Rejection Mechanism of Ionic Solute Removal by Nanofiltration Membranes: An Overview
Abstract
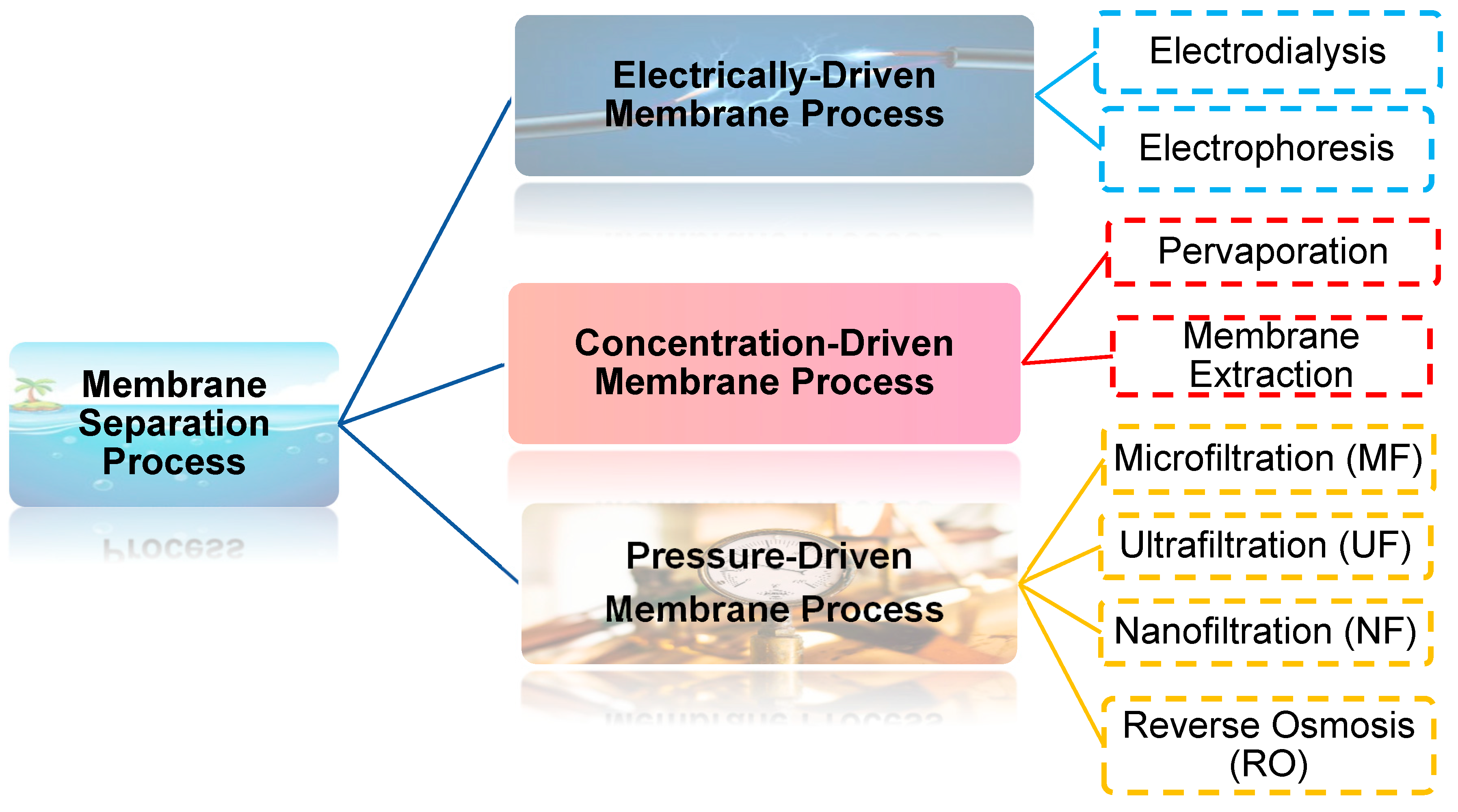
:1. Introduction
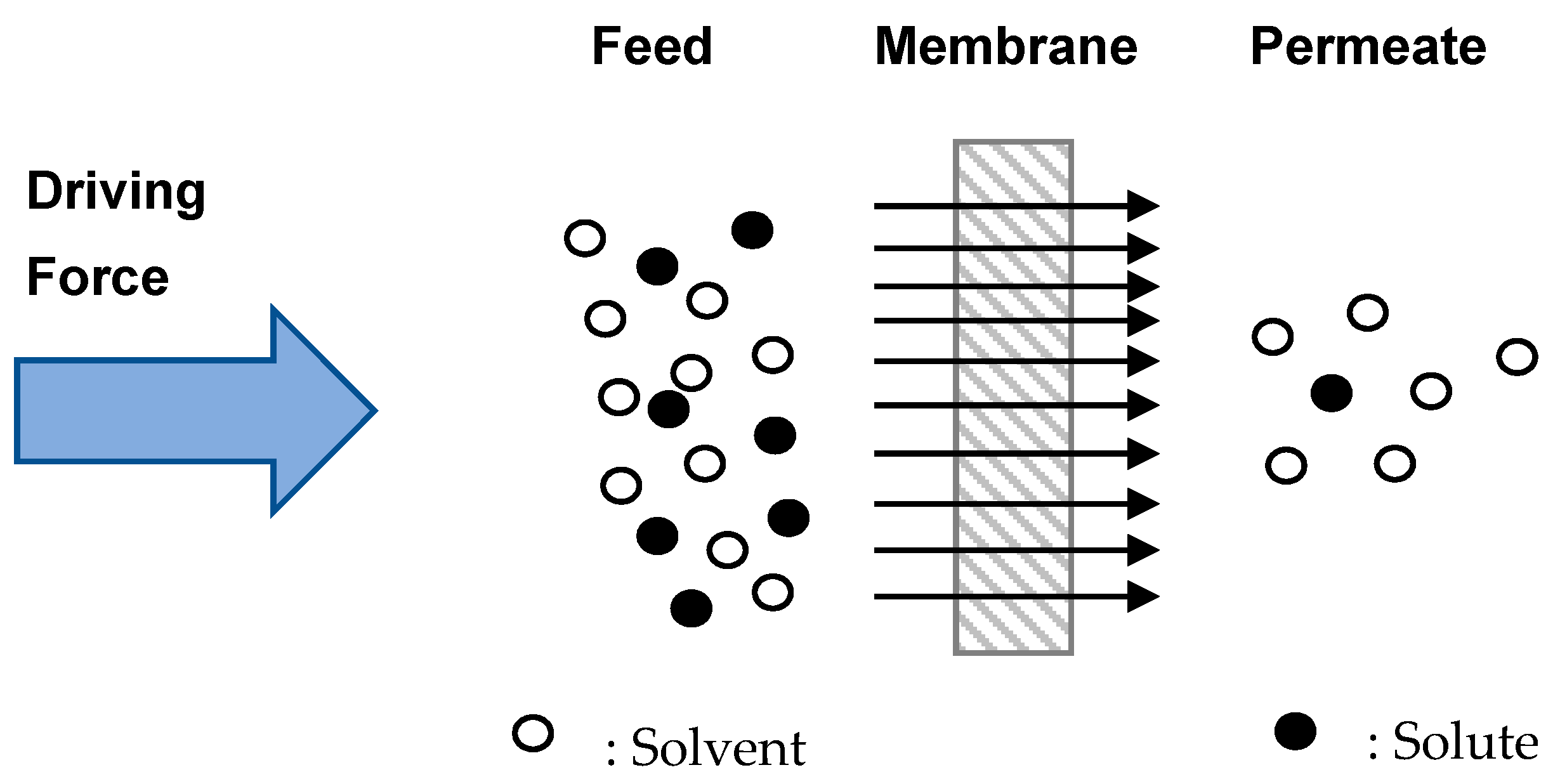
2. Nanofiltration Membrane
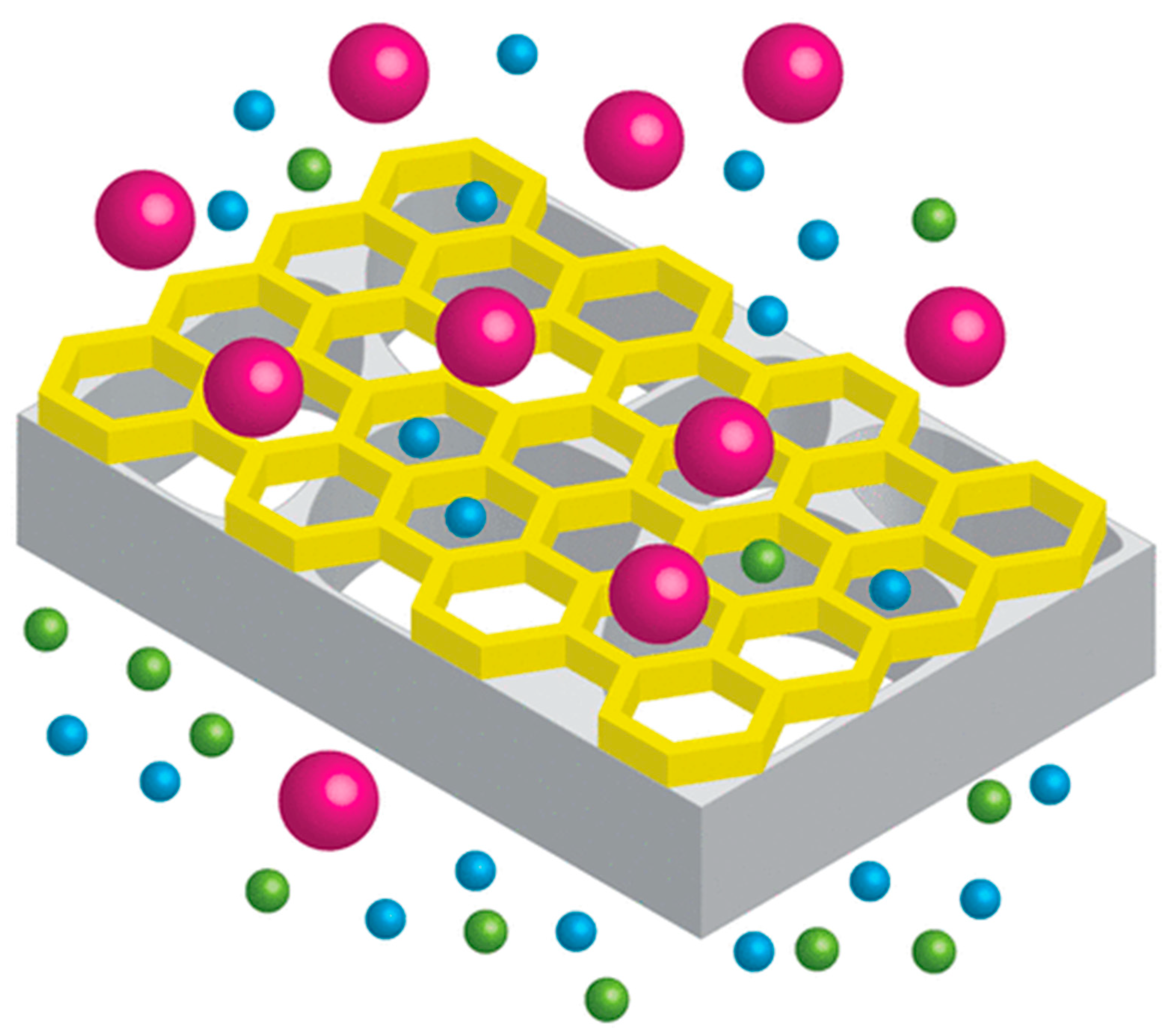
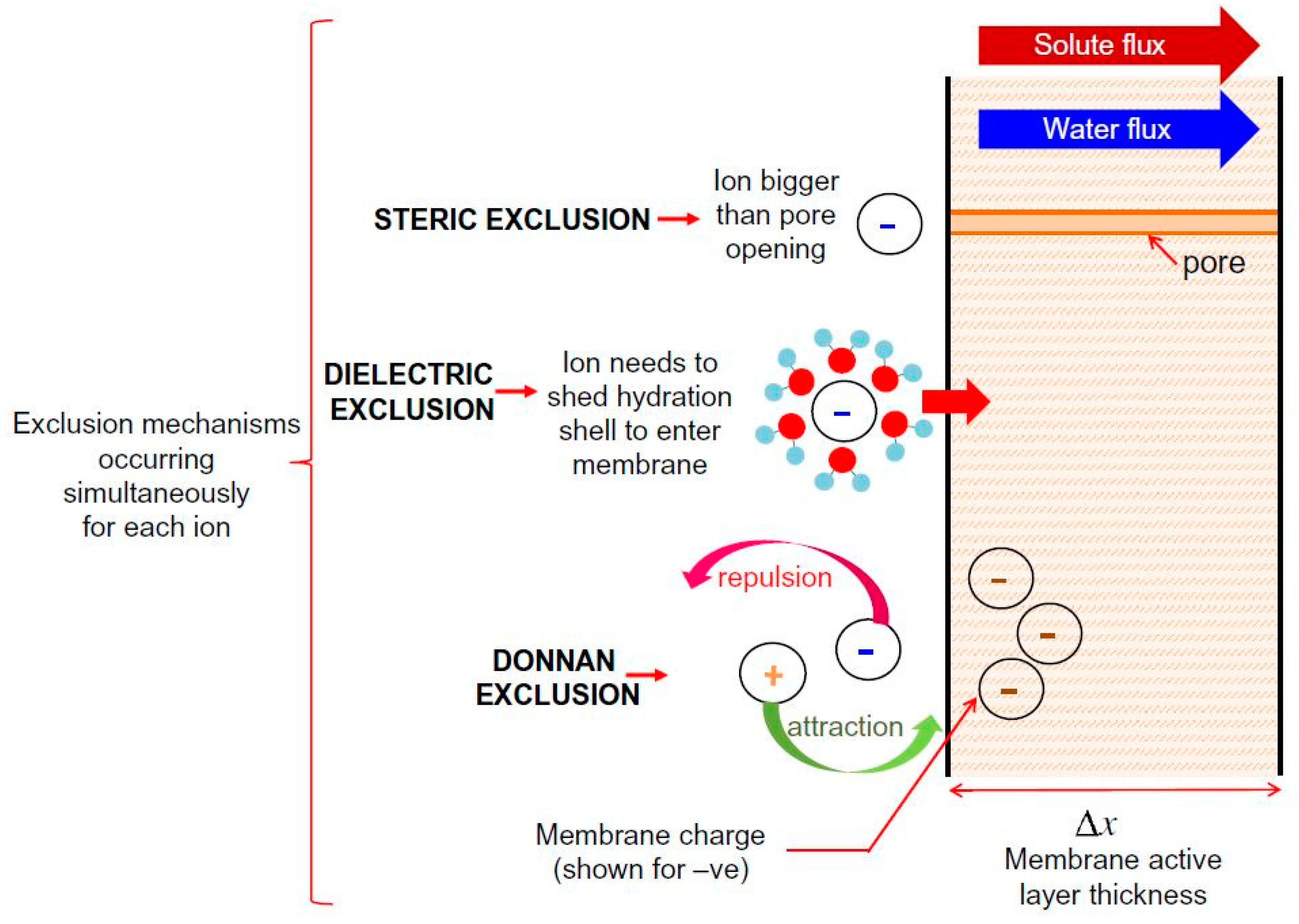
3. Sieving Mechanism: Size Exclusion (Steric Hindrance)
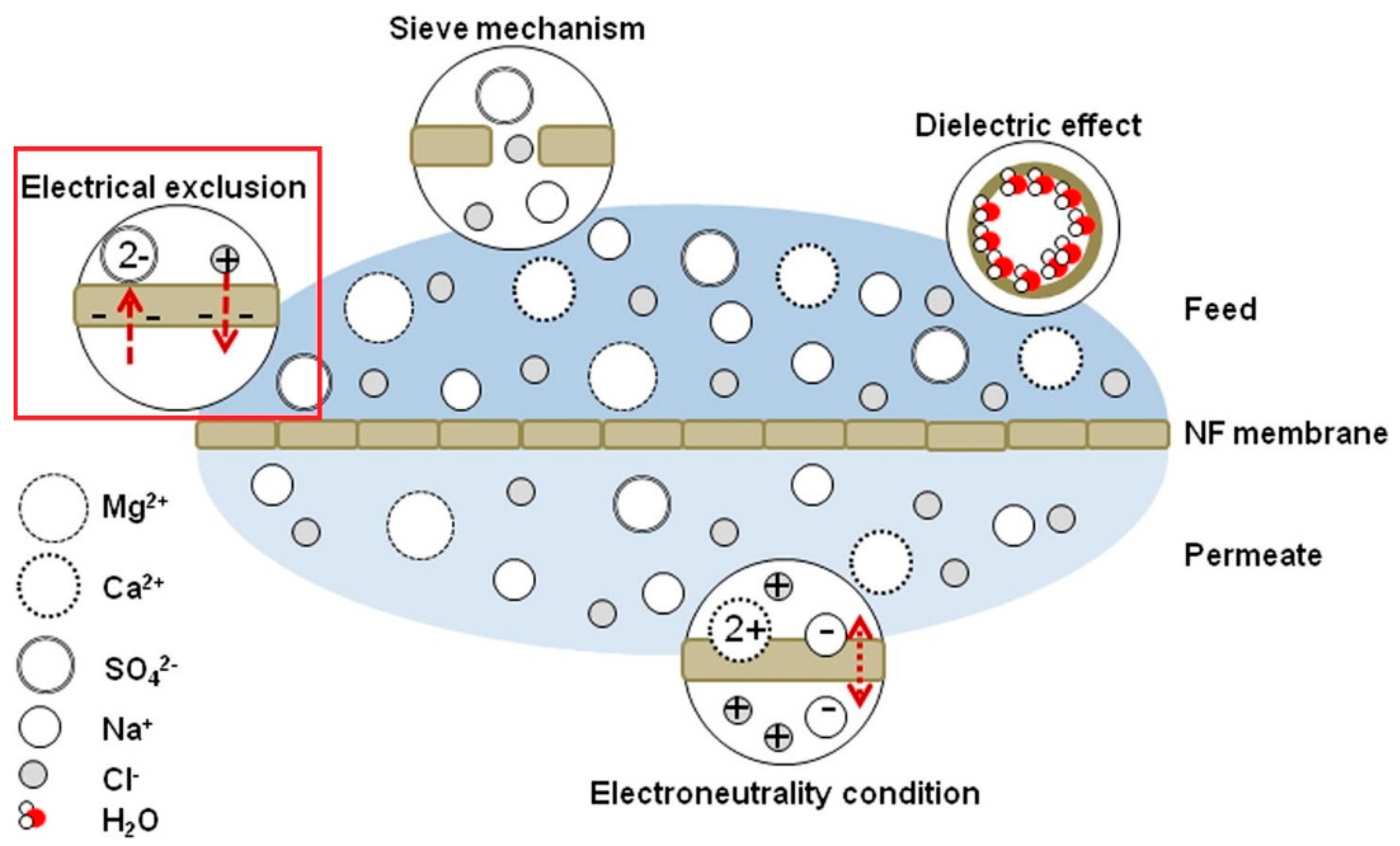
4. Non-Sieving Mechanism
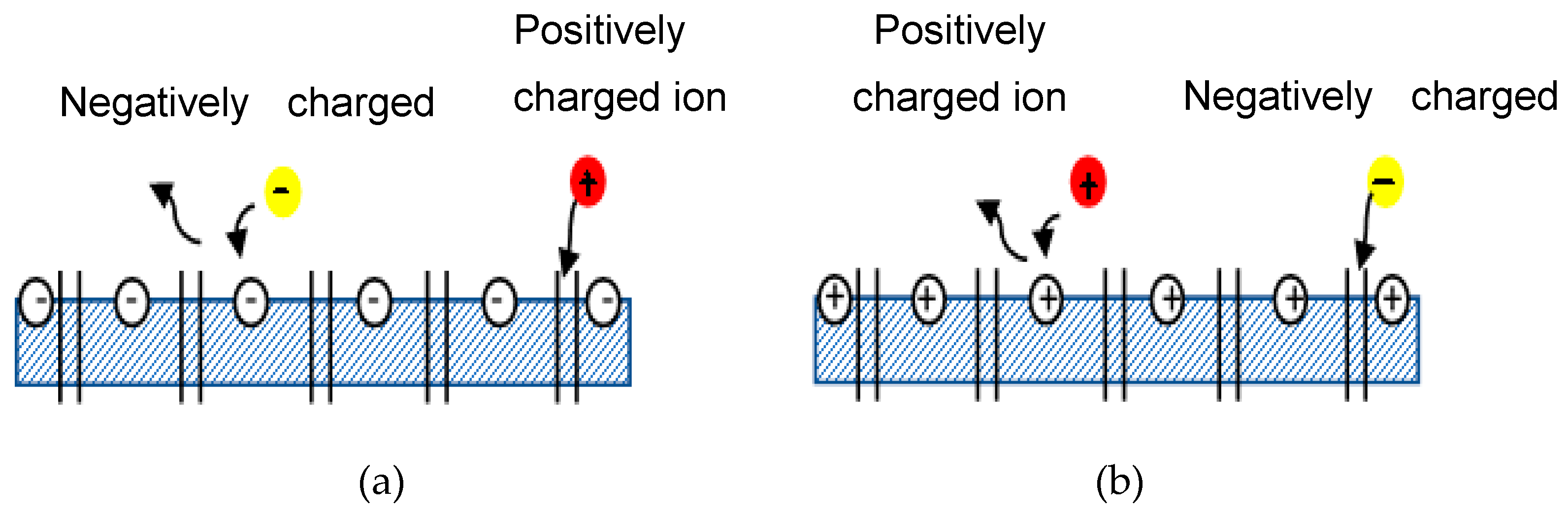
4.1. Donnan Exclusion
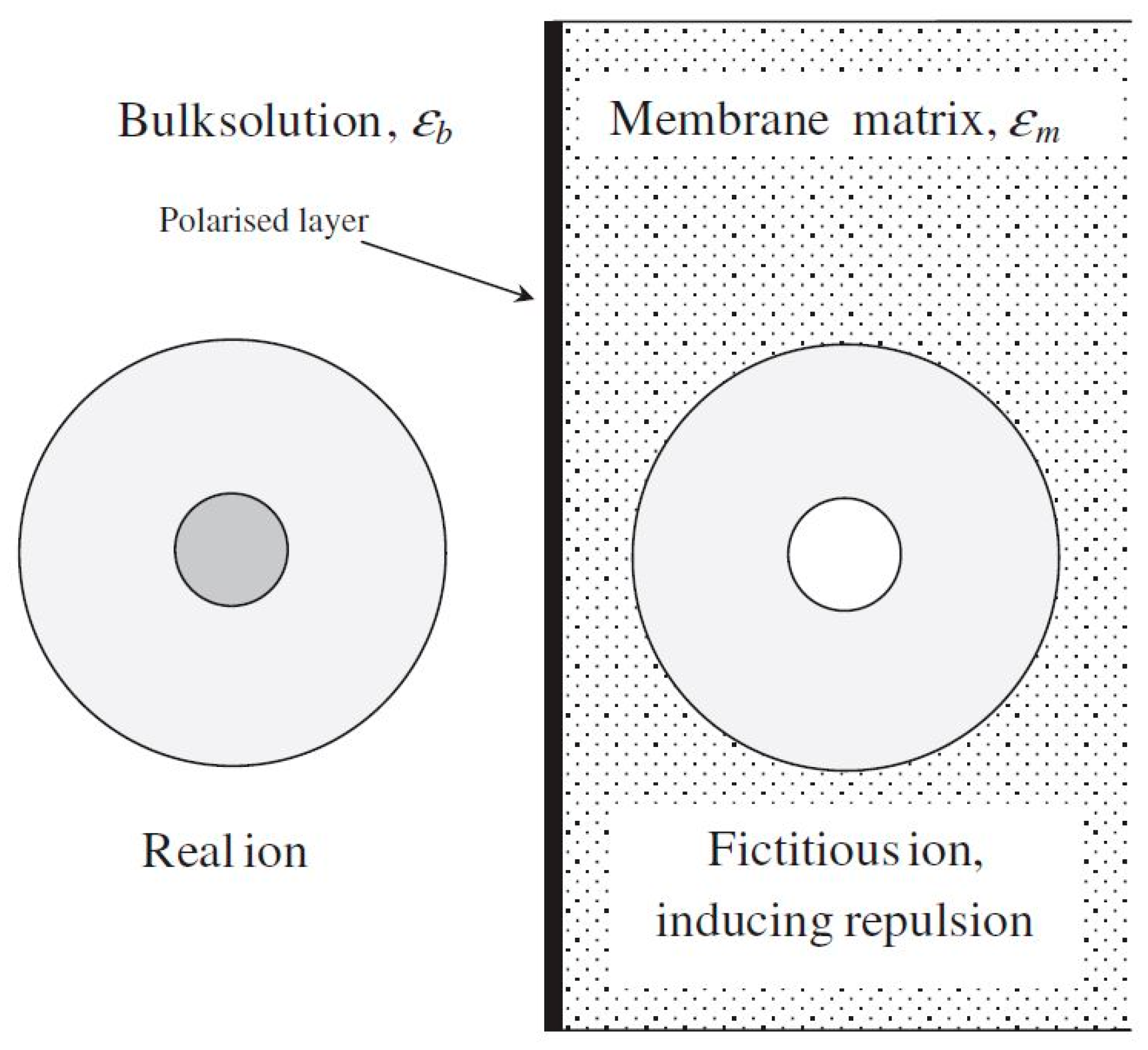
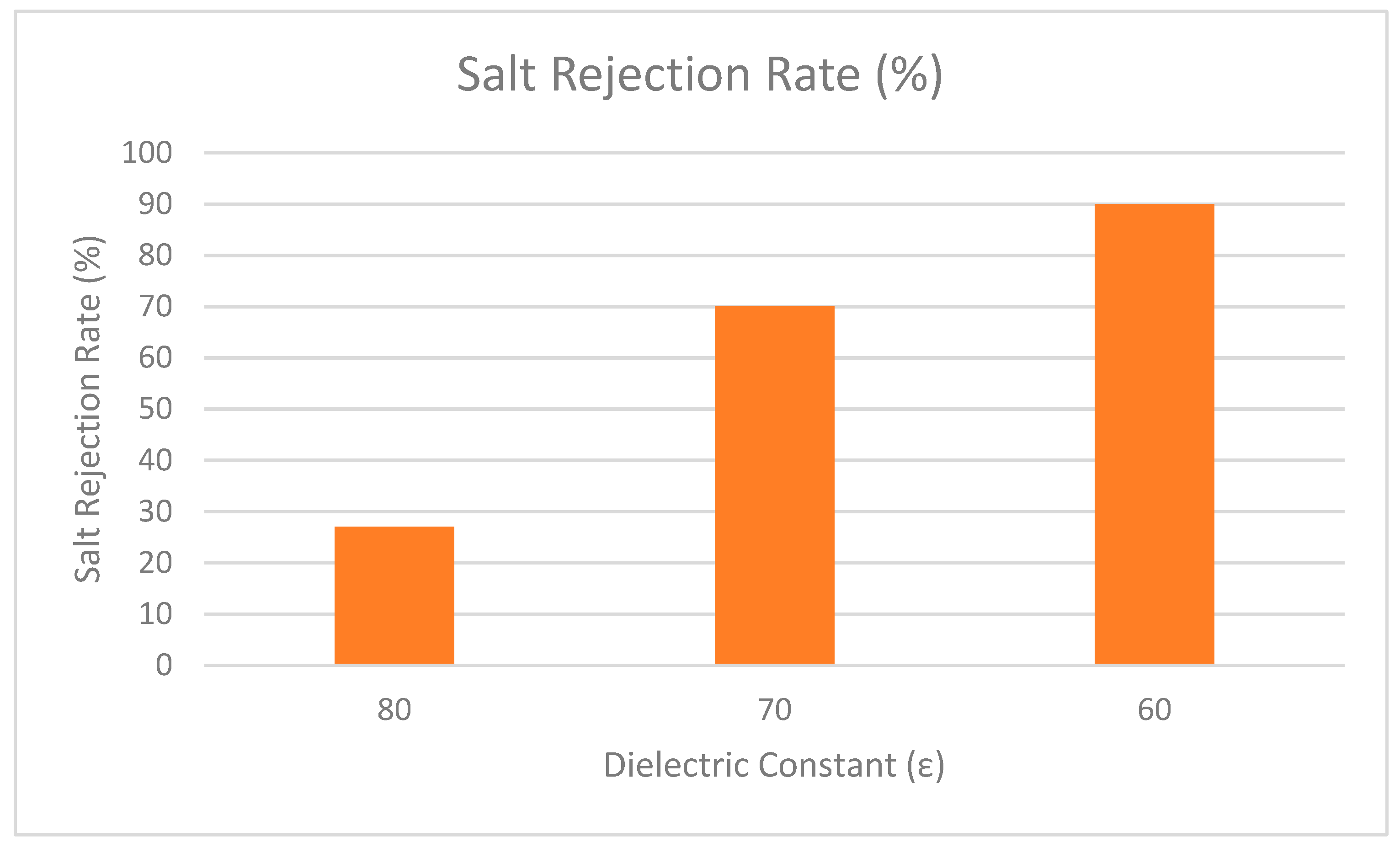
4.2. Dielectric Exclusion
4.3. Hydration Mechanism
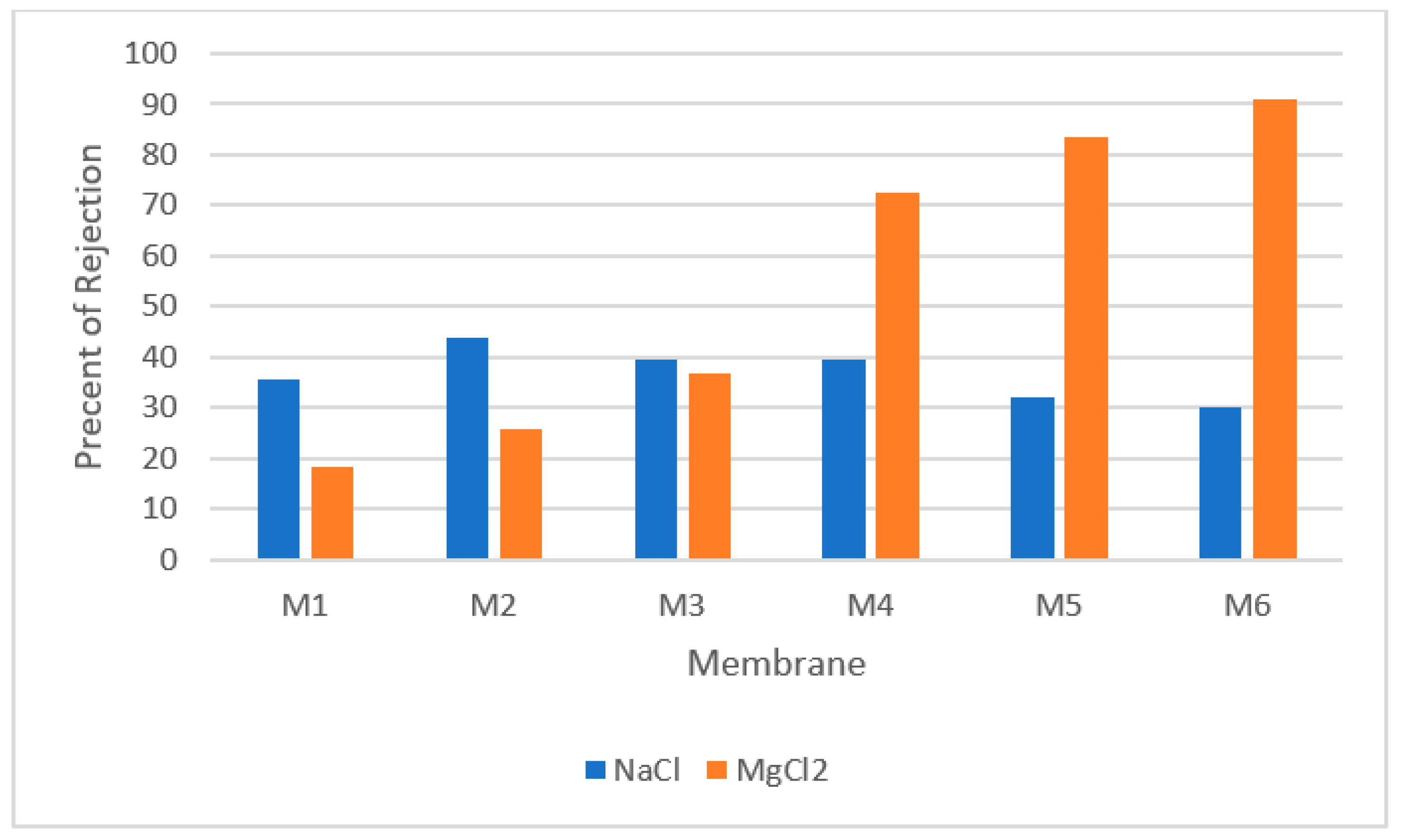
5. Donnan Exclusion as the Main Non-Sieving Rejection Mechanism
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Crittenden, J.C.; Trussell, R.R.; Hand, D.W.; Howe, K.; Tchobanoglous, G. Water Treatment: Principles and Design, 3rd ed.; MWH: Hoboken, NJ, USA, 2012; ISBN 978-0-470-40539-0. [Google Scholar]
- Ezugbe, E.O.; Rathilal, S. Membrane technologies in wastewater treatment: A review. Membranes 2020, 10, 89. [Google Scholar] [CrossRef] [PubMed]
- Peters, T. Membrane technology for water treatment. Chem. Eng. Technol. 2010, 33, 1233–1240. [Google Scholar] [CrossRef]
- Singh, R.; Hankins, N. Emerging Membrane Technology for Sustainable Water Treatment, 1st ed.; Elsevier Science: Amsterdam, The Netherlands, 2016; ISBN 978044463316. [Google Scholar]
- Malliga, P.; Bela, R.B.; Shanmugapriya, N. Conversion of Textile Effluent Wastewater into Fertilizer Using Marine Cyanobacteria along with Different Agricultural Waste; Elsevier Inc.: Amsterdam, The Netherlands, 2020; ISBN 9780128179512. [Google Scholar]
- Siddiqui, M.U.; Arif, A.F.M.; Bashmal, S. Permeability-selectivity analysis of microfiltration and ultrafiltration membranes: Effect of pore size and shape distribution and membrane stretching. Membranes 2016, 6, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasim, N.; Mahmoudi, E.; Mohammad, A.W.; Abdullah, S.R.S. Influence of feed concentration and pH on iron and manganese rejection via nanohybrid polysulfone/Ag-GO ultrafiltration membrane. Desalin. Water Treat. 2017, 61, 29–41. [Google Scholar] [CrossRef]
- Xie, Z.; Li, N.; Wang, Q.; Bolto, B. Desalination by pervaporation. Emerg. Technol. Sustain. Desalin. Handb. 2018, 205–226. [Google Scholar] [CrossRef]
- Kasim, N.; Mahmoudi, E.; Mohammad, A.W.; Sheikh Abdullah, S.R. Study on the Effect of Applied Pressure on Iron and Manganese Rejection by Polyamide and Polypiperazine Amide Nanofiltration Membranes. Solid State Phenom. 2021, 317, 283–290. [Google Scholar] [CrossRef]
- Pirsaheb, M.; Khosravi, T.; Sharafi, K.; Moradi, M. Comparing operational cost and performance evaluation of electrodialysis and reverse osmosis systems in nitrate removal from drinking water in Golshahr, Mashhad. Desalin. Water Treat. 2016, 57, 5391–5397. [Google Scholar] [CrossRef]
- Patel, S.K.; Biesheuvel, P.M.; Elimelech, M. Energy Consumption of Brackish Water Desalination: Identifying the Sweet Spots for Electrodialysis and Reverse Osmosis. ACS ES&T Eng. 2021, 1, 851–864. [Google Scholar] [CrossRef]
- Kei, L.M. Characterization of Cellulose Tri Acetate (Cta) forward Osmosis Membrane for Nom Removal. Ph.D. Thesis, Universiti Malaysia Pahang, Pekan, Malaysia, 2015. [Google Scholar]
- Root, T. Arsenic Speciation and Form in a Glacial Aquifer. 2008. Available online: https://www.researchgate.net/profile/Tara-Root/publication/235224795_Arsenic_Speciation_and_Form_in_a_Glacial_Aquifer_in_the_Midwestern_United_States/links/02bfe5109242d35a9f000000/Arsenic-Speciation-and-Form-in-a-Glacial-Aquifer-in-the-Midwestern-United-States.pdf (accessed on 21 November 2021).
- Singh, N.A.; Kumar, N.; Vishweswaraiah, R.H. Microbial aspects of drinking water quality. Microb. Res. 2011, 117–153. [Google Scholar]
- Shon, H.K.; Phuntsho, S.; Chaudhary, D.S.; Vigneswaran, S.; Cho, J. Nanofiltration for water and wastewater treatment—A mini review. Drink. Water Eng. Sci. 2013, 6, 47–53. [Google Scholar] [CrossRef] [Green Version]
- Kotrappanavar, N.S.; Hussain, A.A.; Abashar, M.E.E.; Al-Mutaz, I.S.; Aminabhavi, T.M.; Nadagouda, M.N. Prediction of physical properties of nanofiltration membranes for neutral and charged solutes. Desalination 2011, 280, 174–182. [Google Scholar] [CrossRef]
- Yaroshcuk, A.E. Rejection mechanisms of NF membranes. Membr. Technol. 1998, 1998, 9–12. [Google Scholar] [CrossRef]
- Labban, O.; Chong, T.H.; Lienhard, J.H. Design and modeling of novel low-pressure nanofiltration hollow fiber modules for water softening and desalination pretreatment. Desalination 2018, 439, 58–72. [Google Scholar] [CrossRef] [Green Version]
- Mohammad, A.W.; Teow, Y.H.; Ang, W.L.; Chung, Y.T.; Oatley-Radcliffe, D.L.; Hilal, N. Nanofiltration membranes review: Recent advances and future prospects. Desalination 2015, 356, 226–254. [Google Scholar] [CrossRef]
- Qadir, D.; Mukhtar, H.B.; Keong, L.K. Rejection of divalent ions in commercial tubular membranes: Effect of feed concentration and anion type. Sustain. Environ. Res. 2017, 27, 103–106. [Google Scholar] [CrossRef]
- Ali, M.E.A. Nanofiltration process for enhanced treatment of RO brine discharge. Membranes 2021, 11, 212. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Li, Q.; He, B.; Liao, B.; Li, X.; Hu, M.; Ji, Y.; Cui, Z.; Younas, M.; Li, J. An ultrahighly permeable-selective nanofiltration membrane mediated by an in situ formed interlayer. J. Mater. Chem. A 2020, 8, 5275–5283. [Google Scholar] [CrossRef]
- Kiamehr, Z.; Farokhi, B.; Hosseini, S.M. Development of a highly-permeable thin-film-based nanofiltration membrane by using surface treatment with Air-Ar plasma. Korean J. Chem. Eng. 2021, 38, 114–120. [Google Scholar] [CrossRef]
- Pal, P. Introduction to membrane materials, processes, and modules. In Membrane-Based Technologies for Environmental Pollution Control; Butterworth-Heinemann: Oxford, UK, 2020; ISBN 9780128194553. [Google Scholar]
- Meng, F.; Chae, S.R.; Drews, A.; Kraume, M.; Shin, H.S.; Yang, F. Recent advances in membrane bioreactors (MBRs): Membrane fouling and membrane material. Water Res. 2009, 43, 1489–1512. [Google Scholar] [CrossRef]
- Du, X.; Shi, Y.; Jegatheesan, V.; Ul Haq, I. A Review on the Mechanism, Impacts and Control Methods of Membrane Fouling in MBR System. Membranes 2020, 10, 24. [Google Scholar] [CrossRef] [Green Version]
- Le-Clech, P.; Chen, V.; Fane, T.A.G. Fouling in membrane bioreactors used in wastewater treatment. J. Membr. Sci. 2006, 284, 17–53. [Google Scholar] [CrossRef]
- Rahimpour, M.R.; Mahmoodi, L. Performance of Reactors with PMs; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780128135495. [Google Scholar]
- Ng, L.Y.; Mohammad, A.W.; Ng, C.Y. A review on nanofiltration membrane fabrication and modification using polyelectrolytes: Effective ways to develop membrane selective barriers and rejection capability. Adv. Colloid Interface Sci. 2013, 197–198, 85–107. [Google Scholar] [CrossRef] [PubMed]
- Abu Seman, M.N.; Hilal, N.; Khayet, M. UV-photografting modification of NF membrane surface for NOM wfouling reduction. Desalin. Water Treat. 2013, 51, 4855–4861. [Google Scholar] [CrossRef] [Green Version]
- Changani, Z.; Razmjou, A.; Taheri-Kafrani, A.; Warkiani, M.E.; Asadnia, M. Surface modification of polypropylene membrane for the removal of iodine using polydopamine chemistry. Chemosphere 2020, 249, 126079. [Google Scholar] [CrossRef]
- Hong Anh Ngo, T.; Dinh Do, K.; Thi Tran, D. Surface modification of polyamide TFC membranes via redox-initiated graft polymerization of acrylic acid. J. Appl. Polym. Sci. 2017, 134, 1–8. [Google Scholar] [CrossRef]
- Chai, P.V.; Law, J.Y.; Mahmoudi, E.; Mohammad, A.W. Development of iron oxide decorated graphene oxide (Fe3O4/GO) PSf mixed-matrix membrane for enhanced antifouling behavior. J. Water Process Eng. 2020, 38, 101673. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhu, H.; Li, A.; Li, G.; Mai, Z.; Gu, Y. Investigating the effect of inhomogeneous fixed charge distribution on dielectric exclusion in nanofiltration membranes. Desalin. Water Treat. 2019, 166, 1–8. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Kong, F.X.; Wang, Z.; Yang, H.W.; Wang, X.M.; Xie, Y.F.; Waite, T.D. Role of membrane and compound properties in affecting the rejection of pharmaceuticals by different RO/NF membranes. Front. Environ. Sci. Eng. 2017, 11, 20. [Google Scholar] [CrossRef]
- Schäfer, A.I.; Akanyeti, I.; Semião, A.J.C. Micropollutant sorption to membrane polymers: A review of mechanisms for estrogens. Adv. Colloid Interface Sci. 2011, 164, 100–117. [Google Scholar] [CrossRef]
- Idress, H.; Zaidi, S.Z.J.; Sabir, A.; Shafiq, M.; Khan, R.U.; Harito, C.; Hassan, S.; Walsh, F.C. Cellulose acetate based Complexation-NF membranes for the removal of Pb(II) from waste water. Sci. Rep. 2021, 11, 1806. [Google Scholar] [CrossRef]
- Wang, J.; Ren, Y.; Zhang, H.; Luo, J.; Woodley, J.M.; Wan, Y. Targeted modification of polyamide nanofiltration membrane for efficient separation of monosaccharides and monovalent salt. J. Membr. Sci. 2021, 628, 119250. [Google Scholar] [CrossRef]
- Hu, P.; He, J.; Tian, B.; Xu, Z.; Yuan, T.; Sun, H.; Li, P.; Niu, Q.J. Application of diazonium-induced anchoring process on ultrafiltration substrate for the fabrication of nanofiltration membrane with enhanced desalination performance. Desalination 2020, 496, 114340. [Google Scholar] [CrossRef]
- Ang, W.L.; Mohammad, A.W.; Benamor, A.; Hilal, N.; Leo, C.P. Hybrid coagulation–NF membrane process for brackish water treatment: Effect of antiscalant on water characteristics and membrane fouling. Desalination 2016, 393, 144–150. [Google Scholar] [CrossRef]
- Sandle, T. Sterilisation by filtration. Steril. Steril. Steril. Assur. Pharm. 2013, 143–155. [Google Scholar] [CrossRef]
- Zhang, R.; Tian, J.; Gao, S.; Van Der Bruggen, B. How to coordinate the trade-off between water permeability and salt rejection in nanofiltration? J. Mater. Chem. A 2020, 8, 8831–8847. [Google Scholar] [CrossRef]
- Seidel, A.; Waypa, J.J.; Elimelech, M. Role of charge (Donnan) exclusion in removal of arsenic from water by a negatively charged porous nanofiltration membrane. Environ. Eng. Sci. 2001, 18, 105–113. [Google Scholar] [CrossRef]
- Zhu, L. Rejection of Organic Micropollutants by Clean and Fouled Nanofiltration Membranes. J. Chem. 2015, 2015, 934318. [Google Scholar] [CrossRef] [Green Version]
- Valentino, L.; Matsumoto, M.; Dichtel, W.R.; Marinas, B.J. Development and Performance Characterization of a Polyimine Covalent Organic Framework Thin-Film Composite Nanofiltration Membrane. Environ. Sci. Technol. 2017, 51, 14352–14359. [Google Scholar] [CrossRef]
- Van der Bruggen, B.; Vandecasteele, C. Modelling of the retention of uncharged molecules with nanofiltration. Water Res. 2002, 36, 1360–1368. [Google Scholar] [CrossRef]
- Van Der Bruggen, B.; Schaep, J.; Wilms, D.; Vandecasteele, C. Influence of molecular size, polarity and charge on the retention of organic molecules by nanofiltration. J. Membr. Sci. 1999, 156, 29–41. [Google Scholar] [CrossRef]
- Schutte, C.F. The rejection of specific organic compounds by reverse osmosis membranes. Desalination 2003, 158, 285–294. [Google Scholar] [CrossRef]
- Yoon, Y.; Lueptow, R.M. Removal of organic contaminants by RO and NF membranes. J. Membr. Sci. 2005, 261, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.E.; Liang, C.H.; Huang, C.P.; Chiang, P.C. A simplified method for elucidating the effect of size exclusion on nanofiltration membranes. Sep. Purif. Technol. 2012, 85, 1–7. [Google Scholar] [CrossRef]
- Miron, S.M.; Dutournié, P.; Ponche, A. Filtration of uncharged solutes: An assessment of steric effect by transport and adsorption modelling. Water 2019, 11, 2173. [Google Scholar] [CrossRef] [Green Version]
- Vrijenhoek, E.M.; Waypa, J.J. Arsenic removal from drinking water by a “loose” nanofiltration membrane. Desalination 2000, 130, 265–277. [Google Scholar] [CrossRef]
- Bhalla, G.; Deen, W.M. Effects of charge on osmotic reflection coefficients of macromolecules in fibrous membranes. Biophys. J. 2009, 97, 1595–1605. [Google Scholar] [CrossRef] [Green Version]
- Suárez, A.; Riera, F.A. Using the spiegler-kedem model to predict solute rejection in the treatment of industrial UHT condensates by reverse osmosis. Desalin. Water Treat. 2016, 57, 24176–24186. [Google Scholar] [CrossRef]
- Velicangil, O.; Howell, J.A. Estimation of the properites of high-flux ultrafiltration membranes. J. Phys. Chem. 1980, 84, 2991–2992. [Google Scholar] [CrossRef]
- Bowen, W.R.; Mohammad, A.W.; Hilal, N. Characterisation of nanofiltration membranes for predictive purposes—Use of salts, uncharged solutes and atomic force microscopy. J. Membr. Sci. 1997, 126, 91–105. [Google Scholar] [CrossRef]
- Geraldes, V.; Brites Alves, A.M. Computer program for simulation of mass transport in nanofiltration membranes. J. Membr. Sci. 2008, 321, 172–182. [Google Scholar] [CrossRef]
- Epsztein, R.; Shaulsky, E.; Dizge, N.; Warsinger, D.M.; Elimelech, M. Role of Ionic Charge Density in Donnan Exclusion of Monovalent Anions by Nanofiltration. Environ. Sci. Technol. 2018, 52, 4108–4116. [Google Scholar] [CrossRef] [PubMed]
- Bellona, C.; Drewes, J.E.; Xu, P.; Amy, G. Factors affecting the rejection of organic solutes during NF/RO treatment—A literature review. Water Res. 2004, 38, 2795–2809. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.-X.; Yang, H.-W.; Wang, X.-M.; Xie, Y.F. Assessment of the hindered transport model in predicting the rejection of trace organic compounds by nanofiltration. J. Membr. Sci. 2016, 498, 57–66. [Google Scholar] [CrossRef]
- Wang, X.-M.; Li, B.; Zhang, T.; Li, X.-Y. Performance of nanofiltration membrane in rejecting trace organic compounds: Experiment and model prediction. Desalination 2015, 370, 7–16. [Google Scholar] [CrossRef]
- Dong, L.X.; Huang, X.C.; Wang, Z.; Yang, Z.; Wang, X.M.; Tang, C.Y. A thin-film nanocomposite nanofiltration membrane prepared on a support with in situ embedded zeolite nanoparticles. Sep. Purif. Technol. 2016, 166, 230–239. [Google Scholar] [CrossRef]
- Fujioka, T.; Khan, S.J.; McDonald, J.A.; Nghiem, L.D. Nanofiltration of trace organic chemicals: A comparison between ceramic and polymeric membranes. Sep. Purif. Technol. 2014, 136, 258–264. [Google Scholar] [CrossRef]
- Roy, Y.; Warsinger, D.M.; Lienhard, J.H. Effect of temperature on ion transport in nanofiltration membranes: Diffusion, convection and electromigration. Desalination 2017, 420, 241–257. [Google Scholar] [CrossRef]
- Tang, C.; Bruening, M.L. Ion separations with membranes. J. Polym. Sci. 2020, 58, 2831–2856. [Google Scholar] [CrossRef]
- Emamjomeh, M.M.; Torabi, H.; Mousazadeh, M.; Alijani, M.H.; Gohari, F. Impact of independent and non-independent parameters on various elements’ rejection by nanofiltration employed in groundwater treatment. Appl. Water Sci. 2019, 9, 71. [Google Scholar] [CrossRef] [Green Version]
- Nicolini, J.V.; Borges, C.P.; Ferraz, H.C. Selective rejection of ions and correlation with surface properties of nanofiltration membranes. Sep. Purif. Technol. 2016, 171, 238–247. [Google Scholar] [CrossRef]
- Liu, Y.; Lin, B.; Liu, W.; Li, J.; Gao, C.; Pan, Q. Preparation and characterization of a novel nanofiltration membrane with chlorine-tolerant property and good separation performance. RSC Adv. 2018, 8, 36430–36440. [Google Scholar] [CrossRef] [Green Version]
- Tsuru, T.; Nakao, S.-I.; Kimura, S. Calculation of ion rejection by extended nernst-planck Equation with charged reverse osmosis membranes for single and mixed electrolyte solutions. J. Chem. Eng. Jpn. 1991, 24, 511–517. [Google Scholar] [CrossRef] [Green Version]
- Teorell, T. Transport Processes and Electrical Phenomena in Ionic Membranes. Prog. Biophys. Biophys. Chem. 1953, 3, 305–369. [Google Scholar] [CrossRef]
- Bowen, W.R.; Mukhtar, H. Characterisation and prediction of separation performance of nanofiltration membranes. J. Membr. Sci. 1996, 112, 263–274. [Google Scholar] [CrossRef]
- Deen, W.M.; Satvat, B.; Jamieson, J.M. Theoretical model for glomerular filtration of charged solutes. Am. J. Physiol. 1980, 7, 126–139. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Lin, S. Pore model for nanofiltration: History, theoretical framework, key predictions, limitations, and prospects. J. Membr. Sci. 2021, 620, 118809. [Google Scholar] [CrossRef]
- Yaroshchuk, A.E. Dielectric exclusion of ions from membranes. Adv. Colloid Interface Sci. 2000, 85, 193–230. [Google Scholar] [CrossRef]
- Oatley, D.L.; Llenas, L.; Pérez, R.; Williams, P.M.; Martínez-Lladó, X.; Rovira, M. Review of the dielectric properties of nanofiltration membranes and verification of the single oriented layer approximation. Adv. Colloid Interface Sci. 2012, 173, 1–11. [Google Scholar] [CrossRef]
- Bowen, W.R.; Welfoot, J.S. Modelling the performance of membrane nanofiltration-critical assessment and model development. Chem. Eng. Sci. 2002, 57, 1121–1137. [Google Scholar] [CrossRef]
- Fadaei, F.; Hoshyargar, V.; Shirazian, S.; Ashrafizadeh, S.N. Mass transfer simulation of ion separation by nanofiltration considering electrical and dielectrical effects. Desalination 2012, 284, 316–323. [Google Scholar] [CrossRef]
- Roy, Y.; Sharqawy, M.H.; Lienhard, J.H. Modeling of flat-sheet and spiral-wound nanofiltration configurations and its application in seawater nanofiltration. J. Membr. Sci. 2015, 493, 360–372. [Google Scholar] [CrossRef]
- Ghorbani, A.; Bayati, B.; Drioli, E.; Macedonio, F.; Kikhavani, T.; Frappa, M. Modeling of nanofiltration process using dspm-de model for purification of amine solution. Membranes 2021, 11, 230. [Google Scholar] [CrossRef] [PubMed]
- Deepak, D.; Arthanareeswaran, G. Modeling and Performance Characteristics of Nanofiltration by DSPM and ARX Model. J. Appl. Membr. Sci. Technol. 2017, 18, 1–7. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhu, H.; Li, G.; Mai, Z.; Gu, Y. The effect of dielectric exclusion on the rejection performance of inhomogeneously charged polyamide nanofiltration membranes. J. Nanopart. Res. 2019, 21, 217. [Google Scholar] [CrossRef]
- Szymczyk, A.; Fievet, P. Investigating transport properties of nanofiltration membranes by means of a steric, electric and dielectric exclusion model. J. Membr. Sci. 2005, 252, 77–88. [Google Scholar] [CrossRef]
- Saliha, B.; Patrick, F.; Anthony, S. Investigating nanofiltration of multi-ionic solutions using the steric, electric and dielectric exclusion model. Chem. Eng. Sci. 2009, 64, 3789–3798. [Google Scholar] [CrossRef]
- Chen, B.; Jiang, H.; Liu, X.; Hu, X. Molecular Insight into Water Desalination across Multilayer Graphene Oxide Membranes. ACS Appl. Mater. Interfaces 2017, 9, 22826–22836. [Google Scholar] [CrossRef]
- Cha-Umpong, W.; Hosseini, E.; Razmjou, A.; Zakertabrizi, M.; Korayem, A.H.; Chen, V. New molecular understanding of hydrated ion trapping mechanism during thermally-driven desalination by pervaporation using GO membrane. J. Membr. Sci. 2020, 598, 117687. [Google Scholar] [CrossRef]
- MacNaughton, S.J.; McCulloch, J.K.; Marshall, K.; Ring, R.J. Application of nanofiltration to the treatment of uranium mill effluents. In Technologies for the Treatment of Effluents from Uranium Mines, Mills and Tailings, Proceedings of a Technical Committee Meeting, Vienna, Austria, 1–4 November 1999; IAEA: Vienna, Austria, 2002; pp. 55–65. [Google Scholar]
- Yang, B.; Gu, K.; Wang, S.; Yi, Z.; Zhou, Y.; Gao, C. Chitosan nanofiltration membranes with gradient cross-linking and improved mechanical performance for the removal of divalent salts and heavy metal ions. Desalination 2021, 516, 115200. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, R.; Ji, M.; Lu, Y.; Zhu, Y.; Jin, J. Polyamide nanofiltration membrane with high mono/divalent salt selectivity via pre-diffusion interfacial polymerization. J. Membr. Sci. 2021, 636, 119478. [Google Scholar] [CrossRef]
- Geng, X.; Wang, J.; Ding, Y.; Zhang, W.; Wang, Y.; Liu, F. Poly(vinyl alcohol)/polydopamine hybrid nanofiltration membrane fabricated through aqueous electrospraying with excellent antifouling and chlorine resistance. J. Membr. Sci. 2021, 632, 119385. [Google Scholar] [CrossRef]
- Fang, L.F.; Zhou, M.Y.; Cheng, L.; Zhu, B.K.; Matsuyama, H.; Zhao, S. Positively charged nanofiltration membrane based on cross-linked polyvinyl chloride copolymer. J. Membr. Sci. 2019, 572, 28–37. [Google Scholar] [CrossRef]
- Mi, Z.; Liu, Z.; Jin, S.; Zhang, D.; Wang, D. Positively charged nanofiltration membrane prepared by polydopamine deposition followed by crosslinking for high efficiency cation separation. Polym. Test. 2021, 93, 107000. [Google Scholar] [CrossRef]
- Wei, X.Z.; Gan, Z.Q.; Shen, Y.J.; Qiu, Z.L.; Fang, L.F.; Zhu, B.K. Negatively-charged nanofiltration membrane and its hexavalent chromium removal performance. J. Colloid Interface Sci. 2019, 553, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Chang, H.; Gao, S.; Zhang, R. How to fabricate a negatively charged NF membrane for heavy metal removal via the interfacial polymerization between PIP and TMC? Desalination 2020, 491, 114499. [Google Scholar] [CrossRef]
- Yun, T.; Kwak, S.Y. Recovery of hydrochloric acid using positively-charged nanofiltration membrane with selective acid permeability and acid resistance. J. Environ. Manag. 2020, 260, 110001. [Google Scholar] [CrossRef]

| RO | NF | UF | MF | |
|---|---|---|---|---|
| Pore Size (µm) | ≥0.0001 | ≥0.001 | ≥0.01 | ≥0.1 |
| Operating pressure (kPa) | 1000–5000 | 500–1000 | 30–50 | 30–50 |
| Components removed | almost all dissolved compounds and suspended particles | polyvalent anions, cations, uncharged compounds, suspended particles | high molecular weight compounds and suspended particles | ideally only suspended particles are removed |
| Retain particulars (MW) | <350 | >150 | 1000–300,000 | >300,000 |
| Scope | Membrane Charged | Results | Refs. |
|---|---|---|---|
| NF-2012-250, polyamide thin-film composite membranes were used for the rejection of high divalent salt | Negative | CaSO4 > Na2SO4 > MgSO4 > MgCl2 > NaCl | [21] |
| Chitosan (CTS) and 1,3,5-triglycidyl isocyanurate (TGIC) were gradiently cross-linked on the polyethersulfone ultrafiltration membrane (PES) | Positive | MgCl2 > MgSO4 > NaCl > Na2SO4 | [87] |
| Polyamide (PA) nanofiltration membranes with high solute-solute selectivity were prepared via a pre-diffusion interfacial polymerization (PDIP) process | Negative | Na2SO4 > MgSO4 > MgCl2 > CaCl2 > NaCl | [88] |
| Poly (vinyl alcohol) (PVA)/polydopamine (PDA) hybrid nanofiltration membrane was fabricated through aqueous electrospraying | Negative | Na2SO4 > MgSO4 > NaCl | [89] |
| A polyvinyl chloride (PVC)-based nanofiltration membrane was fabricated from polyvinyl chloride-graft-poly(N,N-dimethylaminoethyl methacrylate) by heating and crosslinking treatment | Positive | MgCl2 > CaCl2 > NaCl > MgSO4 > Na2SO4 | [90] |
| Nanofiltration membranes were prepared by polydopamine (PDA) deposition followed by crosslinking on the polyethersulfone (PES) ultrafiltration (UF) membrane substrate. | Positive | MgCl2 > CaCl2 > NaCl > MgSO4 > Na2SO4 | [91] |
| A membrane was fabricated via introducing 2, 5 diaminobenzenesulfonic acid (DABSA) into the polyamide layer | Negative | Na2SO4 > MgSO4 > NaCl > MgCl2 | [92] |
| Polyamide (PA) thin film composite (TFC) membranes were prepared by interfacial polymerisation (IP) technique with trimesoyl chloride (TMC) and three acyl chloride groups | Negative | MgSO4 > Na2SO4 > MgCl2 > NaCl | [93] |
| Thermally cross-linked branched-polyethyleneimine (b-PEI) layer was introduced to a loose polyethersulfone NF membrane by dip-coating b-PEI and an epoxy linker and heat treatment in a sealed oven with a high-humidity atmosphere | Positive | MgCl2 > MgSO4 > NaCl > Na2SO4 | [94] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suhalim, N.S.; Kasim, N.; Mahmoudi, E.; Shamsudin, I.J.; Mohammad, A.W.; Mohamed Zuki, F.; Jamari, N.L.-A. Rejection Mechanism of Ionic Solute Removal by Nanofiltration Membranes: An Overview. Nanomaterials 2022, 12, 437. https://doi.org/10.3390/nano12030437
Suhalim NS, Kasim N, Mahmoudi E, Shamsudin IJ, Mohammad AW, Mohamed Zuki F, Jamari NL-A. Rejection Mechanism of Ionic Solute Removal by Nanofiltration Membranes: An Overview. Nanomaterials. 2022; 12(3):437. https://doi.org/10.3390/nano12030437
Chicago/Turabian StyleSuhalim, Nur Syahirah, Norherdawati Kasim, Ebrahim Mahmoudi, Intan Juliana Shamsudin, Abdul Wahab Mohammad, Fathiah Mohamed Zuki, and Nor Laili-Azua Jamari. 2022. "Rejection Mechanism of Ionic Solute Removal by Nanofiltration Membranes: An Overview" Nanomaterials 12, no. 3: 437. https://doi.org/10.3390/nano12030437
APA StyleSuhalim, N. S., Kasim, N., Mahmoudi, E., Shamsudin, I. J., Mohammad, A. W., Mohamed Zuki, F., & Jamari, N. L.-A. (2022). Rejection Mechanism of Ionic Solute Removal by Nanofiltration Membranes: An Overview. Nanomaterials, 12(3), 437. https://doi.org/10.3390/nano12030437










