Synthesis of a Lignin/Zinc Oxide Hybrid Nanoparticles System and Its Application by Nano-Priming in Maize
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of LNP, ZnO, and Hybrid ZnO@LNP
2.2. Characterization of LNP and Hybrid ZnO@LNP
2.3. DPPH Activity of ZnO@LNP
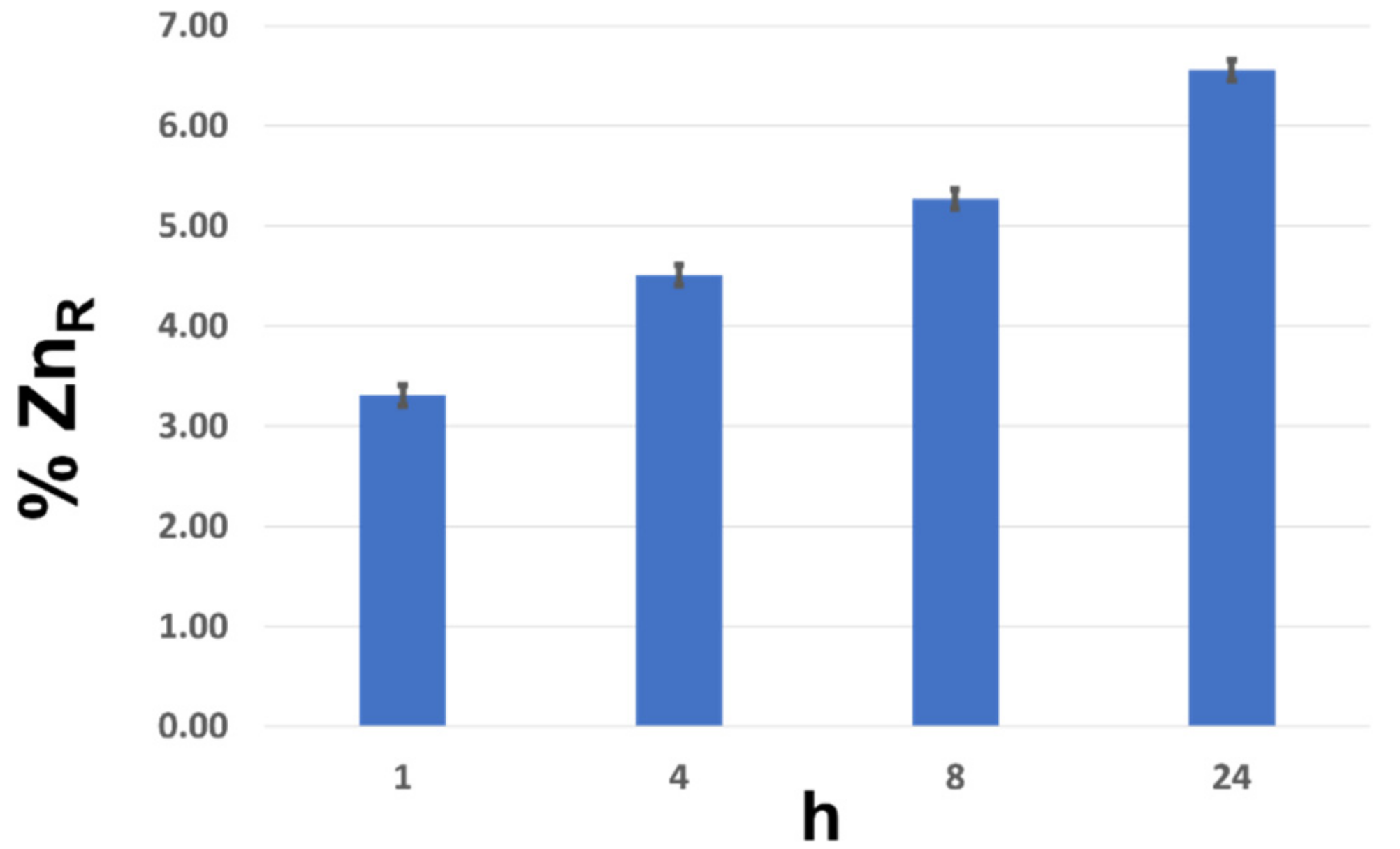
2.4. In Vitro Release Profile of Zn
2.5. Seed Nano-Priming and Measurements
- 🗸
- Control: only water
- 🗸
- T1: 8 mg of ZnO@LNP containing 0.32 mg of ZnO in water at a final volume of 100 mL to reach a ZnO concentration of 3.2 mg L−1;
- 🗸
- T2: 32 mg of ZnO@LNP containing 1.28 mg of ZnO in water at a final volume of 100 mL to reach a ZnO concentration of 12.8 mg L−1;
- 🗸
- T3: 128 mg of ZnO@LNP containing 1.28 mg of ZnO in water at a final volume of 100 mL to reach a ZnO concentration of 51.2 mg L−1;
- 🗸
- T4: 512 mg of ZnO@LNP containing 20.48 mg of ZnO in water at a final volume of 100 mL to reach the concentration of 204.8 mg L−1;
- 🗸
- T5: 2.048 mg of ZnO@LNP containing 81.92 mg of ZnO in water at a final volume of 100 mL to reach the concentration of 819.2 mg L−1.
2.6. Plant Materials and Growth Conditions
2.7. Estimation of Chlorophyll a and b, Carotenoid, Anthocyanin, Total Phenols, DPPH, MDA
2.8. Statistical Analysis
3. Results
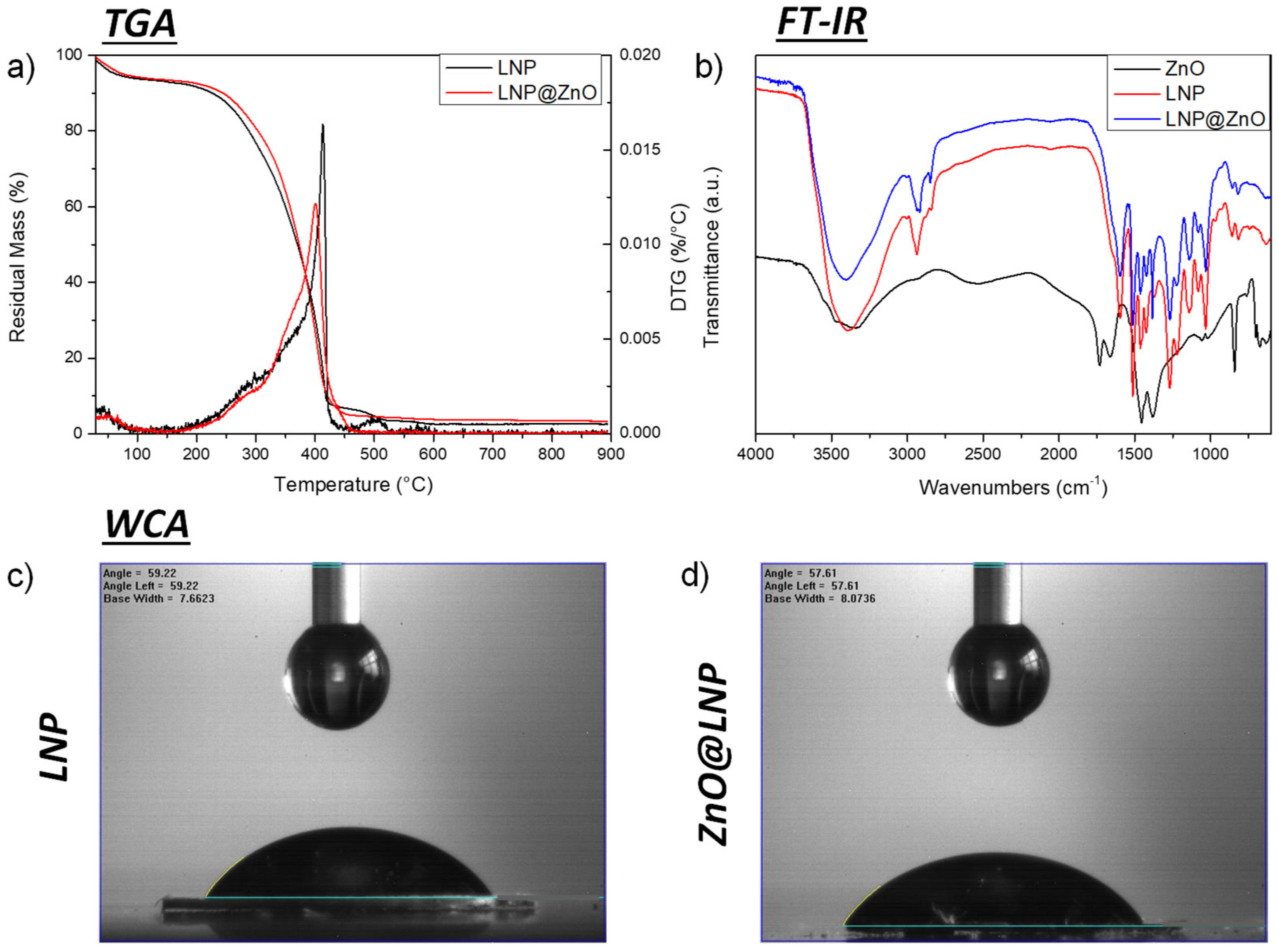
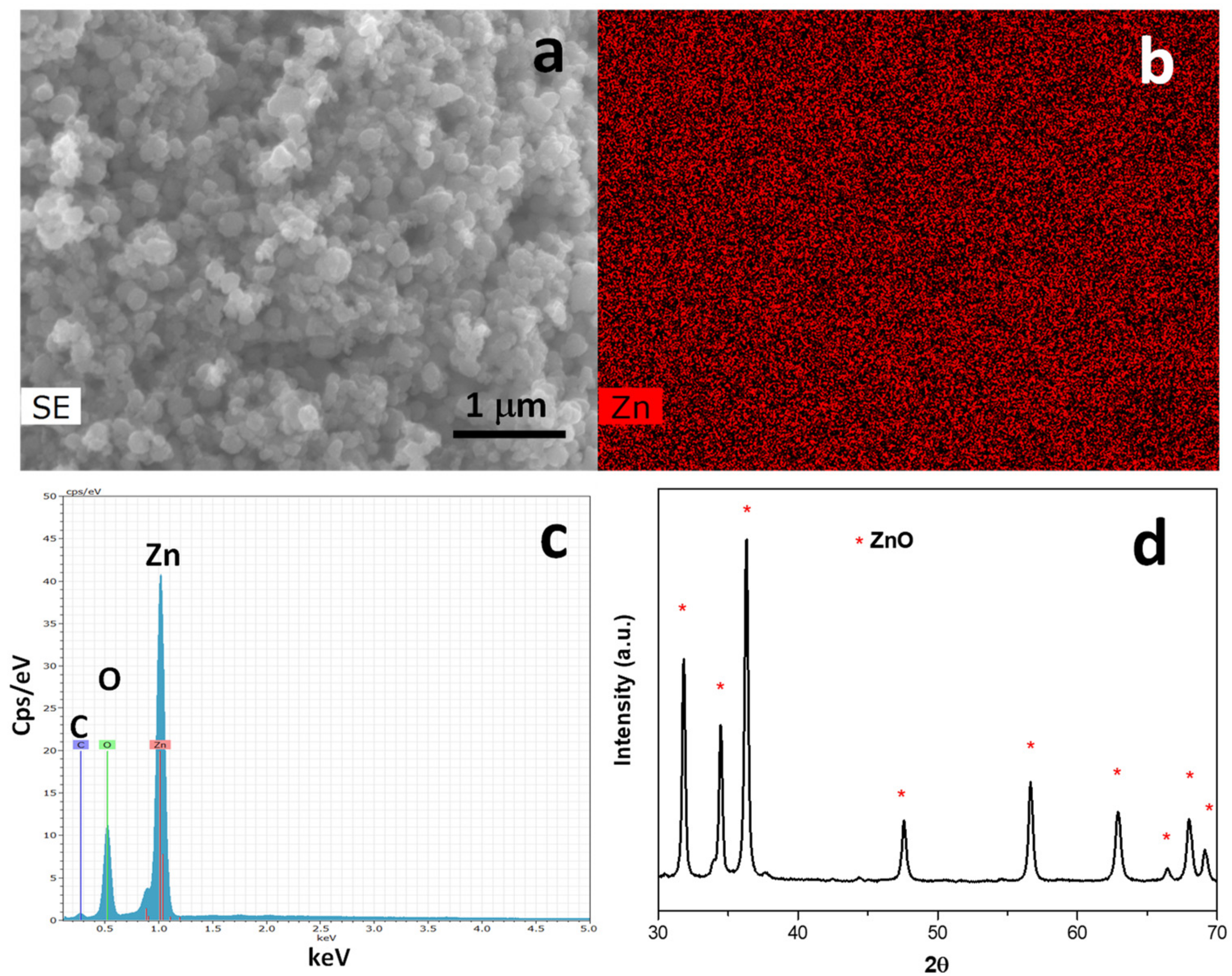
3.1. ZnO@LNP Characterization
3.2. Effect of ZnO@LNP on Seed Development and Maize Growth
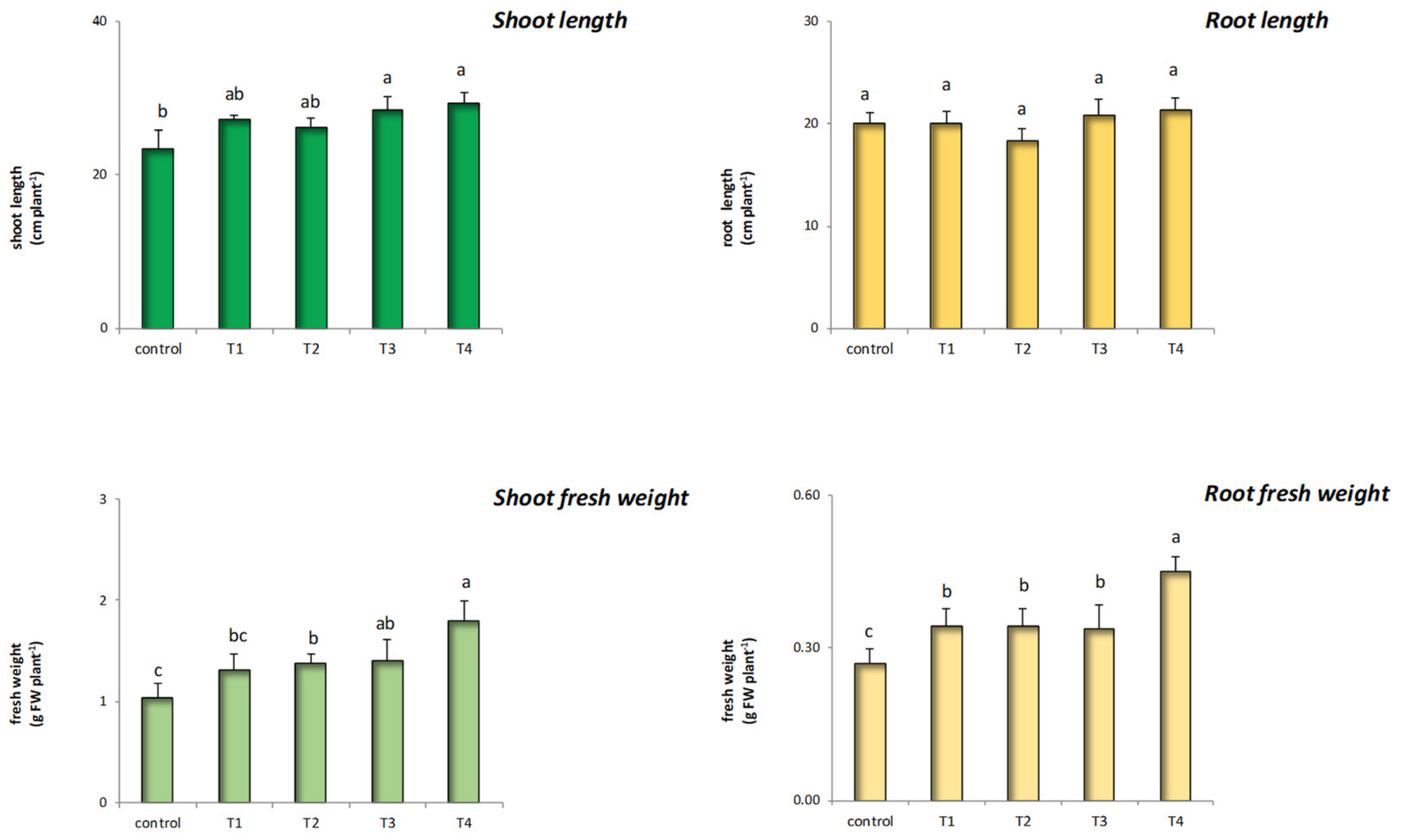
3.3. Effect of ZnO@LNP on Plant Growth
3.4. Effect of ZnO@LNP on Pigment and Soluble Protein
3.5. Effect of ZnO@LNP on Antioxidants and Oxidative Status of Nano-Primed Maize Seedlings
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Puglia, D.; Pezzolla, D.; Gigliotti, G.; Torre, L.; Bartucca, M.L.; Del Buono, D. The Opportunity of Valorizing Agricultural Waste, through Its Conversion into Biostimulants, Biofertilizers, and Biopolymers. Sustainability 2021, 13, 2710. [Google Scholar] [CrossRef]
- Del Buono, D. Can Biostimulants Be Used to Mitigate the Effect of Anthropogenic Climate Change on Agriculture? It Is Time to Respond. Sci. Total Environ. 2021, 751, 141763. [Google Scholar] [CrossRef]
- Kasivelu, G.; Selvaraj, T.; Malaichamy, K.; Kathickeyan, D.; Shkolnik, D.; Chaturvedi, S. Nano-Micronutrients [γ-Fe2O3(Iron) and ZnO (Zinc)]: Green Preparation, Characterization, Agro-Morphological Characteristics and Crop Productivity Studies in Two Crops (Rice and Maize). New J. Chem. 2020, 44, 11373–11383. [Google Scholar] [CrossRef]
- Ahmed, S.; Annu; Chaudhry, S.A.; Ikram, S. A Review on Biogenic Synthesis of ZnO Nanoparticles Using Plant Extracts and Microbes: A Prospect towards Green Chemistry. J. Photochem. Photobiol. B 2017, 166, 272–284. [Google Scholar] [CrossRef]
- Sturikova, H.; Krystofova, O.; Huska, D.; Adam, V. Zinc, Zinc Nanoparticles and Plants. J. Hazard. Mater. 2018, 349, 101–110. [Google Scholar] [CrossRef]
- Guha, T.; Gopal, G.; Kundu, R.; Mukherjee, A. Nanocomposites for Delivering Agrochemicals: A Comprehensive Review. J. Agric. Food Chem. 2020, 68, 3691–3702. [Google Scholar] [CrossRef]
- Luzi, F.; Tortorella, I.; Di Michele, A.; Dominici, F.; Argentati, C.; Morena, F.; Torre, L.; Puglia, D.; Martino, S. Novel Nanocomposite PLA Films with Lignin/Zinc Oxide Hybrids: Design, Characterization, Interaction with Mesenchymal Stem Cells. Nanomaterials 2020, 10, 2176. [Google Scholar] [CrossRef]
- Chi, Z.; Hao, L.; Dong, H.; Yu, H.; Liu, H.; Yu, H. The Innovative Application of Organosolv Lignin for Nanomaterial Modification to Boost Its Heavy Metal Detoxification Performance in the Aquatic Environment. Chem. Eng. J. 2020, 382, 122789. [Google Scholar] [CrossRef]
- Schneider, W.D.H.; Dillon, A.J.P.; Camassola, M. Lignin Nanoparticles Enter the Scene: A Promising Versatile Green Tool for Multiple Applications. Biotechnol. Adv. 2021, 47, 107685. [Google Scholar] [CrossRef]
- Yang, W.; Fortunati, E.; Gao, D.; Balestra, G.M.; Giovanale, G.; He, X.; Torre, L.; Kenny, J.M.; Puglia, D. Valorization of Acid Isolated High Yield Lignin Nanoparticles as Innovative Antioxidant/Antimicrobial Organic Materials. ACS Sustain. Chem. Eng. 2018, 6, 3502–3514. [Google Scholar] [CrossRef]
- Del Buono, D.; Luzi, F.; Puglia, D. Lignin Nanoparticles: A Promising Tool to Improve Maize Physiological, Biochemical, and Chemical Traits. Nanomaterials 2021, 11, 846. [Google Scholar] [CrossRef]
- Figueiredo, P.; Lintinen, K.; Hirvonen, J.T.; Kostiainen, M.A.; Santos, H.A. Properties and Chemical Modifications of Lignin: Towards Lignin-Based Nanomaterials for Biomedical Applications. Prog. Mater. Sci. 2018, 93, 233–269. [Google Scholar] [CrossRef]
- Wang, H.; Qiu, X.; Liu, W.; Yang, D. Facile Preparation of Well-Combined Lignin-Based Carbon/ZnO Hybrid Composite with Excellent Photocatalytic Activity. Appl. Surf. Sci. 2017, 426, 206–216. [Google Scholar] [CrossRef]
- Sharma, D.; Sabela, M.I.; Kanchi, S.; Mdluli, P.S.; Singh, G.; Stenström, T.A.; Bisetty, K. Biosynthesis of ZnO Nanoparticles Using Jacaranda Mimosifolia Flowers Extract: Synergistic Antibacterial Activity and Molecular Simulated Facet Specific Adsorption Studies. J. Photochem. Photobiol. B 2016, 162, 199–207. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Li, Q.; Jiang, J.; Mao, Y. Morphology-Tunable Synthesis of ZnO Nanoforest and Its Photoelectrochemical Performance. Nanoscale 2014, 6, 8769–8780. [Google Scholar] [CrossRef]
- Pineda-Reyes, A.M.; Olvera, M.D.L.L. Synthesis of ZnO Nanoparticles from Water-in-Oil (w/o) Microemulsions. Mater. Chem. Phys. 2018, 203, 141–147. [Google Scholar] [CrossRef]
- Klapiszewski, Ł.; Bula, K.; Dobrowolska, A.; Czaczyk, K.; Jesionowski, T. A high-density polyethylene container based on ZnO/lignin dual fillers with potential antimicrobial activity. Polymer Test. 2019, 73, 51–59. [Google Scholar] [CrossRef]
- Klapiszewski, Ł.; Grząbka-Zasadzińska, A.; Borysiak, S.; Jesionowski, T. Preparation and characterization of polypropylene composites reinforced by functional ZnO/lignin hybrid materials. Polymer Test. 2019, 79, 106058. [Google Scholar] [CrossRef]
- Klapiszewska, I.; Parus, A.; Ławniczak, Ł.; Jesionowski, T.; Klapiszewski, Ł.; Ślosarczyk, A. Production of antibacterial cement composites containing ZnO/lignin and ZnO–SiO2/lignin hybrid admixtures. Cem. Concr. Compos. 2021, 124, 104250. [Google Scholar] [CrossRef]
- Kumar, S.; Nehra, M.; Dilbaghi, N.; Marrazza, G.; Hassan, A.A.; Kim, K.-H. Nano-Based Smart Pesticide Formulations: Emerging Opportunities for Agriculture. J. Control. Release 2019, 294, 131–153. [Google Scholar] [CrossRef]
- Raliya, R.; Saharan, V.; Dimkpa, C.; Biswas, P. Nanofertilizer for Precision and Sustainable Agriculture: Current State and Future Perspectives. J. Agric. Food Chem. 2018, 66, 6487–6503. [Google Scholar] [CrossRef]
- Sharma, A.; Sood, K.; Kaur, J.; Khatri, M. Agrochemical Loaded Biocompatible Chitosan Nanoparticles for Insect Pest Management. Biocatal. Agric. Biotechnol. 2019, 18, 101079. [Google Scholar] [CrossRef]
- Parisi, C.; Vigani, M.; Rodríguez-Cerezo, E. Agricultural Nanotechnologies: What Are the Current Possibilities? Nano Today 2015, 10, 124–127. [Google Scholar] [CrossRef]
- Sun, L.; Wang, Y.; Wang, R.; Wang, R.; Zhang, P.; Ju, Q.; Xu, J. Physiological, Transcriptomic, and Metabolomic Analyses Reveal Zinc Oxide Nanoparticles Modulate Plant Growth in Tomato. Environ. Sci. Nano 2020, 7, 3587–3604. [Google Scholar] [CrossRef]
- Salama, D.M.; Osman, S.A.; Abd El-Aziz, M.E.; Abd Elwahed, M.S.A.; Shaaban, E.A. Effect of Zinc Oxide Nanoparticles on the Growth, Genomic DNA, Production and the Quality of Common Dry Bean (Phaseolus Vulgaris). Biocatal. Agric. Biotechnol. 2019, 18, 101083. [Google Scholar] [CrossRef]
- do Espirito Santo Pereira, A.; Caixeta Oliveira, H.; Fernandes Fraceto, L.; Santaella, C. Nanotechnology Potential in Seed Priming for Sustainable Agriculture. Nanomaterials 2021, 11, 267. [Google Scholar] [CrossRef]
- Navarro-López, E.; Ruíz-Nieto, A.; Ferreira, A.; Acién, F.G.; Gouveia, L. Biostimulant Potential of Scenedesmus Obliquus Grown in Brewery Wastewater. Molecules 2020, 25, 664. [Google Scholar] [CrossRef] [Green Version]
- Panfili, I.; Bartucca, M.L.; Marrollo, G.; Povero, G.; Del Buono, D. Application of a Plant Biostimulant To Improve Maize (Zea Mays) Tolerance to Metolachlor. J. Agric. Food Chem. 2019, 67, 12164–12171. [Google Scholar] [CrossRef]
- Venkatachalam, P.; Priyanka, N.; Manikandan, K.; Ganeshbabu, I.; Indiraarulselvi, P.; Geetha, N.; Muralikrishna, K.; Bhattacharya, R.C.; Tiwari, M.; Sharma, N.; et al. Enhanced Plant Growth Promoting Role of Phycomolecules Coated Zinc Oxide Nanoparticles with P Supplementation in Cotton (Gossypium Hirsutum L.). Plant Physiol. Biochem. 2017, 110, 118–127. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and Carotenoids: Measurement and Characterization by UV-VIS Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Parađiković, N.; Vinković, T.; Vinković Vrček, I.; Žuntar, I.; Bojić, M.; Medić-Šarić, M. Effect of Natural Biostimulants on Yield and Nutritional Quality: An Example of Sweet Yellow Pepper (Capsicum Annuum L.) Plants. J. Sci. Food Agric. 2011, 91, 2146–2152. [Google Scholar] [CrossRef]
- Chollet, B.; Lopez-Cuesta, J.-M.; Laoutid, F.; Ferry, L. Lignin Nanoparticles as a Promising Way for Enhancing Lignin Flame Retardant Effect in Polylactide. Materials 2019, 12, 2132. [Google Scholar] [CrossRef] [Green Version]
- Prieur, B.; Meub, M.; Wittemann, M.; Klein, R.; Bellayer, S.; Fontaine, G.; Bourbigot, S. Phosphorylation of Lignin: Characterization and Investigation of the Thermal Decomposition. RSC Adv. 2017, 7, 16866–16877. [Google Scholar] [CrossRef] [Green Version]
- Bajwa, D.S.; Shojaeiarani, J.; Liaw, J.D.; Bajwa, S.G. Role of Hybrid Nano-Zinc Oxide and Cellulose Nanocrystals on the Mechanical, Thermal, and Flammability Properties of Poly (Lactic Acid) Polymer. J. Compos. Sci. 2021, 5, 43. [Google Scholar] [CrossRef]
- Nagaraju, G.; Prashanth, S.A.; Shastri, M.; Yathish, K.V.; Anupama, C.; Rangappa, D. Electrochemical Heavy Metal Detection, Photocatalytic, Photoluminescence, Biodiesel Production and Antibacterial Activities of Ag–ZnO Nanomaterial. Mater. Res. Bull. 2017, 94, 54–63. [Google Scholar] [CrossRef]
- Khalafi, T.; Buazar, F.; Ghanemi, K. Phycosynthesis and Enhanced Photocatalytic Activity of Zinc Oxide Nanoparticles Toward Organosulfur Pollutants. Sci. Rep. 2019, 9, 6866. [Google Scholar] [CrossRef]
- Sowri Babu, K.; Ramachandra Reddy, A.; Sujatha, C.; Venugopal Reddy, K.; Mallika, A.N. Synthesis and Optical Characterization of Porous ZnO. J. Adv. Ceram. 2013, 2, 260–265. [Google Scholar] [CrossRef] [Green Version]
- Jose, L.M.; Kuriakose, S.; Thomas, S. Fabrication, Characterization and In Vitro Antifungal Property Evaluation of Biocompatible Lignin-Stabilized Zinc Oxide Nanoparticles Against Selected Pathogenic Fungal Strains. BioNanoScience 2020, 10, 583–596. [Google Scholar] [CrossRef]
- Kaur, R.; Thakur, N.S.; Chandna, S.; Bhaumik, J. Development of Agri-Biomass Based Lignin Derived Zinc Oxide Nanocomposites as Promising UV Protectant-Cum-Antimicrobial Agents. J. Mater. Chem. B 2020, 8, 260–269. [Google Scholar] [CrossRef]
- Gerbin, E.; Rivière, G.N.; Foulon, L.; Frapart, Y.M.; Cottyn, B.; Pernes, M.; Marcuello, C.; Godon, B.; Gainvors-Claisse, A.; Crônier, D.; et al. Tuning the Functional Properties of Lignocellulosic Films by Controlling the Molecular and Supramolecular Structure of Lignin. Int. J. Biol. Macromol. 2021, 181, 136–149. [Google Scholar] [CrossRef]
- Martins, N.C.T.; Avellan, A.; Rodrigues, S.; Salvador, D.; Rodrigues, S.M.; Trindade, T. Composites of Biopolymers and ZnO NPs for Controlled Release of Zinc in Agricultural Soils and Timed Delivery for Maize. ACS Appl. Nano Mater. 2020, 3, 2134–2148. [Google Scholar] [CrossRef]
- Ye, Y.; Cota-Ruiz, K.; Hernández-Viezcas, J.A.; Valdés, C.; Medina-Velo, I.A.; Turley, R.S.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Manganese Nanoparticles Control Salinity-Modulated Molecular Responses in Capsicum Annuum L. Through Priming: A Sustainable Approach for Agriculture. ACS Sustain. Chem. Eng. 2020, 8, 1427–1436. [Google Scholar] [CrossRef]
- Oracz, K.; Karpiński, S. Phytohormones Signaling Pathways and ROS Involvement in Seed Germination. Front. Plant Sci. 2016, 7, 864. [Google Scholar] [CrossRef] [Green Version]
- Choudhary, R.C.; Kumaraswamy, R.V.; Kumari, S.; Sharma, S.S.; Pal, A.; Raliya, R.; Biswas, P.; Saharan, V. Zinc Encapsulated Chitosan Nanoparticle to Promote Maize Crop Yield. Int. J. Biol. Macromol. 2019, 127, 126–135. [Google Scholar] [CrossRef]
- Večeřová, K.; Večeřa, Z.; Dočekal, B.; Oravec, M.; Pompeiano, A.; Tříska, J.; Urban, O. Changes of Primary and Secondary Metabolites in Barley Plants Exposed to CdO Nanoparticles. Environ. Pollut. 2016, 218, 207–218. [Google Scholar] [CrossRef]
- Del Buono, D.; Di Michele, A.; Costantino, F.; Trevisan, M.; Lucini, L. Biogenic ZnO Nanoparticles Synthesized Using a Novel Plant Extract: Application to Enhance Physiological and Biochemical Traits in Maize. Nanomaterials 2021, 11, 1270. [Google Scholar] [CrossRef]
- García-López, J.; Zavala-García, F.; Olivares-Sáenz, E.; Lira-Saldívar, R.; Díaz Barriga-Castro, E.; Ruiz-Torres, N.; Ramos-Cortez, E.; Vázquez-Alvarado, R.; Niño-Medina, G. Zinc Oxide Nanoparticles Boosts Phenolic Compounds and Antioxidant Activity of Capsicum Annuum L. during Germination. Agronomy 2018, 8, 215. [Google Scholar] [CrossRef] [Green Version]
- Adhikari, S.; Adhikari, A.; Ghosh, S.; Roy, D.; Azahar, I.; Basuli, D.; Hossain, Z. Assessment of ZnO-NPs Toxicity in Maize: An Integrative MicroRNAomic Approach. Chemosphere 2020, 249, 126197. [Google Scholar] [CrossRef]
- Lee, C.W.; Mahendra, S.; Zodrow, K.; Li, D.; Tsai, Y.-C.; Braam, J.; Alvarez, P.J.J. Developmental Phytotoxicity of Metal Oxide Nanoparticles to Arabidopsis Thaliana. Environ. Toxicol. Chem. 2010, 29, 669–675. [Google Scholar] [CrossRef]
- Narendhran, S.; Rajiv, P.; Sivaraj, R. Toxicity of ZnO Nanoparticles on Germinating Sesamum Indicum (Co-1) and Their Antibacterial Activity. Bull. Mater. Sci. 2016, 39, 415–421. [Google Scholar] [CrossRef] [Green Version]
- Guha, T.; Ravikumar, K.V.G.; Mukherjee, A.; Mukherjee, A.; Kundu, R. Nanopriming with Zero Valent Iron (NZVI) Enhances Germination and Growth in Aromatic Rice Cultivar (Oryza Sativa Cv. Gobindabhog L.). Plant Physiol. Biochem. 2018, 127, 403–413. [Google Scholar] [CrossRef]
- Das, C.K.; Jangir, H.; Kumar, J.; Verma, S.; Mahapatra, S.S.; Philip, D.; Srivastava, G.; Das, M. Nano-Pyrite Seed Dressing: A Sustainable Design for NPK Equivalent Rice Production. Nanotechnol. Environ. Eng. 2018, 3, 14. [Google Scholar] [CrossRef]
- Poynton, H.C.; Lazorchak, J.M.; Impellitteri, C.A.; Smith, M.E.; Rogers, K.; Patra, M.; Hammer, K.A.; Allen, H.J.; Vulpe, C.D. Differential Gene Expression in Daphnia Magna Suggests Distinct Modes of Action and Bioavailability for Zno Nanoparticles and Zn Ions. Environ. Sci. Technol. 2011, 45, 762–768. [Google Scholar] [CrossRef]
- El-Saadony, M.T.; Desoky, E.-S.M.; Saad, A.M.; Eid, R.S.M.; Selem, E.; Elrys, A.S. Biological Silicon Nanoparticles Improve Phaseolus Vulgaris L. Yield and Minimize Its Contaminant Contents on a Heavy Metals-Contaminated Saline Soil. J. Environ. Sci. China 2021, 106, 1–14. [Google Scholar] [CrossRef]
- Manzoor, N.; Ahmed, T.; Noman, M.; Shahid, M.; Nazir, M.M.; Ali, L.; Alnusaire, T.S.; Li, B.; Schulin, R.; Wang, G. Iron Oxide Nanoparticles Ameliorated the Cadmium and Salinity Stresses in Wheat Plants, Facilitating Photosynthetic Pigments and Restricting Cadmium Uptake. Sci. Total Environ. 2021, 769, 145221. [Google Scholar] [CrossRef]
- Singh, P.; Arif, Y.; Siddiqui, H.; Sami, F.; Zaidi, R.; Azam, A.; Alam, P.; Hayat, S. Nanoparticles Enhances the Salinity Toxicity Tolerance in Linum Usitatissimum L. by Modulating the Antioxidative Enzymes, Photosynthetic Efficiency, Redox Status and Cellular Damage. Ecotoxicol. Environ. Saf. 2021, 213, 112020. [Google Scholar] [CrossRef]
- Changlian, P.; Zhifang, L.; Guizhu, L.; Shaowei, C. The Anti-Photooxidation of Anthocyanins-Rich Leaves of a Purple Rice Cultivar. Sci. China Ser. C Life Sci. 2006, 49, 543–551. [Google Scholar] [CrossRef]
- Sonobe, R.; Yamashita, H.; Mihara, H.; Morita, A.; Ikka, T. Estimation of Leaf Chlorophyll a, b and Carotenoid Contents and Their Ratios Using Hyperspectral Reflectance. Remote Sens. 2020, 12, 3265. [Google Scholar] [CrossRef]
- Bartucca, M.L.; Guiducci, M.; Falcinelli, B.; Del Buono, D.; Benincasa, P. Blue:Red LED Light Proportion Affects Vegetative Parameters, Pigment Content, and Oxidative Status of Einkorn (Triticum Monococcum L. Ssp. Monococcum) Wheatgrass. J. Agric. Food Chem. 2020, 68, 8757–8763. [Google Scholar] [CrossRef]
- Gan, R.-Y.; Chan, C.-L.; Yang, Q.-Q.; Li, H.-B.; Zhang, D.; Ge, Y.-Y.; Gunaratne, A.; Ge, J.; Corke, H. Bioactive Compounds and Beneficial Functions of Sprouted Grains. In Sprouted Grains: Nutritional Value, Production, and Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 191–246. [Google Scholar]
- Ibrahim, A.B.M.; Mahmoud, G.A. Chemical- vs. Sonochemical-assisted Synthesis of ZnO Nanoparticles from a New Zinc Complex for Improvement of Carotene Biosynthesis from Rhodotorula Toruloides MH023518. Appl. Organomet. Chem. 2021, 35, e6086. [Google Scholar] [CrossRef]
- Sofo, A.; Bochicchio, R.; Amato, M.; Rendina, N.; Vitti, A.; Nuzzaci, M.; Altamura, M.M.; Falasca, G.; Rovere, F.D.; Scopa, A. Plant Architecture, Auxin Homeostasis and Phenol Content in Arabidopsis Thaliana Grown in Cadmium- and Zinc-Enriched Media. J. Plant Physiol. 2017, 216, 174–180. [Google Scholar] [CrossRef]
- Michalak, A. Phenolic Compounds and Their Antioxidant Activity in Plants Growing under Heavy Metal Stress. Pol. J. Environ. Stud. 2006, 15, 523–530. [Google Scholar]
- Abbasi, B.H.; Zahir, A.; Ahmad, W.; Nadeem, M.; Giglioli-Guivarc’h, N.; Hano, C. Biogenic Zinc Oxide Nanoparticles-Enhanced Biosynthesis of Lignans and Neolignans in Cell Suspension Cultures of Linum Usitatissimum L. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1367–1373. [Google Scholar] [CrossRef] [Green Version]
- Bustos-Torres, K.A.; Vazquez-Rodriguez, S.; la Cruz, A.M.; Sepulveda-Guzman, S.; Benavides, R.; Lopez-Gonzalez, R.; Torres-Martínez, L.M. Influence of the Morphology of ZnO Nanomaterials on Photooxidation of Polypropylene/ZnO Composites. Mater. Sci. Semicond. Process. 2017, 68, 217–225. [Google Scholar] [CrossRef]
- Xiang, L.; Zhao, H.-M.; Li, Y.-W.; Huang, X.-P.; Wu, X.-L.; Zhai, T.; Yuan, Y.; Cai, Q.-Y.; Mo, C.-H. Effects of the Size and Morphology of Zinc Oxide Nanoparticles on the Germination of Chinese Cabbage Seeds. Environ. Sci. Pollut. Res. 2015, 22, 10452–10462. [Google Scholar] [CrossRef]
- Javed, R.; Usman, M.; Yücesan, B.; Zia, M.; Gürel, E. Effect of Zinc Oxide (ZnO) Nanoparticles on Physiology and Steviol Glycosides Production in Micropropagated Shoots of Stevia Rebaudiana Bertoni. Plant Physiol. Biochem. 2017, 110, 94–99. [Google Scholar] [CrossRef]
- Del Buono, D.; Regni, L.; Del Pino, A.M.; Bartucca, M.L.; Palmerini, C.A.; Proietti, P. Effects of Megafol on the Olive Cultivar ’Arbequina’ Grown Under Severe Saline Stress in Terms of Physiological Traits, Oxidative Stress, Antioxidant Defenses, and Cytosolic Ca2+. Front. Plant Sci. 2021, 11, 603576. [Google Scholar] [CrossRef]
- Falsini, S.; Clemente, I.; Papini, A.; Tani, C.; Schiff, S.; Salvatici, M.C.; Petruccelli, R.; Benelli, C.; Giordano, C.; Gonnelli, C.; et al. When Sustainable Nanochemistry Meets Agriculture: Lignin Nanocapsules for Bioactive Compound Delivery to Plantlets. ACS Sustain. Chem. Eng. 2019, 7, 19935–19942. [Google Scholar] [CrossRef] [Green Version]

| Germination | RSG | Radicle Length | GI | |
|---|---|---|---|---|
| (%) | (%) | (cm) | (%) | |
| Control | 77 ± 5 b | - | 2.10 ± 0.30 b | - |
| T1 | 83 ± 10 ab | 109 ± 7 b | 2.56 ± 0.50 ab | 133 ± 9 c |
| T2 | 97 ± 10 a | 126 ± 2 a | 2.83 ± 0.28 a | 170 ± 3 a |
| T3 | 93 ± 6 a | 122 ± 5 a | 2.80 ± 0.27 a | 165 ± 6 a |
| T4 | 87 ± 8 ab | 113 ± 1 b | 2.81 ± 0.25 a | 151 ± 2 b |
| T5 | 56 ± 5 c | 54 ± 10 c | 1.67 ± 0.40 b | 49 ± 9 d |
| Chl a | Chl b | Chl a + Chl b | Chl a/Chl b | Carotenoids | |
|---|---|---|---|---|---|
| (mg g−1 FW) | (mg g−1 FW) | (mg g−1 FW) | (mg g−1 FW) | ||
| Control | 3.88 ± 0.30 b | 1.16 ± 0.05 b | 5.04 ± 0.28 b | 3.34 ± 0.34 b | 0.35 ± 0.02 b |
| T1 | 3.99 ± 0.22 b | 1.09 ± 0.14 b | 5.08 ± 0.35 b | 3.66 ± 0.30 ab | 0.33 ± 0.04 b |
| T2 | 4.62 ± 0.24 a | 1.19 ± 0.06 b | 5.81 ± 0.31 a | 3.88 ± 0.11 a | 0.41 ± 0.03 a |
| T3 | 4.86 ± 0.41 a | 1.17 ± 0.22 b | 6.03 ± 0.50 a | 4.15 ± 0.61 a | 0.44 ± 0.01 a |
| T4 | 4.63 ± 0.28 a | 1.26 ± 0.28 b | 5.89 ± 0.32 a | 3.67 ± 0.11 b | 0.41 ± 0.03 a |
| Anthocyanin | TP (mg g−1 FW) | DPPH | MDA | |
|---|---|---|---|---|
| (mg g−1 FW) | (Gallic Acid Equivalents) | (Scavenging Rate %) | (nmol g−1 FW) | |
| Control | 0.14 ± 0.01 c | 0.76 ± 0.04 c | 65.3 ± 1.6 b | 12.2 ± 1.0 a |
| T1 | 0.16 ± 0.03 abc | 0.79 ± 0.02 c | 72.8 ± 2.9 a | 13.1 ± 1.2 a |
| T2 | 0.17 ± 0.01 ab | 0.86 ± 0.02 b | 74.1 ± 1.3 a | 10.1 ± 0.7 b |
| T3 | 0.18 ± 0.01 ab | 0.85 ± 0.04 b | 73.9 ± 0.9 a | 9.2 ± 0.6 bc |
| T4 | 0.20 ± 0.02 a | 0.95 ± 0.02 a | 76.4 ± 1.0 a | 8.8 ± 0.6 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Del Buono, D.; Luzi, F.; Tolisano, C.; Puglia, D.; Di Michele, A. Synthesis of a Lignin/Zinc Oxide Hybrid Nanoparticles System and Its Application by Nano-Priming in Maize. Nanomaterials 2022, 12, 568. https://doi.org/10.3390/nano12030568
Del Buono D, Luzi F, Tolisano C, Puglia D, Di Michele A. Synthesis of a Lignin/Zinc Oxide Hybrid Nanoparticles System and Its Application by Nano-Priming in Maize. Nanomaterials. 2022; 12(3):568. https://doi.org/10.3390/nano12030568
Chicago/Turabian StyleDel Buono, Daniele, Francesca Luzi, Ciro Tolisano, Debora Puglia, and Alessandro Di Michele. 2022. "Synthesis of a Lignin/Zinc Oxide Hybrid Nanoparticles System and Its Application by Nano-Priming in Maize" Nanomaterials 12, no. 3: 568. https://doi.org/10.3390/nano12030568
APA StyleDel Buono, D., Luzi, F., Tolisano, C., Puglia, D., & Di Michele, A. (2022). Synthesis of a Lignin/Zinc Oxide Hybrid Nanoparticles System and Its Application by Nano-Priming in Maize. Nanomaterials, 12(3), 568. https://doi.org/10.3390/nano12030568










