Minimizing the Silver Free Ion Content in Starch Coated Silver Nanoparticle Suspensions with Exchange Cationic Resins
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Equipment
2.2. Synthesis of Starch Stabilized Silver Nanoparticles
2.3. Quantification of AgNPs and Free Ag+
2.4. Assays with Cationic Exchange Resins
3. Results and Discussion
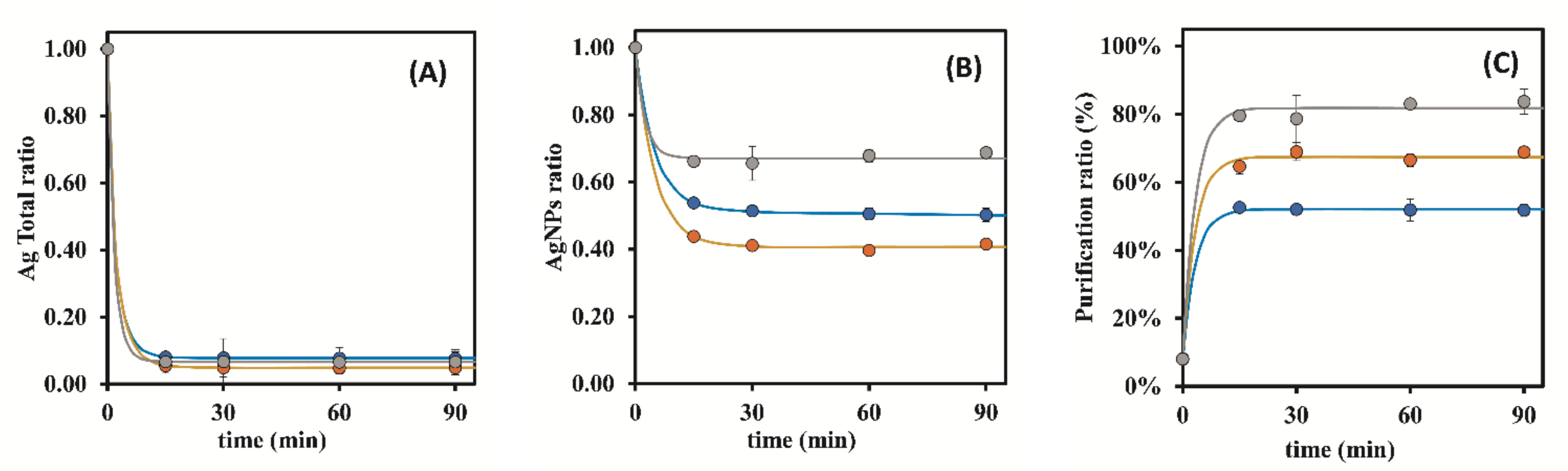
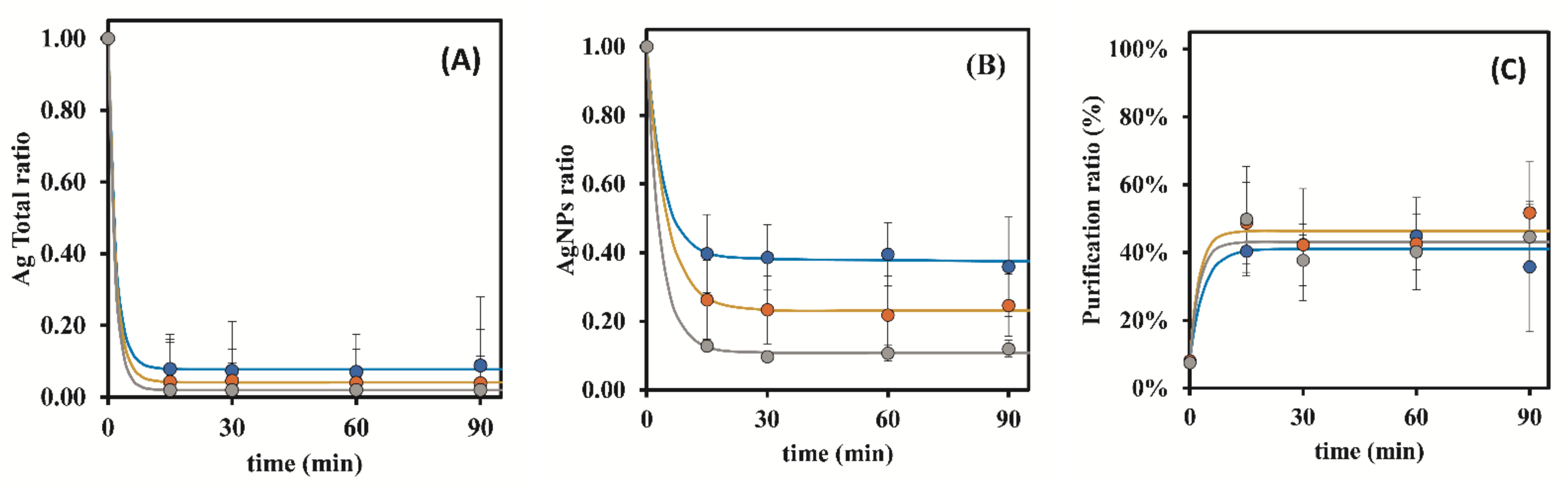
3.1. Preliminary Assays
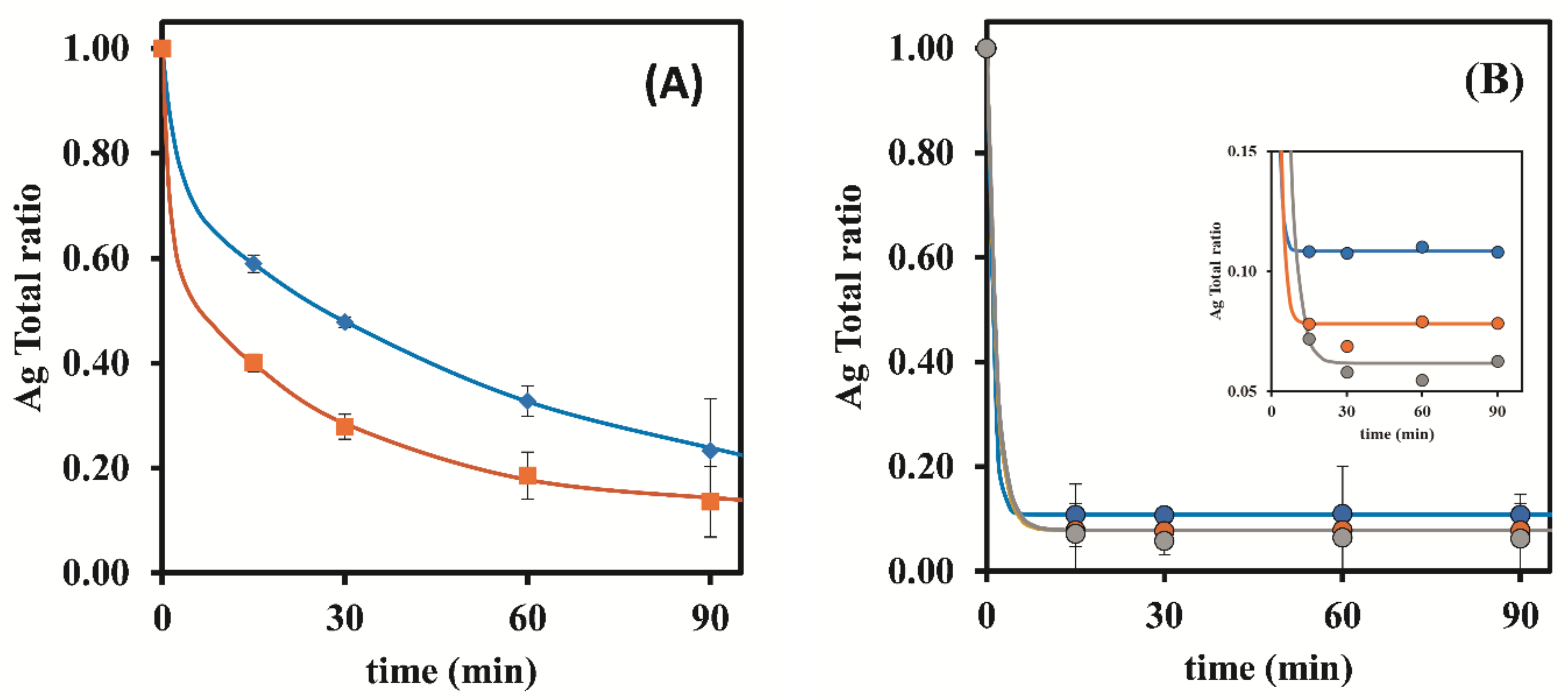
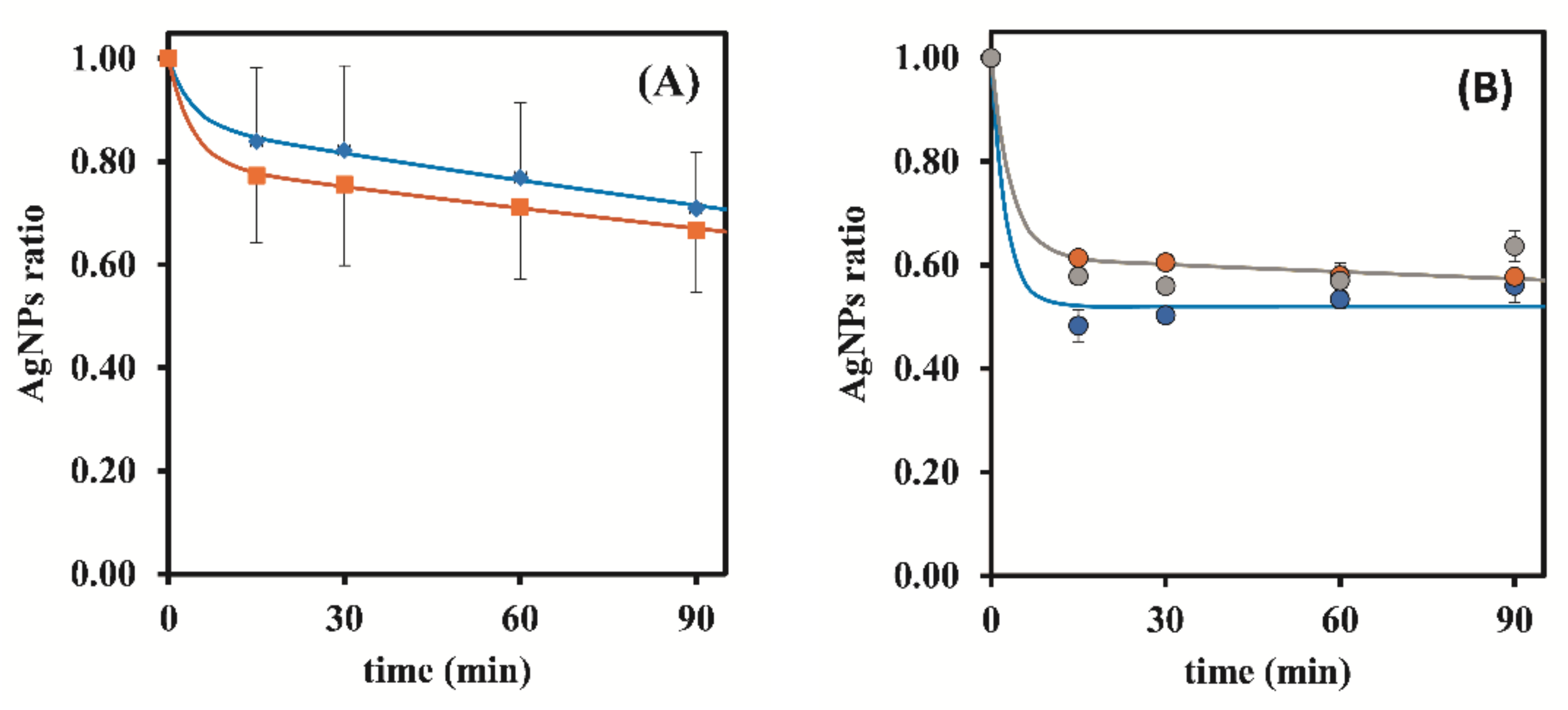
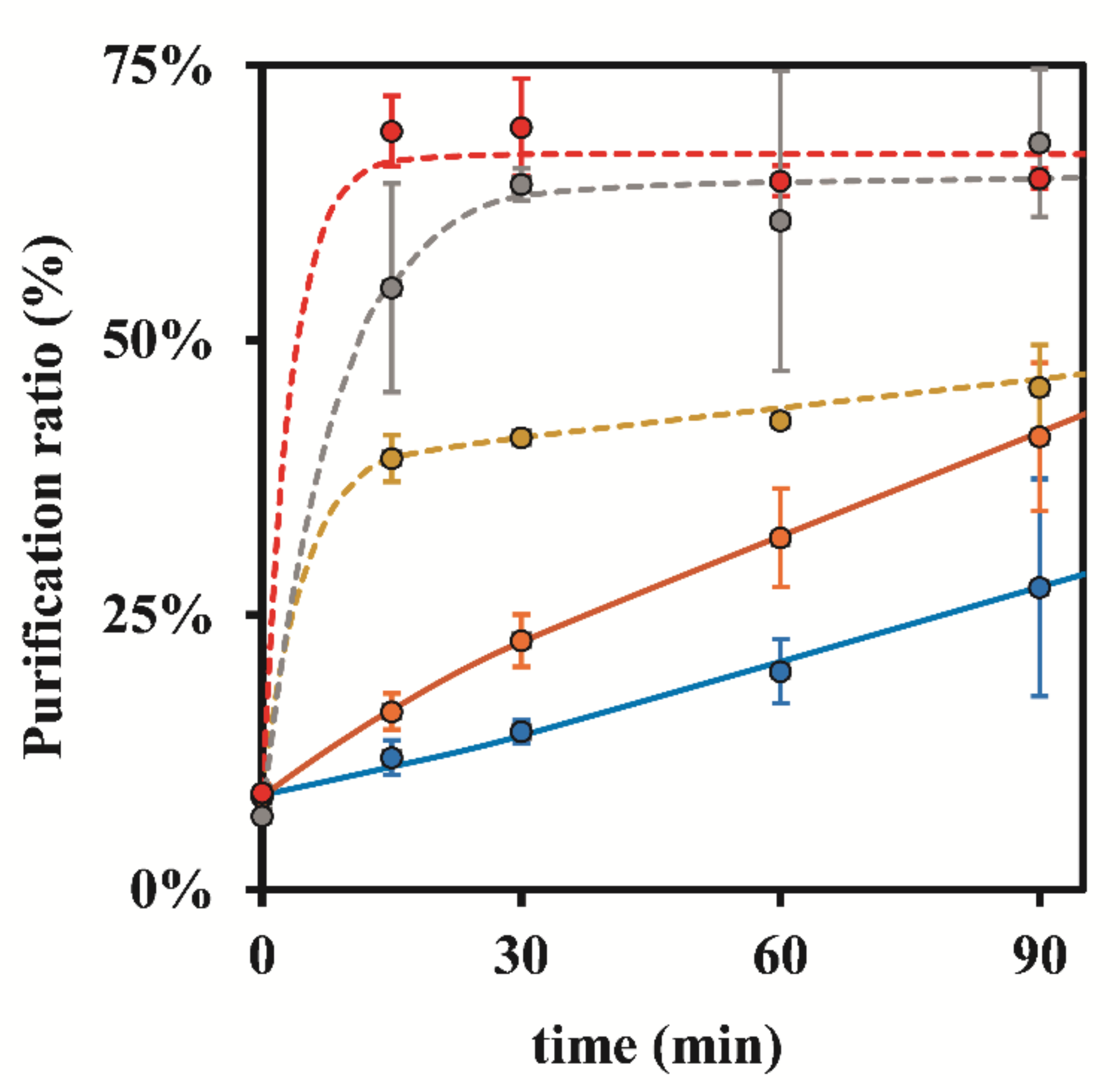
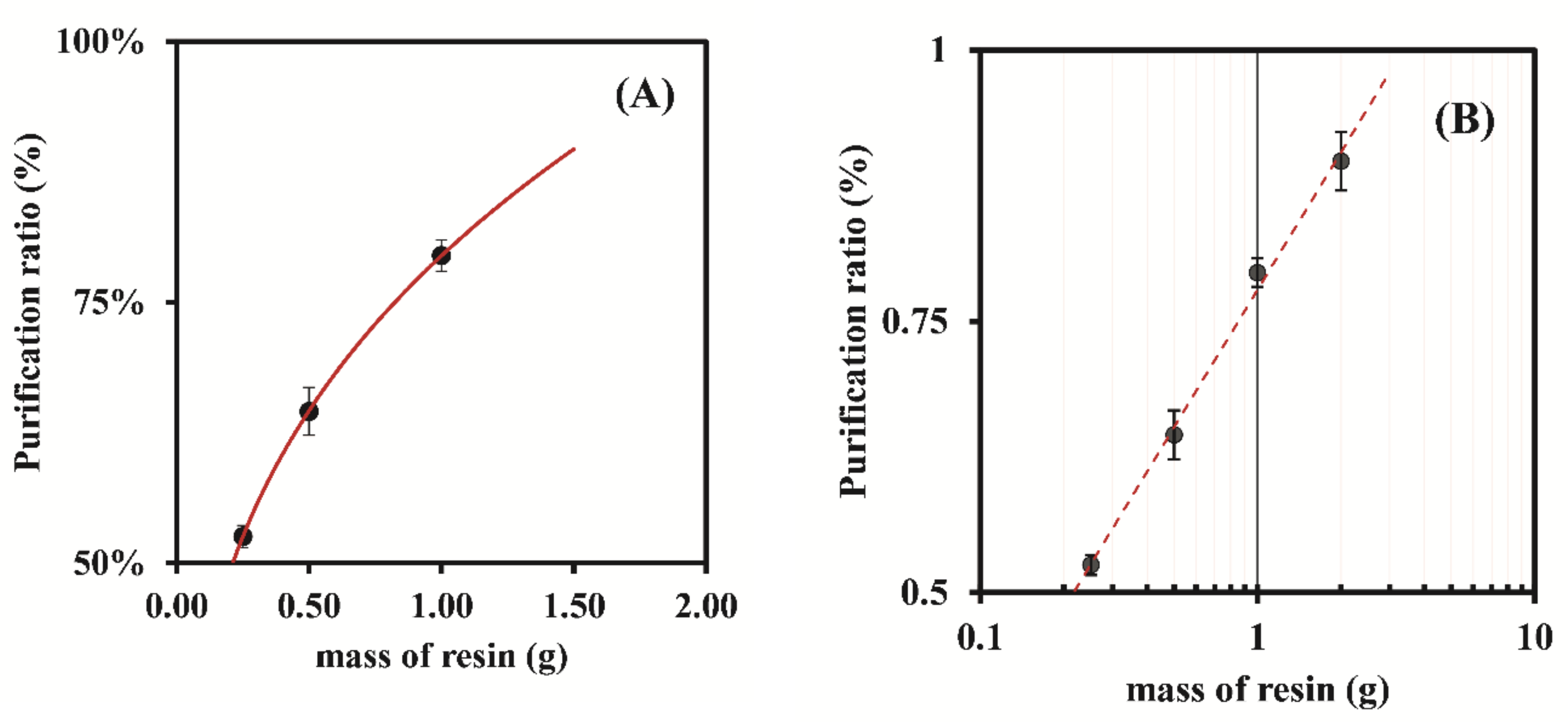
3.1.1. Dowex 50W-X8 50–100 Mesh
3.1.2. Bio-Rad 50W-X8 100–200 Mesh
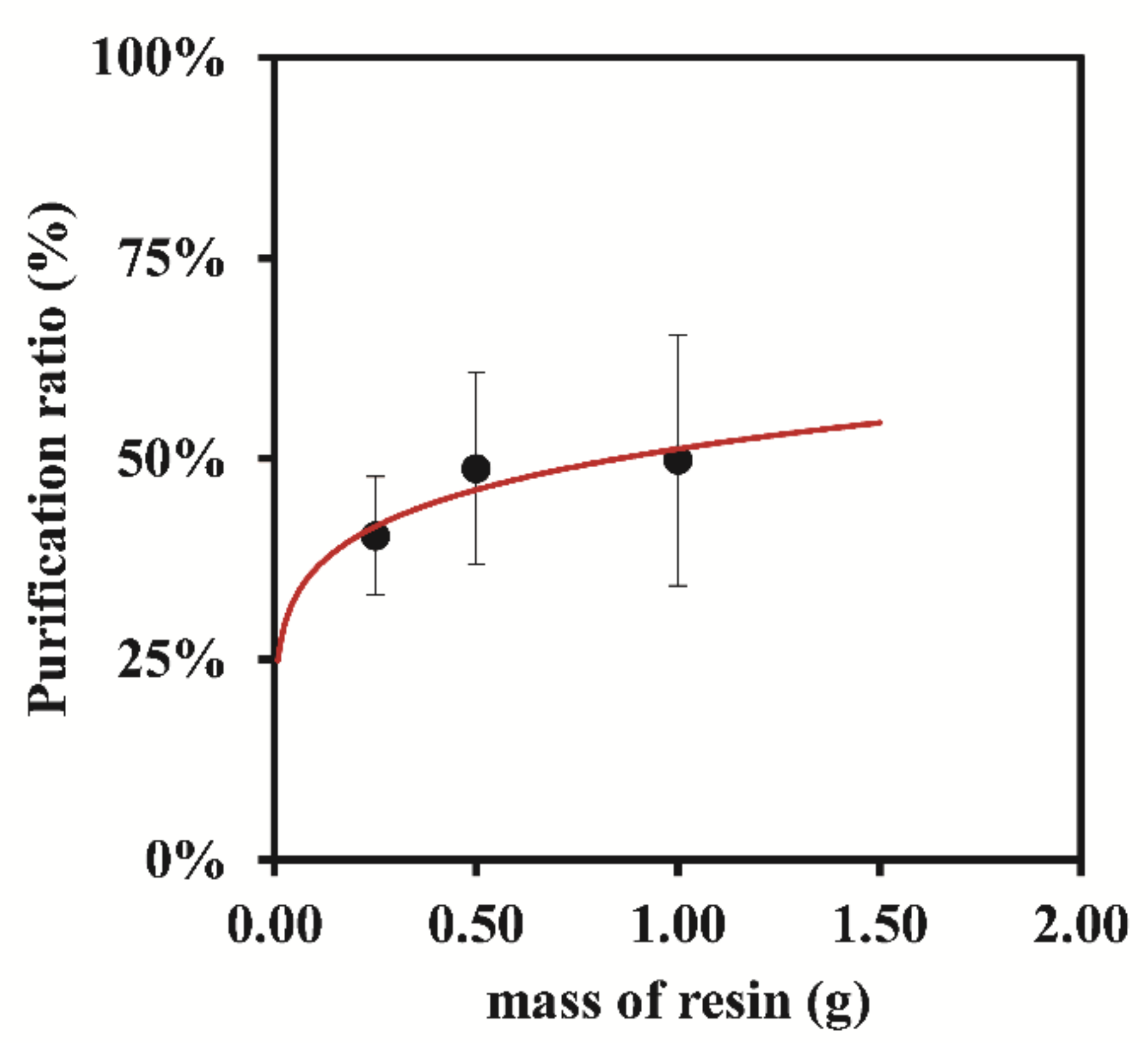
3.1.3. Bio-Rad 50W-X8 200–400 Mesh
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yu, S.-J.; Yin, Y.-G.; Liu, J.-F. Silver nanoparticles in the environment. Environ. Sci. Process. Impacts 2013, 15, 78–92. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-F.; Yu, S.-J.; Yin, Y.-G.; Chao, J.-B. Methods for separation, identification, characterization and quantification of silver nanoparticles. TrAC Trends Anal. Chem. 2012, 33, 95–106. [Google Scholar] [CrossRef]
- Martins, C.S.M.; Sousa, H.B.A.; Prior, J.A.V. From Impure to Purified Silver Nanoparticles: Advances and Timeline in Separation Methods. Nanomaterials 2021, 11, 3407. [Google Scholar] [CrossRef] [PubMed]
- Kahnouji, Y.A.; Mosaddegh, E.; Bolorizadeh, M.A. Detailed analysis of size-separation of silver nanoparticles by density gradient centrifugation method. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 103, 109817. [Google Scholar] [CrossRef]
- Lee, S.H.; Salunke, B.K.; Kim, B.S. Sucrose density gradient centrifugation separation of gold and silver nanoparticles synthesized using Magnolia kobus plant leaf extracts. Biotechnol. Bioprocess Eng. 2014, 19, 169–174. [Google Scholar] [CrossRef]
- Sun, X.; Tabakman, S.M.; Seo, W.-S.; Zhang, L.; Zhang, G.; Sherlock, S.; Bai, L.; Dai, H. Separation of Nanoparticles in a Density Gradient: FeCo@C and Gold Nanocrystals. Angew. Chem. Int. Ed. Engl. 2009, 48, 939–942. [Google Scholar] [CrossRef]
- Mwilu, S.K.; Siska, E.; Baig, R.N.; Varma, R.S.; Heithmar, E.; Rogers, K.R. Separation and measurement of silver nanoparticles and silver ions using magnetic particles. Sci. Total Environ. 2014, 472, 316–323. [Google Scholar] [CrossRef] [Green Version]
- Luo, L.; Yang, Y.; Li, H.; Ding, R.; Wang, Q.; Yang, Z. Size characterization of silver nanoparticles after separation from silver ions in environmental water using magnetic reduced graphene oxide. Sci. Total Environ. 2018, 612, 1215–1222. [Google Scholar] [CrossRef]
- Inglezakis, V.; Kurbanova, A.; Molkenova, A.; Zorpas, A.; Atabaev, T. Magnetic Fe3O4-Ag0 Nanocomposites for Effective Mercury Removal from Water. Sustainability 2020, 12, 5489. [Google Scholar] [CrossRef]
- Kato, H. Field-flow fractionation (FFF) with various detection systems. In Characterization of Nanoparticles; Hodoroaba, V.-D., Unger, W.E.S., Shard, A.G., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 249–264. [Google Scholar]
- António, D.C.; Cascio, C.; Gilliland, D.; Nogueira, A.J.A.; Rossi, F.; Calzolai, L. Characterization of silver nanoparticles-alginate complexes by combined size separation and size measurement techniques. Biointerphases 2016, 11, 04B309. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.-C.; Lee, B.-T.; Lee, S.; Hwang, Y.S.; Jo, E.; Eom, I.-C.; Lee, S.-W.; Kim, S.-O. Optimisation, evaluation and application of asymmetrical flow field-flow fractionation with single particle inductively coupled plasma mass spectrometry (SP-ICP-MS) to characterise silver nanoparticles in environmental media. Microchem. J. 2016, 129, 219–230. [Google Scholar] [CrossRef]
- Müller, D.; Cattaneo, S.; Meier, F.; Welz, R.; De Mello, A.J. Nanoparticle separation with a miniaturized asymmetrical flow field-flow fractionation cartridge. Front. Chem. 2015, 3, 45. [Google Scholar] [CrossRef] [Green Version]
- Marassi, V.; Casolari, S.; Roda, B.; Zattoni, A.; Reschiglian, P.; Panzavolta, S.; Tofail, S.A.; Ortelli, S.; Delpivo, C.; Blosi, M.; et al. Hollow-fiber flow field-flow fractionation and multi-angle light scattering investigation of the size, shape and metal-release of silver nanoparticles in aqueous medium for nano-risk assessment. J. Pharm. Biomed. Anal. 2015, 106, 92–99. [Google Scholar] [CrossRef]
- Saenmuangchin, R.; Mettakoonpitak, J.; Shiowatana, J.; Siripinyanond, A. Separation of silver nanoparticles by hollow fiber flow field-flow fractionation: Addition of tannic acid into carrier liquid as a modifier. J. Chromatogr. A 2015, 1415, 115–122. [Google Scholar] [CrossRef]
- Ramos, K.; Ramos, L.; Cámara, C.; Gómez-Gómez, M. Characterization and quantification of silver nanoparticles in nutraceuticals and beverages by asymmetric flow field flow fractionation coupled with inductively coupled plasma mass spectrometry. J. Chromatogr. A 2014, 1371, 227–236. [Google Scholar] [CrossRef] [Green Version]
- Hanley, T.A.; Saadawi, R.; Zhang, P.; Caruso, J.A.; Landero-Figueroa, J. Separation of silver ions and starch modified silver nanoparticles using high performance liquid chromatography with ultraviolet and inductively coupled mass spectrometric detection. Spectrochim. Acta Part B At. Spectrosc. 2014, 100, 173–179. [Google Scholar] [CrossRef]
- Konop, M.; Kłodzińska, E.; Borowiec, J.; Laskowska, A.; Czuwara, J.; Konieczka, P.; Cieślik, B.; Waraksa, E.; Rudnicka, L. Application of micellar electrokinetic chromatography for detection of silver nanoparticles released from wound dressing. Electrophoresis 2019, 40, 1565–1572. [Google Scholar] [CrossRef]
- Proulx, K.; Hadioui, M.; Wilkinson, K.J. Separation, detection and characterization of nanomaterials in municipal wastewaters using hydrodynamic chromatography coupled to ICPMS and single particle ICPMS. Anal. Bioanal. Chem. 2016, 408, 5147–5155. [Google Scholar] [CrossRef]
- Sötebier, C.A.; Weidner, S.M.; Jakubowski, N.; Panne, U.; Bettmer, J. Separation and quantification of silver nanoparticles and silver ions using reversed phase high performance liquid chromatography coupled to inductively coupled plasma mass spectrometry in combination with isotope dilution analysis. J. Chromatogr. A 2016, 1468, 102–108. [Google Scholar] [CrossRef]
- Soto-Alvaredo, J.; Montes-Bayón, M.; Bettmer, J. Speciation of Silver Nanoparticles and Silver(I) by Reversed-Phase Liquid Chromatography Coupled to ICPMS. Anal. Chem. 2013, 85, 1316–1321. [Google Scholar] [CrossRef]
- Kowalczyk, B.; Lagzi, I.; Grzybowski, B.A. Nanoseparations: Strategies for size and/or shape-selective purification of nanoparticles. Curr. Opin. Colloid Interface Sci. 2011, 16, 135–148. [Google Scholar] [CrossRef]
- Zhou, X.-X.; Liu, R.; Liu, J.-F. Rapid Chromatographic Separation of Dissoluble Ag(I) and Silver-Containing Nanoparticles of 1–100 Nanometer in Antibacterial Products and Environmental Waters. Environ. Sci. Technol. 2014, 48, 14516–14524. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Zhou, X.; Hu, L.; Yin, Y.; Liu, J. Simultaneous size characterization and mass quantification of the in vivo core-biocorona structure and dissolved species of silver nanoparticles. J. Environ. Sci. 2018, 63, 227–235. [Google Scholar] [CrossRef] [PubMed]
- González Fá, A.J.; Cerutti, I.; Springer, V.; Girotti, S.; Centurión, M.E.; Di Nezio, M.S.; Pistonesi, M.F. Simple Characterization of Green-Synthesized Silver Nanoparticles by Capillary Electrophoresis. Chromatographia 2017, 80, 1459–1466. [Google Scholar] [CrossRef]
- Michalke, B.; Vinković-Vrček, I. Speciation of nano and ionic form of silver with capillary electrophoresis-inductively coupled plasma mass spectrometry. J. Chromatogr. A 2018, 1572, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Franze, B.; Engelhard, C. Fast Separation, Characterization, and Speciation of Gold and Silver Nanoparticles and Their Ionic Counterparts with Micellar Electrokinetic Chromatography Coupled to ICP-MS. Anal. Chem. 2014, 86, 5713–5720. [Google Scholar] [CrossRef] [PubMed]
- Mozhayeva, D.; Engelhard, C. Separation of Silver Nanoparticles with Different Coatings by Capillary Electrophoresis Coupled to ICP-MS in Single Particle Mode. Anal. Chem. 2017, 89, 9767–9774. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.-K.; Ko, F.-H.; Huang, P.-W.; Wu, C.-H.; Chu, T.-C. Studying the size/shape separation and optical properties of silver nanoparticles by capillary electrophoresis. J. Chromatogr. A 2005, 1062, 139–145. [Google Scholar] [CrossRef]
- Hanauer, M.; Pierrat, S.; Zins, I.; Lotz, A.; Sönnichsen, C. Separation of Nanoparticles by Gel Electrophoresis According to Size and Shape. Nano Lett. 2007, 7, 2881–2885. [Google Scholar] [CrossRef]
- Mudalige, T.K.; Qu, H.; Van Haute, D.; Ansar, S.M.; Linder, S.W. Capillary electrophoresis and asymmetric flow field-flow fractionation for size-based separation of engineered metallic nanoparticles: A critical comparative review. TrAC Trends Anal. Chem. 2018, 106, 202–212. [Google Scholar] [CrossRef]
- Herrero, M.; Mendiola, J.A.; Ibáñez, E. Gas expanded liquids and switchable solvents. Curr. Opin. Green Sustain. Chem. 2017, 5, 24–30. [Google Scholar] [CrossRef]
- McLeod, M.C.; Anand, M.; Kitchens, C.L.; Roberts, C.B. Precise and Rapid Size Selection and Targeted Deposition of Nanoparticle Populations Using CO2 Gas Expanded Liquids. Nano Lett. 2005, 5, 461–465. [Google Scholar] [CrossRef]
- Mori, Y. Size-Selective Separation Techniques for Nanoparticles in Liquid. KONA Powder Part. J. 2015, 32, 102–114. [Google Scholar] [CrossRef] [Green Version]
- Tovstun, S.A.; Razumov, V.F. Theory of size-selective precipitation. J. Nanopart. Res. 2016, 19, 8. [Google Scholar] [CrossRef]
- Yu, S.-J.; Chao, J.-B.; Sun, J.; Yin, Y.-G.; Liu, J.-F.; Jiang, G.-B. Quantification of the Uptake of Silver Nanoparticles and Ions to HepG2 Cells. Environ. Sci. Technol. 2013, 47, 3268–3274. [Google Scholar] [CrossRef]
- Liu, J.-F.; Chao, J.-B.; Liu, R.; Tan, Z.-Q.; Yin, Y.-G.; Wu, Y.; Jiang, G.-B. Cloud Point Extraction as an Advantageous Preconcentration Approach for Analysis of Trace Silver Nanoparticles in Environmental Waters. Anal. Chem. 2009, 81, 6496–6502. [Google Scholar] [CrossRef]
- Wu, Z.-H.; Tseng, W.-L. Combined cloud point extraction and Tween 20-stabilized gold nanoparticles for colorimetric assay of silver nanoparticles in environmental water. Anal. Methods 2011, 3, 2915–2920. [Google Scholar] [CrossRef]
- Chao, J.-B.; Liu, J.-F.; Yu, S.-J.; Feng, Y.-D.; Tan, Z.-Q.; Liu, R.; Yin, Y.-G. Speciation Analysis of Silver Nanoparticles and Silver Ions in Antibacterial Products and Environmental Waters via Cloud Point Extraction-Based Separation. Anal. Chem. 2011, 83, 6875–6882. [Google Scholar] [CrossRef]
- Arya, S.S.; Kaimal, A.M.; Chib, M.; Sonawane, S.K.; Show, P.L. Novel, energy efficient and green cloud point extraction: Technology and applications in food processing. J. Food Sci. Technol. 2019, 56, 524–534. [Google Scholar] [CrossRef]
- Chen, X.; Sun, S.; Wang, X.; Wen, J.; Wang, Y.; Cao, X.; Yuan, T.; Wang, S.; Shi, Q.; Sun, R. One-pot preparation and characterization of lignin-based cation exchange resin and its utilization in Pb (II) removal. Bioresour. Technol. 2020, 295, 122297. [Google Scholar] [CrossRef]
- Silva, R.A.; Hawboldt, K.; Zhang, Y. Application of resins with functional groups in the separation of metal ions/species—A review. Miner. Process. Extr. Met. Rev. 2018, 39, 395–413. [Google Scholar] [CrossRef]
- Vagliasindi, F.G.; Belgiorno, V.; Napoli, R.M. Water treatment in remote and rural areas: A conceptual screening protocol for appropriate pou/poe technologies. In Environmental Engineering and Renewable Energy; Gavasci, R., Zandaryaa, S., Eds.; Elsevier: Oxford, UK, 1998; pp. 329–336. [Google Scholar]
- Naushad, M.; Alothman, Z.A. Separation of toxic Pb2+ metal from aqueous solution using strongly acidic cation-exchange resin: Analytical applications for the removal of metal ions from pharmaceutical formulation. Desalin. Water Treat. 2015, 53, 2158–2166. [Google Scholar] [CrossRef]
- Iglesias, M.; Torrent, L. Silver Nanoparticles and Ionic Silver Separation Using a Cation-Exchange Resin. Variables Affecting Their Separation and Improvements of AgNP Characterization by SP-ICPMS. Nanomaterials 2021, 11, 2626. [Google Scholar] [CrossRef]
- Hadioui, M.; Leclerc, S.; Wilkinson, K.J. Multimethod quantification of Ag+ release from nanosilver. Talanta 2013, 105, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Hyung, H.; Fortner, J.D.; Hughes, J.B.; Kim, J.-H. Natural Organic Matter Stabilizes Carbon Nanotubes in the Aqueous Phase. Environ. Sci. Technol. 2007, 41, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Leopold, K.; Schuster, M. Effective and selective extraction of noble metal nanoparticles from environmental water through a noncovalent reversible reaction on an ionic exchange resin. Chem. Commun. 2012, 48, 9165–9167. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, M.; Prior, J.A.V. Microwave Aqueous Dissolution of Potato Starch for the Synthesis of Starch Capped Silver Nanoparticles. Starch-Stärke 2021, 73, 2000205. [Google Scholar] [CrossRef]
- Cooke, J.; Hebert, D.; Kelly, J.A. Sweet Nanochemistry: A Fast, Reliable Alternative Synthesis of Yellow Colloidal Silver Nanoparticles Using Benign Reagents. J. Chem. Educ. 2015, 92, 345–349. [Google Scholar] [CrossRef]

| Resin. | Matrix | Particle Size (Mesh) | Cross-Linkage | Capacity (meq/mL) |
|---|---|---|---|---|
| Dowex 50W-X8 | styrene divinylbenzene | 50–100 | 8% | 1.1 |
| Bio-Rad 50W-X8 | styrene divinylbenzene | 100–200 | 8% | 1.7 |
| Bio-Rad 50W-X8 | styrene divinylbenzene | 200–400 | 8% | 1.7 |
| Contact Time (h) | 0.50 g | 1.00 g | ||
|---|---|---|---|---|
| CAg total (mg/L) | CAg in AgNPs (mg/L) | CAg total (mg/L) | CAg in AgNPs (mg/L) | |
| 0 | 1176.80 | 66.94 | 959.20 | 69.97 |
| 1 | 90.09 | 38.84 | 121.59 | 58.50 |
| 2 | 45.56 | 40.34 | 68.03 | 63.16 |
| Resin Mass (g) | Time of Contact (min) | |||||
|---|---|---|---|---|---|---|
| 0 | 15 | 30 | 60 | 90 | ||
| S-OFF | 0.25 | 8.44 ± 1.18 | 12.01 ± 1.60 | 14.36 ± 1.05 | 19.85 ± 2.91 | 27.49 ± 9.91 |
| 0.50 | 8.44 ± 1.18 | 16.20 ± 1.66 | 22.66 ± 2.37 | 32.00 ± 4.48 | 41.21 ± 6.72 | |
| S-ON | 0.25 | 8.80 ± 0.14 | 39.25 ± 2.11 | 41.12 ± 0.29 | 42.65 ± 0.30 | 45.70 ± 3.86 |
| 0.50 | 8.80 ± 0.14 | 69.02 ± 3.20 | 69.36 ± 4.43 | 64.49 ± 1.39 | 64.68 ± 0.93 | |
| 1.00 | 6.68 ± 0.03 | 54.75 ± 9.48 | 64.16 ± 1.46 | 60.84 ± 13.64 | 67.95 ± 6.72 | |
| Resin Mass (g) | Time of Contact (min) | ||||
|---|---|---|---|---|---|
| 0 | 15 | 30 | 60 | 90 | |
| 0.25 | 7.90 ± 0.06 | 52.55 ± 1.07 | 51.96 ± 1.60 | 51.71 ± 2.38 | 51.74 ± 2.27 |
| 0.50 | 8.05 ± 0.05 | 64.55 ± 2.02 | 68.91 ± 2.43 | 66.48 ± 1.74 | 68.81 ± 0.54 |
| 1.00 | 8.01 ± 0.10 | 79.48 ± 1.81 | 78.58 ± 6.48 | 82.98 ± 2.05 | 83.62 ± 2.80 |
| Resin Mass (g) | Time of Contact (min) | ||||
|---|---|---|---|---|---|
| 0 | 15 | 30 | 60 | 90 | |
| 0.25 | 8.10 ± 0.92 | 40.39 ± 7.37 | 42.39 ± 6.09 | 44.92 ± 6.27 | 35.80 ± 19.18 |
| 0.50 | 8.10 ± 0.92 | 48.75 ± 11.94 | 42.30 ± 16.50 | 42.62 ± 13.55 | 51.74 ± 15.00 |
| 1.00 | 7.50 ± 1.00 | 49.80 ± 15.62 | 37.72 ± 7.45 | 40.26 ± 5.30 | 44.66 ± 9.45 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martins, C.S.M.; Araújo, A.N.; de Gouveia, L.P.; Prior, J.A.V. Minimizing the Silver Free Ion Content in Starch Coated Silver Nanoparticle Suspensions with Exchange Cationic Resins. Nanomaterials 2022, 12, 644. https://doi.org/10.3390/nano12040644
Martins CSM, Araújo AN, de Gouveia LP, Prior JAV. Minimizing the Silver Free Ion Content in Starch Coated Silver Nanoparticle Suspensions with Exchange Cationic Resins. Nanomaterials. 2022; 12(4):644. https://doi.org/10.3390/nano12040644
Chicago/Turabian StyleMartins, Catarina S. M., Alberto N. Araújo, Luís Pleno de Gouveia, and João A. V. Prior. 2022. "Minimizing the Silver Free Ion Content in Starch Coated Silver Nanoparticle Suspensions with Exchange Cationic Resins" Nanomaterials 12, no. 4: 644. https://doi.org/10.3390/nano12040644
APA StyleMartins, C. S. M., Araújo, A. N., de Gouveia, L. P., & Prior, J. A. V. (2022). Minimizing the Silver Free Ion Content in Starch Coated Silver Nanoparticle Suspensions with Exchange Cationic Resins. Nanomaterials, 12(4), 644. https://doi.org/10.3390/nano12040644







