Slow-Relaxation Behavior of a Mononuclear Co(II) Complex Featuring Long Axial Co-O Bond
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
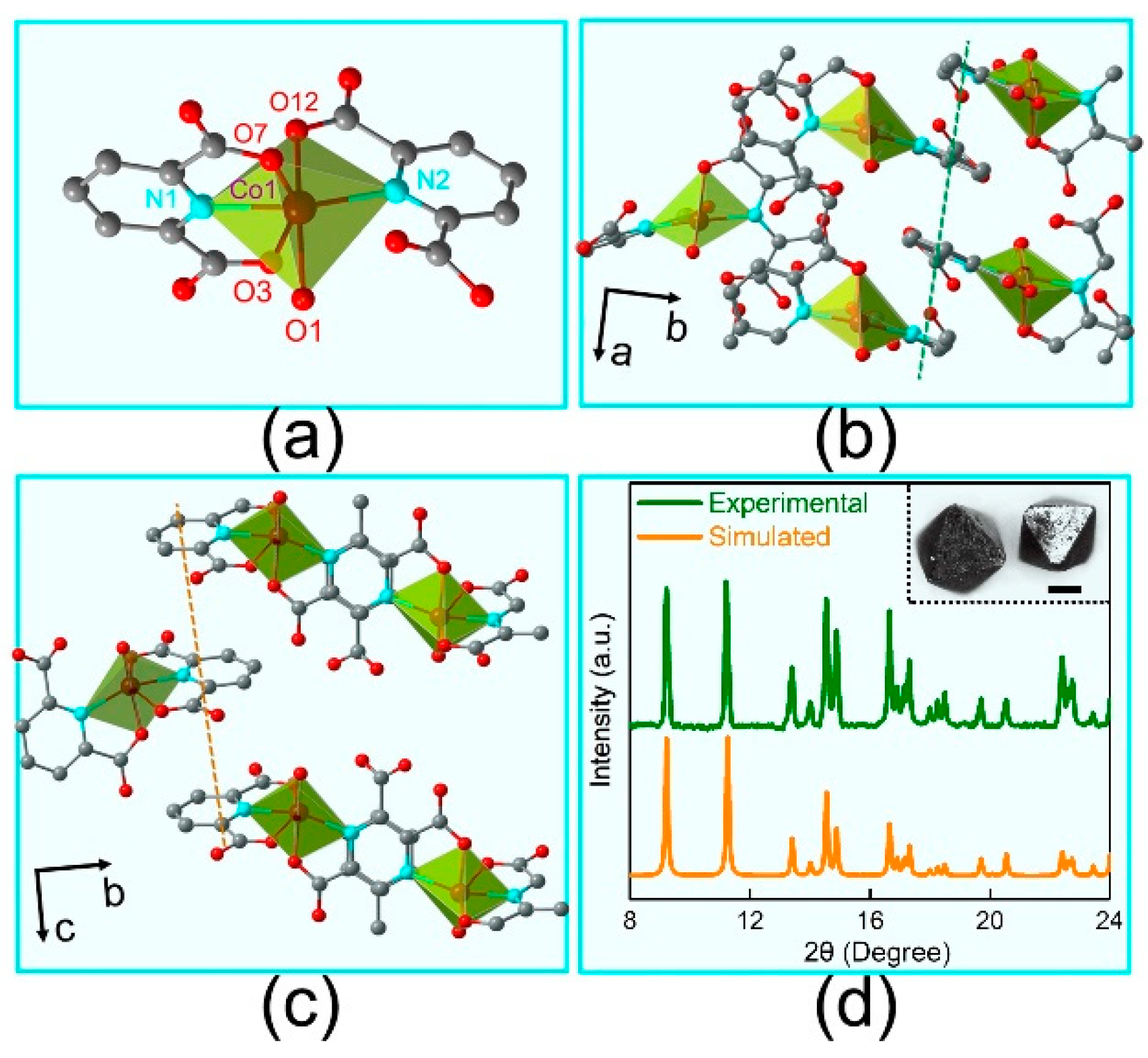
3.1. Structure
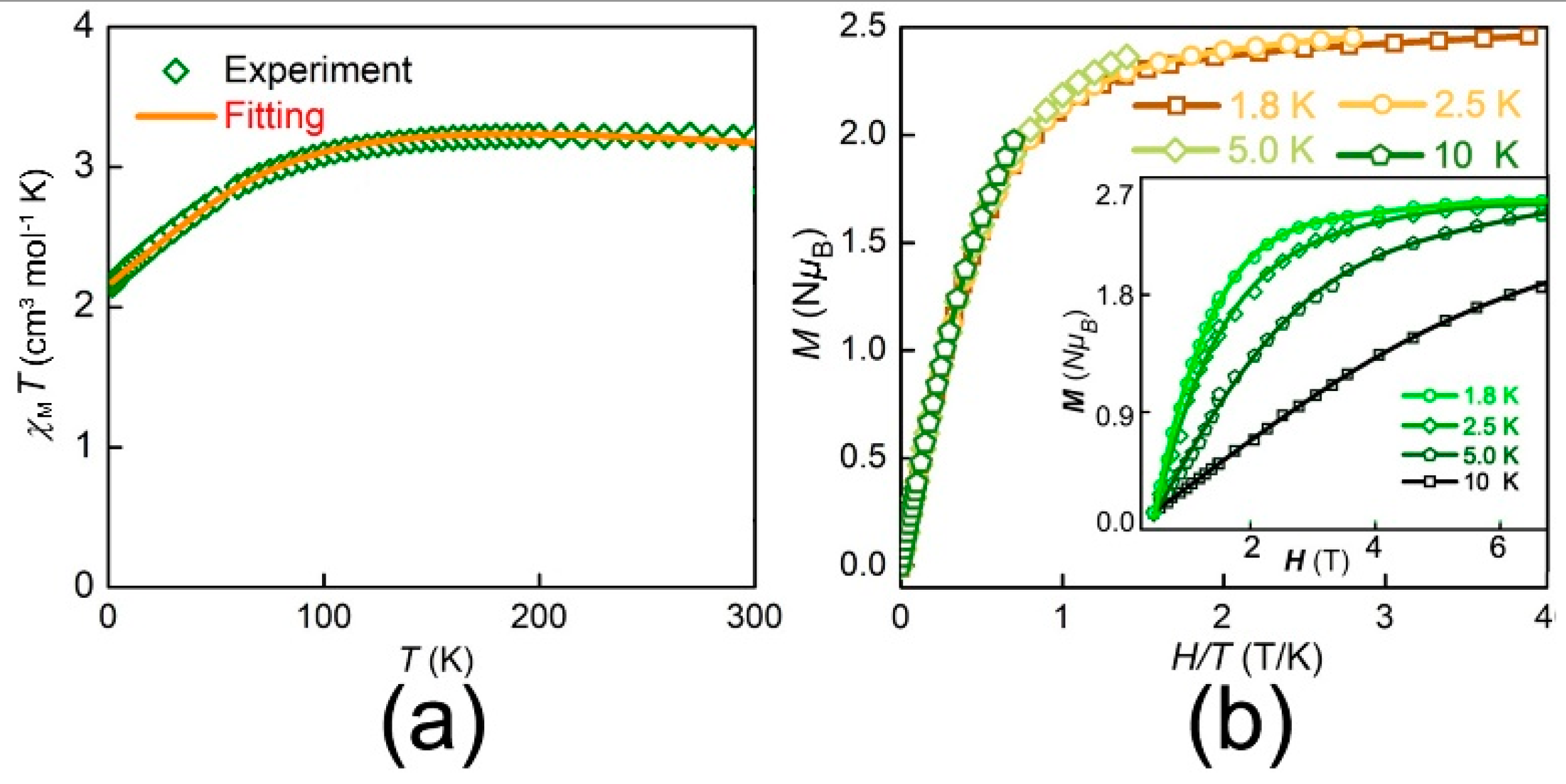
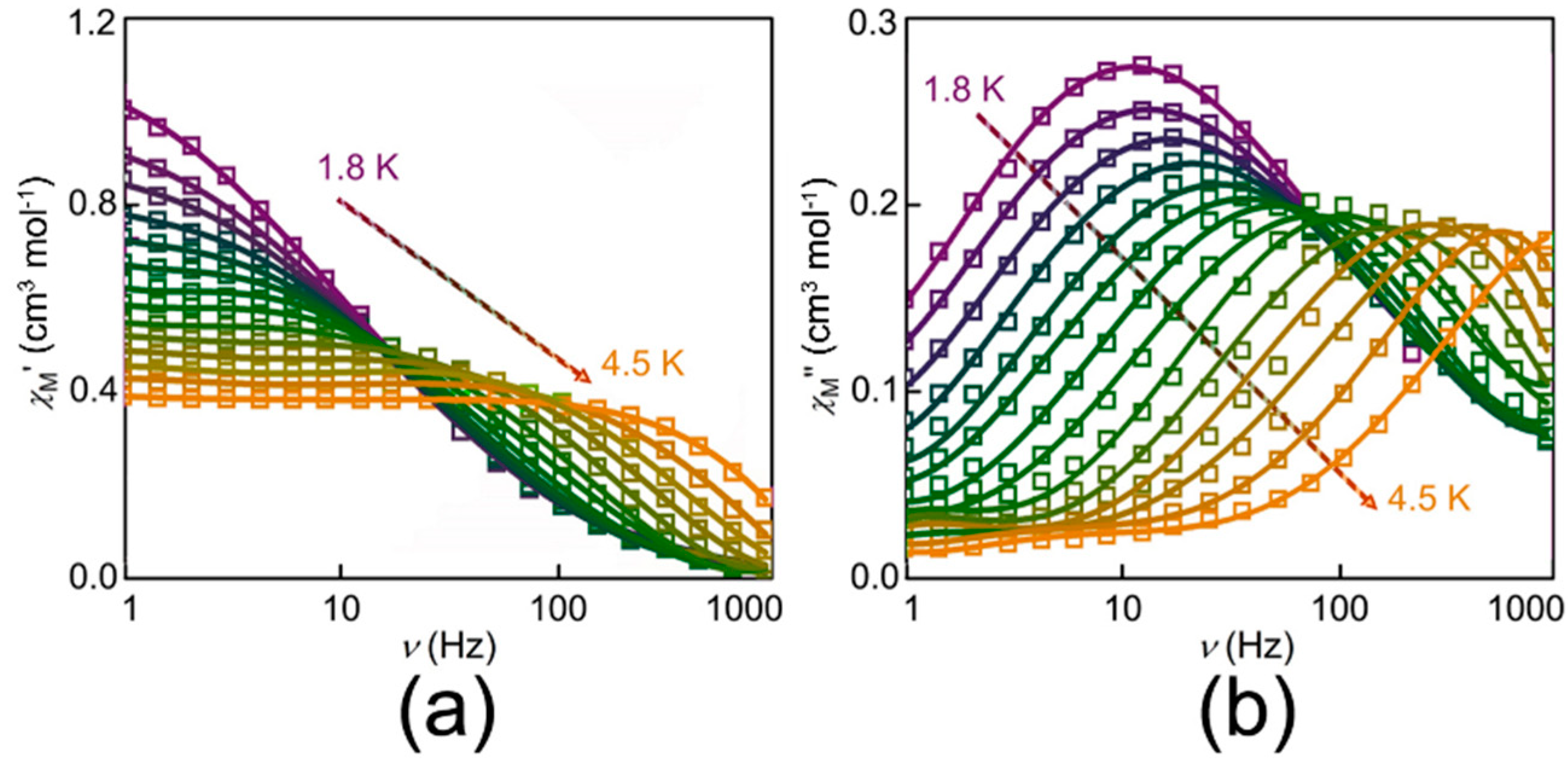
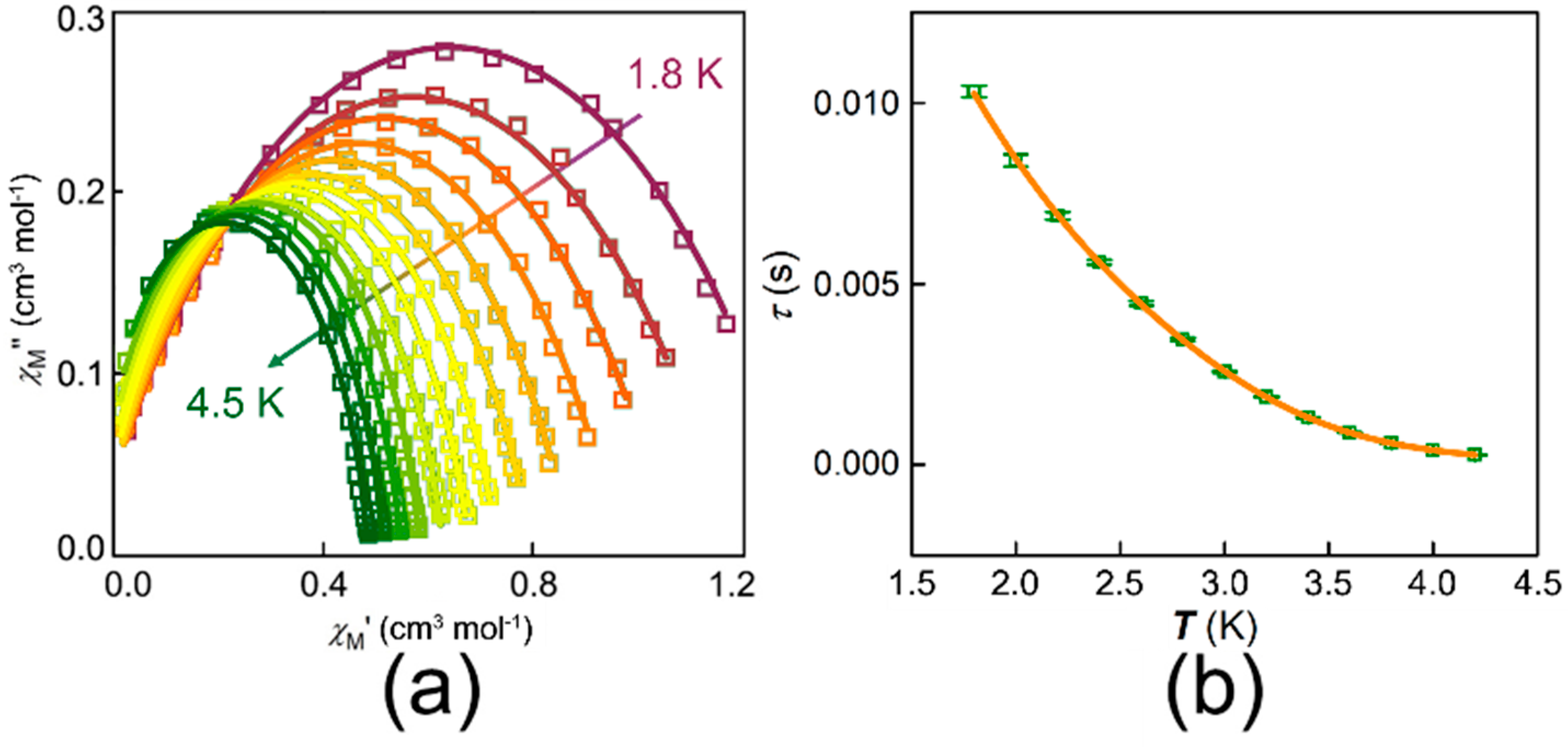
3.2. Magnetic Properties
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ishikawa, N.; Sugita, M.; Ishikawa, T.; Koshihara, S.Y.; Kaizu, Y. Lanthanide double-decker complexes functioning as magnets at the single-molecular level. J. Am. Chem. Soc. 2003, 125, 8694–8695. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, X.L.; Wang, T.W.; Song, Y.; You, X.Z. Slow relaxation processes and single-ion magnetic behaviors in dysprosium-containing complexes. Inorg. Chem. 2010, 49, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.S.; Chen, Y.; Hu, Z.B.; Long, Q.Q.; Yin, C.L.; Zhang, Z.C.; Pan, Z.Q. A Dy-based complex with the magnetic relaxation behavior regulated by enclosing one DyIII ion into a Calixarene ligand. Inorg. Chem. Commun. 2019, 105, 76–81. [Google Scholar] [CrossRef]
- Yu, S.; Chen, Z.; Hu, H.; Li, B.; Liang, Y.; Liu, D.; Zou, H.; Yao, D.; Liang, F. Two mononuclear Dysprosium(III) complexes with their slow magnetic relaxation behaviors tuned by coordination geometry. Dalton Trans. 2019, 48, 16679–16686. [Google Scholar] [CrossRef]
- Chen, Z.; Yu, S.; Wang, R.; Li, B.; Yin, B.; Liu, D.; Liang, Y.; Yao, D.; Liang, F. Three Dy(III) single-ion magnets bearing the tropolone ligand: Structure, magnetic properties and theoretical elucidation. Dalton Trans. 2019, 48, 6627–6637. [Google Scholar] [CrossRef]
- Liu, J.; Chen, Y.C.; Liu, J.L.; Vieru, V.; Ungur, L.; Jia, J.H.; Chibotaru, L.; Lan, F.Y.; Wernsdorfer, W.; Gao, S.; et al. A stable pentagonal bipyramidal Dy(III) single-ion magnet with a record magnetization reversal barrier over 1000 K. J. Am. Chem. Soc. 2016, 138, 5441–5450. [Google Scholar] [CrossRef]
- Li, J.; Yuan, C.; Yang, L.; Kong, M.; Zhang, J.; Ge, J.Y.; Zhang, Y.Q.; Song, Y. Magnetic anisotropy along a series of lanthanide polyoxometalates with pentagonal bipyramidal symmetry. Inorg. Chem. 2017, 56, 7835–7841. [Google Scholar] [CrossRef]
- Guo, F.S.; Day, B.M.; Chen, Y.C.; Tong, M.L.; Mansikkamäki, A.; Layfield, R.A. Magnetic hysteresis up to 80 kelvin in a dysprosium metallocene single-molecule magnet. Science 2018, 362, 1400–1403. [Google Scholar] [CrossRef] [Green Version]
- Murrie, M. Cobalt(II) single-molecule magnets. Chem. Soc. Rev. 2010, 39, 1986–1995. [Google Scholar] [CrossRef]
- Craig, G.A.; Murrie, M. 3d single-ion magnets. Chem. Soc. Rev. 2015, 44, 2135–2147. [Google Scholar] [CrossRef] [Green Version]
- Yao, X.N.; Du, J.Z.; Zhang, Y.Q.; Leng, X.B.; Yang, M.W.; Jiang, S.D.; Wang, Z.X.; Ouyang, Z.W.; Deng, L.; Wang, B.W.; et al. Two-coordinate Co(II) imido complexes as outstanding single molecule magnets. J. Am. Chem. Soc. 2017, 139, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.F.; Han, T.; Yin, B.; Zheng, Y.Z. On balancing the QTM and the direct relaxation processes in single-ion magnets—The importance of symmetry control. Inorg. Chem. Front. 2017, 4, 1141–1148. [Google Scholar] [CrossRef]
- Yao, X.; Yan, P.F.; An, G.H.; Li, Y.X.; Li, W.Z.; Li, G.M. Investigation of magneto-structural correlation based on a series of seven-coordinated -diketone Dy(III) single-ion magnets with C2v and C3v local symmetry. Dalton Trans. 2018, 47, 3976–3984. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.K.; Feng, J.Q.; Ren, M.; Gu, Y.W.; Song, Y.; Ward, M.D. A mononuclear cobalt(II)-dithienylethene complex showing slow magnetic relaxation and photochromic behavior. Chem. Commun. 2013, 49, 8863–8865. [Google Scholar] [CrossRef]
- Hu, Z.B.; Feng, X.; Li, J.; Zhang, Y.Q.; Yin, L.; Wang, Z.; Ouyang, Z.; Kurmoo, M.; Song, Y. Optimal diamagnetic dilution concentration for suppressing the dipole-dipole interaction in single-ion magnets. Dalton Trans. 2020, 49, 2159–2167. [Google Scholar] [CrossRef]
- Vallejo, J.; Castro, I.; Ruiz-García, R.; Cano, J.; Julve, M.; Lloret, F.; De Munno, G.; Wernsdorfer, W.; Pardo, E. Field-induced slow magnetic relaxation in a six-coordinate mononuclear cobalt(II) complex with a positive anisotropy. J. Am. Chem. Soc. 2012, 134, 15704–15707. [Google Scholar] [CrossRef]
- Colacio, E.; Ruiz, J.; Ruiz, E.; Cremades, E.; Krzystek, J.; Carretta, S.; Cano, J.; Guidi, T.; Wernsdorfer, W.; Brechinm, E.K. Slow magnetic relaxation in a CoIIYIII single-ion magnet with positive axial zero-field splitting. Angew. Chem. Int. Ed. 2013, 52, 9130–9134. [Google Scholar] [CrossRef] [Green Version]
- Hu, Z.B.; Jing, Z.Y.; Li, M.M.; Yin, L.; Gao, Y.D.; Yu, F.; Hu, T.P.; Wang, Z.; Song, Y. Important Role of Intermolecular Interaction in Cobalt(II) Single-Ion Magnet from Single Slow Relaxation to Double Slow Relaxation. Inorg. Chem. 2018, 57, 10761–10767. [Google Scholar] [CrossRef]
- Huang, X.C.; Zhou, C.D.; Shao, J.; Wang, X.Y. Field-induced slow magnetic relaxation in Cobalt(II) compounds with pentagonal bipyramid geometry. Inorg. Chem. 2014, 53, 12671–12673. [Google Scholar] [CrossRef]
- Chen, L.; Wang, J.; Wei, J.M.; Wernsdorfer, W.; Chen, X.T.; Zhang, Y.Q.; Song, Y.; Xue, Z.L. Slow magnetic relaxation in a mononuclear eight-coordinate Cobalt(II)complex. J. Am. Chem. Soc. 2014, 136, 12213–12216. [Google Scholar] [CrossRef]
- Zhang, J.; Li, J.; Yang, L.; Yuan, C.; Zhang, Y.Q.; Song, Y. Magnetic anisotropy from trigonal prismatic to trigonal antiprismatic Co(II) complexes:experimental observation and theoretical prediction. Inorg. Chem. 2018, 57, 3903–3912. [Google Scholar] [CrossRef] [PubMed]
- Bunting, P.C.; Atanasov, M.E.; Damgaard-Møller, M.; Perfetti, I.; Crassee, M.; Orlita, J.; Overgaard, M.; Slageren-Van, J.; Neese, F.; Long, J.R. A linear cobalt(II) complex with maximal orbital angular momentum from a non-Aufbau ground state. Science 2018, 362, 7319. [Google Scholar] [CrossRef] [PubMed]
- Woods, T.J.; Ballesteros-Rivas, M.F.; Gómez-Coca, S.; Ruiz, E.; Dunbar, K.R. Relaxation dynamics of identical trigonal bipyramidal Cobalt molecules with different local symmetries and packing arrangements: Magnetostructural correlations and ab inito calculations. J. Am. Chem. Soc. 2016, 138, 16407–16416. [Google Scholar] [CrossRef] [Green Version]
- Collet, A.; Craig, G.A.; Heras-Ojea, M.J.; Bhaskaran, L.; Wilson, C.; Hill, S.; Murrie, M. Slow magnetic relaxation in a {(CoIICoIII2)} complex containing a high magnetic anisotropy trigonal bipyramidal CoII centre. Dalton Trans. 2018, 47, 9237–9240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, X.; Wang, X.; Liu, X.; Wang, J.; Tang, L.; Ju, P. Fine-tuning the effects of auxiliary ligands on two trigonal-bipyramid cobalt(II) complexes exhibiting field-induced slow magnetic relaxation. New J. Chem. 2018, 42, 8583–8590. [Google Scholar] [CrossRef]
- Cui, H.H.; Wang, J.; Chen, X.T.; Xue, Z.L. Slow magnetic relaxation in five-coordinate spin-crossover cobalt(II) complexes. Chem. Commun. 2017, 53, 9304–9307. [Google Scholar] [CrossRef]
- Shaffer, D.; Bhowmick, W.I.; Rheingold, A.L.; Tsay, C.; Livesay, B.N.; Shores, M.P.; Yang, J.Y. Spin-state diversity in a series of Co(II) PNP pincer bromide complexes. Dalton Trans. 2016, 45, 17910–17917. [Google Scholar] [CrossRef]
- Brachňaková, B.; Matejová, S.; Moncol, J.; Herchel, R.; Pavlik, J.; Moreno-Pineda, E.; Ruben, M.; Šalitroš, I. Stereochemistry of coordination polyhedra vs. single ion magnetism in penta- and hexacoordinated Co(II) complexes with tridentate rigid ligands. Dalton Trans. 2020, 49, 1249–1264. [Google Scholar] [CrossRef]
- Shao, F.; Cahier, B.; Guihéry, N.; Rivière, E.; Guillot, R.; Barra, A.L.; Lan, Y.; Wernsdorfer, W.; Campbell, V.E.; Mallah, T. Tuning the Ising-type anisotropy in trigonal bipyramidal Co(II) complexes. Chem. Commun. 2015, 51, 16475–16478. [Google Scholar] [CrossRef]
- Ruamps, R.L.; Batchelor, J.; Guillot, R.; Zakhia, G.; Barra, A.L.; Wernsdorfer, W.; Guihéry, N.; Mallah, T. Ising-type magnetic anisotropy and single molecule magnet behaviour in mononuclear trigonal bipyramidal Co(II) complexes. Chem. Sci. 2014, 5, 3418–3424. [Google Scholar] [CrossRef]
- Nemec, I.; Herchel, R.; Trávníček, Z. Ferromagnetic coupling mediated by Co center dot center dot center dot pi non-covalent contacts in a pentacoordinate Co(II) compound showing field-induced slow relaxation of magnetization. Dalton Trans. 2016, 45, 12479–12482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajnák, C.; Titiš, J.; Fuhr, O.; Ruben, M.; Boča, R. Single-molecule magnetism in a pentacoordinate Cobalt(II) complex supported by an antenna ligand. Inorg. Chem. 2014, 53, 8200–8202. [Google Scholar] [CrossRef] [PubMed]
- Mondal, A.K.; Goswami, T.; Misra, A.; Konar, S. Probing the effects of ligand field and coordination geometry on magnetic anisotropy of pentacoordinate Cobalt(II) single-ion magnets. Inorg. Chem. 2017, 56, 6870–6878. [Google Scholar] [CrossRef] [PubMed]
- Świtlicka, A.; Machura, B.; Penkala, M.; Bieńko, A.; Bieńko, D.C.; Titiš, J.; Rajnák, C.; Boča, R.; Ozarowski, A.; Ozerov, M. Slow magnetic relaxation in Cobalt(II) field-induced single-ion magnets with positive large anisotropy. Inorg. Chem. 2018, 57, 12740–12755. [Google Scholar] [CrossRef]
- Schweinfurt, D.; Sommer, M.G.; Atanasov, M.; Demeshko, S.; Hohloch, S.; Meyer, F.; Neese, F.; Sarkar, B. The ligand field of the azido ligand: Insights into bonding parameters and magnetic anisotropy in a Co(II)-azido complex. J. Am. Chem. Soc. 2015, 137, 1993–2005. [Google Scholar] [CrossRef]
- Mondal, A.; Mondal, A.; Konar, S. Field induced single ion magnetic behaviour in square-pyramidal Cobalt(II) complexes with easy-plane magnetic anisotropy. Magnetochemistry 2019, 5, 12. [Google Scholar] [CrossRef] [Green Version]
- Mondal, A.K.; Jover, J.; Ruiz, E.; Konar, S. Investigation of easy-plane magnetic anisotropy in P-ligand square-pyramidal CoII single ion magnets. Chem. Commun. 2017, 53, 5338–5341. [Google Scholar] [CrossRef] [Green Version]
- Nemec, I.; Liu, H.; Herchel, R.; Zhang, X.; Trávníček, Z. Magnetic anisotropy in pentacoordinate 2,6-bis(arylazanylidene-1-chloromethyl)pyridine cobalt(II) complexes with chlorido co-ligands. Synth. Met. 2016, 215, 158–163. [Google Scholar] [CrossRef]
- Zeng, H.P.; Qi, W.; Zhai, L.X.; Wang, F.S.; Zhang, J.; Li, D. Preparation and characterization of sludge-based magnetic biochar by pyrolysis for methylene blue removal. Nanomaterials 2021, 11, 2473. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, X.; Wang, Y.; Quan, G.X.; Han, X.Y.; Yan, J.L. Facile synthesis of magnetic nitrogen-doped porous carbon from bimetallic metal–organic frameworks for efficient norfloxacin removal. Nanomaterials 2018, 8, 664. [Google Scholar] [CrossRef] [Green Version]
- Matias, I.A.; Ribeiro, A.C.; Ferraria, A.M.; Rego, A.M.; Martins, L.M. Catalytic performance of a magnetic core-shell Iron(II) c-scorpionate under unconventional oxidation conditions. Nanomaterials 2020, 10, 2111. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Dou, J.; Wang, D.; Li, D.; Gao, Z.; Chem, J. Synthesis and crystal structures of two new complexescenter dot [M(OH)2(HDPA)2]·3H2O (M = Mn, Co). J. Chem. Crystallogr. 2006, 36, 613–618. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history. Acta. Crystallogr. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Wu, S.; Zhu, X.; Shen, J.; Ji, K.; Zhang, R. Low-magnetic-feld controlled magnetodielectric coupling at room temperature in a chiral layered diphenylalanine-based 1D-coordination-polymer. J. Phys. D Appl. Phys. 2020, 53, 15LT01. [Google Scholar] [CrossRef]
- Jiang, Y.C.; He, A.P.; Zhao, R.; Chen, Y.; Liu, G.Z.; Lu, H.; Zhang, J.L.; Zhang, Q.; Wang, Z.; Zhao, C.; et al. Coexistence of photoelectric conversion and storage in van der Waals heterojunctions. Phys. Rev. Lett. 2021, 127, 217401. [Google Scholar] [CrossRef]
- Chilton, N.F.; Anderson, R.P.; Turner, L.D.; Soncini, A.; Murray, K.S. PHI: A powerful new program for the analysis of anisotropic monomeric and exchange-coupled polynuclear d- and f-block complexes. J. Comput. Chem. 2013, 34, 1164–1175. [Google Scholar] [CrossRef]
- Guo, Y.N.; Xu, G.F.; Guo, Y.; Tang, J. Relaxation dynamics of dysprosium(III) single molecule magnets. Dalton Trans. 2011, 40, 9953–9963. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [Green Version]
- Hohenberg, P.; Kohn, W. Inhomogeneous electron gas. Phys. Rev. B 1964, 136, B864–B865. [Google Scholar] [CrossRef] [Green Version]
- Kresse, G.; Furthmuller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Zhou, F.; Cococcioni, M.; Marianetti, C.A.; Morgan, D.; Ceder, G. First-principles prediction of redox potentials in transition-metal compounds with LDA + U. Phys. Rev. B: Condens. Matter Mater. Phys. 2004, 70, 235121. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, Z.; Li, Y.; Ji, C.; Jiang, Y.; Ma, C.; Gao, J.; Zhang, J. Slow-Relaxation Behavior of a Mononuclear Co(II) Complex Featuring Long Axial Co-O Bond. Nanomaterials 2022, 12, 707. https://doi.org/10.3390/nano12040707
Xia Z, Li Y, Ji C, Jiang Y, Ma C, Gao J, Zhang J. Slow-Relaxation Behavior of a Mononuclear Co(II) Complex Featuring Long Axial Co-O Bond. Nanomaterials. 2022; 12(4):707. https://doi.org/10.3390/nano12040707
Chicago/Turabian StyleXia, Zhengyao, Yan Li, Cheng Ji, Yucheng Jiang, Chunlan Ma, Ju Gao, and Jinlei Zhang. 2022. "Slow-Relaxation Behavior of a Mononuclear Co(II) Complex Featuring Long Axial Co-O Bond" Nanomaterials 12, no. 4: 707. https://doi.org/10.3390/nano12040707
APA StyleXia, Z., Li, Y., Ji, C., Jiang, Y., Ma, C., Gao, J., & Zhang, J. (2022). Slow-Relaxation Behavior of a Mononuclear Co(II) Complex Featuring Long Axial Co-O Bond. Nanomaterials, 12(4), 707. https://doi.org/10.3390/nano12040707





