A pH Dual-Responsive Multifunctional Nanoparticle Based on Mesoporous Silica with Metal-Polymethacrylic Acid Gatekeeper for Improving Plant Protection and Nutrition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Pro@BMMs−PMAA/Fe3+ Nps
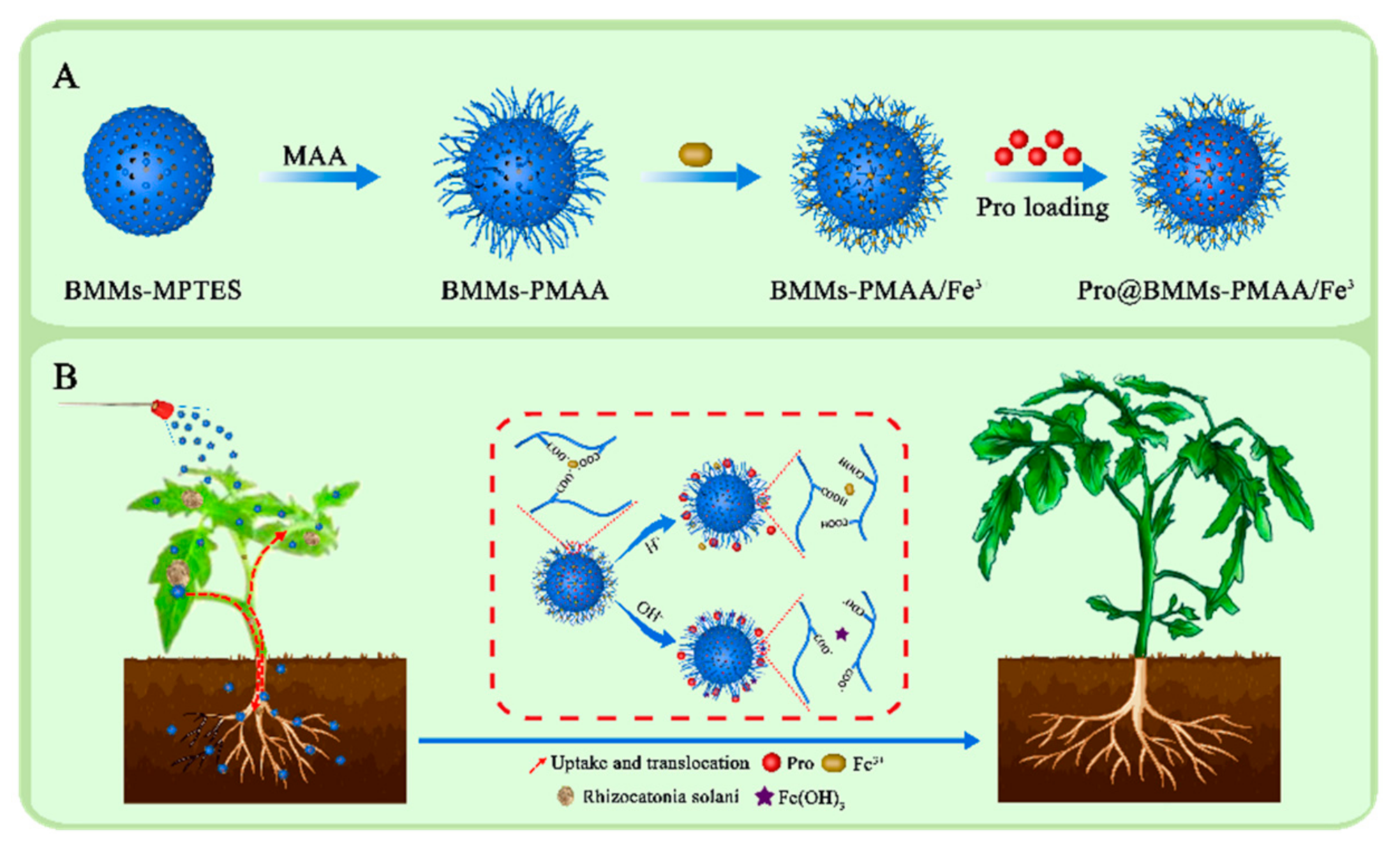
2.2.1. Synthesis of BMMs
2.2.2. Synthesis of Poly-Methacrylic Acid-Coated BMMs (BMMs−PMAA)
2.2.3. Synthesis of Pesticide-Loaded Iron-Chelated Nps (Pro@BMMs−PMAA/Fe3+)
2.3. Preparation of 5-AF-Functionalized Nps (BMMs−PMAA/5-AF)
2.4. Characterizations of Pro@BMMs−PMAA/Fe3+ Nps
2.5. Pro Loading Content
2.6. In Vitro Release Behavior of Pro
2.7. Stability Test
2.7.1. Storage Stability of Pro@BMMs−PMAA/Fe3+ Nps
2.7.2. Photostability of Pro@BMMs−PMAA/Fe3+ Nps
2.8. Bioactivity Evaluation
2.9. Uptake and Translocation of Nps
2.10. Nutritional Function of Pro@BMMs−PMAA/Fe3+ Nps
2.11. Biosafety Evaluation
2.12. Data Analysis
3. Results and Discussion
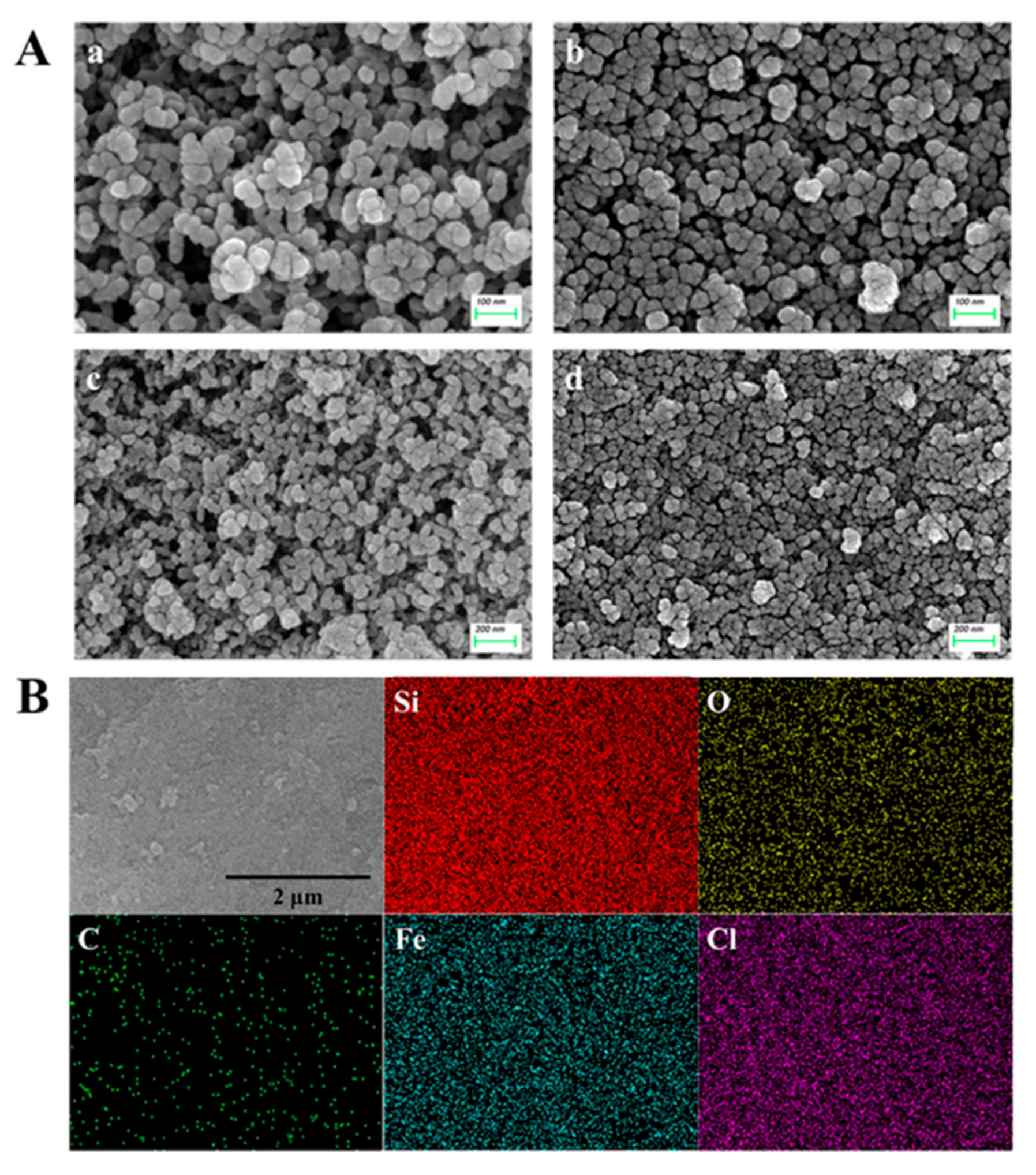
3.1. Preparation and Characterization of Pro@BMMs−PMAA/Fe3+ Nps
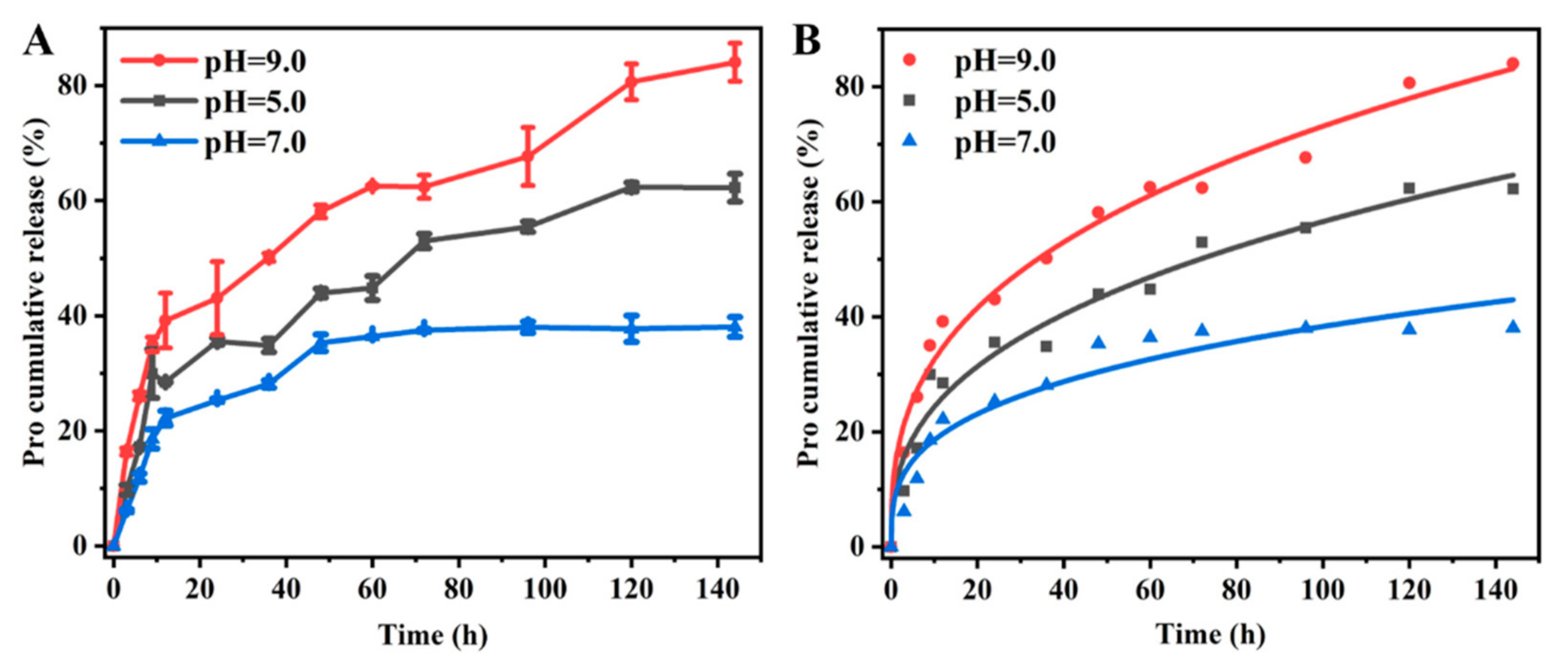
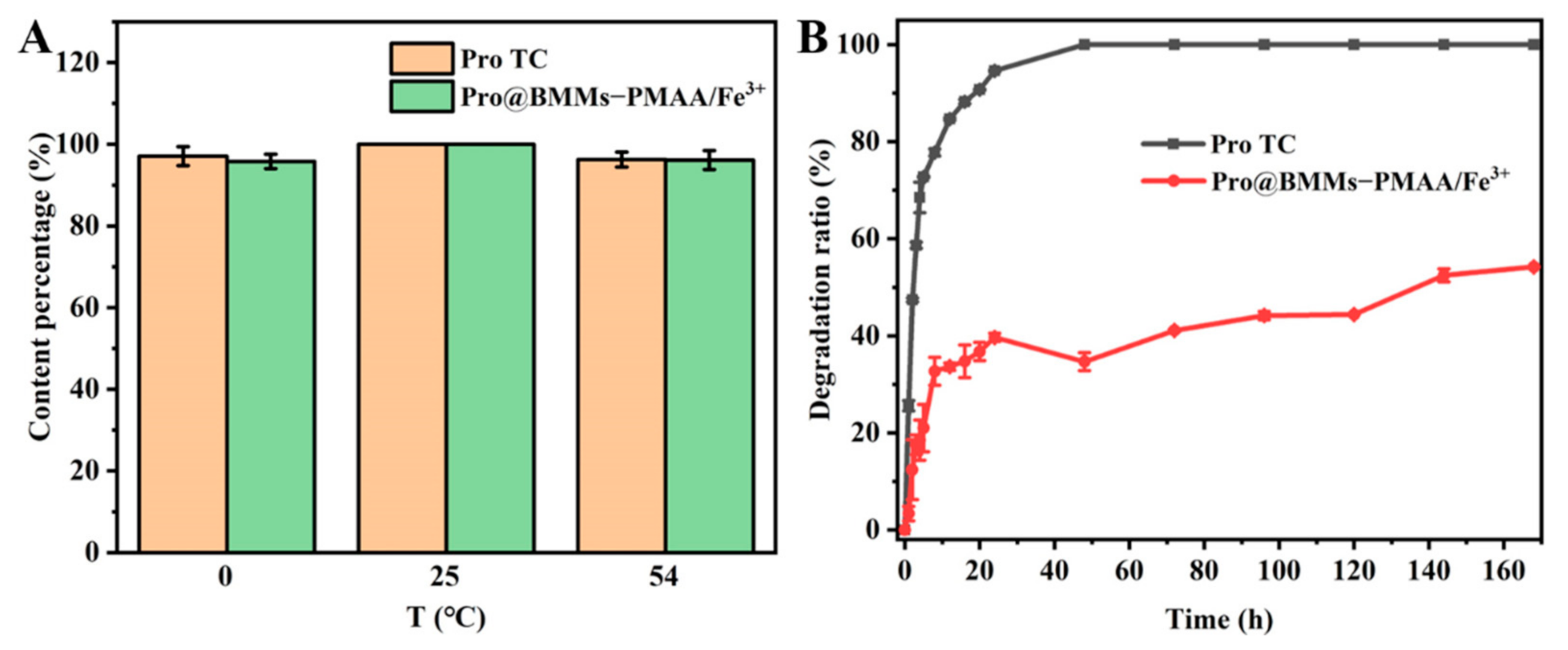
3.2. Release Behavior
3.3. Stability Study
3.4. Bioactivity Evaluation
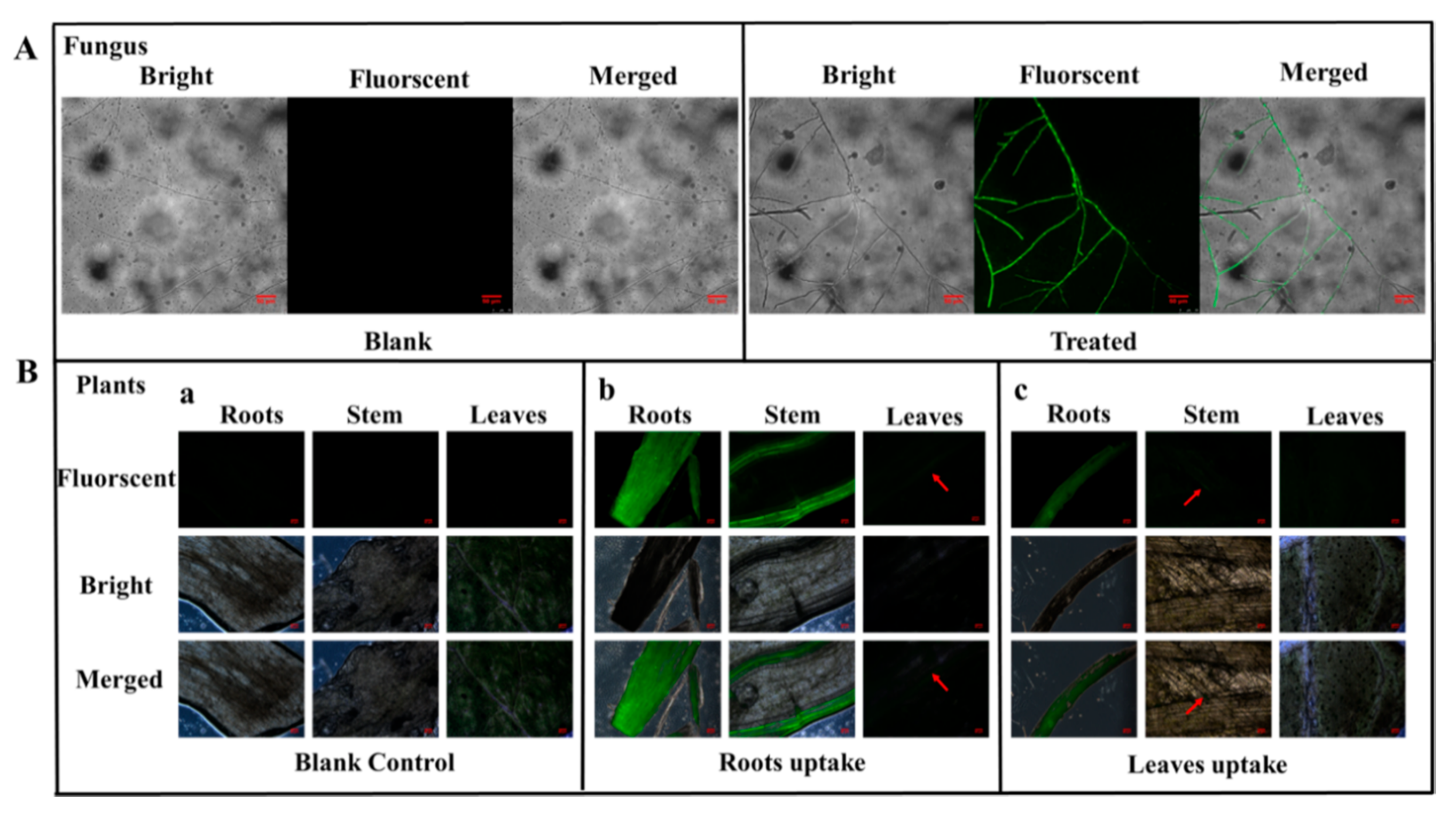
3.5. Uptake and Translocation of BMMs−PMAA
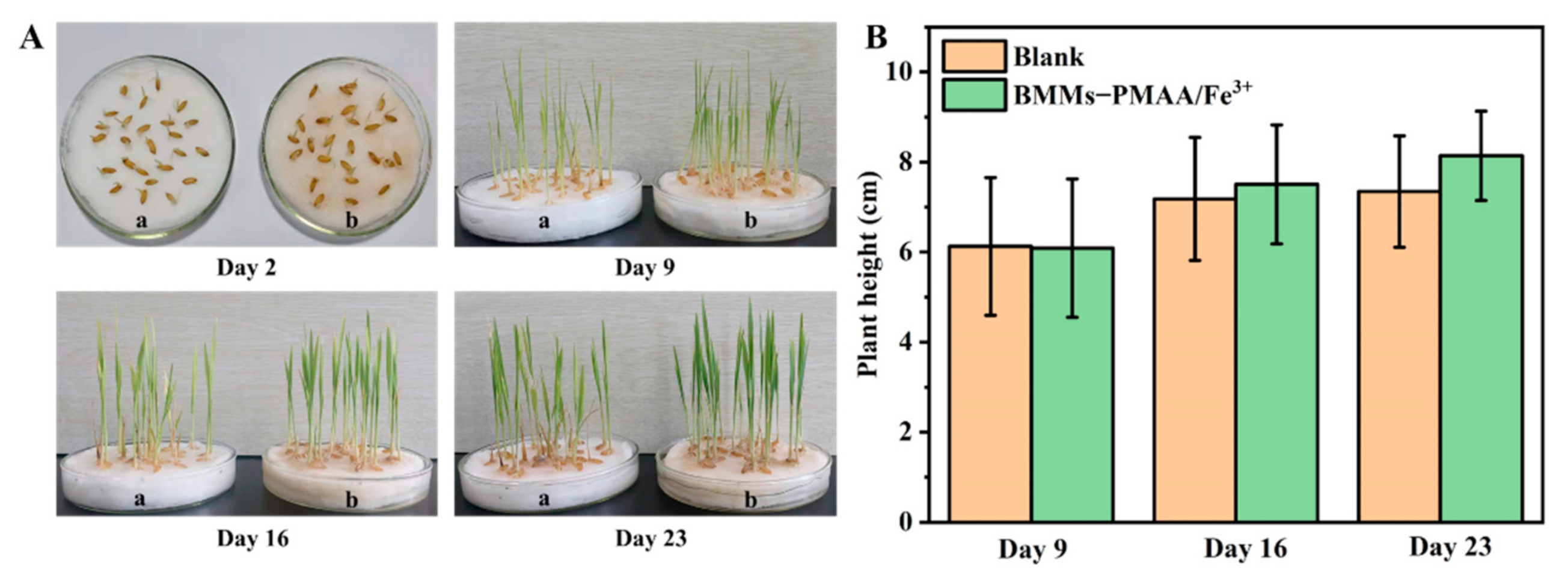
3.6. Nutritional Function of BMMs−PMAA/Fe3+
3.7. Biosafety Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Godfray, H.C.; Beddington, J.R.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Pretty, J.; Robinson, S.; Thomas, S.M.; Toulmin, C. Food security: The challenge of feeding 9 billion people. Science 2010, 327, 812–818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camara, M.C.; Campos, E.V.R.; Monteiro, R.A.; do Espirito Santo Pereira, A.; de Freitas Proenca, P.L.; Fraceto, L.F. Development of stimuli-responsive nano-based pesticides: Emerging opportunities for agriculture. J. Nanobiotechnol. 2019, 17, 100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, X.; Cui, H.; Wang, Y.; Sun, C.; Cui, B.; Zeng, Z. Development Strategies and Prospects of Nano-based Smart Pesticide Formulation. J. Agric. Food Chem. 2018, 66, 6504–6512. [Google Scholar] [CrossRef] [PubMed]
- Lamberth, C.; Jeanmart, S.; Luksch, T.; Plant, A. Current challenges and trends in the discovery of agrochemicals. Science 2013, 341, 742–746. [Google Scholar] [CrossRef] [PubMed]
- Adisa, I.O.; Pullagurala, V.L.R.; Peralta-Videa, J.R.; Dimkpa, C.O.; Elmer, W.H.; Gardea-Torresdey, J.L.; White, J.C. Recent advances in nano-enabled fertilizers and pesticides: A critical review of mechanisms of action. Environ. Sci. Nano 2019, 6, 2002–2030. [Google Scholar] [CrossRef]
- Kah, M.; Tufenkji, N.; White, J.C. Nano-enabled strategies to enhance crop nutrition and protection. Nat. Nanotechnol. 2019, 14, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, T.; Lowry, G.V.; Ghoshal, S.; Tufenkji, N.; Brambilla, D.; Dutcher, J.R.; Gilbertson, L.M.; Giraldo, J.P.; Kinsella, J.M.; Landry, M.P.; et al. Technology readiness and overcoming barriers to sustainably implement nanotechnology-enabled plant agriculture. Nat. Food 2020, 1, 416–425. [Google Scholar] [CrossRef]
- Nuruzzaman, M.; Rahman, M.M.; Liu, Y.; Naidu, R. Nanoencapsulation, Nano-guard for Pesticides: A New Window for Safe Application. J. Agric. Food Chem. 2016, 64, 1447–1483. [Google Scholar] [CrossRef]
- Kumar, S.; Nehra, M.; Dilbaghi, N.; Marrazza, G.; Hassan, A.A.; Kim, K.-H. Nano-based smart pesticide formulations: Emerging opportunities for agriculture. J. Control. Release 2019, 294, 131–153. [Google Scholar] [CrossRef]
- Kookana, R.S.; Boxall, A.B.; Reeves, P.T.; Ashauer, R.; Beulke, S.; Chaudhry, Q.; Cornelis, G.; Fernandes, T.F.; Gan, J.; Kah, M.; et al. Nanopesticides: Guiding principles for regulatory evaluation of environmental risks. J. Agric. Food Chem. 2014, 62, 4227–4240. [Google Scholar] [CrossRef] [Green Version]
- Li, N.; Sun, C.; Jiang, J.; Wang, A.; Wang, C.; Shen, Y.; Huang, B.; An, C.; Cui, B.; Zhao, X.; et al. Advances in Controlled-Release Pesticide Formulations with Improved Efficacy and Targetability. J. Agric. Food Chem. 2021, 69, 12579–12597. [Google Scholar] [CrossRef] [PubMed]
- Beckers, S.J.; Staal, A.H.J.; Rosenauer, C.; Srinivas, M.; Landfester, K.; Wurm, F.R. Targeted Drug Delivery for Sustainable Crop Protection: Transport and Stability of Polymeric Nanocarriers in Plants. Adv. Sci. 2021, 8, e2100067. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.; Zhou, H.; Hao, L.; Chen, H.; Xu, H.; Zhou, X. Enzyme cum pH dual-responsive controlled release of avermectin from functional polydopamine microcapsules. Colloids Surf. B 2020, 186, 110699–1106701. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhu, X.; Chen, X.; Liu, Y.; Gong, Y.; Yuan, G.; Liu, J.; Chen, L. Defense and inhibition integrated mesoporous nanoselenium delivery system against tomato gray mold. Environ. Sci. Nano 2020, 7, 210–227. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, J.; Chen, C.; Wang, D.; Tian, G.; Zhang, G.; Cai, D.; Wu, Z. Infrared-Light-Responsive Controlled-Release Pesticide Using Hollow Carbon Microspheres@Polyethylene Glycol/alpha-Cyclodextrin Gel. J. Agric. Food Chem. 2021, 69, 6981–6988. [Google Scholar] [CrossRef]
- Song, S.; Jiang, X.; Shen, H.; Wu, W.; Shi, Q.; Wan, M.; Zhang, J.; Mo, H.; Shen, J. MXene (Ti3C2) Based Pesticide Delivery System for Sustained Release and Enhanced Pest Control. ACS Appl. Bio Mater. 2021, 4, 6912–6923. [Google Scholar] [CrossRef]
- Dong, J.; Chen, W.; Feng, J.; Liu, X.; Xu, Y.; Wang, C.; Yang, W.; Du, X. Facile, Smart, and Degradable Metal-Organic Framework Nanopesticides Gated with Fe (III)-Tannic Acid Networks in Response to Seven Biological and Environmental Stimuli. ACS Appl. Mater. Interfaces 2021, 13, 19507–19520. [Google Scholar] [CrossRef]
- Huang, G.; Deng, Y.; Zhang, Y.; Feng, P.; Xu, C.; Fu, L.; Lin, B. Study on long-term pest control and stability of double-layer pesticide carrier in indoor and outdoor environment. Chem. Eng. J. 2021, 403, 126342. [Google Scholar] [CrossRef]
- Xia, X.; Shi, B.; Wang, L.; Liu, Y.; Zou, Y.; Zhou, Y.; Chen, Y.; Zheng, M.; Zhu, Y.; Duan, J.; et al. From mouse to mouse-ear cress: Nanomaterials as vehicles in plant biotechnology. Exploration 2021, 1, 9–20. [Google Scholar] [CrossRef]
- Avellan, A.; Yun, J.; Morais, B.P.; Clement, E.T.; Rodrigues, S.M.; Lowry, G.V. Critical Review: Role of Inorganic Nanoparticle Properties on Their Foliar Uptake and in Planta Translocation. Environ. Sci. Technol. 2021, 55, 13417–13431. [Google Scholar] [CrossRef]
- Kong, X.P.; Zhang, B.H.; Wang, J. Multiple Roles of Mesoporous Silica in Safe Pesticide Application by Nanotechnology: A Review. J. Agric. Food Chem. 2021, 69, 6735–6754. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Sun, J.; Li, Y.; Zhang, L. Bimodal mesoporous silicas functionalized with different level and species of the amino groups for adsorption and controlled release of aspirin. J. Nanosci. Nanotechnol. 2011, 11, 6690–6697. [Google Scholar] [CrossRef] [PubMed]
- Avellan, A.; Yun, J.; Zhang, Y.; Spielman-Sun, E.; Unrine, J.M.; Thieme, J.; Li, J.; Lombi, E.; Bland, G.; Lowry, G.V. Nanoparticle Size and Coating Chemistry Control Foliar Uptake Pathways, Translocation, and Leaf-to-Rhizosphere Transport in Wheat. ACS Nano 2019, 13, 5291–5305. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, J.; Zhan, X.; Li, A.; White, J.C.; Gardea-Torresdey, J.L.; Xing, B. Role of Charge and Size in the Translocation and Distribution of Zinc Oxide Particles in Wheat Cells. ACS Sustain. Chem. Eng. 2021, 9, 11556–11564. [Google Scholar] [CrossRef]
- Wu, H.; Hu, P.; Xu, Y.; Xiao, C.; Chen, Z.; Liu, X.; Jia, J.; Xu, H. Phloem Delivery of Fludioxonil by Plant Amino Acid Transporter-Mediated Polysuccinimide Nanocarriers for Controlling Fusarium Wilt in Banana. J. Agric. Food Chem. 2021, 69, 2668–2678. [Google Scholar] [CrossRef]
- Zhang, Y.; Fu, L.; Li, S.; Yan, J.; Sun, M.; Giraldo, J.P.; Matyjaszewski, K.; Tilton, R.D.; Lowry, G.V. Star Polymer Size, Charge Content, and Hydrophobicity Affect their Leaf Uptake and Translocation in Plants. Environ. Sci. Technol. 2021, 55, 10758–10768. [Google Scholar] [CrossRef]
- Li, W.; Wang, Q.; Zhang, F.; Shang, H.; Bai, S.; Sun, J. pH-sensitive thiamethoxam nanoparticles based on bimodal mesoporous silica for improving insecticidal efficiency. R. Soc. Open Sci. 2021, 8, 201967. [Google Scholar] [CrossRef]
- Li, W.; Xu, X.; Pan, H.; Wu, L.; Bai, S.; Sun, J.; Zhang, F. Comparative study on two different methods for fabrication of sustained release boscalid based on mesoporous silica. Mater. Res. Express 2021, 8, 045018. [Google Scholar] [CrossRef]
- Shan, Y.; Cao, L.; Muhammad, B.; Xu, B.; Zhao, P.; Cao, C.; Huang, Q. Iron-based porous metal-organic frameworks with crop nutritional function as carriers for controlled fungicide release. J. Colloid Interface Sci. 2020, 566, 383–393. [Google Scholar] [CrossRef]
- Xu, C.; Cao, L.; Bilal, M.; Cao, C.; Zhao, P.; Zhang, H.; Huang, Q. Multifunctional manganese-based carboxymethyl chitosan hydrogels for pH-triggered pesticide release and enhanced fungicidal activity. Carbohydr. Polym. 2021, 262, 117933. [Google Scholar] [CrossRef]
- Ji, Y.; Ma, S.; Lv, S.; Wang, Y.; Lu, S.; Liu, M. Nanomaterials for Targeted Delivery of Agrochemicals by an All-in-One Combination Strategy and Deep Learning. ACS Appl. Mater. Interfaces 2021, 13, 43374–43386. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Shan, Y.; Bilal, M.; Xu, B.; Cao, L.; Huang, Q. Copper ions chelated mesoporous silica nanoparticles via dopamine chemistry for controlled pesticide release regulated by coordination bonding. Chem. Eng. J. 2020, 395, 125093. [Google Scholar] [CrossRef]
- Liang, Y.; Song, J.; Dong, H.; Huo, Z.; Gao, Y.; Zhou, Z.; Tian, Y.; Li, Y.; Cao, Y. Fabrication of pH-responsive nanoparticles for high efficiency pyraclostrobin delivery and reducing environmental impact. Sci. Total Environ. 2021, 787, 147422. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Zhao, X. Glutathione-Induced Structural Transform of Double-Cross-Linked PEGylated Nanogel for Efficient Intracellular Anticancer Drug Delivery. Mol. Pharm. 2019, 16, 2826–2837. [Google Scholar] [CrossRef] [PubMed]
- Tarn, D.; Yu, C.-J.; Lu, J.; Hartz, A.; Tamanoi, F.; Zink, J.I. In vitro delivery of calcium ions by nanogated mesoporous silica nanoparticles to induce cancer cellular apoptosis. Mol. Syst. Des. Eng. 2017, 2, 384–392. [Google Scholar] [CrossRef]
- Zhao, P.; Cao, L.; Ma, D.; Zhou, Z.; Huang, Q.; Pan, C. Translocation, distribution and degradation of prochloraz-loaded mesoporous silica nanoparticles in cucumber plants. Nanoscale 2018, 10, 1798–1806. [Google Scholar] [CrossRef] [PubMed]
- Ning, Y.; Zhang, X.; Li, B.; Wang, Y.; Guo, J. Distribution of Cd and Cu Fractions in Chinese Soils and Their Relationships with Soil pH: A Meta-Analysis. Sustainability 2019, 11, 337. [Google Scholar] [CrossRef] [Green Version]
- Liang, W.; Xie, Z.; Cheng, J.; Xiao, D.; Xiong, Q.; Wang, Q.; Zhao, J.; Gui, W. A Light-Triggered pH-Responsive Metal-Organic Framework for Smart Delivery of Fungicide to Control Sclerotinia Diseases of Oilseed Rape. ACS Nano 2021, 15, 6987–6997. [Google Scholar] [CrossRef]
- Gao, Y.; Liang, Y.; Dong, H.; Niu, J.; Tang, J.; Yang, J.; Tang, G.; Zhou, Z.; Tang, R.; Shi, X.; et al. A Bioresponsive System Based on Mesoporous Organosilica Nanoparticles for Smart Delivery of Fungicide in Response to Pathogen Presence. ACS Sustain. Chem. Eng. 2020, 8, 5716–5723. [Google Scholar] [CrossRef]
- Baumann, L.; Knorr, S.; Keiter, S.; Nagel, T.; Segner, H.; Braunbeck, T. Prochloraz causes irreversible masculinization of zebrafish (Danio rerio). Environ. Sci. Pollut. Res. Int. 2015, 22, 16417–16422. [Google Scholar] [CrossRef]
- Abdelrahman, T.M.; Qin, X.; Li, D.; Senosy, I.A.; Mmby, M.; Wan, H.; Li, J.; He, S. Pectinase-responsive carriers based on mesoporous silica nanoparticles for improving the translocation and fungicidal activity of prochloraz in rice plants. Chem. Eng. J. 2021, 404, 126440. [Google Scholar] [CrossRef]
- Wang, Q.; Shen, M.; Li, W.; Li, W.; Zhang, F. Controlled-release of fluazinam from biodegradable PLGA-based microspheres. J. Environ. Sci. Health Part B 2019, 54, 810–816. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, A.; Wang, C.; Cui, B.; Sun, C.; Zhao, X.; Zeng, Z.; Shen, Y.; Gao, F.; Liu, G.; et al. Synthesis and characterization of emamectin-benzoate slow-release microspheres with different surfactants. Sci. Rep. 2017, 7, 12761–12769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, C.; Cao, L.; Zhao, P.; Zhou, Z.; Cao, C.; Li, F.; Huang, Q. Emulsion-based synchronous pesticide encapsulation and surface modification of mesoporous silica nanoparticles with carboxymethyl chitosan for controlled azoxystrobin release. Chem. Eng. J. 2018, 348, 244–254. [Google Scholar] [CrossRef]
- Tang, J.; Ding, G.; Niu, J.; Zhang, W.; Tang, G.; Liang, Y.; Fan, C.; Dong, H.; Yang, J.; Li, J.; et al. Preparation and characterization of tebuconazole metal-organic framework-based microcapsules with dual-microbicidal activity. Chem. Eng. J. 2019, 359, 225–232. [Google Scholar] [CrossRef]
- Qi, M.; Pan, H.; Shen, H.; Xia, X.; Wu, C.; Han, X.; He, X.; Tong, W.; Wang, X.; Wang, Q. Nanogel Multienzyme Mimics Synthesized by Biocatalytic ATRP and Metal Coordination for Bioresponsive Fluorescence Imaging. Angew. Chem. Int. Ed. Engl. 2020, 59, 11748–11753. [Google Scholar] [CrossRef]
- Mattos, B.D.; Greca, L.G.; Tardy, B.L.; Magalhaes, W.L.E.; Rojas, O.J. Green Formation of Robust Supraparticles for Cargo Protection and Hazards Control in Natural Environments. Small 2018, 14, e1801256. [Google Scholar] [CrossRef] [Green Version]
- Anstoetz, M.; Sharma, N.; Clark, M.; Yee, L.H. Characterization of an oxalate-phosphate-amine metal–organic framework (OPA-MOF) exhibiting properties suited for innovative applications in agriculture. J. Mater. Sci. 2016, 51, 9239–9252. [Google Scholar] [CrossRef]
- Xiang, Y.; Zhang, G.; Chi, Y.; Cai, D.; Wu, Z. Fabrication of a controllable nanopesticide system with magnetic collectability. Chem. Eng. J. 2017, 328, 320–330. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Y.; Li, W.; Ma, L.; Wang, E.; Xing, M.; Zhou, Y.; Huan, Z.; Guo, F.; Chang, J. Curcumin/Fe-SiO2 nano composites with multi-synergistic effects for scar inhibition and hair follicle regeneration during burn wound healing. Appl. Mater. Today 2021, 23, 101065. [Google Scholar] [CrossRef]
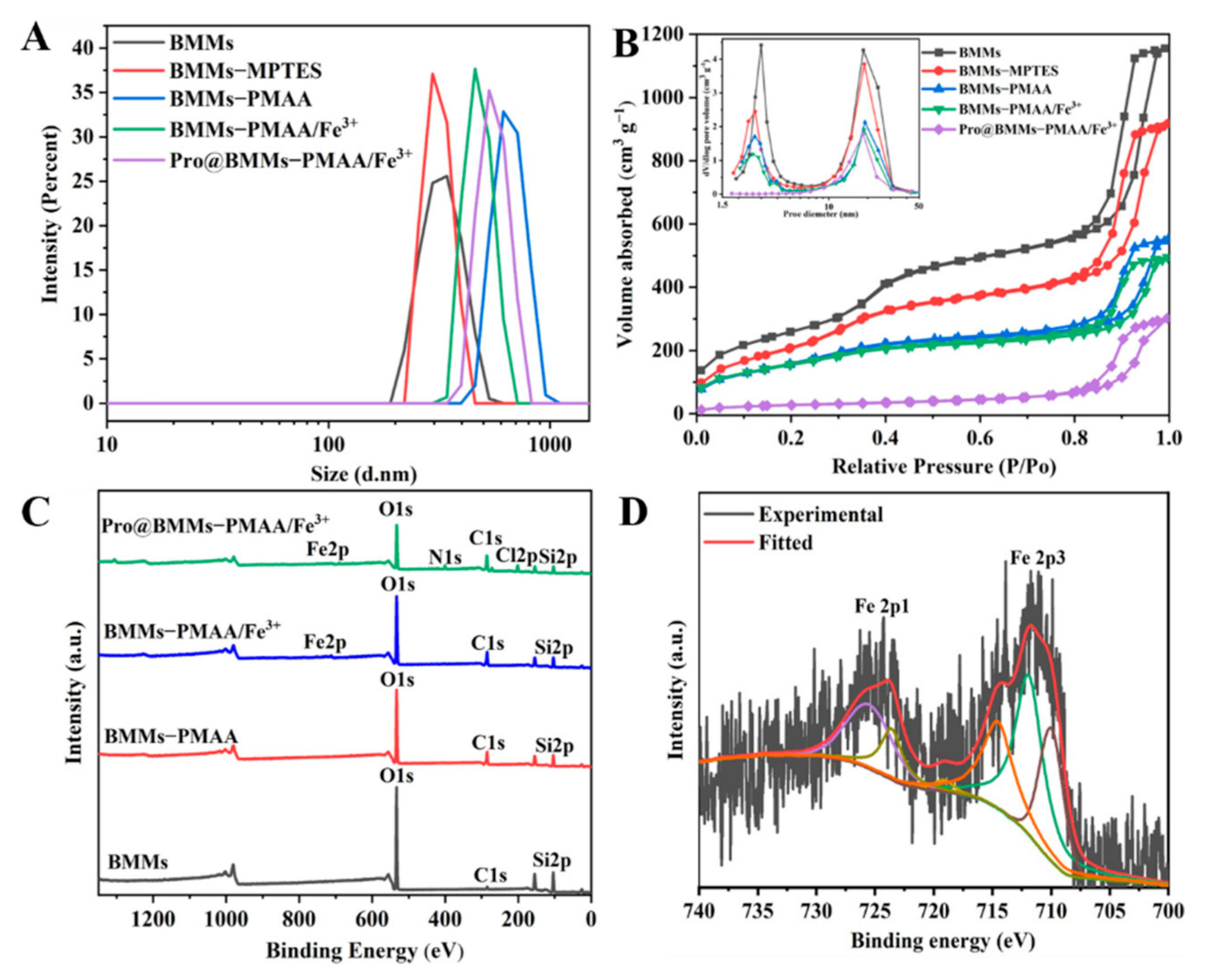



| Sample | SBET (m2·g−1) | Pore Volume (cm3·g−1) | Small Pore (nm) | Large Pore (nm) |
|---|---|---|---|---|
| BMMs | 1161.66 | 1.84 | 2.99 | 18.52 |
| BMMs−MPTES | 978.04 | 1.47 | 2.69 | 18.81 |
| BMMs−PMAA | 622.73 | 0.91 | 2.67 | 18.73 |
| BMMs−PMAA/Fe3+ | 505.09 | 0.75 | 2.57 | 18.69 |
| Pro@BMMs−PMAA/Fe3+ | 116.77 | 0.47 | - | 18.42 |
| Fitting Models | pH Values | Kinetic Equations | R2 |
|---|---|---|---|
| Zero-order | 5.0 | Q = 0.37t + 18.65 | 0.80 |
| 7.0 | Q = 0.23t + 14.79 | 0.63 | |
| 9.0 | Q = 0.47t + 25.10 | 0.82 | |
| First-order | 5.0 | Q = 1–1.22 e0.006t | 0.89 |
| 7.0 | Q = 1–1.27 e0.011t | 0.95 | |
| 9.0 | Q = 1–1.18 e0.003t | 0.68 | |
| Higuchi | 5.0 | Q = 5.10 t1/2 + 6.32 | 0.95 |
| 7.0 | Q = 3.29 t1/2 + 6.16 | 0.86 | |
| 9.0 | Q = 6.47 t1/2 + 9.52 | 0.96 | |
| Ritger–Peppas | 5.0 | Q = 10.47 t0.37 | 0.97 |
| 7.0 | Q = 9.02 t0.31 | 0.92 | |
| 9.0 | Q = 14.49 t0.35 | 0.99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, H.; Huang, W.; Wu, L.; Hong, Q.; Hu, Z.; Wang, M.; Zhang, F. A pH Dual-Responsive Multifunctional Nanoparticle Based on Mesoporous Silica with Metal-Polymethacrylic Acid Gatekeeper for Improving Plant Protection and Nutrition. Nanomaterials 2022, 12, 687. https://doi.org/10.3390/nano12040687
Pan H, Huang W, Wu L, Hong Q, Hu Z, Wang M, Zhang F. A pH Dual-Responsive Multifunctional Nanoparticle Based on Mesoporous Silica with Metal-Polymethacrylic Acid Gatekeeper for Improving Plant Protection and Nutrition. Nanomaterials. 2022; 12(4):687. https://doi.org/10.3390/nano12040687
Chicago/Turabian StylePan, Hua, Weilan Huang, Litao Wu, Qihao Hong, Zhongxuan Hu, Meijing Wang, and Fang Zhang. 2022. "A pH Dual-Responsive Multifunctional Nanoparticle Based on Mesoporous Silica with Metal-Polymethacrylic Acid Gatekeeper for Improving Plant Protection and Nutrition" Nanomaterials 12, no. 4: 687. https://doi.org/10.3390/nano12040687
APA StylePan, H., Huang, W., Wu, L., Hong, Q., Hu, Z., Wang, M., & Zhang, F. (2022). A pH Dual-Responsive Multifunctional Nanoparticle Based on Mesoporous Silica with Metal-Polymethacrylic Acid Gatekeeper for Improving Plant Protection and Nutrition. Nanomaterials, 12(4), 687. https://doi.org/10.3390/nano12040687






