Biostimulation of Anaerobic Digestion Using Iron Oxide Nanoparticles (IONPs) for Increasing Biogas Production from Cattle Manure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
- Determination of pH: The pH of the cattle dung, the influents, and the effluents was determined using an Elico pH meter (Elico ltd., Hyderabad, India) and was 6.17.
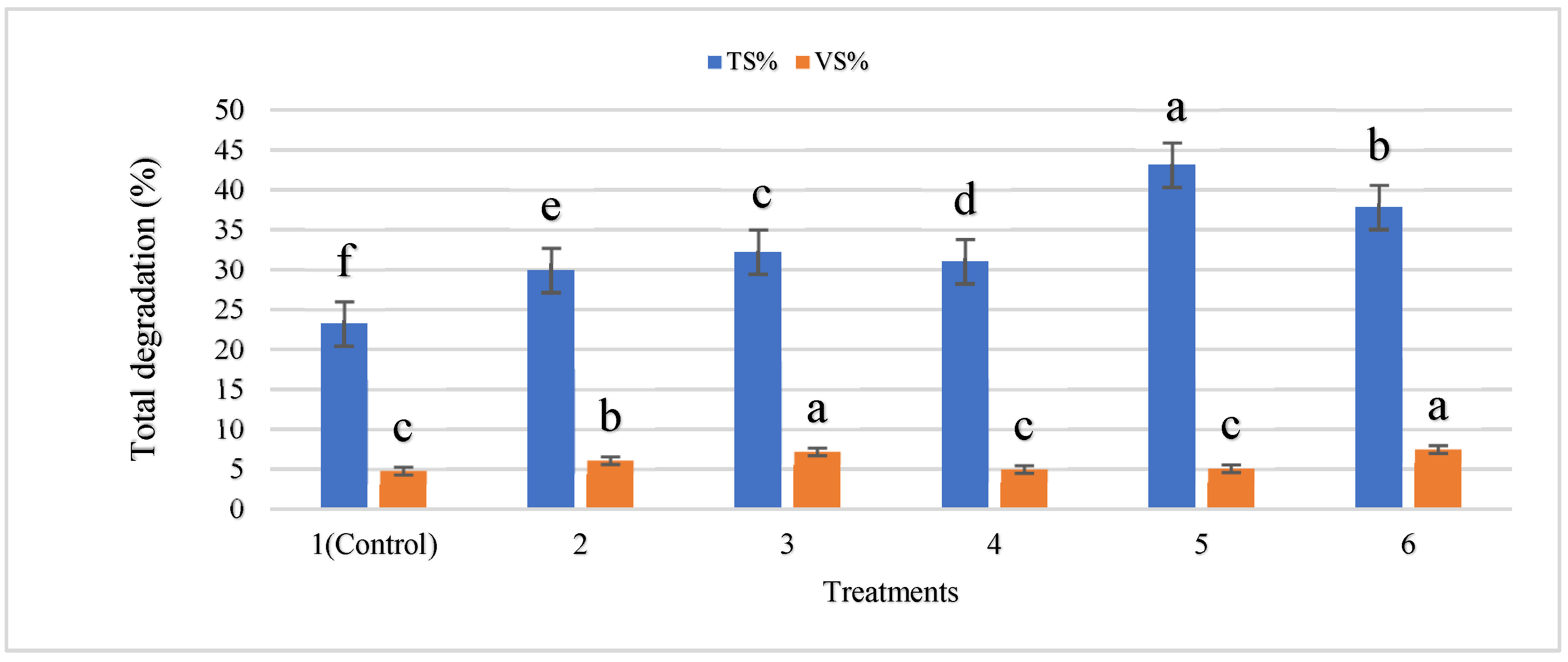
- Determination of Total Solids (TS): 100 g sample was dried at 80 °C to a constant weight for determination of total solids. The residue left was weighed and taken as total solids. We arrived at a figure of 18.42% TS.
- Determination of Volatile Solids (VS): 1.0 g of fine dried powder sample was taken in a pre-weighed china crucible and incinerated in a muffle furnace at 550 °C for 1 h. The loss in weight of TS (total solids) to combustion was taken as volatile solids, which was found to be 82.0%.
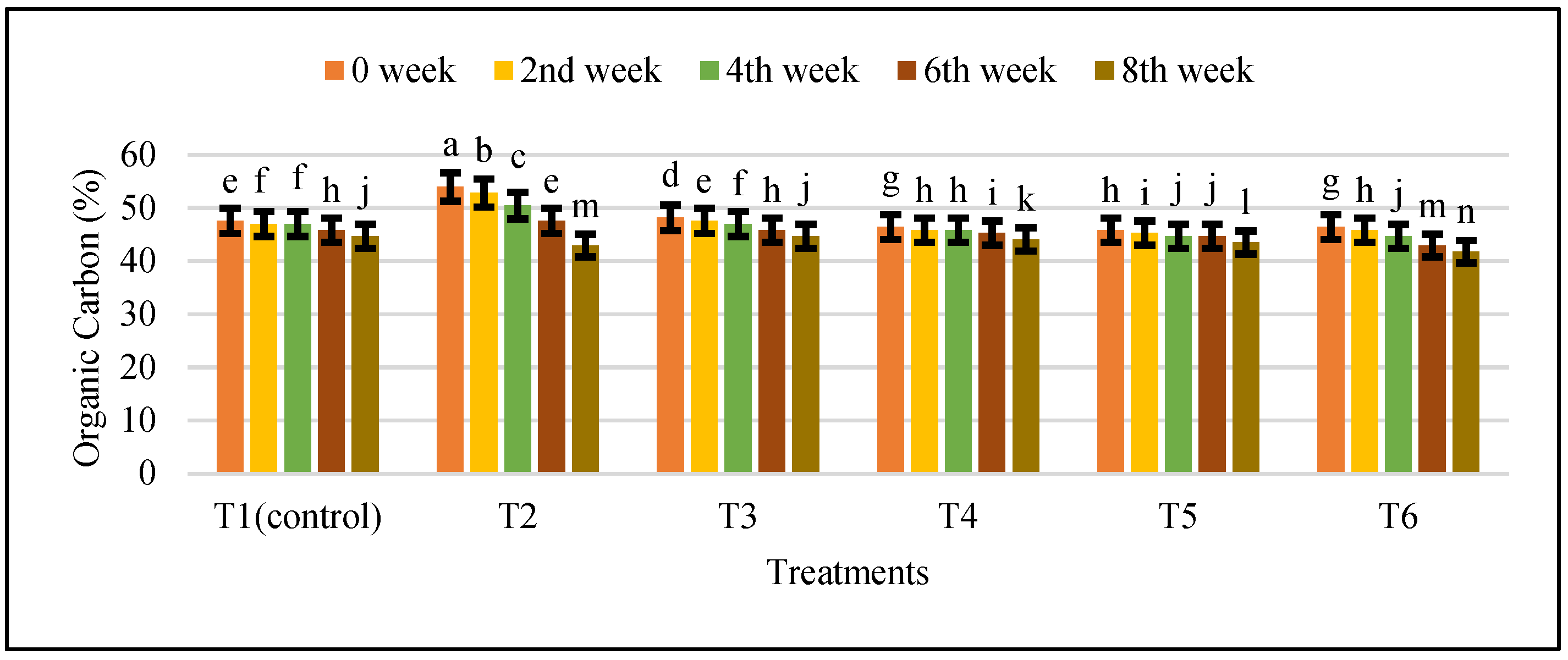
- Determination of Organic Carbon Content: Organic carbon content of the samples was calculated as:Organic carbon was found to be 47.16%.
- Determination of cellulose, hemicellulose, and lignin: Cellulose, lignin, and hemicellulose contents were estimated by determining acid detergent fibre (ADF) and neutral detergent fibre (NDF) in the sample powder (AOAC, 2000) and were found to be 39.2, 16.5, and 11.82%, respectively.
- TVFA, Nitrogen, and C/N were analysed using standard protocols and were found to be 695 mg/kg, 1.32% (N) and 20.1 (C/N), respectively.
2.2. Chemicals
2.3. Preparation of Neem (Azadirachta Indica) Leaves Extract
2.4. Biosynthesis of IONP
2.5. Characterization of IONPs
2.6. Experimental Setup
2.7. Statistical Analysis
3. Results and Discussion
3.1. Biosynthesis of IONPs
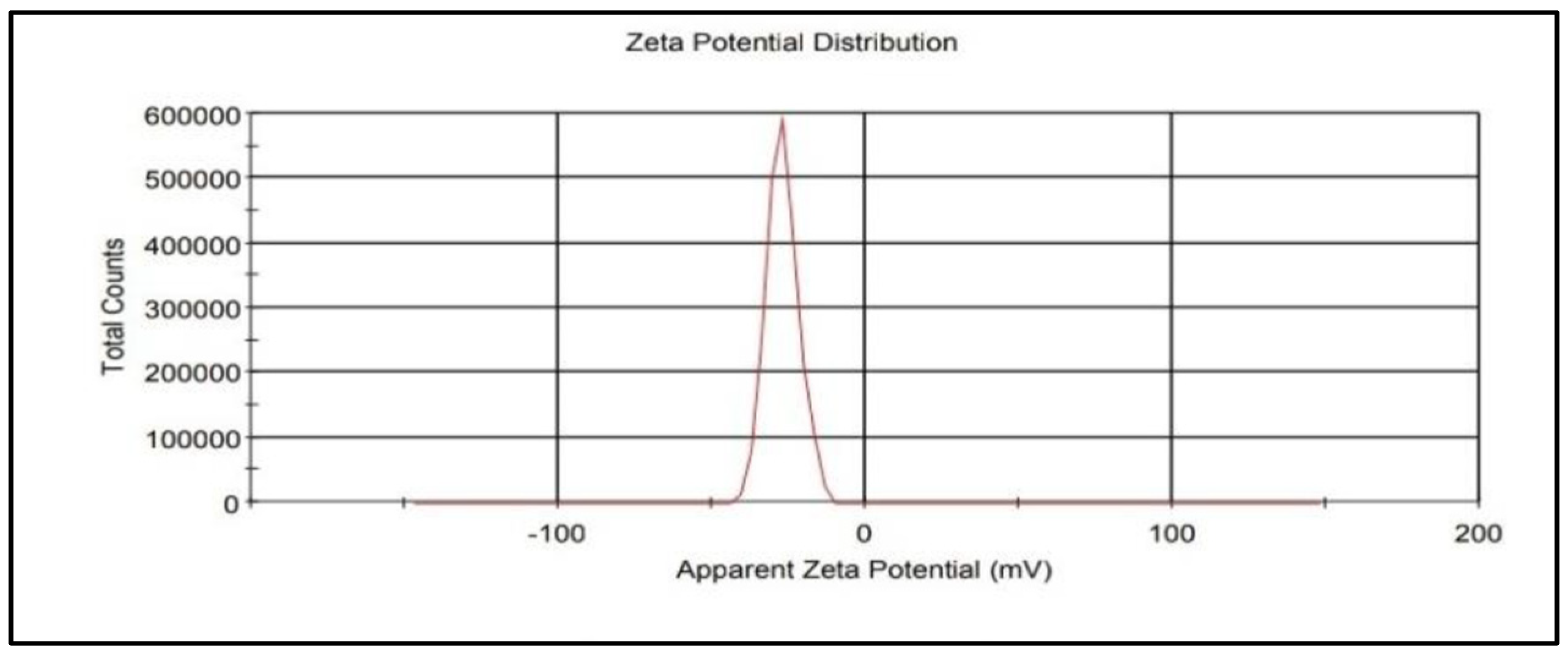
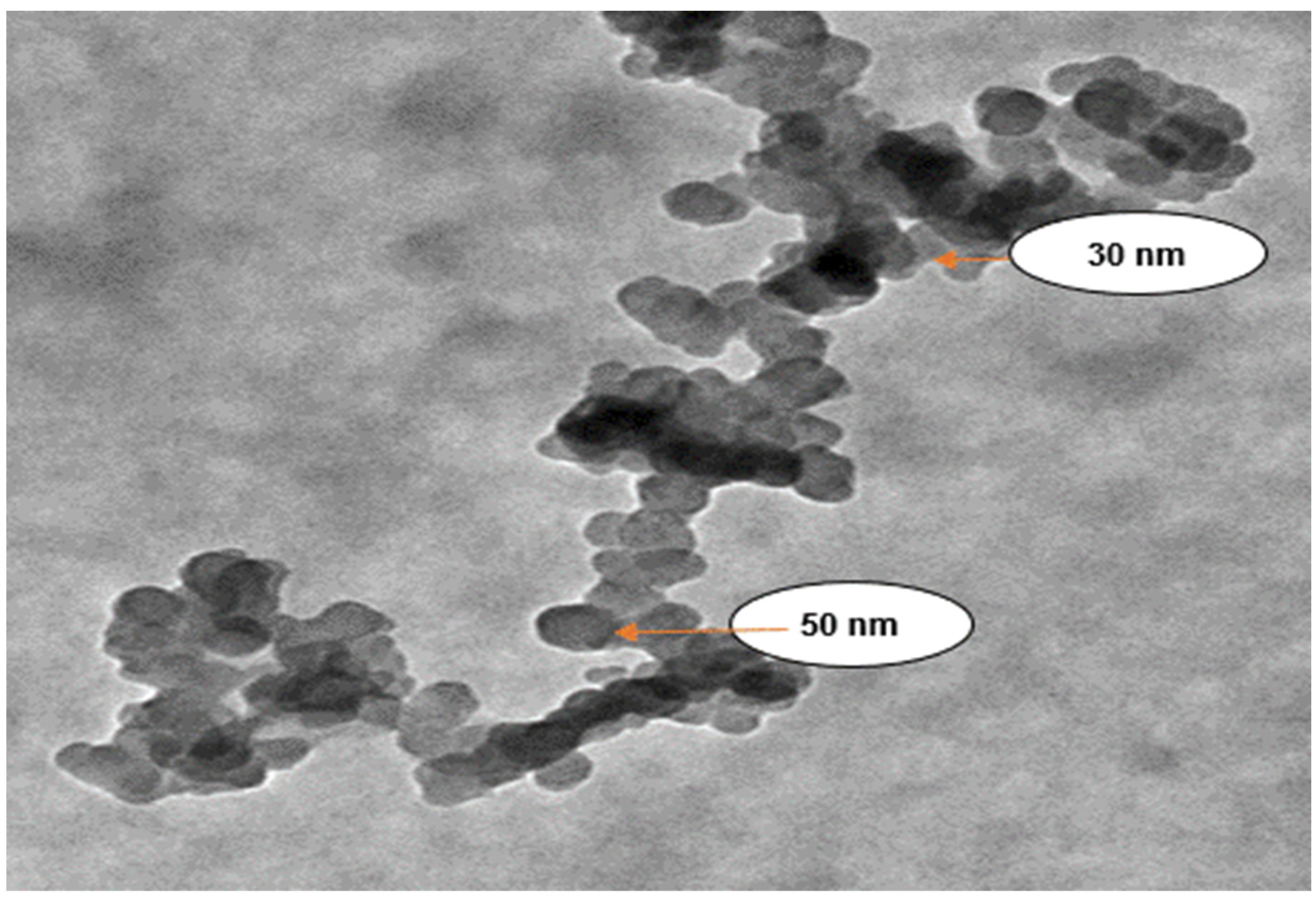
3.2. Characterization of IONPs
3.3. Effect of IONPs on Anaerobic Digestion (AD) of Cattle Manure
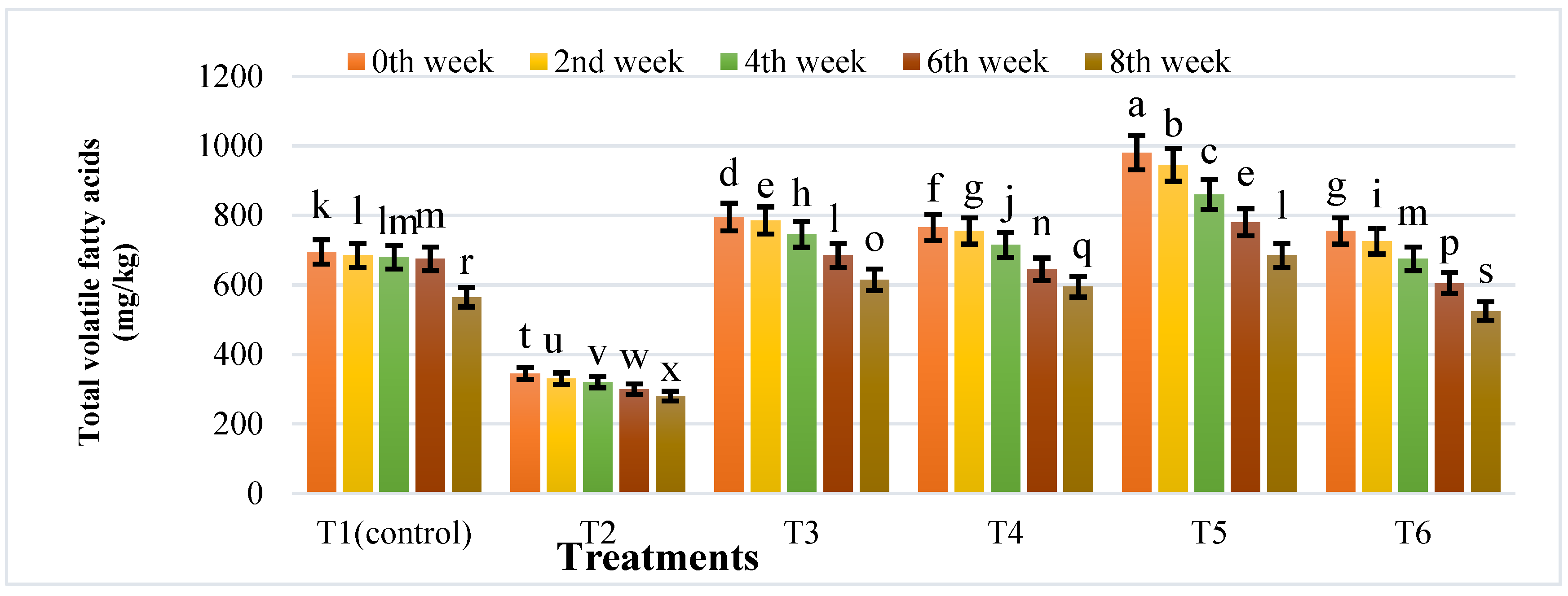
3.4. Changes in Physicochemical Properties during Anaerobic Digestion of Cattle Manure
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meegoda, J.N.; Li, B.; Patel, K.; Wang, L.B. A review of the processes, parameters, and optimization of anaerobic digestion. Int. J. Environ. Res. Public Health 2018, 15, 2224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, Y.; Zhang, Y.; Quan, X.; Chen, S. Enhanced anaerobic digestion of waste activated sludge digestion by the addition of zero valent iron. Water Res. 2014, 52, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhao, J.; Krooneman, J.; Gert, J.; Willem, E. Strategies to boost anaerobic digestion of cow manure: Laboratory achievement and their full-scale application potential. Sci. Total Environ. 2021, 755, 142940. [Google Scholar] [CrossRef] [PubMed]
- Romero-Güiza, M.; Vila, J.; Mata-Alvarez, J.; Chimenos, J.; Astals, S. The role of additives on anaerobic digestion: A review. Renew. Sustain. Energy Rev. 2016, 58, 1486–1499. [Google Scholar] [CrossRef]
- Ajay, C.M.; Mohan, S.; Dinesha, P.; Rosen, M.A. Review of impact of nanoparticle additives on anaerobic digestion and methane generation. Fuel 2020, 277, 118234. [Google Scholar] [CrossRef]
- Mody, V.; Siwale, R.; Singh, A.; Mody, H.R. Introduction to metallic nanoparticles. J. Pharm. Bioallied Sci. 2010, 2, 282–289. [Google Scholar] [CrossRef]
- Kamla, M.; Sushil, A.; Karmal, M. Nanotecology: A sustainable solution for bioenergy and biofuel production. J. Nanosci. Nanotechnol. 2021, 21, 3481–3494. [Google Scholar] [CrossRef]
- Machado, S.; Pinto, S.L.; Grosso, J.P.; Nouws, H.P.A.; Albergaria, J.T.; Delerue-Matos, C. Green production of zero-valent iron nanoparticles using tree leaf extracts. Sci. Total Environ. 2013, 445, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Abdelsalam, M.; Samer, Y.A.; Attia, M.A.; Abdel-Hadi, H.E.; Hassan, Y.; Badr, Y. Comparison of nanoparticles effects on biogas and methane production from anaerobic digestion of cattle dung slurry. Renew. Energy 2016, 87, 592–598. [Google Scholar] [CrossRef]
- Gupta, S.C.; Prasad, S.; Tyagi, A.K.; Kunnumakkara, A.B.; Aggarwal, B.B. Neem (Azadirachta indica): An Indian traditional panacea with modern molecular basis. Phytomedicine 2017, 34, 14–20. [Google Scholar] [CrossRef]
- Zambri, N.D.S.; Taib, N.I.; Latif, F.A.; Mohammed, Z. Utilization of neem leaf extract on biosynthesis of iron oxide nanoparticles. Molecules 2019, 24, 3803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srivastava, S.K.; Agrawal, B.; Kumar, A.; Pandey, A. Phytochemicals of Azadirachta indica source of active medicinal constituent used for cure of various diseases: A Review. J. Sci. Res. 2020, 64, 385–390. [Google Scholar] [CrossRef]
- Saleem, S.; Muhammad, G.; Hussain, M.A.; Bukhari, S.N.A. A comprehensive review of phytochemical profile, bioactive for pharmaceuticals and pharmacological attributes of Azadirachta indica. Phytother. Res. 2018, 32, 1241–1272. [Google Scholar] [CrossRef]
- Linton, Y.M.; Nisbet, A.J.; Mordue, A.J. The effects of azadirachtin on the testes of the desert locust, Schistocerca gregaria (Forskål). J. Insect Physiol. 1997, 43, 1077–1084. [Google Scholar] [CrossRef]
- Chutulo, E.C.; Chalannavar, R.K. Endophytic mycoflora and their bioactive compounds from Azadirachta indica: A comprehensive review. J. Fungi 2018, 4, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nisbet, A.J.; Mordue, A.J.; Grossman, R.B.; Jennens, L.; Ley, S.V.; Mordue, W. Characterization of azadirachtin binding to Sf9 nuclei in vitro. Arch. Insect. Biochem. Physiol. 2001, 46, 78–86. [Google Scholar] [CrossRef]
- Tabatabaei, M.; Aghbashlo, M.; Valijanian, E.; Panahi, H.K.S.; Nizami, A.S.; Ghanavati, H.; Sulaiman, A.; Mirmohamadsadeghi, S.; Karimi, K. A comprehensive review on recent biological innovations to improve biogas production, part 2: Mainstream and downstream strategies. Renew. Energy 2020, 146, 1392–1407. [Google Scholar] [CrossRef]
- Ambuchi, J.J.; Zhang, Z.; Feng, Y. Biogas enhancement using iron oxide nanoparticles and multi-wall carbon nanotubes. Int. J. Chem. Mol. Nucl. Mat. Metall. Eng. 2016, 10, 1239–1246. [Google Scholar]
- Abdelsalam, E.; Samer, M.; Attia, Y.A.; Abdel-Hadi, M.A.; Hassan, H.E.; Badr, Y. Influence of zero valent iron nanoparticles and magnetic iron oxide nanoparticles on biogas and methane production from anaerobic digestion of manure. Energy 2017, 120, 842–853. [Google Scholar] [CrossRef]
- Casals, E.; Barrena, R.; García, A.; González, E.; Delgado, L.; Busquets-Fité, M.; Font, X.; Arbiol, J.; Glatzel, P.; Kvashnina, K.; et al. Programmed iron oxide nanoparticles disintegration in anaerobic digesters boosts biogas production. Small 2014, 10, 2801–2808. [Google Scholar] [CrossRef] [Green Version]
- Farghali, M.; Andriamanohiarisoamanana, F.J.; Ahmed, M.M.; Kotb, S.; Yamashiro, T.; Iwasaki, M.; Umetsu, K. Impacts of iron oxide and titanium dioxide nanoparticles on biogas production: Hydrogen sulfide mitigation, process stability, and prospective challenges. J. Environ. Manag. 2019, 240, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Bhavika, T.; Paras, T. Green synthesis of zero valent iron nanoparticles from Coriandrum sativum and its application in reduction chemical oxygen demand and biological oxygen demand in waste water. South-Asian J. Multidiscip. Stud. 2017, 6, 132–139. [Google Scholar]
- Bibi, I.; Nazar, N.; Ata, S.; Sultan, M.; Ali, A.; Abbas, A.; Jilani, K.; Kamal, S.; Sarim, F.M.; Khan, M.I.; et al. Green synthesis of iron oxide nanoparticles using pomegranate seeds extract and photocatalytic activity evaluation for the degradation of textile dye. J. Mater. Res. Technol. 2019, 8, 6115–6124. [Google Scholar] [CrossRef]
- Sharma, A.K.; Pawar, C.A.; Prasad, N.R.; Yewale, M.A.; Kamble, D.B. Antimicrobial efficacy of green synthesized iron oxide nanoparticles. Mater. Res. Express 2018, 5, 075402. [Google Scholar] [CrossRef]
- Horwitz, W. Official Methods of Analysis of the Association of Official Analytical Chemists; The Association of Official Analytical Chemists: Washington, DC, USA, 2000. [Google Scholar]
- Casida, L.E., Jr.; Klein, D.A.; Santoro, T. Soil dehydrogenase activity. Soil Sci. 1964, 98, 371–376. [Google Scholar] [CrossRef]
- Christensen, L.; Vivekanandhan, S.; Misra, M.; Mohanty, A.K. Biosynthesis of silver nanoparticles using Murraya koenigii (curry leaf): An investigation on the effect of broth concentration in reduction mechanism and particle size. Adv. Mat. Lett. 2011, 2, 429–434. [Google Scholar] [CrossRef]
- Verma, A.; Mehata, M.S. Controllable synthesis of silver nanoparticles using Neem leaves and their antimicrobial activity. J. Radiat. Res. Appl. Sci. 2016, 9, 109–115. [Google Scholar] [CrossRef] [Green Version]
- Devatha, C.P.; Thalla, A.K.; Katte, S.Y. Green synthesis of iron nanoparticles using different leaf extracts for treatment of domestic waste water. J. Clean. Prod. 2016, 139, 1425–1435. [Google Scholar] [CrossRef]
- Singh, J.; Dutta, T.; Kim, K.H.; Rawat, M.; Samddar, P.; Kumar, P. ‘Green’ synthesis of metals and their oxide nanoparticles: Applications for environmental remediation. J. Nanotechnol. 2018, 16, 84. [Google Scholar] [CrossRef] [PubMed]
- Turakhia, B.; Turakhia, P.; Shah, S. Green synthesis of zero valent iron nanoparticles from Spinacia oleracea (spinach) and its application in waste water treatment. J. Adv. Res. Appl. Sci. 2018, 5, 46–51. [Google Scholar]
- Kumar, A.; Sawant, K. Encapsulation of exemestane in polycaprolactone nanoparticles: Optimization, characterization, and release kinetics. Cancer Nanotechnol. 2013, 4, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Mystrioti, C.; Xanthopoulou, T.D.; Tsakiridis, P.; Papassiopi, N.; Xenidis, A. Comparative evaluation of five plant extracts and juices for nano-iron synthesis and application for hexavalent chromium reduction. Sci. Total Environ. 2016, 539, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Yazdani, M.; Ebrahimi-Nik, M.; Heidari, A.; Abbaspour-Fard, M.H. Improvement of biogas production from slaughterhouse wastewater using biosynthesized iron nanoparticles from water treatment sludge. Renew. Energy 2019, 135, 496–501. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, J.; Hu, Z. Impact of nano zero valent iron (NZVI) on methanogenic activity and population dynamics in anaerobic digestion. Water Res. 2013, 47, 6790–6800. [Google Scholar] [CrossRef] [PubMed]
- Hijazi, O.; Abdelsalam, E.; Samer, M.; Attia, Y.A.; Amer, B.M.A.; Amer, M.A.; Badr, M.; Bernhardt, H. Life cycle assessment of the use of nanomaterials in biogas production from anaerobic digestion of manure. Renew. Energy 2020, 148, 417–424. [Google Scholar] [CrossRef]
- Men, Y.; Zheng, L.; Zhang, L.; Li, Z.; Wang, X.; Zhou, X.; Cheng, S.; Bao, W. Effect of adding zero valent iron on the anaerobic digestion of cow manure and lignocellulose. Front. Bioeng. Biotechnol. 2020, 28, 590200. [Google Scholar] [CrossRef]
- Emmanuel Kweinor, T.; Amo-Duodu, G.; Rathilal, S. Synergistic effects of magnetic nanomaterials on post-digestate for biogas production. Molecules 2020, 26, 6434. [Google Scholar] [CrossRef]
- Zupančič, G.D.; Uranjek-Ževart, N.; Roš, M. Full-scale anaerobic co-digestion of organic waste and municipal sludge. Biomass Bioenergy 2008, 32, 162–167. [Google Scholar] [CrossRef]
- Noonari, A.A.; Mahar, R.B.; Sahito, A.R.; Brohi, K.M. Anaerobic co-digestion of canola straw and banana plant wastes with buffalo dung: Effect of Fe3O4 nanoparticles on methane yield. Renew. Energy 2019, 133, 1046–1054. [Google Scholar] [CrossRef]




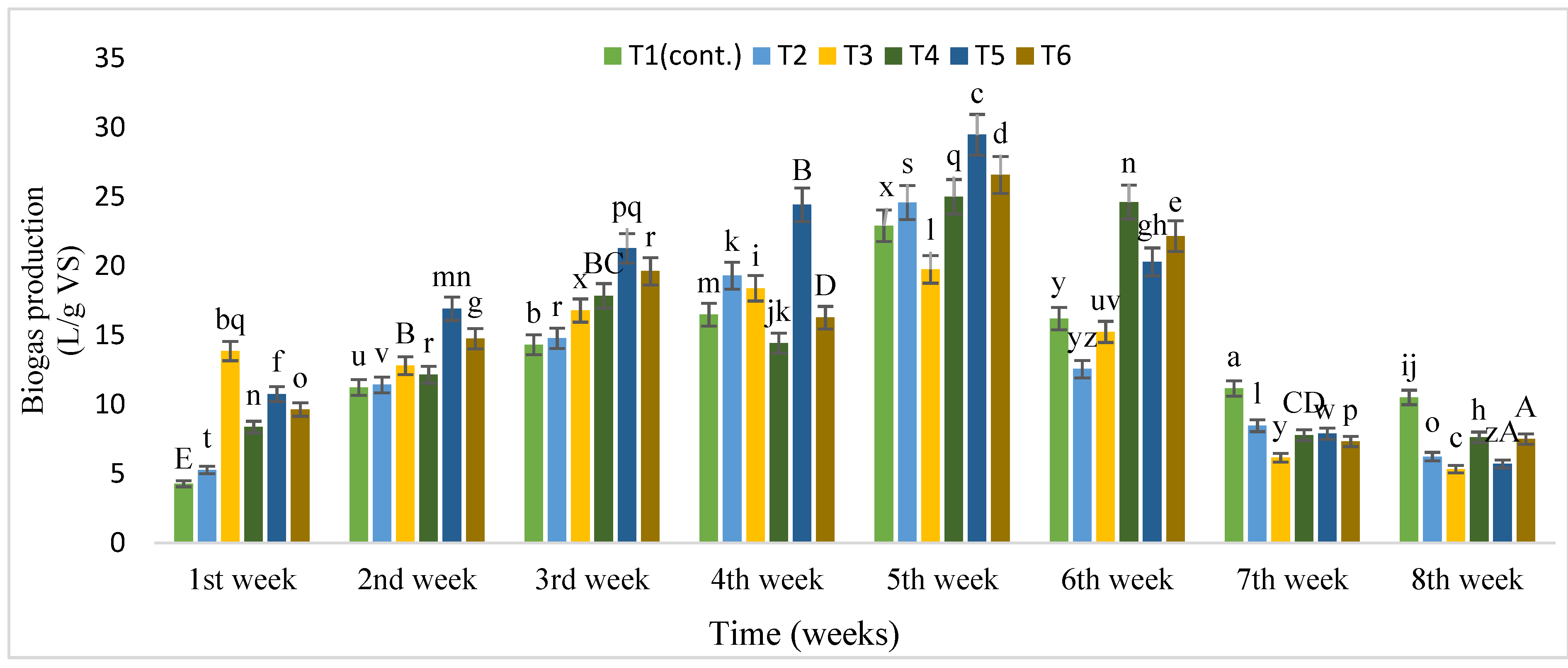



| Treatments | Description |
|---|---|
| T1 | Cattle manure (Control) |
| T2 | Cattle manure + 9 mg/L IONPs |
| T3 | Cattle manure + 12 mg/L IONPs |
| T4 | Cattle manure + 15 mg/L IONPs |
| T5 | Cattle manure + 18 mg/L IONPs |
| T6 | Cattle manure + 21 mg/L IONPs |
| Time (Weeks) | 1st | 2nd | 4th | 6th | 8th | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Treatments/ Parameters (%) | TS | VS | TS | VS | TS | VS | TS | VS | TS | VS |
| T1 | 18.42 | 82.0 | 17.80 | 81.0 | 16.90 | 80.0 | 15.4 | 79.0 | 14.15 | 78.0 |
| T2 | 19.15 | 83.0 | 18.70 | 11.0 | 17.65 | 87.0 | 15.23 | 82.0 | 13.41 | 74.0 |
| T3 | 18.73 | 82.3 | 17.88 | 82.0 | 16.25 | 81.0 | 14.10 | 80.0 | 12.70 | 77.0 |
| T4 | 17.65 | 80.0 | 16.44 | 79.0 | 15.51 | 79.0 | 13.96 | 78.0 | 12.27 | 76.0 |
| T5 | 17.83 | 79.0 | 16.77 | 78.0 | 15.03 | 77.0 | 12.50 | 77.0 | 10.14 | 75.0 |
| T6 | 18.15 | 81.0 | 16.25 | 80.0 | 15.09 | 78.0 | 13.85 | 74.0 | 11.28 | 72.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, D.; Malik, K.; Sindhu, M.; Kumari, N.; Rani, V.; Mehta, S.; Malik, K.; Ranga, P.; Sharma, K.; Dhull, N.; et al. Biostimulation of Anaerobic Digestion Using Iron Oxide Nanoparticles (IONPs) for Increasing Biogas Production from Cattle Manure. Nanomaterials 2022, 12, 497. https://doi.org/10.3390/nano12030497
Singh D, Malik K, Sindhu M, Kumari N, Rani V, Mehta S, Malik K, Ranga P, Sharma K, Dhull N, et al. Biostimulation of Anaerobic Digestion Using Iron Oxide Nanoparticles (IONPs) for Increasing Biogas Production from Cattle Manure. Nanomaterials. 2022; 12(3):497. https://doi.org/10.3390/nano12030497
Chicago/Turabian StyleSingh, Dilbag, Kamla Malik, Meena Sindhu, Nisha Kumari, Vijaya Rani, Shikha Mehta, Karmal Malik, Poonam Ranga, Kashish Sharma, Neeru Dhull, and et al. 2022. "Biostimulation of Anaerobic Digestion Using Iron Oxide Nanoparticles (IONPs) for Increasing Biogas Production from Cattle Manure" Nanomaterials 12, no. 3: 497. https://doi.org/10.3390/nano12030497
APA StyleSingh, D., Malik, K., Sindhu, M., Kumari, N., Rani, V., Mehta, S., Malik, K., Ranga, P., Sharma, K., Dhull, N., Malik, S., & Arya, N. (2022). Biostimulation of Anaerobic Digestion Using Iron Oxide Nanoparticles (IONPs) for Increasing Biogas Production from Cattle Manure. Nanomaterials, 12(3), 497. https://doi.org/10.3390/nano12030497







