Supramolecular Self-Assembly of Atomically Precise Silver Nanoclusters with Chiral Peptide for Temperature Sensing and Detection of Arginine
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Self-Assembly of Ag9-NCs/DD-5 Hydrogel
2.3. The Detection of L-Arg and D-Arg
2.4. Characterizations
3. Results
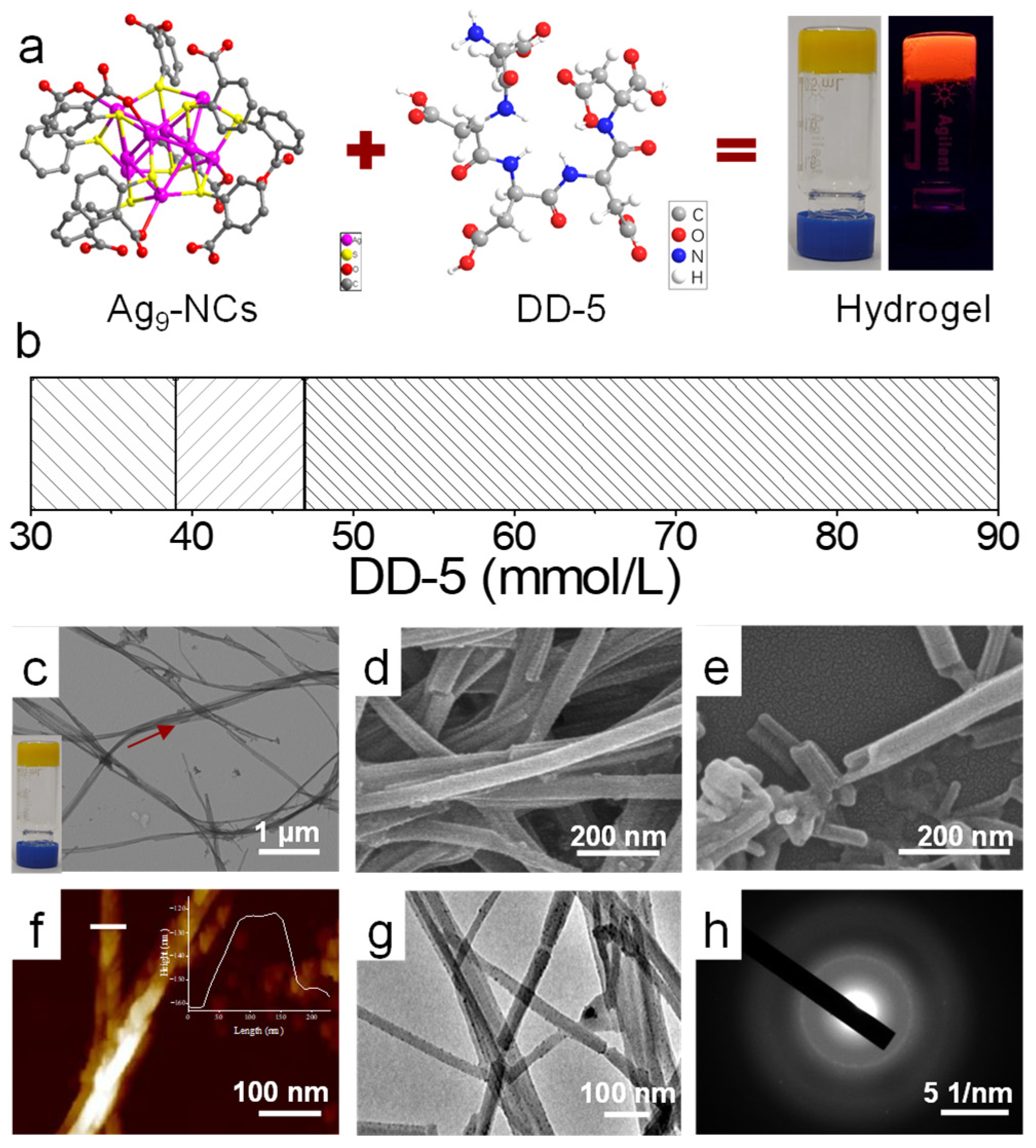
3.1. Self-Assembly of Ag9-NCs/DD-5 Hydrogel
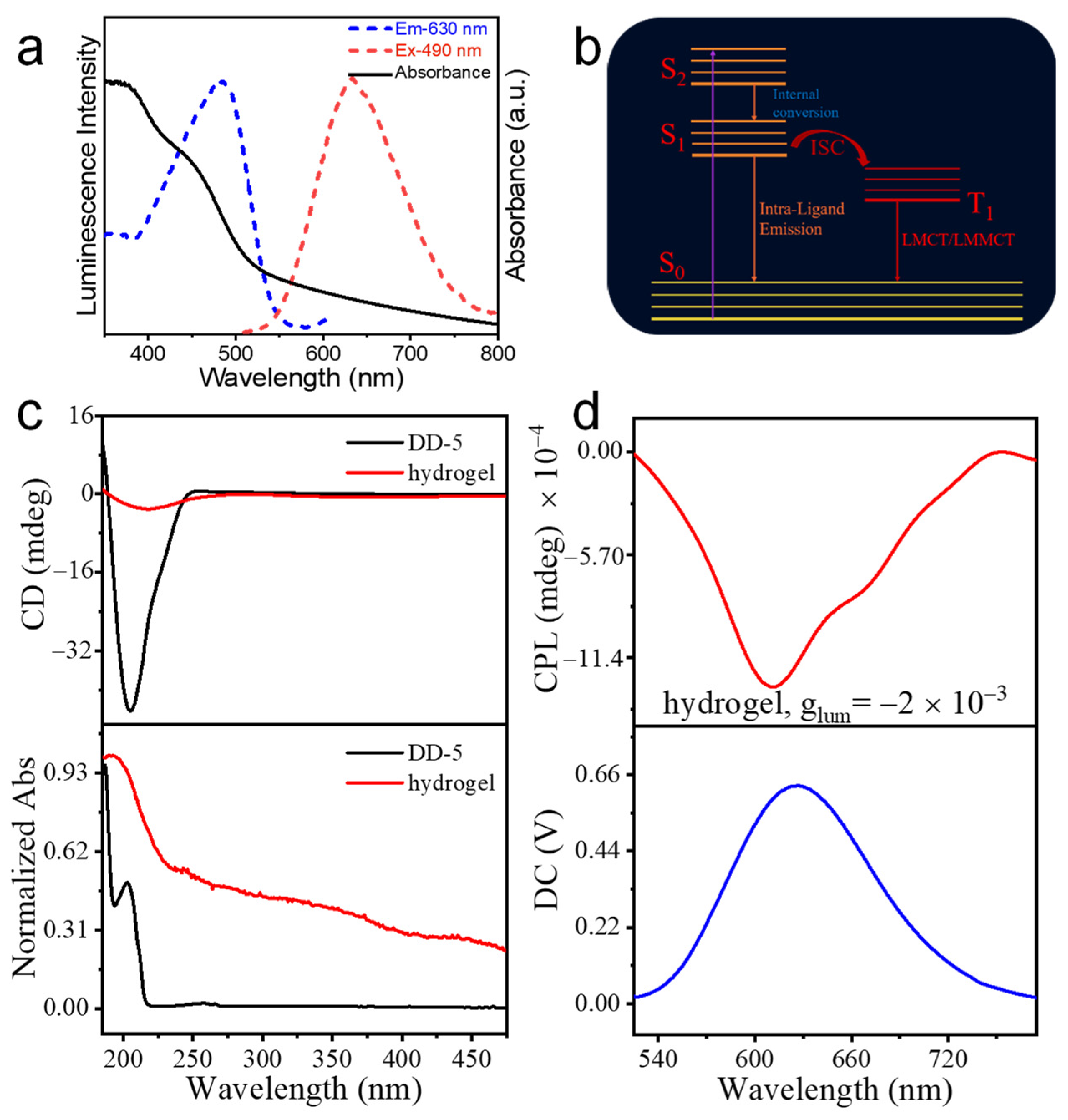
3.2. Fluorescence and Chirality of Ag9-NCs/DD-5 Hydrogel
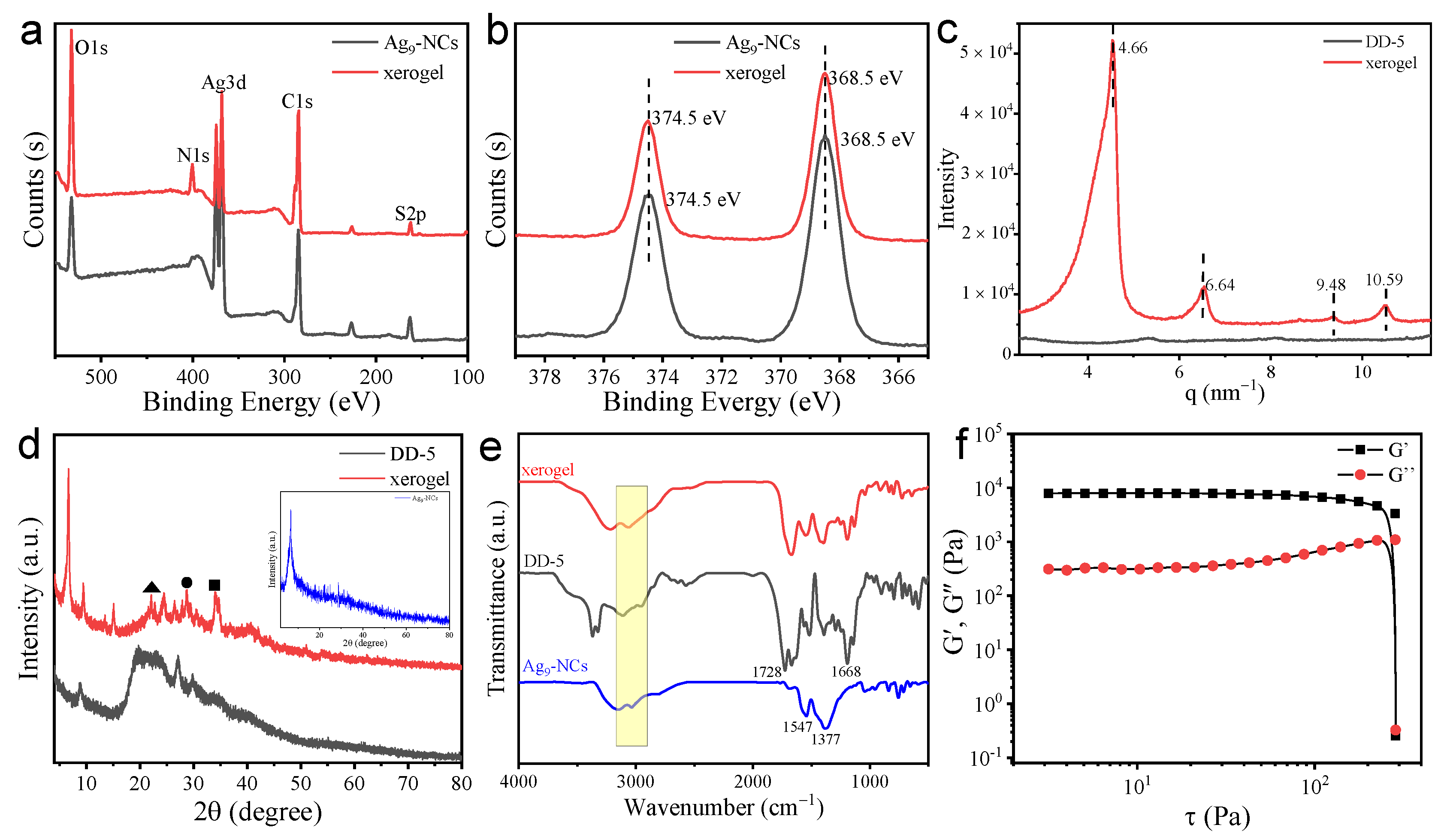
3.3. Structure and Mechanism Analysis of the Hydrogel
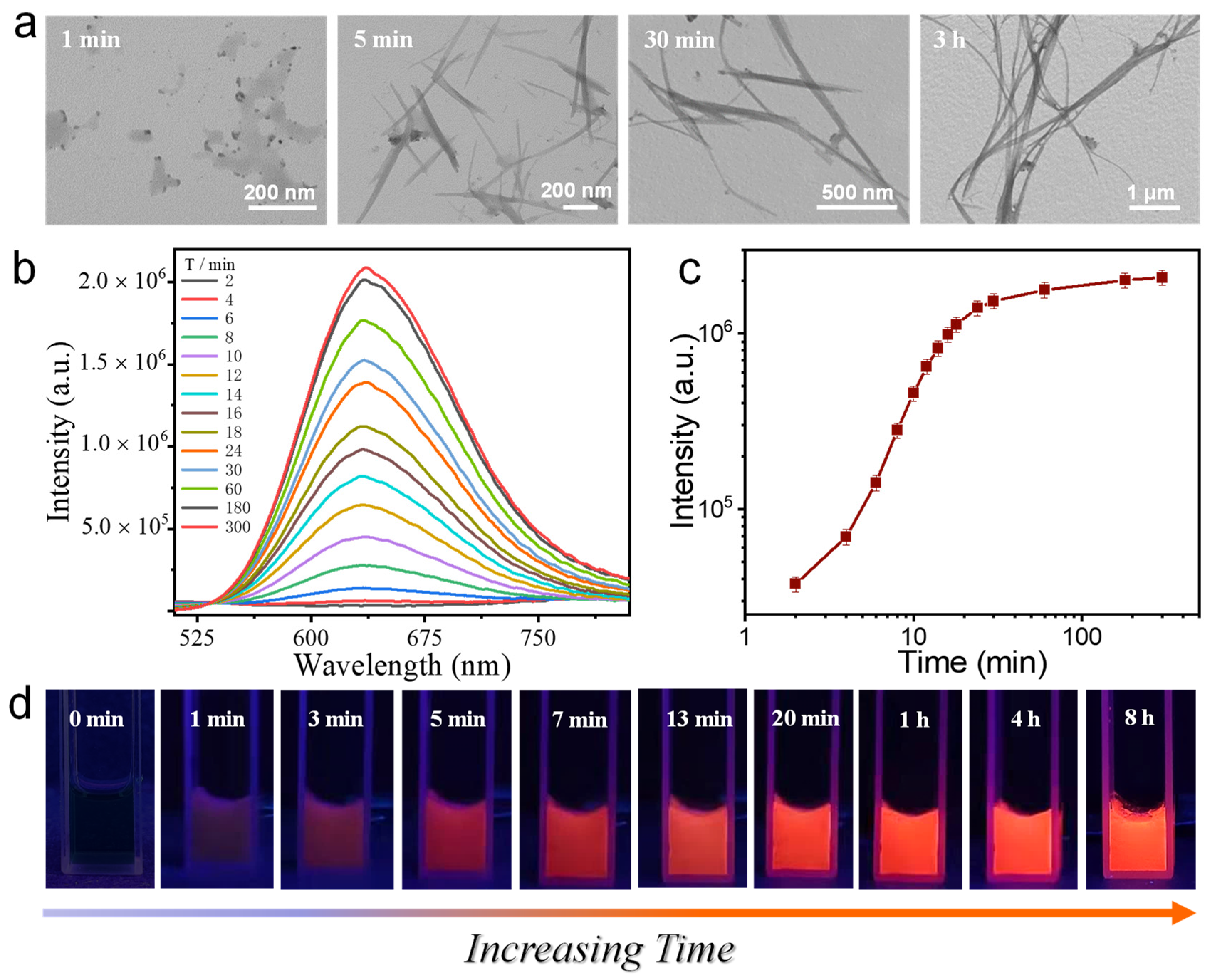
3.4. Kinetic Tracing of the Formation of Ag9-NCs/DD-5 Hydrogel
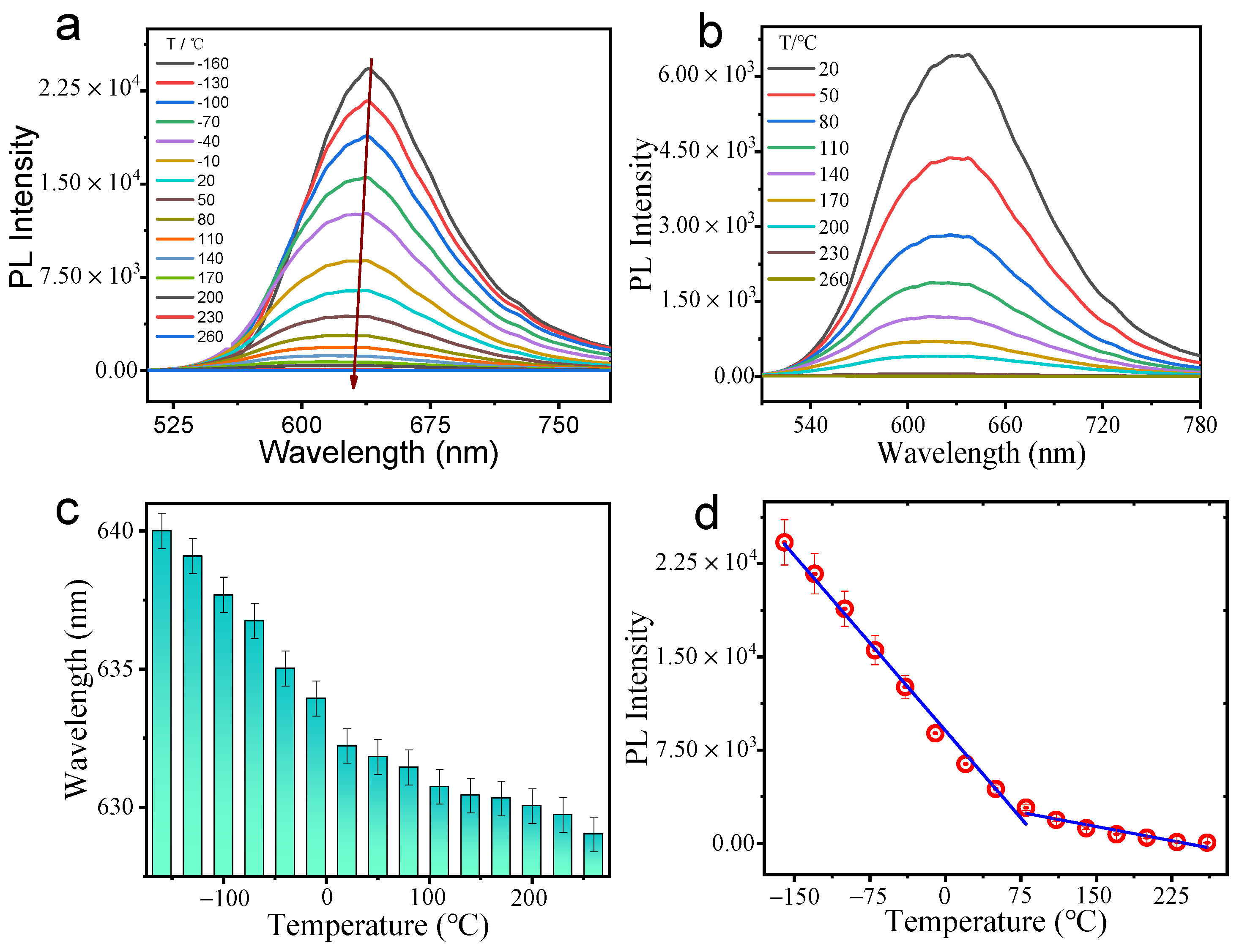
3.5. Temperature Sensing
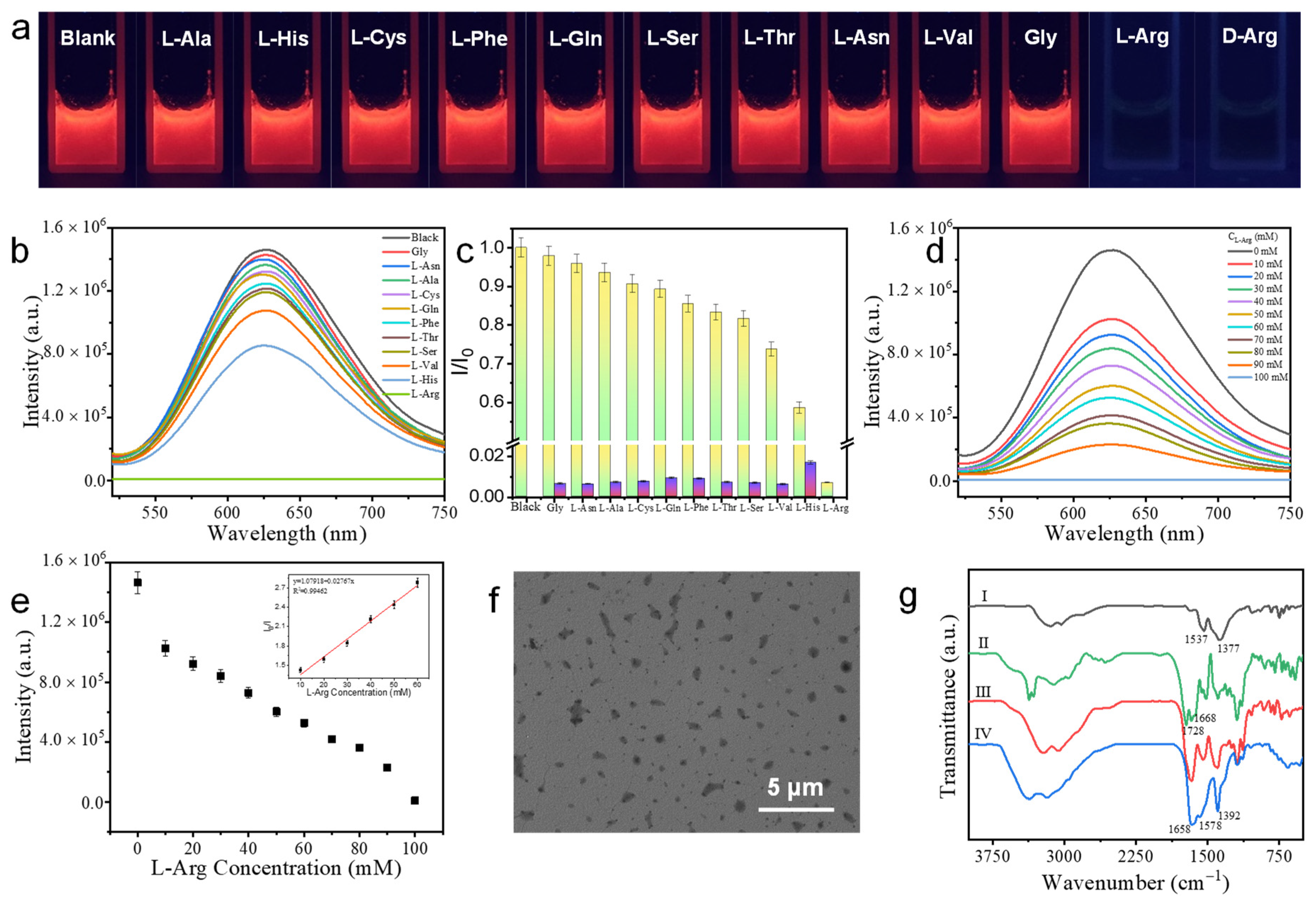
3.6. The Detection of L-Arg and D-Arg
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kailasa, S.; Borse, S.; Koduru, J.; Muthy, Z. Biomolecules as Promising Ligands in the Synthesis of Metal Nanoclusters: Sensing, Bioimaging and Catalytic Applications. Trends Environ. Anal. Chem. 2021, 32, e00104. [Google Scholar] [CrossRef]
- Han, S.; Zhao, Y.; Zhang, Z.; Xu, G. Recent Advances in Electrochemiluminescence and Chemiluminescence of Metal Nanoclusters. Molecules 2020, 20, 5208. [Google Scholar] [CrossRef] [PubMed]
- Shan, P.; Yang, J.; Zang, Z.; Zhao, Q.; Cheng, Y.; Li, L.; Yang, X.; Yu, X.; Lu, Z.; Zhang, X. Effects of Silver Nanoclusters on the Spectral Properties for Fluorescein Isothiocyanate with Restrained Photobleaching. Appl. Surf. Sci. 2021, 548, 14928–14938. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, C.; Xu, C.; Wang, X.; Liu, C.; Waterhouse, G.I.N.; Wang, Y.; Yin, H. Ultrasmall Au Nanoclusters for Biomedical and Biosensing Applications: A Mini-review. Talanta 2019, 200, 432–442. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhu, J.; Xu, K. Fluorescent Metal Nanoclusters: From Synthesis to Applications. TrAC Trends Anal. Chem. 2014, 58, 90–98. [Google Scholar] [CrossRef]
- Xie, Z.; Sun, P.; Wang, Z.; Li, H.; Yu, L.; Sun, D.; Chen, M.; Bi, Y.; Xin, X.; Hao, H. Metal-Organic Gels from Silver Nanoclusters with Aggregation-Induced Emission and Fluorescence-to-Phosphorescence Switching. Angew. Chem. Int. Ed. 2020, 59, 9922–9927. [Google Scholar] [CrossRef]
- Sun, P.; Wang, Z.; Bi, Y.; Sun, D.; Zhao, T.; Zhao, F.; Wang, W.; Xin, X. Self-assembly Driven Aggregation-Induced Emission of Silver Nanoclusters for Light Conversion and Temperature Sensing. ACS Appl. Nano Mater. 2020, 3, 2038–2046. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, X.; Gao, H.; Huang, Y. Cu-Based Metal-Organic Frameworks-Derived Copper Nanoclusters with Tunable Emission for Ratiometric pH Sensing. Sens. Actuators B Chem. 2022, 353, 131130–131140. [Google Scholar] [CrossRef]
- Desai, M.; Basu, H.; Saha, S.; Singhal, R.; Kailasa, S. Fluorescence Enhancement of Bovine Serum Albumin Gold Nanoclusters from La3+ Ion: Detection of Four Divalent Metal Ions (Hg2+, Cu2+, Pb2+ and Cd2+). J. Mol. Liq. 2021, 336, 116239–116249. [Google Scholar] [CrossRef]
- Borse, S.; Murthy, Z.; Park, T.; Kailasa, S. Lysozyme-Decorated Gold and Molybdenum Bimetallic Nanoclusters for the Selective Detection of Bilirubin as a Jaundice Biomarker. ACS Appl. Nano Mater. 2021, 4, 11949–11959. [Google Scholar] [CrossRef]
- Wen, Q.; Peng, J.; Liu, A.; Hu, Y.; Wang, J.; Ling, J.; Cao, Q. Fluorescent Silver Nanoclusters Stabilized in Guanine-Enhanced DNA Hybridization for Recognizing Different Small Biological Molecules. J. Lumin. 2020, 221, 117038–117046. [Google Scholar] [CrossRef]
- Zhang, S.; Su, H.; Wang, Z.; Wang, L.; Zhao, Q.; Tung, C.; Sun, D.; Zheng, L. Anion-Templated Nanosized Silver Alkynyl Clusters: Cluster Engineering and Solution Behavior. Chem. Eur. J. 2017, 23, 3432–3437. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Li, J.; Wang, E. Supramolecular Self-assembly of Morphology-Dependent Luminescent Ag Nanoclusters. Chem. Commun. 2014, 50, 9565–9568. [Google Scholar] [CrossRef] [PubMed]
- Khanra, S.; Abdullah-Al Mamun, M.; Ferreira, F.F.; Ghosh, K.; Guha, S. Functionalized Self-Assembled Peptide Nanotubes with Cobalt Ferrite Nanoparticles for Applications in Organic Electronics. ACS Appl. Nano Mater. 2018, 1, 1175–1187. [Google Scholar] [CrossRef]
- Khan, J.M.; Khan, M.S.; Qadeer, A.; Alsenaidy, M.A.; Ahmed, A.; Al-Shabib, N.A.; Khan, R.H. Cationic Gemini Surfactant (16-4-16) Interact Electrostatically with Anionic Plant Lectin and Facilitates Amyloid Fibril Formation at Neutral pH. Colloids Surf. A Physicochem. Eng. Asp. 2017, 522, 494–502. [Google Scholar] [CrossRef]
- Fatouros, D.G.; Lamprou, D.A.; Urquhart, A.J.; Yannopoulos, S.N.; Viziranakis, I.S.; Zhang, S.; Koutsopoulos, S. Lipid-like Self-Assembling Peptide Nanovesicles for Drug Delivery. ACS Appl. Mater. Interfaces 2014, 6, 8184–8189. [Google Scholar] [CrossRef]
- Xing, R.; Li, S.; Zhang, N.; Shen, G.; Mohwald, H.; Yan, X. Self-Assembled Injectable Peptide Hydrogels Capable of Triggering Antitumor Immune Response. Biomacromolecules 2017, 18, 3514–3523. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, M.; Wang, X.; Chen, Y.; Yan, Y.; Zhang, L.; Zhang, L. Peptide-Modified Chitosan Hydrogels Promote Skin Wound Healing by Enhancing Wound Angiogenesis and Inhibiting Inflammation. Am. J. Transl. Res. 2017, 9, 2352–2362. [Google Scholar]
- Xing, R.; Zou, Q.; Yan, X. Peptide-Based Supramolecular Colloids. Acta Phys. Chim. Sin. 2020, 36, 1909048–1909064. [Google Scholar]
- Shen, J.; Xiao, Q.; Sun, P.; Feng, J.; Xin, X.; Yu, Y.; Qi, W. Self-Assembled Chiral Phosphorescent Microflowers from Au Nanoclusters with Dual-Mode pH Sensing and Information Encryption. ACS Nano 2017, 15, 4947–4955. [Google Scholar] [CrossRef]
- Liu, J.; Yu, Y.; Wang, C.; Shen, J.; Feng, J.; Qi, W. Fabrication of a Chiral Luminescent Hydrogel from Gold Nanoclusters via Molecular Recognition. Chem. Commun. 2021, 57, 10202–10205. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, P.; Ghosh, M.; Schnaider, L.; Adadi, N.; Ji, W.; Bychenko, D.; Dvir, T.; Adler-Abramovich, L.; Gazit, E. Composite of Peptide-Supramolecular Polymer and Covalent Polymer Comprises a New Multifunctional, Bio-Inspired Soft Material. Macromol. Rapid Commun. 2019, 40, 1900175. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Sun, Y.; Wang, J.; Xu, H.; Lu, J. Copper (II)-Mediated Self-Assembly of Hairpin Peptides and Templated Synthesis of CuS Nanowires. Chem. Asian J. 2015, 10, 1953–1958. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Wang, Z.; Sun, D.; Bai, H.; Zhu, Z.; Bi, Y.; Zhao, T.; Xin, X. pH-Guided Self-Assembly of Silver Nanoclusters with Aggregation-Induced Emission for Rewritable Fluorescent Platform and White Light Emitting Diode Application. J. Colloid Interface Sci. 2020, 564, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.; Wang, Z.; Liu, T.; Sun, D.; Godbert, N.; Li, H.; Hao, J.; Xin, X. Supramolecular Chirality from Hierarchical Self-assembly of Atomically Precise Silver Nanoclusters Induced by Secondary Metal Coordination. ACS Nano 2021, 15, 15910–15919. [Google Scholar] [CrossRef] [PubMed]
- Heaven, M.W.; Dass, A.; White, P.S.; Holt, K.M.; Murray, R.W. Crystal Structure of the Gold Nanoparticle [N(C8H17)4] [Au25(SCH2CH2Ph)18]. J. Am. Chem. Soc. 2008, 130, 3754–3755. [Google Scholar] [CrossRef] [PubMed]
- Bolton, O.; Lee, K.; Kim, H.; Lin, K.; Kim, J. Activating Efficient Phosphorescence from Purely Organic Materials by Crystal Design. Nat. Chem. 2011, 3, 205–210. [Google Scholar] [CrossRef]
- Ni, W.; Qiu, Y.; Li, M.; Zheng, J.; Sun, R.W.Y.; Zhan, S.; Ng, S.W.; Li, D. Metallophilicity-Driven Dynamic Aggregation of a Phosphorescent Gold(I)-Silver(I) Cluster Prepared by Solution-Based and Mechanochemical Approaches. J. Am. Chem. Soc. 2014, 136, 9532–9535. [Google Scholar] [CrossRef]
- Pyo, K.; Thanthirige, V.D.; Kwak, K.; Pandurangan, P.; Ramakrishna, P.; Lee, D. Ultrabright Luminescence from Gold Nanoclusters: Rigidifying the Au(I)-Thiolate Shell. J. Am. Chem. Soc. 2015, 137, 8244–8250. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, J.; Deng, L.; Zhou, P.; Wang, S.; Wang, Y.; Xu, H.; Lu, J.R. Tuning the Self-Assembly of Short Peptides via Sequence Variations. Langmuir 2013, 29, 13457–13464. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, Y.; Guo, Z.; Ren, J.; Wang, Y.; Peng, H. Flexible, Stretchable, and Rechargeable Fiber-Shaped Zinc-Air Battery Based on Cross-Stacked Carbon Nanotube Sheets. Angew. Chem. Int. Ed. 2015, 54, 15390–15394. [Google Scholar] [CrossRef] [PubMed]
- Barreiro, E.; Casas, J.S.; Couce, M.D.; Laguna, A.; Lopez-de-Luzuriaga, J.M.; Monge, M.; Sanchez, A.; Sordo, J.; Lopez, E.M.V. A Novel Hexanuclear Silver(I) Cluster Containing a Regular Ag-6 Ring with Short Ag-Ag Distances and an Argentophilic Interaction. Dalton Trans. 2013, 42, 5916–5923. [Google Scholar] [CrossRef]
- Wu, Z.; Du, Y.; Liu, J.; Yao, Q.; Chen, T.; Cao, Y.; Zhang, H.; Xie, J. Aurophilic Interactions in the Self-Assembly of Gold Nanoclusters into Nanoribbons with Enhanced Luminescence. Angew. Chem. Int. Ed. 2019, 58, 8139–8144. [Google Scholar] [CrossRef] [PubMed]
- Cursino, A.C.T.; Gardolinski, J.E.F.D.; Wypch, F. Intercalation of Anionic Organic Ultraviolet Ray Absorbers into Layered Zinc Hydroxide Nitrate. J. Colloid Interface Sci. 2010, 347, 49–55. [Google Scholar] [CrossRef]
- Moitessier, N.; Chapleur, Y. Modulation of the Relative Reactivities of Carbohydrate Secondary Hydroxyl Groups Modification of the Hydrogen Bond Network. Tetrahedron Lett. 2003, 44, 1731–1735. [Google Scholar] [CrossRef]
- Shen, J.; Wang, Z.; Sun, D.; Liu, G.; Yuan, S.; Kurmoo, M.; Xin, X. Self-Assembly of Water-Soluble Silver Nanoclusters: Superstructure Formation and Morphological Evolution. Nanoscale 2017, 9, 19191–19200. [Google Scholar] [CrossRef]
- Shen, X.; Wang, Y.; Zhao, E.; Yuan, W.; Liu, Y.; Lu, P.; Qin, A.; Ma, Y.; Sun, J.; Tang, B. Effects of Substitution with Donor-Acceptor Groups on the Properties of Tetraphenylethene Trimer: Aggregation-Induced Emission, Solvatochromism, and Mechanochromism. J. Phys. Chem. C 2013, 117, 7334–7347. [Google Scholar] [CrossRef]
- Li, J.; Zhang, H.; Zhang, Y.; Zhou, W.; Liu, Y. Room-Temperature Phosphorescence and Reversible White Light Switch Based on a Cyclodextrin Polypseudorotaxane Xerogel. Adv. Opt. Mater. 2019, 7, 1900589. [Google Scholar] [CrossRef]
- Zhu, Y.; Guan, Y.; Niu, Y.; Wang, P.; Chen, R.; Wang, Y.; Wang, P.; Xie, H. Ultralong Polymeric Room Temperature Phosphorescence Materials Fabricated by Multiple Hydrogen Bondings Resistant to Temperature and Humidity. Adv. Opt. Mater. 2021, 9, 2100782. [Google Scholar] [CrossRef]
- Wu, H.; Gu, L.; Baryshnikov, G.V.; Wang, H.; Minaev, B.F.; Agren, H.; Zhao, Y. Molecular Phosphorescence in Polymer Matrix with Reversible Sensitivity. ACS Appl. Mater. Interfaces 2020, 12, 20765–20774. [Google Scholar] [CrossRef]
- Gorren, A.C.F.; Schmidt, K.; Mayer, B. Binding of L-arginine and Imidazole Suggests Heterogeneity of Rat Brain Neuronal Nitric Oxide Synthase. Biochemistry 2002, 41, 7819–7829. [Google Scholar] [CrossRef] [PubMed]
- Senkevitch, E.; Cabrera-Luque, J.; Morizono, H.; Caldovic, L.; Tuchman, M. A Novel Biochemically Salvageable Animal Model of Hyperammonemia Devoid of N-acetylglutamate Synthase. Mol. Genet. Genom. 2012, 106, 160–168. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kapila, S.; Saba, M.; Lin, C.; Bawle, E.V. Arginine Deficiency-Induced Hyperammonemia in a Home Total Parenteral Nutrition-Dependent Patient: A Case Report. JPEN J. Parenter. Enter. Nutr. 2001, 25, 286–288. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Wang, Z.; Sun, D.; Li, S.; Deng, Q.; Xin, X. Supramolecular Self-Assembly of Atomically Precise Silver Nanoclusters with Chiral Peptide for Temperature Sensing and Detection of Arginine. Nanomaterials 2022, 12, 424. https://doi.org/10.3390/nano12030424
Wang W, Wang Z, Sun D, Li S, Deng Q, Xin X. Supramolecular Self-Assembly of Atomically Precise Silver Nanoclusters with Chiral Peptide for Temperature Sensing and Detection of Arginine. Nanomaterials. 2022; 12(3):424. https://doi.org/10.3390/nano12030424
Chicago/Turabian StyleWang, Wenjuan, Zhi Wang, Di Sun, Shulin Li, Quanhua Deng, and Xia Xin. 2022. "Supramolecular Self-Assembly of Atomically Precise Silver Nanoclusters with Chiral Peptide for Temperature Sensing and Detection of Arginine" Nanomaterials 12, no. 3: 424. https://doi.org/10.3390/nano12030424
APA StyleWang, W., Wang, Z., Sun, D., Li, S., Deng, Q., & Xin, X. (2022). Supramolecular Self-Assembly of Atomically Precise Silver Nanoclusters with Chiral Peptide for Temperature Sensing and Detection of Arginine. Nanomaterials, 12(3), 424. https://doi.org/10.3390/nano12030424






