Influence of Cryomilling on Crystallite Size of Aluminum Powder and Spark Plasma Sintered Component
Abstract
:1. Introduction
2. Experimental Procedures
2.1. Sample Preparation
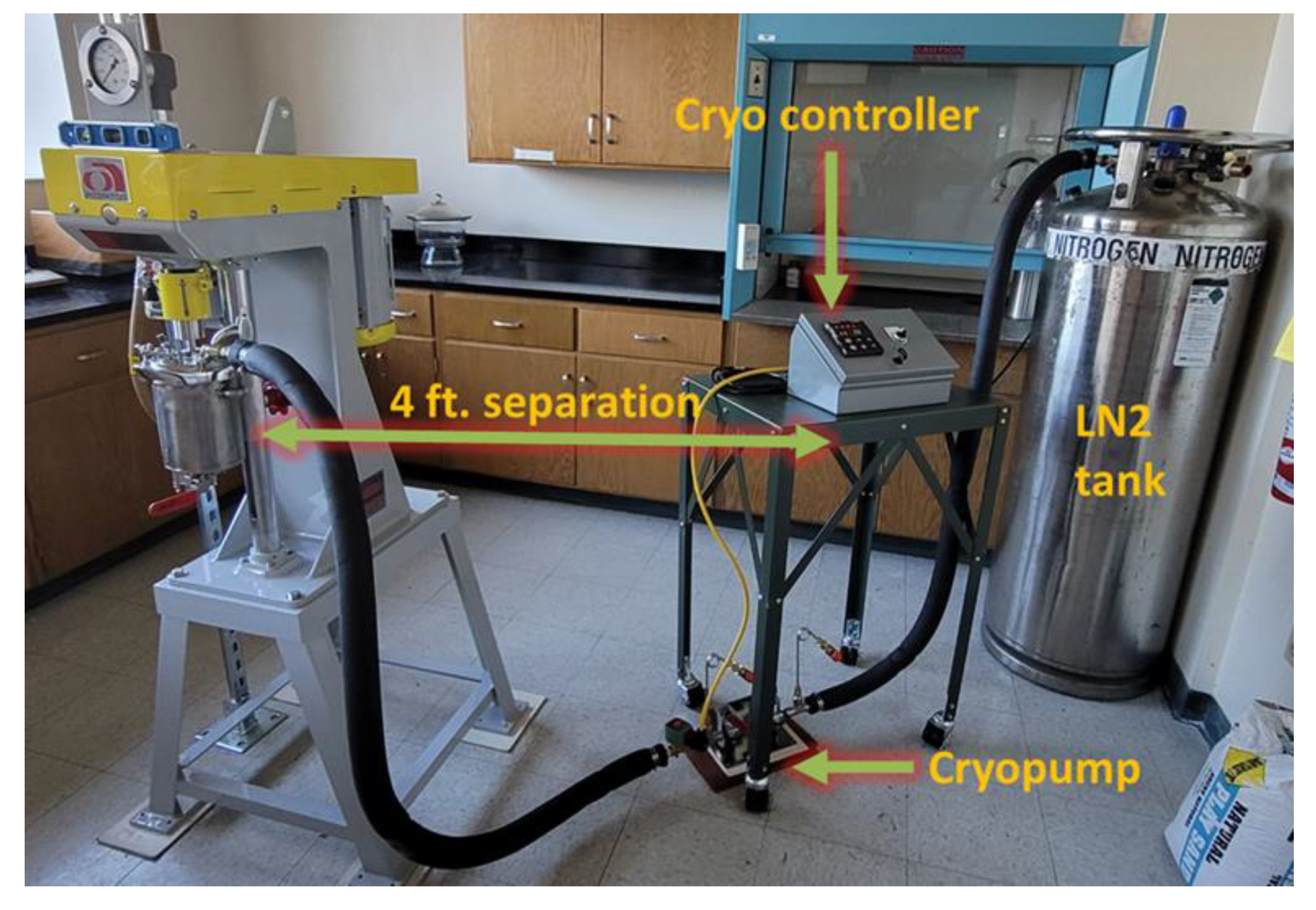
2.1.1. Powders
2.1.2. Bulk SPS Consolidated Samples
2.2. Characterization
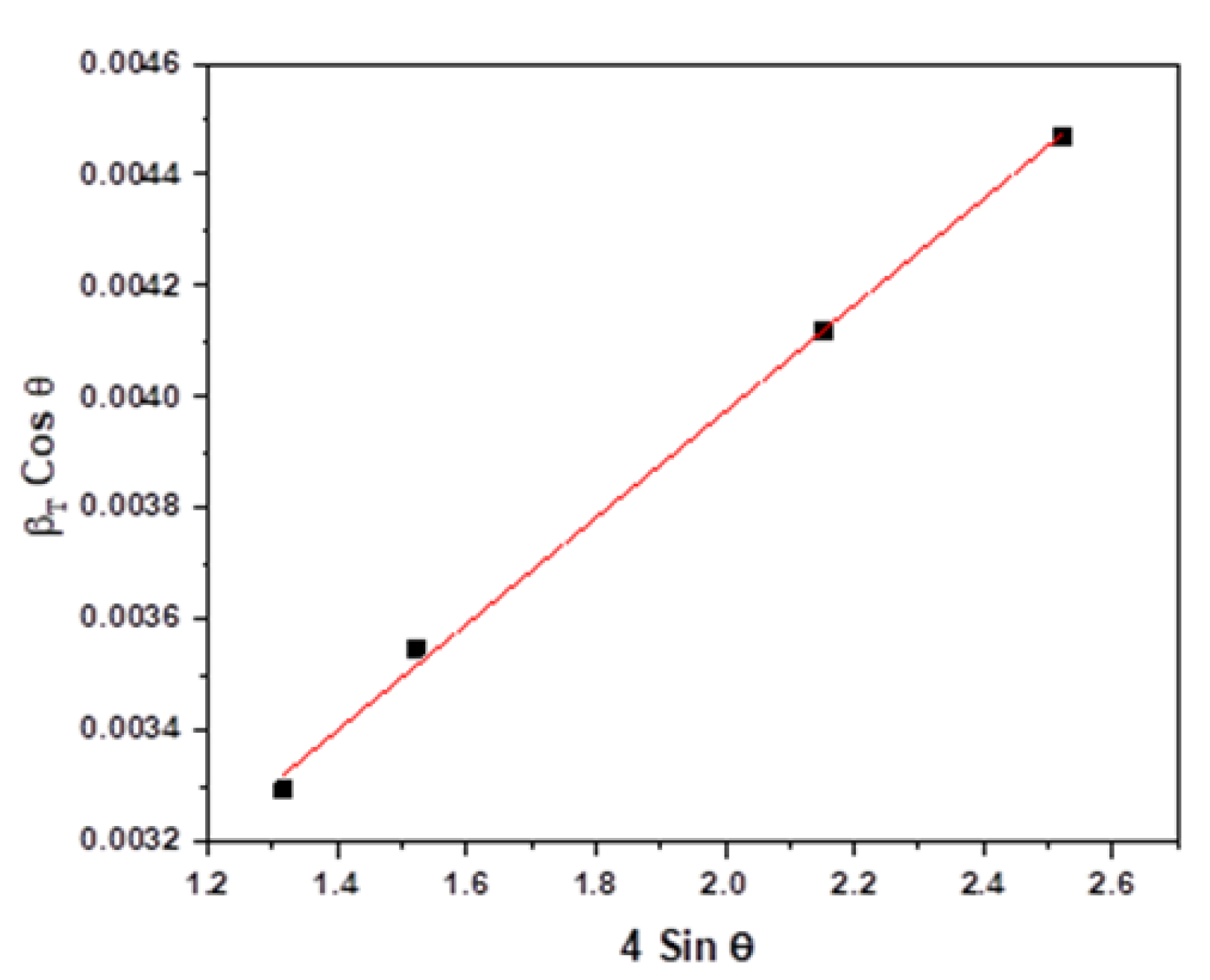
2.2.1. X-ray Diffraction Analysis
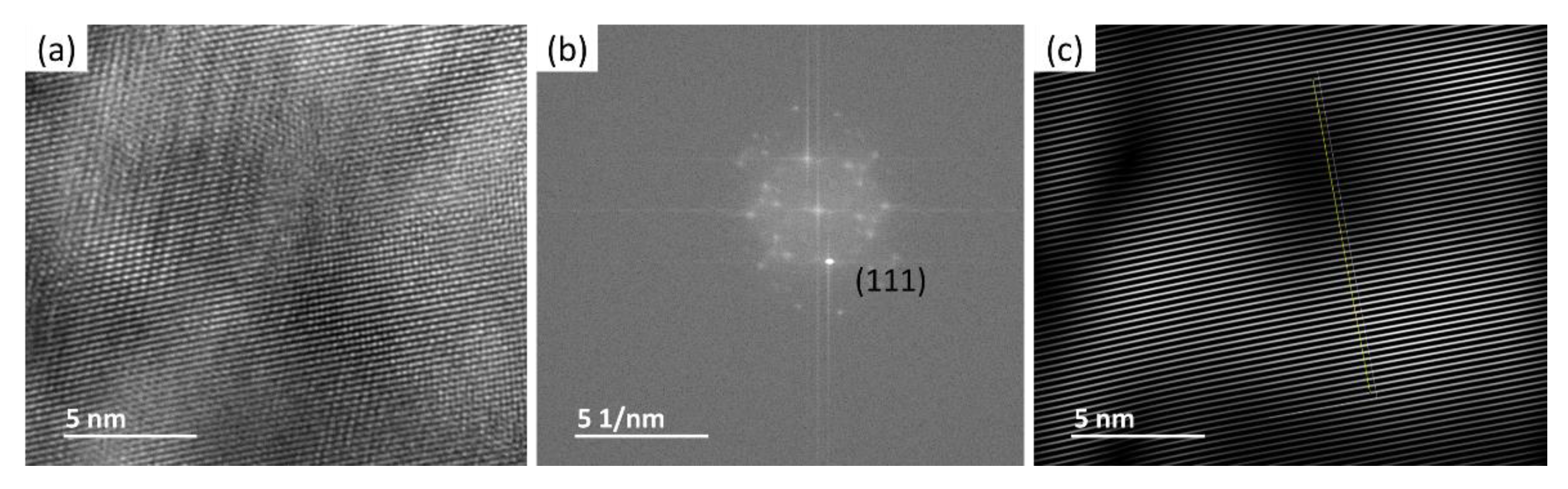
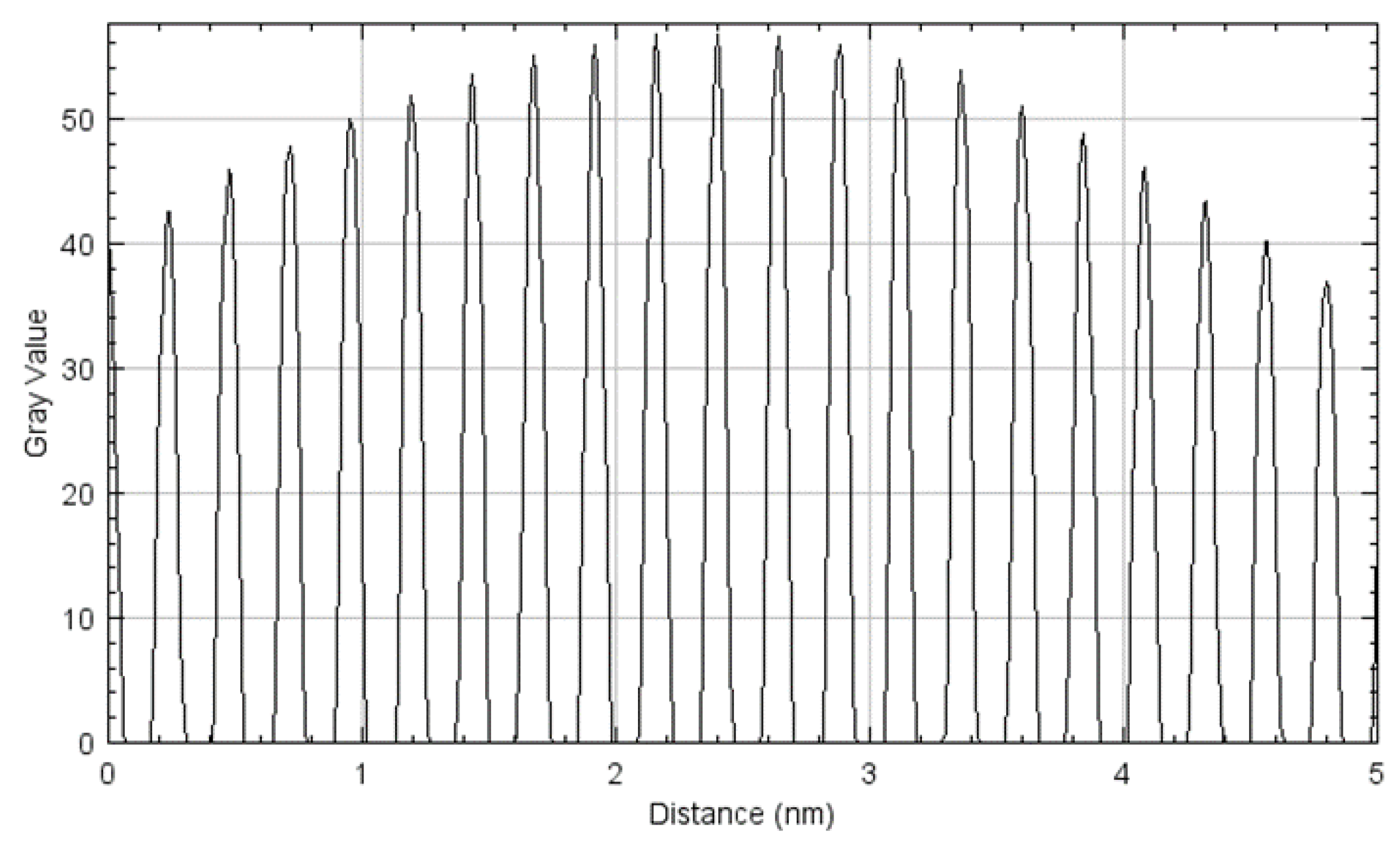
2.2.2. Transmission Electron Microscopy Analysis
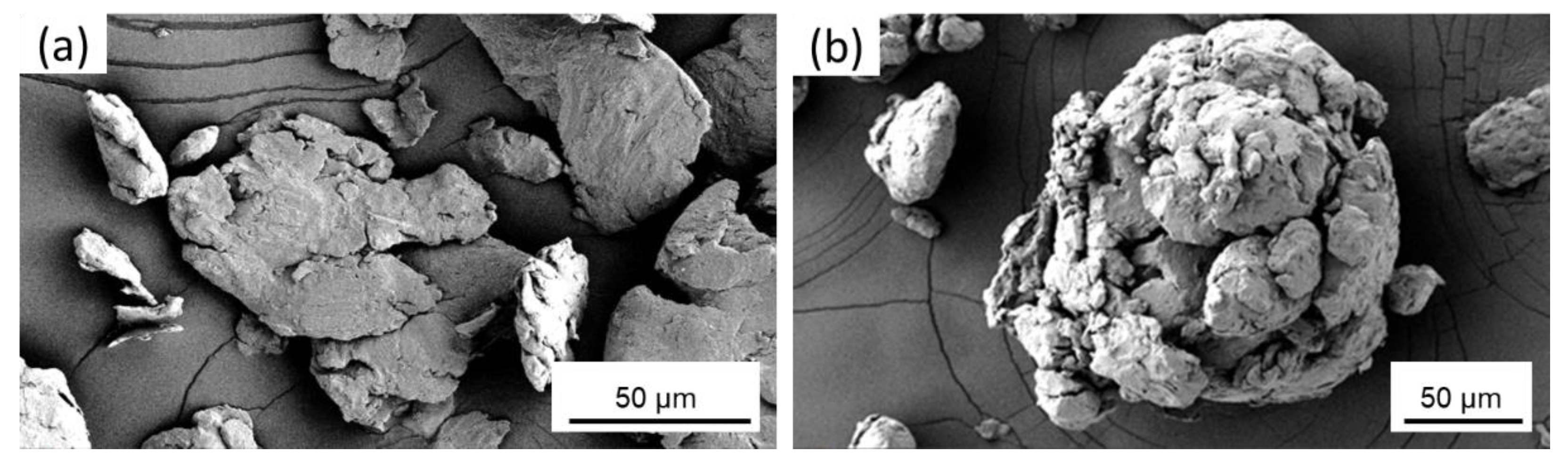
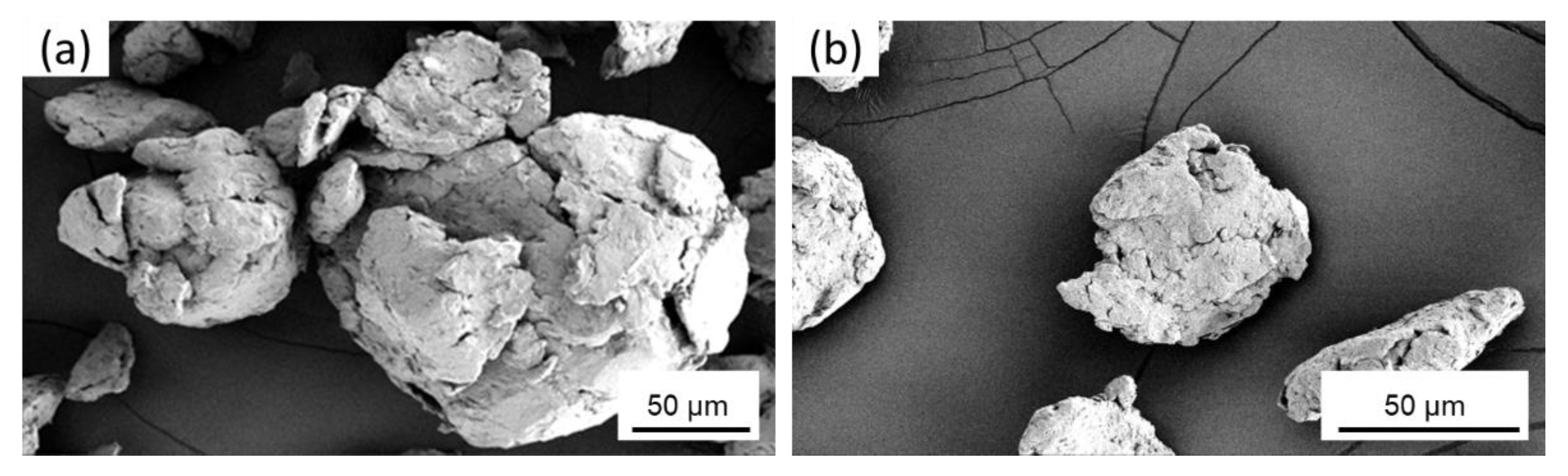
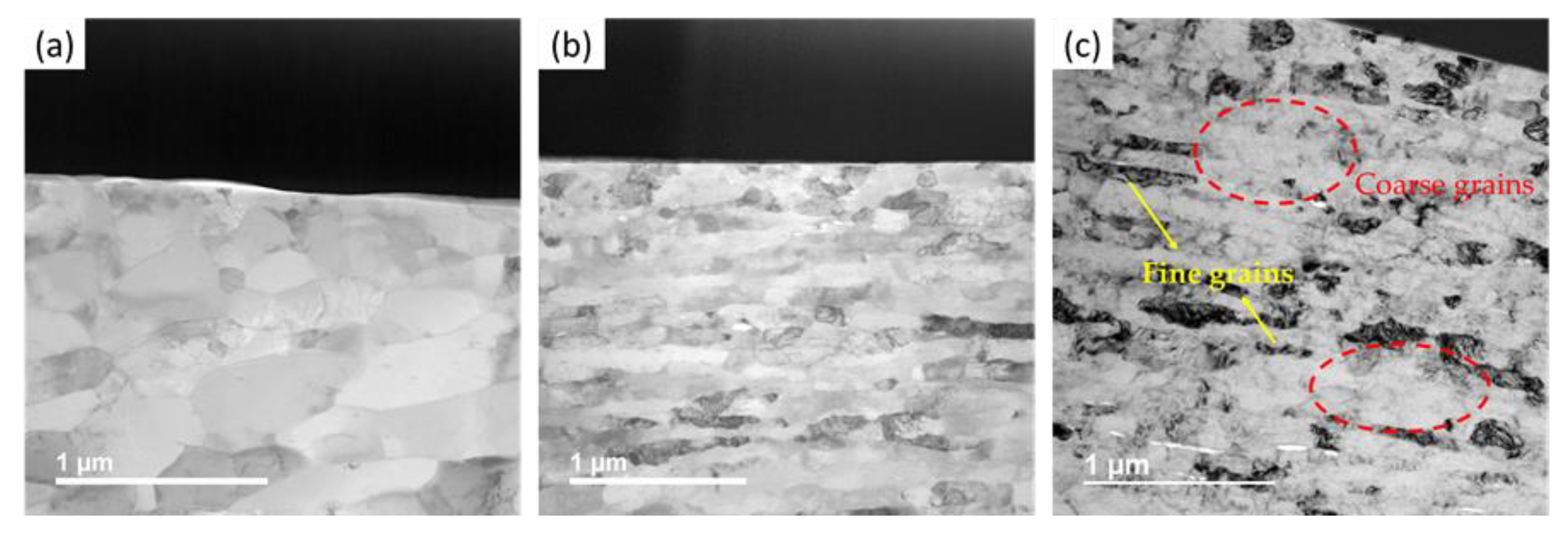
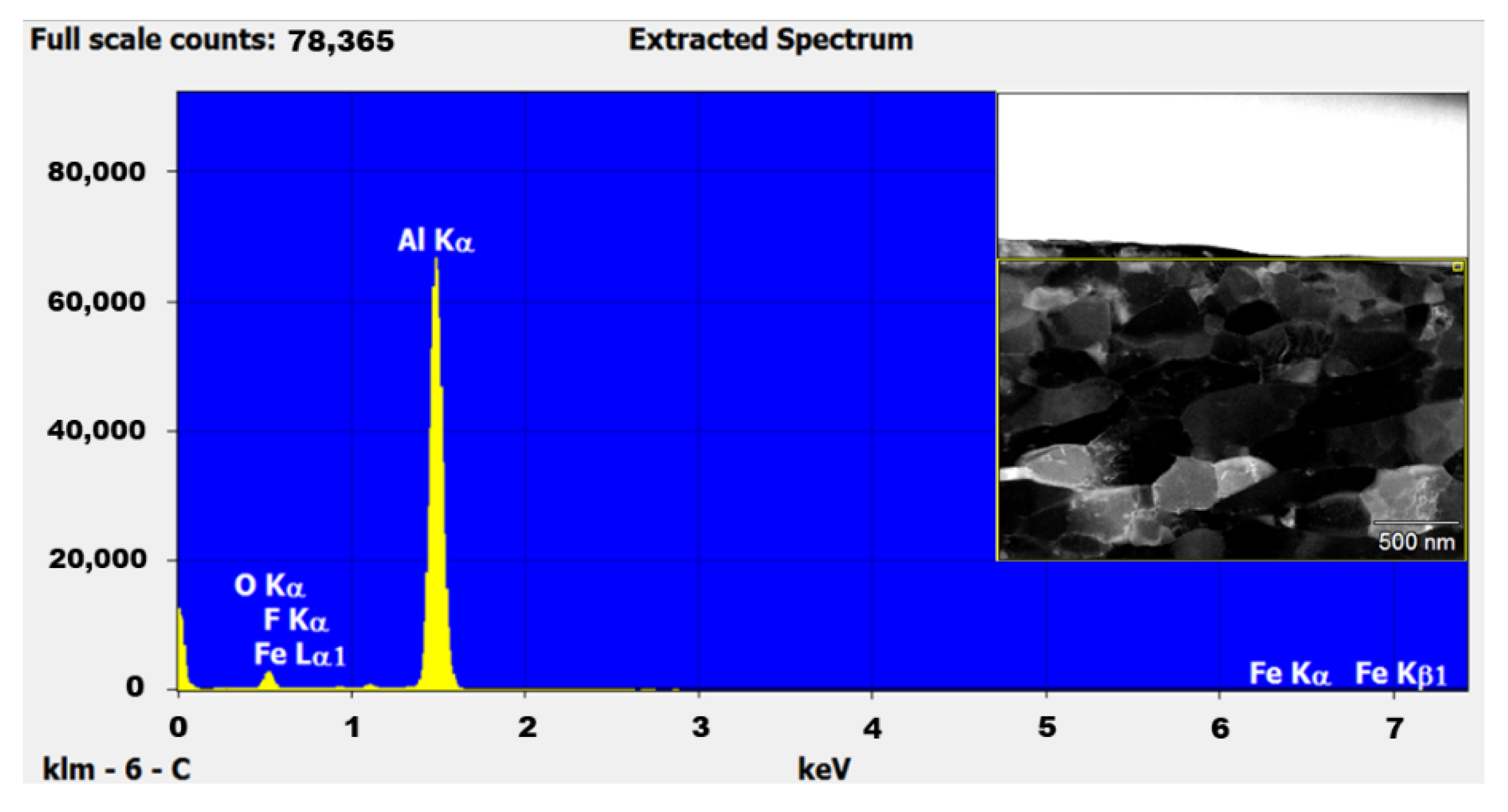
2.2.3. Scanning Electron Microscopy Analysis
2.2.4. Study the Effect of Annealing Temperature on Grain Coarsening
2.2.5. Microhardness Testing
3. Results and Discussion
3.1. Cryomilled Powders
Effect of Annealing Temperatures on Grain Coarsening of Cryomilled Powders
3.2. Bulk SPS Consolidated Samples
Mechanical Properties of the Bulk SPS Consolidated Samples
4. Conclusions
- Pure Al powders showed a 35% decrement in the crystallite size after 8 h of cryomilling determined using XRD. TEM studies also showed a decrease of about 72% in crystallite size. Thus, both XRD and TEM studies confirm the reduction in the crystallite size with increasing cryomilling time.
- There was an increase in crystallite size with the increase in annealing temperatures due to grain coarsening, however, the crystallite sizes were still very well in the NC range. This ensured that the samples could withstand short heat treatment cycles without undergoing much change in the crystallite size.
- Sintering temperature and pressure are crucial factors during the SPS process that ensures the uniform density and preservation of NC grains in the manufactured parts. The crystallite size increased by 55% after SPS for the 8 h cryomilled sample. The high sintering temperature helps in breaking down the oxide film on the powder particle surface, which reduces the oxide composition in the bulk sample.
- There was a three-fold increase in the hardness for the 8 h cryomilled SPS sample, which is attributed to the combined effect of reduced crystallite size during the cryomilling process and densification of the SPS sample due to severe plastic deformation, diffusion, viscous flow, and creep. No hardness gradient was observed in the radial direction of the SPS samples, suggesting uniform heating during the SPS consolidation process.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EDS | Energy dispersive X-ray spectroscopy |
| FCC | Face centered cubic |
| FFT | Fast fourier transform |
| FWHM | Full width at half maximum |
| HEBM | High energy ball milling |
| HIP | Hot isostatic pressing |
| HRTEM | High resolution transmission electron microscopy |
| HV | Vickers microhardness |
| NC | Nanocrystalline |
| RPM | Rotations per minute |
| SAD | Selected area diffraction |
| SEM | Scanning electron microscopy |
| SPD | Severe plastic deformation |
| SPS | Spark plasma sintering/sintered |
| SS | Stainless steel |
| TD | Theoretical density |
| TEM | Transmission electron microscopy |
References
- Mao, B.; Siddaiah, A.; Liao, Y.; Menezes, P.L. Laser surface texturing and related techniques for enhancing tribological performance of engineering materials: A review. J. Manuf. Process. 2020, 53, 153–173. [Google Scholar] [CrossRef]
- John, M.; Kalvala, P.R.; Misra, M.; Menezes, P.L. Peening Techniques for Surface Modification: Processes, Properties, and Applications. Materials 2021, 14, 3841. [Google Scholar] [CrossRef]
- John, M.; Ralls, A.M.; Dooley, S.C.; Thazhathidathil, A.K.V.; Perka, A.K.; Kuruveri, U.B.; Menezes, P.L. Ultrasonic Surface Rolling Process: Properties, Characterization, and Applications. Appl. Sci. 2021, 11, 10986. [Google Scholar] [CrossRef]
- Chen, A.; Wang, C.; Jiang, J.; Ruan, H.; Lu, J. Microstructure Evolution and Mechanical Properties of Austenite Stainless Steel with Gradient Twinned Structure by Surface Mechanical Attrition Treatment. Nanomaterials 2021, 11, 1624. [Google Scholar] [CrossRef] [PubMed]
- Kushwaha, A.K.; John, M.; Misra, M.; Menezes, P.L. Nanocrystalline Materials: Synthesis, Characterization, Properties, and Applications. Crystals 2021, 11, 1317. [Google Scholar] [CrossRef]
- Birringer, R.; Gleiter, H.; Klein, H.P.; Marquardt, P. Nanocrystalline materials an approach to a novel solid structure with gas-like disorder? Phys. Lett. A 1984, 102, 365–369. [Google Scholar] [CrossRef]
- Ovid’ko, I.A.; Valiev, R.Z.; Zhu, Y.T. Review on superior strength and enhanced ductility of metallic nanomaterials. Prog. Mater. Sci. 2018, 94, 462–540. [Google Scholar] [CrossRef]
- Han, B.Q.; Mohamed, F.A.; Bampton, C.C.; Lavernia, E.J. Improvement of toughness and ductility of a cryomilled Al-Mg alloy via microstructural modification. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2005, 36, 2081–2091. [Google Scholar] [CrossRef]
- Wei, Y.; Bower, A.F.; Gao, H. Enhanced strain-rate sensitivity in fcc nanocrystals due to grain-boundary diffusion and sliding. Acta Mater. 2008, 56, 1741–1752. [Google Scholar] [CrossRef]
- Hohl, J.; Kumar, P.; Misra, M.; Menezes, P.; Mushongera, L.T. Thermodynamic stabilization of nanocrystalline aluminum. J. Mater. Sci. 2021, 56, 14611–14623. [Google Scholar] [CrossRef]
- Liu, J.; Khan, A.S.; Takacs, L.; Meredith, C.S. Mechanical behavior of ultrafine-grained/nanocrystalline titanium synthesized by mechanical milling plus consolidation: Experiments, modeling and simulation. Int. J. Plast. 2015, 64, 151–163. [Google Scholar] [CrossRef] [Green Version]
- Roy, D.; Mahesh, B.V.; Atwater, M.A.; Chan, T.E.; Scattergood, R.O.; Koch, C.C. Grain size stability and hardness in nanocrystalline Cu-Al-Zr and Cu-Al-Y alloys. Mater. Sci. Eng. A 2014, 598, 217–223. [Google Scholar] [CrossRef]
- Ertorer, O.; Topping, T.; Li, Y.; Moss, W.; Lavernia, E.J. Enhanced tensile strength and high ductility in cryomilled commercially pure titanium. Scr. Mater. 2009, 60, 586–589. [Google Scholar] [CrossRef]
- Deng, H.; Chen, A.; Chen, L.; Wei, Y.; Xia, Z.; Tang, J. Bulk nanostructured Ti-45Al-8Nb alloy fabricated by cryomilling and Spark Plasma Sintering. J. Alloys Compd. 2019, 772, 140–149. [Google Scholar] [CrossRef]
- Zhao, Y.; Xu, J.; Peng, S. Synthesis and evaluation of TaC nanocrystalline coating with excellent wear resistance, corrosion resistance, and biocompatibility. Ceram. Int. 2021, 47, 20032–20044. [Google Scholar] [CrossRef]
- Peng, R.; Fu, L.; Zhou, L. Improved wear resistance by phase transformation of surface nanocrystalline 1090 steel prepared by sandblasting technique. Appl. Surf. Sci. 2016, 388, 406–411. [Google Scholar] [CrossRef]
- Lu, L.; Pan, Q.; Hattar, K.; Boyce, B.L. Fatigue and fracture of nanostructured metals and alloys. MRS Bull. 2021, 46, 258–264. [Google Scholar] [CrossRef]
- An, X.; Lin, Q.; Wu, S.; Zhang, Z. Improved Fatigue Strengths of Nanocrystalline Cu and Cu–Al Alloys. Mater. Res. Lett. 2015, 3, 135–141. [Google Scholar] [CrossRef] [Green Version]
- Meyers, M.A.; Mishra, A.; Benson, D.J. Mechanical properties of nanocrystalline materials. Prog. Mater. Sci. 2006, 51, 427–556. [Google Scholar] [CrossRef]
- Divinski, S.; Rösner, H.; Wilde, G. Functional Nanostructured Materials – Microstructure, Thermodynamic Stability and Atomic Mobility. In Frontiers of Nanoscience; Elsevier: Amsterdam, The Netherlands, 2009; Volume 1, pp. 1–50. [Google Scholar] [CrossRef]
- Estevesa, L.; Witharamage, C.S.; Christudasjustus, J.; Walunj, G.; O’Brien, S.P.; Ryu, S.; Borkarc, T.; Akans, R.E.; Gupta, R.K. Corrosion behavior of AA5083 produced by high-energy ball milling. J. Alloys Compd. 2021, 857, 158268. [Google Scholar] [CrossRef]
- Esteves, L.; Christudasjustus, J.; O’Brien, S.; Witharamage, C.; Darwish, A.; Walunj, G.; Stack, P.; Borkar, T.; Akans, R.; Gupta, R. Effect of V content on corrosion behavior of high-energy ball milled AA5083. Corros. Sci. 2021, 186, 109465. [Google Scholar] [CrossRef]
- Tellkamp, V.; Lavernia, E.J. Processing and mechanical properties of nanocrystalline 5083 al alloy. Nanostructured Mater. 1999, 12, 249–252. [Google Scholar] [CrossRef]
- Gleiter, H. Nanostructured materials: Basic concepts and microstructure. Acta Mater. 2000, 48, 1–29. [Google Scholar] [CrossRef] [Green Version]
- Katiyar, N.K.; Biswas, K.; Tiwary, C.S. Cryomilling as environmentally friendly synthesis route to prepare nanomaterials. Int. Mater. Rev. 2020, 66, 1–40. [Google Scholar] [CrossRef]
- Guan, D.; Gao, J.; Rainforth, W.M. Effect of cryomilling time on microstructure evolution and hardness of cryomilled AZ31 powders. Mater. Charact. 2021, 178, 111311. [Google Scholar] [CrossRef]
- Kumar, N.; Biswas, K. Cryomilling: An environment friendly approach of preparation large quantity ultra refined pure aluminium nanoparticles. J. Mater. Res. Technol. 2019, 8, 63–74. [Google Scholar] [CrossRef]
- Elangovan, H.; Sengupta, S.; Narayanan, R.; Chattopadhyay, K. Silicon nanoparticles with UV range photoluminescence synthesized through cryomilling induced phase transformation and etching. J. Mater. Sci. 2020, 56, 1515–1526. [Google Scholar] [CrossRef]
- Guan, D.; Rainforth, W.M.; Sharp, J.; Gao, J.; Todd, I. On the use of cryomilling and spark plasma sintering to achieve high strength in a magnesium alloy. J. Alloys Compd. 2016, 688, 1141–1150. [Google Scholar] [CrossRef]
- Milligan, J.; Vintila, R.; Brochu, M. Nanocrystalline eutectic Al–Si alloy produced by cryomilling. Mater. Sci. Eng. A 2009, 508, 43–49. [Google Scholar] [CrossRef]
- Li, J.; Xiong, Y.; Wang, X.; Yan, S.; Yang, C.; He, W.; Chen, J.; Wang, S.; Zhang, X.; Dai, S. Microstructure and tensile properties of bulk nanostructured aluminum/graphene composites prepared via cryomilling. Mater. Sci. Eng. A 2015, 626, 400–405. [Google Scholar] [CrossRef]
- Kumar, N.; Tiwary, C.S.; Biswas, K. Preparation of nanocrystalline high-entropy alloys via cryomilling of cast ingots. J. Mater. Sci. 2018, 53, 13411–13423. [Google Scholar] [CrossRef]
- Tang, F.; Hagiwara, M.; Schoenung, J. Formation of coarse-grained inter-particle regions during hot isostatic pressing of nanocrystalline powder. Scr. Mater. 2005, 53, 619–624. [Google Scholar] [CrossRef]
- Kishimoto, H.; Alinger, M.; Odette, G.; Yamamoto, T. TEM examination of microstructural evolution during processing of 14CrYWTi nanostructured ferritic alloys. J. Nucl. Mater. 2004, 329–333, 369–371. [Google Scholar] [CrossRef]
- Ye, J.; Ajdelsztajn, L.; Schoenung, J. Bulk nanocrystalline aluminum 5083 alloy fabricated by a novel technique: Cryomilling and spark plasma sintering. Met. Mater. Trans. A 2006, 37, 2569–2579. [Google Scholar] [CrossRef]
- Shkodich, N.; Kovalev, I.; Kuskov, K.; Kovalev, D.; Vergunova, Y.; Scheck, Y.; Vadchenko, S.; Politano, O.; Baras, F.; Rogachev, A. Fast mechanical synthesis, structure evolution, and thermal stability of nanostructured CoCrFeNiCu high entropy alloy. J. Alloys Compd. 2021, 893, 161839. [Google Scholar] [CrossRef]
- Pandey, V.K.; Shadangi, Y.; Shivam, V.; Basu, J.; Chattopadhyay, K.; Majumdar, B.; Sarma, B.N.; Mukhopadhyay, N.K. Synthesis, Characterization and Thermal Stability of Nanocrystalline MgAlMnFeCu Low-Density High-Entropy Alloy. Trans. Indian Inst. Met. 2020, 74, 33–44. [Google Scholar] [CrossRef]
- Yang, Y.F.; Qian, M. Spark plasma sintering and hot pressing of titanium and titanium alloys. In Titanium Powder Metallurgy; Elsevier: Amsterdam, The Netherlands, 2015; pp. 219–235. [Google Scholar] [CrossRef]
- Munir, Z.A.; Anselmi-Tamburini, U.; Ohyanagi, M. The effect of electric field and pressure on the synthesis and consolidation of materials: A review of the spark plasma sintering method. J. Mater. Sci. 2006, 41, 763–777. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, F.; Lee, S.; Liu, Y.; Cheng, J.; Liang, Y. Microstructure characteristic, mechanical properties and sintering mechanism of nanocrystalline copper obtained by SPS process. Mater. Sci. Eng. A 2009, 523, 134–138. [Google Scholar] [CrossRef]
- Xie, G.; Ohashi, O.; Chiba, K.; Yamaguchi, N.; Song, M.; Furuya, K.; Noda, T. Frequency effect on pulse electric current sintering process of pure aluminum powder. Mater. Sci. Eng. A 2003, 359, 384–390. [Google Scholar] [CrossRef]
- Reddy, K.M.; Kumar, N.; Basu, B. Inhibition of grain growth during the final stage of multi-stage spark plasma sintering of oxide ceramics. Scr. Mater. 2010, 63, 585–588. [Google Scholar] [CrossRef]
- Ye, J.; He, J.; Schoenung, J. Cryomilling for the fabrication of a particulate B4C reinforced Al nanocomposite: Part I. Effects of process conditions on structure. Met. Mater. Trans. A 2006, 37, 3099–3109. [Google Scholar] [CrossRef]
- Mote, V.; Purushotham, Y.; Dole, B. Williamson-Hall analysis in estimation of lattice strain in nanometer-sized ZnO particles. J. Theor. Appl. Phys. 2012, 6, 6. [Google Scholar] [CrossRef] [Green Version]
- Ungár, T. Microstructural parameters from X-ray diffraction peak broadening. Scr. Mater. 2004, 51, 777–781. [Google Scholar] [CrossRef]
- Oshida, K.; Nakazawa, T.; Miyazaki, T.; Endo, M. Application of image processing techniques for analysis of nano- and micro-spaces in carbon materials. Synth. Met. 2001, 125, 223–230. [Google Scholar] [CrossRef]
- He, J.; Lavernia, E.J. Development of nanocrystalline structure during cryomilling of Inconel 625. J. Mater. Res. 2001, 16, 2724–2732. [Google Scholar] [CrossRef]
- Zhou, F.; Liao, X.; Zhu, Y.; Dallek, S.; Lavernia, E. Microstructural evolution during recovery and recrystallization of a nanocrystalline Al-Mg alloy prepared by cryogenic ball milling. Acta Mater. 2003, 51, 2777–2791. [Google Scholar] [CrossRef]
- Kwon, H.; Park, D.H.; Park, Y.; Silvain, J.-F.; Kawasaki, A. Spark plasma sintering behavior of pure aluminum depending on various sintering temperatures. Met. Mater. Int. 2010, 16, 71–75. [Google Scholar] [CrossRef]
- Roy, I.; Chauhan, M.; Mohamed, F.A.; Lavernia, E.J. Thermal stability in bulk cryomilled ultrafine-grained 5083 Al alloy. Met. Mater. Trans. A 2006, 37, 721–730. [Google Scholar] [CrossRef]
- Langford, R.M.; Rogers, M. In situ lift-out: Steps to improve yield and a comparison with other FIB TEM sample preparation techniques. Micron 2008, 39, 1325–1330. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Malis, T.; Dionne, S. Recent advances in FIB–TEM specimen preparation techniques. Mater. Charact. 2006, 57, 64–70. [Google Scholar] [CrossRef]
- Gourdet, S.; Montheillet, F. An experimental study of the recrystallization mechanism during hot deformation of aluminium. Mater. Sci. Eng. A 2000, 283, 274–288. [Google Scholar] [CrossRef]
- Rokni, M.R.; Widener, C.A.; Nardi, A.T.; Champagne, V.K. Nano crystalline high energy milled 5083 Al powder deposited using cold spray. Appl. Surf. Sci. 2014, 305, 797–804. [Google Scholar] [CrossRef]
- Bouaziz, O.; Estrin, Y.; Bréchet, Y.; Embury, J.D. Critical grain size for dislocation storage and consequences for strain hardening of nanocrystalline materials. Scr. Mater. 2010, 63, 477–479. [Google Scholar] [CrossRef]


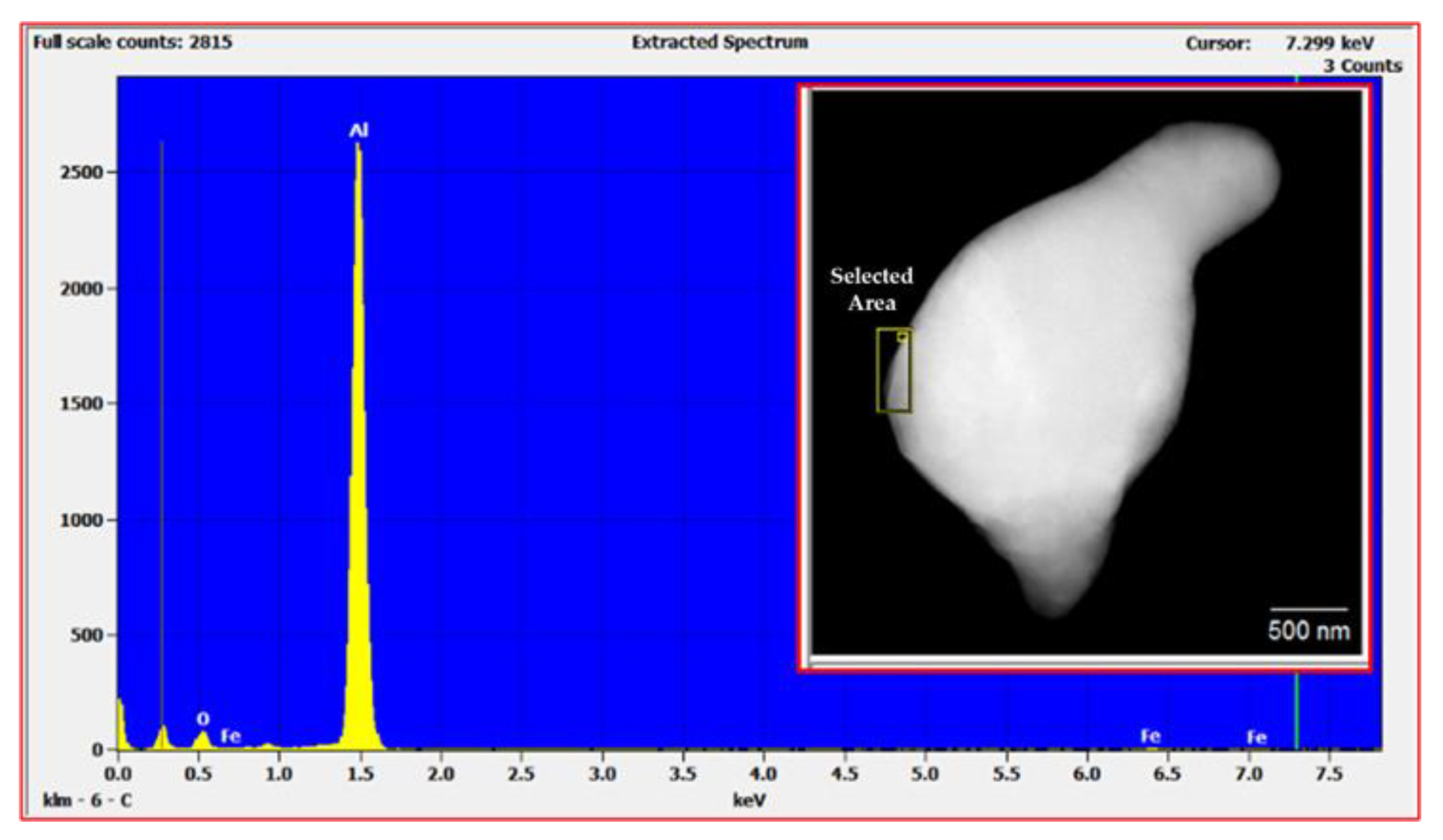
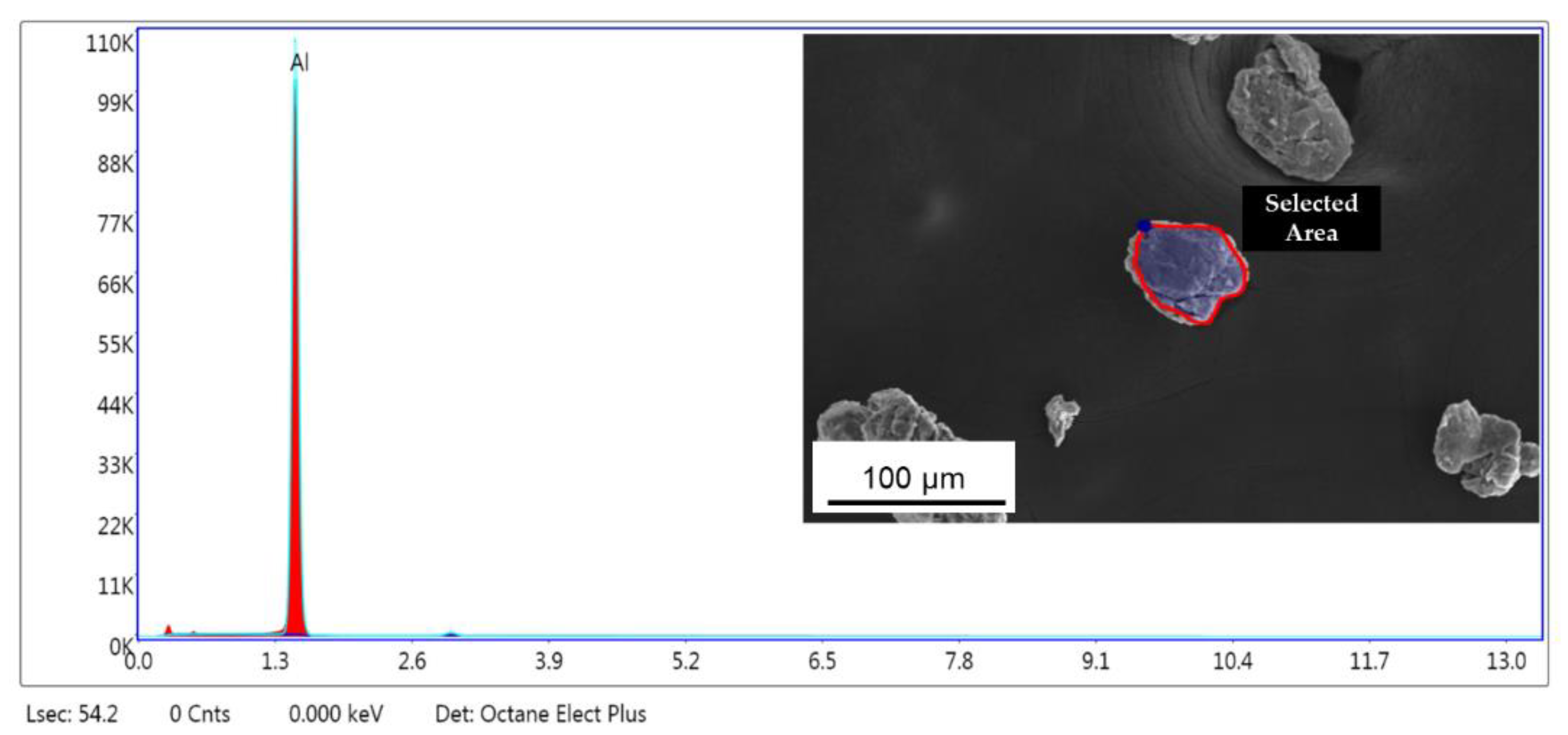
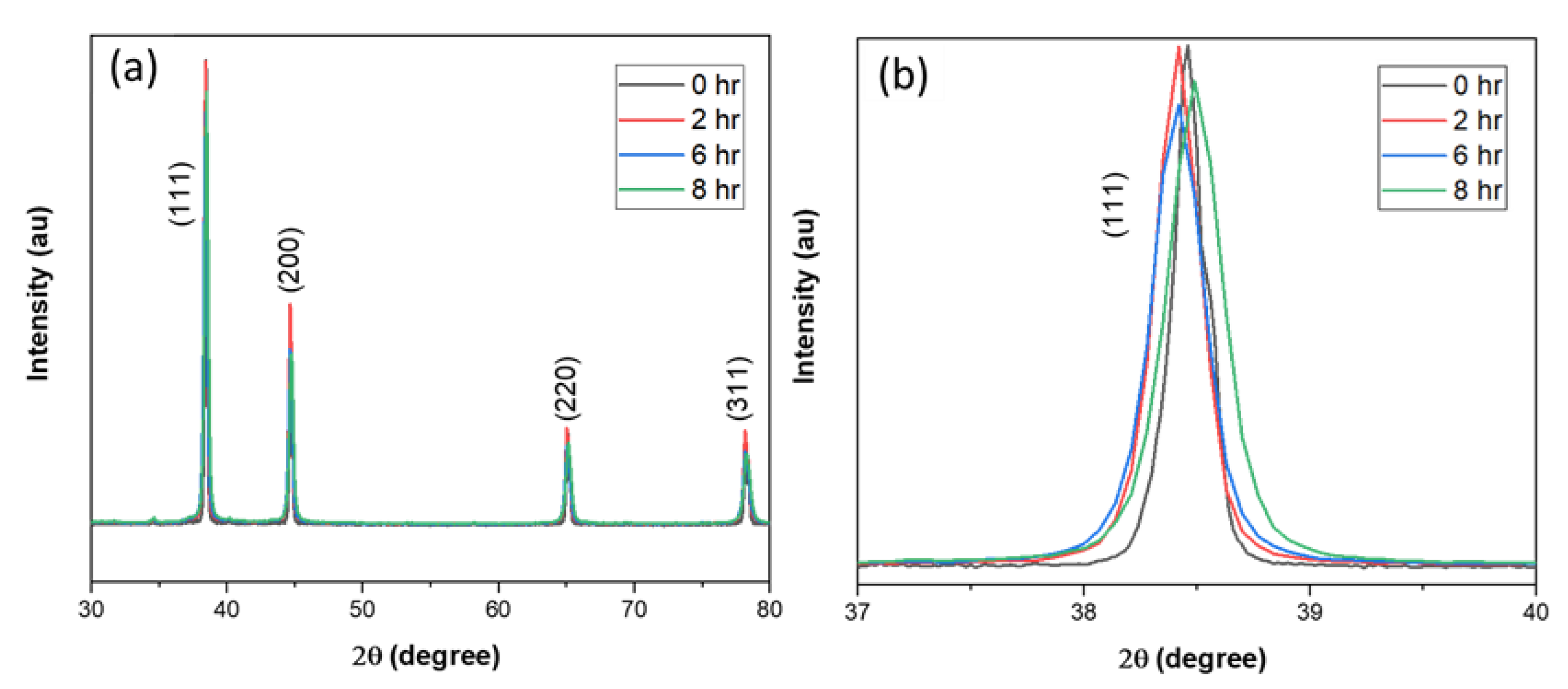
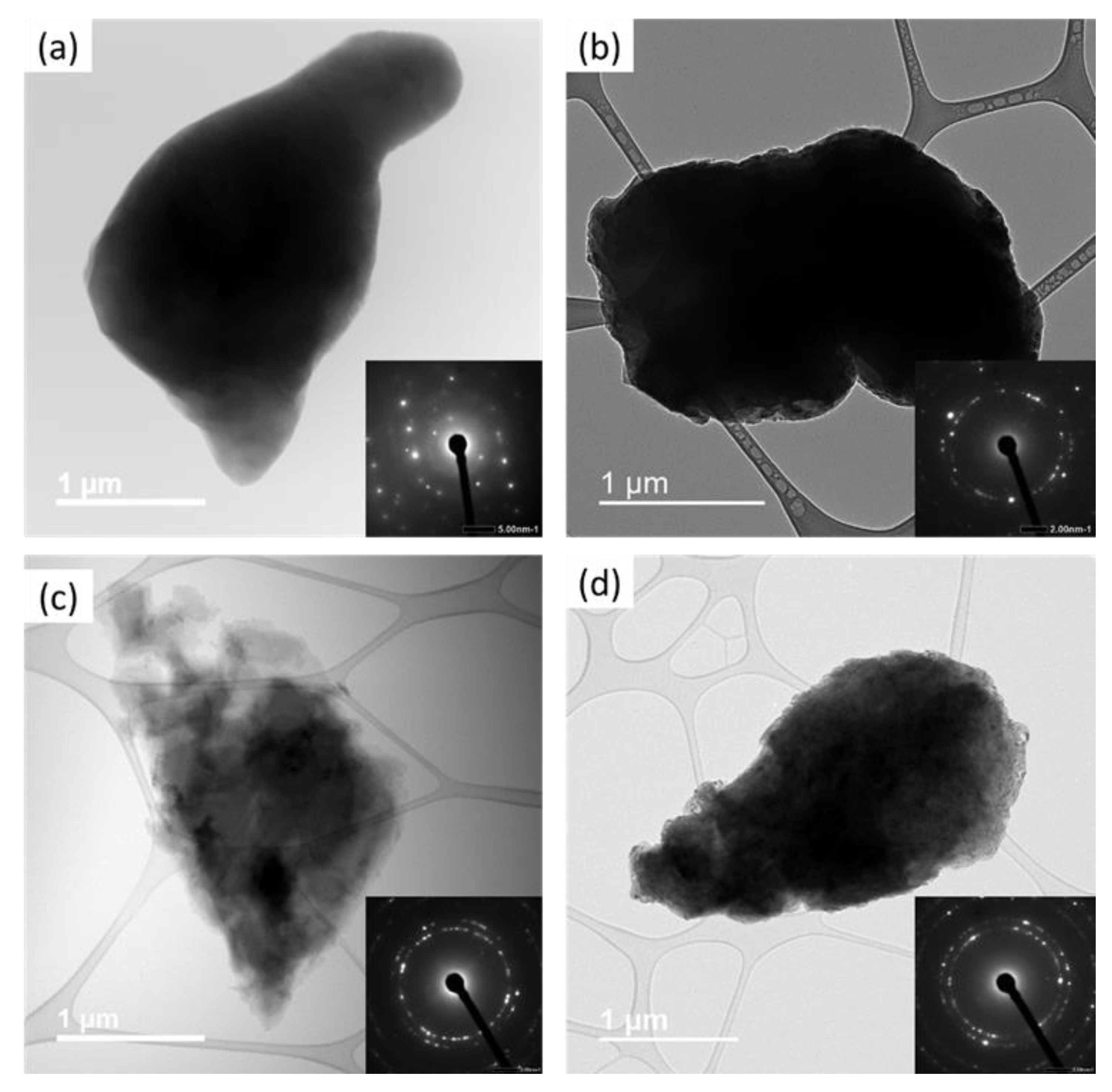

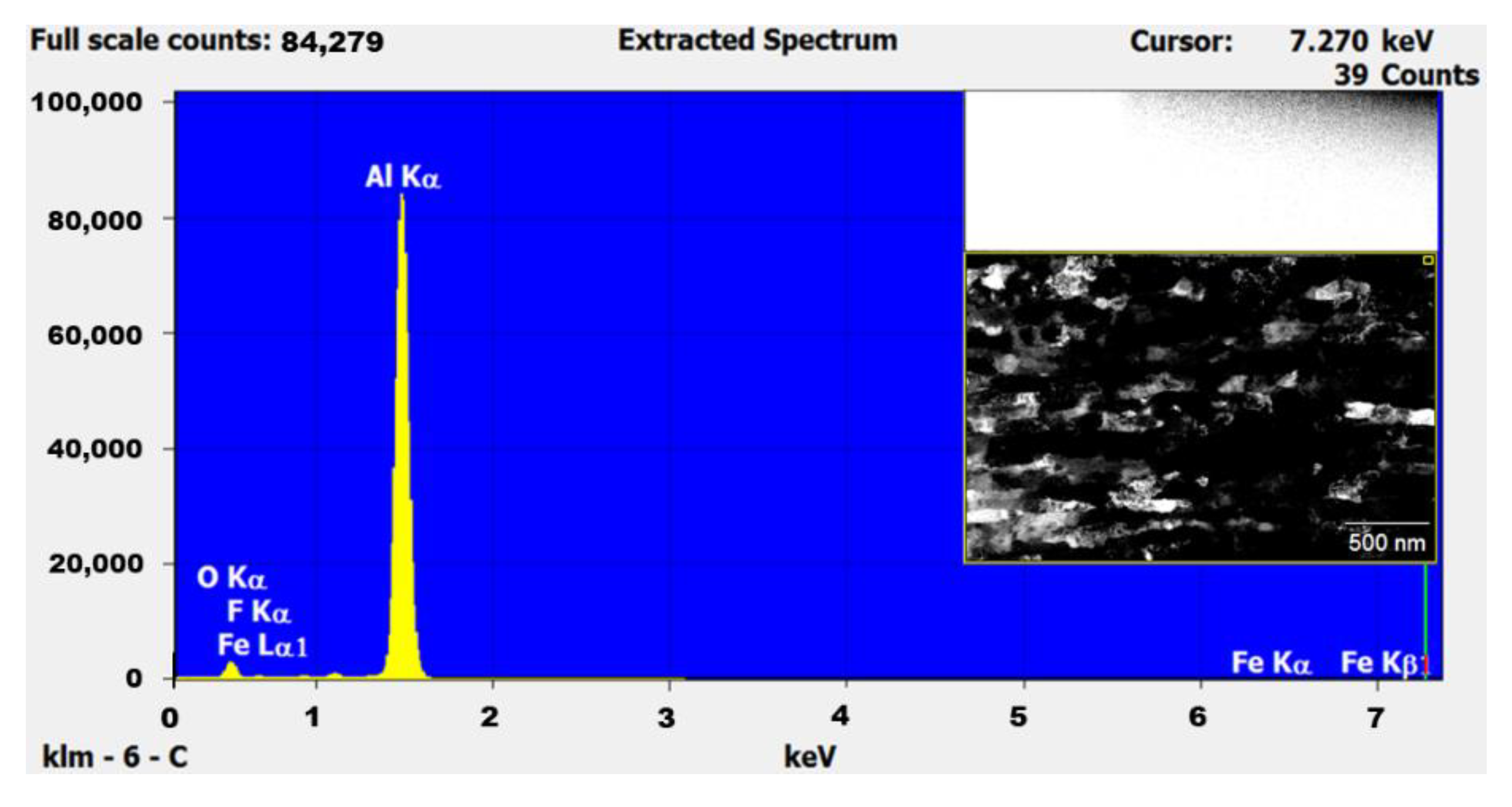
| Milling Time | L–XRD (nm) | L–TEM (nm) |
|---|---|---|
| Unmilled | 43 | 67 |
| 2 h | 35 | 27 |
| 6 h | 31 | 26 |
| 8 h | 28 | 19 |
| Annealing Temperature | L111–XRD (nm) | L200–XRD (nm) | L220–XRD (nm) | L311–XRD (nm) | L222–XRD (nm) | Average L (nm) |
|---|---|---|---|---|---|---|
| 300 °C | 36 | 31 | 25 | 22 | 23 | 27 |
| 400 °C | 39 | 34 | 26 | 23 | 24 | 29 |
| 500 °C | 44 | 38 | 30 | 26 | 27 | 33 |
| 550 °C | 46 | 40 | 31 | 27 | 27 | 34 |
| Milling Time | L111–XRD (nm) | L200–XRD (nm) | L220–XRD (nm) | L311–XRD (nm) | L222–XRD (nm) | Average L (nm) |
|---|---|---|---|---|---|---|
| Unmilled SPS | 57 | 53 | 73 | 65 | 68 | 63 |
| 2 h SPS | 59 | 57 | 64 | 56 | 62 | 60 |
| 8 h SPS | 48 | 40 | 34 | 50 | 44 | 43 |
| Sample | Vickers Microhardness (HV) |
|---|---|
| Unmilled Al SPS | 52 ± 1 |
| 8 h cryomilled Al SPS | 142 ± 12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kushwaha, A.K.; Maccione, R.; John, M.; Lanka, S.; Misra, M.; Menezes, P.L. Influence of Cryomilling on Crystallite Size of Aluminum Powder and Spark Plasma Sintered Component. Nanomaterials 2022, 12, 551. https://doi.org/10.3390/nano12030551
Kushwaha AK, Maccione R, John M, Lanka S, Misra M, Menezes PL. Influence of Cryomilling on Crystallite Size of Aluminum Powder and Spark Plasma Sintered Component. Nanomaterials. 2022; 12(3):551. https://doi.org/10.3390/nano12030551
Chicago/Turabian StyleKushwaha, Amanendra K., Raven Maccione, Merbin John, Sridhar Lanka, Manoranjan Misra, and Pradeep L. Menezes. 2022. "Influence of Cryomilling on Crystallite Size of Aluminum Powder and Spark Plasma Sintered Component" Nanomaterials 12, no. 3: 551. https://doi.org/10.3390/nano12030551
APA StyleKushwaha, A. K., Maccione, R., John, M., Lanka, S., Misra, M., & Menezes, P. L. (2022). Influence of Cryomilling on Crystallite Size of Aluminum Powder and Spark Plasma Sintered Component. Nanomaterials, 12(3), 551. https://doi.org/10.3390/nano12030551









