Morphological and Electrochemical Properties of ZnMn2O4 Nanopowders and Their Aggregated Microspheres Prepared by Simple Spray Drying Process
Abstract
:1. Introduction
2. Materials and Methods
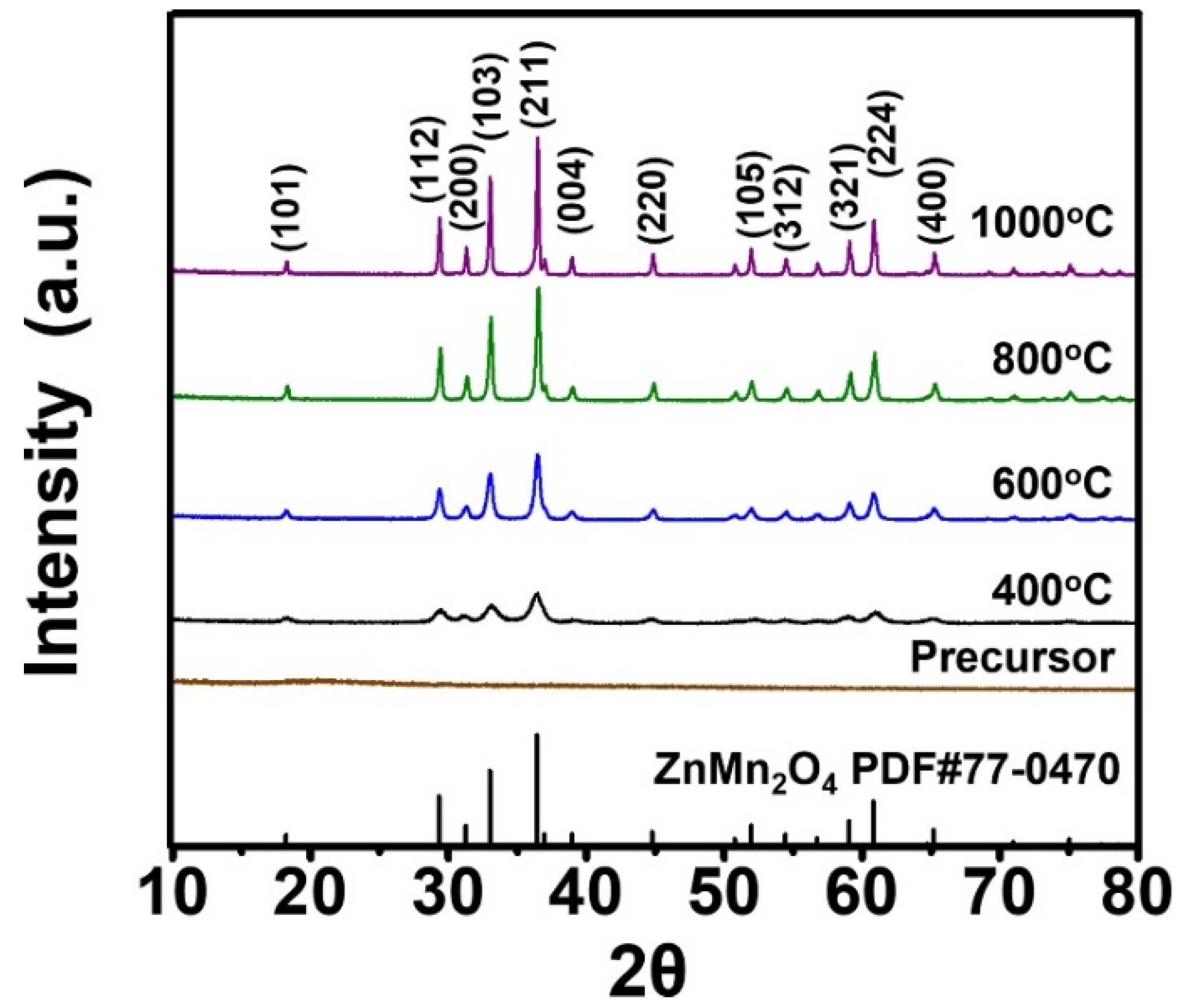
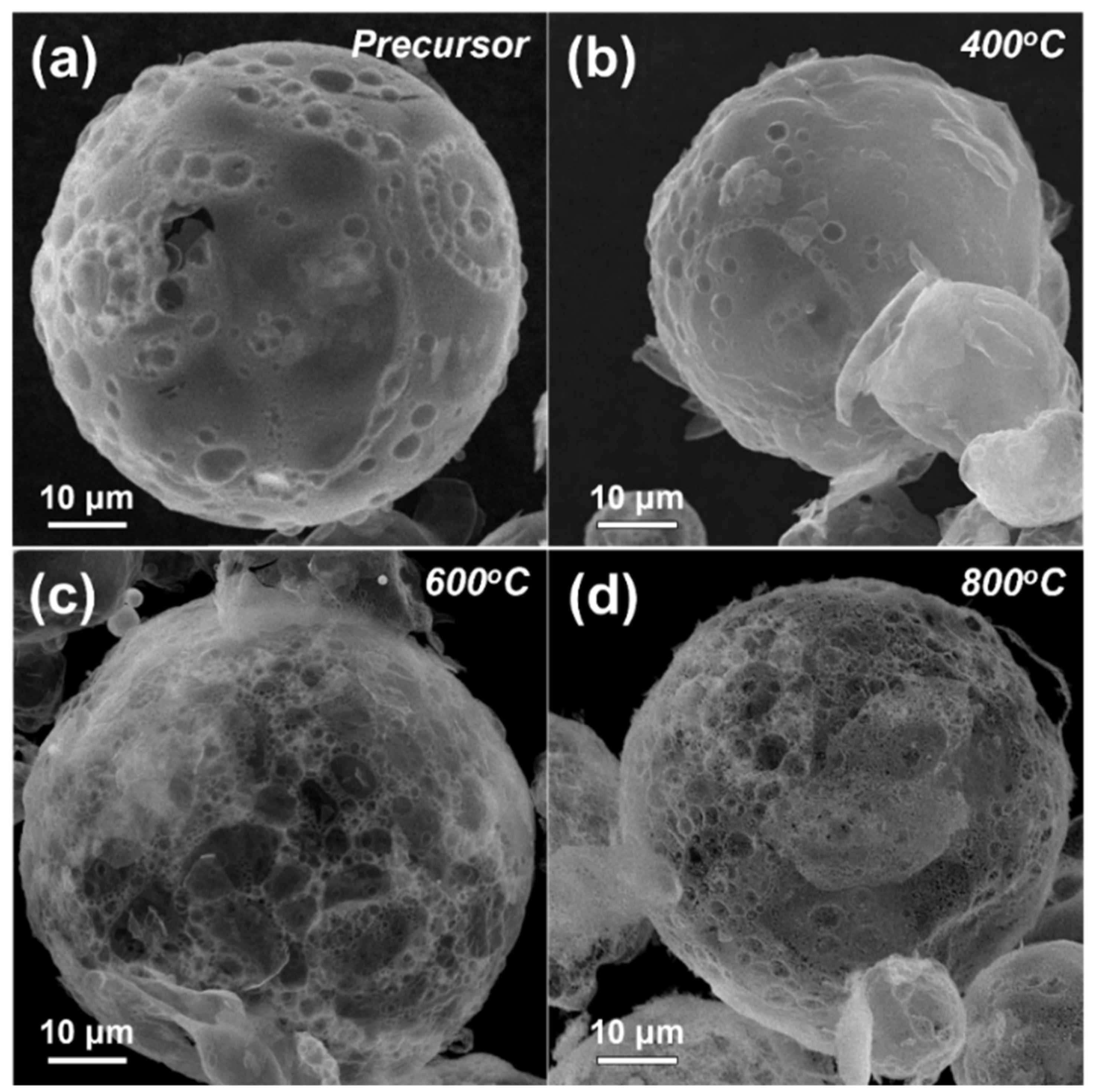
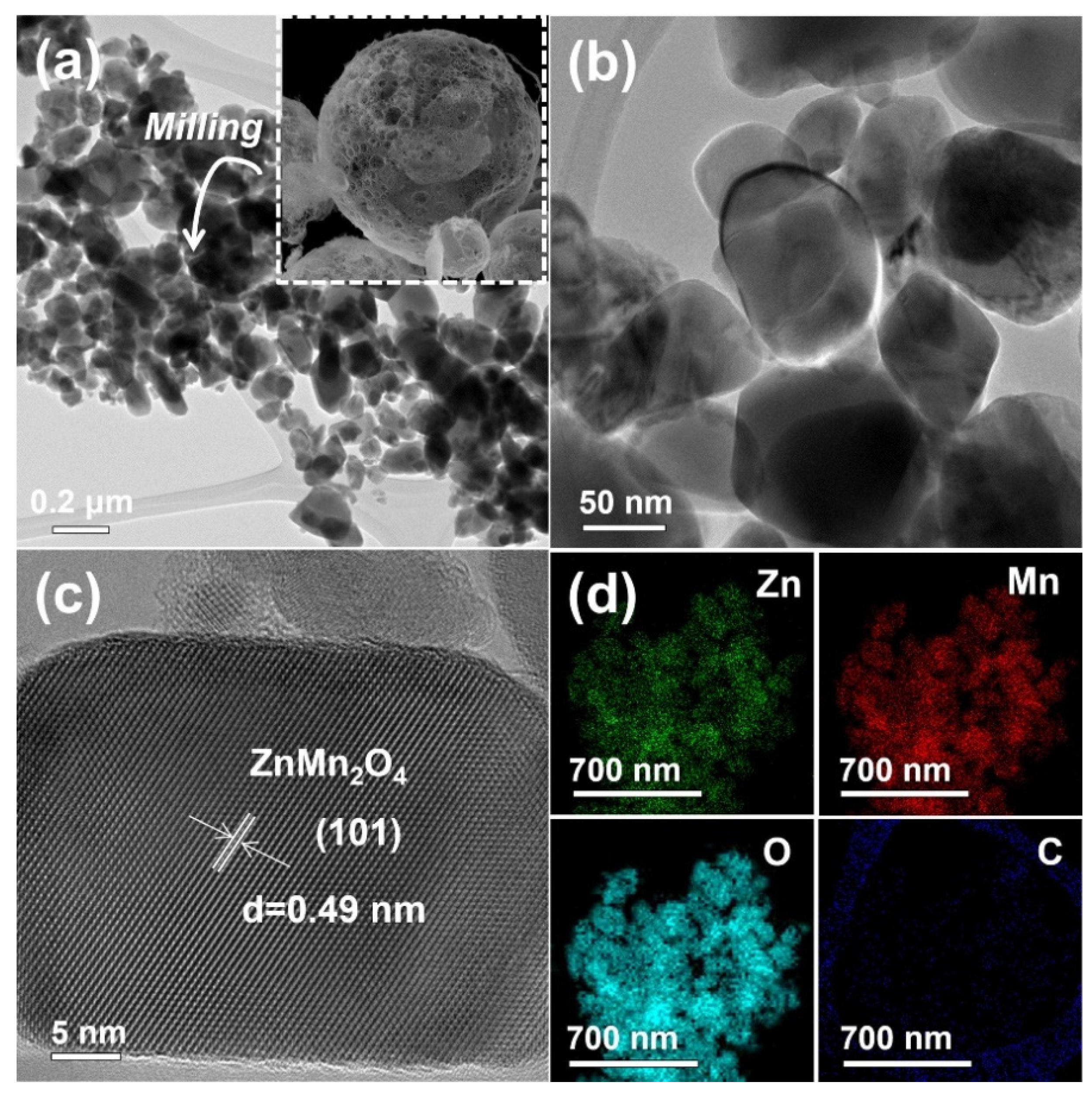
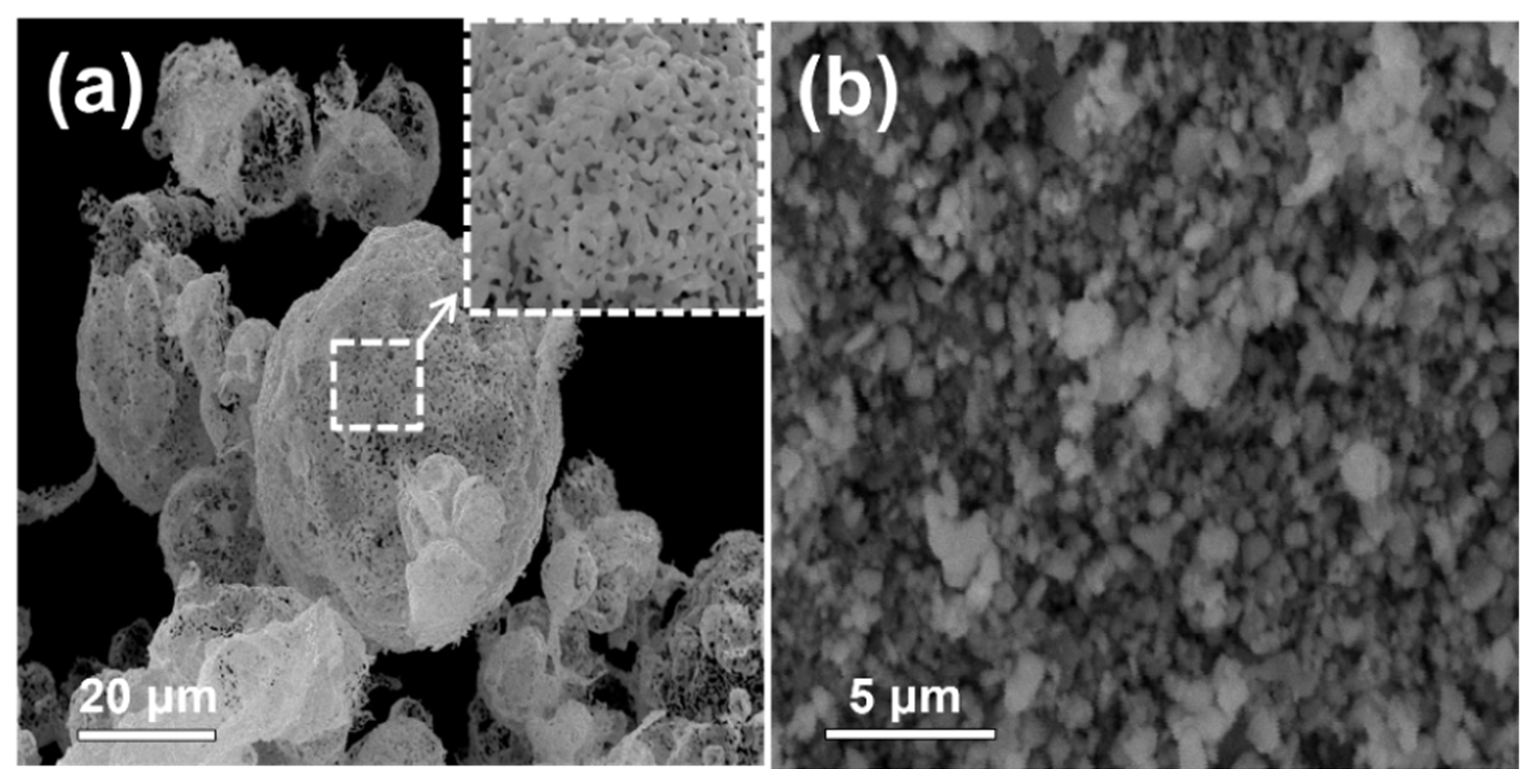
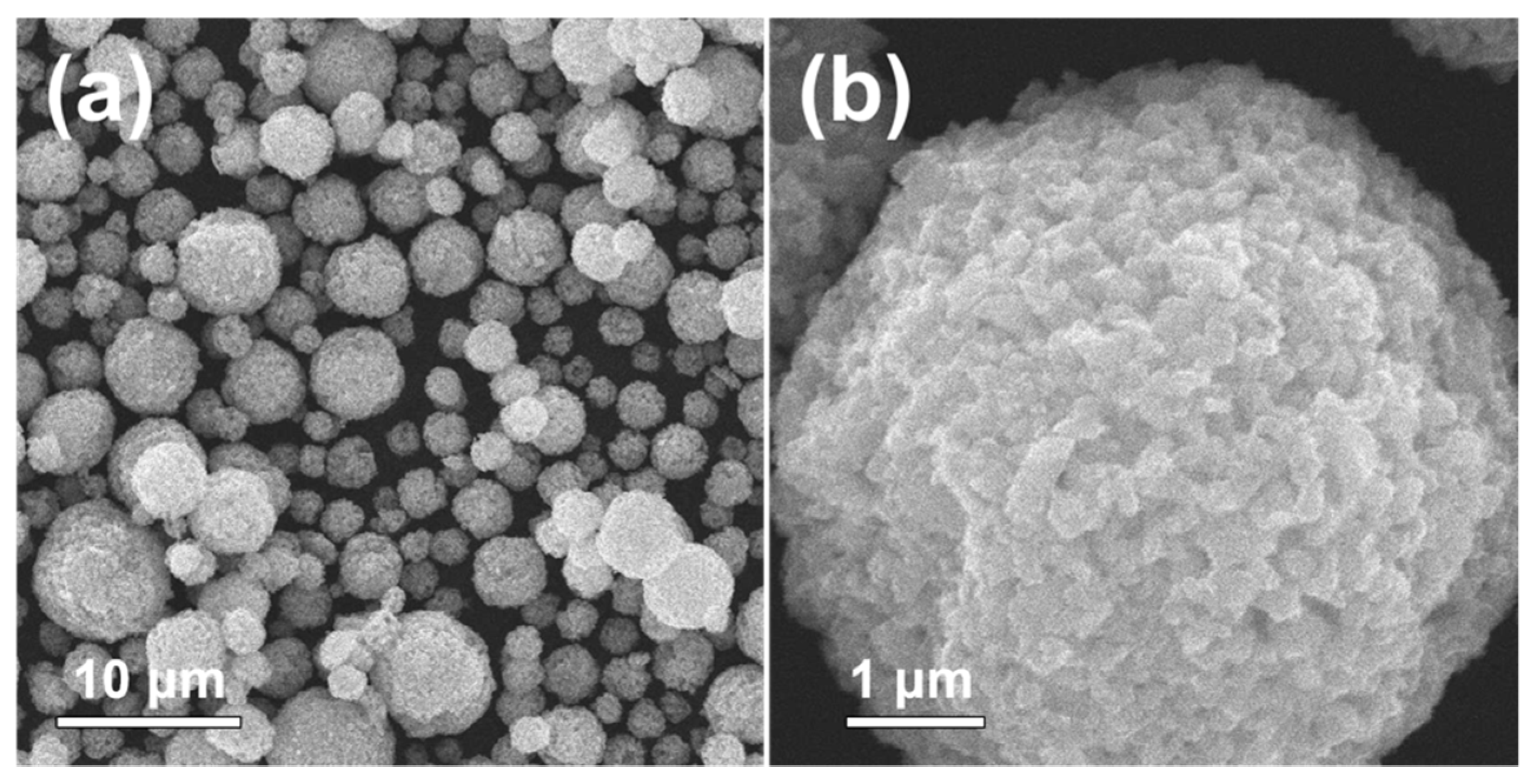
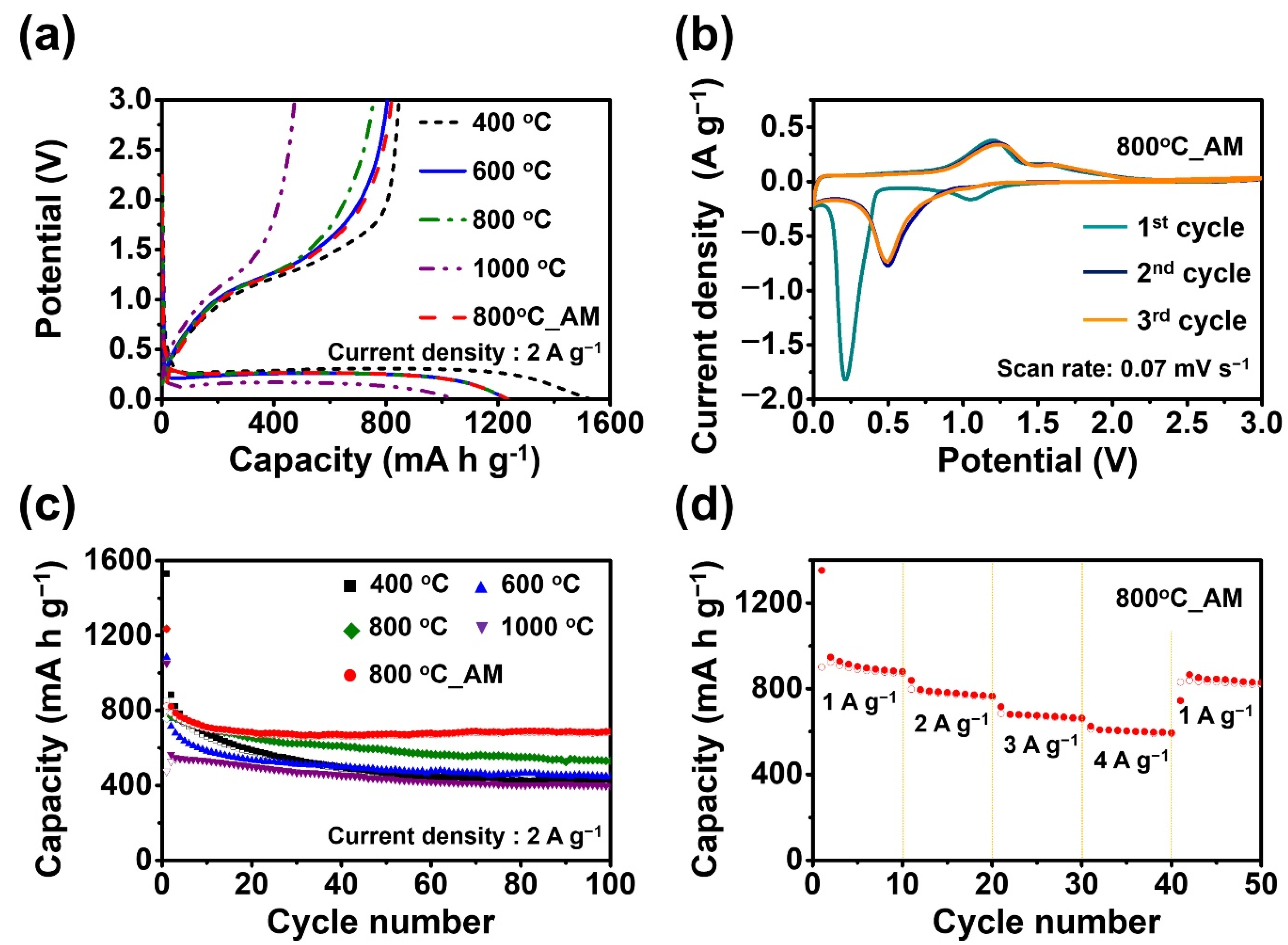
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Poizot, P.; Laruelle, S.; Grugeon, S.; Dupont, L.; Tarascon, J.M. Nano-sized transition-metal oxides as negative-electrode materials for lithium-ion batteries. Nature 2000, 407, 496–499. [Google Scholar] [CrossRef] [PubMed]
- Lou, X.W.; Archer, L.A.; Yang, Z. Hollow micro-/nanostructures: Synthesis and applications. Adv. Mater. 2008, 20, 3987–4019. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Zhou, L.; Lou, X.W. Metal oxide hollow nanostructures for lithium-ion batteries. Adv. Mater. 2012, 24, 1903–1911. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xue, D. Hollow nanostructured anode materials for Li-ion batteries. Nanoscale 2010, 5, 1525–1534. [Google Scholar] [CrossRef] [Green Version]
- Kang, N.R.; Park, J.H.; Choi, J.W.; Jin, J.W.; Chun, J.S.; Jung, I.G.; Jeong, J.H.; Park, J.G.; Lee, S.M.; Kim, H.J.; et al. Nanoparticulate iron oxide tubes from microporous organic nanotubes as stable anode materials for lithium-ion batteries. Angew. Chem. Int. Ed. 2012, 51, 6626–6630. [Google Scholar] [CrossRef]
- Wu, Z.S.; Ren, W.C.; Wen, L.; Gao, L.B.; Zhao, J.P.; Chen, Z.P.; Zhou, G.M.; Li, F.; Cheng, H.M. Graphene anchored with Co3O4 nanoparticles as anode of lithium-ion batteries with enhanced reversible capacity and cyclic performance. ACS Nano. 2010, 4, 3187–3194. [Google Scholar] [CrossRef]
- Cha, H.G.; Sohn, J.H.; Park, Y.J.; Lee, K.J.; Jung, M.H.; Lee, J.W.; Shin, W.S.; Kang, M.J.; Kim, D.Y.; Kang, Y.S. Hierarchical NiO hollow microspheres: Electrochemical and magnetic properties. RSC Adv. 2012, 2, 9786–9790. [Google Scholar] [CrossRef]
- Reddy, M.V.; Yu, C.; Jiahuan, F.; Loh, K.P.; Chowdari, B.V.R. Molten salt synthesis and energy storage studies on CuCo2O4 and CuO·Co3O4. RSC Adv. 2012, 2, 9619–9625. [Google Scholar] [CrossRef]
- Wang, Y.; Su, D.W.; Ung, A.; Ahn, J.H.; Wang, G.X. Hollow CoFe2O4 nanospheres as a high capacity anode material for lithium-ion batteries. Nanotechnology 2012, 23, 055402. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhang, J.; Wang, X.F.; Chen, G.; Chen, D.; Zhou, C.W.; Shen, G.Z. Hierarchical three-dimensional ZnCo2O4 nanowire arrays/carbon cloth anodes for a novel class of high-performance flexible lithium-ion batteries. Nano Lett. 2012, 12, 3005–3011. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Lee, H.W.; Muralidharan, P.; Seo, D.H.; Yoon, W.S.; Kim, D.K.; Kang, K.S. Electrochemical performance and ex situ analysis of ZnMn2O4 nanowires as anode materials for lithium rechargeable batteries. Nano. Res. 2011, 4, 505–510. [Google Scholar] [CrossRef]
- Kim, C.J.; Noh, M.J.; Choi, M.S.; Cho, J.P.; Park, B.W. Critical size of a nano SnO2 electrode for Li-secondary battery. Chem. Mater. 2005, 17, 3297–3301. [Google Scholar] [CrossRef]
- Ye, J.F.; Zhang, H.J.; Yang, R.; Li, X.G.; Qi, L.M. Morphology-controlled synthesis of SnO2 nanotubes by using 1D silica mesostructures as sacrificial templates and their applications in lithium-ion batteries. Small 2010, 6, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Courtel, F.M.; Duncan, H.; Abu-Lebdeh, Y.; Davidson, I.J. High capacity anode materials for Li-ion batteries based on spinel metal oxides AMn2O4 (A = Co, Ni, and Zn). J. Mater. Chem. 2011, 21, 10206–10218. [Google Scholar] [CrossRef] [Green Version]
- Courtel, F.M.; Abu-Lebdeh, Y.; Davidson, I.J. ZnMn2O4 nanoparticles synthesized by a hydrothermal method as an anode material for Li-ion batteries. Electrochim. Acta 2012, 71, 123–127. [Google Scholar] [CrossRef]
- Luo, X.; Zhang, X.; Chen, L.; Li, L.; Zhu, G.; Chen, G.; Yan, D.; Xu, H.; Yu, A. Mesoporous ZnMn2O4 microtubules derived from a biomorphic strategy for high-performance lithium/sodium ion batteries. ACS Appl. Mater. Interfaces 2018, 10, 33170–33178. [Google Scholar] [CrossRef]
- Xiao, L.; Yang, Y.; Yin, J.; Li, Q.; Zhang, L. Low temperature synthesis of flower-like ZnMn2O4 superstructures with enhanced electrochemical lithium storage. J. Power Sources 2009, 194, 1089–1093. [Google Scholar] [CrossRef]
- Deng, Y.F.; Tang, S.D.; Zhang, Q.M.; Shi, Z.C.; Zhang, L.T.; Zhan, S.Z.; Chen, G.H. Controllable synthesis of spinel nano-ZnMn2O4 via a single source precursor route and its high capacity retention as anode material for lithium ion batteries. J. Mater. Chem. 2011, 21, 11987–11995. [Google Scholar] [CrossRef]
- Zhou, L.; Wu, H.B.; Zhu, T.; Lou, X.W. Facile preparation of ZnMn2O4 hollow microspheres as high-capacity anodes for lithium-ion batteries. J. Mater. Chem. 2012, 22, 827–829. [Google Scholar] [CrossRef]
- Park, Y.S.; Oh, H.; Lee, S.M. Mechanochemical synthesis of ZnMn2O4 and its electrochemical properties as an anode material for lithium-ion batteries. Bull. Korean Chem. Soc. 2011, 32, 3333–3337. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.H.; Kang, Y.C. Characteristics of ZnMn2O4 nanopowders prepared by flame spray pyrolysis for use as anode material in lithium-ion batteries. Int. J. Electrochem. Sci. 2013, 8, 6281–6290. [Google Scholar]
- Tran, N.; Bramnik, K.G.; Hibst, H.; Prölß, J.; Mronga, N.; Holzapfel, M.; Scheifele, W.; Novák, P. Spray-drying synthesis and electrochemical performance of lithium vanadates as positive electrode materials for lithium batteries. J. Electrochem. Soc. 2008, 155, A384–A389. [Google Scholar] [CrossRef]
- Jung, D.S.; Hwang, T.H.; Park, S.B.; Choi, J.W. Spray drying method for large-scale and high-performance silicon negative electrodes in Li-ion batteries. Nano Lett. 2013, 13, 2092–2097. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Jang, Y.S.; Park, B.K.; Kang, Y.C. Electrochemical properties of C-coated Cu6Sn5 nanoparticles dispersed on carbon matrix prepared by spray drying process. Int. J. Electrochem. Sci. 2013, 8, 1067–1078. [Google Scholar]
- Hou, A.; Liu, Y.; Ma, L.; Chai, F.; Zhang, P.; Fan, Y. High-rate LiNi0.815Co0.15Al0.035O2 cathode material prepared by spray drying method for Li-ion batteries. J. Mater. Sci. Mater. Electron. 2020, 31, 1159–1167. [Google Scholar] [CrossRef]
- Asenbauer, J.; Binder, J.R.; Mueller, F.; Kuenzel, M.; Geiger, D.; Kaiser, U.; Passerini, S.; Bresser, D. Scalable synthesis of microsized, nanocrystalline Zn0.9Fe0.1O-C secondary particles and their use in Zn0.9Fe0.1O-C/LiNi0.5Mn1.5O4 lithium-ion full cells. ChemSusChem 2020, 13, 3504–3513. [Google Scholar] [CrossRef]
- Min, X.; Zhang, Y.; Yu, M.; Wang, Y.; Yuan, A.; Xu, J. A hierarchical dual-carbon supported ZnMn2O4/C composite as an anode material for Li-ion batteries. J. Alloys Compd. 2021, 877, 160242. [Google Scholar] [CrossRef]
- Zhang, G.; Yu, L.; Wu, H.B.; Hoster, H.E.; Lou, X.W. Formation of ZnMn2O4 ball-in-ball hollow microspheres as a high-performance anode for lithium-ion batteries. Adv. Mater. 2012, 24, 4609–4613. [Google Scholar] [CrossRef]
- Song, M.S.; Cho, Y.J.; Yoon, D.Y.; Nahm, S.; Oh, S.H.; Woo, K.; Ko, J.M.; Cho, W.I. Solvothermal synthesis of ZnMn2O4 as an anode material in lithium ion battery. Electrochim. Acta 2014, 137, 266–272. [Google Scholar] [CrossRef]
- Dang, W.; Wang, F.; Ding, Y.; Feng, C.; Guo, Z. Synthesis and electrochemical properties of ZnMn2O4 microspheres for lithium-ion battery application. J. Alloys Compd. 2017, 690, 72–79. [Google Scholar] [CrossRef]
- Islam, M.; Ali, G.; Akbar, M.; Ali, B.; Jeong, M.-G.; Kim, J.-Y.; Chung, K.Y.; Nam, K.-W.; Jung, H.-G. Investigating the energy storage performance of the ZnMn2O4 anode for its potential application in lithium-ion batteries. Int. J. Energy Res. 2021. [Google Scholar] [CrossRef]
- Feng, T.-T.; Yang, J.; Dai, S.-Y.; Wang, J.-C.; Wu, M.-Q. Microemulsion synthesis of ZnMn2O4/Mn3O4 sub-microrods for Li-ion batteries and their conversion reaction mechanism. Trans. Nonferrous Met. Soc. China 2021, 31, 265–276. [Google Scholar] [CrossRef]
- Park, G.D.; Park, S.-K.; Kang, Y.C. Superior electrochemical properties of composite microspheres consisting of hollow Fe2O3 nanospheres and graphitic carbon. ACS Sustain. Chem. Eng. 2018, 6, 111759–111767. [Google Scholar] [CrossRef]
- Jo, M.S.; Park, G.D.; Kang, Y.C.; Cho, J.S. Design and synthesis of interconnected hierarchically porous anatase titanium dioxide nanofibers as high-rate and long-cycle-life anodes for lithium-ion batteries. Nanoscale 2018, 10, 13539–13547. [Google Scholar] [CrossRef] [PubMed]
- Park, G.D.; Kang, Y.C. Design and synthesis of spherical multicomponent aggregates composed of core–shell, yolk–shell, and hollow nanospheres and their lithium-ion storage performances. Small 2018, 14, 1703957. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Ma, X.; Xu, H.; Chen, L.; Yue, J.; Niu, F.; Yang, J.; Qian, Y. Porous ZnMn2O4 microspheres as a promising anode material for advanced lithium-ion batteries. Nano Energy 2014, 6, 193–199. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, S.; Cao, H.; Hou, L.; Yuan, C. Hierarchical Porous ZnMn2O4Hollow Nanotubes with Enhanced Lithium Storage toward Lithium-Ion Batteries. Chem. A Eur. J. 2015, 21, 10771–10777. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Y.; Lin, H.; Xia, P.; Cai, X.; Li, X.; Li, X.; Li, W. Porous ZnMn2O4 nanospheres: Facile synthesis through microemulsion method and excellent performance as anode of lithium ion battery. J. Power Sources 2016, 312, 137–145. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Guo, C.; Tang, B.; Wang, X.; Bai, Z. Porous ZnMn2O4 nanowires as an advanced anode material for lithium ion battery. Electrochim. Acta 2015, 182, 1140–1144. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, G.D.; Kang, Y.C.; Cho, J.S. Morphological and Electrochemical Properties of ZnMn2O4 Nanopowders and Their Aggregated Microspheres Prepared by Simple Spray Drying Process. Nanomaterials 2022, 12, 680. https://doi.org/10.3390/nano12040680
Park GD, Kang YC, Cho JS. Morphological and Electrochemical Properties of ZnMn2O4 Nanopowders and Their Aggregated Microspheres Prepared by Simple Spray Drying Process. Nanomaterials. 2022; 12(4):680. https://doi.org/10.3390/nano12040680
Chicago/Turabian StylePark, Gi Dae, Yun Chan Kang, and Jung Sang Cho. 2022. "Morphological and Electrochemical Properties of ZnMn2O4 Nanopowders and Their Aggregated Microspheres Prepared by Simple Spray Drying Process" Nanomaterials 12, no. 4: 680. https://doi.org/10.3390/nano12040680





