Computational Design of Gas Sensors Based on V3S4 Monolayer
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
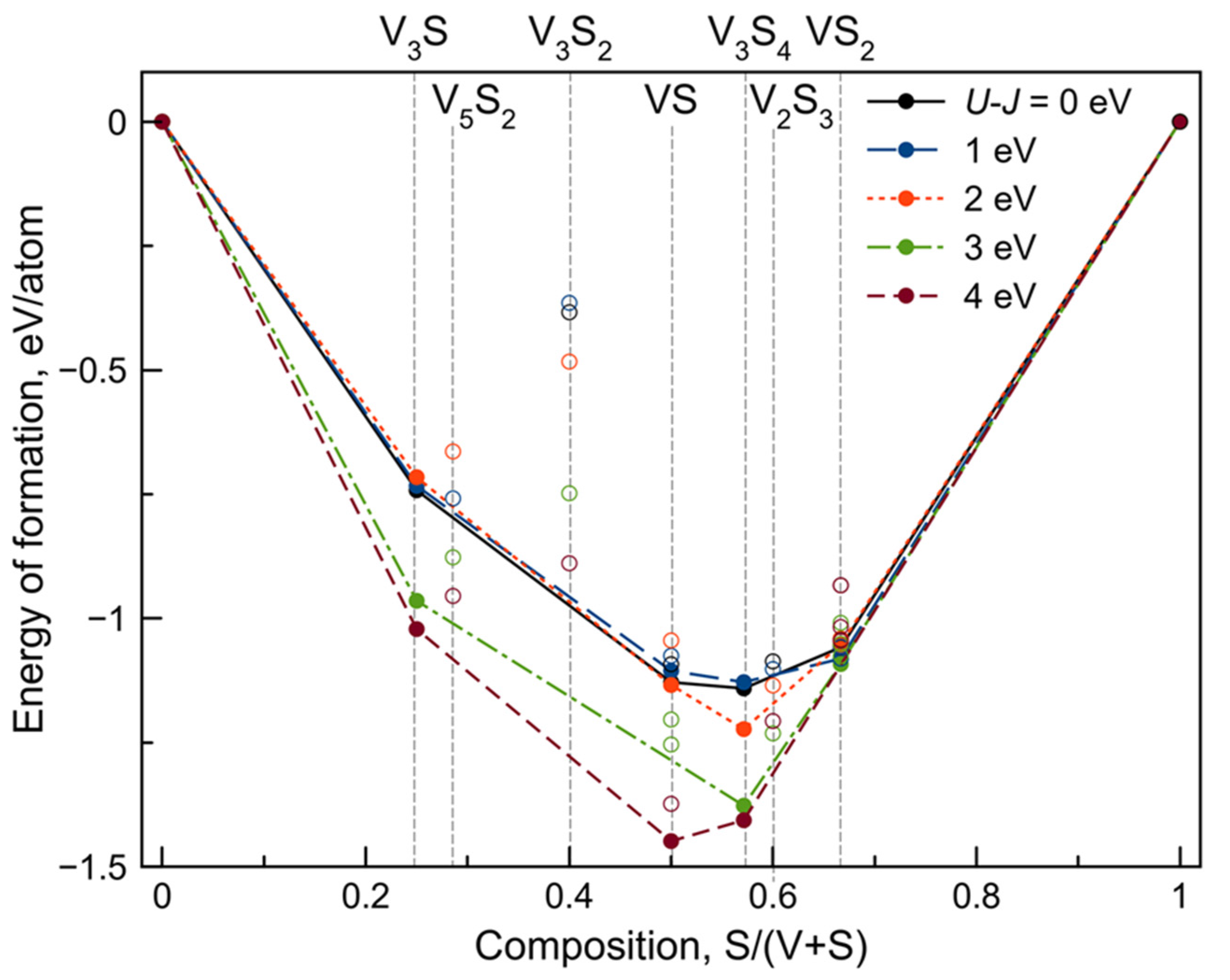
3.1. Crystal Structure Prediction
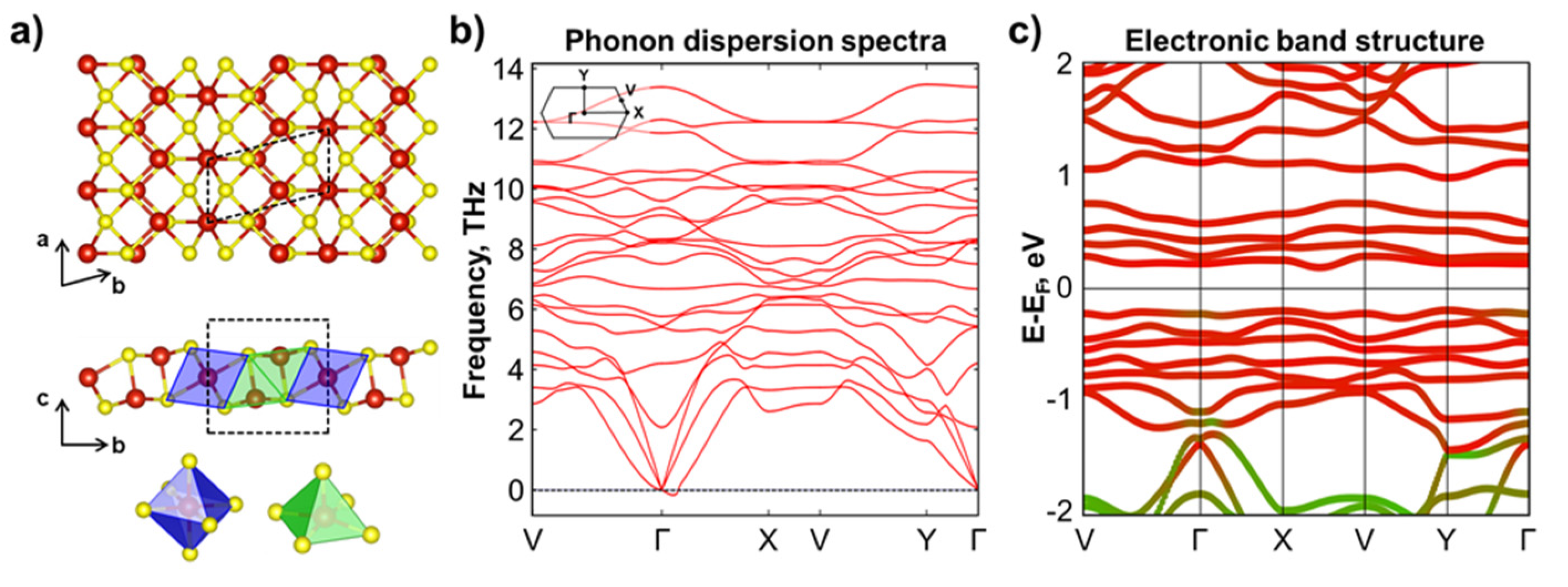
3.2. Novel V3S4 Phase
3.3. Sensing Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shinde, P.V.; Rout, C.S. Magnetic gas sensing: Working principles and recent developments. Nanoscale Adv. 2021, 3, 1551–1568. [Google Scholar] [CrossRef]
- Kiani, M.; Rehman, M.U.; Tian, X.; Yakobson, B. Two-dimensional nanomaterials for the development of efficient gas sensors: Recent advances, challenges, and future perspectives. Adv. Mater. Technol. 2021, 2101252. [Google Scholar] [CrossRef]
- Lin, T.; Lv, X.; Hu, Z.; Xu, A.; Feng, C. Semiconductor metal oxides as chemoresistive sensors for detecting volatile organic compounds. Sensors 2019, 19, 233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Liu, X.; Neri, G.; Pinna, N. Nanostructured materials for room-temperature gas sensors. Adv. Mater. 2016, 28, 795–831. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ma, T.; Pinna, N.; Zhang, J. Two-dimensional nanostructured materials for gas sensing. Adv. Funct. Mater. 2017, 27, 1702168. [Google Scholar] [CrossRef]
- Joshi, N.; Hayasaka, T.; Liu, Y.; Liu, H.; Oliveira, O.N.; Lin, L. A review on chemiresistive room temperature gas sensors based on metal oxide nanostructures, graphene and 2D transition metal dichalcogenides. Microchim. Acta 2018, 185, 213. [Google Scholar] [CrossRef]
- Matatagui, D.; Kolokoltsev, O.V.; Qureshi, N.; Mejía-Uriarte, E.V.; Saniger, J.M. A magnonic gas sensor based on magnetic nanoparticles. Nanoscale 2015, 7, 9607–9613. [Google Scholar] [CrossRef]
- Sukhanova, E.; Visotin, M.A.; Popov, Z.I.; Sorokin, P.B. Stability and gas sensing properties of Ta2X3M8 (X = Pd, Pt; M = S, Se) nanoribbons: A first-principles investigation. Phys. Chem. Chem. Phys. 2020, 22, 14651–14659. [Google Scholar] [CrossRef]
- Ciprian, R.; Baratto, C.; Giglia, A.; Koshmak, K.; Vinai, G.; Donarelli, M.; Ferroni, M.; Campanini, M.; Comini, E.; Ponzoni, A. Magnetic gas sensing exploiting the magneto-optical kerr effect on ZnO nanorods/Co layer system. RSC Adv. 2016, 6, 42517–42521. [Google Scholar] [CrossRef]
- Lin, G.; Makarov, D.; Schmidt, O.G. Magnetic sensing platform technologies for biomedical applications. Lab Chip 2017, 17, 1884–1912. [Google Scholar] [CrossRef]
- Zheng, W.; Liu, X.; Xie, J.; Lu, G.; Zhang, J. Emerging van der Waals junctions based on TMDs materials for advanced gas sensors. Coord. Chem. Rev. 2021, 447, 214151. [Google Scholar] [CrossRef]
- Zheng, W.; Xu, Y.; Zheng, L.; Yang, C.; Pinna, N.; Liu, X.; Zhang, J. MoS2 Van der Waals p–n junctions enabling highly selective room-temperature NO2 sensor. Adv. Funct. Mater. 2020, 30, 2000435. [Google Scholar] [CrossRef]
- Zhao, R.; Wang, T.; Zhao, M.; Xia, C.; An, Y.; Wei, S.; Dai, X. External electric field and strains facilitated nitrogen dioxide gas sensing properties on 2D monolayer and bilayer SnS2 nanosheets. Appl. Surf. Sci. 2019, 491, 128–137. [Google Scholar] [CrossRef]
- Zhao, R.; Wang, T.; An, Y.; Dai, X.; Xia, C. VS2 nanosheet as a promising candidate of recycle and reuse NO2 gas sensor and capturer: A DFT study. J. Phys. Condens. Matter 2021, 33, 165501. [Google Scholar] [CrossRef]
- Ataca, C.; Şahin, H.; Ciraci, S. Stable, single-layer MX2 transition-metal oxides and dichalcogenides in a honeycomb-like structure. J. Phys. Chem. C 2012, 116, 8983–8999. [Google Scholar] [CrossRef]
- Lv, R.; Robinson, J.A.; Schaak, R.E.; Sun, D.; Sun, Y.; Mallouk, T.E.; Terrones, M. Transition metal dichalcogenides and beyond: Synthesis, properties, and applications of single- and few-layer nanosheets. Acc. Chem. Res. 2015, 48, 56–64. [Google Scholar] [CrossRef]
- Shidpour, R.; Manteghian, M. A density functional study of strong local magnetism creation on MoS2 nanoribbon by sulfur vacancy. Nanoscale 2010, 2, 1429–1435. [Google Scholar] [CrossRef]
- He, J.; Wu, K.; Sa, R.; Li, Q.; Wei, Y. Magnetic properties of nonmetal atoms absorbed MoS2 monolayers. Appl. Phys. Lett. 2010, 96, 082504. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, K.; Li, H.; Liu, Z.; Shi, Z.; Lu, J.; Suenaga, K.; Joung, S.-K.; Okazaki, T.; Jin, Z.; et al. Ultra-narrow WS2 nanoribbons encapsulated in carbon nanotubes. J. Mater. Chem. 2010, 21, 171–180. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, Z.; Zhang, S.; Chen, Z. MoS2 nanoribbons: High stability and unusual electronic and magnetic properties. J. Am. Chem. Soc. 2008, 130, 16739–16744. [Google Scholar] [CrossRef]
- Ma, Y.; Dai, Y.; Guo, M.; Niu, C.; Lu, J.; Huang, B. Electronic and magnetic properties of perfect, vacancy-doped, and nonmetal adsorbed MoSe2, MoTe2 and WS2 monolayers. Phys. Chem. Chem. Phys. 2011, 13, 15546–15553. [Google Scholar] [CrossRef]
- Ma, Y.; Dai, Y.; Guo, M.; Niu, C.; Huang, B. Graphene adhesion on MoS2 monolayer: An ab initio study. Nanoscale 2011, 3, 3883–3887. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Xue, Q.; Mao, X.; Wang, W.; Xu, Q.; Xue, D. Ferromagnetism in ultrathin VS2 nanosheets. J. Mater. Chem. C 2013, 1, 5909–5916. [Google Scholar] [CrossRef]
- Ma, Y.; Dai, Y.; Guo, M.; Niu, C.; Zhu, Y.; Huang, B. Evidence of the existence of magnetism in pristine VX2 monolayers (X = S, Se) and their strain-induced tunable magnetic properties. ACS Nano 2012, 6, 1695–1701. [Google Scholar] [CrossRef] [PubMed]
- Coelho, P.M.; Nguyen Cong, K.; Bonilla, M.; Kolekar, S.; Phan, M.-H.; Avila, J.; Asensio, M.C.; Oleynik, I.I.; Batzill, M. Charge density wave state suppresses ferromagnetic ordering in VSe2 monolayers. J. Phys. Chem. C 2019, 123, 14089–14096. [Google Scholar] [CrossRef]
- Ma, Y.; Dai, Y.; Guo, M.; Niu, C.; Huang, B. Intriguing behavior of halogenated two-dimensional tin. J. Phys. Chem. C 2012, 116, 12977–12981. [Google Scholar] [CrossRef]
- Zhong, M.; Li, Y.; Xia, Q.; Meng, X.; Wu, F.; Li, J. Ferromagnetism in VS2 nanostructures: Nanoflowers versus ultrathin nanosheets. Mater. Lett. 2014, 124, 282–285. [Google Scholar] [CrossRef]
- Joseph, T.; Ghorbani-Asl, M.; Kvashnin, A.G.; Larionov, K.V.; Popov, Z.I.; Sorokin, P.B.; Krasheninnikov, A.V. Nonstoichiometric phases of two-dimensional transition-metal dichalcogenides: From chalcogen vacancies to pure metal membranes. J. Phys. Chem. Lett. 2019, 10, 6492–6498. [Google Scholar] [CrossRef]
- Zhu, Q.; Xiao, Q.; Zhang, B.; Yan, Z.; Liu, X.; Chen, S.; Ren, Z.; Yu, Y. VS4 with a chain crystal structure used as an intercalation cathode for aqueous Zn-ion batteries. J. Mater. Chem. A 2020, 8, 10761–10766. [Google Scholar] [CrossRef]
- Kozlova, M.N.; Mironov, Y.V.; Grayfer, E.D.; Smolentsev, A.I.; Zaikovskii, V.I.; Nebogatikova, N.A.; Podlipskaya, T.Y.; Fedorov, V.E. Synthesis, crystal structure, and colloidal dispersions of vanadium tetrasulfide (VS4). Chem. Eur. J. 2015, 21, 4639–4645. [Google Scholar] [CrossRef]
- Knecht, M.; Ebert, H.; Bensch, W. Electronic and magnetic properties of V5S8. J. Phys. Condens. Matter 1998, 10, 9455–9463. [Google Scholar] [CrossRef]
- Kitaoka, Y.; Yasuoka, H. NMR investigations on the spin fluctuations in itinerant antiferromagnets. II. V3S4 and V5S8. J. Phys. Soc. Jpn. 1980, 48, 1949–1956. [Google Scholar] [CrossRef]
- Jellinek, F. Structural study of vanadium sulfides. Rev. Chim. Minér. 1974, 11, 624–636. [Google Scholar]
- Kawada, I.; Nakano-Onoda, M.; Ishii, M.; Saeki, M.; Nakahira, M. Crystal structures of V3S4 and V5S8. J. Solid State Chem. 1975, 15, 246–252. [Google Scholar] [CrossRef]
- Materials Project. Available online: https://materialsproject.org/ (accessed on 20 June 2021).
- Bensch, W.; Ebert, H. The electronic bandstructure of hexagonal V3S4 isotypic with Nb3S4. Solid State Commun. 1994, 89, 235–238. [Google Scholar] [CrossRef]
- Oganov, A.R.; Glass, C.W. Crystal structure prediction using ab initio evolutionary techniques: Principles and applications. J. Chem. Phys. 2006, 124, 244704. [Google Scholar] [CrossRef] [Green Version]
- Oganov, A.R.; Lyakhov, A.O.; Valle, M. How evolutionary crystal structure prediction works—And why. Acc. Chem. Res. 2011, 44, 227–237. [Google Scholar] [CrossRef]
- Lyakhov, A.O.; Oganov, A.R.; Stokes, H.T.; Zhu, Q. New developments in evolutionary structure prediction algorithm USPEX. Comput. Phys. Commun. 2013, 184, 1172–1182. [Google Scholar] [CrossRef]
- Hohenberg, P.; Kohn, W. Inhomogeneous electron gas. Phys. Rev. 1964, 136, B864–B871. [Google Scholar] [CrossRef] [Green Version]
- Kohn, W.; Sham, L.J. Self-consistent equations including exchange and correlation effects. Phys. Rev. 1965, 140, A1133–A1138. [Google Scholar] [CrossRef] [Green Version]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kresse, G.; Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 1993, 47, 558–561. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular-dynamics simulation of the liquid-metal—Amorphous-semiconductor transition in germanium. Phys. Rev. B 1994, 49, 14251–14269. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [Green Version]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Anisimov, V.I.; Zaanen, J.; Andersen, O.K. Band theory and Mott insulators: Hubbard U instead of Stoner I. Phys. Rev. B 1991, 44, 943–954. [Google Scholar] [CrossRef] [Green Version]
- Dudarev, S.L.; Botton, G.A.; Savrasov, S.Y.; Humphreys, C.J.; Sutton, A.P. Electron-energy-loss spectra and the structural stability of nickel oxide: An LSDA + U study. Phys. Rev. B 1998, 57, 1505–1509. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [Green Version]
- Togo, A.; Tanaka, I. First principles phonon calculations in materials science. Scr. Mater. 2015, 108, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Togo, A.; Oba, F.; Tanaka, I. First-principles calculations of the ferroelastic transition between rutile-type and CaCl2-type SiO2 at high pressures. Phys. Rev. B 2008, 78, 134106. [Google Scholar] [CrossRef] [Green Version]
- Ganose, A.M.; Jackson, A.J.; Scanlon, D.O. Sumo: Command-line tools for plotting and analysis of periodic*ab initio* calculations. J. Open Source Softw. 2018, 3, 717. [Google Scholar] [CrossRef]
- Zhuang, H.L.; Hennig, R.G. Stability and magnetism of strongly correlated single-layer VS2. Phys. Rev. B 2016, 93, 054429. [Google Scholar] [CrossRef] [Green Version]
- Esters, M.; Hennig, R.G.; Johnson, D.C. Dynamic instabilities in strongly correlated VSe2 monolayers and bilayers. Phys. Rev. B 2017, 96, 235147. [Google Scholar] [CrossRef] [Green Version]
- Bensch, W.; Koy, J. Structure of hexagonal V3S4 determined at three different temperatures. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1993, 49, 1133–1135. [Google Scholar] [CrossRef]
- Bader, R.F. Atoms in molecules. Acc. Chem. Res. 1985, 18, 9–15. [Google Scholar] [CrossRef]
- Henkelman, G.A.; Arnaldsson, A.; Jónsson, H. A fast and robust algorithm for Bader decomposition of charge density. Comput. Mater. Sci. 2006, 36, 354–360. [Google Scholar] [CrossRef]
- Wang, X.; Du, X.; Yang, G.; Xue, J.; Chen, Y.; Zhang, L. Chemisorption of NO2 on V-based SCR catalysts: A fundamental study toward the mechanism of “Fast SCR” reaction. J. Phys. Chem. C 2019, 123, 20451–20458. [Google Scholar] [CrossRef]
- Sun, H.; Wang, M.; Zhang, S.; Liu, S.; Shen, X.; Qian, T.; Niu, X.; Xiong, J.; Yan, C. Boosting oxygen dissociation over bimetal sites to facilitate oxygen reduction activity of zinc-air battery. Adv. Funct. Mater. 2021, 31, 2006533. [Google Scholar] [CrossRef]
- Wan, H.; Jensen, A.W.; Escudero-Escribano, M.; Rossmeisl, J. Insights in the oxygen reduction reaction: From metallic electrocatalysts to diporphyrins. ACS Catal. 2020, 10, 5979–5989. [Google Scholar] [CrossRef]
- Gonzalez, D.J.X.; Francis, C.K.; Shaw, G.M.; Cullen, M.R.; Baiocchi, M.; Burke, M. Upstream oil and gas production and ambient air pollution in California. Sci. Total Environ. 2022, 806, 150298. [Google Scholar] [CrossRef] [PubMed]
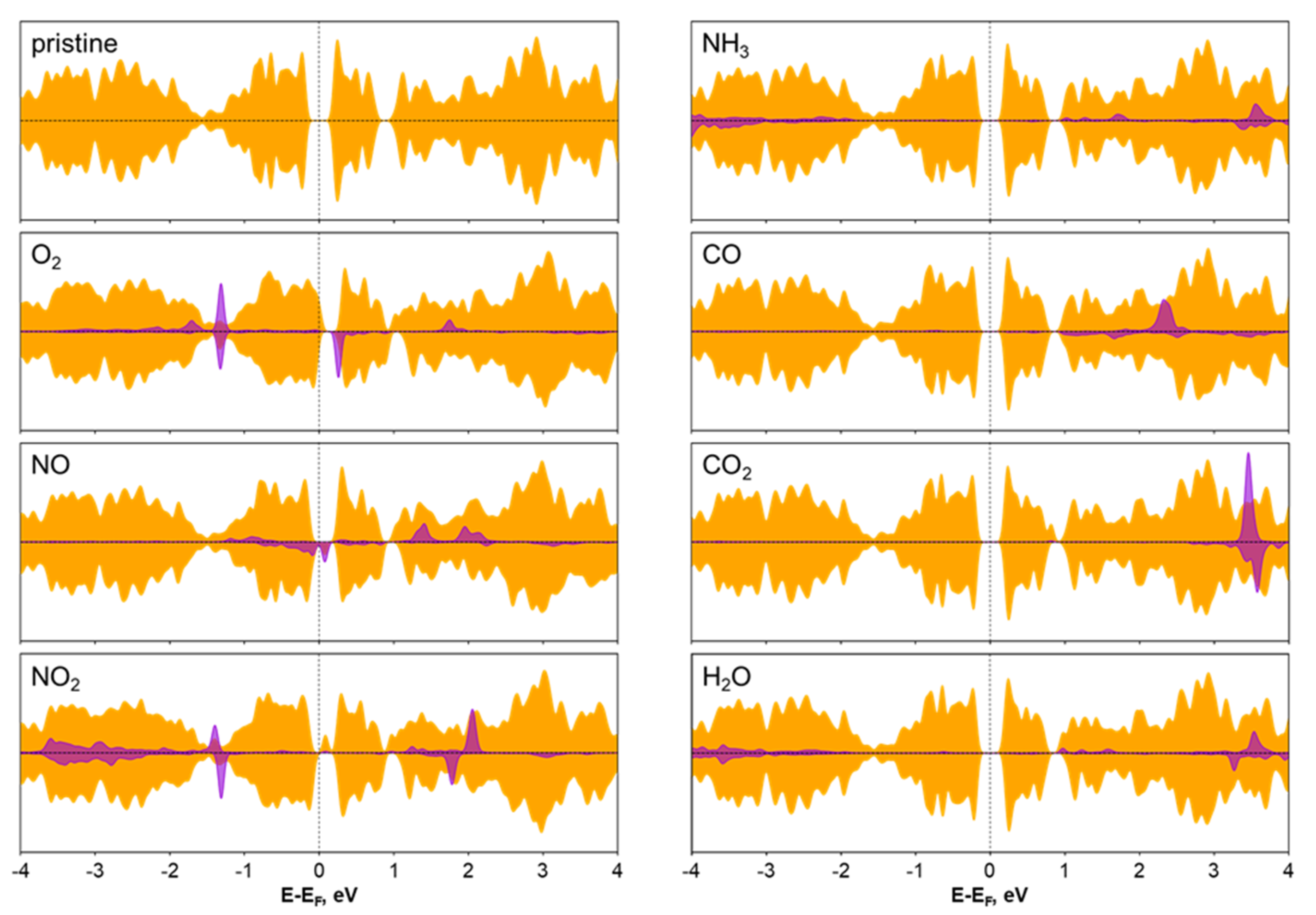
| Molecule | Ea (eV) | D (Å) | α0 (°) | α (°) | d0 (Å) | d (Å) | Δq (e) |
|---|---|---|---|---|---|---|---|
| CO | −0.82 | 2.11 | - | - | 1.14 | 1.14 | 0.069 |
| CO2 | −0.26 | 2.39 | 179.97 | 179.48 | 1.17 | 1.17 | 0.007 |
| H2O | −0.83 | 2.23 | 104.35 | 106.48 | 0.97 | 0.97 | −0.051 |
| O2 | −0.59 | 2.04 | - | - | 1.23 | 1.32 | 0.473 |
| NH3 | −1.25 | 2.22 | 106.58/106.57/106.56 | 108.49/108.63/107.59 | 1.02 | 1.02 | −0.114 |
| NO | −0.93 | 1.87 | - | - | 1.16 | 1.17 | 0.255 |
| NO2 | −0.91 | 1.91 | 133.9 | 111.37 | 1.21 | 1.42/1.19 | 0.506 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chepkasov, I.V.; Sukhanova, E.V.; Kvashnin, A.G.; Zakaryan, H.A.; Aghamalyan, M.A.; Mamasakhlisov, Y.S.; Manakhov, A.M.; Popov, Z.I.; Kvashnin, D.G. Computational Design of Gas Sensors Based on V3S4 Monolayer. Nanomaterials 2022, 12, 774. https://doi.org/10.3390/nano12050774
Chepkasov IV, Sukhanova EV, Kvashnin AG, Zakaryan HA, Aghamalyan MA, Mamasakhlisov YS, Manakhov AM, Popov ZI, Kvashnin DG. Computational Design of Gas Sensors Based on V3S4 Monolayer. Nanomaterials. 2022; 12(5):774. https://doi.org/10.3390/nano12050774
Chicago/Turabian StyleChepkasov, Ilya V., Ekaterina V. Sukhanova, Alexander G. Kvashnin, Hayk A. Zakaryan, Misha A. Aghamalyan, Yevgeni Sh. Mamasakhlisov, Anton M. Manakhov, Zakhar I. Popov, and Dmitry G. Kvashnin. 2022. "Computational Design of Gas Sensors Based on V3S4 Monolayer" Nanomaterials 12, no. 5: 774. https://doi.org/10.3390/nano12050774
APA StyleChepkasov, I. V., Sukhanova, E. V., Kvashnin, A. G., Zakaryan, H. A., Aghamalyan, M. A., Mamasakhlisov, Y. S., Manakhov, A. M., Popov, Z. I., & Kvashnin, D. G. (2022). Computational Design of Gas Sensors Based on V3S4 Monolayer. Nanomaterials, 12(5), 774. https://doi.org/10.3390/nano12050774









