Using a Dugdale Model. J. Colloid Interface Sci. 1991, 150, 243–269. [Google Scholar] [CrossRef]
- Li, Y.; Huang, S.; Wei, C.; Wu, C.; Mochalin, V.N. Adhesion of Two-Dimensional Titanium Carbides (MXenes) and Graphene to Silicon. Nat. Commun. 2019, 10, 3014. [Google Scholar] [CrossRef] [Green Version]
- Plummer, G.; Anasori, B.; Gogotsi, Y.; Tucker, G.J. Nanoindentation of Monolayer Tin+1CnTx MXenes via Atomistic Simulations: The Role of Composition and Defects on Strength. Comput. Mater. Sci. 2019, 157, 168–174. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, Y.; Zhang, M. Excellent Catalytic Activity of Two-Dimensional Ti2C and Ti2CT2 (T = O, F, OH) Monolayers on Hydrogen Storage of MgH2: First-Principles Calculations. Int. J. Hydrog. Energy 2021, 46, 33176–33185. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Wang, Y.; Jiang, Q.; El-Demellawi, J.K.; Kim, H.; Alshareef, H.N. MXene Printing and Patterned Coating for Device Applications. Adv. Mater. 2020, 32, 1908486. [Google Scholar] [CrossRef] [PubMed]

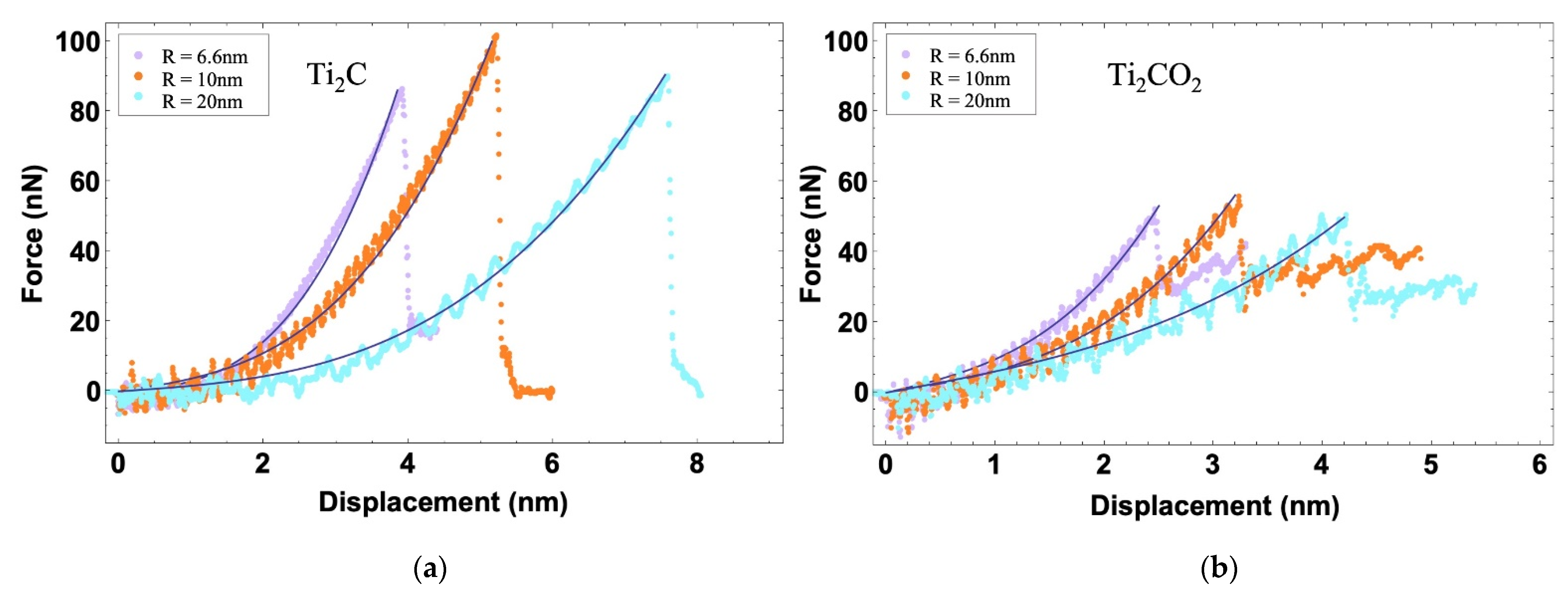
| MXenes | |||||
|---|---|---|---|---|---|
| Ti2C | 6.6 | 91 | 62 | 111 | 10.7 |
| 10 | 105 | 63 | 113 | 11.5 | |
| 20 | 93 | 65 | 116 | 11.0 | |
| Average | - | 96 | 63 | 113 | 11.1 |
| Ti2CO2 | 6.6 | 55 | 93 | 155 | 10.1 |
| 10 | 58 | 118 | 197 | 11.7 | |
| 20 | 52 | 133 | 222 | 11.8 | |
| Average | - | 55 | 115 | 191 | 11.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, Y.; Chen, Y.; Liu, H.; Yan, X. The Effects of the Temperature and Termination(-O) on the Friction and Adhesion Properties of MXenes Using Molecular Dynamics Simulation. Nanomaterials 2022, 12, 798. https://doi.org/10.3390/nano12050798
Deng Y, Chen Y, Liu H, Yan X. The Effects of the Temperature and Termination(-O) on the Friction and Adhesion Properties of MXenes Using Molecular Dynamics Simulation. Nanomaterials. 2022; 12(5):798. https://doi.org/10.3390/nano12050798
Chicago/Turabian StyleDeng, Yao, Yu Chen, Hanxu Liu, and Xin Yan. 2022. "The Effects of the Temperature and Termination(-O) on the Friction and Adhesion Properties of MXenes Using Molecular Dynamics Simulation" Nanomaterials 12, no. 5: 798. https://doi.org/10.3390/nano12050798
APA StyleDeng, Y., Chen, Y., Liu, H., & Yan, X. (2022). The Effects of the Temperature and Termination(-O) on the Friction and Adhesion Properties of MXenes Using Molecular Dynamics Simulation. Nanomaterials, 12(5), 798. https://doi.org/10.3390/nano12050798






