SnO2 Anchored in S and N Co-Doped Carbon as the Anode for Long-Life Lithium-Ion Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
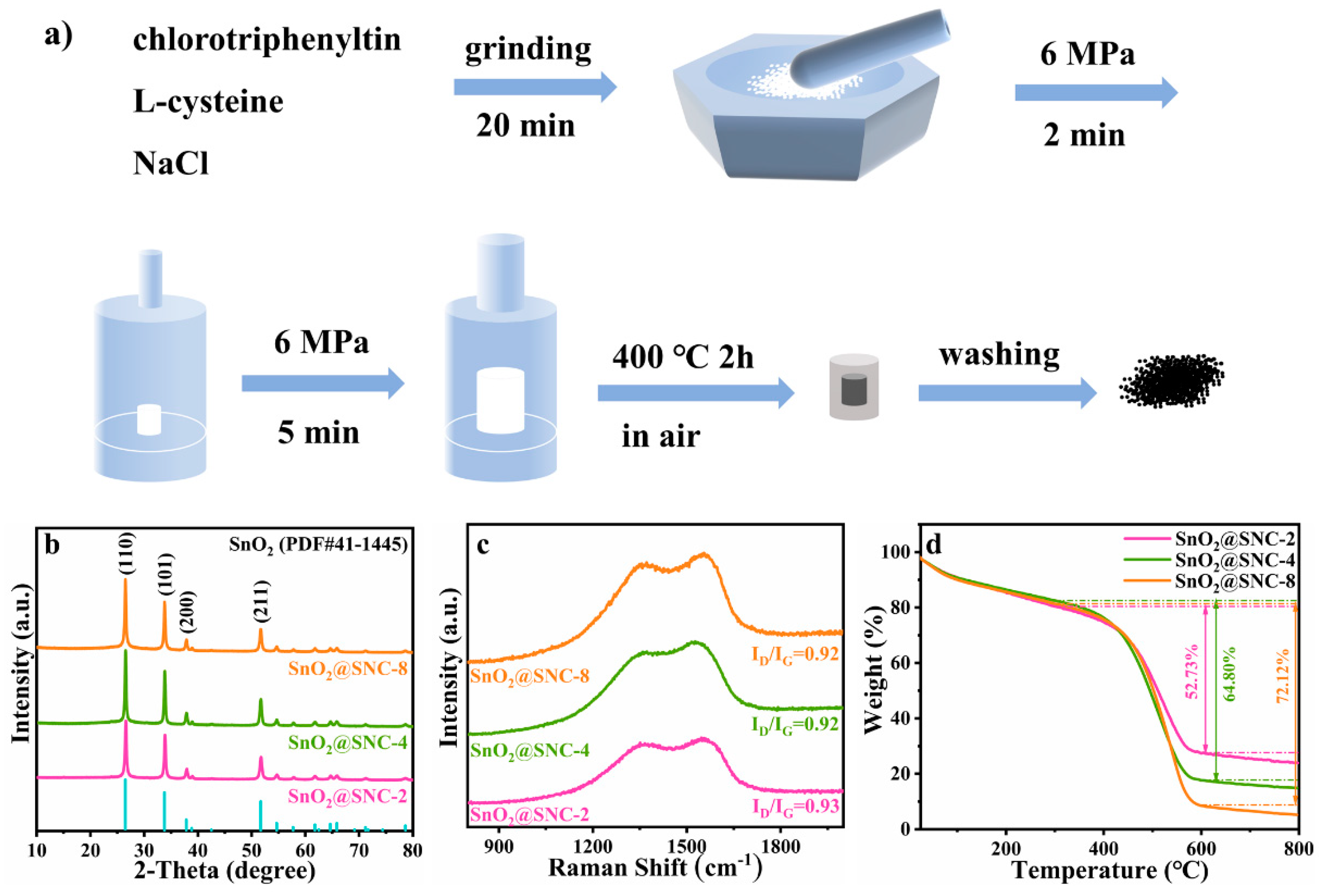
2.2. Synthesis of SnO2@SNC Composite Structures
2.3. Materials Characterization
2.4. Electrochemical Measurement
3. Results and Discussions
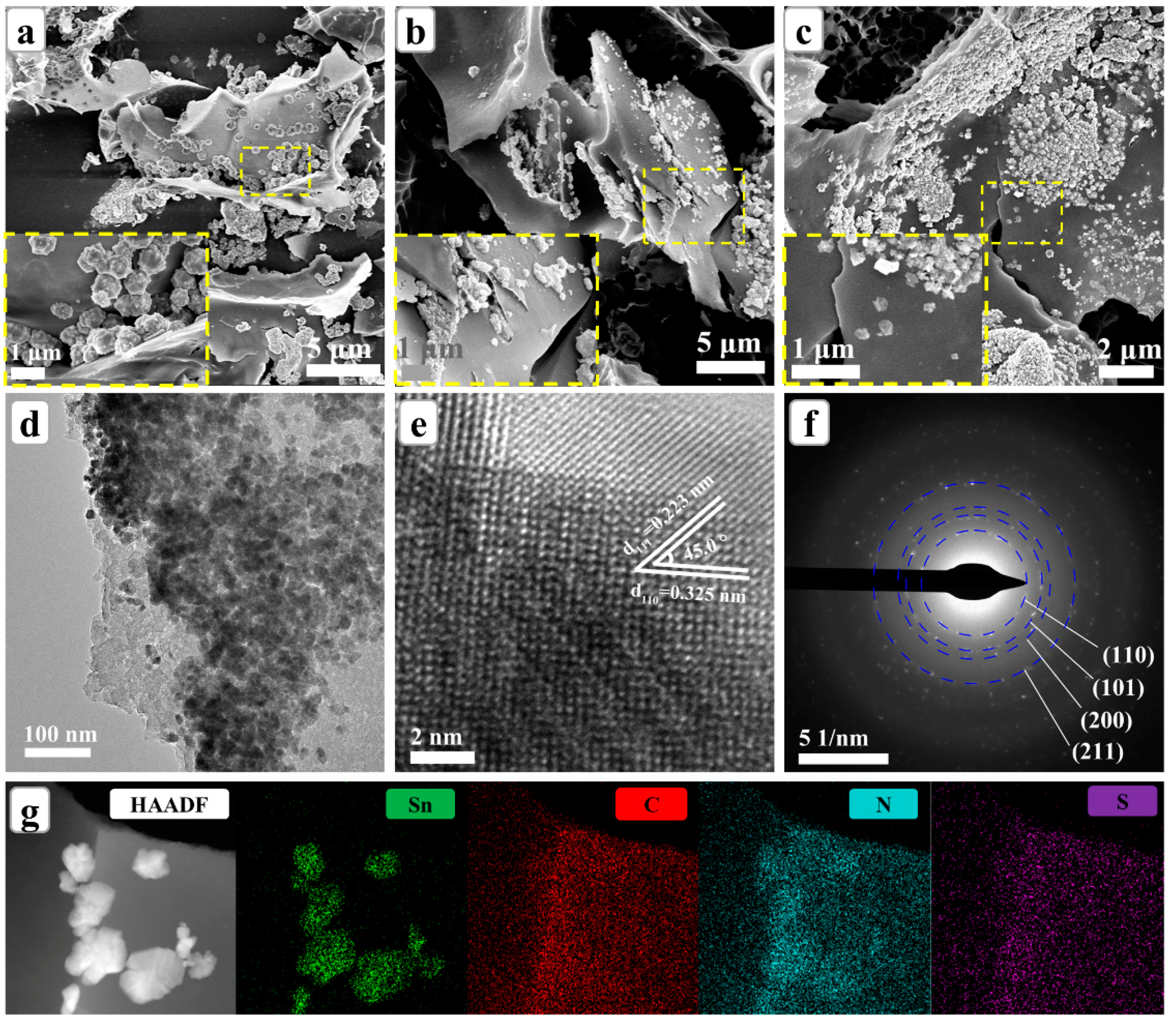
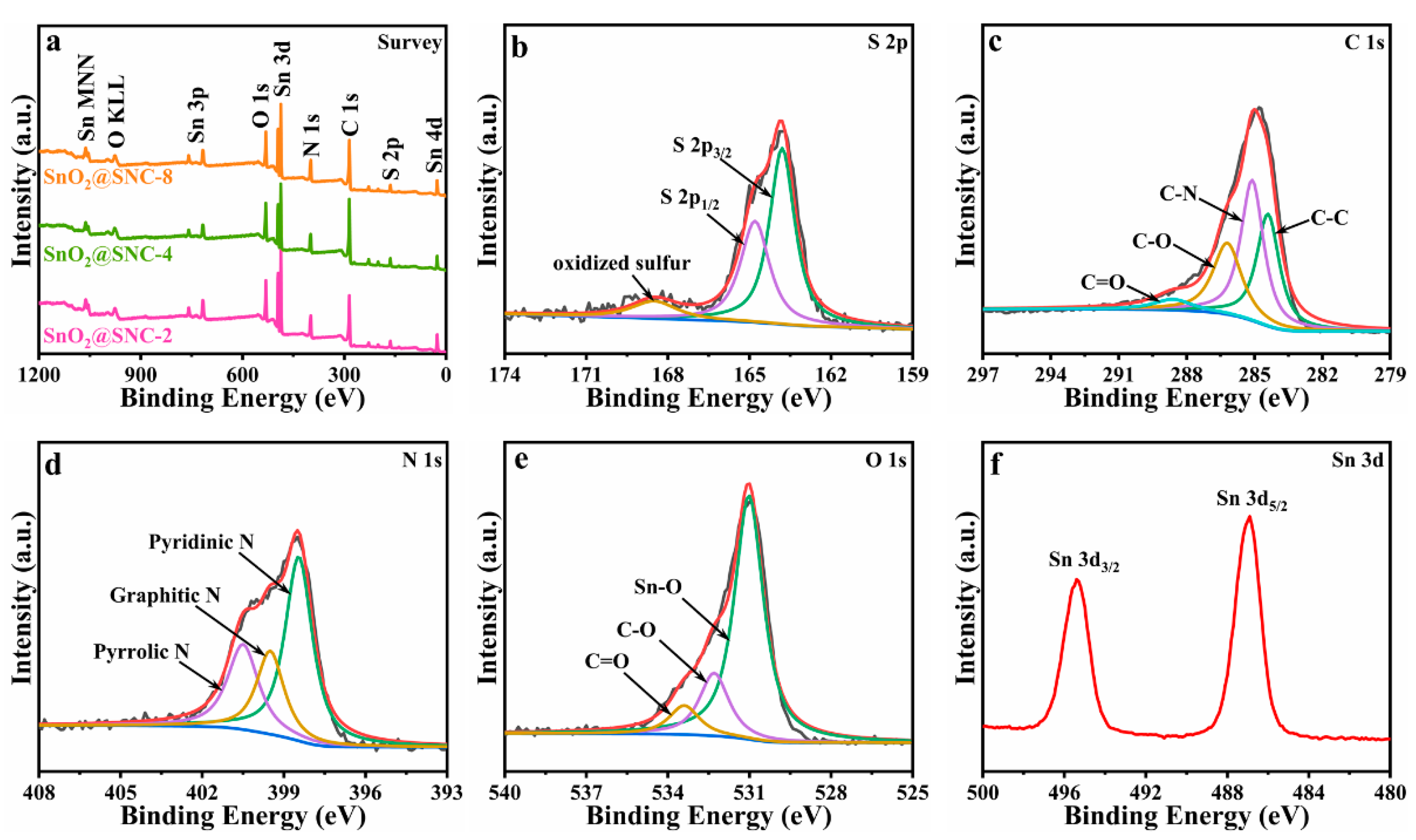
3.1. Composition and Microstructures of SnO2@SNC Composite Materials
3.2. Electrochemical Property in Half-Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, M.; Deng, Z.-P.; Zhang, X.-F.; Huo, L.-H.; Gao, S. Biomass-Derived Graphitic Carbon/Co3O4 Nanocomposites with Pseudocapacitance for Lithium Storage. ACS Appl. Nano Mater. 2021, 4, 1340–1350. [Google Scholar] [CrossRef]
- Bernard, P.; Alper, J.P.; Haon, C.; Herlin-Boime, N.; Chandesris, M. Electrochemical analysis of silicon nanoparticle lithiation–Effect of crystallinity and carbon coating quantity. J. Power Sources 2019, 435, 226769. [Google Scholar] [CrossRef]
- Chen, H.; He, J.; Ke, G.; Sun, L.; Chen, J.; Li, Y.; Ren, X.; Deng, L.; Zhang, P. MoS2 nanoflowers encapsulated into carbon nanofibers containing amorphous SnO2 as an anode for lithium-ion batteries. Nanoscale 2019, 11, 16253–16261. [Google Scholar] [CrossRef]
- Chen, R.; Xue, X.; Hu, Y.; Kong, W.; Lin, H.; Chen, T.; Jin, Z. Intermetallic SnSb nanodots embedded in carbon nanotubes reinforced nanofabric electrodes with high reversibility and rate capability for flexible Li-ion batteries. Nanoscale 2019, 11, 13282–13288. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Huang, Y.; Wang, M. Three-dimensional hierarchical porous MnCo2O4@MnO2 network towards highly reversible lithium storage by unique structure. Chem. Eng. J. 2019, 378, 122207. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, B.; Luo, H.; Jin, F.; Ruan, T.; Wang, D. MoO2 nanobelts modified with an MOF-derived carbon layer for high performance lithium-ion battery anodes. J. Alloys Compd. 2019, 803, 664–670. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, S.; Yang, J.; Bai, T.; Ren, Y.; Tian, H. Metal-Organic Frameworks Derived Okra-like SnO2 Encapsulated in Nitrogen-Doped Graphene for Lithium Ion Battery. ACS Appl. Mater. Interfaces 2017, 9, 14309–14318. [Google Scholar] [CrossRef]
- Zoller, F.; Bohm, D.; Bein, T.; Fattakhova-Rohlfing, D. Tin Oxide Based Nanomaterials and Their Application as Anodes in Lithium-Ion Batteries and Beyond. ChemSusChem 2019, 12, 4140–4159. [Google Scholar] [CrossRef] [Green Version]
- Zhao, S.; Sewell, C.D.; Liu, R.; Jia, S.; Wang, Z.; He, Y.; Yuan, K.; Jin, H.; Wang, S.; Liu, X.; et al. SnO2 as Advanced Anode of Alkali-Ion Batteries: Inhibiting Sn Coarsening by Crafting Robust Physical Barriers, Void Boundaries, and Heterophase Interfaces for Superior Electrochemical Reaction Reversibility. Adv. Energy Mater. 2019, 10, 1902657. [Google Scholar] [CrossRef]
- Zhang, S.; Liang, B.; Fan, Y.; Wang, J.; Liang, X.; Huang, H.; Huang, D.; Zhou, W.; Guo, J. Ferrocene as a Novel Additive to Enhance the Lithium-Ion Storage Capability of SnO2/Graphene Composite. ACS Appl. Mater. Interfaces 2019, 11, 31943–31953. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, S.; Zhou, L.; Chang, L.; Liu, W.; Yin, D.; Yi, Z.; Wang, L. SnO2 Quantum Dots: Rational Design to Achieve Highly Reversible Conversion Reaction and Stable Capacities for Lithium and Sodium Storage. Small 2020, 16, e2000681. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, C. Environment-friendly synthesis of carbon-encapsulated SnO2 core-shell nanocubes as high-performance anode materials for lithium ion batteries. Mater. Today Energy 2020, 16, 100406. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, H.B.; Liu, B.; Lou, X.W. Formation of porous SnO2 microboxes via selective leaching for highly reversible lithium storage. Energy Environ. Sci. 2014, 7, 1013–1017. [Google Scholar] [CrossRef]
- Zhang, J.; Ren, H.; Wang, J.; Qi, J.; Yu, R.; Wang, D.; Liu, Y. Engineering of multi-shelled SnO2 hollow microspheres for highly stable lithium-ion batteries. J. Mater. Chem. A 2016, 4, 17673–17677. [Google Scholar] [CrossRef]
- Zhang, J.; Wan, J.; Wang, J.; Ren, H.; Yu, R.; Gu, L.; Liu, Y.; Feng, S.; Wang, D. Hollow Multi-Shelled Structure with Metal-Organic-Framework-Derived Coatings for Enhanced Lithium Storage. Angew. Chem. Int. Ed. Engl. 2019, 58, 5266–5271. [Google Scholar] [CrossRef]
- Wu, N.; Du, W.; Gao, X.; Zhao, L.; Liu, G.; Liu, X.; Wu, H.; He, Y.B. Hollow SnO2 nanospheres with oxygen vacancies entrapped by a N-doped graphene network as robust anode materials for lithium-ion batteries. Nanoscale 2018, 10, 11460–11466. [Google Scholar] [CrossRef]
- Zhang, F.; Teng, X.; Shi, W.; Song, Y.; Zhang, J.; Wang, X.; Li, H.; Li, Q.; Li, S.; Hu, H. SnO2 nanoflower arrays on an amorphous buffer layer as binder-free electrodes for flexible lithium-ion batteries. Appl. Surf. Sci. 2020, 527, 146910. [Google Scholar] [CrossRef]
- Kamali, A.R.; Fray, D.J. Solid phase growth of tin oxide nanostructures. Mater. Sci. Eng. B 2012, 177, 819–825. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, L.P.; Xi, S.; Du, Y.; Yao, Q.; Guan, L.; Xu, Z.J. Encapsulating porous SnO2 into a hybrid nanocarbon matrix for long lifetime Li storage. J. Mater. Chem. A 2017, 5, 25609–25617. [Google Scholar] [CrossRef]
- Xie, W.; Wang, Q.; Xu, J.; Yu, Y.; Zhao, R.; Li, N.; Li, M.; Du, Y.; Peng, S.; Cao, G. Microbelt–void–microbelt-structured SnO2@C as an advanced electrode with outstanding rate capability and high reversibility. J. Mater. Chem. A 2019, 7, 10523–10533. [Google Scholar] [CrossRef]
- He, Z.-K.; Sun, Q.; Xie, K.; Lu, P.; Shi, Z.; Kamali, A.R. Reactive molten salt synthesis of natural graphite flakes decorated with SnO2 nanorods as high performance, low cost anode material for lithium ion batteries. J. Alloy. Compd. 2019, 792, 1213–1222. [Google Scholar] [CrossRef]
- Shin, H.-J.; Kim, T.H.; Abbas, S.; Cho, J.; Ha, H.Y. Petal-shaped SnO2 free-standing electrodes with electrically conducting layers via a plasma-activated nitrogen doping process for high performance lithium-ion batteries. Chem. Eng. J. 2021, 412, 128614. [Google Scholar] [CrossRef]
- Cai, D.; Wang, C.; Shi, C.; Tan, N. Facile synthesis of N and S co-doped graphene sheets as anode materials for high-performance lithium-ion batteries. J. Alloys Compd. 2018, 731, 235–242. [Google Scholar] [CrossRef]
- Dong, X.; Deng, Z.-P.; Huo, L.-H.; Zhang, X.-F.; Gao, S. Large-scale synthesis of NiS@N and S co-doped carbon mesoporous tubule as high performance anode for lithium-ion battery. J. Alloys Compd. 2019, 788, 984–992. [Google Scholar] [CrossRef]
- Dwivedi, P.K.; Nair, A.; Mehare, R.S.; Chaturvedi, V.; Joshi, K.; Shelke, M.V. Experimental and theoretical investigations of the effect of heteroatom-doped carbon microsphere supports on the stability and storage capacity of nano-Co3O4 conversion anodes for application in lithium-ion batteries. Nanoscale Adv. 2020, 2, 2914–2924. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, M.; Wang, Q.; Yang, J.; Luo, X.; Li, Y.; Du, R.; Yan, X.; Sun, X.; Dong, C.; et al. Pseudocapacitance boosted N-doped carbon coated Fe7S8 nanoaggregates as promising anode materials for lithium and sodium storage. Nano Res. 2020, 13, 691–700. [Google Scholar] [CrossRef]
- Yuan, Y.; Chen, Z.; Yu, H.; Zhang, X.; Liu, T.; Xia, M.; Zheng, R.; Shui, M.; Shu, J. Heteroatom-doped carbon-based materials for lithium and sodium ion batteries. Energy Storage Mater. 2020, 32, 65–90. [Google Scholar] [CrossRef]
- Shan, J.; Liu, Y.; Liu, P.; Huang, Y.; Su, Y.; Wu, D.; Feng, X. Nitrogen-doped carbon-encapsulated SnO2–SnS/graphene sheets with improved anodic performance in lithium ion batteries. J. Mater. Chem. A 2015, 3, 24148–24154. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Jiao, R.; Zuo, X.; Tang, R.; Su, H.; Xu, D.; Sun, D.; Zeng, S.; Zhang, X. Novel Bake-in-Salt Method for the Synthesis of Mesoporous Mn3O4@C Networks with Superior Cycling Stability and Rate Performance. ACS Appl. Mater. Interfaces 2016, 8, 35163–35171. [Google Scholar] [CrossRef]
- Zhou, F.; Li, S.; Han, K.; Li, Y.; Liu, Y.N. Polymerization inspired synthesis of MnO@carbon nanowires with long cycling stability for lithium ion battery anodes: Growth mechanism and electrochemical performance. Dalton Trans. 2021, 50, 535–545. [Google Scholar] [CrossRef]
- Hao, J.; Bai, J.; Wang, X.; Wang, Y.; Guo, Q.; Yang, Y.; Zhao, J.; Chi, C.; Li, Y. S, O dual-doped porous carbon derived from activation of waste papers as electrodes for high performance lithium ion capacitors. Nanoscale Adv. 2021, 3, 738–746. [Google Scholar] [CrossRef]
- Yao, W.; Wu, S.; Zhan, L.; Wang, Y. Two-dimensional porous carbon-coated sandwich-like mesoporous SnO2/graphene/mesoporous SnO2 nanosheets towards high-rate and long cycle life lithium-ion batteries. Chem. Eng. J. 2019, 361, 329–341. [Google Scholar] [CrossRef]
- Cheng, Y.; Huang, J.; Qi, H.; Cao, L.; Luo, X.; Li, J.; Xu, Z.; Yang, J. Controlling the Sn-C bonds content in SnO2@CNTs composite to form in situ pulverized structure for enhanced electrochemical kinetics. Nanoscale 2017, 9, 18681–18689. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Chen, Z.; Hou, J.; Xu, T.; Liu, D.; Leng, S.; Guo, H.; Chen, C.; Yang, J.; Wu, M. SnO2 nano-crystals anchored on N-doped porous carbon with enhanced lithium storage properties. Appl. Surf. Sci. 2020, 515, 145902. [Google Scholar] [CrossRef]
- Tian, Q.; Chen, Y.; Chen, F.; Chen, J.; Yang, L. Walnut core-like hollow carbon micro/nanospheres supported SnOx@C composite for high performance lithium-ion battery anode. J. Colloid Interface Sci. 2019, 554, 424–432. [Google Scholar] [CrossRef]
- Tian, Q.; Chen, Y.; Sui, Z.; Chen, J.; Yang, L. The sandwiched buffer zone enables porous SnO2@C micro-/nanospheres to toward high-performance lithium-ion battery anodes. Electrochim. Acta 2020, 354, 136699. [Google Scholar] [CrossRef]
- Cheng, Y.; Huang, J.; Qi, H.; Cao, L.; Yang, J.; Xi, Q.; Luo, X.; Yanagisawa, K.; Li, J. Adjusting the Chemical Bonding of SnO2 @CNT Composite for Enhanced Conversion Reaction Kinetics. Small 2017, 13, 656. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, L.; Zhao, K.; Yan, W.; Liu, M.; Wei, D.; Xi, L.; Zhang, J. 3D branched rutile TiO2 @ rutile SnO2 nanorods array heteroarchitectures/carbon cloth with an adjustable band gap to enhance lithium storage reaction kinetics for flexible lithium-ion batteries. Electrochim. Acta 2020, 354, 136727. [Google Scholar] [CrossRef]
- Liu, X.; Zhu, S.; Liang, Y.; Li, Z.; Wu, S.; Luo, S.; Chang, C.; Cui, Z. 3D N-doped mesoporous carbon/SnO2 with polypyrrole coating layer as high-performance anode material for Li-ion batteries. J. Alloys Compd. 2022, 892, 162083. [Google Scholar] [CrossRef]
- Yang, D.; Ren, H.; Wu, D.; Zhang, W.; Lou, X.; Wang, D.; Cao, K.; Gao, Z.; Xu, F.; Jiang, K. Bi-functional nitrogen-doped carbon protective layer on three-dimensional RGO/SnO2 composites with enhanced electron transport and structural stability for high-performance lithium-ion batteries. J. Colloid Interface Sci. 2019, 542, 81–90. [Google Scholar] [CrossRef]
- Han, J.; Zheng, J. Coaxial single-walled CNT@SnO2@N-doped carbon with high rate capability and cycling stability for lithium ion batteries. Solid State Ion. 2021, 369, 115723. [Google Scholar] [CrossRef]
- Fang, S.; Tong, Z.; Zhang, X. 3D nitrogen-doped carbon foam supported Ge@C composite as anode for high performance lithium-ion battery. Chem. Eng. J. 2017, 322, 188–195. [Google Scholar] [CrossRef]
- Wei, J.-L.; Wang, Z.-Y.; Sun, Y.-H.; Zhang, G.-L.; Guan, D.-C.; Nan, J.-M. The kinetics investigation of nitrogen/sulfur co-doped reduced graphene oxide@spinel SnFe2O4/Sn0.205Fe1.727O3 as high performance anode for lithium-ion batteries and its application in full cells. Electrochim. Acta 2021, 375, 138026. [Google Scholar] [CrossRef]
- Liu, B.; Liu, Z.; Li, D.; Guo, P.; Liu, D.; Shang, X.; Lv, M.; He, D. Nanoscale α-MnS crystallites grown on N-S co-doped rGO as a long-life and high-capacity anode material of Li-ion batteries. Appl. Surf. Sci. 2017, 416, 858–867. [Google Scholar] [CrossRef]
- Liang, X.; Wang, J.; Zhang, S.; Wang, L.; Wang, W.; Li, L.; Wang, H.; Huang, D.; Zhou, W.; Guo, J. Fabrication of uniform Si-incorporated SnO2 nanoparticles on graphene sheets as advanced anode for Li-ion batteries. Appl. Surf. Sci. 2019, 476, 28–35. [Google Scholar] [CrossRef]



| SnO2@SNC−8 | SnO2 | |
|---|---|---|
| Rct (Ω) | 166.9 | 391.5 |
| Rs (Ω) | 2.286 | 6.569 |
| Zw (Ω s−1/2) | 127.3 | 320.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, S.; Zhou, H.; Zhang, Y.; Zhu, K.; Zhai, Y.; Wei, D.; Zeng, S. SnO2 Anchored in S and N Co-Doped Carbon as the Anode for Long-Life Lithium-Ion Batteries. Nanomaterials 2022, 12, 700. https://doi.org/10.3390/nano12040700
Zhou S, Zhou H, Zhang Y, Zhu K, Zhai Y, Wei D, Zeng S. SnO2 Anchored in S and N Co-Doped Carbon as the Anode for Long-Life Lithium-Ion Batteries. Nanomaterials. 2022; 12(4):700. https://doi.org/10.3390/nano12040700
Chicago/Turabian StyleZhou, Shuli, Hongyan Zhou, Yunpeng Zhang, Keke Zhu, Yanjun Zhai, Denghu Wei, and Suyuan Zeng. 2022. "SnO2 Anchored in S and N Co-Doped Carbon as the Anode for Long-Life Lithium-Ion Batteries" Nanomaterials 12, no. 4: 700. https://doi.org/10.3390/nano12040700
APA StyleZhou, S., Zhou, H., Zhang, Y., Zhu, K., Zhai, Y., Wei, D., & Zeng, S. (2022). SnO2 Anchored in S and N Co-Doped Carbon as the Anode for Long-Life Lithium-Ion Batteries. Nanomaterials, 12(4), 700. https://doi.org/10.3390/nano12040700





