Electrochemical Detection of Ascorbic Acid in Oranges at MWCNT-AONP Nanocomposite Fabricated Electrode
Abstract
:1. Introduction
2. Reagents and Experimental Procedure
2.1. Reagents
2.2. Antimony Oxide Nanoparticles (AONPs) Synthetic Method
2.3. Synthesis of f-MWCNTs
2.4. MWCNT-AONP Nanocomposite Synthesis
2.5. Real-Sample Analysis
3. Results and Discussion
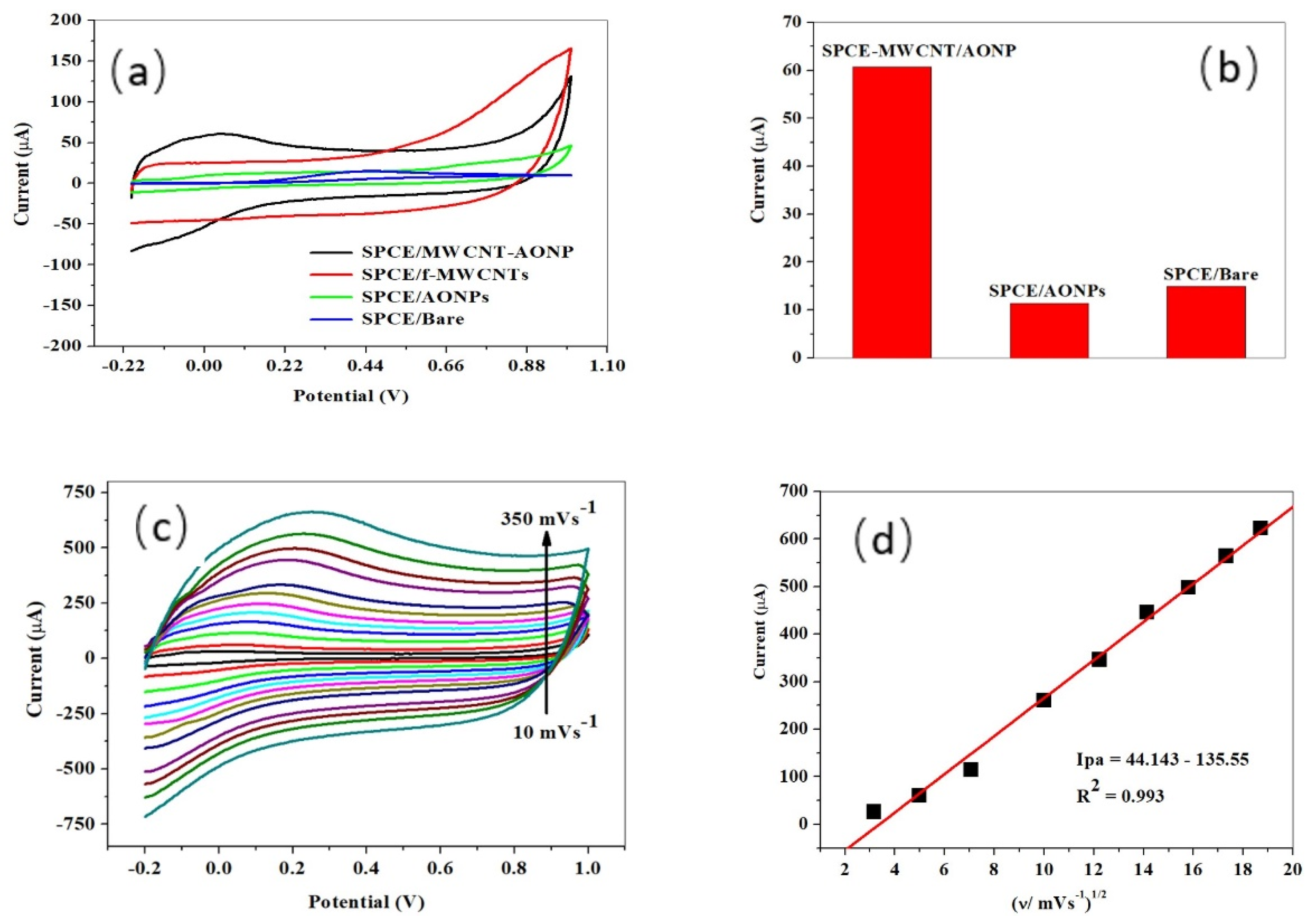
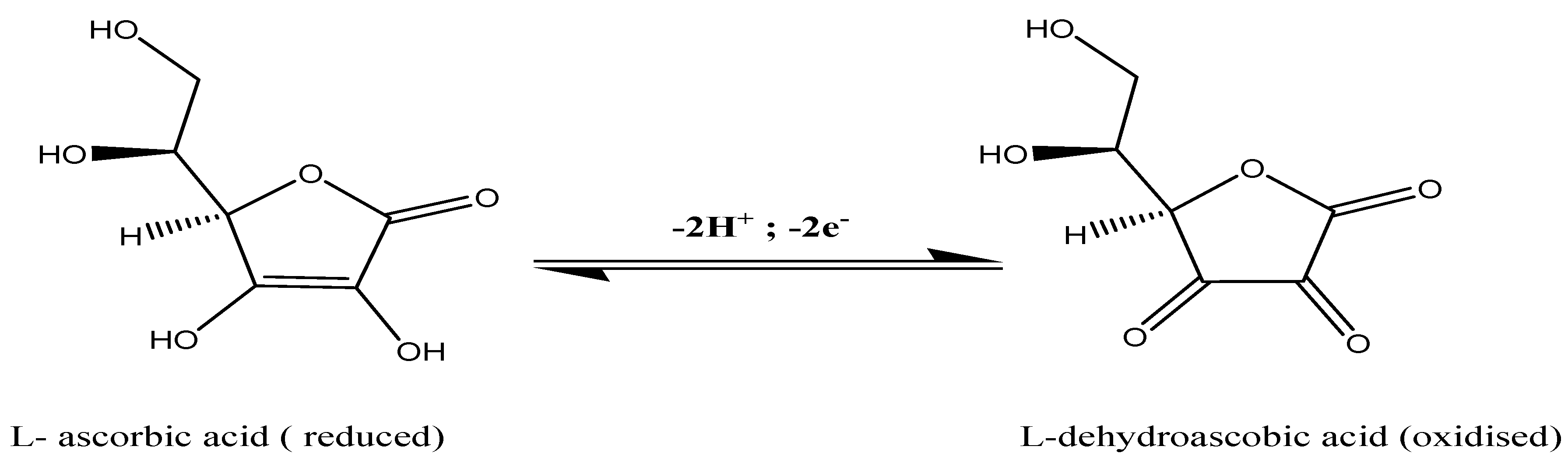
3.1. Electrocatalytic Experiements
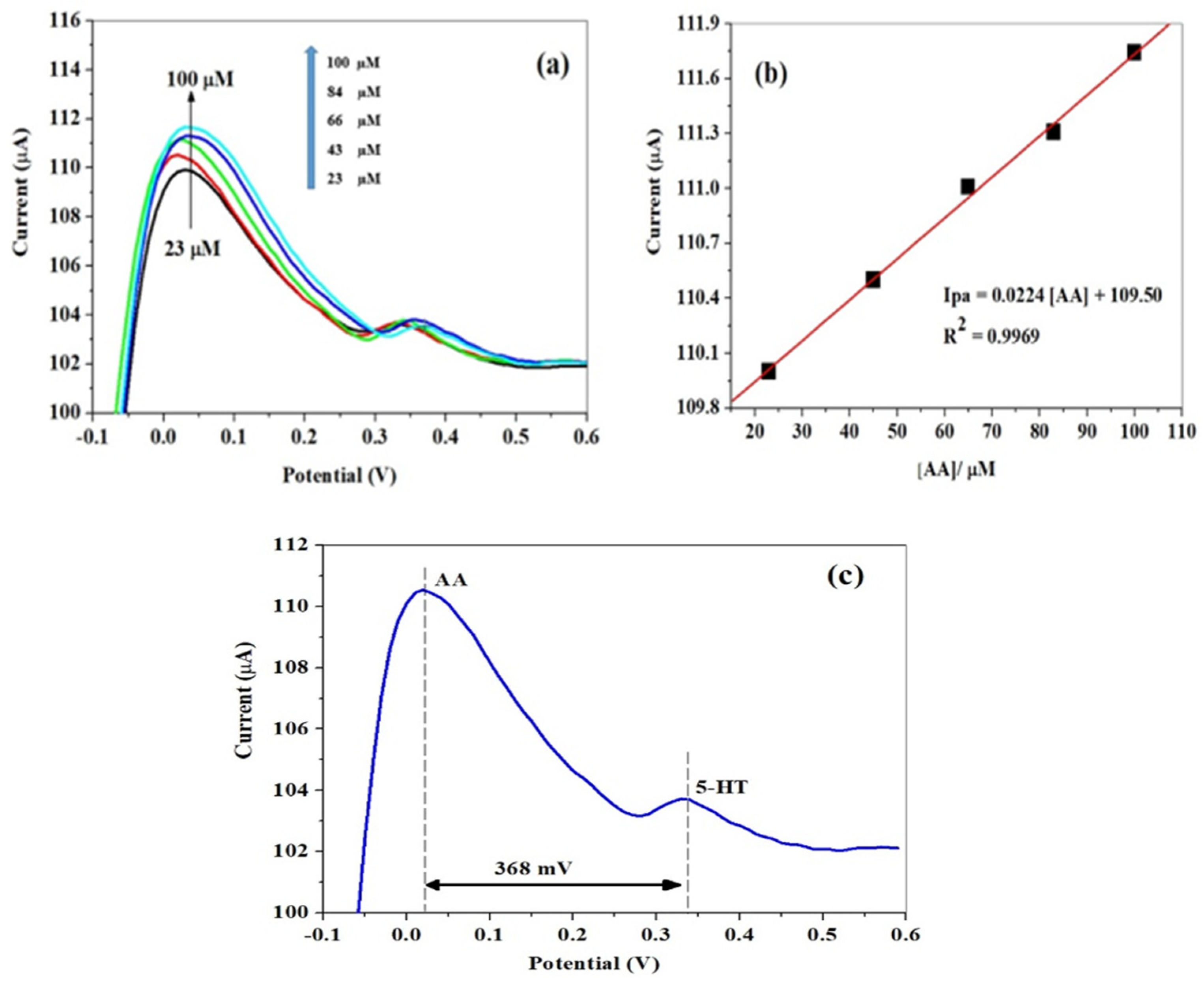
3.2. Electro-Analytic Experiments
3.3. Simultaneous Detection of AA and 5-HT
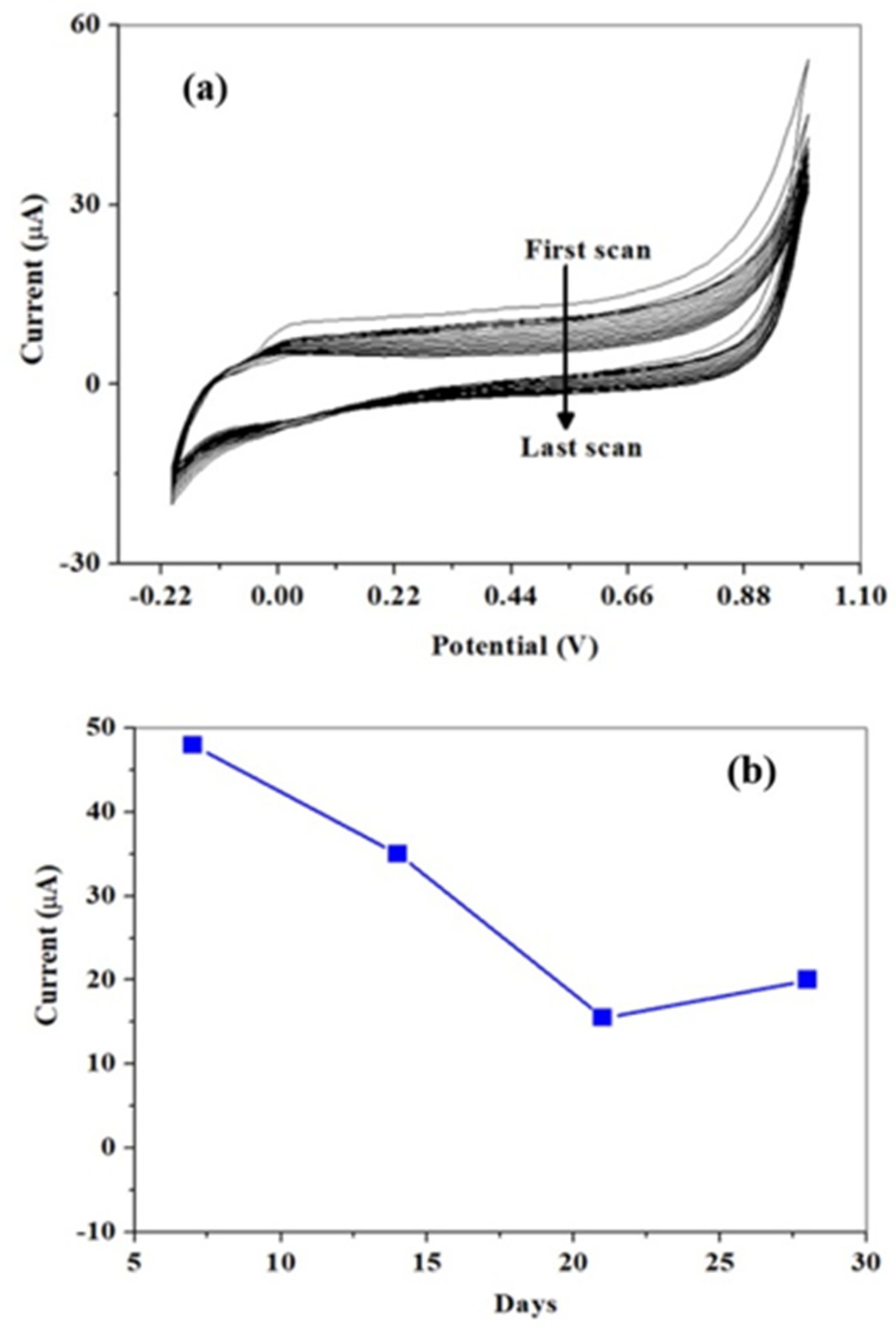
3.4. Reproducibility and Shelve-Life Study of The Proposed Sensor
3.5. Real-Aample Analysis
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Fadhel, D.H. Spectrophotometric determination of ascorbic acid in aqueous solutions. Al-Nahrain J. Sci. 2012, 15, 88–94. [Google Scholar] [CrossRef] [Green Version]
- Carr, A.C.; Maggini, S. Vitamin C and immune function. Nutrients 2017, 9, 1211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tajima, S.; Pinnell, S.R. Ascorbic acid preferentially enhances type I and III collagen gene transcription in human skin fibroblasts. J. Dermatol. Sci. 1996, 11, 250–253. [Google Scholar] [CrossRef]
- Johnston, C.S.; Huang, S. Effect of ascorbic acid nutriture on blood histamine and neutrophil chemotaxis in guinea pigs. J. Nutr. 1991, 121, 126–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohammed, B.M.; Fisher, B.J.; Kraskauskas, D.; Farkas, D.; Brophy, D.F.; Natarajan, R. Vitamin C: A novel regulator of neutrophil extracellular trap formation. Nutrients 2013, 5, 3131–3150. [Google Scholar] [CrossRef] [Green Version]
- Stankova, L.; Gerhardt, N.B.; Nagel, L.; Bigley, R.H. Ascorbate and phagocyte function. Infect. Immun. 1975, 12, 252–256. [Google Scholar] [CrossRef] [Green Version]
- Sharma, P.; Raghavan, S.A.; Saini, R.; Dikshit, M. Ascorbate-mediated enhancement of reactive oxygen species generation from polymorphonuclear leukocytes: Modulatory effect of nitric oxide. J. Leukoc. Biol. 2004, 75, 1070–1078. [Google Scholar] [CrossRef] [Green Version]
- Ganguly, R.; Durieux, M.; Waldman, R.J. Macrophage function in vitamin C-deficient guinea pigs. Am. J. Clin. Nutr. 1976, 29, 762–765. [Google Scholar] [CrossRef]
- Desneves, K.J.; Todorovic, B.E.; Cassar, A.; Crowe, T.C. Treatment with supplementary arginine, vitamin C and zinc in patients with pressure ulcers: A randomised controlled trial. Clin. Nutr. 2005, 24, 979–987. [Google Scholar] [CrossRef] [Green Version]
- Chauhan, N.; Soni, S.; Agrawal, P.; Balhara, Y.P.S.; Jain, U. Recent advancement in nanosensors for neurotransmitters detection: Present and future perspective. Process Biochem. 2020, 91, 241–259. [Google Scholar] [CrossRef]
- Gupta, P.; Tiwari, S.; Haria, J. Relationship between depression and vitamin C status: A study on rural patients from western uttar pradesh in India. Int. J. Sci. Study 2014, 1, 37–39. [Google Scholar]
- Manjunatha, J.G.; Swamy, B.K.; Deraman, M.; Mamatha, G.P. Simultaneous determination of ascorbic acid, dopamine and uric acid at poly (aniline blue) modified carbon paste electrode: A cyclic voltammetric study. Int. J. Pharm. Pharm. Sci. 2013, 5, 355–362. [Google Scholar]
- Pisoschi, A.M.; Pop, A.; Serban, A.I.; Fafaneata, C. Electrochemical methods for ascorbic acid determination. Electrochim. Acta 2014, 121, 443–460. [Google Scholar] [CrossRef]
- Guo, X.; Yue, G.; Huang, J.; Liu, C.; Zeng, Q.; Wang, L. Label-free simultaneous analysis of Fe (III) and ascorbic acid using fluorescence switching of ultrathin graphitic carbon nitride nanosheets. ACS Appl. Mater. Interfaces 2018, 10, 26118–26127. [Google Scholar] [CrossRef] [PubMed]
- Hodkinson, A. Oxalic Acid in Biology and Medicine; Academic Press: London, UK, 1977; pp. 254–319. [Google Scholar]
- Drouin, G.; Godin, J.R.; Pagé, B. The genetics of vitamin C loss in vertebrates. Curr. Genom. 2011, 12, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Elgailani, I.E.H.; Elkareem, M.; Noh, E.; Adam, O.; Alghamdi, A. Comparison of two methods for the determination of vitamin C (ascorbic acid) in some fruits. Am. J. Chem. 2017, 2, 1–7. [Google Scholar] [CrossRef]
- Vissers, M.C.; Carr, A.C.; Pullar, J.M.; Bozonet, S.M. The bioavailability of vitamin C from kiwifruit. Adv. Food Nutr. Res. 2013, 68, 125–147. [Google Scholar]
- Kyaw, A. A simple colorimetric method for ascorbic acid determination in blood plasma. Clin. Chim. Acta 1978, 86, 153–157. [Google Scholar] [CrossRef]
- Washko, P.W.; Hartzell, W.O.; Levine, M. Ascorbic acid analysis using high-performance liquid chromatography with coulometric electrochemical detection. Anal. Biochem. 1989, 181, 276–282. [Google Scholar] [CrossRef]
- Badea, M.; Florescu, M.; Veregut, V.; Chelmea, L.; Corcan, O.; Floroian, L.; Restani, P.; Marty, J.; Moga, M. Optimization of electrochemical detection of L-ascorbic acid from plant food supplements using screen printed transducers. Adv. Anal. Chem. 2015, 5, 69–73. [Google Scholar]
- De Faria, L.V.; Lisboa, T.P.; de Farias, D.M.; Araujo, F.M.; Machado, M.M.; de Sousa, R.A.; Matos, M.A.C.; Muñoz, R.A.A.; Matos, R.C. Direct analysis of ascorbic acid in food beverage samples by flow injection analysis using reduced graphene oxide sensor. Food Chem. 2020, 319, 126509. [Google Scholar] [CrossRef] [PubMed]
- Versari, A.; Mattioli, A.; Parpinello, G.P.; Galassi, S. Rapid analysis of ascorbic and isoascorbic acids in fruit juice by capillary electrophoresis. Food Control 2004, 15, 355–358. [Google Scholar] [CrossRef]
- Silva, F.O. Total ascorbic acid determination in fresh squeezed orange juice by gas chromatography. Food Control 2005, 16, 55–58. [Google Scholar] [CrossRef]
- Oliveira, E.J.; Watson, D.G. Chromatographic techniques for the determination of putative dietary anticancer compounds in biological fluids. J. Chromatogr. B Biomed. Sci. Appl. 2001, 764, 3–25. [Google Scholar] [CrossRef]
- Habibi, B.; Jahanbakhshi, M.; Pournaghi-Azar, M.H. Differential pulse voltammetric simultaneous determination of acetaminophen and ascorbic acid using single-walled carbon nanotube-modified carbon–ceramic electrode. Anal. Biochem. 2011, 411, 167–175. [Google Scholar] [CrossRef]
- López-Pastor, J.A.; Martínez-Sánchez, A.; Aznar-Poveda, J.; García-Sánchez, A.J.; García-Haro, J.; Aguayo, E. Quick and Cost-Effective Estimation of Vitamin C in Multifruit Juices Using Voltammetric Methods. Sensors 2020, 20, 676. [Google Scholar] [CrossRef] [Green Version]
- Aryal, K.P.; Jeong, H.K. Electrochemical detection of ascorbic acid with chemically functionalized carbon nanofiber/beta-cyclodextrin composite. Chem. Phys. Lett. 2020, 757, 137881. [Google Scholar] [CrossRef]
- Das, T.R.; Sharma, P.K. Hydrothermal-assisted green synthesis of Ni/Ag@rGO nanocomposite using Punica granatum juice and electrochemical detection of ascorbic acid. Microchem. J. 2020, 156, 104850. [Google Scholar] [CrossRef]
- Fernandes, D.S.; do Carmo, D.R. Silsesquioxane Modified with PAMAM Dendrimer and a Bimetallic Complex for Electrochemical Detection of Ascorbic Acid. Electroanalysis 2020, 33, 365–374. [Google Scholar] [CrossRef]
- Gai, P.; Zhang, H.; Zhang, Y.; Liu, W.; Zhu, G.; Zhang, X.; Chen, J. Simultaneous electrochemical detection of ascorbic acid, dopamine and uric acid based on nitrogen doped porous carbon nanopolyhedra. J. Mater. Chem. B 2013, 1, 2742–2749. [Google Scholar] [CrossRef]
- Ibarlucea, B.; Roig, A.P.; Belyaev, D.; Baraban, L.; Cuniberti, G. Electrochemical detection of ascorbic acid in artificial sweat using a flexible alginate/CuO-modified electrode. Microchim. Acta 2020, 187, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Park, S.G.; Park, J.E.; Cho, E.I.; Hwang, J.H.; Ohsaka, T. Electrochemical detection of ascorbic acid and serotonin at a boron-doped diamond electrode modified with poly(N,N-dimethylaniline). Res. Chem. Intermed. 2006, 32, 595–601. [Google Scholar] [CrossRef]
- Sun, C.L.; Lee, H.H.; Yang, J.M.; Wu, C.C. The simultaneous electrochemical detection of ascorbic acid, dopamine, and uric acid using graphene/size-selected Pt nanocomposites. Biosens. Bioelectron. 2011, 26, 3450–3455. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, Y.-C.; Ma, L.X. One-pot facile fabrication of graphene-zinc oxide composite and its enhanced sensitivity for simultaneous electrochemical detection of ascorbic acid, dopamine and uric acid. Sens. Actuators B Chem. 2016, 227, 488–496. [Google Scholar] [CrossRef]
- Zestos, A.G. Carbon nanoelectrodes for the electrochemical detection of neurotransmitters. Int. J. Electrochem. 2018, 2018, 3679627. [Google Scholar] [CrossRef] [Green Version]
- Chang, P.R.; Yu, J.; Ma, X.J.M.M. Fabrication and characterization of Sb2O3/carboxymethyl cellulose sodium and the properties of plasticized starch composite films. Macromol. Mater. Eng. 2009, 294, 762–767. [Google Scholar] [CrossRef]
- Falcaro, P.; Ricco, R.; Yazdi, A.; Imaz, I.; Furukawa, S.; Maspoch, D.; Ameloot, R.; Evans, J.D.; Doonan, C. Application of metal and metal oxide nanoparticles@ MOFs. Coord. Chem. Rev. 2016, 307, 237–254. [Google Scholar] [CrossRef]
- Karak, N.; Maiti, S. Antimony polymers. III. Flame retardant behavior of chloroprene and natural rubber vulcanizates with antimony polymer. J. Appl. Polym. Sci. 1998, 68, 927–935. [Google Scholar] [CrossRef]
- Mostashari, S.; Baie, S. Thermogravimetry studies of cotton fabric’s flame-retardancy by means of synergism of lithium bromide and antimony trioxide. J. Therm. Calorim. 2008, 94, 97–101. [Google Scholar] [CrossRef]
- Miura, N.; Mizuno, H.; Yamazoe, N.J. Humidity sensor using antimony phosphate operative at a medium temperature of 150–250 °C. Jpn. J. Appl. Phys. 1988, 27, L931. [Google Scholar] [CrossRef]
- Badawy, W.; El-Taher, E.A. Preparation and electrochemical behaviour of some metal oxide films. Thin Solid Films 1988, 158, 277–284. [Google Scholar] [CrossRef]
- Sahoo, N.K.; Apparao, K.V. Process-parameter optimization of Sb2O3 films in the ultraviolet and visible region for interferometric applications. Appl. Phys. A 1996, 63, 195–202. [Google Scholar] [CrossRef]
- El-Diasty, F.; Wahab, F.A.; Abdel-Baki, M. Optical band gap studies on lithium aluminum silicate glasses doped with Cr3+ ions. J. Appl. Phys. 2006, 100, 093511. [Google Scholar] [CrossRef]
- Tigau, N.; Ciupina, V.; Prodan, G. The effect of substrate temperature on the optical properties of polycrystalline Sb2O3 thin films. J. Cryst. Growth 2005, 277, 529–535. [Google Scholar] [CrossRef]
- Chin, H.S.; Cheong, K.Y.; Razak, K.A. Review on oxides of antimony nanoparticles: Synthesis, properties, and applications. J. Mater. Sci. 2010, 45, 5993–6008. [Google Scholar] [CrossRef]
- Wang, Z.H.; Liang, Q.l.; Wang, Y.M.; Luo, G.A. Carbon nanotube-intercalated graphite electrodes for simultaneous determination of dopamine and serotonin in the presence of ascorbic acid. J. Electroanal. Chem. 2003, 540, 129–134. [Google Scholar] [CrossRef]
- McCreery, R.L. Advanced carbon electrode materials for molecular electrochemistry. Chem. Rev. 2008, 108, 2646–2687. [Google Scholar] [CrossRef]
- Li, G.; Xia, Y.; Tian, Y.; Wu, Y.; Liu, J.; He, Q.; Chen, D. Recent developments on graphene-based electrochemical sensors toward nitrite. J. Electrochem. Soc. 2019, 166, B881. [Google Scholar] [CrossRef]
- Saleh, T.A.; Siddiqui, M.N.; Al-Arfaj, A.A. Synthesis of multi-walled carbon nanotubes-titania nanomaterial for desulfurization of model fuel. J. Nanomater. 2014, 2014, 194. [Google Scholar] [CrossRef]
- Puangjan, A.; Chaiyasith, S.; Wichitpanya, S.; Daengduang, S.; Puttota, S. Electrochemical sensor based on PANI/MnO2-Sb2O3 nanocomposite for selective simultaneous voltammetric determination of ascorbic acid and acetylsalicylic acid. J. Electroanal. Chem. 2016, 782, 192–201. [Google Scholar] [CrossRef]
- Düzmen, Ş.; Aslanoglu, M. An electrochemical platform of bimetallic oxide (Sb2O3@ WO3) nanoparticles and carbon nanotubes for the sensitive determination of metaproterenol in pharmaceuticals and biological samples. Measurement 2021, 173, 108572. [Google Scholar] [CrossRef]
- Masibi, K.K.; Fayemi, O.E.; Adekunle, A.S.; Al-Mohaimeed, A.M.; Fahim, A.M.; Mamba, B.B.; Ebenso, E.E. Electrochemical detection of endosulfan using an AONP-PANI-SWCNT modified glassy carbon electrode. Materials 2021, 14, 723. [Google Scholar] [CrossRef] [PubMed]
- Masibi, K.K.; Fayemi, O.E.; Adekunle, A.S.; Sherif, E.M.; Ebenso, E.E. Electrocatalysis of Lindane Using Antimony Oxide Nanoparticles Based-SWCNT/PANI Nanocomposites. Front. Chem. 2018, 6, 423. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Huh, H.; Lee, S.W. Hydrothermal synthesis of antimony oxychloride and oxide nanocrystals: Sb4O5Cl2, Sb8O11Cl2, and Sb2O3. J. Solid State Chem. 2008, 181, 2127–2132. [Google Scholar] [CrossRef]
- Ba Hashwan, S.S.; Fatin, M.; Ruslinda, A.R.; Md Arshad, M.; Hashim, U.; Ayub, R. Functionalization of Multi Wall Carbon Nanotubes Using Nitric Acid Oxidation. Appl. Mech. Mater. 2015, 754–755, 1156–1160. [Google Scholar] [CrossRef]
- Uwaya, G.E.; Fayemi, O.E. Electrochemical Detection of Ascorbic acid in Orange on Iron (III) Oxide Nanoparticles Modified Screen Printed Carbon Electrode. J. Clust. Sci. 2021, 32, 1–9. [Google Scholar] [CrossRef]
- Wu, Y.; Deng, P.; Tian, Y.; Feng, J.; Xiao, J.; Li, J.; Liu, J.; Li, G.; He, Q. Simultaneous and sensitive determination of ascorbic acid, dopamine and uric acid via an electrochemical sensor based on PVP-graphene composite. J. Nanobiotechnol. 2020, 18, 1–13. [Google Scholar] [CrossRef]
- Choukairi, M.; Bouchta, D.; Bounab, L.; Elkhamlichi, R.; Chaouket, F.; Raissouni, I.; Rodriguez, I. Electrochemical detection of uric acid and ascorbic acid: Application in serum. J. Electroanal. Chem. 2015, 758, 117–124. [Google Scholar] [CrossRef]
- Gu, B.; Liu, Z.; Wang, X.; Dong, X. RF magnetron sputtering synthesis of carbon fibers/ZnO coaxial nanocable microelectrode for electrochemical sensing of ascorbic acid. Mater. Lett. 2016, 181, 265–267. [Google Scholar] [CrossRef]
- Mazzara, F.; Patella, B.; Aiello, G.; O’Riordan, A.; Torino, C.; Vilasi, A.; Inguanta, R. Electrochemical detection of uric acid and ascorbic acid using r-GO/NPs based sensors. Electrochim. Acta 2021, 388, 138652. [Google Scholar] [CrossRef]

| Modified Electrode | Method | Linearity(µM) | LOD (µM) | R2 | Ref |
|---|---|---|---|---|---|
| 1 PVP-GR/GCE | LSV | 4.0–1000 | 0.80 | 0.989 | [58] |
| RGO–ZnO/GCE | DPV | 50–2350 | 3.71 | 0.997 | [35] |
| Sonigel-carbon@ME 2 CF/ZnO CN-ME | SWV DPV | 50–1000 0.6–1800 | 50.0 156.7 | 0.995 0.992 | [59] [60] |
| 3 ITO-rGO-AuNPs | LSV | 20–100 | 5.63 | 0.996 | [61] |
| Fe3O4NPs@SPCE | SWV | 10–100 | 15.7 | 0.981 | [57] |
| MWCNT-AONP@SPCE | SWV | 0.04–0.64 | 0.14 | 0.985 | This work |
| Sample | Added (µM) | Detected (µM) | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| Oranges | 400 | 431.03 | 107.76 | 5.71 |
| 600 | 594.69 | 99.12 | 3.89 | |
| 900 | 919.52 | 102.17 | 0.97 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Motsaathebe, P.C.; Fayemi, O.E. Electrochemical Detection of Ascorbic Acid in Oranges at MWCNT-AONP Nanocomposite Fabricated Electrode. Nanomaterials 2022, 12, 645. https://doi.org/10.3390/nano12040645
Motsaathebe PC, Fayemi OE. Electrochemical Detection of Ascorbic Acid in Oranges at MWCNT-AONP Nanocomposite Fabricated Electrode. Nanomaterials. 2022; 12(4):645. https://doi.org/10.3390/nano12040645
Chicago/Turabian StyleMotsaathebe, Pholoso Calvin, and Omolola Ester Fayemi. 2022. "Electrochemical Detection of Ascorbic Acid in Oranges at MWCNT-AONP Nanocomposite Fabricated Electrode" Nanomaterials 12, no. 4: 645. https://doi.org/10.3390/nano12040645






